Abstract
Background
Phlebotomine insects harbor bacterial, viral and parasitic pathogens that can cause diseases of public health importance. Lutzomyia longipalpis is the main vector of visceral leishmaniasis in the New World. Insects can mount a powerful innate immune response to pathogens. Defensin peptides take part in this response and are known to be active against Gram-positive and Gram-negative bacteria, and some parasites. We studied the expression of a defensin gene from Lutzomyia longipalpis to understand its role in sand fly immune response.
Methods
We identified, sequenced and evaluated the expression of a L. longipalpis defensin gene by semi-quantitative RT-PCR. The gene sequence was compared to other vectors defensins and expression was determined along developmental stages and after exposure of adult female L. longipalpis to bacteria and Leishmania.
Results
Phylogenetic analysis showed that the L. longipalpis defensin is closely related to a defensin from the Old World sand fly Phlebotomus duboscqi. Expression was high in late L4 larvae and pupae in comparison to early larval stages and newly emerged flies. Defensin expression was modulated by oral infection with bacteria. The Gram-positive Micrococcus luteus induced early high defensin expression, whilst the Gram-negative entomopathogenic Serratia marcescens induced a later response. Bacterial injection also induced defensin expression in adult insects. Female sand flies infected orally with Leishmania mexicana showed no significant difference in defensin expression compared to blood fed insects apart from a lower defensin expression 5 days post Leishmania infection. When Leishmania was introduced into the hemolymph by injection there was no induction of defensin expression until 72 h later.
Conclusions
Our results suggest that L. longipalpis modulates defensin expression upon bacterial and Leishmania infection, with patterns of expression that are distinct among bacterial species and routes of infection.
Keywords: Lutzomyia longipalpis, Defensin, Leishmania, Bacteria
Background
Sand flies are vectors of bacterial and parasitic diseases such as bartonellosis and leishmaniasis [1,2]. Lutzomyia longipalpis is the main vector of Leishmania infantum chagasi, the causative agent of visceral leishmaniasis in South America [2]. Although little is known about sand fly responses to bacterial infection, several studies have focused on molecular events that occur during the establishment of Leishmania infection in the insect [3]. Leishmania molecules such as lipophosphoglycan (LPG) [4] and chitinase [5] have been shown to contribute to the success of Leishmania infections in sand flies. Additionally, sand fly molecules such as galectin receptors [6], digestive proteases [7,8] and a physical barrier such as the peritrophic matrix [9] have been shown to have an important role in Leishmania survival within the sand fly gut.
Several studies have described the natural gut microbiota in Old World [10-12] and New World sand flies [13-16] although mechanisms by which sand flies control the microbial balance in the gut are still unknown.
Insects are capable of mounting a complex repertoire of immune responses to maintain gut homeostasis and eliminate pathogens. Cellular responses include phagocytosis by hemocytes and melanotic encapsulation of pathogenic microorganisms through the activation of the phenoloxidase cascade [17]. Humoral responses, on the other hand, lead to the synthesis of a wide range of effector molecules, including antimicrobial peptides (AMPs) [18-20]. AMPs have been described in many insects as having a central role in innate immune responses against bacterial and parasitic infections [21-23]. Among these, defensin (a 4 kDa cationic peptide) has been identified in several insects [23-26] and shown to have a deleterious effect on bacteria [26], Plasmodium[27] and Leishmania[28,29]. Defensin was shown to be upregulated in Phlebotomus duboscqi upon Leishmania major infection [29]. Here we identified, sequenced and investigated the expression profile of a L. longipalpis defensin throughout the sand fly developmental stages, after Gram-positive and Gram-negative bacterial challenges and after sand fly infection with Leishmania mexicana.
Methods
Defensin gene sequence analysis
Partial L. longipalpis defensin gene (LlDef1) sequences were obtained from our previous database [30,31] and the full genomic sequence was obtained using primers designed to target the 5'UTR and 3'UTR regions (LlDef1F 5’-TTGGTCATAGCGTGCAGAAG-3’ and LlDef1R 5’-AAAAACATTGAAACATGCGACTT-3’). Sequence identity was determined by similarity using BLAST searches [32] against the NCBI database. Multiple alignments were performed using the MAFFT software [33]. Phylogenetic tree analysis was done using MEGA5 software [34] with Neighbor-Joining test, using the p-distance method with complete deletion and 10,000 replicates for bootstrap value. The molecular model of the L. longipalpis defensin was built based on the tertiary structure of the Anopheles gambiae [PDB:2NY8] [35] and Phormia terranovae [PDB:1ICA] [36] peptides present in the Protein Data Bank (PDB) [37]. The defensin sequence of L. longipalpis and A. gambiae were deposited on the molecular modeling server of the SWISS-MODEL (Automated Comparative Protein Modeling Server) [38,39] for the creation of a 3D prediction structure. The two structures were visually analyzed using the Swiss PDB Viewer 3.7 [40].
Insects
All experiments were performed using insects from a laboratory colony of L. longipalpis established from sand flies caught in Jacobina (Bahia, Brazil) using standard methods [41]. Insects were fed on 70% sucrose ad libitum and fed on rabbit blood once a week. The insectary was kept under controlled conditions of temperature (27 ± 1°C), humidity (80–95%), and photoperiod (12 h/12 h). All procedures involving animals were performed in accordance with the UK Government (Home Office), HSE and EC regulations.
Experimental bacterial feeds
Escherichia coli (K12 RM148), Micrococcus luteus (A270), Ochrobactrum sp. (OM1,198 Jacobina colony isolate), Pantoea agglomerans (NCIMB11392), and Serratia marcescens (NCIMB 1377) were inoculated on Luria-Bertani (LB) agar plates and incubated overnight for 24 hours at 37°C. Single colonies were transferred to polypropylene tubes, grown overnight in LB liquid medium, centrifuged at 13,200 rpm, re-suspended in 20% sucrose to OD600 = 0.2 and offered daily to female L. longipalpis through cotton wool. All bacteria were viable under these conditions over the duration of the experiment. Bacterial feed experiments were performed in parallel, one for each bacteria species, collecting 3 pools of 3 females each.
Leishmania infections
Leishmania infections were performed as previously described [42]. In brief, L. mexicana (strain MNYC/BZ/62/M379) were cultured at 26°C in M199 medium supplemented with 25 μg/mL gentamicin sulphate (Sigma), 1X BME vitamins (Gibco) and 10% fetal calf serum (PAA). In preparation for infection, 2 mL of heat-inactivated (56°C for 1 hour) rabbit blood was used to re-suspend cultured promastigotes to a final concentration of 2 × 106 promastigotes/mL. Rabbit blood seeded with parasites was offered to L. longipalpis through chick skin feeders and fully engorged flies were transferred to fresh cages. Sand flies were dissected at 5 days post-infection to confirm successful infections. Control flies were fed on rabbit blood only. The infection experiment was performed once, collecting 3 pools of 3 females each.
Microinjections
Newly emerged L. longipalpis were microinjected in the thorax with 18 nL of E. coli culture in LB medium at OD600 = 0.2 or 2 × 106/mL L. mexicana promastigotes using a Nanoject II microinjector. Control flies were either pricked in the thorax with a borosilicate needle or injected with 18 nL of autoclaved LB medium.
RNA extractions and RT-PCR
Total RNA was extracted from triplicate samples derived from pools of 3 whole larvae or adult L. longipalpis using TRI Reagent (Ambion). Semi-quantitative RT-PCR was performed using SuperScript III One-Step RT-PCR Platinum TaqHiFi (Invitrogen) according to manufacturer’s instructions, with 10 ng of template RNA and defensin-specific primers [30] (Defensin F 5’-GCCTGTGTGTTGTGGTTCT-3’; Defensin R 5’- GCATCTCCCCATCCTGTT-3’). Gene transcription was normalized based on the 60S ribosomal protein L3 gene from L. longipalpis [GenBank: AM088777]. RT-PCR products were resolved on 2% agarose/ethidium bromide gels and band intensity was determined by densitometric measurement using the Image J software [43]. Differential transcription of genes was determined by the ratio between target gene band intensity and the corresponding 60S L3 products obtained from multiplex RT-PCR reactions.
Statistical analysis
Statistical t-test analysis was performed using the GraphPad Prism software (San Diego, CA, USA). Results were expressed as mean ± SEM. Significance was considered when P < 0.05.
Results
Defensin gene sequence and phylogeny
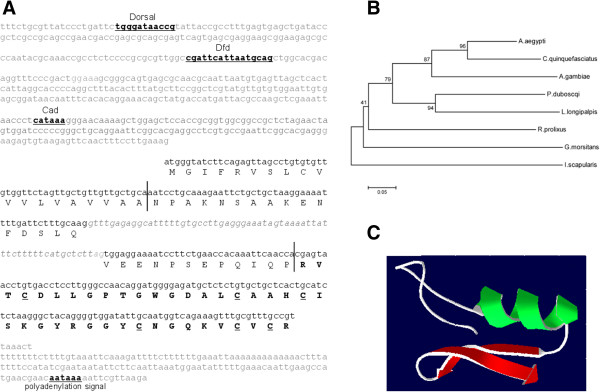
The LlDef1 sequence was shown to contain 1034 nucleotides (nt) with the coding region between nucleotides 512 and 837 and an intron located between nucleotides 617 and 681 (Figure 1A). The 5'UTR sequence displayed putative binding sites for dorsal, caudal and HSF transcription factors and a polyadenylation signal site was found in the 3'UTR. The amino acid prediction indicates an 87 residues peptide, from which 40 correspond to the mature peptide (4.23 kDa) (Figure 1A).
Figure 1.
Sequence, phylogenetic analysis, and molecular modeling of Lutzomyia longipalpis defensin 1 (LlDef1). (A) The complete genomic sequence of the Lutzomyia longipalpis LlDef1 defensin gene containing 1034 nucleotides (nt) is shown. The 5'UTR region contains 518 nt and the 3'UTR 153 nt (lower case gray letters). In the 5'UTR region, potential binding sites for transcription factors Dorsal, Dfd and Caudal are shown (underlined bold lower case letters). The LlDef1 coding region contains 363 nucleotides with a 63 nt intron (gray lower case italic letters). The amino acid prediction indicates an 87 residues peptide (upper case letters), from which 40 correspond to the mature peptide (bold upper case letters). The first vertical bar limits the signal peptide and the second vertical bar divides the pre and pro-peptide. The 6 cysteines of the pro-peptide with the potential to generate 3 disulfide bonds are underlined and the polyadenylation site is indicated. (B) Neighbour-joining tree based on multi alignment created from defensins predicted amino acid sequences of L. longipalpis [JQ970473], A. aegypti [P81602.2], A. gambiae [AAC18575.1], P. duboscqi [P83404.3], Rhodnius prolixus [AAO74624.1], Glossina morsitans [Q8WTD4.1], Culex quinquefasciatus [AEQ27735.1] and Ixodes scapularis [XP_002401521.1], showing the phylogenetic relationship between L. longipalpis and other insect defensins. (C) Putative tertiary structure of the L. longipalpis defensin showing the characteristic architecture of arthropod defensins with two anti-parallel β-sheets (red) and an α-helix (green).
Multiple alignment analysis indicated conserved regions among all defensin sequences selected from blood feeding arthropods (data not shown) and the phylogenetic analysis showed that the L. longipalpis defensin sequence is closely related to defensins obtained from P. duboscqi and other nematocerans (Figure 1B). The putative L. longipalpis defensin tertiary structure was developed based on other insect defensins present in PDB. The analysis of the structure showed the expected architecture with two anti-parallel β-sheets and one α-helix (Figure 1C).
Transcription of defensin in L. longipalpis developmental stages
RT-PCR was performed with RNA samples obtained from both immature and newly emerged sand flies. Actively feeding larvae (L3 and L4-f) expressed low defensin levels compared to non-feeding L4 larvae, although the difference was not statistically significant (Figure 2). Defensin expression was significantly lower in L3 and L4-f when compared to pupae. Similarly, newly emerged females showed a trend towards lower levels of defensin expression in comparison to pupae (Figure 2).
Figure 2.
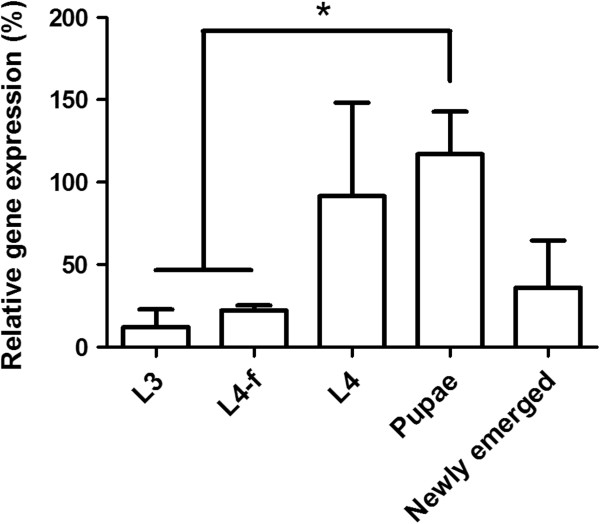
Defensin expression analysis in L. longipalpis developmental stages. Relative defensin gene expression (normalized against the housekeeping internal control gene 60S rRNA) was determined for feeding larval stages (L3 and L4-f), non-feeding stages (L4 and pupae) and recently emerged female L. longipalpis. Bar charts represent mean ± SEM of 3 pools of 3 insects. Asterisk indicates statistical significance at P < 0.05.
Transcription of defensin in L. longipalpis fed on bacteria or Leishmania
Defensin expression increased in female sand flies fed on four out of five bacteria tested when compared to sugar fed controls. This increased defensin expression was statistically significant 48 and 72 h after E. coli, Ochrobactrum sp. or S. marcescens ingestion (Figure 3A, B and C), and 24, 48 and 72 h after M. luteus ingestion (Figure 3E). Defensin expression decreased significantly 24 and 48 h after P. agglomerans ingestion and was unchanged in relation to controls at 72 h (Figure 3D). At the latest time point tested (96 h after infection), defensin expression returned to control levels observed in sugar-fed sand flies for all bacteria tested.
Figure 3.
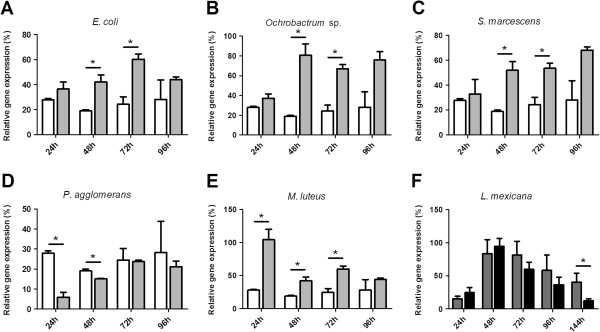
Defensin expression analysis in L. longipalpis fed with bacteria or orally infected with Leishmania. Female L. Longipalpis fed on suspensions of (A) E. coli; (B) Ochrobactrum sp.; (C) S. marcescens; (D) P. agglomerans; (E) M. luteus, were collected at 24, 48, 72 and 96 h after feeding (gray bars). Insects fed on sterile sucrose solution were used as control (white bars). (F) Female L. longipalpis fed on blood seeded with L. mexicana were collected at 24, 48, 72, 96, and 144 h after infection (black bars). Insects fed on blood were used as control (dark gray bars). The relative defensin gene expression was normalized against the housekeeping internal control gene 60S-rRNA. Bar charts represent mean ± SEM of 3 pools of 3 insects. Asterisks represent statistical significance at P < 0.05.
In adult females fed both on blood or blood containing L. mexicana, defensin expression increased sharply at 48 h and then slowly decreased from that time point until 144 h post-feed. In insects fed on blood containing Leishmania defensin expression decreased significantly at 144 h in comparison to blood fed controls (Figure 3F).
Expression of defensin was also investigated in insects exposed to E. coli and L. mexicana through injection into the hemocoel. Pricking the insects (data not shown) or injecting female sand flies with autoclaved LB media generated an increase in defensin expression at 24 and 48 h after injection in comparison to uninjected sugar-fed control sand flies (Figure 4). Similarly, female sand flies expressed higher levels of defensin mRNA at 24 and 72 h after E. coli injection when compared to the mock-injected control group (Figure 4). Insects injected with L. mexicana initially expressed significantly reduced levels of defensin mRNA at 24 and 48 h after injections, showing increased defensin expression at 72 h after injections when compared to the corresponding control group (Figure 4).
Figure 4.
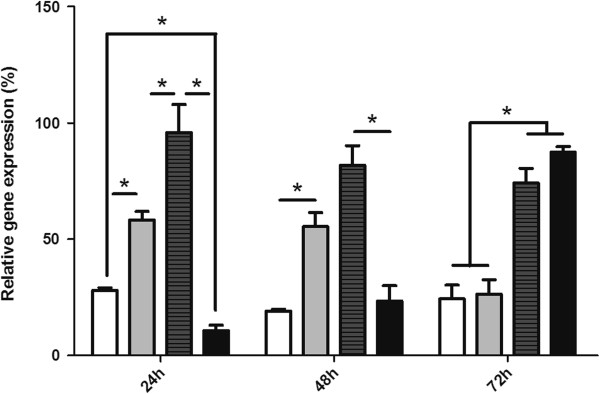
Defensin expression analysis upon bacterial and Leishmania injection into the hemocoel of female L. longipalpis: Female L. longipalpis were microinjected with E. coli (dark gray bars) or L. mexicana suspensions (black bars) and collected at 24, 48 and 72 h after pathogen inoculation. Insects fed on sugar (white bars) or injected with LB medium (light gray bars) were used as controls. The relative defensin gene expression was normalized against the housekeeping internal control gene 60S-rRNA. Bar charts represent mean ± SEM of 3 pools of 3 insects. Asterisks represent statistical significance at P < 0.05.
Discussion
In the present study we investigated and analyzed the expression profile of a defensin gene in L. longipalpis developmental stages, adult females infected orally with Gram-positive or negative bacteria and L. mexicana, or injected with E. coli or L. mexicana.
The L. longipalpis LlDef1 defensin gene contains two exons (134 and 172 nt respectively) interspersed with a 63 nt intron. The presence of six cysteines at positions 52, 57, 61, 71, 77 and 79 on the predicted amino acid sequence, with the potential to create three disulfide bonds, characterizes a defensin signature sequence [Pfam 01097]. We also sequenced 511 nt of the LlDef1 5'UTR and the analysis revealed that this gene is potentially under the control of at least two immune-related transcription factors: caudal and dorsal. Caudal encodes a DNA-binding nuclear transcription factor that plays a crucial role during development and innate immune response in Drosophila[44]. In Drosophila, Dorsal has its nuclear localization enhanced upon microbial challenge, interacting with Pelle, Tube, and Cactus during Toll activation to translocate and bind to NFκB-related sequences of AMP genes inside the nucleus [45]. The phylogenetic analysis showed that LlDef1 is similar to defensin sequences from other nematoceran diptera, being closely related to a P. duboscqi defensin [29].
High transcription levels were detected in non-feeding L. longipalpis L4 larvae and pupae. In Anopheles gambiae, defensin expression was detected in non-challenged third and fourth instar larvae and pupae, reaching high expression levels after E. coli injections [46]. A Drosophila defensin was detected in third instar larvae only after bacterial challenge, although expression was detected in non-challenged pupae [47], similarly to what was observed in L. longipalpis and A. gambiae. No previous study explored the immune response in naturally feeding versus non-feeding larvae in the Diptera group. Since transstadial passage of bacteria from larvae to pupae and adult flies has been already reported for sandflies [12,48,49], L. longipalpis non-feeding L4 and pupae may trigger defensin expression to control and select gut microbiota during late L4 through pupation to emerged adult.
L. longipalpis were orally exposed to five different Gram-positive and Gram-negative bacteria. Defensin expression was found to increase with time upon infection with the Gram-negative E. coli, Ochrobactrum sp. and S. marcescens. Ochrobactrum sp. is acquired by P. duboscqi larvae from the environment [12] and it is plausible to consider that it is recognized by the insect immune system as a foreign antigen as much as E. coli. S. marcescens is entomopathogenic and was shown to trigger the L. longipalpis immune system through ROS increase [50]. Interestingly, infection with the Gram-negative P. agglomerans showed an initial decrease of defensin expression and a very constant level of expression over time matching control levels. This may be due to the fact that this bacterium, commonly found in Anopheles stephensi gut, is not pathogenic [51] and may not be recognized as a hazard by L. longipalpis. Insect defensins are known to be active mainly against Gram-positive bacteria [23,26,52]. Accordingly, flies exposed to the Gram-positive M. luteus showed a sharp up-regulation of defensin mRNA during the early stages of infection (24 h post-feeding). Although defensin gene expression dropped considerably during the following 3 days, transcription was still significantly increased at 48 and 72 h post-feeding in comparison to controls.
These results suggest that sand flies are capable of mounting different innate immune responses against distinct bacterial species. A previous study that used the synergistic effects of lysozyme with antibacterial peptides revealed that L. longipalpis can successfully mount a humoral response against bacterial challenge and this response specifically discriminates between M. luteus and E. coli[53]. Although an increase of expression of a 4 kDa peptide was detected in the hemolymph of both M. luteus and E. coli-injected L. longipalpis in comparison to mock-injected controls, an unknown 33 kDa peptide could be detected in the hemolymph of the sand fly only when insects were challenged with M. luteus but not with E. coli[53]. These findings, and our present results, suggest that specific and discriminating immune responses are probably produced against the Gram-positive and Gram-negative bacteria in L. longipalpis.
At 48 h after artificial blood feeding and artificial infection with L. mexicana adult female sand flies showed a dramatic increase of defensin expression that slowly decreased over time. This initial increase in defensin expression may be a response to the proliferation of sand fly gut microbiota caused by the ingestion of a nutrient-rich blood meal as it was seen in P. duboscqi[12] and Aedes aegypti[54,55]. Interestingly, a defensin down regulation was observed starting at 72 h after Leishmania infection, reaching statistical significance at 144 h in comparison to blood-fed controls. Late infections were previously correlated with high numbers of Leishmania promastigotes within the sand fly gut [56]. Our present results indicate that high parasite number is correlated to low defensin expression. One explanation of this may be due to low levels of defensin expression at later time points after bloodfeeding, allowing for parasite survival and multiplication. On the other hand, if the defensin expression response is primarily towards bacterial molecular factors then the significant fall in defensin expression may be due to suppression of the gut bacterial population, via a competitive exclusion effect, in the presence of Leishmania.
A different transcription profile was reported in P. duboscqi infected with Leishmania major, where low levels of defensin expression were observed in the first day of infection whereas expression was strongly induced at four days after the Leishmania infection [29]. It is plausible that different phlebotomine sand flies and different Leishmania species may trigger diverse immune responses. This has been reported in mosquitoes, where different immune-related genes were modulated upon infection with various Plasmodium species [57,58].
Expression of defensin in L. longipalpis after L. mexicana or E. coli intra-thoracic injection was also investigated. Pricked and LB medium-injected sand flies showed an increase in defensin expression in comparison to uninjected sugar-fed controls at 24 and 48 h post-injection. These results indicate that trauma by injection was sufficient to activate the innate immunity and induce defensin transcription in L. longipalpis. Cuticle pricking and mock-injection of dsRNA into the sand flies’ hemocoel was shown to reduce the number of L. mexicana promastigotes within the midgut of L. longipalpis, possibly by nonspecific activation of the IMD pathway [59]. In A. aegypti, the injection of sterile saline induced the mosquito immune response and produced low but detectable levels of defensin mRNA [60]. Previous work in L. longipalpis showed that antimicrobial activity increased in sham-injected insects when compared to non-injected controls [53]. Similarly, our results demonstrated that control L. longipalpis microinjected with medium showed a significant increase in defensin expression at 24 h in comparison to controls, which was maintained until 48 h post-injection. In Drosophila Toll and IMD pathways can regulate different AMPs [61] and both can act synergistically [62]. This much is not yet explored in L. longipalpis.
Nimmo et al. [53] observed a significant increase in L. longipalpis humoral response against E. coli or M. luteus estimated by inhibition zone assays using hemolymph from bacteria-challenged insects. In addition, P. duboscqi inoculated with Erwinia carotovora showed higher defensin expression in comparison to naive insects and bacteria-fed sand flies [29]. Although in line with results obtained for P. duboscqi, our results show a much subtler defensin expression in L. longipalpis upon bacterial injection. Similar results were obtained in A. aegypti inoculated with E. coli and M. luteus which showed 3 times higher levels of defensin peptides in their hemolymph when compared to sterile saline-injected insects [63]. These results confirm that mosquitoes and sand flies can mount an immune response through defensin expression upon bacterial challenge in their hemolymph.
L. longipalpis injected with L. mexicana showed a significant increase of defensin expression at 72 h post infection. Although the presence of Leishmania in the hemolymph does not occur in nature, it is possible that the ectopic presence of parasites within the hemolymph induced an immune response. It has been shown that Drosophila is capable of producing an immune response against injected Plasmodium gallinaceum oocytes [64]. Defensin reduction at 24 and 48 h after Leishmania injection may be a counterbalance caused by activation of the IMD pathway triggering other AMP, but not DefLl1. Later, at 72 h, L. longipalpis is able to express high levels of defensin. To our knowledge, this is the first report of an immune response in sandflies after parasite injection. Investigation of other Toll or IMD related AMPs could address and clarify this hypothesis related to the sandflies immune response to Leishmania injection in hemolymph, but none has been described up to date.
Conclusion
Here, we have described a L. longipalpis defensin gene similar to a P. duboscqi defensin, modulated by bacterial feed and injection and Leishmania infections. These genes are the only defensins so far described for both sand fly species but the presence of multiple defensin genes and other AMPs co-existing in sand flies is possible. Defensin isoforms with distinct transcriptional patterns and putative distinct roles were previously described in A. gambiae [65]. The difference in defensin expression levels upon bacterial challenge observed for the New and Old World species may therefore be due to expression of different defensin isoforms acting concertedly to control bacterial proliferation within the sand fly midgut and hemolymph. Our results suggest that L. longipalpis is able to mount a differential response of defensin expression upon bacterial feeding and bacterial injection into the hemocoel and Leishmania gut infection.
Competing interests
The authors declare no competing interests.
Authors’ contributions
RJD and YMT designed the experiments. ELT and MOA carried out the biological and molecular experiments. ANP performed sequence and phylogenetic analysis. ELT, MRVS, ANP, RJD and YMT wrote the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Erich L Telleria, Email: erich.loza-telleria@yale.edu.
Maurício R Viana Sant’Anna, Email: m.santanna@lancaster.ac.uk.
Mohammad O Alkurbi, Email: kurbi@liverpool.ac.uk.
André N Pitaluga, Email: pitaluga@ioc.fiocruz.br.
Rod J Dillon, Email: r.dillon@lancaster.ac.uk.
Yara M Traub-Csekö, Email: ytraub@ioc.fiocruz.br.
Acknowledgements
This work was funded by The Leverhulme Trust (http://www.leverhulme.co.uk) ref F/00 808/C; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES-PDEE; Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ; Instituto Oswaldo Cruz and PAPES VI-FIOCRUZ.
References
- Cohnstaedt LW, Beati L, Caceres AG, Ferro C, Munstermann LE. Phylogenetics of the phlebotomine sand fly group Verrucarum (Diptera: Psychodidae: Lutzomyia) AmJTrop Med Hyg. 2011;84:913–922. doi: 10.4269/ajtmh.2011.11-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready PD. Should sand fly taxonomy predict vectorial and ecological traits? J Vector Ecol. 2011;36(Suppl 1):S17–S22. doi: 10.1111/j.1948-7134.2011.00108.x. [DOI] [PubMed] [Google Scholar]
- Ramalho-Ortigao M, Saraiva EM, Traub-Cseko YM. Sand Fly-Leishmania Interactions: Long Relationships are Not Necessarily Easy. The Open Parasitology Journal. 2010;4:195–204. doi: 10.2174/1874421401004010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svarovska A, Ant TH, Seblova V, Jecna L, Beverley SM, Volf P. Leishmania major glycosylation mutants require phosphoglycans (lpg2-) but not lipophosphoglycan (lpg1-) for survival in permissive sand fly vectors. PLoS Negl Trop Dis. 2010;4:e580. doi: 10.1371/journal.pntd.0000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Hajmova M, Joshi MB, Sadlova J, Dwyer DM, Volf P, Bates PA. Leishmania chitinase facilitates colonization of sand fly vectors and enhances transmission to mice. Cell Microbiol. 2008;10:1363–1372. doi: 10.1111/j.1462-5822.2008.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, Barillas-Mury C, Sacks DL, Valenzuela JG. A role for insect galectins in parasite survival. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sant'anna MR, Diaz-Albiter H, Mubaraki M, Dillon RJ, Bates PA. Inhibition of trypsin expression in Lutzomyia longipalpis using RNAi enhances the survival of Leishmania. Parasit Vectors. 2009;2:62. doi: 10.1186/1756-3305-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telleria EL, de Araujo AP, Secundino NF, d'Avila-Levy CM, Traub-Cseko YM. Trypsin-like serine proteases in Lutzomyia longipalpis--expression, activity and possible modulation by Leishmania infantum chagasi. PLoS One. 2010;5:e10697. doi: 10.1371/journal.pone.0010697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta PF, Modi GB, Pereira ST, Shahabuddin M, Sacks DL. A novel role for the peritrophic matrix in protecting Leishmania from the hydrolytic activities of the sand fly midgut. Parasitology. 1997;115(Pt 4):359–369. doi: 10.1017/s0031182097001510. [DOI] [PubMed] [Google Scholar]
- Guernaoui S, Garcia D, Gazanion E, Ouhdouch Y, Boumezzough A, Pesson B, Fontenille D, Sereno D. Bacterial flora as indicated by PCR-temperature gradient gel electrophoresis (TGGE) of 16S rDNA gene fragments from isolated guts of phlebotomine sand flies (Diptera: Psychodidae) J Vector Ecol. 2011;36(Suppl 1):S144–S147. doi: 10.1111/j.1948-7134.2011.00124.x. [DOI] [PubMed] [Google Scholar]
- Hillesland H, Read A, Subhadra B, Hurwitz I, McKelvey R, Ghosh K, Das P, Durvasula R. Identification of aerobic gut bacteria from the kala azar vector, Phlebotomus argentipes: a platform for potential paratransgenic manipulation of sand flies. AmJTrop Med Hyg. 2008;79:881–886. [PubMed] [Google Scholar]
- Volf P, Kiewegova A, Nemec A. Bacterial colonisation in the gut of Phlebotomus duboseqi (Diptera: Psychodidae): transtadial passage and the role of female diet. Folia Parasitol. 2002;49:73–77. doi: 10.14411/fp.2002.014. [DOI] [PubMed] [Google Scholar]
- Azpurua J, De La Cruz D, Valderama A, Windsor D. Lutzomyia sand fly diversity and rates of infection by Wolbachia and an exotic Leishmania species on Barro Colorado Island, Panama. PLoS Negl Trop Dis. 2010;4:e627. doi: 10.1371/journal.pntd.0000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia C, Asensi MD, Zahner V, Rangel EF, Oliveira SM. Study on the bacterial midgut microbiota associated to different Brazilian populations of Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae) Neotrop Entomol. 2008;37:597–601. doi: 10.1590/S1519-566X2008000500016. [DOI] [PubMed] [Google Scholar]
- Oliveira SM, Moraes BA, Goncalves CA, Giordano-Dias CM, D'Almeida JM, Asensi MD, Mello RP, Brazil RP. Prevalence of microbiota in the digestive tract of wild females of Lutzomyia longipalpis Lutz & Neiva, 1912) (Diptera: Psychodidae) Rev Soc Bras Med Trop. 2000;33:319–322. doi: 10.1590/s0037-86822000000300012. [DOI] [PubMed] [Google Scholar]
- McCarthy CB, Diambra LA, Rivera Pomar RV. Metagenomic analysis of taxa associated with Lutzomyia longipalpis, vector of visceral leishmaniasis, using an unbiased high-throughput approach. PLoS Negl Trop Dis. 2011;5:e1304. doi: 10.1371/journal.pntd.0001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azambuja P, Garcia ES, Ratcliffe NA. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005;21:568–572. doi: 10.1016/j.pt.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaar H, Gross R. Immune reactions of insects on bacterial pathogens and mutualists. Microbes Infect. 2008;10:1082–1088. doi: 10.1016/j.micinf.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Welchman DP, Aksoy S, Jiggins F, Lemaitre B. Insect immunity: from pattern recognition to symbiont-mediated host defense. Cell Host Microbe. 2009;6:107–114. doi: 10.1016/j.chom.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Boulanger N, Bulet P, Lowenberger C. Antimicrobial peptides in the interactions between insects and flagellate parasites. Trends Parasitol. 2006;22:262–268. doi: 10.1016/j.pt.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Bulet P, Hetru C, Dimarcq JL, Hoffmann D. Antimicrobial peptides in insects; structure and function. Dev Comp Immunol. 1999;23:329–344. doi: 10.1016/S0145-305X(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Lamberty M, Ades S, Uttenweiler-Joseph S, Brookhart G, Bushey D, Hoffmann JA, Bulet P. Insect immunity. Isolation from the lepidopteran Heliothis virescens of a novel insect defensin with potent antifungal activity. J Biol Chem. 1999;274:9320–9326. doi: 10.1074/jbc.274.14.9320. [DOI] [PubMed] [Google Scholar]
- Mandrioli M, Bugli S, Saltini S, Genedani S, Ottaviani E. Molecular characterization of a defensin in the IZD-MB-0503 cell line derived from immunocytes of the insect Mamestra brassicae (Lepidoptera) Biol Cell. 2003;95:53–57. doi: 10.1016/S0248-4900(02)01219-4. [DOI] [PubMed] [Google Scholar]
- Vizioli J, Richman AM, Uttenweiler-Joseph S, Blass C, Bulet P. The defensin peptide of the malaria vector mosquito Anopheles gambiae: antimicrobial activities and expression in adult mosquitoes. Insect Biochem Mol Biol. 2001;31:241–248. doi: 10.1016/S0965-1748(00)00143-0. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, Raikhel AS. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2010;107:8111–8116. doi: 10.1073/pnas.1003056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni MM, McMaster WR, Kamysz W, McGwire BS. Antimicrobial peptide-induced apoptotic death of leishmania results from calcium-de pend ent, caspase-independent mitochondrial toxicity. J Biol Chem. 2009;284:15496–15504. doi: 10.1074/jbc.M809079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger N, Lowenberger C, Volf P, Ursic R, Sigutova L, Sabatier L, Svobodova M, Beverley SM, Spath G, Brun R. et al. Characterization of a defensin from the sand fly Phlebotomus duboscqi induced by challenge with bacteria or the protozoan parasite Leishmania major. Infect Immun. 2004;72:7140–7146. doi: 10.1128/IAI.72.12.7140-7146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaluga AN, Mason PW, Traub-Cseko YM. Non-specific antiviral response detected in RNA-treated cultured cells of the sandfly, Lutzomyia longipalpis. Dev Comp Immunol. 2008;32:191–197. doi: 10.1016/j.dci.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Pitaluga AN, Beteille V, Lobo AR, Ortigao-Farias JR, Davila AM, Souza AA, Ramalho-Ortigao JM, Traub-Cseko YM. EST sequencing of blood-fed and Leishmania-infected midgut of Lutzomyia longipalpis, the principal visceral leishmaniasis vector in the Americas. Mol Genet Genomics. 2009;282:307–317. doi: 10.1007/s00438-009-0466-2. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon C, Barbault F, Legrain M, Guenneugues M, Vovelle F. Rational design of peptides active against the gram positive bacteria Staphylococcus aureus. Proteins. 2008;72:229–239. doi: 10.1002/prot.21912. [DOI] [PubMed] [Google Scholar]
- Cornet B, Bonmatin JM, Hetru C, Hoffmann JA, Ptak M, Vovelle F. Refined three-dimensional solution structure of insect defensin A. Structure. 1995;3:435–448. doi: 10.1016/S0969-2126(01)00177-0. [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Modi GB. In: The molecular biology of insect disease vectors: a methods manual. Crampton JM, Beard CB, Louis C, editor. London (United Kingdom): Chapman and Hall Ltd; 1997. Care and maintenance of phlebotomine sandfly colonies; pp. 21–30. [Google Scholar]
- Sant'Anna MR, Alexander B, Bates PA, Dillon RJ. Gene silencing in phlebotomine sand flies: Xanthine dehydrogenase knock down by dsRNA microinjections. Insect Biochem Mol Biol. 2008;38:652–660. doi: 10.1016/j.ibmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Han SH, Ryu JH, Oh CT, Nam KB, Nam HJ, Jang IH, Brey PT, Lee WJ. The moleskin gene product is essential for Caudal-mediated constitutive antifungal Drosomycin gene expression in Drosophila epithelia. Insect Mol Biol. 2004;13:323–327. doi: 10.1111/j.0962-1075.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- Valanne S, Wang JH, Ramet M. The Drosophila Toll signaling pathway. J Immunol. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- Dimopoulos G, Richman A, Muller HM, Kafatos FC. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci U S A. 1997;94:11508–11513. doi: 10.1073/pnas.94.21.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimarcq JL, Hoffmann D, Meister M, Bulet P, Lanot R, Reichhart JM, Hoffmann JA. Characterization and transcriptional profiles of a Drosophila gene encoding an insect defensin. A study in insect immunity. Eur J Biochem. 1994;221:201–209. doi: 10.1111/j.1432-1033.1994.tb18730.x. [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Hillesland H, Fieck A, Das P, Durvasula R. The paratransgenic sand fly: a platform for control of Leishmania transmission. Parasit Vectors. 2011;4:82. doi: 10.1186/1756-3305-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkova-Koci K, Robles-Murguia M, Ramalho-Ortigao M, Zurek L. Significance of bacteria in oviposition and larval development of the sand fly Lutzomyia longipalpis. Parasit Vectors. 2012;5:145. doi: 10.1186/1756-3305-5-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Albiter H, Sant' Anna MR, Genta FA, Dillon RJ. Reactive oxygen species-mediated immunity against Leishmania mexicana and Serratia marcescens in the phlebotomine sand fly Lutzomyia longipalpis. J Biol Chem. 2012;287:23995–24003. doi: 10.1074/jbc.M112.376095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle MA, Moreira CK, Lampe D, Lauzon C, Jacobs-Lorena M. Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int J Parasitol. 2007;37:595–603. doi: 10.1016/j.ijpara.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liao M, Ueda M, Gong H, Xuan X, Fujisaki K. Sequence characterization and expression patterns of two defensin-like antimicrobial peptides from the tick Haemaphysalis longicornis. Peptides. 2007;28:1304–1310. doi: 10.1016/j.peptides.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Nimmo DD, Ham PJ, Ward RD, Maingon R. The sandfly Lutzomyia longipalpis shows specific humoral responses to bacterial challenge. Med Vet Entomol. 1997;11:324–328. doi: 10.1111/j.1365-2915.1997.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Antonova Y, Alvarez KS, Kim YJ, Kokoza V, Raikhel AS. The role of NF-kappaB factor REL2 in the Aedes aegypti immune response. Insect Biochem Mol Biol. 2009;39:303–314. doi: 10.1016/j.ibmb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JH, Goncalves RL, Lara FA, Dias FA, Gandara AC, Menna-Barreto RF, Edwards MC, Laurindo FR, Silva-Neto MA, Sorgine MH, Oliveira PL. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011;7:e1001320. doi: 10.1371/journal.ppat.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol. 2007;37:1097–1106. doi: 10.1016/j.ijpara.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia AC, Kubota MS, Tempone AJ, Pinheiro WD, Tadei WP, Secundino NF, Traub-Cseko YM, Pimenta PF. Anopheles aquasalis Infected by Plasmodium vivax displays unique gene expression profiles when compared to other malaria vectors and plasmodia. PLoS One. 2010;5:e9795. doi: 10.1371/journal.pone.0009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telleria EL, Sant'anna MR, Ortigao-Farias JR, Pitaluga AN, Dillon VM, Bates PA, Traub-Cseko YM, Dillon RJ. Caspar-like Gene Depletion Reduces Leishmania Infection in Sand Fly Host Lutzomyia longipalpis. J Biol Chem. 2012;287:12985–12993. doi: 10.1074/jbc.M111.331561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenberger CA, Kamal S, Chiles J, Paskewitz S, Bulet P, Hoffmann JA, Christensen BM. Mosquito-Plasmodium interactions in response to immune activation of the vector. Exp Parasitol. 1999;91:59–69. doi: 10.1006/expr.1999.4350. [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Hu X, Weber AN, Ip YT. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol Cell Biol. 2007;27:4578–4588. doi: 10.1128/MCB.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenberger C, Bulet P, Charlet M, Hetru C, Hodgeman B, Christensen BM, Hoffmann JA. Insect immunity: isolation of three novel inducible antibacterial defensins from the vector mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1995;25:867–873. doi: 10.1016/0965-1748(95)00043-U. [DOI] [PubMed] [Google Scholar]
- Schneider D, Shahabuddin M. Malaria parasite development in a Drosophila model. Science. 2000;288:2376–2379. doi: 10.1126/science.288.5475.2376. [DOI] [PubMed] [Google Scholar]
- Meredith JM, Hurd H, Lehane MJ, Eggleston P. The malaria vector mosquito Anopheles gambiae expresses a suite of larval-specific defensin genes. Insect Mol Biol. 2008;17:103–112. doi: 10.1111/j.1365-2583.2008.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]