Abstract
Background
The serotonin system is thought to play a role in the aetiology of antisocial and aggressive behaviour in both adults and children however previous findings have been inconsistent. Recently, research has suggested that the function of the serotonin system may be specifically altered in a sub-set of antisocial populations – those with psychopathic (callous-unemotional) personality traits. We explored the relationships between callous-unemotional traits and functional polymorphisms of selected serotonin-system genes, and tested the association between callous-unemotional traits and serum serotonin levels independently of antisocial and aggressive behaviour.
Method
Participants were boys with antisocial behaviour problems aged 3–16 years referred to University of New South Wales Child Behaviour Research Clinics. Participants volunteered either a blood or saliva sample from which levels of serum serotonin (N = 66) and/or serotonin-system single nucleotide polymorphisms (N = 157) were assayed.
Results
Functional single nucleotide polymorphisms from the serotonin 1b receptor gene (HTR1B) and 2a receptor gene (HTR2A) were found to be associated with callous-unemotional traits. Serum serotonin level was a significant predictor of callous-unemotional traits; levels were significantly lower in boys with high callous-unemotional traits than in boys with low callous-unemotional traits.
Conclusion
Results provide support to the emerging literature that argues for a genetically-driven system-wide alteration in serotonin function in the aetiology of callous-unemotional traits. The findings should be interpreted as preliminary and future research that aims to replicate and further investigate these results is required.
Introduction
Of all the childhood psychopathologies, antisocial and aggressive behaviour problems such as Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD) account for the greatest cost to psychological, psychiatric and social services [1]. ODD and CD include behaviours such as; spitefulness, arguing with adults, aggression towards others, destruction of property and violation of rules. The diagnoses identify heterogeneous groups; however, a characteristic that can be used to identify more homogenous groupings is that of callous-unemotional (CU) traits. These traits comprise low levels of guilt and shame, and a lack of empathy, and can be thought of as the downward extension of the affective and interpersonal characteristics of adult psychopathic personality traits [2]. Childhood antisocial behaviour problems characterised by high levels of CU traits have been found to be highly heritable [3], [4]. Viding et al. [4] found that genetic variability accounted for 71% of the variance in antisocial behaviour problems in 9 year old children with high levels of CU traits but only 36% in those with low levels of CU traits. High CU traits are also related to more severe and chronic antisocial behaviour [5], [6], greater use of proactive aggression [7], and specific patterns of neural dysfunction, specifically with regards to the amygdala [8]–[11]. Furthermore, people with high levels of CU traits (psychopathic personality) have been shown to have three specific cognitive and emotional deficits; a poor conditioned fear response, reduced ability to recognise fear, and deficits in stimulus-reinforcement tasks (see Moul et al. [12] for a review). These finding suggest that antisocial behaviour problems characterised by high levels of CU traits may have unique aetiological mechanisms associated with specific cognitive and affective impairments that are heavily dependent on genetics.
The Differential Amygdala Activation Model (DAAM) [12] is a recently developed model of amygdala function that has proposed a mechanism by which the subtle cognitive and emotional deficits characteristic of people with high CU traits, may develop. The DAAM posits that reduced serotonin neurotransmission may be integral to the pattern of amygdala activation it describes and hence to the development of the three deficits which, in turn, lead to high levels of CU traits. There is evidence to support the role of serotonin in the cognitive and emotional deficits characteristic of people with high levels of CU traits. Studies by Harmer et al. [13] and Attenburrow et al. [14] respectively found that acute administration of a serotonin selective reuptake inhibitor (SSRI) and ingestion of tryptophan (the natural precursor of serotonin) improved recognition of both fear and happiness. It has been shown that serotonin depletion in the prefrontal cortex of monkeys results in an impairment in stimulus-reinforcement learning [15]. Furthermore, low levels of serotonin in the basolateral amygdala are related to reductions in the conditioned fear response – a response which is reliably impaired in adults with high levels of psychopathic personality (CU traits) [16], [17].
Other researchers [18]–[21] have suggested that the serotonin system may be important in CU traits but we are aware of only two studies that have directly investigated the relationship between serotonergic function and CU traits. Fowler et al. (2009) found that a variable number tandem repeat (VNTR) polymorphism in the gene encoding monoamine oxidase A (responsible for catabolism of serotonin and other catecholamine neurotransmitters), and an insertion/deletion polymorphism in the SLC6A4 promoter (5-HTTLPR) were associated with what the authors term the “emotional dysfunction” aspect of psychopathy (a construct that corresponds with CU traits) in a sample of adolescents with attention deficit hyperactivity disorder (ADHD) [18]. Sadeh et al. (2010) found that a gene-environment interaction between 5-HTTLPR and socioeconomic resources was associated with CU traits in adolescents. CU traits increased as SES decreased only among adolescents with the homozygous-long (l/l) genotype [22].
Serotonin has, however, been implicated in behaviours often found to be positively associated with CU traits; that is, aggression, antisocial behaviour and impulsivity. Genes encoding the serotonin 2a receptor (HTR2A) and tryptophan hydroxylase 1 (TPH1) have been associated with antisocial personality disorder (ASPD) in adult males [21]. Single nucleotide polymorphisms (SNPs) of the gene encoding the serotonin 1b receptor (HTR1B) have also been associated with anger and hostility [23]. A number of studies have used peripheral measures of serotonin and neuroendocrine challenge to demonstrate that the function of the serotonergic system may be altered in aggressive populations. For example, a negative relationship has been found between peripheral levels of serotonin (and its metabolite 5-HIAA) and antisocial/aggressive behaviours in both criminal and non-criminal males [24], [25]. Adults with heightened levels of aggression and antisocial behaviour have been shown to have a reduced reaction to fenfluramine challenge (a test of serotonin function in the hypothalamic-pituitary-adrenal axis) in comparison with healthy controls [26], [27]. In contrast Moffitt et al., [28] found that whole blood serotonin levels were positively associated with violence in adult males. In all, the vast majority of research using adult samples has demonstrated an inverse relationship between aggressive behaviour and serotonin system function (see [29] for a review).
The evidence to implicate low serotonergic function in childhood antisocial behaviour problems is less clear. Some studies have found a negative relationship between antisocial/aggressive behaviour and serotonin function [30], [31] that support the majority of findings from adult populations. Other studies, however, have found either no significant relationship [32], [33] or a positive relationship [34]–[37]. Interestingly, Halperin et al. [38] demonstrated that aggressive children with low-prolactin responses to fenfluramine challenge had significantly greater numbers of first- and second-degree relatives with aggressive and antisocial characteristics compared to both non-aggressive children and aggressive children with high-prolactin responses. The authors propose that these results suggest that there are two forms of aggression in children and only aggression that has its basis in familial biology is associated with diminished serotonin function. As commented by Manuck, Kaplan and Lotrich [29] this hypothesis is consistent with the identification by Moffitt [39] of different trajectories of childhood antisocial behaviour, one of which is described as “life-course-persistent” and thought to be strongly influenced by neurobiology. It is possible, therefore, as Manuck, Kaplan and Lotrich suggest, that it is this group of children with a familial, biological predisposition towards antisocial and aggressive behaviour that later comprise adult antisocial samples thereby offering a possible explanation for the general homogeneity of findings linking serotonin and aggression in adults.
It should be noted that heightened levels of aggression and antisocial behaviour do not demarcate high levels of CU traits. High levels of CU traits have, however, been shown to be a risk factor for the greater use of aggression and antisocial behaviours [5]–[7]. As such, it is possible that unmeasured, varying levels of CU traits within antisocial child samples may provide an explanation for the diversity of findings regarding the relationship between serotonin and antisocial and aggressive behaviours. Furthermore, the high heritability of CU traits and their association with more chronic and serious aggression and antisocial behaviour problems make them a strong candidate for the driving force behind the familial transmission of aggressive behaviour that Halperin et al. [38] argue is mediated, in part, by reduced central serotonin function.
In all, there is some indication that the function of the serotonin system may be important in CU traits. Current evidence, however, is inconsistent and the precise role of CU traits versus antisocial and aggressive behaviour, and other comorbidities, is unclear. Further, existing research has been limited to one or two functional polymorphisms at the expense of a more comprehensive assessment of the serotonin system.
This study explored the relationships between the serotonin system and CU traits in an antisocial sample with the general hypothesis that high levels of CU traits would be associated with indicators of reduced serotonin-system function. The majority of research into the role of serotonin has compared antisocial to non-antisocial groups and, as such, the unique association, if any, between CU traits and the serotonin system is unclear. As such, this research aimed to investigate the association between the serotonin system and CU traits within an entirely antisocial population. To this end, data regarding both serum serotonin levels and functional SNPs of serotonin-system genes were collected from a sample of boys with antisocial behaviour problems and varying levels of CU traits. It was predicted that boys with antisocial behaviour problems and high levels of CU traits would have lower levels of serum serotonin than boys with antisocial behaviour problems and low levels of CU traits. In addition, given the high heritability of antisocial behaviour problems in the presence of high levels of CU traits, it was hypothesized that CU traits would be significantly associated with functional polymorphisms of the serotonin-system. Given the exploratory nature of this research, 15 SNPs were selected from 7 genes with functions pertaining to the regulation of serotonin neurotransmission. SNPs were chosen on the basis of prior research regarding their functionality and association with psychopathologies. As the actual associations between SNPs and serotonin neurotransmission are dependent on factors other than just the functionality of the genotype there were no directional hypotheses made regarding the relationship between genotypes and CU traits. Two SNPs from the TPH1 gene were included even though their functionality is not yet clarified because this gene is involved in the regulation of serotonin synthesis in the body (as opposed to the brain) and given previous research to implicate low levels of peripheral serotonin this gene was an important inclusion. Details of the SNPs and their functionality are shown in table 1. The dominance of genotypes was inferred from previous research.
Table 1. Serotonin-System Gene Single Nucleotide Polymorphisms and their Functions.
| Gene function | rs number | Major/minor (Inferred dominance) | Function of SNP | Associated with: | Influence of variant |
| HTR1A Codes for the 5-HT1A receptor - regulation of serotonin neurotransmission | rs6295 | C/G (G dominant) | Promoter polymorphism – blocks the function of repressors resulting in increased 5-HT1A expression | Amygdala volume [65]; amygdala reactivity to threat [66] | G allele increases 5-HT1A autoreceptor expression and reduces 5-HT release [66] |
| HTR1B Codes for presynaptic autoreceptor 5-HT1B – inhibition of serotonin release | rs13212041 | A/G (G dominant) | Attenuates microRNA mediated repression of gene expression | Conduct disorder [67]; variance in self-reported aggression and hostility [23] | AA genotypes have increased potential for the suppression of 5-HT1B expression. [67] |
| rs6296 | G/C (C dominant) | Unknown – but in high LD with variants associated with transcriptional activity [63] | Aggressiveness and impulsivity [68]Aggression in children [69] | C allele has been associated with lower binding potential of 5HT1B receptors in the brain but this is thought to be due to high LD with functional SNPs | |
| rs130058 | A/T (T dominant) | Promoter polymorphism: in balance with rs11568817 regulates activity of 5-HT1B [62]. | Suicide and hostility in suicide completers [70] | A allele linked with higher transcriptional activity. | |
| rs11568817 | T/G (Co-dominant) | Promoter polymorphism: in balance with rs130058 regulates activity of 5-HT1B | Alcohol dependence [71] | G allele linked with higher transcriptional activity [62] | |
| HTR2A Codes for the 5-HT2A receptor - regulation of serotonin neurotransmission | rs6314 | C/T (T dominant) | Polymorphism results in a missense substitution at the 452nd amino acid. | Antisocial behaviour and rule breaking in adolescents [61] | T allele results in amino acid substitution thought to result in changes to the secondary structure of the receptor [72] |
| rs6311 | C/T (T dominant) | Promoter polymorphism: involved in gene expression. | Aggression and impulsive behaviour [73] | T allele associated with increased 5-HT2A receptor binding [74] | |
| HTR3B Codes for the 5-HT3B receptor – mediates fast excitatory serotonin transmission | rs1176744 | T/G (G dominant) | Polymorphism results in a missense substitution which leads to an increased receptor response to 5-HT | Alcohol dependence [75]; receptor response to 5-HT [76] | G allele results in amino acid substitution which leads to increased receptor response to 5-HT |
| TPH1 Codes for TPH - the rate limiting enzyme of serotonin synthesis external to the brain | rs1800532 | C/A (Dominance unclear) | Function not yet clarified | Bipolar disorder and alcohol dependence [77] | Not yet clarified. A allele associated with blunted prolactin response to fenfluramine [78] and low levels of cerebrospinal fluid 5-Hydroxyindoleacetic acid [79] |
| rs211105 | T/G (Dominance unclear) | Function not yet clarified | Haplotype including rs211105 associated with schizophrenia [80] | Not yet clarified | |
| TPH2 Codes for the majority of TPH in the human brain [81] | rs4570625 | G/T (T dominant) | Polymorphism involved in gene expression | Associated with major depressive disorder [82] amygdala reactivity to emotional stimuli increased in T allele carriers [83] | T allele thought to down-regulate gene expression [84] |
| rs11178997 | T/A (A dominant) | Polymorphism influences TPH2 transcriptional activity | Personality disorders [85] | A allele associated with reduced gene transcription [86] | |
| rs7305115 | G/A (A dominant) | Polymorphism involved in gene expression | TPH2 expression [87] | A allele associated with greater gene expression [87] | |
| rs4565946 | C/T (C dominant) | Function not yet clarified | Early-onset obsessive compulsive disorder [88] | Not yet clarified | |
| SLC6A4 Codes for 5HTT – responsible for the reuptake of serotonin into the presynaptic membrane. | rs2066713 | C/T (T dominant) | Polymorphism associated with serum serotonin levels in males | Autism [89] | T allele associated with lower serum serotonin levels in males [90] |
Note: 5-HT = 5 hydroxytryptamine (serotonin); TPH = tryptophan hydroxylase; 5HTT = serotonin transporter protein.
Materials and Methods
Ethics Statement
Ethics approval was from the Human Research Ethics Committee of the University of New South Wales (UNSW). Primary caregivers provided written informed consent to take part in the research and also provide written informed consent on behalf of their participating child/children. Adolescents (over the age of 12) were required to provide independent written informed consent. The donation of a blood/saliva sample was voluntary and participants and their parents were informed that choosing not to donate a biological sample would have no impact on their relationship with UNSW or their inclusion in the research program.
Participants
All participants were referred to UNSW Child Behaviour Research Clinic (CBRC) or Royal Far West Children's Hospital (RFW), Sydney. Participants were a subset of a larger sample who met formal criteria for: 1) DSM-IV diagnosis [40] of antisocial behaviour problems (ODD or CD) using DISCAP structured interview [41]; 2) aged from 3–16 years for the genetic sample (M = 7.61, SD = 3.12) and aged 4–12 years for the serum sample (M = 6.89, SD = 2.25); 3) no major neurological/physical illness; 4) IQ>75; 5) have at least one set of measures of serotonin system SNPs or serum serotonin levels; 6) all known (at least 3) grandparents of Caucasian background (for participants included in the genetics sample); 7) provided written parental consent. Collection and analysis of DNA and serum levels evolved over several years and complete data were not available for all children; once all exclusions were in place, the sample sizes used for the main measures in the study were: serotonin system SNPs – Caucasian only (N = 157); serotonin serum levels – no ethnicity restrictions (N = 66); both genetic and serum serotonin level data – Caucasian only (N = 35). Boys using prescription drugs were included as it was predicted that their exclusion may have resulted in a biased sample. See table 2 for information regarding the types and usage of medications.
Table 2. The Percentage of Participants Using Medications in the Serum Serotonin Sample.
| Medication type | Whole sample (N = 66) | Low CU (N = 47) | High CU (N = 19) |
| Psychostimulant | 9.1 | 10.6 | 5.3 |
| SSRI/MAOI | 4.5 | 4.3 | 5.3 |
| Antipsychotic | 0.0 | 0.0 | 0.0 |
| Bronchodilator | 4.5 | 4.3 | 5.3 |
| Other | 3.0 | 4.3 | 0.0 |
| Combinations# | 3.0 | 2.1 | 5.3 |
| Nil | 75.8 | 74.5 | 78.9 |
Note:
some participants were taking more than one type of medication from the list above. Some participants were taking antipsychotics but only in combination with other medications.
(SSRI = Selective Serotonin Reuptake Inhibitor, MAOI = Monoamine Oxidase Inhibitor).
Measures
Parents completed the Antisocial Processes Screening Device (APSD) [42] and the Strengths and Difficulties Questionnaire [43]. These measures were chosen as they measure traits and behaviours associated with psychopathy and have been shown to be reliable in measuring these traits in child populations [43]–[46]. Family function was indexed via the Family Assessment Device-brief version (FAD) [47]. The FAD comprises a 12-item questionnaire that is based on the McMaster Model of Family Functioning and provides a measure of the structural and organizational properties of the family that have been found to distinguish between healthy and unhealthy families [47]–[49]. Current parental psychopathology was assessed via the short version of the Depression Anxiety Stress Scales (DASS) [50], [51]. The DASS is a self-report measure that indexes the emotional states of depression, anxiety and depression via three sub-scales of 7 items each. In addition, the parent also provided information regarding their home address. This information was used in conjunction with the Australian Bureau of Statistics (ABS) Socio-Economic Index of Area [52] to estimate the quality of the child's current surrounding environment. This scale provides a rating of the average standard of living for a given area on a ten-point scale with 1 indicating disadvantage and 10 indicating advantage.
CU traits were rated by parents using the UNSW system of combining items from the APSD [42] and prosocial scale of the SDQ [43]. This method has been validated by factor analysis [44] and has been used in previous research [44], [53], [54]. Cronbach's alpha coefficient for the UNSW CU traits scale in the sample of children that included both the genetic and the neurochemical samples was 0.81. Participants were divided into two groups for further analysis. Previous research suggests a range between the top 45% and 20% of aggressive/antisocial groups to represent high CU traits [6], [44], [55]; thus, boys with a CU traits score equal to or less than the 33rd percentile were categorised as the low CU group. Boys with a CU traits score greater than this value were categorised into the high CU group.
Diagnoses were made using DSM-IV criteria by the assessing psychiatrist/psychologist using the DISCAP [41] with parents, and the child for those older than 8 years. Diagnoses were checked by having a second diagnostic team make an independent diagnosis. Kappa agreement across UNSW child mental health services on primary and secondary diagnoses were 0.772 and 0.770 respectively. Participants were rated by clinical psychologists, blind to levels of CU traits, on levels of antisocial behaviour problems, ADHD, autism spectrum disorders and anxiety and depression. Ratings were made on a scale of 0 to 6 with a rating of 4 or more indicating clinical levels of severity and a rating of 3 indicating borderline clinical severity. Boys with borderline ratings of antisocial behaviour problems were included in this study.
Adversity for the child was measured using the Quality of the Family Environment (QFE) [56], a clinician rating scale of the lowest quality of family environment to which the child was exposed during a substantial period (at least 1 year) before the age of 12. Ratings were made by a second naïve clinician on a subset of cases (r = 0.96). Sample characteristics are shown in table 3.
Table 3. Characteristics (mean (SD)) of the Genetic and Serum Samples.
| Genetics sample (N = 133) | Serum Sample (N = 66) | |
| Age | 7.56 (3.20) | 6.89 (2.25) |
| QFE | 74.28 (12.87) | 74.69 (13.30) |
| ABS | 7.80 (2.48) | 8.61 (1.72) |
| Antisocial severity | 4.05 (0.77) | 4.06 (0.82) |
| ADHD severity | 2.07 (2.02) | 1.98 (2.09) |
| Anx/Dep severity | 0.65 (1.37) | 0.53 (1.28) |
| ASD severity | - | 0.20 (0.81) |
| CU traits | 7.26 (2.84) | 7.50 (2.81) |
| CU traits 66th percentile | 8 | 8 |
| Ethnicity | Caucasian 100% | Caucasian 66.66% |
| Asian 16.66% | ||
| Other/Unknown 16.66% |
Note: QFE = Quality of the Family Environment, ABS = Australian Bureau of Statistics, Antisocial = Conduct Disorder/Oppositional Defiant Disorder, ADHD = Attention Deficit Hyperactivity Disorder, Anx/Dep = Anxiety/Depression, ASD = Autism Spectrum Disorder, CU = Callous-unemotional.
Serum Serotonin Levels
66 participants gave blood samples between 8am and 10.45am (M = 9.03) at commercial pathology collection centres. 4 ml of blood was collected and left at room temperature for 30 minutes before spinning at 3000 rpm for 5–10 minutes. Serum was then poured off and kept frozen at −40 degrees centigrade until analysis. Serotonin levels were determined at the General Biochemistry Laboratory, Sydpath, St Vincent's Hospital, Sydney. Following solid phase extraction through Merck extrelut columns, serotonin in serum was measured by gas chromatography/mass spectrometry (GC/MS). This assay has a minimum detection level of 50 nmol/L and the assay is linear up to 7000 nmol/L. Intra-assay and inter-assay variation was 4% and 8.5%, respectively. All analyses were carried out blind to the participant's demographic information and diagnostic group. While relationships between peripheral blood concentrations and brain levels of serotonin are not fully understood, there is evidence that blood levels are a reliable marker of levels in the cerebral spinal fluid (CSF). Yan et al. [57] found large correlations between the concentration of serotonin in CSF and blood in 7 non-human primates; (e.g., at 9am r = 0.81). Furthermore, previous research has successfully documented significant relationships between aggression/antisocial behaviour and serotonin using peripheral blood measures [33], [37], [58].
Serotonin-System Genotypes
122 participants chose to donate a saliva sample via Oragene saliva collection kits (http://www.dnagenotek.com/). DNA extraction rates were >95% for both blood and saliva methods. Genotypes were determined using iPLEX Gold™ primer extension followed by mass spectrometry analysis on the Sequenom MassARRAY system (Sequenom, San Diego, CA) by the Australian Genome Research Facility (AGRF: http://www.agrf.org.au/).
Serotonin-system SNPs were selected using dbSNP (www.ncbi.nlm.nih.gov/snp/). Genes that have been implicated in psychopathy (HTR1B, HTR2A, TPH1, TPH2) were prioritised so that at least two SNPs were selected for each of these genes. As this was an exploratory analysis of serotonin-system genetics it was important that the SNPs selected had known and relevant functionality. As such, with the exception of SNPs encoding TPH genes, all SNPs were functionally linked to serotonin-system gene expression by prior research (see table 1 for details). The SNPs were then reduced in number according to inclusion limitations of the Sequenom MassARRAY and the exclusion of SNPs in 100% linkage disequilibrium. The serotonin transporter promoter insertion/deletion polymorphism (5HTTLPR) has been implicated in psychopathy. However, due to funding and technical restraints, only SNP analyses were included in this research. The SNPs are shown in table 1.
Statistical Analyses
Statistical significance levels for each comparison were adjusted to maintain p = 0.05 across multiple comparisons using False Discovery Rate (FDR) methods [59], [60]. All SNPs were in Hardy-Weinberg equilibrium with the exception of rs2066713 from the 5HTT gene (p<0.01). 12 samples were genotyped twice, once from saliva and once from blood, to provide a quality check. There was a perfect correlation of genotypes (r = 1.00, p<0.001) between samples which suggests that rs2066713 was not out of Hardy-Weinberg equilibrium due to genotyping error. This lack of Hardy-Weinberg equilibrium may be related to the fact that all children included in the analysis had antisocial behaviour problems and thus represent a stratified sample of the population.
The CU groups were first compared, by means of a univariate analysis of variance, on a number of possible covariates. These covariates were; age, antisocial behaviour severity, autism spectrum disorder (ASD) severity, attention deficit hyperactivity disorder (ADHD) severity, anxiety/depression severity and the Quality of the Family Environment (QFE) measure. The groups were found to differ significantly with regards to ASD severity (F(1,135) = 5.03, p = 0.03). The differences in all other variables between groups were found to be non-significant (ps>0.10). As such, children with ASD severity greater than zero (N = 24) were excluded from all further analyses leaving a sample of 133 boys.
Chi-square tests were conducted to compare frequencies of genotypes of all 15 SNPs between the high and low CU groups. As this was an exploratory analysis, all three genotypes (homozygous minor, heterozygous, homozygous major) were included in the Chi-square analyses where possible. For SNPs for which the expected distribution of genotypes violated the assumptions of Chi-square, genotypes were combined in accordance with knowledge regarding dominant/recessive alleles inferred from the existing literature (see table 1). In all cases, this meant combining minor homozygotes with heterozygotes to form “carrier” groups of the dominant allele.
Logistic regression was used to determine whether serum serotonin levels were a significant predictor of CU traits group after controlling for possible confounding variables. CU traits group was used as the dependent variable, covariates (age, time of blood collection, ABS Economic Index of Area, QFE, parental psychopathology (FAD and DASS scores), comorbid diagnosis severity) were entered in step 1 and serum serotonin level was entered in step 2.
Results
Relationship of CU traits to Serotonin-System Genotypes
Genotypes of HTR1B SNP rs11568817 (χ2(2, N = 133) = 11.63, p = 0.003) and HTR2A SNP rs6314 (χ2 (1, N = 133) = 7.88, p = 0.005) were found to differ significantly in frequency between the high and low CU groups. Participants with high levels of CU traits were significantly more likely to be heterozygous (G/T) for SNP rs11568817 than participants low in CU traits. Participants homozygous for the major allele (CC) of SNP rs6314 were more likely to have high CU traits than heterozygotes (C/T) or minor homozygotes (TT). Results for all SNPs are shown in table 4. Haplotype analyses were conducted using PHASE software for SNPs from the 1b receptor gene, TPH1 and TPH2. The results of these analyses did not add to the results from the individual SNPs and, as such, are not described.
Table 4. Percentages and Chi-Square Statistic of Serotonin System Genotypes according to CU Group.
| Gene | SNP | Low CU (N = 92) | High CU (N = 41) | χ2 | ||||
| Minor Homozygote | Heterozygote | Major Homozygote | Minor Homozygote | Heterozygote | Major Homozygote | (N = 133) | ||
| HTR1A | rs6295 | 26 | 54 | 20 | 34 | 39 | 27 | 2.49 |
| HTR1B | rs13212041 | 46 | 54 | 43 | 57 | 0.11 | ||
| rs6296 | 11 | 31 | 58 | 5 | 49 | 46 | 4.08 | |
| rs130058 | 11 | 37 | 52 | 5 | 56 | 39 | 4.57 | |
| rs11568817 | 22 | 37 | 41 | 7 | 68 | 24 | 11.63* # | |
| HTR2A | rs6314 | 29 | 71 | 7 | 93 | 7.88* # | ||
| rs6311 | 20 | 45 | 35 | 20 | 34 | 46 | 1.54 | |
| HTR3B | rs1176744 | 15 | 39 | 46 | 18 | 37 | 45 | 0.18 |
| TPH1 | rs1800532 | 12 | 38 | 50 | 20 | 44 | 37 | 2.49 |
| rs211105 | 6 | 47 | 47 | 10 | 37 | 53 | 1.18 | |
| TPH2 | rs4570625 | 40 | 60 | 40 | 60 | 0.00 | ||
| rs11178997 | 10 | 90 | 15 | 85 | 0.72 | |||
| rs7305115 | 21 | 42 | 37 | 15 | 56 | 29 | 2.17 | |
| rs4565946 | 19 | 52 | 29 | 13 | 52 | 35 | 0.64 | |
| SLC6A4 | rs2066713 | 25 | 35 | 40 | 22 | 46 | 32 | 1.64 |
Note:
represents p<0.01 (2-tailed),
represents p<0.05 after FDR adjustment for 15 comparisons. SNPs located on the same chromosome are listed in order of their position along the gene.
Relationship of CU traits to Serum Serotonin Levels
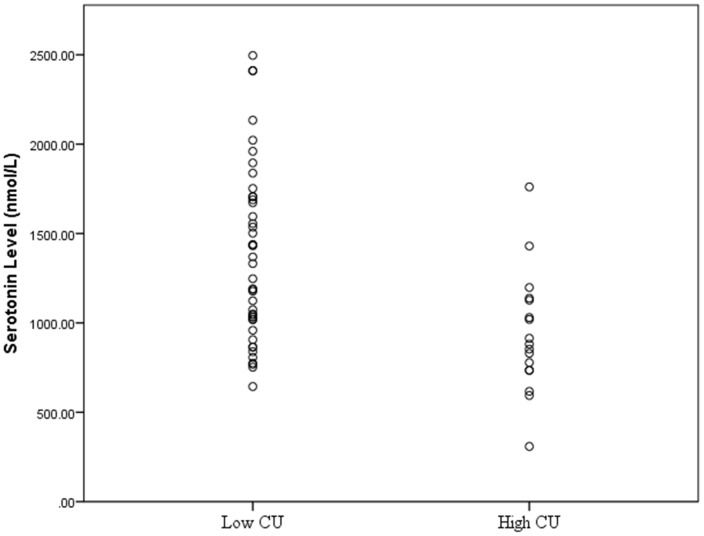
The regression model at step 1 did not significantly predict CU group membership (χ2 = 14.81, df = 12, p = 0.25). Of all the variables entered, only antisocial behaviour severity was a significant predictor of CU group membership (β = 1.15, Wald criteria = 5.05, p = 0.025, Exp (B) = 3.15). The inclusion of serotonin level at step 2 resulted in a significant regression model (χ2 = 27.98, df = 13, p = 0.009). At step 2, serum serotonin level (β = −0.004, Wald criteria = 7.44, p = 0.006, Exp (B) = 0.996) was the only significant predictor of CU traits group. All other variables were non-significant (p>0.05). The low CU group (N = 47) had a mean serotonin level (M = 1324.9 nmol/L) that was significantly higher than that of the high CU group (N = 19) (M = 861.6 nmol/L). Figure 1 shows the distribution of serum serotonin levels in the high and low CU groups.
Figure 1. Distribution of serotonin levels in each of the CU trait groups.
To be cautious and in order to be sure that the relationship between serotonin and CU traits was not being driven by medication effects, the regression analysis was repeated with boys using SSRIs/MAOIs (or a combination including SSRIs/MAOIs) (N = 5) excluded. Serum serotonin remained a significant predictor of CU traits group (β = −0.004, Wald criteria = 6.86, p = 0.009, Exp (B) = 0.996). None of the other variables were significant predictors of CU traits group when serotonin was included as a predictor.
Genetics and Serotonin Levels
In order to explore the nature of the relationships between serotonin-system genetics and peripheral serotonin levels analyses of variance were conducted with serotonin level as the dependent variable and HTR1B rs11568817 and HTR2A rs6314 as the independent factors. It should be noted that sample sizes for these analyses were small; the number of participants for whom both serotonin level and genetic data were available (Caucasian only) and who were not taking SSRI/MAOIs was N = 35. As such, these data should be viewed as exploratory pilot results requiring replication in larger samples.
Serotonin level was not significantly correlated with any of the diagnostic variables, age or the Quality of the Family Environment measure in the sample (ps>0.20). As such, no variables were included as covariates in the analyses. rs11568817 was significantly associated with serum serotonin levels ((F(2,32) = 3.55, p = 0.04). Pairwise comparisons revealed that minor homozygotes had significantly lower mean levels of serum serotonin (M = 853.5, SD = 370) than major homozygotes (M = 1790.9, SD = 711.4, p = 0.037). There were, however, only 4 minor homozygotes and when the data from these participants were grouped with those from the heterozygotes the association between genotypes and serum serotonin levels was no longer significant (F(1,33) = 2.98, p = 0.09). rs6314 showed unequal variances between genotypes (Levene's test of equality of error variance (F(1,33) = 11.38, p<0.01)) and so a Kruskall-Wallis test was conducted. The test showed that rs6314 was not a significant predictor of serum serotonin levels (H = 0.36, df = 1, p = 0.55).
Discussion
The results from analyses of both SNPs and peripheral serotonin levels suggest that differences in function of the serotonin system may be associated with high levels of CU traits in boys with antisocial/aggressive behaviours.
Two functional serotonin-system SNPs were significantly associated with CU traits. Genotypes of HTR1B rs11568817 were found to be significantly differently distributed in high versus low CU groups. Participants with high levels of CU traits were significantly more likely to be heterozygous (G/T) than participants low in CU traits. There thus appears to be a deleterious effect of the heterozygous genotype on CU traits: however, as true heterozygous effects are rare and the numbers of minor homozygotes in the high CU group are small, replication is required in larger cohorts to determine whether this is a true effect. Genotypes of HTR2A SNP rs6314 were also significantly differently distributed in high versus low CU groups. Participants homozygous for the major allele (CC) were more likely to have high CU traits than heterozygotes (C/T) or minor homozygotes (TT). The direction of this results is concordant with previous research that demonstrated that adolescents homozygous for the major (C) allele of rs6314 had significantly higher levels of antisocial behaviour and rule breaking, although notably not aggression, than heterozygotes and minor homozygotes [61].
The results from this exploratory study provide preliminary support to implicate the serotonin system in the aetiology of CU traits as proposed by the DAAM [12] and provide data to suggest that future research may benefit from comprehensive analyses of the serotonin 1b and 2a receptor genes.
This study also demonstrated that within a sample of children with antisocial behaviour problems there is a relationship between CU traits and peripheral serotonin levels that is independent from antisocial behaviour severity. It was found that serotonin level was a significant predictor of high CU traits even when antisocial behaviour severity was included as a covariate. This result supports the idea that previously reported mixed findings regarding the relationship between serotonin and antisocial and aggressive behaviour in children may be linked to variation in the levels of CU traits within and between samples.
With regards to the relationships between serotonin-system genetics and peripheral serotonin levels, the results provide tentative preliminary evidence to suggest that the G allele of HTR1B rs11568817 may be associated with lower peripheral serotonin levels. This analysis was, however, insufficiently powered and the results should be interpreted with appropriate caution. This direction of the association, however, is concordant with the idea that the minor allele of SNP rs11568817 engenders a risk for higher levels of CU traits, which in turn were found to be associated with lower levels of peripheral serotonin. Theoretically, this relationship between rs11568817 and peripheral serotonin levels is plausible. SNP rs11568817 is situated in the promoter region of the gene. Research has demonstrated that the minor allele of this SNP is associated with increased binding of transcription factors in the promoter region of the gene which produces a 2.3-fold increase in gene transcription [62]. As an autoreceptor, the serotonin 1b receptor helps to regulate the amount of serotonin released by the raphe nucleus. If gene transcription were increased, the density of 1b receptors would be amplified, which could lead to an increased inhibition of serotonin release. This is a possible mechanism by which the minor allele of SNP rs11568817 might lead to reduced peripheral serotonin levels and diminished serotonin-induced activation of the BLA as proposed by the DAAM [12]. It should be noted, however, that the effect of increased HTR1B gene expression on serotonin neurotransmission may vary depending on the location of the 5-HT1B receptor in the brain [63]. As such, any interpretation of the influence of changes in gene expression on serotonin neurotransmission should be made with caution.
There were a number of limitations to this research that require comment. First, this study was restricted by sample size. As the donation of a biological sample was a voluntary component of the overarching study the majority of donors chose to give a saliva sample from which peripheral levels of serotonin could not be obtained. As such, these results require replication with a larger sample for which both genetic data and peripheral serotonin levels would be necessary. In addition, power restrictions related to the sample size limited the number of SNPs that could be assessed. As such, while the results demonstrated that HTR1B and HTR2B warrant further investigation they do not rule out the possibility that genes for which few or only one SNP was tested may be important for CU traits. The research was also restricted by the use of a male-only sample. As such, no conclusions can be made about the relationship between serotonin-system function and CU traits in young girls with antisocial and aggressive behaviour. There is very limited research regarding the aetiology of CU traits in girls and this study requires replication in a female sample. The third limitation was that the genetic sample comprised children of Caucasian ethnicity only. While this was a necessary limitation in order to have a homogenous sample for genetic analyses it does, of course, mean that the results from this study cannot reliably be applied to children of other ethnic backgrounds. Ancestry was ascertained by self-report, rather than the more accurate method of genotyping ancestry-informative markers. However, it has been indicated that using self-reported ethnicity is an adequate substitute for ancestry markers in European ancestry populations, especially in studies examining a relatively small number of SNPs such as the current study [64]. Finally, it should be noted that the age range of children included in this research was large (3–16 years). Behavioural problems can present very differently across this age range and future research aimed at replicating these findings would benefit from testing restricted age ranges.
Future research should aim to replicate these findings in populations of adolescents and adults (both male and female). While CU traits in children are thought of by some as analogous to psychopathic personality traits in adults, the relationship between these traits and the function of neurochemical systems may not be the same in child and adult populations. The findings from this study may only apply to young male antisocial populations. Adolescence may significantly alter the associations between CU traits and the functions of the serotonin system. It is also likely that interaction effects of androgens may change the nature of associations between CU traits and neurochemical systems between genders. In order to comprehend the aetiology and development of CU traits it is necessary to get a better understanding of how these traits are characterised by the function of neurochemical systems in males and females across all developmental stages. It is also important for future research to go beyond exploratory association studies such as the one presented here and test specific hypotheses regarding functional SNPs and their impact on serotonin neurotransmission. We suggest that the HTR1B and HTR2A genes may be appropriate starting points.
In all, the results from this study suggest that the function of serotonin-system genes is important in the aetiology of CU traits. Functional SNPs from HTR1B and HTR2A were identified as significant predictors of CU traits in a sample of young boys with antisocial and aggressive behaviours. Future research should aim to replicate and further assess these results.
Acknowledgments
The authors would like to thank; Australian Genome Research Facility, Adelaide; General Biochemistry Laboratory, Sydpath, St Vincent's Hospital, Sydney; South Eastern Area Laboratory Services at Sydney Children's Hospital and Douglass Hanly Moir Pathology at Manly for their excellent services and support. The authors would also like to thank all staff and families involved with this research.
Funding Statement
Prof. Dadds is supported by grants from the Australian Research Council (DP120102296 and LP100200150) (http://www.arc.gov.au/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Scott S, Knapp M, Henderson J, Maughan B (2001) Financial cost of social exclusion: follow up study of antisocial children into adulthood. BMJ 323: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lynam DR, Caspi A, Moffitt TE, Loeber R, Stouthamer-Loeber M (2007) Longitudinal evidence that psychopathy scores in early adolescence predict adult psychopathy. Journal of Abnormal Psychology 116: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Viding E, Blair RJR, Moffitt TE, Plomin R (2005) Evidence for substantial genetic risk for psychopathy in 7-years-olds. Journal of Child Psychology and Psychiatry and Allied Disciplines 46: 592–597. [DOI] [PubMed] [Google Scholar]
- 4. Viding E, Jones AP, Paul JF, Moffitt TE, Plomin R (2008) Heritability of antisocial behaviour at 9: Do callous-unemotional traits matter? Developmental Science 11: 17–22. [DOI] [PubMed] [Google Scholar]
- 5. Frick PJ, Stickle TR, Dandreaux DM, Farrell JM, Kimonis ER (2005) Callous-unemotional traits in predicting the severity and stability of conduct problems and delinquency. Journal of Abnormal Child Psychology 33: 471–487. [DOI] [PubMed] [Google Scholar]
- 6. Rowe R, Maughan B, Moran P, Ford T, Briskman J, et al. (2010) The role of callous and unemotional traits in the diagnosis of conduct disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines 51: 688–695. [DOI] [PubMed] [Google Scholar]
- 7. Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE (2003) Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. Journal of Abnormal Child Psychology 31: 457–470. [DOI] [PubMed] [Google Scholar]
- 8. Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, et al. (2005) Deficient fear conditioning in psychopathy: A functional magnetic resonance imaging study. Archives of General Psychiatry 62: 799–805. [DOI] [PubMed] [Google Scholar]
- 9. Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E (2009) Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry 166: 95–102. [DOI] [PubMed] [Google Scholar]
- 10. Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, et al. (2008) Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry 165: 712–720. [DOI] [PubMed] [Google Scholar]
- 11. Müller JL, Sommer M, Wagner V, Lange K, Taschler H, et al. (2003) Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry 54: 152–162. [DOI] [PubMed] [Google Scholar]
- 12. Moul C, Killcross AS, Dadds MR (2012) A Model of Differential Amygdala Activation in Psychopathy. Psychological Review [DOI] [PubMed] [Google Scholar]
- 13. Harmer CJ, Bhagwagar Z, Perrett DI, Völlm BA, Cowen PJ, et al. (2003) Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 28: 148–152. [DOI] [PubMed] [Google Scholar]
- 14. Attenburrow MJ, Williams C, Odontiadis J, Reed A, Powell J, et al. (2003) Acute administration of nutritionally sourced tryptophan increases fear recognition. Psychopharmacology 169: 104–107. [DOI] [PubMed] [Google Scholar]
- 15. Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, et al. (2005) Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. Journal of Neuroscience 25: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newman JP, Curtin JJ, Bertsch JD, Baskin-Sommers AR (2010) Attention Moderates the Fearlessness of Psychopathic Offenders. Biological Psychiatry 67: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patrick CJ (1994) Emotion and psychopathy: Startling new insights. Psychophysiology 31: 319–330. [DOI] [PubMed] [Google Scholar]
- 18. Fowler T, Langley K, Rice F, Van Den Bree MBM, Ross K, et al. (2009) Psychopathy trait scores in adolescents with childhood ADHD: The contribution of genotypes affecting MAOA, 5HTT and COMT activity. Psychiatric Genetics 19: 312–319. [DOI] [PubMed] [Google Scholar]
- 19. Glenn AL (2011) The other allele: Exploring the long allele of the serotonin transporter gene as a potential risk factor for psychopathy: A review of the parallels in findings. Neuroscience and Biobehavioral Reviews 35: 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glenn AL, Raine A (2008) The Neurobiology of Psychopathy. Psychiatric Clinics of North America 31: 463–475. [DOI] [PubMed] [Google Scholar]
- 21. Cuartas Arias JM, Palacio Acosta CA, Valencia JG, Montoya GJ, Arango Viana JC, et al. (2011) Exploring epistasis in candidate genes for antisocial personality disorder. Psychiatric Genetics 21: 115–124. [DOI] [PubMed] [Google Scholar]
- 22. Sadeh N, Javdani S, Jackson JJ, Reynolds EK, Potenza MN, et al. (2010) Serotonin transporter gene associations with psychopathic traits in youth vary as a function of socioeconomic resources. Journal of Abnormal Psychology 119: 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conner TS, Jensen KP, Tennen H, Furneaux HM, Kranzler HR, et al. (2010) Functional polymorphisms in the serotonin 1B receptor gene (HTR1B) predict self-reported anger and hostility among young men. Am J Med Genet B Neuropsychiatr Genet 5: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moore TM, Scarpa A, Raine A (2002) A meta-analysis of serotonin metabolite 5-HIAA and antisocial behavior. Aggressive Behavior 28: 299–316. [Google Scholar]
- 25. Virkkunen M, Goldman D, Nielsen DA, Linnoila M (1995) Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. Journal of psychiatry & neuroscience: JPN 20: 271–275. [PMC free article] [PubMed] [Google Scholar]
- 26. O'Keane V, Moloney E, O'Neill H, O'Connor A, Smith C, et al. (1992) Blunted prolactin responses to d-fenfluramine in sociopathy. Evidence for subsensitivity of central serotonergic function. The British Journal of Psychiatry 160: 643–646. [DOI] [PubMed] [Google Scholar]
- 27. Moss HB, Yao JK, Panzak GL (1990) Serotonergic responsivity and behavioral dimensions in antisocial personality disorder with substance abuse. Biological Psychiatry 28: 325–338. [DOI] [PubMed] [Google Scholar]
- 28. Moffitt TE, Brammer GL, Caspi A, Fawcett JP, Raleigh M, et al. (1998) Whole Blood Serotonin Relates to Violence in an Epidemiological Study. Biological Psychiatry 43: 446–457. [DOI] [PubMed] [Google Scholar]
- 29.Manuck SB, Kaplan JR, Lotrich FE (2006) Brain serotonin and aggressive disposition in humans and nonhuman primates. In: Nelson RJ, editor. Biology of aggression. New York: Oxford University Press. pp. 65–113.
- 30. Van Goozen SHM, Matthys W, Cohen-Kettenis PT, Westenberg H, Van Engeland H (1999) Plasma monoamine metabolites and aggression: Two studies of normal and oppositional defiant disorder children. European Neuropsychopharmacology 9: 141–147. [DOI] [PubMed] [Google Scholar]
- 31. Halperin JM, Kalmar JH, Schulz KP, Marks DJ, Sharma V, et al. (2006) Elevated childhood serotonergic function protects against adolescent aggression in disruptive boys. Journal of the American Academy of Child and Adolescent Psychiatry 45: 833–840. [DOI] [PubMed] [Google Scholar]
- 32. Stoff DM, Pasatiempo AP, Yeung J, Cooper TB, Bridger WH, et al. (1992) Neuroendocrine responses to challenge with dl-fenfluramine and aggression in disruptive behavior disorders of children and adolescents. Psychiatry Research 43: 263–276. [DOI] [PubMed] [Google Scholar]
- 33. Cook EH Jr (1995) Attention deficit hyperactivity disorder and whole-blood serotonin levels: Effects of comorbidity. Psychiatry Research 57: 13–20. [DOI] [PubMed] [Google Scholar]
- 34. Unis AS, Cook EH, Vincent JG, Gjerde DK, Perry BD, et al. (1997) Platelet serotonin measures in adolescents with conduct disorder. Biological Psychiatry 42: 553–559. [DOI] [PubMed] [Google Scholar]
- 35. Pine DS, Coplan JD, Wasserman GA, Miller LS, Fried JE, et al. (1997) Neuroendocrine Response to Fenfluramine Challenge in Boys: Associations With Aggressive Behavior and Adverse Rearing. Arch Gen Psychiatry 54: 839–846. [DOI] [PubMed] [Google Scholar]
- 36. Castellanos FX, Elia J, Kruesi MJP, Gulotta CS, Mefford IN, et al. (1994) Cerebrospinal fluid monoamine metabolites in boys with attention-deficit hyperactivity disorder. Psychiatry Research 52: 305–316. [DOI] [PubMed] [Google Scholar]
- 37. Hughes CW, Petty F, Sheikha S, Kramer GL (1996) Whole-blood serotonin in children and adolescents with mood and behavior disorders. Psychiatry Research 65: 79–95. [DOI] [PubMed] [Google Scholar]
- 38. Halperin JM, Schulz KP, McKay KE, Sharma V, Newcorn JH (2003) Familial correlates of central serotonin function in children with disruptive behavior disorders. Psychiatry Research 119: 205–216. [DOI] [PubMed] [Google Scholar]
- 39.Moffitt TE (2003) Life-course-persistent and adolescence-limited antisocial behavior: A 10-year research review and a research agenda. In: Lahey BB, Moffitt TE, Caspi A, editors. Causes of conduct disorder and juvenile delinquency. New York, NY, US: Guilford Press. pp. 49–75.
- 40.(1994) Diagnostic and Statistical Manual of Mental Disorders. In: Association AP, editor.
- 41.Holland D, Dadds MR (1997) The Diagnostic Interview Schedule for Children, Adolescents, and Parents. In: Brisbane QGU, editor. Australia.
- 42.Frick PJ, Hare RD (2001) The Antisocial Process Screening Device. Toronto: Multi-Health Systems
- 43. Goodman R (1997) The strengths and difficulties questionnaire: A research note. Journal of Child Psychology and Psychiatry and Allied Disciplines 38: 581–586. [DOI] [PubMed] [Google Scholar]
- 44. Dadds MR, Frost A, Fraser J, Hawes DJ (2005) Disentangling the underlying dimensions of psychopathy and conduct problems in childhood: A community study. Journal of Consulting and Clinical Psychology 73: 400–410. [DOI] [PubMed] [Google Scholar]
- 45. Dadds MR, Hawes DJ, Frost AD, Vassallo S, Bunn P, et al. (2009) Learning to 'talk the talk: the relationship of psychopathic traits to deficits in empathy across childhood. Journal of child psychology and psychiatry, and allied disciplines 50: 599–606. [DOI] [PubMed] [Google Scholar]
- 46. Koglin U, Petermann F (2012) Callous-unemotional traits: Behavioral problems und prosocial behavior in kindergarten children. Kindheit und Entwicklung 21: 141–150. [Google Scholar]
- 47. Epstein NB, Baldwin LM, Bishop DS (1983) The McMaster Family Assessment Device. Journal of Marital and Family Therapy 9: 171–180. [Google Scholar]
- 48. Walrath CM, Franco E, Liao Q, Holden EW (2004) Measures of child emotional and behavioral strengths and family functioning: A preliminary report on the reliability and validity of their spanish translations. Journal of Psychoeducational Assessment 22: 209–219. [Google Scholar]
- 49. Waller G, Slade P, Calam R (1990) Who knows best? Family interaction and eating disorders. British Journal of Psychiatry 156: 546–550. [DOI] [PubMed] [Google Scholar]
- 50.Lovibond SH, Lovibond PF (1995) Manual for the Depression Anxiety Stress Scales.
- 51. Szabó M (2010) The short version of the Depression Anxiety Stress Scales (DASS-21): Factor structure in a young adolescent sample. Journal of Adolescence 33: 1–8. [DOI] [PubMed] [Google Scholar]
- 52.(2001) Information Paper: Census of Population and Housing: Socio-Economic Indexes for Areas, Australia. Information Paper: Census of Population and Housing - Socio-Economic Indexes for Areas, Australia.
- 53. Dadds MR, El Masry Y, Wimalaweera S, Guastella AJ (2008) Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry 47: 455–463. [DOI] [PubMed] [Google Scholar]
- 54.Pasalich DS, Dadds MR, Hawes DJ, Brennan J (2012) Attachment and callous-unemotional traits in children with early-onset conduct problems. Journal of Child Psychology and Psychiatry and Allied Disciplines. [DOI] [PubMed]
- 55.Frick PJ, Moffitt TE (2010) A proposal to the DSM–V Childhood Disorders and the ADHD and Disruptive Behavior Disorders Work Groups to include a specifier to the diagnosis of conduct disorder based on the presence of callous-unemotional traits.
- 56. Rey JM, Singh M, Hung SF, Dossetor DR, Newman L, et al. (1997) A global scale to measure the quality of the family environment. Archives of General Psychiatry 54: 817–822. [DOI] [PubMed] [Google Scholar]
- 57. Yan D, Urano T, Pietraszek MH, Shimoyama I, Uemura K, et al. (1993) Correlation between serotonergic measures in cerebrospinal fluid and blood of subhuman primate. Life Sciences 52: 745–749. [DOI] [PubMed] [Google Scholar]
- 58. Gao XP, Su LY, Xie GR, Huang CX, Li XR (2005) Intellectual and behavioral characteristics and their relations with serum 5-hydroxytryptamine level in children with attention deficit hyperactivity disorder complicated by oppositional defiant disorder. Chinese Journal of Clinical Rehabilitation 9: 221–223. [Google Scholar]
- 59. Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 57: 289–300. [Google Scholar]
- 60. Keselman HJ, Cribbie R, Holland B (1999) The pairwise multiple comparison multiplicity problem: An alternative approach to familywise and comparison wise Type I error control. Psychological Methods 4: 58–69. [Google Scholar]
- 61. Burt SA, Mikolajewski AJ (2008) Preliminary evidence that specific candidate genes are associated with adolescent-onset antisocial behavior. Aggressive Behavior 34: 437–445. [DOI] [PubMed] [Google Scholar]
- 62. Duan J, Sanders AR, Molen JE, Martinolich L, Mowry BJ, et al. (2003) Polymorphisms in the 5′-untranslated region of the human serotonin receptor 1B (HTR1B) gene affect gene expression. Mol Psychiatry 8: 901–910. [DOI] [PubMed] [Google Scholar]
- 63. Drago A, Alboni S, Nicoletta B, De Ronchi D, Serretti A (2010) HTR1B as a risk profile maker in psychiatric disorders: A review through motivation and memory. European Journal of Clinical Pharmacology 66: 5–27. [DOI] [PubMed] [Google Scholar]
- 64. Divers J, Redden D, Rice K, Vaughan L, Padilla M, et al. (2011) Comparing self-reported ethnicity to genetic background measures in the context of the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Genetics 12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Willemsen J, De Ganck J, Verhaeghe P (2012) Psychopathy, traumatic exposure, and lifetime posttraumatic stress. International Journal of Offender Therapy and Comparative Criminology 56: 505–524. [DOI] [PubMed] [Google Scholar]
- 66. Albert PR (2012) Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos Trans R Soc Lond B Biol Sci 367: 2402–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jensen KP, Covault J, Conner TS, Tennen H, Kranzler HR, et al. (2009) A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Molecular Psychiatry 14: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Clark MS, Neumaier JF (2001) The 5-HT1B receptor: behavioral implications. Psychopharmacology Bulletin 35: 170–185. [PubMed] [Google Scholar]
- 69. Krischer MK, Sevecke K (2008) Early traumatization and psychopathy in female and male juvenile offenders. International Journal of Law and Psychiatry 31: 253–262. [DOI] [PubMed] [Google Scholar]
- 70. Zouk H, McGirr A, Lebel V, Benkelfat C, Rouleau G, et al. (2007) The effect of genetic variation of the serotonin 1B receptor gene on impulsive aggressive behavior and suicide. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics 144: 996–1002. [DOI] [PubMed] [Google Scholar]
- 71. Hicks BM, Vaidyanathan U, Patrick CJ (2010) Validating female psychopathy subtypes: Differences in personality, antisocial and violent behavior, substance abuse, trauma, and mental health. Personality Disorders: Theory, Research, and Treatment 1: 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Heiser P, Dempfle A, Friedel S, Konrad K, Hinney A, et al. (2007) Family-based association study of serotonergic candidate genes and attention-deficit/hyperactivity disorder in a German sample. J Neural Transm 114: 513–521. [DOI] [PubMed] [Google Scholar]
- 73. Giegling I, Hartmann AM, Möller H-J, Rujescu D (2006) Anger- and aggression-related traits are associated with polymorphisms in the 5-HT-2A gene. Journal of Affective Disorders 96: 75–81. [DOI] [PubMed] [Google Scholar]
- 74. Turecki G, Brière R, Dewar K, Antonetti T, Lesage AD, et al. (1999) Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. American Journal of Psychiatry 156: 1456–1458. [DOI] [PubMed] [Google Scholar]
- 75. Enoch MA, Gorodetsky E, Hodgkinson C, Roy A, Goldman D (2011) Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence. Mol Psychiatry 16: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Walstab J, Hammer C, Bönisch H, Rappold G, Niesler B (2008) Naturally occurring variants in the HTR3B gene significantly alter properties of human heteromeric 5-hydroxytryptamine-3A/B receptors. Pharmacogenetics and Genomics 18: 793–802. [DOI] [PubMed] [Google Scholar]
- 77. Chen D, Liu F, Yang C, Liang X, Shang Q, et al. (2012) Association between the TPH1 A218C polymorphism and risk of mood disorders and alcohol dependence: Evidence from the current studies. Journal of Affective Disorders 138: 27–33. [DOI] [PubMed] [Google Scholar]
- 78. Davidson RJ, Putnam KM, Larson CL (2000) Dysfunction in the Neural Circuitry of Emotion Regulation–A Possible Prelude to Violence. Science 289: 591–594. [DOI] [PubMed] [Google Scholar]
- 79. Jönsson E, Goldman D, Spurlock G, Gustavsson JP, Nielsen D, et al. (1997) Tryptophan hydroxylase and catechol-O-methyltransferase gene polymorphisms: relationships to monoamine metabolite concentrations in CSF of healthy volunteers. European Archives of Psychiatry and Clinical Neuroscience 247: 297–302. [DOI] [PubMed] [Google Scholar]
- 80. Gaysina D, Leopardi R, Gizatullin R, Juriev E, Gabdulhakov R, et al. (2006) Haplotype analysis of the TPH gene and association with suicidal behaviour in Russian males and females. Annals of General Psychiatry 5: S213. [Google Scholar]
- 81. Zill P, Büttner A, Eisenmenger W, Möller H-J, Ackenheil M, et al. (2007) Analysis of tryptophan hydroxylase I and II mRNA expression in the human brain: A post-mortem study. Journal of Psychiatric Research 41: 168–173. [DOI] [PubMed] [Google Scholar]
- 82. Gao J, Pan Z, Jiao Z, Li F, Zhao G, et al. (2012) TPH2 Gene Polymorphisms and Major Depression – A Meta-Analysis. PLoS ONE 7: e36721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, et al. (2005) A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Molecular Psychiatry 10: 884–888. [DOI] [PubMed] [Google Scholar]
- 84. Chen GL, Vallender EJ, Miller GM (2008) Functional characterization of the human TPH2 5′ regulatory region: Untranslated region and polymorphisms modulate gene expression in vitro. Human Genetics 122: 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Müller J, et al. (2007) Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. International Journal of Neuropsychopharmacology 10: 309–320. [DOI] [PubMed] [Google Scholar]
- 86. Scheuch K, Lautenschlager M, Grohmann M, Stahlberg S, Kirchheiner J, et al. (2007) Characterization of a functional promoter polymorphism of the human tryptophan hydroxylase 2 gene in serotonergic raphe neurons. Biol Psychiatry 62: 1288–1294. [DOI] [PubMed] [Google Scholar]
- 87. Lim JE, Pinsonneault J, Sadee W, Saffen D (2007) Tryptophan hydroxylase 2 (TPH2) haplotypes predict levels of TPH2 mRNA expression in human pons. Molecular Psychiatry 12: 491–501. [DOI] [PubMed] [Google Scholar]
- 88. Mossner R, Walitza S, Geller F, Scherag A, Gutknecht L, et al. (2006) Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in children and adolescents with obsessive-compulsive disorder. Int J Neuropsychopharmacol 9: 437–442. [DOI] [PubMed] [Google Scholar]
- 89. Ma DQ, Rabionet R, Konidari I, Jaworski J, Cukier HN, et al. (2010) Association and gene–gene interaction of SLC6A4 and ITGB3 in autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 153B: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Weiss LA, Abney M, Cook EH Jr, Ober C (2005) Sex-Specific Genetic Architecture of Whole Blood Serotonin Levels. The American Journal of Human Genetics 76: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]