Abstract
Background
The lung clearance index (LCI), measured by multiple breath washout (MBW), reflects global ventilation inhomogeneity and is a sensitive marker of early cystic fibrosis (CF) lung disease. Current evidence is based on a customized mass spectrometry system that uses sulfur hexafluoride (SF6) as a tracer gas, which is not widely available. Nitrogen (N2) washout may be better suited for clinical use and multi-center trials.
Objective
To compare the results obtained from a N2 washout system to those generated by the SF6 based system in healthy children and children with CF.
Methods
Children with CF were recruited from outpatient clinics; healthy children were recruited from the Research4Kids online portal. Participants performed MBWSF6 (Amis 2000, Innovision, Denmark) and MBWN2 (ExhalyzerD, EcoMedics, Switzerland) in triplicate, in random order on the same day. Agreement between systems was assessed by Bland-Altman plot.
Results
Sixty-two healthy and 61 children with CF completed measurements on both systems. In health there was good agreement between systems (limits of agreement −0.7 to 1.9); on average N2 produced higher values of LCI (mean difference 0.58 (95% CI 0.42 to 0.74)). In CF the difference between systems was double that in health with a clear bias towards disproportionately higher LCIN2 compared to LCISF6 at higher mean values of LCI.
Conclusion
LCIN2 and LCISF6 have similar discriminative power and intra-session repeatability but are not interchangeable. MBWN2 offers a valid new tool to investigate early obstructive lung disease in CF, but requires independent normative values.
Introduction
Pathologic changes associated with cystic fibrosis (CF) lung disease occur in early childhood, but have historically gone undetected until the onset of clinical symptoms, at which point irreversible lung damage may have already occurred [1]. Consequently, over the last ten years the focus of clinical care in CF has shifted to early intervention and prevention of these structural changes. To facilitate early intervention there is a pressing need for surrogate markers of early obstructive lung disease that are also sensitive enough to detect treatment effects. [2]
Spirometric measures, such as forced expired volume in one second (FEV1), have traditionally been used in the assessment of CF lung disease due to their direct correlation with morbidity and mortality.[3] However, FEV1 tends to remain within normal limits in a high percentage of children, despite radiographic evidence of airway damage. [4], [5], [6], [7] This is likely due to the fact that these measures are primarily influenced by resistive changes in the large airways and thus not reflective of the patchy distribution of small airway pathology characteristic of early CF lung disease. [8] In addition to this inherent insensitivity, young children are also often not developmentally advanced enough to perform complicated respiratory maneuvers. The lung clearance index (LCI), as measured by multiple breath washout (MBW), reflects global ventilation inhomogeneity (VI) and as such is a highly sensitive marker of early obstructive lung disease.[9], [10], [11] Furthermore, LCI is more sensitive than other measures of lung function in detecting structural changes identified by high resolution computed tomography (HRCT) imaging [4], [6], [7]. MBW is performed during tidal breathing and requires only passive co-operation, it is therefore feasible during infancy and early childhood. Importantly, LCI tracks from preschool to school-age and has been found to precede subsequent abnormalities in spirometric indices [12].
To date most evidence for LCI has been collected using mass spectrometry based MBW systems. [9], [10], [11] The equipment is immobile, expensive and uses sulfur hexafluoride (SF6) as its inert tracer gas. Therefore, the current customized system is neither suitable for multi-center clinical research nor clinical practice. Multiple breath nitrogen washout (MBWN2) offers a possible alternative to mass spectrometry based SF6 washout (MBWSF6). N2 is a resident gas and permeates even poorly ventilated lung units, which may not be the case during MBWSF6. Thus, the physiological attributes of the respective tracer gases may lead to differences in measurements obtained with the two systems. The aim of this study was to determine whether the results of MBWN2 and MBWSF6 can be used interchangeably in both healthy children and children with CF. In addition, we aimed to quantify the discriminatory power of LCI, as measured by MBWN2 and MBWSF6, to differentiate health and disease throughout a range of pulmonary function abnormalities in CF.
Methods
This study was approved by the research ethics board (REB) at the Hospital for Sick Children (HSC), Toronto, Canada (REB# 1000019945). Informed written consent was obtained from the parents or guardians of healthy children and children with CF. Assent was obtained from subjects when appropriate.
Study Subjects
Families with eligible children between the ages of 3 and 18 years attending a routine visit to the CF outpatient clinic of the HSC were invited to participate in our study. Eligibility was defined as a diagnosis of CF by a positive newborn screening test or at least one clinical feature of CF in combination with either a documented sweat chloride ≥60 mEq/L by quantitative pilocarpine iontophoresis or a genotype with two CF-causing mutations. Children with acute respiratory symptoms, inter-current respiratory infections, or chronic lung disease not related to CF were excluded from participation; as were patients requiring supplemental oxygen.
Healthy controls were recruited from siblings of children attending our Respiratory Medicine outpatient clinics, children of staff members and through the Research4Kids online portal supported by the SickKids Research Institute. Health was defined as no history of chronic use of bronchodilator or controller medication for asthma symptoms, no chronic lung disease and no active or passive exposure to cigarette smoke. All subjects were free of acute respiratory tract symptoms for at least four weeks prior to testing. Children with any history of wheeze within the previous two years were excluded from the study.
Participants performed MBWSF6 and MBWN2 in triplicate, in random order on the same day. All children attempted to perform spirometry, while plethysmographic lung volume measurement was attempted by children age seven and older. Lung function testing was performed according to American Thoracic Society (ATS) standards using the Vmax system (VIASYS CareFusion San Diego, California, USA). [13], [14] Children between the ages of 3 and 6 years performed spirometry to ATS ERS standards for pre-school lung function testing [15] using the Easy-on-PC system (ndd, Zurich, Switzerland). Height, weight, BMI and spirometry outcomes were standardized for age, body size and sex.[16], [17], [18]
MBW Testing
MBWSF6
A mass spectrometer (AMIS 2000; Innovision A/S, Odense, Denmark) based set up and technique was used to perform MBW testing with a SF6/He gas mixture as previously described.[9], [10], [11] Briefly, subjects breathed a gas mixture containing 4% SF6, 4% He, 21% O2, balance N2 via an open circuit bias flow system through either a mask or mouthpiece and an attached heated pneumotachograph (3700 series Hans Rudolph, Shawnee, KS, USA) which measures flow by pressure differential, until equilibrium was reached. Once the inert tracer gas (SF6) stabilized at 4%, the gas source was removed during the start of exhalation and the subject breathed room air until end-tidal SF6 concentration reached below 1/40th of its starting concentration for at least three breaths. Depending on individual feasibility, either a mask (Silkomed, Rendell Baker Masks size 3, Rusch Canada Inc., Benson Medical Industries, Markham, Ontario) filled with therapeutic putty (Air Putty, Sammons Preston Canada Inc., Mississauga, Ontario) or mouthpiece (VacuMed model #1004, Ventura, CA, USA) with nose clips was used. All subjects used the same size pneumotachograph with a total post gas sampling point dead space of 15.4 ml; pre-gas sampling point dead space was considered to be zero for mouthpiece and 10 mls for mask and putty [19]. Calculation of signal delay and subsequent alignment of flow and gas concentration signals with appropriate BTPS correction was performed as previously described. [9], [10], [11]
MBWN2
MBWN2 was performed using an open circuit, bias flow system (Exhalyzer D®, EcoMedics AG, and Duernten, Switzerland) and associated software (Spiroware® 3.1 EcoMedics AG). This MBWN2 device uses an indirect technique to determine N2 concentration. Oxygen (O2) and carbon dioxide (CO2) were measured during testing; N2 was then calculated based on Dalton's law of partial pressures.[20] CO2 was measured using a mainstream infrared CO2 sensor (Capnostat® 5, Respironics Novametrix LLC, Wallingford CT, USA). Incorporated into the CO2 sensor was a sampling port where O2 was measured side stream at a rate of approximately 3 ml/s to an internal O2 analyzer (Oxigraf Inc, Mountain View, CA, USA). Flow was measured by an ultrasonic flow head [21] inline along the breathing circuit, and volume was derived from the flow signal by integration. Due to differences in O2 and CO2 sensor response times a speeding algorithm was applied to the O2 signal to reduce the response time to approximately 110 ms in order to align gas signals. Synchronized gas signals were time-shifted to align with flow as described by Singer et al, 2012.[20]
In contrast to MBWSF6, a wash-in phase using a test gas was not required. The subject breathed 100% O2 during wash out to reduce the concentration of N2 in the lungs to below 1/40th of the starting concentration. The switch from room air to 100% O2 was automated, eliminating the need for manual disconnect as was done during MBWSF6. As there was no parallel wash-in phase during MBWN2 subjects were allowed to re-equilibrate in room air between test trials. Time between trials was at minimum the time required to washout on the previous trial.
Offline Data Analysis
Synchronized data files from both systems were analyzed by trained observers using custom written analysis software (TestPoint, Capital Equipment Corp., Billerica, MA, USA). To assess inter-observer variability of offline MBW results, the N2 data files from 40 subjects (20 HC and 20 CF) were independently over-read by two observers. Quality control standards, as proposed by the ERS working group [19], were used as guidelines for technical acceptability during offline data analysis.
Indices calculated
Functional residual capacity (FRC) is calculated by dividing the net amount of inert tracer gas exhaled over the course of the washout by the difference in end-tidal marker gas concentration (Cet) from the beginning to the end of washout. [22] LCI represents the number of FRC turnovers required to reduce the end-tidal concentration of tracer gas to 1/40th of the starting concentration and is calculated by dividing the sum of exhaled tidal breaths (cumulative exhaled volume (CEV)) by simultaneously measured FRC. [22]
Statistical Analysis
For each outcome, agreement between the SF6 and N2 systems was assessed using Bland-Altman plots. [23] A t-test was used to test whether MBW outcomes in healthy controls were different from children with CF. Additional analysis used simple linear regression to determine whether the differences between the two systems could be explained by body size and/or lung function. A p-value <0.05 was regarded as statistically significant.
Results
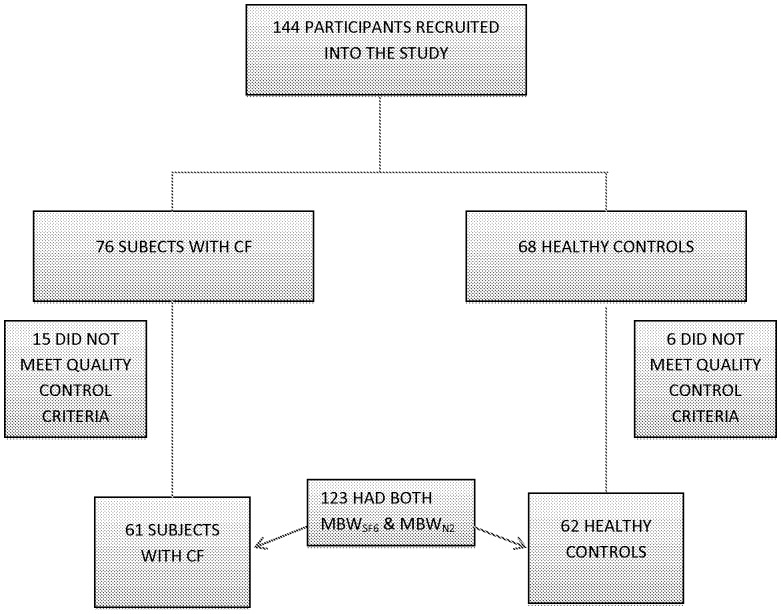
144 children (68 healthy controls and 76 CF) were enrolled into this study (Figure 1). Subjects who failed to meet MBWSF6 and or MBWN2 quality control criteria were excluded from analysis (Figure 1). In most cases, subjects failed to meet quality control criteria due to inability to maintain stable breathing pattern, leak around interface, or incomplete washout. In total 62 HC (91%) and 61 CF (80%) had paired measurements on both systems available for analysis. Both groups were well matched for age and sex. As expected the healthy group were taller and heavier than CF subjects (Table 1). Spirometry (FEV1 z-scores) was reduced in the CF group compared to healthy controls, whereas FRC measured by plethysmography (percent predicted) was elevated in CF compared to healthy controls (Table 1). Each subject completed at least two acceptable MBW trials. Overall the within test occasion variability (coefficient of variation (CV) of all trials) was similar for both systems, and similar in health and disease (Table 2). There was no evidence that the CV was affected by increased ventilation inhomogeneity as CV was constant across the range of LCI.
Figure 1. Study Participant Flow Diagram.
Table 1. Characteristics of the study population (presented as mean (SD) unless otherwise indicated).
| CF n = 61 | Health n = 62 | |
| % Females | 41% | 39% |
| Age (years) mean (range) | 11.0 (3–17) | 10.9 (3–18) |
| Weight (kg) Centile-for-age | 45.7 (27.7) | 69.0 (22.4) |
| BMI Centile-for-age | 46.5 (25.3) | 57.2 (27.7) |
| Height (cm) Centile-for-age | 47.6 (29.5) | 75.8 (21.1) |
| * FRCpleth (% pred) | 118.8 (19.9) | 105.5 (14.6) |
| ** FEV1 (Z-score) | −1.2 (1.5) | −0.2 (0.8) |
| ** FEV1 (% pred) | 85.9 (18.2) | 97.8 (10.2) |
FRCpleth measurements were obtained in n = 44 HC and n = 30 CF.
FEV1 measured in n = 53 HC and n = 56 CF.
Table 2. Summary of MBW outcomes (presented as mean (CV) unless otherwise indicated).
| HC mean (CV) | CF mean (CV) | P-value | |
| Sample Size | 61 | 62 | |
| LCISF6 | 6.19 (0.05) | 10.05 (0.05) | <0.001 |
| LCIN2 | 6.81 (0.05) | 11.29 (0.05) | <0.001 |
| FRCSF6 (L) | 1.60 (0.06) | 1.41 (0.06) | 0.185 |
| FRCN2 (L) | 1.92 (0.07) | 1.89 (0.05) | 0.948 |
| * FRCpleth (L) | 2.25 (0.79) | 2.31(0.97) | 0.471 |
FRCpleth measurements were obtained in n = 44 HC and n = 30 CF; results presented as mean (SD).
LCI comparison between systems
In both systems LCI identified the same proportion (96%) and the same subjects as abnormal. On average, in healthy subjects MBWN2 generated higher values of LCI (mean difference (LCIN2−LCISF6) = 0.61 (95% CI 0.45 to 0.78), but there was good agreement between systems with uniform scatter around the mean difference (limits of agreement −0.7 to 1.9) (Figure 2a). In CF, the mean difference between systems (LCIN2−LCISF6) was double that in health (1.41 (95% CI 0.92 to 1.90), with a clear bias such that LCIN2 was disproportionately higher than LCISF6 as the average LCI values increased (Figure 2b).
Figure 2. Bland Altman Plot of the agreement between LCI N2 and LCISF6 in a) healthy controls and b) subjects with Cystic Fibrosis.
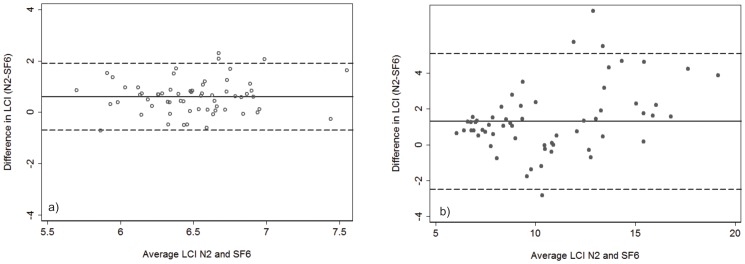
The solid horizontal line represents the mean difference, and the dashed lines represent the limits of agreement (mean difference+/−2SD). In health, there was good agreement between the systems, the mean difference (LCIN2−LCISF6 was 0.61 (95% CI 0.45 to 0.78), limits of agreement (−0.7 to 1.9)); whereas in CF there was an obvious bias (mean difference = 1.41 (95% CI 0.92 to 1.90), limits of agreement (−2.4 to 5.2)) such that LCIN2 increased disproportionately to LCISF6 as mean LCI increased.
The same bias was not observed when LCISF6 was compared to LCI measured using another low density gas, helium (LCIHe). While the variability in the difference between LCISF6 and LCIHe increased as the average LCI increased, the scatter was uniform on both sides of the mean difference (data not shown).
FRC comparison between systems
As a crude way to adjust for body size, FRC measurements from both systems were adjusted for height (FRC/height)*100 and expressed as relative FRC. In health MBWN2 produced higher values of FRC (mean difference (FRCN2−RCSF6) = 0.21 (95% CI 0.16; 0.25)), with no bias observed between systems (limits of agreement −0.15; 0.56) (Figure 3a). In CF the difference between the two systems was greater than in health (mean difference = 0.33 (95%CI 0.27; 0.38)), and the difference was disproportionately greater with higher average adjusted FRC (Figure 3b).
Figure 3. Bland Altman Plot of the agreement between FRC N2 and FRCSF6 in a) healthy controls and b) subjects with Cystic Fibrosis.
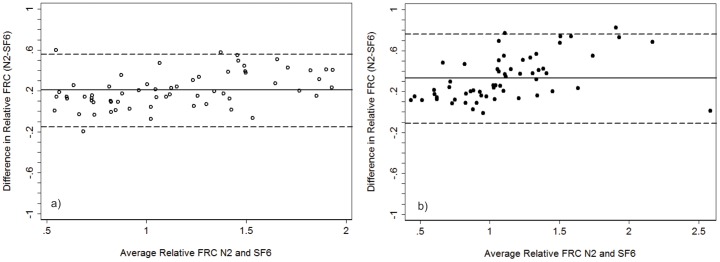
The solid horizontal line represents the mean difference, and the dashed lines represent the limits of agreement (mean difference+/−2SD). FRC was crudely corrected for body size (FRC/height*100). In health N2 produced higher values of FRC; the mean difference (FRCN2−FRCSF6) was 0.21 (95%CI 0.16; 0.25), limits of agreement (−0.15; 0.56) with no bias observed between systems. In CF the mean difference was 0.33 (95%CI 0.27; 0.38), limits of agreement (−0.11; 0.76) with the difference between systems becoming disproportionately greater with higher adjusted FRC.
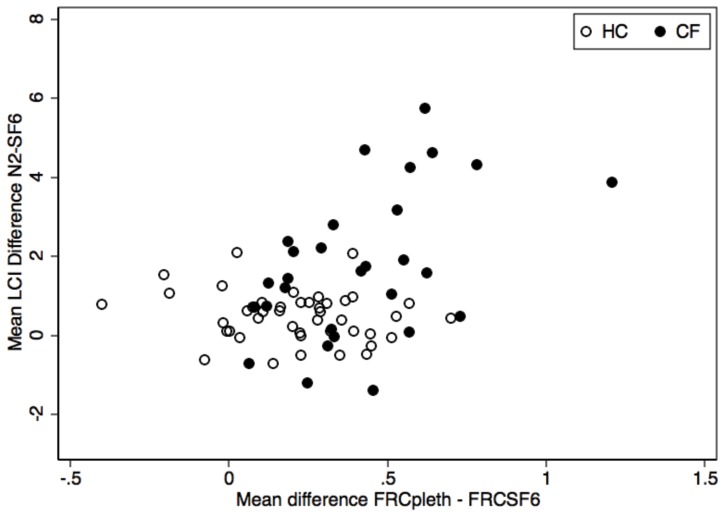
Thirty CF and 44 HC had measurements of all three FRC outcomes (FRCpleth, FRCSF6 and FRCN2) (Table 2); for comparison each FRC measure was corrected for body size in the same manner (FRC/height*100). FRCN2 more closely agreed with FRCpleth (Figure 4). As the difference between FRCpleth and FRCSF6 may represent the volume of gas in extremely slowly ventilated lung units, we compared the difference in LCI between systems to trapped gas volume (FRCpleth−FRCSF6). We observed that the volume of trapped gas increased as LCIN2 increased disproportionately to LCISF6 suggesting that the N2 system is measuring volume not captured using SF6 (Figure 5).
Figure 4. Bland Altman Plot of the agreement between a) FRCpleth and FRCSF6 and b) FRCpleth and FRCN2.
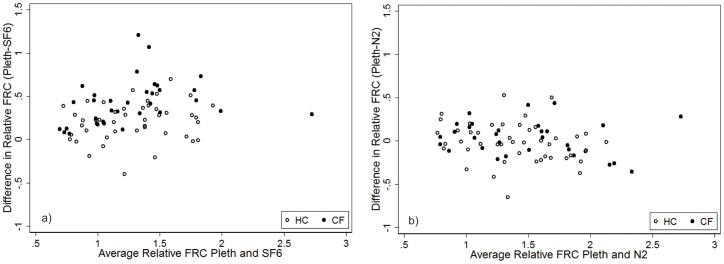
Healthy controls are represented by the open circles, and subjects with CF by the solid circles. FRC was crudely corrected for body size (FRC/height*100). FRCN2 more closely agreed with FRCpleth with the difference between FRCpleth and FRCSF6 suggestive of trapped gas volume.
Figure 5. Comparison of the mean difference in LCI between systems to volume of trapped gas (FRCpleth−FRCSF6).
The volume of trapped gas increased as LCIN2 increased disproportionately to LCISF6 suggesting that the N2 system was measuring volume not captured during MBWSF6.
Additional comparisons between systems
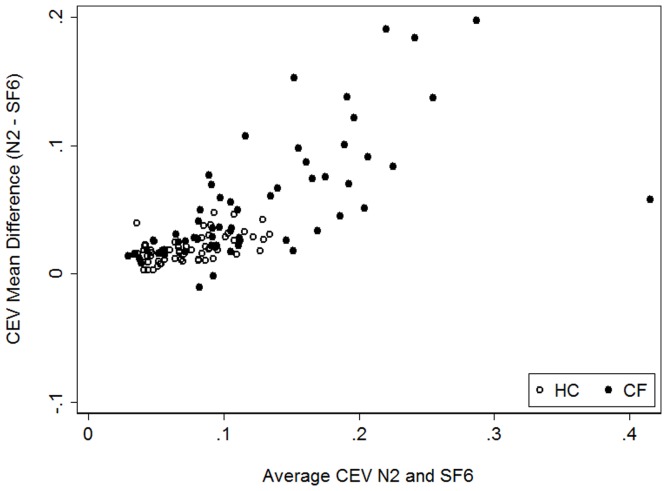
As LCI is the cumulative expiratory volume (CEV) divided by FRC, we examined the agreement of CEVN2 and CEVSF6, corrected for pre and post gas sampling point dead space, between systems and found good agreement in health with no bias observed (limits of agreement −0.001 to 0.041) (Figure 6). In CF, there was a strong bias such that CEVN2 was disproportionately higher than CEVSF6 with increasing mean values of CEV (limits of agreement −0.041 to 0.150).
Figure 6. Bland Altman Plot of the agreement between CEVN2 and CEVSF6.
Healthy controls are represented by the open circles, and subjects with CF by the solid diamonds. CEV was adjusted for body size (CEV/height*100). In health there was good agreement between systems, mean difference (CEVN2−CEVSF6) was 0.20 (95% CI 0.017; 0.022), limits of agreement (−0.001; 0.041) with no bias observed between systems. In CF there was a strong bias such that CEVN2 became disproportionately higher than CEVSF6 with increasing mean values of CEV (mean difference (0.054 (95% CI 0.042; 0.067), limits of agreement (−0.041; 0.150)).
Since CEV is the product of tidal volume (Vt) and number of breaths required to complete washout, we compared the Vt/FRC ratio between systems. Both variables were corrected for pre and post gas sampling point dead space. While the variability of Vt/FRC was greater in health than in CF, there was minimal difference and no bias observed when the two systems were compared (data not shown). Healthy subjects required an additional 5 breaths to complete washout during MBWN2 compared to MBWSF6 (mean (SD): 35(14) vs. 30(13), p<0.001). CF subjects required an additional 18 breaths to complete washout using the N2 system (mean (SD): 56 (26) vs. 38(14), p<0.001). This indicates that the bias observed in CEV between systems is related to number of breaths. When the difference in breath number was compared to volume of trapped gas we found that number of breaths required to complete washout using N2 increases proportionally to volume of trapped gas (data not shown).
Respiratory rate was lower during MBWN2 compared to MBWSF6 in both health (17 breaths/minute vs. 19; p<0.001) and disease (18 breaths/minute vs. 21; p<0.001)), but was constant across the range of LCI; there was no bias observed in respiratory rate between the two systems (data not shown).
Comparison between systems and disease severity
To determine whether the difference in LCI between systems was related to lung function we compared the difference in LCI across a range of lung function abnormalities. The difference in LCI between the two systems was greater as lung function worsened (i.e. lower values of FEV1 (Figure 7) and higher values of FRCpleth (data not shown)), such that on average LCIN2 was disproportionately higher than LCISF6 in subjects with abnormal lung function compared to those with normal spirometric and plethysmographic findings (data not shown). The observed differences could not be explained by differences in age or body size (height, weight, BMI (data not shown)).
Figure 7. Comparison of difference in LCI (LCIN2−LCISF6) to FEV1 (% predicted).
Healthy controls are represented by the open circles and subjects with CF by the solid circles. The difference in LCI was greater as FEV1 became lower such that on average LCIN2 was disproportionately higher than LCISF6 in subjects with abnormal spirometric findings.
Finally, to investigate the contribution of factors explaining the observed differences in LCI between systems, a linear regression was performed for each factor separately (Table 3). Greater breath number during MBWN2 compared to MBWSF6 explained most of the variability (24%) in the difference in LCI while trapped gas and zFEV1 explained 15% and 13% of the variability respectively.
Table 3. Linear univariate regression analysis investigating difference in LCI between the two systems.
| R2 | |
| Difference in breath number | 0.242 |
| zFEV1 | 0.129 |
| FRCpleth percent predicted | 0.097 |
| Difference in tidal volume | 0.001 |
| Trapped Gas (FRCpleth−FRCSF6) | 0.147 |
Discussion
To the best of our knowledge, no other study has directly compared outcomes measured by MBWN2 to those measured by both MBWSF6 and traditional lung function tests in healthy children and children with CF. LCIN2 and LCISF6 had similar discriminative power and intra-session repeatability but are not interchangeable as LCIN2 was on average higher than LCISF6. As such, interpretation of parameters measured by MBWN2 will require independent normative values to define an appropriate upper limit of normal.
The feasibility of using MBWN2 in a pediatric clinical setting has recently been described but this study did not include head to head comparison to other technologies[24]. Two studies have previously compared alternative MBW systems to mass spectrometry based MBWSF6.[25],[26]. However, both used SF6 as the tracer gas and neither performed between system comparisons in the same individual nor compared MBW based lung volume measurements to plethysmographic FRC measurements; therefore results are not directly comparable to our study.
Although the LCI and FRC were comparable between systems in health, albeit higher using N2, the bias observed in CF subjects clearly demonstrates that the two systems cannot be used interchangeably. These observed differences could potentially be explained by differing physiological properties of SF6 and N2. SF6 is a heavy gas and thus may behave differently in the periphery of the lung than a lighter gas (He or N2); however comparison of LCISF6 to LCIHe in CF did not demonstrate the same bias observed between LCISF6 and LCIN2. The endogenous nature of N2 results in the contribution of gas from very slowly ventilated lung units not captured by MBWSF6 as evidenced by the relationship between trapped gas, number of breaths and difference in LCI between systems. However, this will also increase washout time in subjects with uneven ventilation distribution as it will take longer to clear endogenous tracer gas from their lungs compared to SF6, which may not equilibrate in extremely slowly ventilated lung units.
FRC measured by MBW is subject to the same limitations as other gas dilution techniques in that only communicating lung units will contribute to measured volume, while FRC measured by body plethysmography includes all compressible gas volume. Thus, in subjects with significant peripheral airway obstruction we would expect differences between FRCpleth and FRCMBW, and indeed FRC measured by both MBW techniques was lower than that measured by plethysmography. However, we observed that FRCN2 more closely agreed with FRCpleth. These results suggest that the difference between FRCpleth and FRCSF6 may reflect trapped gas volume and that the volume contribution of slowly ventilated lung regions, not captured during MBWSF6, results in lower FRCSF6 values. Consequently, during MBWN2 subjects with CF required significantly more breaths to complete washout leading to the disproportionately higher CEVN2 compared to CEVSF6. Our data demonstrate that these differences are progressively more pronounced with worsening obstructive lung disease. LCIN2 was shown to increase disproportionately more than LCISF6 with greater disease severity (increased FRCpleth and lower FEV1) and as such may be able to more accurately reflect the degree of VI than LCISF6.
These interpretations are based on the assumption that the additional gas volume measured during MBWN2 can be attributed to measurement of gas in extremely slowly ventilated lung units. However, a further unquantifiable amount of tissue dissolved N2 will diffuse from the blood into the alveoli during MBWN2, particularly during long washouts seen in subjects with significant VI. Most evidence would suggest unless lung disease is severe the tissue N2 contribution will be relatively low.[19] The close correspondence of FRCN2 and FRCpleth observed in this study would support this hypothesis.
While it would appear that MBWN2 is better able to reflect the degree of peripheral airway disease than MBWSF6, washout times will be substantially longer in subjects with significant VI. Long washout times may limit the feasibility of MBWN2 in the clinical setting. This limitation could potentially be overcome by choosing higher cut-off concentrations earlier in the washout. Preliminary evidence [27] would suggest that this is possible without compromising the sensitivity of MBWN2. Investigation into the minimal number of trials required to achieve reproducible results; another option to shorten the test duration, is ongoing.
In conclusion, MBWN2 offers a valid tool to investigate obstructive lung disease in CF. Furthermore, future studies in younger patients are required to better understand the sensitivity of multiple breath N2 washout in this age group. In addition, interventional studies similar to those performed with MBWSF6 are needed to further clarify the role of MBWN2 as an outcome measure in clinical trials in CF patients.
Acknowledgments
We would like to thank Dasiga Sundaralingam, Theo Tackey, Katie LePage, Sophon Kang, Colleen Keast, Nadia Rampersad, Rajdip Grewal and Anouk Benseler for their help with measurements and to the families and children who volunteered to participate in our study.
Funding Statement
This study was funded by Cystic Fibrosis Foundation Therapeutics (www.cff.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bush A, Davies J (2005) Early detection of lung disease in preschool children with cystic fibrosis. Curr Opin Pulm Med 11: 534–538. [DOI] [PubMed] [Google Scholar]
- 2. Rosenfeld M (2007) An overview of endpoints for cystic fibrosis clinical trials: one size does not fit all. Proc Am Thorac Soc 4: 299–301. [DOI] [PubMed] [Google Scholar]
- 3. Kerem E, Reisman J, Corey M, Canny GJ, Levison H (1992) Prediction of mortality in patients with cystic fibrosis. N Engl J Med 326: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 4. Ellemunter H, Fuchs SI, Unsinn KM, Freund MC, Waltner-Romen M, et al. (2010) Sensitivity of Lung Clearance Index and chest computed tomography in early CF lung disease. Respir Med 104: 1834–1842. [DOI] [PubMed] [Google Scholar]
- 5. Fuchs SI, Toussaint S, Edlhaimb B, Ballmann M, Gappa M (2010) Short-term effect of physiotherapy on variability of the lung clearance index in children with cystic fibrosis. Pediatr Pulmonol 45: 301–306. [DOI] [PubMed] [Google Scholar]
- 6. Gustafsson PM, De Jong PA, Tiddens HA, Lindblad A (2008) Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax 63: 129–134. [DOI] [PubMed] [Google Scholar]
- 7. Owens CM, Aurora P, Stanojevic S, Bush A, Wade A, et al. (2011) Lung Clearance Index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax 66: 481–488. [DOI] [PubMed] [Google Scholar]
- 8. Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, et al. (2012) Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax 67: 509–516. [DOI] [PubMed] [Google Scholar]
- 9. Aurora P, Gustafsson P, Bush A, Lindblad A, Oliver C, et al. (2004) Multiple breath inert gas washout as a measure of ventilation distribution in children with cystic fibrosis. Thorax 59: 1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aurora P, Bush A, Gustafsson P, Oliver C, Wallis C, et al. (2005) Multiple-breath washout as a marker of lung disease in preschool children with cystic fibrosis. Am J Respir Crit Care Med 171: 249–256. [DOI] [PubMed] [Google Scholar]
- 11. Gustafsson PM, Aurora P, Lindblad A (2003) Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur Respir J 22: 972–979. [DOI] [PubMed] [Google Scholar]
- 12. Aurora P, Stanojevic S, Wade A, Oliver C, Kozlowska W, et al. (2011) Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med 183: 752–758. [DOI] [PubMed] [Google Scholar]
- 13. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. (2005) Standardisation of spirometry. Eur Respir J 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 14. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, et al. (2005) Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522. [DOI] [PubMed] [Google Scholar]
- 15. Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, et al. (2007) An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 175: 1304–1345. [DOI] [PubMed] [Google Scholar]
- 16. Corey M, Levison H, Crozier D (1976) Five- to seven-year course of pulmonary function in cystic fibrosis. Am Rev Respir Dis 114: 1085–1092. [DOI] [PubMed] [Google Scholar]
- 17. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, et al. (2000) CDC growth charts: United States. Adv Data 1–27. [PubMed] [Google Scholar]
- 18. Stanojevic S, Wade A, Cole TJ, Lum S, Custovic A, et al. (2009) Spirometry centile charts for young Caucasian children: the Asthma UK Collaborative Initiative. Am J Respir Crit Care Med 180: 547–552. [DOI] [PubMed] [Google Scholar]
- 19. Robinson PD, Latzin P, Verbanck S, Hall GL, Horsley A, et al. (2012) Guidelines for Inert Gas Washout Measurement using Multiple and Single Breath Tests. Eur Respir J in press [DOI] [PubMed] [Google Scholar]
- 20. Singer F, Houltz B, Latzin P, Robinson P, Gustafsson P (2012) A realistic validation study of a new nitrogen multiple-breath washout system. PLoS One 7: e36083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latzin P, Sauteur L, Thamrin C, Schibler A, Baldwin D, et al. (2007) Optimized temperature and deadspace correction improve analysis of multiple breath washout measurements by ultrasonic flowmeter in infants. Pediatr Pulmonol 42: 888–897. [DOI] [PubMed] [Google Scholar]
- 22. Gustafsson PM (2005) Inert gas washout in preschool children. Paediatr Respir Rev 6: 239–245. [DOI] [PubMed] [Google Scholar]
- 23. Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310. [PubMed] [Google Scholar]
- 24. Singer F, Kieninger E, Abbas C, Yammine S, Fuchs O, et al. (2012) Practicability of nitrogen multiple-breath washout measurements in a pediatric cystic fibrosis outpatient setting. Pediatr Pulmonol [DOI] [PubMed] [Google Scholar]
- 25. Fuchs SI, Buess C, Lum S, Kozlowska W, Stocks J, et al. (2006) Multiple breath washout with a sidestream ultrasonic flow sensor and mass spectrometry: a comparative study. Pediatr Pulmonol 41: 1218–1225. [DOI] [PubMed] [Google Scholar]
- 26. Pillow JJ, Ljungberg H, Hulskamp G, Stocks J (2004) Functional residual capacity measurements in healthy infants: ultrasonic flow meter versus a mass spectrometer. Eur Respir J 23: 763–768. [DOI] [PubMed] [Google Scholar]
- 27. Yammine S, Singer F, Abbas C, Roos M, Latzin P (2012) Multiple-breath washout measurements can be significantly shortened in children. Thorax [DOI] [PubMed] [Google Scholar]