Abstract
Rhinovirus (RV) infections account for approximately two thirds of all virus-induced asthma exacerbations and often result in an impaired response to β2 agonist therapy. Using an in vitro model of RV infection, we investigated the mechanisms underlying RV-induced β2 adrenoceptor desensitization in primary human airway smooth muscle cells (ASMC). RV infection of primary human bronchial epithelial cells (HBEC) for 24 hours produced conditioned medium that caused β2 adrenoceptor desensitization on ASMCs without an effect on ASMCs viability. Less than 3 kDa size fractionation together with trypsin digestion of RV-induced conditioned medium did not prevent β2 adrenoceptor desensitization, suggesting it could potentially be mediated by a small peptide or lipid. RV infection of BECs, ASMCs and fibroblasts produced prostaglandins, of which PGE2, PGF2α and PGI2 had the ability to cause β2 adrenoceptor desensitization on ASMCs. RV-induced conditioned medium from HBECs depleted of PGE2 did not prevent ASMC β2 adrenoceptor desensitization; however this medium induced PGE2 from ASMCs, suggesting that autocrine prostaglandin production may be responsible. Using inhibitors of cyclooxygenase and prostaglandin receptor antagonists, we found that β2 adrenoceptor desensitization was mediated through ASMC derived COX-2 induced prostaglandins. Since ASMC prostaglandin production is unlikely to be caused by RV-induced epithelial derived proteins or lipids we next investigated activation of toll-like receptors (TLR) by viral RNA. The combination of TLR agonists poly I:C and imiquimod induced PGE2 and β2 adrenoceptor desensitization on ASMC as did the RNA extracted from RV-induced conditioned medium. Viral RNA but not epithelial RNA caused β2 adrenoceptor desensitization confirming that viral RNA and not endogenous human RNA was responsible. It was deduced that the mechanism by which β2 adrenoceptor desensitization occurs was by pattern recognition receptor activation of COX-2 induced prostaglandins.
Introduction
Acute exacerbations of asthma are the major cause of morbidity, mortality and health costs related to the disease. Respiratory viral infections trigger approximately 85% of asthma exacerbation in adults and children and the mechanisms by which this occurs remain unclear [1]. Human rhinovirus (RV) belongs to the Picornaviridae family of positive single stranded RNA viruses and is implicated in a variety of respiratory disorders ranging from the common cold to the induction of exacerbations of respiratory diseases. Of the respiratory viruses that cause asthma exacerbations, RV accounts for about two thirds of all viral-induced asthma exacerbations [1]. Asthma medications such as corticosteroids and the epinephrine analogues such as selective β2 agonists are the most common therapies for asthma management and, during acute exacerbations, including those caused by respiratory viruses, β2 agonists are a commonly used rescue medication [2].
Under normal circumstances, airway obstruction in asthma improves in response to inhaled β2 agonists, however there have been reports that airway obstruction does not improve with β2 agonists during virally induced asthma exacerbations [3], [4]. Reddel and colleagues reported that in asthmatic adults, during a respiratory viral infection their exacerbation was characterized by reduced response to β2 agonists despite having good asthma control prior to infection, and a good response to β2 agonists prior to achieving good asthma control [3]. Similarly, Rueter et al. reported that asthmatic children responded less effectively to β2 agonist therapy in response to a viral-induced exacerbation in which RV was the most frequently identified virus [4]. These reports indicate that the underlying cause of this reduced response to β2 agonists during these exacerbations of asthma may be unique to a viral infection. The exact causes of exacerbations of asthma are unknown, however it possible that functional impairment of the β2 adrenoceptor (β2 AR) may disrupt intrinsic bronchodilation through circulating epinephrine and thus result in airflow limitation characteristic of an exacerbation.
In vivo, the epithelial cells form a physiological barrier in the airways and are the principal cell type infected by RV in the lower airways [5] even though there is evidence that underlying submucosal cells can be infected, perhaps as a consequence of viremia or compromised epithelial barrier as occurs in asthma or COPD [6], [7]. Since RV-induced inflammation is thought to contribute to asthma exacerbations, it is likely that the viral-epithelial infection and the subsequent interaction within the epithelial mesenchymal trophic unit are critical in mediating viral-induced exacerbations.
Previous research has suggested that clinical impairment to β2 agonist therapy may be due to desensitization of the β2 AR on airway smooth muscle cells (ASMCs) [8]. Our group have previously developed an in vitro model to show that RV infection of epithelial cells produces a conditioned medium, containing unknown substances, that when applied to ASMCs, causes internalisation of the β2 AR, and results in reduced generation of cyclic adenosine monophosphate (cAMP) in response to a β2 agonist [8]. Furthermore, the effect observed was not due to the impaired ability to generate cAMP as the adenylate cyclase activator forskolin induced cAMP response was not reduced. This in vitro phenomenon may translate to the possible reason why asthmatic patients with RV-induced asthma exacerbations do not respond to β2 agonists clinically, however the mechanism by which it occurs, or the identity of the RV-induced epithelial derived substance remains unknown.
Eicosanoids are lipid mediators which incorporate the two large families of prostaglandins and leukotrienes, and their levels are increased in asthma and during clinical RV infections [9], [10]. It has been shown that of the prostaglandin (PG) family, PGE2 can cause ASMC relaxation by the induction of cAMP [11]. In doing so, PGE2 can cause heterologous desensitization of the β2 AR by either activation of common G protein coupled receptor (GPCR) kinases, for example GPCR that share common Gs alpha subunits, or alternatively by direct activation of protein kinase A (PKA) [11]–[14]. In contrast, within the leukotriene (LT) family, LTD4 causes ASMC contraction by activating cystLT1 receptors which are coupled to Gq alpha subunit proteins and activate the protein kinase C (PKC) pathway which ultimately increases intracellular calcium. Surprisingly, LTD4 can also cause desensitization of the β2 AR [15].
In this study, the identity of the unknown substance/s responsible for or the mechanism that causes β2 AR desensitization in ASMCs were investigated using an in vitro epithelial-ASM β2 AR model using human primary bronchial epithelial cells (HBEC) and human primary ASMCs. In the airways, fibroblasts are structural support cells that lie in close proximity to the epithelium and smooth muscle, and during RV infection could contribute to RV induced β2 AR desensitization by their release of mediators such as prostaglandins. For this reason, the ability of airway structural cells including ASMCs, epithelial cells and lung fibroblasts to produce prostaglandins in response to RV infection and the ability of prostaglandins to cause β2 AR desensitization in ASMCs were also investigated. We found that RV infection of epithelial cells resulted in an increase in viral RNA which could activate toll-like receptors (TLRs) on the ASMC. This resulted in the generation of COX-2 induced prostaglandins from ASMCs which in turn caused β2 AR desensitization. Since RV infection of various airway structural cells also produces prostaglandins, this could further contribute to β2 AR desensitization.
Materials and Methods
Ethics Statement
The study was approved by the Ethics Review Committee of the Sydney South West Area Health Service, Royal Prince Alfred Hospital and The University of Sydney human research ethics committee. All volunteers provided written informed consent.
Cell culture: Human Bronchial Epithelial Cells, Airway Smooth Muscle and Fibroblasts
Primary HBEC, ASMC and parenchymal fibroblasts were isolated from macroscopically normal tissue of patients undergoing lung resection for thoracic carcinoma or transplantation for end stage lung disease. All patient details are shown in Table 1 and 2.
Table 1. Demographic data of study patients in RV infection of airway structural cells and prostaglandin production.
| Patient # | Cell Type | Sex | Age | Disease | Sample |
| 1 | Fibroblasts | F | 43 | Emphysema | Transplant |
| 2 | Fibroblasts | F | 65 | Ca | Resection |
| 3 | Fibroblasts | M | 68 | SCCA | Resection |
| 4 | Fibroblasts | F | 38 | Emphysema | Transplant |
| 5 | ASMC | F | 65 | Ca | Resection |
| 6 | ASMC | M | 76 | SCCA | Resection |
| 7 | ASMC | F | 59 | NSCCA | Resection |
| 8 | ASMC | F | 66 | Ca | Resection |
| 9 | ASMC | M | 62 | Emphysema | Transplant |
| 10 | ASMC | F | 56 | Bronchiectasis | Transplant |
| 11 | ASMC | M | 47 | Emphysema | Transplant |
| 12 | ASMC | F | 43 | Emphysema | Transplant |
| 13 | ASMC | M | 71 | Asthma | Biopsy |
| 14 | ASMC | F | 49 | Asthma | Biopsy |
| 15 | ASMC | F | 47 | Asthma | Biopsy |
| 16 | ASMC | M | 45 | Asthma | Biopsy |
| 17 | HBEC | F | 54 | Emphysema | Biopsy |
| 18 | HBEC | M | 73 | Ca | Resection |
| 19 | HBEC | M | 64 | NSCCA | Resection |
| 20 | HBEC | F | 72 | NSCCA | Resection |
| 21 | HBEC | M | 63 | Emphysema | Transplant |
Table 2. Demographic data of study patients in the characterisation of the mechanism of RV-induced β2 adrenoceptor desensitization.
| Patient # | Cell Type | Sex | Age | Disease | Sample |
| 1 | HBEC | F | 71 | Ca | Resection |
| 2 | HBEC | M | 58 | Ca | Resection |
| 3 | HBEC | F | 60 | Emphysema | Transplant |
| 4 | HBEC | M | 74 | Ca | Resection |
| 5 | HBEC | F | 44 | Emphysema | Transplant |
| 6 | HBEC | M | 66 | Ca | Resection |
| 7 | HBEC | F | 43 | Emphysema | Transplant |
| 8 | HBEC | M | 57 | Emphysema | Transplant |
| 9 | HBEC | M | 58 | No disease | Transplant |
| 10 | HBEC | F | 68 | COPD/Ca | Resection |
| 11 | HBEC | M | 55 | Emphysema | Transplant |
| 12 | HBEC | F | 60 | Emphysema | Transplant |
| 13 | HBEC | M | 54 | NSCCA | Resection |
| 14 | HBEC | M | 58 | Emphysema | Transplant |
| 15 | HBEC | M | 30 | Cystic Fibrosis | Transplant |
| 16 | HBEC | M | 61 | Pulmonary fibrosis | Transplant |
| 17 | HBEC | M | 63 | Asthma | Biopsy |
| 18 | HBEC | F | 51 | Emphysema | Transplant |
| 19 | HBEC | M | 57 | NSCCA/COPD | Resection |
| 20 | HBEC | F | 55 | Ca | Resection |
| 21 | HBEC | F | 59 | NSCCA/COPD | Resection |
| 22 | ASMC | M | 56 | α1 anti-trypsin deficiency | Transplant |
| 23 | ASMC | M | 57 | α1 anti-trypsin deficiency | Transplant |
| 24 | ASMC | M | 71 | Ca | Resection |
| 25 | ASMC | M | 49 | Emphysema | Transplant |
| 26 | ASMC | M | 33 | Asthma | Biopsy |
| 27 | ASMC | M | 66 | SCCA | Resection |
| 28 | ASMC | M | 38 | Asthma | Biopsy |
| 29 | ASMC | M | 22 | No disease | Biopsy |
| 30 | ASMC | M | 22 | Asthma | Biopsy |
| 31 | ASMC | M | 20 | No disease | Biopsy |
| 32 | ASMC | M | 65 | Ca | Resection |
| 33 | ASMC | M | 21 | Asthma | Biopsy |
| 34 | ASMC | M | 61 | Emphysema | Transplant |
| 35 | ASMC | M | 56 | Emphysema | Transplant |
| 36 | ASMC | M | 22 | Asthma | Biopsy |
| 37 | ASMC | F | 43 | Emphysema | Transplant |
| 38 | ASMC | F | 19 | Asthma | Biopsy |
| 39 | ASMC | M | 65 | NSCCA | Resection |
| 40 | ASMC | M | 41 | Emphysema | Transplant |
| 41 | ASMC | M | 59 | Emphysema | Transplant |
| 42 | ASMC | M | 57 | Sarcoidosis | Transplant |
| 43 | ASMC | M | 66 | NSCCA | Resection |
| 44 | ASMC | M | 70 | NSCCA | Resection |
| 45 | ASMC | F | 45 | Emphysema | Transplant |
| 46 | ASMC | M | 70 | Ca | Resection |
| 47 | ASMC | M | 58 | NSCCA/COPD | Resection |
| 48 | ASMC | M | 59 | Ca | Resection |
| 49 | ASMC | M | 61 | Ca | Resection |
| 50 | ASMC | M | 80 | Ca | Resection |
| 51 | ASMC | M | 75 | NSCCA | Resection |
| 52 | ASMC | M | 72 | NSCCA | Resection |
| 53 | ASMC | M | 64 | NSCCA | Resection |
| 54 | ASMC | M | 43 | Pulmonary fibrosis | Transplant |
| 55 | ASMC | M | 52 | Ca | Resection |
| 56 | ASMC | M | 52 | Pulmonary fibrosis | Transplant |
| 57 | ASMC | M | 59 | Emphysema | Transplant |
| 58 | ASMC | F | 62 | Emphysema | Transplant |
| 59 | ASMC | M | 74 | SCCA | Resection |
| 60 | ASMC | M | 58 | Ca | Resection |
| 61 | ASMC | F | 71 | Ca | Resection |
| 62 | ASMC | F | 29 | Pulmonary hypertension | Transplant |
| 63 | ASMC | F | 44 | Emphysema | Transplant |
| 64 | ASMC | M | 70 | Ca | Resection |
| 65 | ASMC | F | 43 | Emphysema | Transplant |
| 66 | ASMC | F | 56 | Emphysema | Transplant |
| 67 | ASMC | F | 53 | Pulmonary fibrosis | Transplant |
| 68 | ASMC | M | 66 | NSCCA | Resection |
| 69 | ASMC | M | 57 | Emphysema | Transplant |
| 70 | ASMC | F | 59 | Ca | Resection |
| 71 | ASMC | F | 59 | Ca | Resection |
| 72 | ASMC | M | 75 | NSCCA/COPD | Resection |
| 73 | ASMC | F | 41 | Ca | Resection |
| 74 | ASMC | F | 62 | Ca | Resection |
| 75 | ASMC | M | 67 | NSCCA | Resection |
| 76 | ASMC | F | 15 | Pulmonary hypertension | Transplant |
| 77 | ASMC | M | 58 | No disease | Transplant |
| 78 | ASMC | F | 68 | NSCCA/COPD | Resection |
| 79 | ASMC | M | 61 | Ca | Resection |
| 80 | ASMC | M | 55 | Emphysema | Transplant |
| 81 | ASMC | F | 60 | Emphysema | Transplant |
| 82 | ASMC | M | 54 | NSCCA | Resection |
| 83 | ASMC | F | 44 | Emphysema | Transplant |
| 84 | ASMC | F | 51 | Pulmonary fibrosis | Transplant |
| 85 | ASMC | M | 81 | No disease | Unknown |
| 86 | ASMC | M | 71 | NSCCA | Resection |
| 87 | ASMC | F | 50 | Emphysema | Transplant |
| 88 | ASMC | M | 35 | Emphysema | Transplant |
| 89 | ASMC | M | 30 | Cystic Fibrosis | Transplant |
| 90 | ASMC | M | 30 | Cystic Fibrosis | Transplant |
| 91 | ASMC | F | 66 | Ca | Resection |
| 92 | ASMC | M | 53 | Emphysema | Transplant |
| 93 | ASMC | M | 54 | Emphysema | Transplant |
| 94 | ASMC | M | 58 | Emphysema | Transplant |
| 95 | ASMC | M | 64 | Emphysema | Transplant |
| 96 | ASMC | F | 68 | Ca | Resection |
| 97 | ASMC | M | 71 | SCCA | Resection |
| 98 | ASMC | F | 51 | Emphysema | Transplant |
| 99 | ASMC | M | 58 | No disease | Biopsy |
| 100 | ASMC | M | 77 | Ca | Resection |
| 101 | ASMC | M | 60 | NSCCA | Resection |
| 102 | ASMC | F | 58 | NSCCA | Resection |
| 103 | ASMC | F | 67 | Ca | Resection |
| 104 | ASMC | M | 76 | Ca | Resection |
| 105 | ASMC | F | 63 | Ca | Resection |
| 106 | ASMC | M | 26 | Bronchiolitis Obliterans | Transplant |
| 107 | ASMC | M | 66 | NSCCA | Resection |
| 108 | ASMC | F | 62 | Emphysema | Transplant |
Key: ASMC = airway smooth muscle cells; HBEC = human bronchial epithelial cells; SCCA = Small cell carcinoma; NSCCA = Non small cell carcinoma; COPD = Chronic obstructive pulmonary disease; Ca = Cancer.
HBEC and ASMC were obtained from human bronchial airways and fibroblasts from lung parenchyma by methods previously described [16]–[18]. Briefly, segments of bronchus were dissected free from the surrounding parenchyma, cut open and washed in Hanks’ balanced salt solution (HBSS) (Invitrogen, Victoria, Australia). The epithelium was removed and collected into 75 cm2 flasks with bronchial epithelial growth medium (BEGM) (Clonetics, California, USA). In doing so this exposes the underlying bands of smooth muscle, which were then gently separated from the underlying connective tissue in small bundles (smooth muscle cell explants). The explants were transferred into 25 cm2 flasks and covered with a minimal amount of Dulbecco’s Modified Eagle Medium (DMEM) containing 1% antibiotics: 20 U/L of penicillin, 20 µg/mL of streptomycin, 2.5 µg/mL of amphotericin B, and 10% Fetal Bovine Serum (FBS) (all purchased from Invitrogen). Fibroblasts were isolated from resected lung tissue by seeding 1–2 mm3 pieces of parenchymal tissue into DMEM supplemented with 10% FBS and 1% antibiotics in 75 mm2 flasks. Both cell types were incubated at 37°C in a humidified atmosphere of 5% CO2-95% air.
The medium was replenished every 5 days for the first 10–20 days, and within this time, cell growth occurred. The cells were passaged (split 1∶3) with a solution of trypsin [0.05% wt/vol in HBSS] containing 1 mM EDTA. The cells were maintained in DMEM supplemented with 1% antibiotics in 10% FBS whilst HBEC were maintained in BEGM. All cells were tested for mycoplasma contamination and only mycoplasma free cells were selected and passaged once they reached confluence. ASMCs and fibroblasts at passages 3–7 and HBEC at passages 2–3 were used for experiments.
RV Propagation and Ultraviolet Inactivation of RV (UVi-RV)
Human RV serotype-16 were propagated in Ohio HeLa cells and purified using a 100,000 kDa molecular weight cut off (MWCO) filter as previously described [19], [20]. RV concentration was determined by virus titration as described previously [21]. In some experiments RV or conditioned medium was UV inactivated in 24 well plates containing 200 µL of sample/well at a distance of 5 cm from a 30 W UV light source (germicidal lamp G30T8, Sankyo Denki, Japan) for 15 minutes. Successful UV inactivation of RV was established by a virus titration and was used as a non-infectious virus control.
Rhinovirus Infection of BEC, Fibroblasts and ASMCs and Prostaglandin Production
HBEC were seeded at a concentration of 6.4×104 cells/mL in BEGM, while fibroblasts and ASMCs were seeded at 3.2×104 cells/mL in 10%FBS/DMEM in 6 well plates for 72 hours. The total number of cells after 72 hours was assessed and wells were then either left uninfected (control) or exposed to UV inactivated RV or live RV at a multiplicity of infection (MOI) of 1. The cells were placed on a shaker (100 rpm) for 15 minutes at room temperature before being incubated at 37°C at 5% CO2. After 1 hour the cells were washed with HBSS and replaced with fresh BEGM or 10%FBS DMEM before being incubated at 37°C in a humidified atmosphere of 5% CO2-95% air. After 24 hours, the supernatant was purged with nitrogen, collected and stored at −80°C for ELISA and the number of remaining viable cells was estimated for each well using trypan blue exclusion and manual cell counting to normalize prostaglandin release to cell number.
ELISAs
PGE2, PGD2, PGF2α, PGI2 metabolite 6ketoPGF1α, CystLT and LTB4 ELISAs were purchased from Cayman Chemicals, Michigan, USA. The assays were carried out according to the manufacturer’s instructions. The detection range for each assay was PGE2 7–1000 pg/mL; PGD2 78–5000 pg/mL; PGF2α 3–500 pg/mL; 6ketoPGF1α 1–1000 pg/mL; CystLT 7–1000 pg/mL and LTB4 3–500 pg/mL. Prostaglandin ELISA results were normalised to cell number and expressed as concentration per 1×106cells.
ELISA kits for IL-6 and IL-8 were purchased from R&D Systems (Minneapolis, USA) and BD Biosciences (California, USA) respectively. ELISAs were carried out according to the manufacturers’ instructions. The detection limits of these assays were: 7–1000 pg/mL (IL-6) and 15–2000 pg/mL (IL-8).
Reagents
Prostaglandins (PG) D2, E2, F2α, the PGI2 analogues: MRE-269 (selective) and Beraprost (non-selective) – both of which are being used in clinical trials for the treatment of pulmonary hypertension, the selective COX-2 inhibitor celecoxib (Cayman Chemicals), indomethacin and 3-isobutyl-1-methylxanthine (IBMX) were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Missouri, USA) and stored at −80°C prior to use. Isoprenaline (Sigma-Aldrich) was dissolved in water before use. The protease trypsin was dissolved in Hank’s balanced salt solution (HBSS) (both from Invitrogen) prior to use and to inhibit its digestive activity during experimentation 0.1% bovine serum albumin (BSA) (Invitrogen) was used. The TLR 3 agonist polyinosinic : polycytidylic acid (Poly I:C) (Sigma-Aldrich) and TLR 7/8 agonist imiquimod (InvivoGen, California, USA) were dissolved in DMSO and H2O respectively and stored at −20°C. The pro-opiomelanocortin (POMC) protein was purchased ready to use from ABCAM (Cambridge, UK) and the 1.2 kDa “substance X” peptide was synthesized by Auspep (Victoria, Australia) and dissolved in H2O. Both protein and peptide were stored at −80°C before use. The prostaglandin receptor antagonists AH6809 (EP1–3, DP1); CAY10441 (IP); AL8810 (FP); BWA868C (DP2); L-161,982 (EP4) were purchased from Cayman Chemicals and dissolved in DMSO and stored at −80°C prior to use. In experiments where a single or mix of prostaglandin receptor antagonists or vehicle were used, the final concentrations and formulation for experimentation were chosen according to its use in previous studies to successfully inhibit their specified receptor and they consisted of AH6809 (10−5 M) [22]; CAY10441 (10−6 M) [23]; AL8810 (10−5 M) [24]; BWA868C (10−5 M) [25]; L-161,982 (10−6 M) [26] or the sum of each corresponding amount of vehicle (DMSO).
Assessment of Cell Viability and Number
In some experiments, cell viability was assessed by means of measuring mitochondrial activity using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) (Sigma) assay as previously described [27]. In other experiments, total cell number was determined by a manual trypan blue exclusion cell count using a haemocytometer and an estimation of the total number of cells was calculated.
Generation of HBEC Derived Conditioned Medium
Primary HBEC were seeded at a concentration of 5×104 cells/mL in 75 cm2 culture flasks with 10 mL of BEGM until >90% confluent. Briefly, RV or UVi-RV (MOI = 2) or BEGM was added to the confluent cell monolayers for 1 hour with orbital shaking at 37°C. The cells were then washed with HBSS and fresh BEGM was added and the cells were incubated at 37°C in a humidified atmosphere of 5% CO2 for 24 hours. In experiments with the COX inhibitor indomethacin, infection occurred in the presence of the drug at 1×10−5 M in BEGM and replacement BEGM also contained the drug at 1×10−5 M. Control, UVi-RV and RV generated conditioned medium was collected 1 day after infection. All conditioned medium (control, UVi-RV and RV) were UV treated by placing 250 µL of conditioned medium into each well of a 12-well tissue culture plate at a distance of 5 cm from a UV lamp for 5 minutes to ensure that all RV was inactivated before being applied undiluted on primary human ASMCs to assess β2 AR function.
Primary human ASMCs were incubated with control, UVi-RV and RV conditioned medium for 3 days. In experiments assessing whether prostaglandins, TLR agonists or total extracted RNA from conditioned medium can cause β2 AR desensitization, treatments were applied in BEGM or control conditioned medium. In experiments utilizing prostaglandin receptor antagonists to determine the prostaglandin responsible for β2 AR desensitization, ASMCs were pretreated with the antagonist 1 hour prior and during the 3 day incubation period with conditioned medium. β2 AR function on ASMCs was assessed by the functional cAMP assay and where appropriate ASMC viability was assessed using an MTT assay.
Evidence of epithelial RV infection was assessed by the measurement of RV induced IL-6 by ELISA. The induction of proteins present in RV conditioned medium was assessed using the Quantipro BCA assay kit (Sigma-Aldrich) and 1-D protein gel electrophoresis with silver staining.
cAMP Assay
To assess β2 AR function cells were stimulated with the β agonist isoprenaline (10−7 M) for 5 minutes in the presence of the phosphodiesterase inhibitor IBMX (10−5 M) in HBSS. The cells were then lysed in a solution of H2O with 0.03% (v/v) Tween-20 and 5 mM HEPES buffer by vigorous pipetting. The amount of isoprenaline induced cAMP in the lysate samples was quantified using an Alphascreen cAMP Assay Kit (Perkin Elmer, Massachusetts, USA) according to the manufacturer’s instructions.
Quantipro BCA Assay Kit
Protein concentration in conditioned medium was determined using the bicinchoninic acid (BCA) assay kit according to the manufacturer’s instruction.
Protein Gel Electrophoresis
Conditioned medium was prepared as follows for gel electrophoresis: 5 µL of loading buffer (2% sodium dodecyl sulphate (SDS), 7.5% glycerol, 31.25 mM Tris-HCl (pH 6.8), 0.0025% bromophenol blue, 200 mM DTT (all from Sigma-Aldrich) was added to 25 µL of conditioned medium and denatured at 95°C for 5 minutes. Five µL of Precision plus Protein Dual Xtra Molecular Standard ladder (Bio-Rad, New South Wales, Australia) was added in a separate lane of each gel to indicate the size of the visualised bands, while 20 µL of prepared protein samples was added to each separate lane.
Twenty µL of protein samples were loaded onto a 4% polyacrylamide stacking gel (25% (v/v) SDS Tris pH 6.8; 10% (v/v) 37.5∶1 acrylamide (Bio-Rad); 0.1% (v/v) tetramethylethylenediamine (Sigma-Aldrich); 0.1% (v/v) ammonium persulfate (Sigma-Aldrich); in Milli-Q H20) and separated by SDS-polyacrylamide electrophoresis on a 10% polyacrylamide gel (25% (v/v) SDS Tris pH 8.8; 25% (v/v) 37.5∶1 acrylamide; 0.1% (v/v) tetramethylethylenediamine; 0.1% (v/v) ammonium persulfate; in Milli-Q H20) at 150 V for 90 minutes before they were prepared for silver staining. Gels were visualized and analysed using an IS4000MM Kodak imaging system and software (Kodak Scientific Imaging Systems, New York, USA).
Silver Staining
After protein electrophoresis, the gels were transferred into a 50 mL fixative solution (50% (v/v) methanol, 12% (v/v) glacial acetic acid, 0.05% (v/v) 37% formaldehyde (Sigma-Aldrich), Milli-Q H2O) for 30 minutes on an orbital shaker. The fixative solution was then removed and three washes of 50% ethanol in Milli-Q H2O were carried out each for 20 minute intervals. The gels were then washed three times with Milli-Q H2O and incubated with 20% w/v thiosulfate solution for 1 minute then washed three times again with Milli-Q H2O. The gels were incubated in 0.2% w/v silver nitrate in the dark for 20 minutes before being washed twice in Milli-Q H2O and then incubated in developer solution (6% (w/v) Na2CO3, 0.05% (v/v) 37% formaldehyde, 2% (v/v) of thiosulfate solution as described above, in Milli-Q H2O) until the bands were clear. The development was stopped by three washes in Milli-Q H2O and the bands were captured using the Kodak imaging system.
Protease Digestion
In order to assess whether RV-induced epithelial derived proteins in the conditioned medium may be responsible for causing β2 AR desensitization, large proteins were digested using the protease trypsin and the conditioned medium was reassessed for its ability to induce β2 AR desensitization on ASMCs.
Initial experiments involved the optimization of multiple variables including the amount of protease required for digestion, time for sufficient digestion of conditioned medium proteins and the appropriate concentration of inhibitor substance to avoid cytotoxic effects on ASMCs. Protein gel electrophoresis followed by silver staining of digested products validated the digestion process, while MTT assays were used to validate the safety of optimized concentrations of protease and time of digestion on ASMC viability over 3 days. As the protein concentration in conditioned medium varied from batch to batch, control and RV conditioned medium from multiple batches were pooled in these experiments.
Since endogenous proteases are necessary for homeostatic cell function, the use of protease inhibitors to stop the exogenous protease digestion may affect ASMC function and viability. Therefore since trypsin digestion can be safely stopped using saturation with BSA instead of the use of protease inhibitors, trypsin instead of other proteases was selected for enzymatic digestion of the conditioned medium to assess β2 AR desensitization.
Briefly, 3–4 batches of control and RV conditioned medium were pooled to a total of 4–6 mL and trypsin was added (500 µg/mL) and the mixture was allowed to incubate in a water bath at 37°C for 24 hours. After the digestion, 0.1% BSA was added to the mixture to saturate and stop the enzymatic digestion. Since the presence of trypsin and BSA may affect the outcome of results, trypsin (>20 kDa) was also inactivated by removal using the Amicon ultra-15 centrifugal filtration unit (Millipore, Massachusetts, USA) with 3 kDa molecular weight cut off (MWCO). The undiluted trypsin digested conditioned medium was applied to ASMCs to assess β2 AR desensitization using a functional cAMP assay.
PGE2 Affinity Chromatography Column
PGE2 columns filled with 1 mL of PGE2 affinity sorbent (mouse anti-PGE2 covalently bound to Sepharose 4B) were purchased from Cayman Chemicals. PGE2 purification and extraction was carried out according to the manufacturer’s instruction. Briefly, the column was first washed with 0.1 M phosphate buffer solution, and then 4 mL of pooled control or RV conditioned medium was loaded onto the column. The flow-through product free of PGE2 (confirmed by PGE2 ELISA) was collected for experimentation. The column was then washed twice with 2 mL of distilled water, and a 2 mL solution containing 95% absolute ethanol and 5% distilled water was used to elute the column bound PGE2. The eluted solution was completely evaporated by vacuum centrifugation for 2 hours at 37°C and purified PGE2 was reconstituted in 1 mL of BEGM for experimentation. Depletion of PGE2 was confirmed by ELISA.
Molecular Weight Fractionation
Amicon ultra-15 centrifugal filtration units of 100, 50, 30, 10, 3 kDa MWCO filters were purchased from Millipore. Initially, 15 mls of control or RV conditioned medium was pooled and loaded into the initial 100 kDa MWCO filter unit and centrifuged at 4000×g for 30 minutes. One mL of the flow-through product was collected for experimentation and the remainder was loaded into the 50 kDa filter unit and centrifuged as before. The sequential filtration, fractionation and sampling were carried out from the 100 kDa to the 3 kDa MWCO filter unit. Flow-through fractions were used to treat ASMCs to assess the relationship between the size fraction and its ability to cause β2 AR desensitization.
Mass Spectrometry
In order to identify whether there was a difference in the peptide profiles between the control and RV conditioned medium that were in the <3 kDa fraction, mass spectrometry (MS) was carried out as previously described by Ly et al [28]. In brief, the samples were purified using Ziptips (Millipore) as per the manufacturer’s instructions. The peptides were then analysed by liquid chromatography-mass spectrometry analysis (LCMS). Sample peptides were concentrated and desalted onto a ZORBAX 300SB-C18 trap (5 µm, 5 × 0.3 mm, Agilent Technologies, Victoria, Australia) with 5% (v/v) acetonitrile at 10 µL/min. After a 10 minute wash the pre-column was switched into line with an in-house prepared fritless nano column which had been packed with ReproSil-Pur 120Å C18 (Dr Maisch GmbH, Ammerbuch-Entringen, Germany) and peptides eluted using a acetonitrile gradient over 30 minutes at 300 nL/min. High voltage (2300 V) was applied through a low volume tee (Upchurch Scientific, Washington, USA) at the column inlet and the outlet positioned 1.5 cm from the orifice of a QSTAR Elite hybrid tandem mass spectrometer (Applied Biosystems, California, USA). Positive ions were generated by electrospray and the QSTAR operated in information dependent acquisition mode. A survey scan was acquired (350–1750 m/z, 0.5 s) and the 3 most abundantly multiplied charged ions (counts >30, charge state 2 to 4) sequentially selected by Q1 for MS/MS analysis. Nitrogen was used as the collision gas and optimum collision energy was automatically chosen based on charge state and mass. Tandem mass spectra were accumulated with smart exit enabled (quality = 20 or up to 2 s (65–2000 m/z)).
Database Searching
Peak lists were generated using Analyst QS 2.0 and were searched using Mascot v2.3 (Matrix Science, Massachusetts, USA) against the LudwigNR 2012 Q1 database. The following criteria were used: precursor and product ion tolerances 0.2 Da; variable modifications of methionine oxidation; trypsin and one allowed missed cleavage specified; and decoy search enabled. Similar searches were performed but with no enzyme specified.
RNA Purification
Total RNA including micro RNA was purified using the miRNeasy Mini purification kit (Qiagen, Victoria, Australia) as per the manufacturer’s instructions. Total RNA concentration and quality was determined using a spectrophotometer (Nanodrop ND-1000).
Analysis of Data
All data were checked for normal distribution and when the results were not parametrically distributed, the dataset was log transformed prior to statistical analysis using GraphPad Prism Version 5 (GraphPad Software, California, USA). Results were analysed by paired Student’s t-tests and 1 or 2-way analysis of variance (ANOVA) where appropriate and Bonferroni post test comparison with corresponding controls. In all cases, a P value of less than or equal to 0.05 was considered statistically significant.
Results
RV-induced Conditioned Medium and β2 Adrenoceptor Desensitization
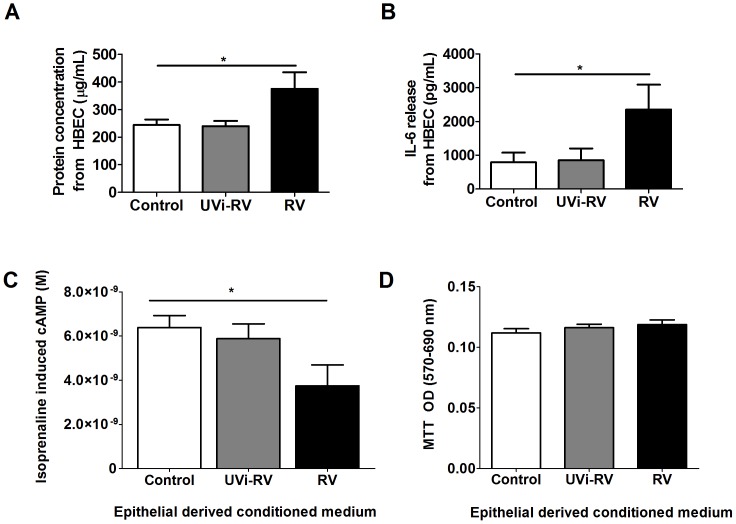
Conditioned medium was initially characterised and is defined as the supernatant obtained from primary HBEC exposed to no virus (control), UVi-RV or replication competent RV. Since RV infection of HBECs induces IL-6, measurement of IL-6 induction was used as a positive control marker to indicate viral infection [8], [29]. Total protein content and IL-6 concentration in the supernatant from HBEC exposed to UVi RV was not different from the non exposed cells. However supernatant from RV infected HBEC, contained a significant increased total protein content as well as IL-6 concentration compared to unstimulated cells (p<0.05, Figure 1 A-B). Primary ASMCs treated with UVi RV conditioned medium from HBEC did not alter isoprenaline induced cAMP levels compared to control conditioned medium. In contrast RV-induced conditioned medium from HBEC resulted in a decreased isoprenaline induced cAMP from ASMCs compared to treatment with control conditioned medium and therefore was indicative of β2 AR desensitization (p<0.05, Figure 1C). RV-induced β2 AR desensitization was independent of an effect on cell viability as assessed by MTT (p>0.05, Figure 1D).
Figure 1. Conditioned medium derived from RV-infected HBEC had increased concentrations of protein and IL-6; and caused a decrease in isoprenaline induced cAMP from ASMCs without an effect on ASMC viability.
(A–B) HBEC (n = 4) were uninfected (Control) or exposed to: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 2 for 24 hours. The concentration of total protein and IL-6 in the supernatant was measured using a BCA assay and ELISA respectively. (C–D) ASMCs (n = 14) were treated with conditioned medium from HBEC (n = 3) that were uninfected, (Control) or exposed to: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 2 for 3 days. Isoprenaline induced cAMP was measured using a cAMP functional assay and ASMC viability was measured using a MTT assay. Data represent mean ± SEM. Statistical differences were examined for using 1-way ANOVA with Bonferroni post test comparison to control treatment *p<0.05.
Molecular Weight Fractionation and Trypsin Digestion of Conditioned Medium
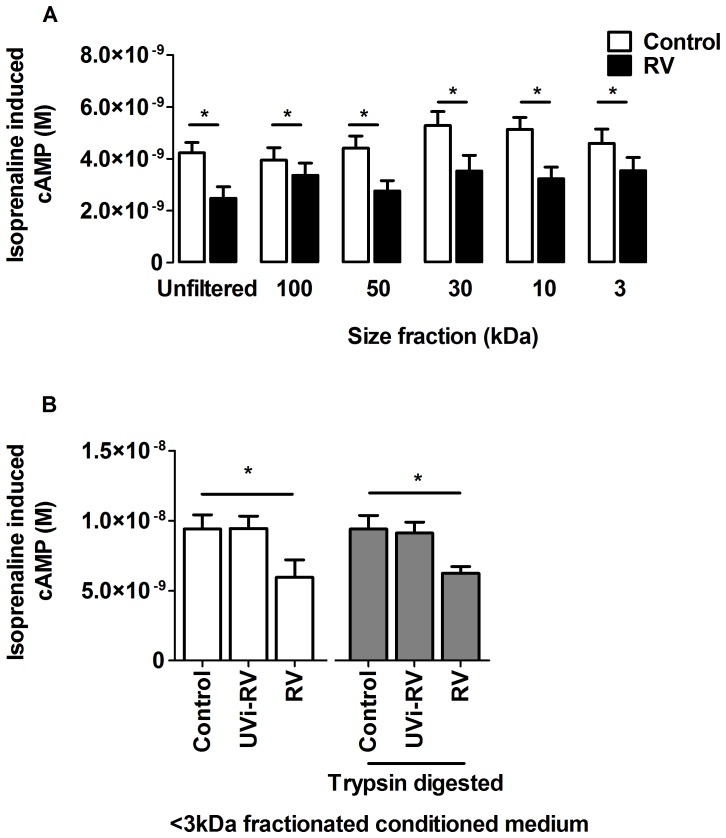
The RV-induced mediator from HBEC responsible for β2 AR desensitization was investigated by size fractionation of the conditioned medium. ASMCs treated with RV-induced conditioned medium that was either not fractioned or was size fractioned through 100, 50, 30, 10 or 3 kDa molecular weight cut off (MWCO) filters, all decreased isoprenaline induced cAMP compared to the control conditioned medium from the corresponding fraction (p<0.05, Figure 2A).
Figure 2. Size fractionation and trypsin digestion of HBEC conditioned medium still resulted in RV-induced β2 adrenoceptor desensitization on ASMCs.
HBEC (n = 6) were uninfected (Control) or infected with replication competent RV (RV) at an MOI = 2 for 24 hours to generate conditioned medium. The conditioned medium was pooled and serially fractionated through ultrafilters with molecular weight cut offs ranging from 100-3 kDa. Alternatively, conditioned medium from HBEC (n = 3) that was uninfected (Control) or exposed to: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 2 for 24 hours was digested in the presence of 500 µg/mL of trypsin for 24 hours at 37°C and trypsin removed using a 3 kDa MWCO ultrafilter. ASMCs (n = 6) were then treated with unfiltered or size fractionated control or RV-induced conditioned medium (A) or 3 kDa fractionated conditioned medium which was undigested or digested with trypsin (B) for 3 days. Isoprenaline induced cAMP was measured using a cAMP functional assay. Data represent mean ± SEM. Statistical differences were detected using multiple paired Student’s t-tests (A) or a 1-way ANOVA with Bonferroni post test (B) comparisons to control conditioned medium *p<0.05.
To determine whether the RV-induced mediator was a protein, the conditioned medium was trypsin digested. Complete trypsin digestion was confirmed by absence of detection by ELISA of two different sized proteins IL-6 (26 kDa) and IL-8 (8 kDa) (Figure S1) and further confirmed by the absence of protein bands using protein gel electrophoresis and silver staining (Data not shown).
ASMCs treated with RV-induced conditioned medium that was trypsin digested and fractionated through the 3 kDa filter to remove trypsin still caused decreased isoprenaline induced cAMP compared to their respective controls (p<0.05, Figure 2B). RV-induced conditioned medium that was digested with trypsin and was not fractionated but saturated with BSA to inhibit the activity of trypsin, also caused reduced isoprenaline induced cAMP (Figure S2). These results imply that the mediator responsible for β2 AR desensitization was not trypsin digestible and had a molecular weight of <3 kDa.
RV-induced Release of Prostaglandins from HBECs, ASMCs and Lung Fibroblasts
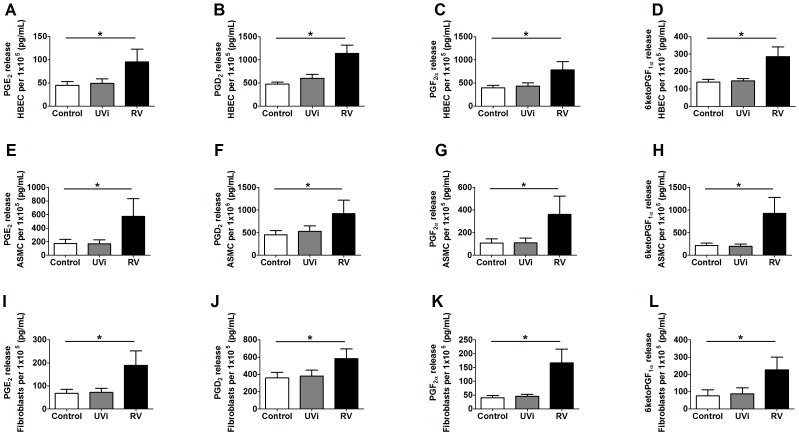
Since the responsible mediator was <3 kDa and not trypsin digestible, prostaglandin release in response to RV infection was investigated. Primary human HBECs, ASMCs and lung fibroblasts exposed to UVi RV at MOI = 1 did not induced further prostaglandin release compared to basal control levels. RV infection with a MOI = 1 induced the release of PGE2, PGF2α, PGD2 and the PGI2 metabolite 6ketoPGF1α from all cell types when compared to basal control levels (p<0.05, Figure 3 A–L).
Figure 3. RV infected primary HBECs, ASMCs and lung fibroblasts induced prostaglandins.
HBECs (n = 5) (A–D), ASMCs (n = 12) (E–H) and lung fibroblasts (n = 4) (I–L) were uninfected (Control) or exposed to: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 1 for 24 hours. Levels of PGE2 (A, E, I), PGF2α (B, F, J), PGD2 (C, G, K) and the PGI2 metabolite 6ketoPGF1α (D, H, L) in the supernatant were measured using ELISA and normalised to total cell number calculated using manual cell counting with trypan blue exclusion after 24 hours. Data represent mean ± SEM. Statistical differences were detected using 1-way ANOVA with Bonferroni post test comparisons to prostaglandin levels in the presence of control conditioned medium *p<0.05.
Effect of Prostaglandins on β2 Adrenoceptor Desensitization
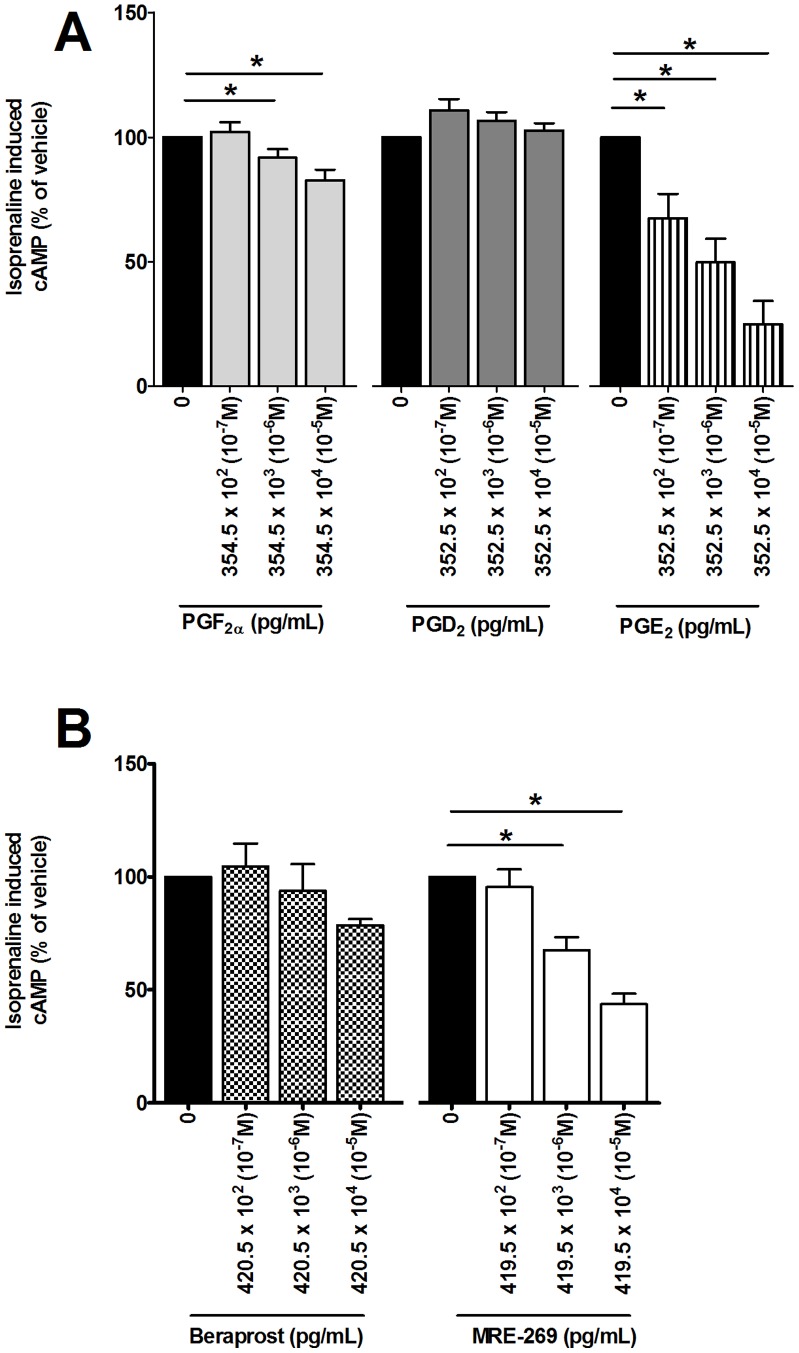
Since RV induced the release of prostaglandins from primary airway structural cells, their effect on β2 adrenoceptor desensitization was investigated. PGE2 and PGF2α, but not PGD2 (10−7-10−5 M), caused a concentration dependent decrease in isoprenaline induced cAMP compared to the respective vehicle controls (p<0.05, Figure 4A). MRE-269, but not beraprost (10−7-10−5 M) caused a concentration dependent decrease in isoprenaline-induced cAMP compared to the respective vehicle controls (p<0.05, Figure 4B).
Figure 4. PGF2α, PGE2, and MRE-269 reduced isoprenaline induced cAMP from ASMCs but not PGD2 and Beraprost.
ASMCs (n = 8) were treated with vehicle (DMSO) (0 M- solid black), PGF2α, PGD2 and PGE2 (10−7-10−5 M) (A) or ASMCs (n = 5) were treated with beraprost and MRE-269 (10−7-10−5 M) (B) in BEGM for 3 days. Isoprenaline induced cAMP was measured using a cAMP functional assay. Data represent mean ± SEM and are expressed as a percentage of the appropriate vehicle. Statistical differences were detected using 1-way ANOVA with Bonferroni post test comparisons to the respective vehicle *p<0.05.
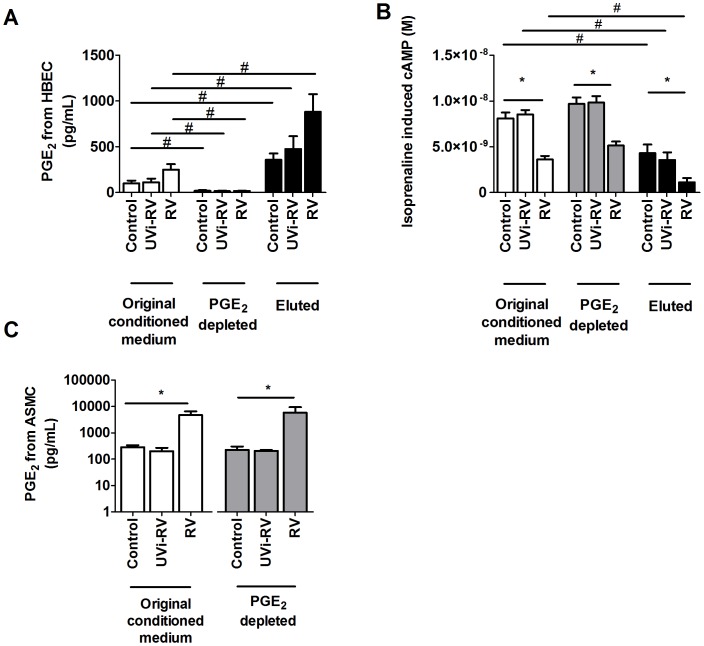
Effect of Depleting PGE2 from Conditioned Medium
Of the RV-induced prostaglandins, only PGE2, PGI2 and PGF2α caused β2 AR desensitization. With PGI2 being unstable and difficult to measure, and the effects of PGF2α on β2 AR desensitization being minimal the investigation focused on the role of PGE2. Conditioned medium from uninfected, UVi-RV or RV infected HBEC was depleted of PGE2 passed through a PGE2 immuno affinity column compared with the original conditioned medium (p<0.05, Figure 5A). RV-induced conditioned medium depleted of PGE2 still caused decreased isoprenaline induced cAMP from ASMCs (p<0.05, Figure 5B). However the PGE2 depleted RV-induced conditioned medium induced the release of PGE2 from ASMCs (p<0.05, Figure 5C). The PGE2 captured by the affinity column was eluted and was found to be concentrated 4 fold by the extraction process (Figure 5A). Even from the control conditioned medium, the PGE2 was sufficient to decrease the isoprenaline induced cAMP from ASMCs and was comparable to that obtained with RV-induced conditioned medium (p<0.05, Figure 5B). Eluted PGE2 from RV-induced conditioned medium caused a greater decrease in isoprenaline induced cAMP from ASMCs compared with ASMCs treated with eluted PGE2 extracted from control conditioned medium (p<0.05, Figure 5B).
Figure 5. PGE2 was successfully depleted from RV-induced conditioned medium from HBEC; but it still caused RV-induced β2 adrenoceptor desensitization and further induced PGE2 from ASMCs.
HBEC (n = 3) were uninfected (Control) or exposed to: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 2 for 24 hours to generate conditioned medium. Conditioned medium was depleted of PGE2 using affinity chromatography. The original conditioned medium, conditioned medium depleted of PGE2 and PGE2 eluted product were collected and the levels of PGE2 (A) was measured using ELISA. ASMCs (n = 6) were then treated with the original conditioned medium, conditioned medium depleted of PGE2 or PGE2 eluted product in BEGM for 3 days. Isoprenaline induced cAMP was measured using a cAMP functional assay (B) and the level of PGE2 released by ASMCs due to each component was measured using ELISA (C). Data represent mean ± SEM. Statistical differences were detected using a 2-way ANOVA (A&B) and 1-way ANOVA (C) with Bonferroni post test comparisons to the respective control conditioned medium *p<0.05; respective components of the original conditioned medium #p<0.05.
Effect of COX Inhibitors and Prostaglandin Receptor Antagonists on RV-induced β2 Adrenoceptor Desensitization
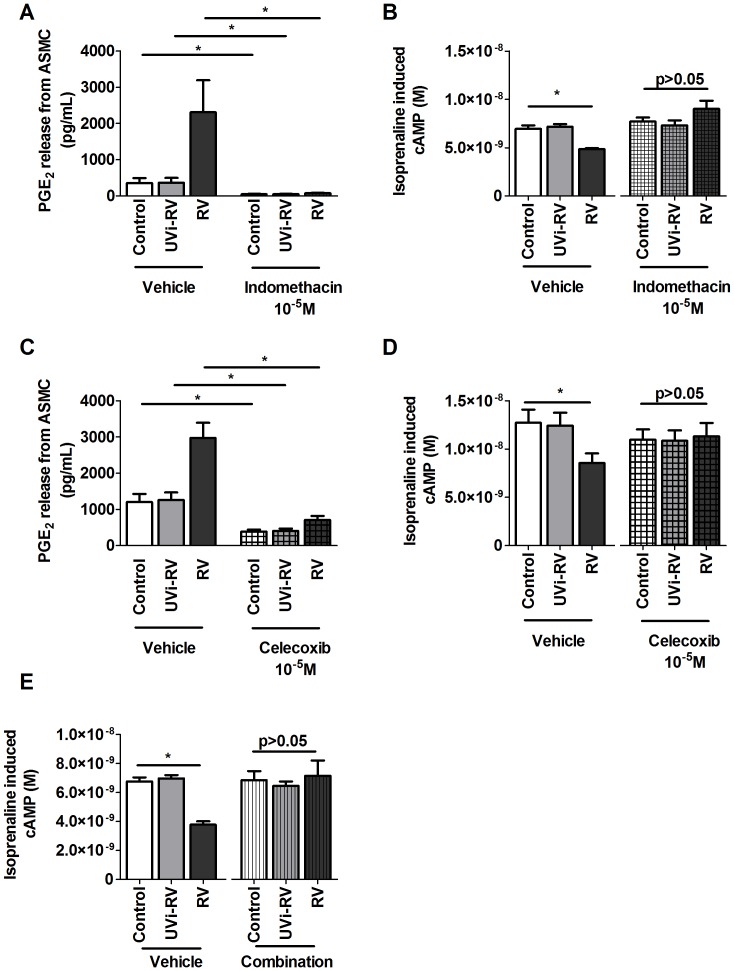
The autocrine action of ASMC derived prostaglandins on β2 AR desensitization was investigated with pharmacological tools using the non selective COX inhibitor indomethacin, a COX-2 selective inhibitor celecoxib and a combination of PGE2, PGD2, PGF2α and PGI2 receptor antagonists.
PGE2 release from ASMCs treated with conditioned medium in the presence of indomethacin and celecoxib was significantly attenuated compared to RV-induced conditioned medium in the presence of vehicle (p<0.05, Figure 6 A and C).
Figure 6. Pharmacological inhibition of prostaglandins prevented the effect of RV-induced β2 adrenoceptor desensitization on ASMCs.
ASMCs (n = 5) were pretreated for 1 hr with appropriate vehicles or indomethacin (10−5 M) (A,B), celecoxib (10−5 M) (C,D) or a combination of prostaglandin receptor antagonists (combination composition: AH6809 (10−5 M); CAY10441 (10−6 M); AL8810 (10−5 M); BWA868C (10−5 M); L-161,982 (10−6 M)) (E) and maintained for a further 3 days in the presence of conditioned medium from HBEC (n = 1) that were uninfected (Control) or exposed to: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 2 for 24 hours. PGE2 (A, C) was measured using ELISA and isoprenaline induced cAMP (B, D, E) was measured using a cAMP functional assay. Data represent mean ± SEM. Statistical differences were detected using 1-way ANOVA with Bonferroni post test comparisons to control conditioned medium pretreatment in each group *p<0.05.
ASMCs treated with RV-induced conditioned medium resulted in a decreased isoprenaline induced rise in cAMP in the presence of vehicle (p<0.05), however this effect was absent when ASMCs was treated with indomethacin, celecoxib or a combination of prostaglandin receptor antagonists in the presence of RV-induced conditioned medium (p>0.05 Figure 6 B, D and E).
Since non specific prostaglandin inhibition prevented RV-induced β2 AR desensitization, individual receptor antagonists of prostaglandin receptors were used to determine which prostaglandin was responsible for RV-induced β2 AR desensitization. However RV-induced conditioned medium still caused β2 adrenoceptor desensitization on ASMCs even in the presence of individual PGE2, PGI2, PGD2 or PGF2α receptor antagonists (p<0.05, Figure S3).
Mass Spectrometry Analysis of Conditioned Medium
In order to investigate whether a small peptide was present in the RV conditioned medium and could be responsible for β2 AR desensitization on ASMCs, an overall peptide profile present in the less than 3 kDa fraction of the uninfected, UVi-RV and RV-induced conditioned medium was obtained using mass spectrometry.
A comparative proteomic analysis of uninfected, UVi-RV and RV-induced conditioned medium resulted in the identification of a 1.2 kDa peptide with an 11 amino acid sequence “GDEQPLTENPR” that was present only in the RV-induced conditioned medium. The peptide is a fragment of the pro-opiomelanocortin (POMC) protein, which is a polypeptide that is cleaved to give rise to a super family of neuro-peptides. The 1.2 kDa peptide was synthesized and along with POMC, did not influence ASMC viability, PGE2 induction or β2 AR desensitization (data not shown).
RNA Levels in Conditioned Medium and Effect of TLR Activation on β2 Adrenoceptor Desensitization on ASMCs
Toll-like receptors (TLR) are pathogen pattern recognition receptors that are present on ASMCs [30]. Activation of TLRs on ASMCs by small synthetic molecules, pure viral RNA and even fragments of RNA only 5 nucleotides long are immuno-stimulatory [31], [32]. Since activation of the bacterial recognition receptors TLRs 2 & 4 result in the induction of COX-2 induced prostaglandins [33], [34], we investigated whether RV RNA as a result of infection can activate the viral recognition receptors TLR 3, 7 and 8 and also induce COX-2 mediated prostaglandins which may then cause β2 AR desensitization on ASMCs.
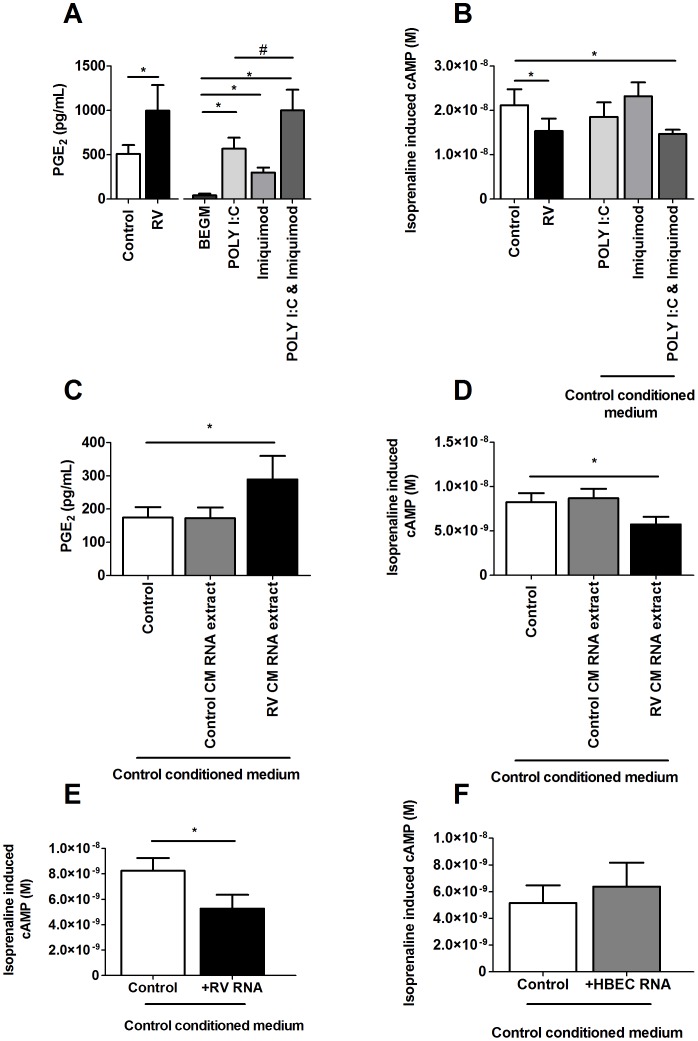
When conditioned medium generated from HBEC (n = 3) was pooled, the total RNA measured in RV-induced conditioned medium (300.25 ng/µL) was almost 6 times higher than RNA in control conditioned medium (53.91 ng/µL). RV infection produces both ssRNA and dsRNA during replication and whether activation of TLRs 3 and 7/8 using synthetic analogues can result in induction of PGE2 and β2 AR desensitization on ASMCs were investigated.
Treatment with poly I:C (50 µg/mL) or imiquimod (30 µg/mL) induced PGE2 from ASMCs compared to BEGM alone (p<0.05) and did not alter isoprenaline induced cAMP (Figure 7 A&B). Treatment with the combination of poly I:C and imiquimod caused an additive induction of PGE2 similar to the levels caused by RV-induced conditioned medium from ASMCs and caused a decrease in isoprenaline induced cAMP (p<0.05, Figure 7 A&B).
Figure 7. The combination of TLR 3 and 7/8 agonists, and RNA extracted from RV-induced conditioned medium or RV stock caused PGE2 induction and β2 adrenoceptor desensitization on ASMCs, but not RNA extracted from HBECs.
Control and RV-induced conditioned medium was generated from HBEC (n = 2) and pooled. ASMCs (n = 6) were treated with this pooled control or RV-induced conditioned medium; or untreated (BEGM), poly I:C (50 µg/mL), imiquimod (30 µg/mL) and poly I:C & imiquimod (50 µg/mL, 30 µg/mL respectively) in BEGM or in the presence of the control conditioned medium for 3 days (A, B). (Figure C-F) Control and RV-induced conditioned medium was generated from HBEC (n = 3) and pooled. Total RNA was extracted from: control- (53.91 ng/µL), RV-induced conditioned medium (300.25 ng/µL), RV stock (567.35 ng/µL) and cell lysate collected from a sub-confluent 75 cm2 flask of HBEC (300 ng/µL) using a miRNeasy Mini purification kit and amount of RNA quantified using a spectrophotometer. ASMCs (n = 6) were treated with pooled control conditioned medium (control), or total extracted RNA collected from those sources in the presence of control conditioned medium for 3 days. PGE2 was measured using an ELISA (A, C) and isoprenaline induced cAMP was measured using a cAMP functional assay (B, D-F). Data represent mean ± SEM. Statistical differences were detected using 1-way ANOVA with Bonferroni post test comparisons to respective BEGM, control conditioned medium only (Control) or Poly I:C *p<0.05, #p<0.05.
Treatment with 300.25ng/µL of RNA extracted from RV-induced conditioned medium induced PGE2 release and decreased isoprenaline induced cAMP from ASMCs compared to the control treatment without RNA (p<0.05, Figure 7 C&D). These effects did not occur with treatment of RNA extracted from control conditioned medium.
Since the extraction of total RNA from RV induced conditioned medium may include endogenous RNA from HBEC as well as viral RNA, the effect of viral RNA and endogenous RNA on β2 AR desensitization was investigated. Treatment with RNA obtained from RV-16 stock in the presence of control conditioned medium decreased isoprenaline induced cAMP from ASMCs compared to the control treatment without RNA (p<0.05, Figure 7E) but treatment with endogenous RNA extracted from HBEC lysate did not alter isoprenaline induced cAMP (p>0.05, Figure 7F) when compared to control conditioned medium without RNA. This suggests that viral RNA in RV induced conditioned medium may be responsible for β2 AR desensitization on ASMCs.
Discussion
Using an in vitro model we previously showed that factor(s) released from RV infected epithelial cells cause β2AR desensitization on ASMCs [8]. In the present study, we aimed to identify the responsible mediators and pathways which are involved and we confirmed that RV infection of primary HBEC produced conditioned medium that when used to treat ASMCs reduces isoprenaline induced cAMP, thus functionally causing β2 AR desensitization. It was deduced that the responsible mediator was less than 3 kDa which meant it could potentially have been a lipid such as a prostaglandin. We showed that RV infected airway cells produce prostaglandins and, since PGE2 is known to desensitize the β2 AR [11], the effect of other members of the prostaglandin family on the β2 AR was examined in vitro. It was found that PGF2α, PGE2 and MRE-269 caused β2 AR desensitization and with the use of pharmacological tools it was deduced that β2 AR desensitization occurred via autocrine prostaglandins from ASMCs. Mass spectrometry analysis yielded a single peptide present in the less than 3 kDa conditioned medium fraction; however it alone had no influence on β2 AR function. Since RNA was detected at greater levels in RV-induced conditioned medium than the control conditioned medium, we investigated RNA as the potential mediator causing β2 AR desensitization via the activation of TLR receptors. Using synthetic analogs of double stranded (ds) and single stranded (ss) RNA it was found that their combination caused PGE2 induction from ASMCs and β2 AR desensitization and similarly, total RNA extracted from RV-induced conditioned medium also caused PGE2 induction from ASMCs and β2 AR desensitization. Since human endogenous RNA extracted from HBEC did not cause this effect but RNA extracted from RV did, it was deduced that viral RNA produced during RV replication was required to cause β2 AR desensitization.
Like all G protein coupled receptors (GPCRs), desensitization of the β2 AR occurs physiologically to prevent over activation of the receptor. This can be achieved by the mechanism of homologous desensitization i.e. activation of the receptor by its own defined agonist, for example, activation of the β2 AR by a β2 agonist causing β2 AR desensitization. Alternatively this can also occur by heterologous desensitization i.e. activation of another GPCR resulting in β2 AR desensitization because of common downstream cross-regulation of the signal pathways of the G protein subunits [12], [14]. Prostaglandin receptors are all GPCRs, and it is well documented that PGE2 can cause heterologous desensitization of the β2 AR by means of downstream cross talk of GPCR kinases (GRK) but also by activation of the PKA pathway through the EP2 and EP4 receptors [12], [13].
Trian et al. first developed this in vitro model as used in this study to explore and understand why patients with naturally occurring RV-induced asthma exacerbations do not respond well to β2 agonists. We previously deduced that the substance was released only from RV infected HBEC [8]. Findings in the current investigation confirmed the result of Trian et al that RV infection of HBEC indeed produced increased levels of mediators in conditioned medium which may cause β2 AR desensitization. In this study our data indicates that the responsible mediator for β2 AR desensitization was less than 3 kDa and not affected by trypsin digestion and therefore could potentially be a lipid or a trypsin-resistant peptide.
It has already been reported by Seymour et al that in vivo RV infection results in the up regulation of COX-2 enzymes as detected by immunostaining of bronchial mucosal biopsies [9]. However they did not examine which prostaglandin was released or which cell the prostaglandins came from. There are a limited number of studies that have investigated RV-induced eicosanoids from airway structural cells and their impact in the respiratory system. Within this context and of the few investigations in this area, Oliver et al reported that RV infection of alveolar macrophages induces the release of PGE2 [35]. Kuo et al found that RV infection of epithelial cells resulted in the induction of PGE2, and RV-induced PGE2 could potentially influence airway remodelling via its effect on altering neighbouring cellular behaviour such as cell migration [17]. In the present study we extended the research beyond just PGE2, to show that RV infection of primary HBECs, ASMCs and lung fibroblasts can induce the release of various prostaglandin isotypes other than just PGE2. The amount of each prostaglandin released in response to RV infection varied amongst the various airway cell types tested, and although this issue is beyond the scope of this study, it could potentially highlight differences in the contribution of each individual prostaglandin as infection progresses from the epithelium to sub-mucosal cells. This raises the possibility of a pool of potential candidates singly or in combination that may be responsible for desensitization of the β2 AR.
The investigation showed that RV infection of airway cells produced prostaglandins and PGE2 can cause desensitization of the β2 AR, which is in keeping with the study by Penn et al. who showed that PGE2 can cause desensitization of the β2 AR, [11]. Since it has not been previously explored, the role of other prostaglandins was investigated and it was found that PGF2α can also cause desensitization of the β2 AR, suggesting it may share common GRKs with the β2 AR. Similarly, the PGI2 analogue MRE-269 also has the ability to cause β2 AR desensitization suggesting this desensitization may involve PKA as well as GRK cross talk. Therefore it is possible that RV infection may result in an increase of prostaglandins in the vicinity and may result in β2 AR desensitization on ASMCs. The ability of PGF2α to induce β2 AR desensitization was minimal compared to those by MRE-269 and PGE2, and since PGI2 is extremely unstable and only its inactive metabolite could be measured, it was considered unlikely to be sufficiently stable in the model to cause β2 AR desensitization on ASMCs. Since we showed PGE2 caused β2 AR desensitization most prominently compared to other prostaglandins, it was investigated whether HBEC derived PGE2 could be responsible by its depletion from conditioned medium. However, removal of PGE2 from conditioned medium did not affect β2 AR desensitization on ASMCs. It was then hypothesized that the autocrine action of ASMC derived prostaglandins may instead be responsible for β2 AR desensitization on the ASMCs, as PGE2 depleted RV-induced conditioned medium further induced approximately 10 fold higher levels of PGE2 in comparison to control conditioned medium. This suggested that the unidentified mediator, although not HBEC derived PGE2, induces PGE2 and potentially other prostaglandins from ASMCs.
To investigate if ASMC derived prostaglandins were responsible for β2 AR desensitization, ASMCs was treated with indomethacin and this prevented β2 AR desensitization from occurring when ASMCs were exposed to RV-induced HBEC conditioned medium. These findings were similar to the study by Guo et al, who showed that exogenously applied interleukin (IL)-1β induced COX-2 induction of PGE2 caused β2 AR desensitization and this was also preventable with indomethacin treatment in primary human ASMCs [12]. However IL-1β is a 17 kDa protein and since RV-induced conditioned medium from HBEC does not contain IL-1β and the unknown mediator is smaller than 3 kDa it was excluded as the mediator in this investigation [8], [36], [37]. It was further verified that COX-2 induced autocrine prostaglandins were responsible for β2 AR desensitization as treatment with celecoxib also prevented this effect. The hypothesis that autocrine prostaglandins were causing β2 AR desensitization was also confirmed when ASMCs treated with a combination of prostaglandin antagonists prevented β2 AR desensitization in response to RV-induced HBEC conditioned medium exposure. Therefore this suggests that RV infection of HBEC may be producing unidentified mediators which cause increased activity of COX-2 and production of prostaglandins from ASMCs which act autocrinely to cause β2 AR desensitization.
An attempt was made to determine which prostaglandin isotype was responsible for β2 AR desensitization by pre-treating ASMCs with different prostaglandin receptor antagonists only to find that none of the antagonists alone prevented β2 AR desensitization. The receptor antagonists available are designed to be selective for individual prostaglandin receptors however, in some circumstances; the antagonists are not selective enough and lack the ability to differentiate between the very similar receptors of PGE2 and PGD2. In addition, prostaglandins are also very pleotropic and can cross activate each other’s receptors [38], [39]. In future, with more defined and specific antagonists available or by genetically knocking down specific prostaglandin receptors, it may be possible to delete individual autocrine prostaglandins and to identify the combination of prostaglandin isotypes involved in RV-induced β2 AR desensitization.
Using mass spectrometry it was found that a 1.2 kDa peptide was present in RV-induced conditioned medium, but not in the control conditioned medium. The 1.2 kDa peptide is a fragment from the POMC protein which is a precursor for various hormones and ultradian rhythm neuro-peptides [40]. However treatment of ASMCs with the 1.2 kDa peptide or the polypeptide POMC did not cause β2 AR desensitization on ASMCs, suggesting they are not the responsible mediators or that a combination of mediators are required.
Since RV-induced conditioned medium cause β2 AR desensitization through an immuno-stimulatory process such as via the process of autocrine COX-2 induction, and the unidentified mediator was small, it was hypothesized that the responsible mediator might be viral RNA or viral RNA fragments.
Results of the study showed that the use of the synthetic TLR 7/8 agonist imiquimod and the TLR 3 agonist poly I:C alone slightly increased PGE2 release from ASMCs but did not alter isoprenaline induced cAMP. This result is in agreement with that of Cooper et al, who also showed that poly I:C promotes inflammatory mediator release [41]. However in order to mimic the presence of both viral ssRNA and dsRNA fragments which would occur during RV replication, the combination of both synthetic agonists of the TLRs 3 and 7/8 was used and it was found that it caused β2 AR desensitization as well as an additive induction of PGE2 from ASMCs equivalent to those levels produced by RV-induced conditioned medium. This was further explored by showing that RNA extracted from an equal volume of RV-induced HBEC conditioned medium was higher than the control conditioned medium, and that RNA collected from RV-induced conditioned medium caused ASMC β2 AR desensitization. Total RNA extracted from RV-induced conditioned medium could include both endogenous human RNA as well as RV RNA and it is difficult to distinguish these small nucleotide fragments. It was confirmed that equal concentrations of RNA extracted from pure RV-16 caused PGE2 induction and β2 AR desensitization on ASMCs but this was not the case for endogenous human RNA extracted from HBEC lysate, which suggests that foreign viral RNA is required.
RV is a positive sense ssRNA virus and during replication, dsRNA are formed in order to generate more copies of the ssRNA genome to be packed into new virions. However, like many RNA viruses, RV replication is susceptible to RNA polymerase error with a rate of 10−3 and 10−4 errors/nucleotide/cycle of replication [42]. This could result in incorrect or incomplete formation of new ssRNA strands which may not be packed into new virion capsules and may accumulate intracellularly. Continuing RV replication would eventually result in the accumulation of large amounts of both incomplete and complete viral ss/dsRNA inside the cell. This viral RNA could escape the cell via transporting vesicles, cellular pores or upon cell lysis which could expose foreign RV RNA as well as new infectious RV progeny to neighbouring cells. In the case of the ASMC, it is possible that RV RNA could potentially trigger TLRs to activate COX-2 induced prostaglandins and therefore result in β2 AR desensitization. Whilst UVi-RV contains RV RNA, the virions cannot replicate and therefore there is no increase in viral RNA. Whether β2 AR desensitization is restricted to asthma, or not is not known. It has been reported that greater RV replication occurs in epithelial cell derived from people with asthma [43] so there is a possibility that there would be greater RV RNA. Alternatively, it is possible that in people without asthma or airway hyper-reactivity, RV-induced β2 AR desensitization can occur during colds and remains clinically unnoticed. It is uncertain whether full length RV RNA or RNA fragments activate ASMCs to cause β2 AR desensitization, however both are possibilities, as it has been shown that nucleotide fragments as small as 5 nucleotides long can activate TLR 7/8 [32].
This in vitro model attempted to simulate the events of an in vivo RV infection of the lung, which occurs principally in the airway epithelium and may result in ASMC β2 AR desensitization. However there are limitations to the current model that need to be acknowledged. Primarily, the in vitro model involved only 2 cell types, whilst physiologically there could be more cell types involved during infection. For example inflammatory cells and or perhaps other structural cells such as fibroblasts could play a role. For this reason it is possible that other cells may be as influential in causing β2 AR desensitization on ASMCs as epithelial cells. Furthermore, when other cells are considered, the system can become much more complex, and protein mediators such as IL-1β which can also cause β2 AR desensitization [12], [44] may also be involved. In addition, as it was shown that each size fraction caused β2 AR desensitization, it does not necessarily mean that it is only one small mediator that was responsible. It is possible that there is a combination of multiple sized mediators trapped in each fraction which could activate GPCRs and cause β2 AR desensitization.
Although it was deduced in the current model that β2 AR desensitization was due to RV RNA activating TLRs, in our investigation RNA was extracted using a trizol methodology and for this reason small RNA mediators such as RV-induced HBEC derived micro (mi) RNA which may be able to activate TLRs [32] and/or have their own undefined effect on β2 AR modulation could also be responsible and requires further investigation. It is theoretically possible to degrade the RNA in RV-induced conditioned medium using exogenous ribonucleases and assess for β2 AR desensitization. The limitation to this is that RNA that has been degraded to as small as 5 nucleotides long may still be or become immuno-stimulatory [32]. In a multicellular environment, production of leukotrienes is also highly likely and can also cause β2 AR desensitization, as it has been shown previously with LTD4 [15]. In addition, cytoplasmic helicase proteins such as retinoic-acid-inducible protein I (RIG-I) and melanoma-differentiation-associated gene 5 (MDA5) have been implicated in viral dsRNA recognition [45]. During RV infection of epithelial cells TLR activation is responsible for co-ordinating the up regulation of RIG-I and MDA-5 which further induces antiviral responses [46]. Therefore, there is a possibility that activation of TLRs but also RIG-I and MDA-5 by RV RNA could potentially contribute in a co-ordinated manner to induce β2 AR desensitization and should be investigated in future studies.
The second limitation of the in vitro model is the use of primary human ASMCs and BEC which were obtained from multiple disease tissue origins. The use of primary human cells is a better representation of human cellular behaviour than some transformed cell lines because they reflect more accurately the true nature of biological heterogeneity of cellular responses, however since the current model utilized two cell types which were not diseased matched, and in some cases, HBEC derived conditioned medium was pooled from multiple diseases, it might be possible that asthma specific effects were missed.
Results from the present study identify the potential mediator responsible for RV-induced β2 AR desensitization as viral RNA. During RV replication, viral RNA increases and activates TLRs and as a result induces COX-2 mediated prostaglandin production which causes β2 AR dysfunction. Interestingly, the findings in this study also raise the possibility that β2 AR desensitization may not be entirely unique to RV infection but could also be caused by other respiratory viruses that actively infect and replicate. By extrapolating the current findings to the clinical situation, the potential use of COX-2 inhibitors to restore β2 agonist efficacy in asthmatic patients during viral infection is possible and could be investigated clinically.
Supporting Information
Trypsin digestion of conditioned medium removes IL-6 and IL-8. Conditioned medium pooled from HBEC (n = 3) that were uninfected (Control) or treated with: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 2 for 24 hours was digested in the presence of 500 µg/mL of trypsin for 24 hours at 37°C and the reaction was stopped with 0.1% BSA. IL-6 and IL-8 were measured using ELISA with n = 1 experimental repeat.
(TIF)
Trypsin digestion of conditioned medium still causes ASMC β2 adrenoceptor desensitization. Conditioned medium pooled from HBEC (n = 3) that were uninfected (Control) or exposed to: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 2 for 24 hours was digested in the presence of 500 µg/mL of trypsin for 24 hours at 37°C and the reaction was stopped with 0.1% BSA. ASMCs (n = 6) were treated with trypsin digested conditioned medium for 3 days. Isoprenaline induced cAMP was measured using a cAMP functional assay. Data represent mean ± SEM. Statistical differences were detected using a 1-way ANOVA with Bonferroni post test comparisons to control conditioned medium *p<0.05.
(TIF)
Individual prostaglandin antagonists do not prevent β2 adrenoceptor desensitization. ASMCs (n = 6) were pretreated for 1 hr with vehicle (0.1% DMSO), CAY10441 (10−6 M) (A), AH6809 (10−5 M) (B), AL8810 (10−5 M) (C), BWA868C (10−5 M) (D) or L-161,982 (10−6 M) (E) and maintained for a further 3 days in the presence of conditioned medium from HBEC (n = 2) that were uninfected (Control) or infected with replication competent RV (RV) at an MOI = 2 for 24 hours. Isoprenaline induced cAMP was measured using a cAMP functional assay. Data represent mean ± SEM. Statistical differences were detected using 1-way ANOVA with Bonferroni post test comparisons to the control conditioned medium pretreatment in each group *p<0.05.
(TIF)
Acknowledgments
We acknowledge the collaborative effort of the cardiopulmonary transplant team and the pathologists at St Vincent’s Hospital, Sydney; the thoracic physicians and pathologists at Royal Prince Alfred Hospital, Strathfield Private Hospital and Rhodes Pathology, Sydney; and Dr Lucy Morgan and Professor Matthew Peters at Concord Repatriation General Hospital, Sydney. The mass spectrometry was facilitated by access to the Sydney University Proteome Research Unit established under the Australian Government’s Major National Research Facilities program and supported by the University of Sydney.
Funding Statement
JKB is supported by a National Health and Medical Research Council Career Development Fellowship #1032695. JLB is supported by a National Health and Medical Research Council Senior Principal Research Fellowship #571098. BGGO is supported by a National Health and Medical Research Council Career Development Fellowship APP1026880. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Edwards MR, Kebadze T, Johnson MW, Johnston SL (2006) New treatment regimes for virus-induced exacerbations of asthma. Pulm Pharmacol Ther 19: 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GINA GIFA (2011) GINA Report, Global Strategy for Asthma Management and Prevention.
- 3. Reddel H, Ware S, Marks G, Salome C, Jenkins C, et al. (1999) Differences between asthma exacerbations and poor asthma control. Lancet 353: 364–369. [DOI] [PubMed] [Google Scholar]
- 4. Rueter K, Bizzintino J, Martin AC, Zhang G, Hayden CM, et al. (2012) Symptomatic viral infection is associated with impaired response to treatment in children with acute asthma. J Pediatr 160: 82–87. [DOI] [PubMed] [Google Scholar]
- 5. Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, et al. (2000) Rhinoviruses infect the lower airways. J Infect Dis 181: 1875–1884. [DOI] [PubMed] [Google Scholar]
- 6.Wos M, Sanak M, Soja J, Olechnowicz H, Busse WW, et al.. (2008) The Presence of Rhinovirus in Lower Airways of Patients with Bronchial Asthma. Am J Respir Crit Care Med. [DOI] [PMC free article] [PubMed]
- 7. Xatzipsalti M, Kyrana S, Tsolia M, Psarras S, Bossios A, et al. (2005) Rhinovirus viremia in children with respiratory infections. Am J Respir Crit Care Med 172: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 8. Trian T, Moir LM, Ge Q, Burgess JK, Kuo C, et al. (2010) Rhinovirus-induced exacerbations of asthma: How is the {beta}2-adrenoceptor implicated? Am J Respir Cell Mol Biol 43: 227–233. [DOI] [PubMed] [Google Scholar]
- 9. Seymour ML, Gilby N, Bardin PG, Fraenkel DJ, Sanderson G, et al. (2002) Rhinovirus infection increases 5-lipoxygenase and cyclooxygenase-2 in bronchial biopsy specimens from nonatopic subjects. J Infect Dis 185: 540–544. [DOI] [PubMed] [Google Scholar]
- 10. Seymour ML, Rak S, Aberg D, Riise GC, Penrose JF, et al. (2001) Leukotriene and prostanoid pathway enzymes in bronchial biopsies of seasonal allergic asthmatics. Am J Respir Crit Care Med 164: 2051–2056. [DOI] [PubMed] [Google Scholar]
- 11. Penn RB, Panettieri RA Jr, Benovic JL (1998) Mechanisms of acute desensitization of the beta2AR-adenylyl cyclase pathway in human airway smooth muscle. Am J Respir Cell Mol Biol 19: 338–348. [DOI] [PubMed] [Google Scholar]
- 12. Guo M, Pascual RM, Wang S, Fontana MF, Valancius CA, et al. (2005) Cytokines regulate beta-2-adrenergic receptor responsiveness in airway smooth muscle via multiple PKA- and EP2 receptor-dependent mechanisms. Biochemistry 44: 13771–13782. [DOI] [PubMed] [Google Scholar]
- 13. Kong KC, Gandhi U, Martin TJ, Anz CB, Yan H, et al. (2008) Endogenous Gs-coupled receptors in smooth muscle exhibit differential susceptibility to GRK2/3-mediated desensitization. Biochemistry 47: 9279–9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly E, Bailey CP, Henderson G (2008) Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol 153 Suppl 1S379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rovati GE, Baroffio M, Citro S, Brichetto L, Ravasi S, et al. (2006) Cysteinyl-leukotrienes in the regulation of beta2-adrenoceptor function: an in vitro model of asthma. Respir Res 7: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roth M, Soler M, Hornung M, Emmons LR, Stulz P, et al. (1992) Cell cultures from cryopreserved human lung tissue. Tissue Cell 24: 455–459. [DOI] [PubMed] [Google Scholar]
- 17. Kuo C, Lim S, King NJ, Bartlett NW, Walton RP, et al. (2011) Rhinovirus infection induces expression of airway remodelling factors in vitro and in vivo. Respirology 16: 367–377. [DOI] [PubMed] [Google Scholar]
- 18. Belsham GJ, Sonenberg N (1996) RNA-protein interactions in regulation of picornavirus RNA translation. Microbiol Rev 60: 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papi A, Johnston SL (1999) Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem 274: 9707–9720. [DOI] [PubMed] [Google Scholar]
- 20. Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, et al. (2008) Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med 14: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Ly D, King NJ, Moir LM, Burgess JK, Black JL, et al. (2011) Effects of beta(2) Agonists, Corticosteroids, and Novel Therapies on Rhinovirus-Induced Cytokine Release and Rhinovirus Replication in Primary Airway Fibroblasts. J Allergy (Cairo) 2011: 457169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clarke DL, Belvisi MG, Hardaker E, Newton R, Giembycz MA (2005) E-ring 8-isoprostanes are agonists at EP2- and EP4-prostanoid receptors on human airway smooth muscle cells and regulate the release of colony-stimulating factors by activating cAMP-dependent protein kinase. Mol Pharmacol 67: 383–393. [DOI] [PubMed] [Google Scholar]
- 23. Gomez E, Schwendemann C, Roger S, Simonet S, Paysant J, et al. (2008) Aging and prostacyclin responses in aorta and platelets from WKY and SHR rats. Am J Physiol Heart Circ Physiol 295: H2198–2211. [DOI] [PubMed] [Google Scholar]
- 24. Griffin BW, Klimko P, Crider JY, Sharif NA (1999) AL-8810: a novel prostaglandin F2 alpha analog with selective antagonist effects at the prostaglandin F2 alpha (FP) receptor. J Pharmacol Exp Ther 290: 1278–1284. [PubMed] [Google Scholar]
- 25. Zhang A, Dong Z, Yang T (2006) Prostaglandin D2 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in MDCK cells. Am J Physiol Renal Physiol 291: F1332–1342. [DOI] [PubMed] [Google Scholar]
- 26. Cuthbert AW (2011) Lubiprostone targets prostanoid EP(4) receptors in ovine airways. Br J Pharmacol 162: 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirst SJ, Barnes PJ, Twort CH (1992) Quantifying proliferation of cultured human and rabbit airway smooth muscle cells in response to serum and platelet-derived growth factor. Am J Respir Cell Mol Biol 7: 574–581. [DOI] [PubMed] [Google Scholar]
- 28. Ly L, Barnett MH, Zheng YZ, Gulati T, Prineas JW, et al. (2011) Comprehensive tissue processing strategy for quantitative proteomics of formalin-fixed multiple sclerosis lesions. J Proteome Res 10: 4855–4868. [DOI] [PubMed] [Google Scholar]
- 29. Wark PA, Grissell T, Davies B, See H, Gibson PG (2009) Diversity in the bronchial epithelial cell response to infection with different rhinovirus strains. Respirology 14: 180–186. [DOI] [PubMed] [Google Scholar]
- 30. Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, et al. (2006) Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol 118: 641–648. [DOI] [PubMed] [Google Scholar]
- 31. Kuo C, Lim S, King NJ, Johnston SL, Burgess JK, et al. (2011) Rhinovirus infection induces extracellular matrix protein deposition in asthmatic and nonasthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L951–957. [DOI] [PubMed] [Google Scholar]
- 32. Judge AD, Sood V, Shaw JR, Fang D, McClintock K, et al. (2005) Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol 23: 457–462. [DOI] [PubMed] [Google Scholar]
- 33. Kuper C, Beck FX, Neuhofer W (2012) Toll-like receptor 4 activates NF-kappaB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am J Physiol Renal Physiol 302: F38–46. [DOI] [PubMed] [Google Scholar]
- 34. Villamon E, Roig P, Gil ML, Gozalbo D (2005) Toll-like receptor 2 mediates prostaglandin E(2) production in murine peritoneal macrophages and splenocytes in response to Candida albicans. Res Microbiol 156: 115–118. [DOI] [PubMed] [Google Scholar]
- 35. Oliver BG, Lim S, Wark P, Laza-Stanca V, King N, et al. (2008) Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax 63: 519–525. [DOI] [PubMed] [Google Scholar]
- 36. Kelsen SG, Anakwe O, Aksoy MO, Reddy PJ, Dhanasekaran N (1997) IL-1 beta alters beta-adrenergic receptor adenylyl cyclase system function in human airway epithelial cells. Am J Physiol 273: L694–700. [DOI] [PubMed] [Google Scholar]
- 37. March CJ, Mosley B, Larsen A, Cerretti DP, Braedt G, et al. (1985) Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature 315: 641–647. [DOI] [PubMed] [Google Scholar]
- 38. Kang KH, Shim JJ, Banerjee M, Newman JH (1996) PGF2 alpha causes bronchoconstriction and pulmonary vasoconstriction via thromboxane receptors in rat lung. Korean J Intern Med 11: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olman MA (2009) Beyond TGF-beta: a prostaglandin promotes fibrosis. Nat Med 15: 1360–1361. [DOI] [PubMed] [Google Scholar]
- 40. Girotti M, Weinberg MS, Spencer RL (2009) Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab 296: E888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, et al. (2009) TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol 297: L530–537. [DOI] [PubMed] [Google Scholar]
- 42. Cordey S, Junier T, Gerlach D, Gobbini F, Farinelli L, et al. (2010) Rhinovirus genome evolution during experimental human infection. PLoS One 5: e10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, et al. (2005) Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 201: 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mak JC, Hisada T, Salmon M, Barnes PJ, Chung KF (2002) Glucocorticoids reverse IL-1beta-induced impairment of beta-adrenoceptor-mediated relaxation and up-regulation of G-protein-coupled receptor kinases. Br J Pharmacol 135: 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, et al. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441: 101–105. [DOI] [PubMed] [Google Scholar]
- 46. Slater L, Bartlett NW, Haas JJ, Zhu J, Message SD, et al. (2010) Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog 6: e1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trypsin digestion of conditioned medium removes IL-6 and IL-8. Conditioned medium pooled from HBEC (n = 3) that were uninfected (Control) or treated with: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 2 for 24 hours was digested in the presence of 500 µg/mL of trypsin for 24 hours at 37°C and the reaction was stopped with 0.1% BSA. IL-6 and IL-8 were measured using ELISA with n = 1 experimental repeat.
(TIF)
Trypsin digestion of conditioned medium still causes ASMC β2 adrenoceptor desensitization. Conditioned medium pooled from HBEC (n = 3) that were uninfected (Control) or exposed to: UV inactivated RV (UVi-RV) or replication competent RV (RV) at an MOI = 2 for 24 hours was digested in the presence of 500 µg/mL of trypsin for 24 hours at 37°C and the reaction was stopped with 0.1% BSA. ASMCs (n = 6) were treated with trypsin digested conditioned medium for 3 days. Isoprenaline induced cAMP was measured using a cAMP functional assay. Data represent mean ± SEM. Statistical differences were detected using a 1-way ANOVA with Bonferroni post test comparisons to control conditioned medium *p<0.05.
(TIF)
Individual prostaglandin antagonists do not prevent β2 adrenoceptor desensitization. ASMCs (n = 6) were pretreated for 1 hr with vehicle (0.1% DMSO), CAY10441 (10−6 M) (A), AH6809 (10−5 M) (B), AL8810 (10−5 M) (C), BWA868C (10−5 M) (D) or L-161,982 (10−6 M) (E) and maintained for a further 3 days in the presence of conditioned medium from HBEC (n = 2) that were uninfected (Control) or infected with replication competent RV (RV) at an MOI = 2 for 24 hours. Isoprenaline induced cAMP was measured using a cAMP functional assay. Data represent mean ± SEM. Statistical differences were detected using 1-way ANOVA with Bonferroni post test comparisons to the control conditioned medium pretreatment in each group *p<0.05.
(TIF)