Abstract
Across bilateral cochlear implants, contralateral threshold shift has been investigated as a function of electrode difference between the masking and probe electrodes. For contralateral electric masking, maximum threshold elevations occurred when the position of the masker and probe electrode was approximately place-matched across ears. The amount of masking diminished with increasing masker-probe electrode separation. Place-dependent masking occurred in both sequentially implanted ears, and was not affected by the masker intensity or the time delay from the masker onset. When compared to previous contralateral masking results in normal hearing, the similarities between place-dependent central masking patterns suggest comparable mechanisms of overlapping excitation in the central auditory nervous system.
INTRODUCTION
Contralateral masking occurs when the threshold of a signal in one ear is elevated by the presence of a masker in the opposite ear. The contralateral masking effect was first reported in the seminal masking study by Wegel and Lane (1924), but was primarily attributed to peripheral masking due to cross-hearing or masker leakage around the head. The theory of central masking postulates that the contralateral masker and probe signals interact in the central auditory nervous system (Zwisklocki, 1972; 1978). This is unlike ipsilateral masking where monaural threshold elevations can be attributed to the physical overlap of masker and probe signals in the auditory periphery. Central masking experiments have shown that hearing thresholds are elevated when spectrally similar sounds are presented in the contralateral ear (Zwisklocki et al., 1968; Zwisklocki, 1972). The amount of central masking has been shown to depend on the masker intensity, duration, and the time delay from the masker onset (Zwisklocki et al., 1968; Dirks and Malmquist, 1965). Psychophysical tuning curves obtained with contralateral masking are smaller in effect but more sharply tuned than with ipsilateral masking (e.g., Mills et al., 1996).
The present study reevaluates the neural mechanisms of central masking by using electric stimulation from bilateral cochlear implants (CIs). Electrode arrays bypass normal hair-cell synapses by directly stimulating afferent auditory neurons at different place locations along the cochlea. The usage of contralateral electric signals completely averts the issue of cross-hearing from masker leakage around the head and transcranial bone conduction (Wegel and Lane, 1924; Bekesy, 1948). The electric masking paradigm can also be used to dissociate the two major efferent feedback pathways to the auditory periphery: (a) the middle ear muscle (MEM) reflex and (b) the medial olivocochlear (MOC) efferent reflex. The MEM reflex can be electrically evoked (Allum et al., 2002; Clement et al., 2002), and is activated when output from the cochlear nucleus excites the stapedius motoneurons, stiffens the ossicular chain, and thereby attenuates the transmission of vibrational energy to the cochlea (McRobert et al., 1969). Efferent feedback projections from crossed MOC neurons can also elevate thresholds in the cochlear periphery by suppressing the gain of the outer hair cell amplifiers (Warren and Liberman, 1989; Puria et al., 1996). The contralateral masking effects of these two efferent feedback pathways are bypassed with bilateral cochlear implants because the suppression of mechanical excitation will have no effect in electrically stimulated inner ears. Central masking patterns obtained with bilateral cochlear implants will therefore reflect interactions solely in the central auditory nervous system.
In an early masking study, two of the first bilateral recipients showed contralateral threshold shift, but not in a place-dependent manner such as in normal hearing (Van Hoesel and Clark, 1997). These findings may have been obscured by the strict CI candidacy requirements at the time since both subjects were only eligible for bilateral implantation because their original implants were shallowly inserted and possessed multiple electrical shorts. The present study reexamines central masking in a larger subject population with fully inserted bilateral arrays to test a wider range of place-pairs. Central masking patterns will be important to consider when fitting bilateral implants for optimal performance. Neural response patterns can vary between ears depending on the distribution of excitable neurons, electrode insertion depths, and current flow geometries (Chatterjee and Shannon, 1998; Kral et al., 1998, McKay et al., 1999). The presence of place-dependent contralateral masking could lead to opportunities to improve frequency-to-place electrode correspondences. Previous bilateral CI studies have shown that matching interaural electrode pairs restores sensitivities for interaural time differences (Long et al., 2003) and binaural masking level differences (Lu et al., 2010). Binaural advantages in sentence perception and vowel identification also depend on both ears receiving spectrally matched speech information (Siciliano et al., 2010).
The present study also compares central masking patterns between ears because many bilateral CI users have one implant that performs considerably better than the other (e.g., van Hoesel and Tyler, 2003; Litovsky et al., 2006). The first implanted ear is often the ear with better performance, at least for sequentially implanted children with long intervals between implantation (Peters et al., 2007). A comparison of the symmetry of masking patterns could reveal whether these outcomes affect bilateral processing and the potential for binaural benefit. Additionally, electric-on-acoustic masking was measured in a unilateral CI subject with single-sided deafness. Contrasting electric and acoustic central masking patterns could further delineate the neural mechanisms involved in the contralateral masking phenomenon.
METHODS
Subjects
Ten bilateral CI subjects participated in the study. All bilateral subjects were sequentially implanted with two Cochlear, Ltd. devices (Table TABLE I.). One unilateral CI subject (UL1) with single-sided deafness also participated to contrast the effects of electric masking on acoustic stimulation. This subject had nearly normal thresholds (≤25 dB HL re ANSI-1996 for octave frequencies between 250 and 8000 Hz, except 35 dB HL at 4000 Hz) in the non-implanted left ear and was implanted in the right ear for the treatment of severe unilateral tinnitus and sensorineural hearing loss (Cullington and Zeng, 2010; Zeng et al., 2011). Both Institutional Review Board approval and written informed consent were obtained at University of California, Irvine.
TABLE I.
Subject's age at testing, duration of CI use, inter-implant interval, bilateral CI usage, first CI type, second CI type, side of first CI, age at hearing loss.
| Subject | Age at testing (years) | Duration of CI use (years) | Inter-implant interval (years) | Bilateral CI usage (years) | First CI type | Second CI type | Side of first CI | Age at hearing loss (years) |
|---|---|---|---|---|---|---|---|---|
| BL1 | 77 | 3 | 1 | 2 | Freedom | Freedom | L | 26–32 |
| BL2 | 49 | 7 | 6 | 1 | N24 | N5 | L | 0 |
| BL3 | 65 | 21 | 12, 4 | 9 | N22, N24 | N24 | R | 26 |
| BL4 | 77 | 5 | 4 | 1 | Freedom | N5 | R | 63 |
| BL5 | 47 | 10 | 8 | 2 | N24 | N5 | L | 9 |
| BL6 | 59 | 9 | 2 | 7 | N24 | N24 | R | 1 |
| BL7 | 10 | 9 | 5 | 4 | N24 | Freedom | R | 0 |
| BL8 | 32 | 12 | 10 | 2 | N24 | N24 | L | 2 |
| BL9 | 16 | 13 | 8 | 5 | N24 | Freedom | R | 0 |
| BL10 | 78 | 6 | 2 | 4 | Freedom | Freedom | L | 71 |
| UL1 | 51 | 6 | — | — | HiRes90k | — | R | 45 |
Stimuli
The binaural electric stimuli presented to the bilateral CI users were trains of biphasic electric pulses delivered from single active electrodes in each cochlear implant. The Spear3 research processor was used to stimulate all electrodes at specified levels using the same pulse rate (900 pps), pulse duration (25 μs), and with monopolar (MP1 + 2) stimulation. Custom Sound 2.0 was used to measure impedances and to set comfort current levels prior to testing. Electric threshold levels were recorded in current level programming units (CL) derived from the clinical software of Cochlear, Ltd. The implanted stimulator delivers current in 256 steps ranging from approximately 10 μA at CL = 0 to approximately 1750 μA at CL = 255. The stimulator output [I(μA)] at any given CL can be derived using the formula:
| (1) |
The appropriate levels and test settings were confirmed with an electrodogram monitor (IF5/PCI and RFStatsNT by Hearworks, Ltd., Australia).
Unilateral subject UL1 was implanted with the Advanced Bionics HiRes 90K device, and electric stimuli were programmed using soundwave 1.6 clinical software and presented via a body-worn Clarion Platinum Series processor. For this subject only, electrodes were stimulated at 5156 pps pulse rates and with 10.8 μs pulse duration. The HiRes 90K array also has fewer electrodes (16 contacts) and wider spacing (0.85 mm) compared to the Cochlear, Ltd. devices. The acoustic stimuli presented to this subject's opposite ear were pure tones generated in matlab. The acoustic stimuli were delivered via Sennheiser HDA-200 headphones. Acoustic sound calibrations were conducted by coupling the Sennheiser HDA-200 headphones to a Bruel & Kjaer (B&K) 4153 artificial ear with a flat-plate coupler. The acoustic output was measured by a B&K 4192 [1/2] in. condenser microphone, and read on a B&K 2260 sound level meter.
Procedure
The detection threshold of the probe stimulation was measured using a three interval forced choice (3IFC) task without visual feedback. A graphical user interface illuminated the three 500 ms intervals in succession with 500 ms of silence in between each. One of the three intervals randomly contained a 200 ms probe signal delivered 150 ms after the onset of the interval. Subjects were instructed to select only the interval that contained the probe. The intensity level of the probe was adapted using a 2-down, 1-up decision rule corresponding to the 70.7% correct point on the subject's psychometric function (Levitt, 1971). Large step sizes of 3 CL were used for the first four reversals and small step sizes of 1 CL were used for the next four reversals. For acoustic probe stimuli, large and small step sizes of 5 and 2 dB were used instead. The detection threshold was determined as the average of the last four reversals. For each electrode, unmasked detection thresholds were calculated as the average threshold from three separate trials, rounding to the nearest CL unit.
In the masked condition, 500 ms masking signals were presented during each interval. The masker intensity was set slightly above the “most-comfortable-level” (MCL) of an electric stimulation as indicated by each subject as a 7 on a 10-interval loudness scale card provided by Advanced Bionics. The MCL was determined prior to testing by repeatedly presenting three 500 ms tones and adjusting the levels by 1–5 CL steps until the subject indicated the “most-comfortable-level.” Masked detection thresholds for each subject were calculated as the average threshold from two separate trials. The amount of masking was defined as the difference between the masked threshold and the unmasked threshold. Threshold elevation was calculated in percentage as the ratio between the amount of masking and the full dynamic range to allow comparison across widely varying electrodes and subjects (Lim et al., 1989). The percentage of dynamic range (%DR) is calculated according to the equation
| (2) |
where θm is the masked threshold level, and θu is the unmasked threshold level.
Central masking with bilateral cochlear implants
The first experiment was designed to determine what effect electric stimulation delivered from a cochlear implant had on the detection of electric stimuli delivered to the opposite ear. Electric detection thresholds were measured in quiet at five electrodes spanning the entire length of the array (electrodes E22, E16, E11, E6, and E1), and then measured in the presence of electric maskers in the opposite ear at five numerically identical electrodes. Although electrode insertion depths could not be confirmed with imaging measurements, identical electrode numbers are estimated to be approximately place-matched across ears because surgical reports indicated normal (i.e., full) insertion depths for all participating subjects. Electrodes are reported in base-to-apex order, such that electrode E22 is the most apical and electrode E1 is the most basal.
The second part of this experiment focused on determining the effects of altering masker intensity or the time delay between masker and probe onsets. In the reduced masker intensity condition, the original procedure was repeated after reducing the masker intensity from 100% DR (MCL) to 50% DR. In the concurrent onset/offset condition, the original procedure was repeated using a 500 ms probe that coincided with the onset and offset of the 500 ms maskers. In these two alternate conditions of the experiment, electric detection thresholds were only measured at three electrodes spanning the lengths of each array (electrodes E22, E11, and E1).
Central masking with acoustic simulation
The second experiment utilized unilateral CI subject UL1 to determine what effect electric stimulations delivered from a cochlear implant had on the detection of acoustic pure tones delivered to a normal hearing ear. Acoustic thresholds were measured in quiet at 250, 1000, 4000, and 8000 Hz, and then measured in the presence of electric maskers in the opposite implanted ear at electrodes E1, E9, and E16. These three electrodes span the entire length of the HiRes90k array and are reported in apex-to-base order, such that electrode E1 is the most apical and electrode E16 is the most basal.
RESULTS
Central masking with bilateral cochlear implants
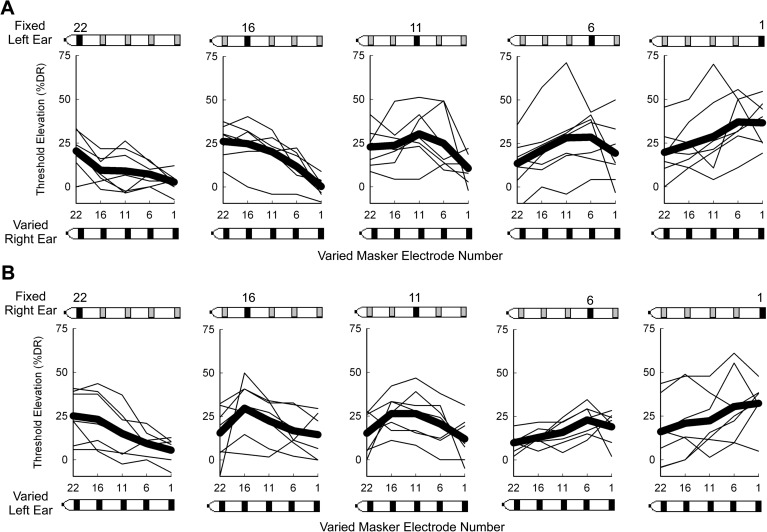
Figure 1 shows the complete set of central masking data where electric masking patterns are represented as the threshold elevation of probe electrodes (%DR) as a function of contralateral masking electrodes. Each of the seven bilateral subjects (BL1–BL7) was tested twice, alternating the masking stimuli from the left to the right ears (n = 7). Electric masking patterns are sorted into panels according to the location of the probe electrode in the fixed left ear [Fig. 1A], or in the fixed right ear [Fig. 1B]. Thin lines show individual data and thick lines show the mean data for each fixed electrode probe location. Contralateral masking electrodes elevated detection thresholds in both the left and the right ears. In spite of individual variability, the threshold elevation peaks generally occurred for interaural pairings with the same number, which corresponds to electrodes with assumed similar insertion depth. Bilateral subject BL4 was the only individual who did not show threshold elevation peaks at any interaural pairings with the same electrode number. This subject was newly implanted at the time of testing and had the least bilateral experience.
Figure 1.
Complete set of central masking data measured for the seven bilateral CI subjects (n = 7), sorted into panels according to the location of the fixed probe electrode (black contact) in the left ear (A), or the right ear (B). Electric masking patterns are represented as the threshold elevation of the probe electrode (%DR) as a function of contralateral masking electrodes. Thin lines show individual data and the thick lines show the mean data for each fixed probe electrode location.
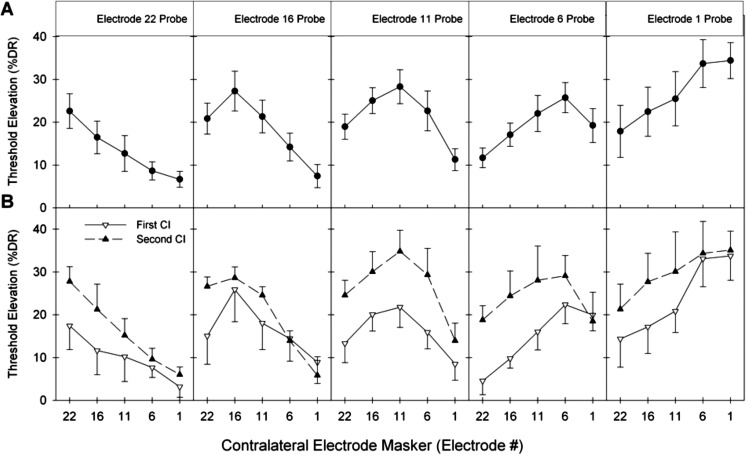
Figure 2A graphs the average electric masking patterns of both ears combined (n = 14). Error bars indicate one standard error of the mean. Maximum threshold elevations occurred in all masking conditions where the masker and probe electrode numbers were identical across ears. For example, masker electrode E22 elevated the threshold of probe electrode E22 on the opposite ear by 23% DR, a greater amount compared to masker electrodes E16, E11, E6, or E1. A two-way repeated measure analysis of variance (ANOVA) was conducted to compare the main effects of both masker and probe electrode on threshold elevation. There was a significant main effect of masker electrode [F(4, 24) = 3.73, p = 0.017], and a significant masker-probe electrode interaction [F(16, 96) = 8.24, p < 0.001]. These results demonstrate that central masking with bilateral cochlear implants was place-dependent.
Figure 2.
(A) Average electric masking patterns of both ears combined (n = 14). Error bars indicate one standard error of the mean. (B) Electric masking patterns with split averages for when the first or second sequentially implanted CI represented the probe.
Figure 2B shows central masking data separated based on whether the probes were presented to the first or second sequentially implanted ear. Even when the data was separated in this manner, maximum threshold elevations occurred in all masking conditions where the masker and probe electrode numbers were identical across ears. A pair of two-way repeated measure ANOVA showed a significant masker-probe electrode interaction when the first implanted CI was used as the probe electrode [F(16, 96) = 4.667, p < 0.001], and also when the second sequentially implanted CI was used as the probe electrode [F(16, 96) = 5.556, p < 0.001]. These results demonstrate that central masking was place-dependent in both sequentially implanted ears. A two-way repeated measure ANOVA was conducted to compare the main effect of the first versus second sequentially implanted ear and threshold elevation. There was a significant main effect of the ear used for the probe electrode [F(1, 6) = 7.657, p = 0.033], and a significant interaction between ear and masker electrode [F(4, 24) = 2.954, p = 0.041]. Average threshold elevations were found to be greater when the probe electrode was from the second sequentially implanted ear. These results demonstrate that while central masking was place-dependent in both sequentially implanted ears, the absolute amount of masking differed on each side.
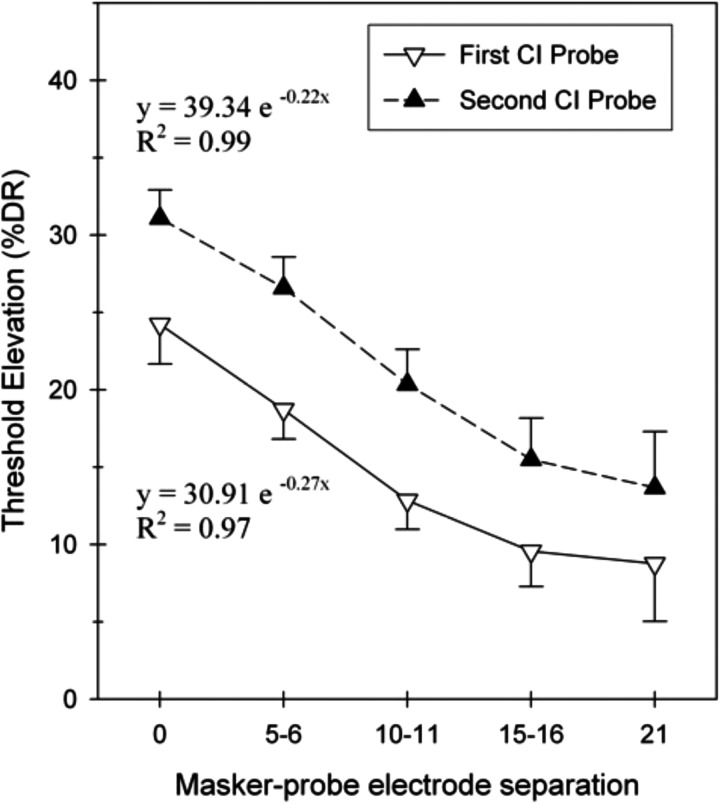
Figure 3 reorganizes the data to analyze the growth of masking as a function of the masker-probe electrode separation across ears. The masker-probe electrode separation was calculated by subtracting the difference between the masker and probe electrode numbers. Place-matched masking conditions were categorized as “0” since both the masker and probe electrode numbers were identical across ears. When categorized in this manner, the amount of central masking diminished with increasing masker-probe electrode separation. The masking growth pattern for each sequentially implanted ear was fitted with an exponential equation and both ears displayed similar spatial constants and significant R2 values (R2 > 0.97). These results demonstrate that central masking diminished with increasing masker-probe electrode separation at similar rates on both sides.
Figure 3.
Threshold elevation as a function of the masker-probe electrode separation across ears (n = 14). Exponential equations were calculated to characterize masking growth.
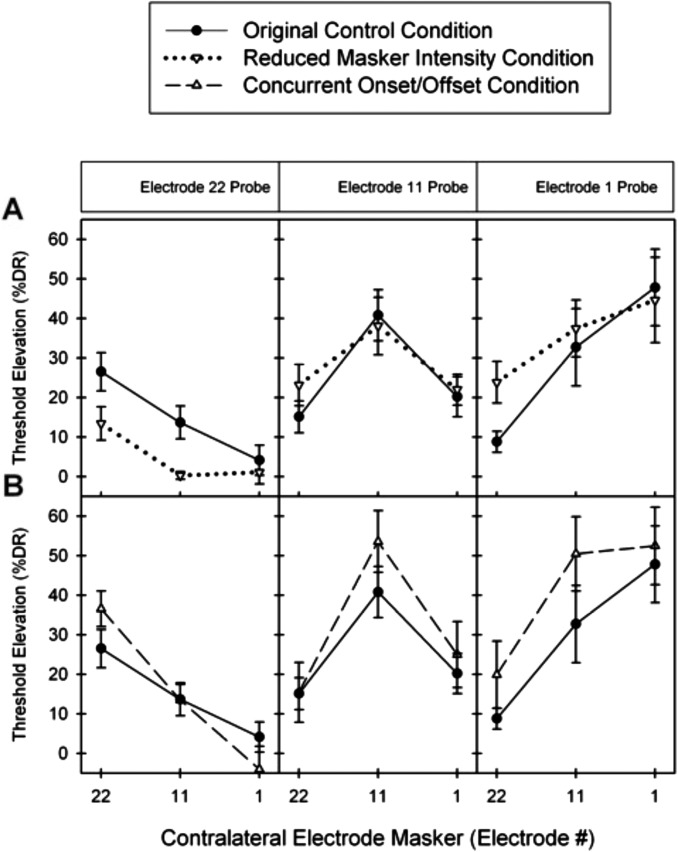
Following these significant findings in seven bilateral CI subjects, three additional bilateral CI subjects (BL8, BL9, and BL10) participated in alternate conditions of this experiment to examine the effects of altering masker intensity or the time delay from the masker onset. Figure 4 compares the average electric masking patterns of these two alternate conditions with the original conditions. In the reduced masker intensity condition [Fig. 4A], there was no significant main effect of masker intensity [F(1, 2) = 0.203, p = 0.697]. In the concurrent onset/offset condition [Fig. 4B], there was no significant main effect of masker-probe onset [F(1, 2) = 1.371, p = 0.362]. These results demonstrate that neither the masker intensity nor the time delay from the masker onset significantly affected the average amount of central masking.
Figure 4.
(A) Compares the average electric masking patterns of the original condition with the reduced masker intensity condition (n = 6). (B) Compares the average electric masking patterns of the original condition with the concurrent onset/offset condition.
Central masking with acoustic simulation
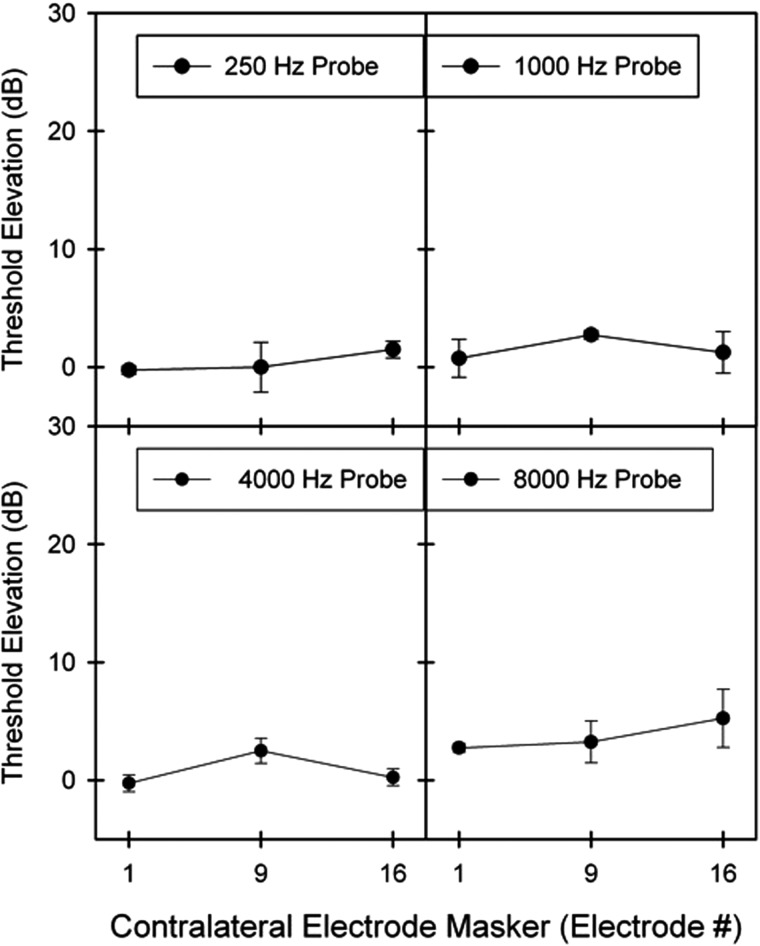
Figure 5 shows electric masking patterns for unilateral CI subject UL1. Unlike electric on electric signals, contralateral electric stimulation could not mask acoustic pure tones in the opposite ear. Threshold elevations ranged from −0.25 to 5.25 dB with a mean [±SD] of 1.65 dB [±1.12]. Unlike acoustic on acoustic signals, acoustic pure tones could not be masked in a place-dependent manner. The range of acoustic pure tones (250–8000 Hz) could not be masked by contralateral electrodes despite similarities in Greenwood frequency-position correspondences (500–3200 Hz) (Boex et al., 2006), and despite being programmed with an overlapping frequency allocation (333–6665 Hz).
Figure 5.
Average electric masking patterns represented as the threshold elevation of pure tones (dB) as a function of contralateral masking electrodes for subject UL1 (n = 1).
DISCUSSION
The place-dependent contralateral masking patterns from bilateral CI subjects are consistent with what was observed in acoustic hearing studies (e.g., Ingham, 1959; Sherrick and Mangabeira-Albernaz, 1961; Dirks and Malmquist, 1965). Previous work showed that for acoustic on acoustic signals, thresholds peaked when the masker and probe were the same frequency across ears and diminished with increasing frequency separation. Here, for electric on electric signals, thresholds peaked when the position of the masker and probe electrodes were approximately place-matched across ears and diminished with increasing electrode separation. The average amount of masking was similar in scale to previous studies (van Hoesel and Clark, 1997) and was not significantly affected by masker intensity or onset delay. Unlike in normal hearing, the bilateral CI subjects also exhibited much broader masking that persisted across distant masker-probe electrode separation. The broad spread of activation in electric stimulation likely explains how these results contrasted with the sharply tuned masking patterns exhibited by normal hearing listeners (e.g., Mills et al., 1996). Additional difficulties in signal detection may have resulted from uncertainty in the stimulus. Qualitative similarities between the signal and masker can lead to informational masking in the absence of energetic overlap (Pollack, 1975; Watson et al., 1976).
A comparison of central masking patterns between ears showed asymmetries in the amount of masking. A greater amount of contralateral masking was found in the second sequentially implanted ear compared to the first, an outcome that likely resulted from a more ingrained reliance and attention to the familiar ear. A reluctance to work and make adjustments to the newly implanted ear has also been cited as an explanation for performance asymmetry (Peters et al., 2007). Cochlear implant experience and auditory plasticity could produce changes in masking outcomes because many aspects of binaural processing continue to develop after implantation (Laszig et al., 2004; Buss et al., 2008).
The neural mechanisms of central masking can be reevaluated using electric stimulation from bilateral implants. The theory of central masking posits a mechanism of overlapping excitation between contralateral signals at some point in the central auditory system (Zwisklocki, 1972; 1978). Contralateral masking with bilateral cochlear implants is contingent upon overlapping excitation between signals in the central auditory system. Binaural interaction has been demonstrated in brainstem-evoked responses of a bilaterally implanted patient (Pelizzone et al., 1990). Subsequent animal studies have shown that the binaural interaction component of the electrically evoked auditory brainstem response peaks for interaural electrode pairs at the same relative cochlear position and drops with increasing cochlear separation in either direction (Smith and Delgutte, 2007). Neural response patterns in the inferior colliculus showed that these peaks occurred when electrodes from both sides maximally activated overlapping neural populations. Contralateral noise has also been shown to reduce the steady-state response of the auditory cortex, indicating central interaction sites beyond the brainstem level (Galambos and Makeig, 1992).
The role of the efferent system in contralateral masking can be reinvestigated after considering the electric masking results. The MOC pathways originate in the brainstem surrounding the ipsilateral and contralateral superior olivary complex and project to the outer hair cells (Warr, 1975). Unmyelinated lateral olivocochlear neurons innervate afferent dendrites of the cochlear nerve near their synapses with inner hair cells (Groff and Liberman, 2003). Contralateral tones suppress responses of auditory-nerve fiber stimuli near characteristic frequency, but only if the olivocochler bundle is intact (Fex, 1967; Warren and Liberman, 1989). A psychoacoustic study with macaque monkeys indicated that the suppressive effects of central masking could be reduced or eliminated when the MOC bundle was sectioned at the floor of the IVth ventricle (Smith et al., 2000). On the other hand, the bilateral CI subjects in the present report demonstrated central masking despite lacking efferent outer hair cell modulation in their electrically stimulated inner ears. These findings are in agreement with a case study on a vestibular neurotomy patient (severed olivocochlear bundle) who showed that a 1000 Hz contralateral masking tone elevated the threshold of the 1000 Hz probe by the same amount in the operated and unoperated ears (Scharf et al., 1994). Functioning efferent feedback pathways could be a requirement for mediating the sharp tuning of contralateral masking since efferent fibers of a given characteristic frequency innervate a restricted cochlear region of similar characteristic frequency (Liberman and Brown, 1986). A reduction in selective auditory attention to expected frequencies has been shown in ears following vestibular neurotomy (Scharf et al., 1997), and the suppression of evoked otoacoustic emissions by a contralateral noise is also reduced in operated ears without efferent input (Giraud et al., 1995).
The electric-on-acoustic masking patterns from the unilateral subject with single-sided deafness contrasts with the bilateral masking data. Despite testing a range of different locations along the cochlea, contralateral electric stimulation could hardly mask acoustic stimulation (250–8000 Hz) in the opposite ear. These results are consistent with a previous contralateral masking study where bimodal CI subjects reported that electrical pulse trains and acoustic sine waves did not fuse or merge well into a single percept (James et al., 2001). The spatial and temporal characteristics of an electrical stimulus can be quite unlike that of any acoustic stimulus. Contralateral cues can improve signal detection, presumably by reducing stimulus uncertainty (Sorkin, 1965; Taylor and Forbes, 1969; Taylor and Clarke, 1971). Therefore, these outcomes could instead be attributed to decision-making based on alternate cues such as interaural level differences, image width, lateral position, and binaural loudness (e.g., Ruotolo et al., 1979; Shub et al., 2008).
On the other hand, ipsilateral electric masking studies have shown that stimulation from deeply inserted electrodes can elevate the threshold of residual acoustic hearing in the same implanted ear (Lin et al., 2011). In the case of ipsilateral masking, the electric fields of neighboring electrodes could stimulate overlapping subsets of nerve fibers at the peripheral level prior to central processing (Shannon, 1983; Tang et al., 2011). Limiting the spatial overlap between combined electric and acoustic stimulation has been shown to reduce the suppressive effects on both electrically and acoustically evoked auditory-nerve responses in guinea pigs (Stronks et al., 2010; Stronks et al., 2012). The temporal response characteristics of combined electric and acoustic stimulation have also been measured in normal hearing cats with intracochlear multichannel electrodes inserted into the scalae tympani. Interspike interval histograms revealed decreased phase locking (decreased synchronization indices) in both the acoustic and electric response properties of primary auditory nerve afferents and the contralateral central nucleus of the inferior colliculus (Tillein et al., 2004; Vollmer et al., 2010). These physiological studies, together with the present results, suggest a role of both place location and temporal firing patterns in central masking.
SUMMARY
The present contralateral masking results from CI subjects show the following.
-
(1)
Bilateral electric stimulation produces a place-dependent central masking pattern similar to normal acoustic hearing.
-
(2)
Central masking persists at various masker intensities, probe durations, and onset delays.
-
(3)
Place-dependent central masking occurs in both sequentially implanted ears, but the amount of masking may differ on each side.
-
(4)
Contralateral electric stimulation did not effectively mask acoustic pure tones of 250–8000 Hz in the unilateral CI subject with contralateral acoustic hearing.
ACKNOWLEDGMENTS
We thank our cochlear implant subjects for their dedication and time. The authors acknowledge data collection help from Yohannes Assefa, Geoffrey Gao, Theodore Lim, and Taaj Raasikh. This project was supported in part by the National Institutes of Health, United States Department of Health and Human Services (Grant Nos. RO1 DC008858 and P30 DC008369).
References
- Allum, J., Greisiger, R., and Probst, R. (2002). “Relationships of intraoperative electrically evoked stapedius reflex thresholds to maximum comfortable loudness levels of children with cochlear implants,” Int. J. Audiol. 41(2), 93–99. 10.3109/14992020209090399 [DOI] [PubMed] [Google Scholar]
- Bekesy, G. (1948). “Vibration of the head in a sound field and its role in hearing by bone conduction,” J. Acoust. Soc. Am. 20, 749–760. 10.1121/1.1906433 [DOI] [Google Scholar]
- Boex, C., Baud, L., Cosendai, G., Sigrist, A., Kos, M. I., and Pelizzone, M. (2006). “Acoustic to electric pitch comparisons in cochlear implant subjects with residual hearing,” J. Assoc. Res. Otolaryngol. 7(2), 110–124. 10.1007/s10162-005-0027-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, E., Pillsbury, H., Buchman, C., Pillsbury, C., Clark, M., Haynes, D., Labadie, R., Amberg, S., Roland, P., Kruger, P., Novak, M., Wirth, J., Black, J., Peters, R., Lake, J., Wackym, P., Firszt, B., Wilson, B., Lawson, D., Schatzer, R., D'Haese, P., and Barco, A. (2008). “Multicenter U.S. bilateral MED-EL cochlear implantation study: Speech perception over the first year of use,” Ear Hear. 29, 20–32. [DOI] [PubMed] [Google Scholar]
- Chatterjee, M., and Shannon, R. V. (1998). “Forward masked excitation patterns in multielectrode electrical stimulation,” J. Acoust. Soc. Am. 105, 2565–2572. 10.1121/1.422777 [DOI] [PubMed] [Google Scholar]
- Clemet, R., Carter, P., and Kipke, D. (2002). “Measuring the electrical stapedius reflex with stapedius muscle electromyogram recordings,” Ann. Biomed. Eng. 30(2), 169–179. 10.1114/1.1454132 [DOI] [PubMed] [Google Scholar]
- Cullington, H., and Zeng, F.-G. (2010). “Bimodal hearing benefit for speech recognition with competing voice in cochlear implant subject with normal hearing in contralateral ear,” Ear Hear. 31(1), 70–73. 10.1097/AUD.0b013e3181bc7722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks, D. D., and Malmquist, C. (1965). “Shifts in air conduction thresholds produced by pulsed and continuous contralateral masking,” J. Acoust. Soc. Am. 37, 631–637. 10.1121/1.1909383 [DOI] [PubMed] [Google Scholar]
- Fex, J. (1967). “Efferent inhibition in the cochlea related to hair cell DC activity: Study of postsynaptic activity of the crossed olivocochlear fibers in the cat,” J. Acoust. Soc. Am. 41, 666–674. 10.1121/1.1910395 [DOI] [PubMed] [Google Scholar]
- Galambos, R., and Makeig, S. (1992). “Physiological studies of central masking in man. I: The effects of noise on the 40-Hz steady state response,” J. Acoust. Soc. Am. 92, 2683–2690. 10.1121/1.404383 [DOI] [PubMed] [Google Scholar]
- Giraud, A., Collet, L., Chery-Croze, S., Magnan, J., and Chays, A. (1995). “Evidence of a medial olivocochlear involvement in the contralateral suppression of otoacoustic emissions, in humans,” Brain Res. 705, 15–23. 10.1016/0006-8993(95)01091-2 [DOI] [PubMed] [Google Scholar]
- Groff, J., and Liberman, C. (2003). “Modulation of cochlear afferent response by the lateral olivocochlear system: Activation via electrical stimulation of the inferior colliculus,” J. Neurophysiol 90, 3178–3200. 10.1152/jn.00537.2003 [DOI] [PubMed] [Google Scholar]
- Ingham, J. C. (1959). “Variations in Cross-masking with Frequency,” J. Exptl. Psychol. 58, 199–205. 10.1037/h0041226 [DOI] [PubMed] [Google Scholar]
- James, C., Blamey, P., Shallop, J. K., Incerti, P. V., and Nicholas, A. M. (2001). “Contralateral masking in cochlear implant users with residual hearing in the non-implanted ear,” Audiol. Neuro-otol. 6, 87–97. 10.1159/000046814 [DOI] [PubMed] [Google Scholar]
- Kral, A., Hartmann, R., Mortazavi, D., and Klinke, R. (1998). “Spatial resolution of cochlear implants: The electric field and excitation of auditory efferents,” Hear. Res. 121, 11–28. 10.1016/S0378-5955(98)00061-6 [DOI] [PubMed] [Google Scholar]
- Laszig, R., Aschendorff, A., Stecker, M., Muller-Deile, J., Maune, S., Dillier, N., Weber, B., Hey, M., Begall, K., Lenarz, T., Battmer, R., Bohm, M., Steffens, T., Strutz, J., Linder, T., Probst, R., Allum, J., Westhofen, M., and Doering, W. (2004). “Benefits of bilateral electrical stimulation with the nucleus cochlear implant in adults: 6-month postoperative results,” Otol. Neurotol. 25, 958–968. 10.1097/00129492-200411000-00016 [DOI] [PubMed] [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Liberman, M., and Brown, M. (1986). “Physiology and anatomy of single olivocochlear neurons in the cat,” Hear. Res. 24, 17–36. 10.1016/0378-5955(86)90003-1 [DOI] [PubMed] [Google Scholar]
- Lim, H. H., Tong, Y. C., and Clark, G. M. (1989). “Forward masking patterns produced by intracochlear electrical stimulation of one and two electrode pairs in the human cochlea,” J. Acoust. Soc. Am. 86, 971–980. 10.1121/1.398732 [DOI] [PubMed] [Google Scholar]
- Lin, P., Turner, C. W., Gantz, G. J., Djalilian, H. R., and Zeng, F.-G. (2011). “Ipsilateral masking between acoustic and electric stimulations,” J. Acoust. Soc. Am. 130(2), 858–865. 10.1121/1.3605294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R., Parkinson, A., Arcaroli, J., and Sammeth, C. (2006). “Simultaneous bilateral cochlear implantation in adults: a multicenter clinical study,” Ear Hear. 27, 714–731. 10.1097/01.aud.0000246816.50820.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C. J., Eddington, D. K., Colburn, H. S., and Rabinowitz, W. M. (2003). “Binaural sensitivity as a function of interaural electrode positions with a bilateral cochlear implant user,” J. Acoust. Soc. Am. 114, 1565–1574. 10.1121/1.1603765 [DOI] [PubMed] [Google Scholar]
- Lu, T., Litovsky, R., and Zeng, F.-G. (2010). “Binaural masking level differences in actual and simulated bilateral cochlear implant listeners,” J. Acoust. Soc. Am. 127(3), 1479–1490. 10.1121/1.3290994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay, C., O'Brien, A., and James, C. (1999). “Effect of current level on electrode discrimination in electrical stimulation.” Hear. Res. 136, 159–164. 10.1016/S0378-5955(99)00121-5 [DOI] [PubMed] [Google Scholar]
- McRobert, H., Bryan, M. E., and Tempest, W. (1969). “The role of middle ear muscle contractions in contralateral remote masking,” J. Sound Vib. 10, 305–316. 10.1016/0022-460X(69)90202-8 [DOI] [Google Scholar]
- Mills, J. H., Dubno, J. R., and He, N. J. (1996). “Masking by ipsilateral and contralateral maskers,” J. Acoust. Soc. Am. 100, 3336–3344. 10.1121/1.416974 [DOI] [PubMed] [Google Scholar]
- Pelizzone, M., Kasper, A., and Montandon, P. (1990). “Binaural interaction in a cochlear implant patient,” Hear. Res. 48, 287–290. 10.1016/0378-5955(90)90069-2 [DOI] [PubMed] [Google Scholar]
- Peters, R., Litovsky, R., Parkinson, A., and Lake, J. (2007). “Importance of age and post implantation experience on performance in children with sequential bilateral cochlear implants,” Otol. Neurotol. 28, 649–657. 10.1097/01.mao.0000281807.89938.60 [DOI] [PubMed] [Google Scholar]
- Pollack, I. (1975). “Auditory informational masking,” J. Acoust. Soc. Am 57, S5. 10.1121/1.1995329 [DOI] [Google Scholar]
- Puria, S., Guinan, J., and Liberman, M. (1996). “Olivocochlear reflex assays: Effects of contralateral sound on compound action potentials versus ear-canal distortion products,” J. Acoust. Soc. Am. 99(1), 500–507. 10.1121/1.414508 [DOI] [PubMed] [Google Scholar]
- Ruotolo, B., Stern, R., and Colburn, H. (1979). “Discrimination of symmetric time-intensity traded binaural stimuli,” J. Acoust. Soc. Am. 66, 1733–1737. 10.1121/1.383646 [DOI] [PubMed] [Google Scholar]
- Scharf, B., Magnan, J., Collet, L., Ulmer, E., and Chays, A. (1994). “On the role of the olivocochlear bundle in hearing: A case study,” Hear. Res. 75, 11–26. 10.1016/0378-5955(94)90051-5 [DOI] [PubMed] [Google Scholar]
- Scharf, B., Magnan, J., and Chays, A. (1997). “On the role of the olivocochlear bundle in hearing: 16 case studies,” Hear. Res. 103, 101–122. 10.1016/S0378-5955(96)00168-2 [DOI] [PubMed] [Google Scholar]
- Shannon, R. (1983). “Multichannel electrical stimulation of the auditory nerve in man. II. Channel interaction,” Hear. Res. 12, 1–16. 10.1016/0378-5955(83)90115-6 [DOI] [PubMed] [Google Scholar]
- Sherrick, C. E., and Mangabeira-Albernaz, P. L. (1961). “Auditory threshold shifts produced by simultaneously pulsed contralateral stimuli,” J. Acoust. Soc. Am. 33, 1381–1385. 10.1121/1.1908445 [DOI] [Google Scholar]
- Shub, D., Durlach, N., and Colburn, H. (2008). “Monaural level discrimination under dichotic conditions,” J. Acoust. Soc. Am. 123, 4421–4433. 10.1121/1.2912828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano, C., Faulkner, A., Rosen, S., and Mair, K. (2010). “Resistance to learning binaurally mismatched frequency-to-place maps: Implications for bilateral stimulation with cochlear implants,” J. Acoust. Soc. Am. 127(3), 1645–1660. 10.1121/1.3293002 [DOI] [PubMed] [Google Scholar]
- Smith, D., Turner, D., and Henson, M. (2000). “Psychological correlates of contralateral efferent suppression. I. The role of the medial olivocochlear system in “central masking” in nonhuman primates,” J. Acoust. Soc. Am. 107, 933–941. 10.1121/1.428274 [DOI] [PubMed] [Google Scholar]
- Smith, Z. M., and Delgutte, B. (2007). “Using evoked potentials to match interaural electrode pairs with bilateral cochlear implants,” J. Assoc. Res. Otolaryngol. 8, 134–151. 10.1007/s10162-006-0069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin, R. (1965). “Uncertain Signal Detection with Simultaneous Contralateral Cues,” J. Acoust. Soc. Am. 38, 207–212. 10.1121/1.1909635 [DOI] [PubMed] [Google Scholar]
- Stronks, H. C., Prijs, V. F., Chimona, T. S., Grolman, W., and Klis, S. F. (2012). “Spatial Overlap of Combined Electroacoustic Stimulation Determines the Electrically Evoked Response in the Guinea Pig Cochlea,” Otol. Neurotol 33(9), 1535–1542. 10.1097/MAO.0b013e318271c0b6 [DOI] [PubMed] [Google Scholar]
- Stronks, H. C., Versnel, H., Prijs, V. F., and Klis, S. F. (2010). “Suppression of the acoustically evoked auditory-nerve response by electrical stimulation in the cochlea of the guinea pig,” Hear. Res. 259(1), 64–74. 10.1016/j.heares.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Tang, Q., Benitez, Raul, and Zeng, F.-G. (2011). “Spatial channel interactions in cochlear implants,” J. Neural Eng. 8, 1–15. 10.1088/1741-2560/8/4/046029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, M., and Clarke, D. (1971). “Monaural Detection with a Contralateral Cue (MDCC). II. Interaural Delay of Cue and Signal,” J. Acoust. Soc. Am. 49, 1243–1253. 10.1121/1.1912487 [DOI] [PubMed] [Google Scholar]
- Taylor, M., and Forbes, S. (1969). “Monaural Detection with a Contralateral Cue (MDCC). I. Better than Energy Detector Performance by Human Observers,” J. Acoust. Soc. Am. 46, 1519–1526. 10.1121/1.1911896 [DOI] [PubMed] [Google Scholar]
- Tillein, J., Vollmer, M., Kral, A., Klinke, R., and Hartmann, R. (2004). “Interactions between electrical and acoustical auditory nerve fiber responses of intracochlear implanted cats with residual hearing,” abstract 565, Association of Research in Otolaryngology.
- van Hoesel, R., and Clark, G. (1997). “Psychophysical studies with two binaural cochlear implant subjects,” J. Acoust. Soc. Am. 102, 495–507. 10.1121/1.419611 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R., and Tyler, R. (2003). “Speech perception, localization, and lateralization with bilateral cochlear implants,” J. Acoust. Soc. Am. 113(3), 1617–1630. 10.1121/1.1539520 [DOI] [PubMed] [Google Scholar]
- Vollmer, M., Hartmann, R., and Tillein, J. (2010). “Neuronal responses in cat inferior colliculus to combined acoustic and electric stimulation,” Adv. Otorhinolaryngol. 67, 61–69. [DOI] [PubMed] [Google Scholar]
- Warr, W. (1975). “Olivocochlear and vestibular efferent neurons of the feline brainstem: their location, morphology and number determined by retrograde axonal transport and acetylcholinesterase histochemistry,” J. Com. Neurol. 161, 159–182. 10.1002/cne.901610203 [DOI] [PubMed] [Google Scholar]
- Warren, E. H., and Liberman, M. C. (1989). “Effects of contralateral sound on auditory-nerve responses. I. Contributions of cochlear efferents,” Hear. Res. 37, 89–104. 10.1016/0378-5955(89)90032-4 [DOI] [PubMed] [Google Scholar]
- Watson, C., Kelly, W., and Wroton, H. (1976). “Factors in the discrimination of tonal patterns: II. Selective attention and learning under various levels of stimulus uncertainty,” J. Acoust. Soc. Am. 60, 1176–1185. 10.1121/1.381220 [DOI] [PubMed] [Google Scholar]
- Wegel, R. L., and Lane, C. E. (1924). “The auditory masking of one pure tone by another and its probable relation to the dynamics of the inner ear,” Phys. Rev. 23, 266–285. 10.1103/PhysRev.23.266 [DOI] [Google Scholar]
- Zeng, F.-G., Tang, Q., Dimitrijevic, A., Starr, A., Larky, J., and Blevins, N. (2011). “Tinnitus suppression by low-rate electric stimulation and its electrophysiological mechanisms,” Hear. Res. 277(1–2), 61–66. 10.1016/j.heares.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwislocki, J. J. (1972). “A Theory of Central Auditory Masking and its Partial Validation,” J. Acoust. Soc. Am. 52(2B), 644–659. 10.1121/1.1913154 [DOI] [Google Scholar]
- Zwislocki, J. J. (1978). “Masking: Experimental and theoretical aspects of simultaneous, forward, backward, and central masking,” in Handbook of Perception, edited by Carterette E. C. and Friedman M. P. (Academic, New York: ), Vol. IV, pp. 283–333. [Google Scholar]
- Zwislocki, J. J., Buining, E., and Glantz, J. (1968). “Frequency distribution of central masking,” J. Acoust. Soc. Am. 43, 1267–1271. 10.1121/1.1910978 [DOI] [PubMed] [Google Scholar]