Abstract
Past work applying otoacoustic emissions to gauge maturational status of the medial olivocochlear (MOC) reflex in human newborns has produced mixed results. The present study revisits the question while considering the dual nature of the 2f1 – f2 distortion product otoacoustic emission (DPOAE) and expanding measures of medial efferent function. Subjects included premature and term-born neonates, 6-month-old infants and young adults. The MOC reflex was elicited with contralateral acoustic stimulation (CAS) while shifts in amplitude and phase of the DPOAE, and its distortion and reflection components, were monitored. Overall, CAS-elicited reductions in DPOAE level did not differ among age groups. For all ages, the MOC reflex was strongest at frequencies below 1.5 kHz, and the reflection component of the DPOAE was most affected, showing maximally reduced amplitude and shallower phase slope when contralateral noise was presented. Results suggest that the MOC reflex likely reaches maturation prior to full-term birth. However, prematurely born neonates show markedly more episodes of CAS-induced DPOAE level enhancement. This may be due to more intrusive component mixing in this age group or disruptions in the formation of the MOC pathway or synapse in the most premature neonates.
INTRODUCTION
Considering the putative role of the medial olivocochlear (MOC) effect in perception, defining its functional status in the developing human auditory system is of strong importance. Activation of the medial efferent system modulates cochlear mechanics by hyperpolarizing outer hair cells (OHCs) and reducing cochlear amplifier gain (Guinan, 1996). Collectively this effect and its influence on auditory nerve fiber discharge patterns and rate is thought to facilitate perception of signals in background noise and may also provide protection from damaging noise (Kawase et al., 1993; Maison et al., 2002).
The first appearance of medial olivocochlear neurons in the human brainstem and beneath the OHCs is noted at approximately 20–22 fetal weeks (Lavigne-Rebillard and Pujol, 1988; Moore et al., 1999). Development of OHC post-synaptic cisternae and the proper formation of axo-somatic MOC-OHC synapses, occur sometime in the third fetal trimester, although post-mortem fetal tissue around the perinatal period is sparse (Lavigne-Rebillard and Pujol, 1988; Pujol et al., 1998). The functional status of the human medial efferent system during this last trimester and around the time of birth remains unclear. Otoacoustic emissions (OAEs), which depend upon activity of the OHCs effectively gauge efferent activation, most typically showing reductions in level. In this report, we follow the convention of Guinan (2010) and refer to MOC-elicited reductions in OAE amplitude as inhibition rather than suppression, to differentiate synaptic effects from cochlear-based suppression.
Revisiting the question
Over a decade ago, we reported that reductions in DPOAE level elicited by contralateral broadband noise averaged ∼1.5 dB and that MOC reflexes were adultlike by term birth (Abdala et al., 1999). Although the magnitude of the inhibition appeared to be adultlike in premature neonates, as a group these newborns showed an atypical pattern of DPOAE level enhancement elicited by contralateral noise. Enhancement was noted in 43% of premature newborn measurements but only 15% of adult observations.
Recent advances in our understanding of OAE generation processes provide at least a partial explanation for the enhancement. The 2f1 – f2 DPOAE includes contributions from two spatially separated sources on the basilar membrane and emerge from two distinct processes (Kim, 1980; Knight and Kemp, 2001; Shera and Guinan, 1999). Nonlinear distortion is generated around the overlap of the traveling waves evoked by primary tones (f1, f2), and at moderate-high levels, dominates the DPOAE recorded in the ear canal. However, backscattering of energy from the 2f1 – f2 place due to randomly distributed impedance perturbations along the cochlear partition also contributes to the ear canal DPOAE (Zweig and Shera, 1995). A vector combination of these two components (termed distortion and reflection) yields the DPOAE recorded at the microphone in the ear canal. Constructive and destructive interference between DPOAE components in the ear canal produces DPOAE fine structure.
The dual nature of DPOAE generation can be problematic for measures of the MOC reflex because the medial efferent reflex shifts the phase relationship between components. DPOAE fine structure often exhibits deep minima at frequencies where the two components of similar magnitude have combined while out-of-phase, effectively cancelling the response. If MOC activation alters the amplitude and phase balance between these two components, it can effect a release from cancellation. Predictably, this produces an increase in DPOAE amplitude.
Past work has confirmed that MOC activation impacts the two DPOAE components differentially (Abdala et al., 2009; Deeter et al., 2009; Henin et al., 2011) and that MOC-induced level enhancement is observed primarily in the dips of DPOAE fine structure. Level enhancements can obscure measures of MOC reflex strength as they are, at their root, artifacts of component interference. When the MOC reflex is measured at peak frequencies in DPOAE fine structure where components are adding in-phase, a reduction of either component by the MOC reflex will decrease DPOAE level and produce more typical reductions. Indeed, one can eliminate much of the level enhancement artifact by recording at peaks in DPOAE fine structure. In our earlier study of the MOC reflex in newborns (Abdala et al., 1999), we did not consider the dual-source nature of the DPOAE and failed to control for component interference.
Maturation of the MOC reflex
Other studies probing maturation of the MOC reflex in human neonates have used mainly click-evoked OAEs (CEOAEs), which are predominantly reflection source emissions comparable to the reflection component of the DPOAE (Kalluri and Shera, 2001, 2007). These studies have produced mixed results as varied methodologies, response criteria and age ranges were utilized. One early report suggested a complete absence of the MOC reflex in prematurely born neonates (Morlet et al., 1993); however, after methodological revision (viewing the response in a restricted time window and monitoring contralateral noise levels in-the-ear), premature neonates under 36 weeks gestational age did, in fact, show reductions of CEOAE amplitude but the effect was smaller than in adults (Morlet et al., 1999, 2004). The strength of the MOC reflex was reported to correlate positively with post-conceptional age (PCA). Others have, likewise, reported reduced CEOAE-based measures of the MOC reflex in premature neonates, though experimental parameters were not always well specified (Gkoritsa et al., 2007; Hamburger et al., 1998). In contrast, Chabert et al. (2006) reported adultlike CEOAE-based MOC reflexes in preterm infants tested between 36 and 37 weeks PCA. By term birth, contralateral inhibition of CEOAEs is adultlike, producing both a reduction in CEOAE amplitude and, in one study, reduced latency (Ryan and Piron, 1994).
There are many methodological inconsistencies among studies that make conclusive statements difficult. Most applied a rather lax 3 dB signal to noise ratio (SNR) criteria for OAE data, a critical consideration as measures of the MOC reflex are influenced by noise in both the baseline and contralateral elicitor condition. The contralateral noise was presented at high levels, which could potentially evoke contractions of the middle ear muscle, i.e., 70–78 dB SPL (e.g., Chabert et al., 2006; Gkoritsa et al., 2007; Morlet et al., 1993, 1999); whereas other studies presented noise elicitors at levels that might be too low to adequately evoke MOC activity (e.g., Chabert et al., 2006; Morlet et al., 1993), in particular because newborns have an inefficient transfer of energy through the middle ear, effectively lessening stimulation to the cochlea. Calibration specific to newborn ears was not always employed and several studies recorded the CEOAE using a conventional nonlinear stimulus paradigm; this method effectively cancels out much of the linear reflection emission, possibly impacting the measured magnitude of the MOC reflex.
In the current experiment we applied methods to control the effect of component interference on DPOAE-based measures of the MOC reflex, and expanded the repertoire of indices. Additionally, we considered the effects of contralateral noise on both level and phase of the 2f1 – f2 DPOAE and on its dual-source components. Our objective was to exploit recent advances in the understanding of OAE generation to better probe the functional status of the contralateral MOC reflex in human newborns. By including both premature and term neonates, we were able to assess integrity of the MOC reflex during both the pre-term period and the perinatal period following term birth.
METHODS
Subjects
Subjects included 96 individuals in four age groups. M, F, R, and L refer to male, female, right, and left ear, respectively: (1) 15 prematurely born neonates (5M, 10F; 8R, 7L) with a mean PCA of 34.2 weeks at test, and gestational ages at birth ranging from 24 to 36 weeks; (2) 31 term neonates born between 37 and 42 gestational weeks (15M, 16F; 27R, 4L); (3) 19 older infants with a mean age of 6.4 months (9M, 10F, 19R); and (4) 31 young adults with an average age of 21 years (6M, 25F; 18R, 13L). With the exception of the 6-month-old group, in which right ears were exclusively used, test ear was chosen in a pseudorandom fashion unless one ear had markedly higher DPOAE levels or was more accessible in the test isolette.
Protocol
Newborns were tested at the Infant Auditory Research Laboratory within the University of Southern California + Los Angeles County Medical Center, Neonatology Unit. All newborns passed a click-evoked auditory brainstem response screen conducted at 35 dB nHL. DPOAEs were measured in a sound-attenuating isolette providing between 25 and 40 dB of attenuation (Eckels ABC-100). Newborn testing was always conducted in pairs; one tester attended to the newborn throughout the test, watching for movement and probe slippage while the other tester implemented the data collection program. If a spike in noise was noted on the spectral display of the ear canal microphone signal, or baby movement was observed, the sweep was manually stopped, rejected in its entirety and re-initiated.
DPOAE testing of 6-month-old infants was conducted at a satellite laboratory at the University of Washington (UW). Their hearing was screened with CEOAEs and data were collected during natural sleep within a double-walled IAC sound-attenuating chamber. Adult subjects were tested at the House Research Institute (HRI) and Northwestern University (NU). They were awake during testing and seated comfortably in a padded armchair, also within a double-walled IAC sound-attenuating chamber. Adult subjects had ≤15 dB HL audiometric thresholds between 0.25 and 8 kHz. A standard clinical tympanogram (Grason-Stadler Instruments, Tympstar) was conducted on the day of test for both adult and 6-month-old infants to ensure normal middle ear status. Laboratories across centers were matched in hardware and software; protocols were matched across sites and all testers received comparable training.
Primary tones, f1, f2, were presented at 65 and 55 dB SPL (L1, L2) with a fixed f2/f1 ratio of 1.22. Tones were logarithmically swept upward in frequency at 8 s/octave between 0.5 and 4 kHz for a total of 24 s per sweep. For adults and some newborns, 16 sweeps were collected in the no-noise condition (NoN) and eight sweeps collected with contralateral acoustic stimulation (broadband noise) presented to the opposite ear through an ER2 insert transducer at 60 dB SPL. Eight NoN sweeps were collected for 6-month-old infants. The level of contralateral acoustic stimulation (CAS) was chosen because it has shown to be an effective elicitor of the MOC reflex (Guinan et al., 2003). The frequency response of the broadband noise in a Zwislocki coupler showed relatively flat energy from 0.2 to 12 kHz. The noise elicitor was turned on 1 s before the primary tones. The NoN and CAS sweeps were interleaved throughout the test protocol in pairs with a 2 s interval between sweeps. For example, a pair of NoN sweeps was presented, followed by a pair of sweeps at CAS = 60 dB SPL, a pair of sweeps at CAS = 65 dB SPL and so on until the targeted number of sweeps was collected in each condition (Note: CAS = 65 dB SPL was not available for all subjects and was not analyzed in this report.).
Signal processing and instrumentation
DPOAEs were recorded using a Macintosh laptop controlling a MOTU 828 Mk II audio device (44.1 kHz, 24 bit). The output of the MOTU was appropriately amplified and fed to either MB Quartz 13.01 HX drivers (NU) or Etymotic Research ER-2 tube phones (HRI, UW). The output of the drivers was coupled to the subjects' ears through the sound tubes of an Etymotic Research ER10B+ probe microphone assembly.
DPOAE level and phase estimates were calculated from averages comprised of eight sweeps each, using a least-squares-fit algorithm (LSF) at frequency intervals of between 2 and 12 Hz. As an initial data inclusion criterion, a median of every three consecutive points in the average was calculated and compared to the noise estimate at the corresponding frequency; if SNR was < 6 dB, the data point was not accepted into the average. Each average included a total of ∼ 400–500 individual data points spanning the three-octave test range. In this implementation of the LSF technique, models for the stimulus tones and DPOAE of interest are created. Signal components are then fitted to these models to minimize the sum of squared errors between the model and the data (Long et al., 2008). The noise floor is estimated by taking the difference between sweep pairs and applying the LSF to this difference. DPOAE phase was unwrapped by sequentially subtracting 360° from all points beyond identifiable discontinuities. The final estimate of DPOAE phase was computed by subtracting 2ϕ1 – ϕ2, (where ϕ1,2 are phases of f1 and f2) from ϕdp (the extracted phase at 2f1 – f2).
Component separation: Inverse FFT
MATLAB-based software was used to separate the DPOAE distortion- and reflection-source components based on their respective phase-gradient delays (developed by C. Talmadge; adapted by P. Luo). During inverse FFT (IFFT), the DPOAE complex pressure measured in the frequency domain was multiplied by a moving Hann window in overlapping 50 Hz steps. The length of the window was adjusted on a logarithmic scale in close approximation to the cochlear frequency map and ranged from 0.4 to 0.9 kHz with increasing frequency. Rectangular time-domain filters were applied to each window to recursively extract the target component. A search range of −2 to 10 ms was applied to window the short-latency (distortion) component, and 3 to 15 ms to window the long-latency (reflection) component. The time domain filters were centered around the maximum or peak energy within each window. The filtered windows of data were then transformed back to the frequency domain by FFT and the level and phase of the distortion and reflection components reconstructed. Data segments equal to half of the length of the analysis window were eliminated at low- and high-frequency boundaries to remove edge effects caused by the time-windowing process.
Reflection component phase was determined to be reliable by the following process: NoN phase was recalculated using an alternative sequence of frequencies. If the two estimates (the original and recalculated phase-frequency function) replicated, the function was considered reliable. If the phase had been adversely impacted by noise, the two functions diverged, in which case either the entire phase frequency function was eliminated or the start frequency was modified to eliminate the noisy segment. Phase-frequency functions from one term newborn, one older infant and one adult were eliminated using this reliability probe. Discontinuities in reflection-component phase remained in some subject data, but these were typically associated with deep minima in DPOAE level fine structure; they included half-cycle shifts that were randomly upward or downward, exerting little bias on the overall trend. In these cases, phase was recalculated using double the number of points to resolve any unwrapping ambiguities.
Calibration
Calibrated stimuli were delivered to each subject after compensating for the depth of probe insertion (Lee et al., 2012). This allowed us to approximate the desired SPL across frequency at the tympanic membrane and in doing so, to eliminate the effects of standing waves. In newborns, a priori measurement of the half-wave resonance in 20 ears provided a reference depth insertion; compensation was applied equally to all neonates based on this normative measure.
Broadband noise was calibrated in an ear simulator (IEC 60318-4; Bruel and Kjaer 4157) for adults and older infants but measured in-the-ear for a reference group of newborns. It is difficult to monitor a probe fit to a newborn subject's contralateral ear because the ear is often concealed from visual inspection. Consequently, CAS was delivered via a foam and silicone ear cover (Natus Halo Ear Muff, Biologic). The Ear Muff was firmly attached to skin around the neonatal pinna of the non-test ear and ER-2 tubing was inserted into an opening in the coupler designed for sound delivery. For calibration purposes, reference noise levels within the Ear Muff were measured in 10 newborn ears using an Etymotic Research ER-7 probe microphone with tubing threaded through a small hole in the silicone, and centered at the external auditory meatus. This set-up provided a stable noise delivery system resistant to slight head movements.
Analysis
DPOAE MOC Indices
The following measures were taken at DPOAE fine structure maxima only. Maxima were identified based on the first and second derivatives of the DPOAE level function and the relationship between them. Data points where the first derivative was equal to 0 and the second derivative was negative were identified as maxima or peaks (Abdala and Dhar, 2012).
-
(a)
MOCRdB = LdpCAS − LdpNoN where Ldp is DPOAE level. MOCRdB was calculated at DPOAE fine structure peaks only; negative values denote reductions in level. For MOCR indices, we restricted ourselves to measuring reductions in level (termed here inhibition). Episodes of increased level evoked by CAS were labeled enhancement if they exceeded + 0.1 dB change (from baseline level) and were counted and analyzed separately. Theoretical and empirical evidence suggests different mechanistic underpinnings for CAS-induced enhancement compared to reductions in DPOAE level (Abdala et al., 2009; Deeter et al., 2009; Henin et al., 2011). We implemented this analysis strategy in an attempt to disentangle MOC effects from artifacts related to component mixing.
-
(b)
MOCRn = (|PdpNoN| − |PdpCAS|)/|PdpNoN| where |Pdp| is DPOAE amplitude. MOCRn was calculated at fine structure peaks only and reported as a fraction of the baseline amplitude.
-
(c)
MOCRVn = |(PdpCAS − PdpNoN)|/|PdpNoN| where Pdp is a complex DPOAE vector. MOCRVn was calculated as the amplitude of the difference vector between NoN and CAS at fine structure peaks only, and reported as a fraction of the baseline amplitude.
Component-specific MOC Indices
-
(a)
MOCRD, MOCRR = (|PDNoN| − |PDCAS|)/|PDNoN| and (|PRNoN| − |PRCAS|)/|PRNoN| where |PD| and |PR| represent distortion and reflection-component amplitude, respectively. MOCRD and MOCRR were reported as a fraction of the baseline amplitude.
-
(b)
Component Phase-Distortion and reflection component phase-frequency functions in both NoN and CAS conditions were fit with loess trend lines (Cleveland, 1993). Loess is a form of locally weighted scatter plot smoothing that is a modern version of classical linear and nonlinear least squares regression. Simple models of linear and nonlinear least squares regression are fitted to localized subsets of the data and adjacent fits are joined to create the overall fit.
-
(c)
MOCRPA = [ϕCAS (0.7) – ϕCAS (3.6)] – [ϕNoN(0.7) −ϕNoN (3.6)]. The phase accumulation in cycles between f = 0.7 kHz and f = 3.6 kHz was measured as an approximate metric of phase slope for the reflection component. The difference in phase accumulation between NoN and CAS conditions was calculated.
Middle ear muscle reflex
Due to limited test time with infants, it was not possible to complete tests of the middle ear muscle reflex (MEMR) in all infant subjects. A complete set of MEMR data was available only in young adult subjects; therefore, this group was used to estimate the potential impact of MEMRs on the MOC reflex. The level of a pure tone (L1) was monitored in the ear canal during NoN and CAS conditions; changes in amplitude produced by CAS were calculated to detect MEMRs. This same calculation was conducted for the difference between NoN and a replicate no-noise average to provide a reference of normal variability. A MEMR was considered present if two criteria were met: the amplitude change in the ear canal was >1.4% and it exceeded the difference between repeated NoN averages (Patrick Feeney, personal communication; Feeney et al., 2003)
Statistical analyses
So as to maximize usage of all data points for the repeated measure, the frequency variable was collapsed into low- (center frequencies = 707–1414 Hz) and high- (center frequencies = 1781–3563 Hz) frequency categories for analysis of variance (ANOVA) tests; however, Figs. 234 display MOC measures at each center frequency individually to provide the reader with detailed frequency information. Age (4) × frequency (2) ANOVAs with repeated measures on frequency were conducted (SPSS ver. 18.0). When appropriate, post hoc age comparisons were conducted using a Bonferroni correction. The alpha level was p = 0.05.
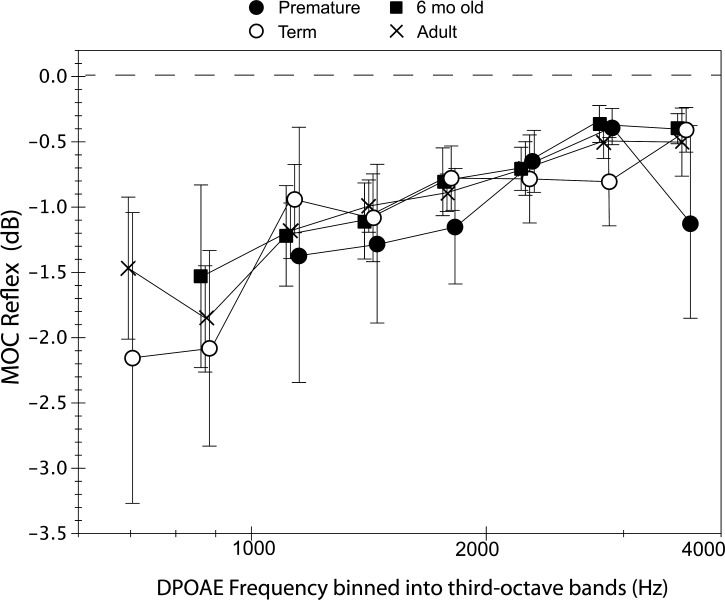
Figure 2.
The MOC reflex calculated in decibels (MOCRdB) as LdpCAS − LdpNoN measured at DPOAE fine structure peaks and binned into third-octave intervals with center frequencies ranging from 707 to 3563 Hz. The dashed line indicates no CAS-induced shift. Mean values were displayed if at least five observations were available. The range of observations contributing to each mean varied with age group and frequency as follows: premature = 6–11, term = 7–25, 6-month-old = 5–12 and adult = 9–27. Frequency was collapsed into only two categories (low, high) for statistical analyses so as to maximize numbers. Error bars represent 95% confidence intervals (CI).
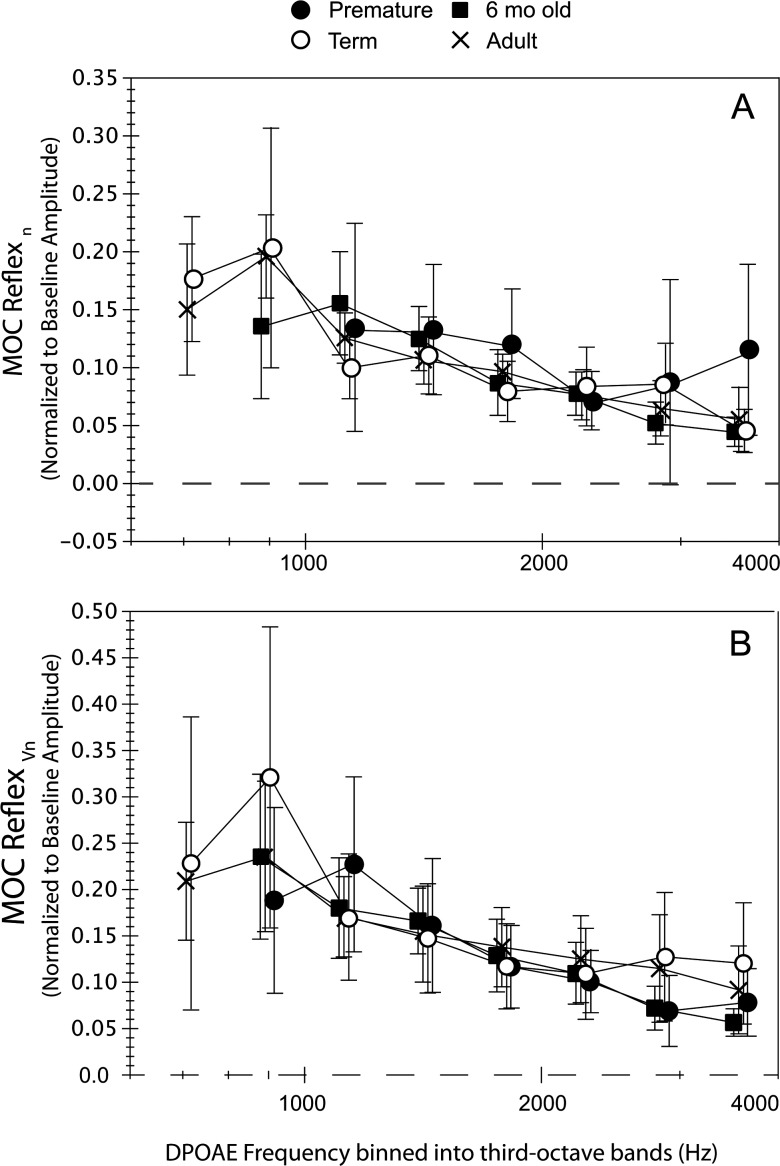
Figure 3.
(A) Normalized MOC reflex, termed MOCRn, calculated as (|PdpNoN| − |PdpCAS|)/|PdpNoN| and (B) normalized MOC reflex as the magnitude of thedifference vector, termed MOCRVn, and calculated as |(PdpCAS − PdpNoN)|/|PdpNoN|. Both indices were measured at fine structure peaks only and binned into third-octave intervals with center frequencies ranging from 707 to 3563 Hz. Mean values were displayed if at least five observations were available. The range of observations contributing to each mean varied with age group and frequency as follows: premature = 6–11, term = 7–25, 6-month-old = 5–12 and adult = 9–27. Frequency was collapsed into only two categories (low, high) for statistical analyses to maximize numbers. Error bars represent 95% CIs.
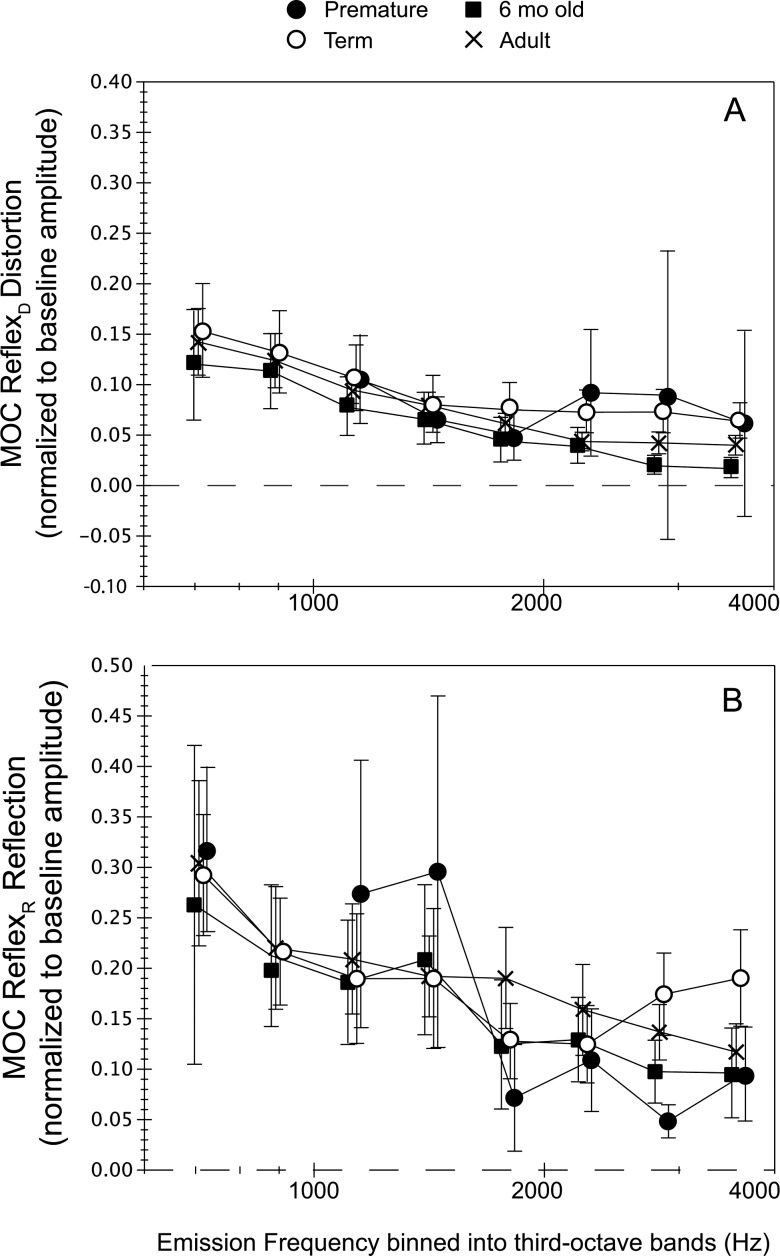
Figure 4.
Normalized component-specific (A) distortion- and (B) reflection-component MOC reflex, termed MOCRD and MOCRR and calculated as: (|PDNoN| − |PDCAS|)/|PDNoN| or (|PRNoN| − |PRCAS|)/|PRNoN|. Values were binned into third-octave intervals for each age group with center frequencies ranging from 707 to 3563 Hz. The dashed line indicates no CAS-induced shift. The range of observations contributing to each mean varied with age group and frequency as follows: premature = 5–10, term = 15–22, 6-month-old = 5–11 and adult = 23–27. Frequency was collapsed into only two categories (low, high) for statistical analyses to maximize numbers. Error bars reflect 95% CIs.
Phase-frequency functions were fit with loess lines to aid in visualizing global phase trends. To reduce non-meaningful phase variance, functions with phase values that were not within ± 0.5 cycles of 0 at 0.7 kHz were shifted up or down by one cycle. Since all frequency points are shifted by the same integer value, the normalization procedure does not affect the frequency dependent phase accumulation measured in any one subject but simply reduces the scatter that comes from the first point.
RESULTS
The number of observations at any given center frequency varied for several reasons: (1) fine structure peaks are distributed idiosyncratically among subjects; (2) SNR measurements that did not meet the minimum criteria were not accepted; and (3) calculations of the MOC reflex were eliminated if deemed outliers. An outlier was ± 2 standard deviations from the mean for that metric, age group, and center frequency. The overall percentage of outlier values eliminated for each age group is as follows: Premature = 5%; Term = 3%; 6-month-old Infant = 7%; Young Adult = 3%.
DPOAE and noise level values were binned into third-octave intervals with center frequencies ranging from 707 to 3563 Hz. The difference between these levels was calculated in each third-octave bin and defined as the signal-to-noise ratio (SNR). Grand mean SNR for each age group was as follows: adult = 28 dB, 6-month-old infant = 22 dB, term newborn = 24 dB, and premature newborn = 20 dB. These SNR values include all data, i.e., at both maxima and minima in fine structure; therefore, for MOC indices calculated at peaks only, SNR was more favorable than these reported values.
DPOAE MOC reflex
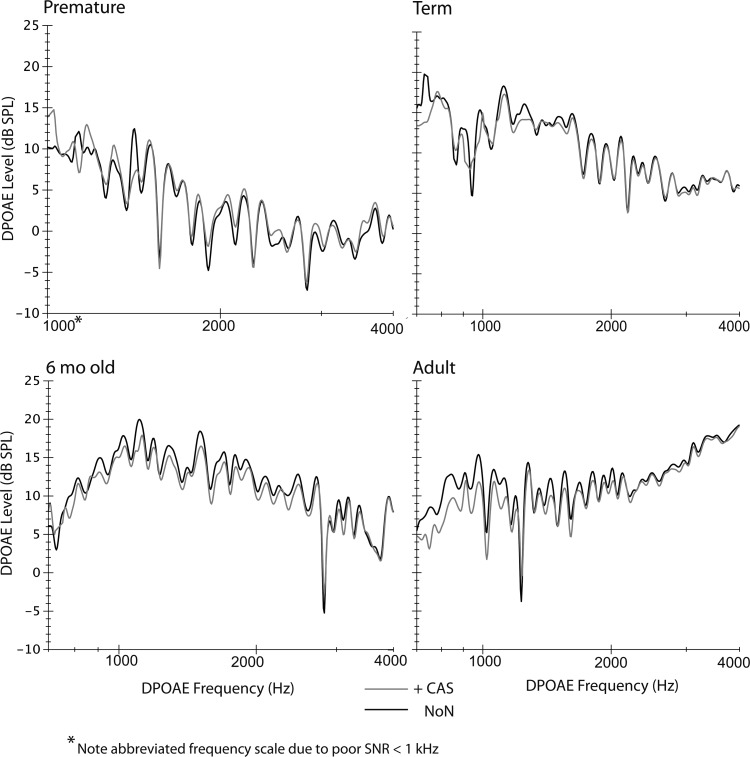
Figure 1 shows an example of DPOAE level recorded at fine frequency intervals in NoN (black line) and CAS = 60 dB SPL (gray line) conditions for each age group. Each trace represents an average of eight sweeps and includes ∼400 to 500 points across frequency. In general, the strongest reductions in level were noted around peaks in fine structure. Peaks were sometimes shifted towards higher frequencies, reflecting CAS-induced changes in emission phase. At deep valleys in fine structure, DPOAE enhancement was often observed due to component interference (See Sec. 1A). The premature newborn subject displayed in Fig. 1 shows increases in DPOAE level at several peaks in fine structure.
Figure 1.
One example of DPOAE level fine structure in no-noise (NoN, black line) and during CAS (contralateral acoustic stimulation, gray line) for each age group. Note: The frequency scale is slightly abbreviated for the premature newborn due to excessive noise at low-frequencies.
Past work has often calculated the MOC reflex in dB; hence for comparison purposes, comparable data are shown in Fig. 2 though these were not statistically analyzed. MOCRdB shows marked overlap among age groups. The MOC reflex is strongest at low frequencies with mean values ranging from 1 to 2 dB. Figure 3A shows the MOC reflex normalized to each subject's own baseline amplitude as MOCRn. Figure 3A shows that MOCRn represents 0.5 to 0.20 of baseline DPOAE amplitude across age groups. Figure 3B displays the amplitude of the difference vector between NoN and CAS conditions normalized by baseline amplitude, MOCRVn. Vector differences were generally larger than measures based solely on amplitude shifts. Although data are shown at each of the eight center frequencies in Figs. 234, frequency was collapsed into two categories (low- and high-frequency) for statistical analyses. Age (4) × frequency (2) ANOVAs with repeated measures on frequency showed no effect of age on either MOCRn or MOCRVn but an effect of frequency on both (F = 69.8; p = 0.0001; F = 68; p = 0.0001) as MOC effects were stronger in the low-frequency interval (< 1.5 kHz). There were no interactions between age and frequency.
DPOAE level enhancement
Between 91% and 96% of DPOAE fine structure peaks from term newborns, 6-month-old infants and adults showed reductions in DPOAE level or no-change when a contralateral noise elicitor was presented. In contrast, only 72% of DPOAE fine structure peaks from the premature newborn group showed level reductions whereas 28% showed increases in DPOAE amplitude with CAS. A one-way ANOVA comparing the percentage of enhanced peaks across age group was significant (F = 7.014; p < 0.0001). The magnitude of the DPOAE enhancement effect in premature neonates averaged 0.67 dB overall, generally smaller than mean reductions in level. Of 62 observations of enhancement, 14 episodes occurred at frequencies <1.5 kHz and 48 above. The association between these enhancement episodes and SNR was examined in the premature newborn group. No significant correlations were observed, suggesting that the quality of the data as gauged by SNR, was not systematically associated with DPOAE enhancement elicited by CAS. In the discussion we posit three potential sources for DPOAE level enhancement, which has been consistently observed in prematurely born neonates tested in our laboratory.
Component-specific MOC reflex
Component amplitude
Independent measures of the MOC effect on distortion- and reflection-components derived from the ear canal DPOAE were calculated as MOCRD and MOCRR. The mean magnitude of distortion-component MOC reflexes ranged from 0.2 to 1.6 dB, whereas mean reflection-component MOC reflexes ranged from 1 to 3 dB (not displayed). Figure 4 shows normalized MOCRD and MOCRR for each age group. The distortion component MOC reflex represents a fraction of baseline component amplitude ranging from 0.02 to 0.24 across frequency; the reflection component MOC reflex ranged from 0.05 to 0.32. As noted in Fig. 4B, the reflection component measures also showed more data scatter.
Age (4) × frequency (2) ANOVAs with repeated measures on frequency were conducted separately for each component-specific MOCR index. There was no effect of age on MOCRD but an effect of frequency (F = 182; p < 0.0001), the low-frequency interval manifesting the most robust MOC effect. There was no significant interaction. The MOCRR measures showed no main effect of age but an interaction between age and frequency (F = 5.3; p = 0.002). Post hoc tests confirmed that an age effect was restricted to the high-frequency interval (>1.4 kHz; F = 4.49; p = 0.006). Paired age comparisons further showed that the premature age group had lower MOCRR values compared to term newborns (p = 0.008) and adults (p = 0.02) but not compared to the 6-month-old infant group.
Component phase
The phase of distortion and reflection components are displayed in Figs. 56, respectively; however, the primary measure of interest is the effect of MOC activation on reflection-component phase as these emissions are associated with low-level hearing—the range at which MOC effects are optimal—and are thought to preferentially gauge medial efferent activity.
Figure 5.
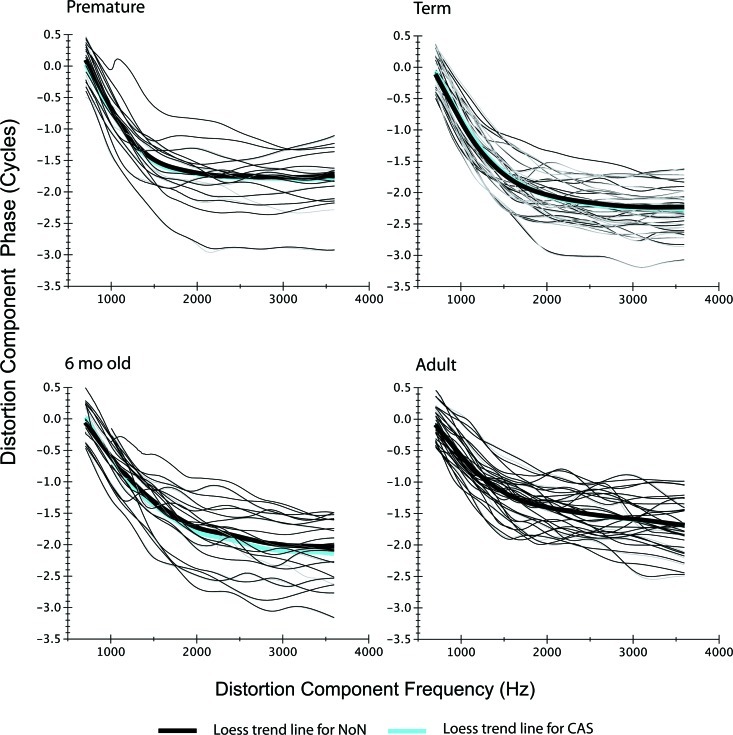
Distortion component phase-frequency functions for each age group. Individual functions are shown with thin lines (black = NoN; gray = CAS), and loess fits are shown as thick lines in black (NoN) and cyan (CAS). If the cyan line is not visible, it indicates overlap between trend lines and a limited effect of CAS on distortion phase. Note: the range shown on the ordinate is only 4 cycles and differs significantly from the range of cycles displayed in Fig. 6 for comparable phase data from the reflection component (30 cycles).
Figure 6.
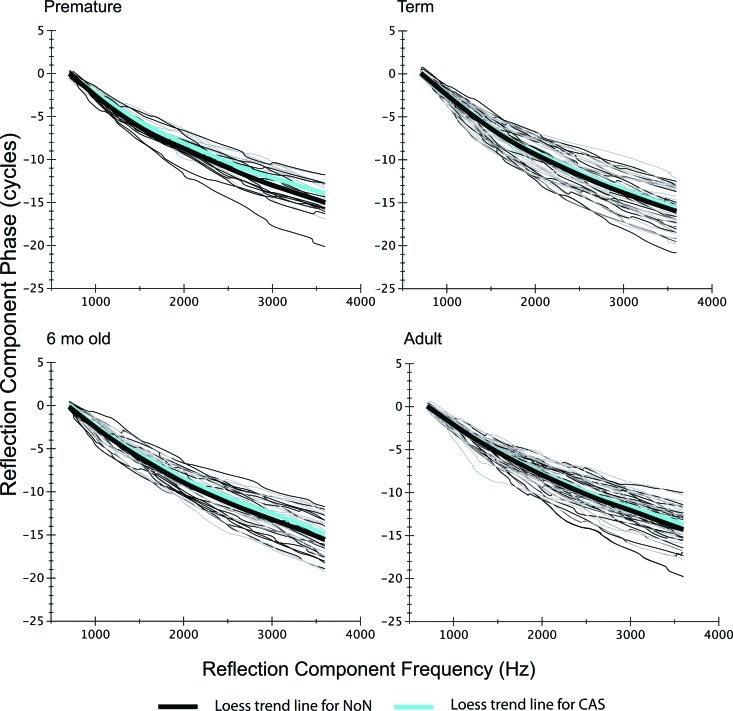
Reflection component phase-frequency functions for each age group. Individual functions are shown with thin lines (black = NoN; gray = CAS), and loess fits are shown as thick lines in black (NoN) and cyan (CAS). Note: the range shown on the ordinate is 30 cycles and differs significantly from the limited range of cycles displayed in Fig. 5 for the distortion component (4 cycles).
Distortion component.
Figure 5 displays individual phase-frequency functions and superimposed trend lines. (Note: The ordinate phase scale spans only 4 cycles compared to reflection-component phase displayed across 30 cycles in Fig. 6.) There was no systematic change in the overall features of distortion-component phase with the presentation of contralateral noise. Distortion component phase is relatively invariant across frequency, providing evidence of approximate local scaling for much of the cochlea (Shera et al., 2000); for frequencies <1.5 kHz, distortion phase deviates from this scale invariance. The thin black (NoN) and gray (CAS) lines are phase-frequency functions from individual subjects. The thick trend lines are superimposed, shown in black (NoN) and cyan (CAS). The superimposition of the trend lines indicates that MOC activation did not alter the frequency at which distortion phase breaks from invariance, often considered the apical-basal transition; nor did it alter the basic two-segment configuration of the DPOAE phase-frequency function for any of the age groups. This result does not indicate that the MOC reflex did not impact the distortion generator, simply that it impacted f1and f2 similarly. In doing so, a constant relationship appears to have been maintained between primary tones, leaving DPOAE phase (which is referenced to the phases of f1 and f2) unchanged.
Reflection component.
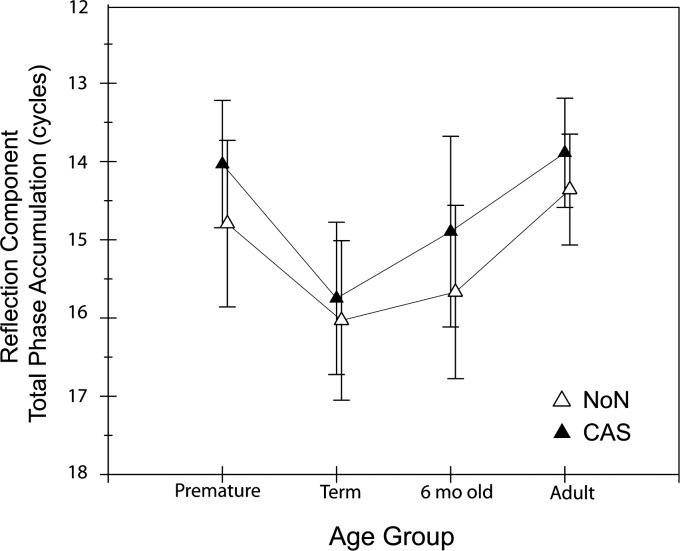
Figure 6 shows the individual reflection-component phase-frequency functions in thin black and gray lines (NoN and CAS respectively); the thick trend lines fit to the group data are shown in black (NoN) and cyan (CAS). The difference between the black and cyan trend lines provides a rough index of the impact of CAS on reflection-component phase. For all age groups, CAS produced slightly shallower slope of phase. The MOC effect was quantified by measuring the difference in total phase accumulation in NoN and CAS conditions and termed, MOCRPA. Figure 7 displays the mean phase accumulation between 0.7 and 3.6 kHz for NoN and CAS conditions in each age group. The reflection-component phase slope became significantly shallower when CAS was presented (F = 17.29; p < 0.0001); however, there was no age effect on MOCRPA.
Figure 7.
Mean number of phase cycles accumulated between 0.7 and 3.6 kHz for the reflection component of the DPOAE in both NoN (open triangle) and CAS (closed triangle) conditions. Error bars reflect 95% CI.
Middle ear muscle reflex
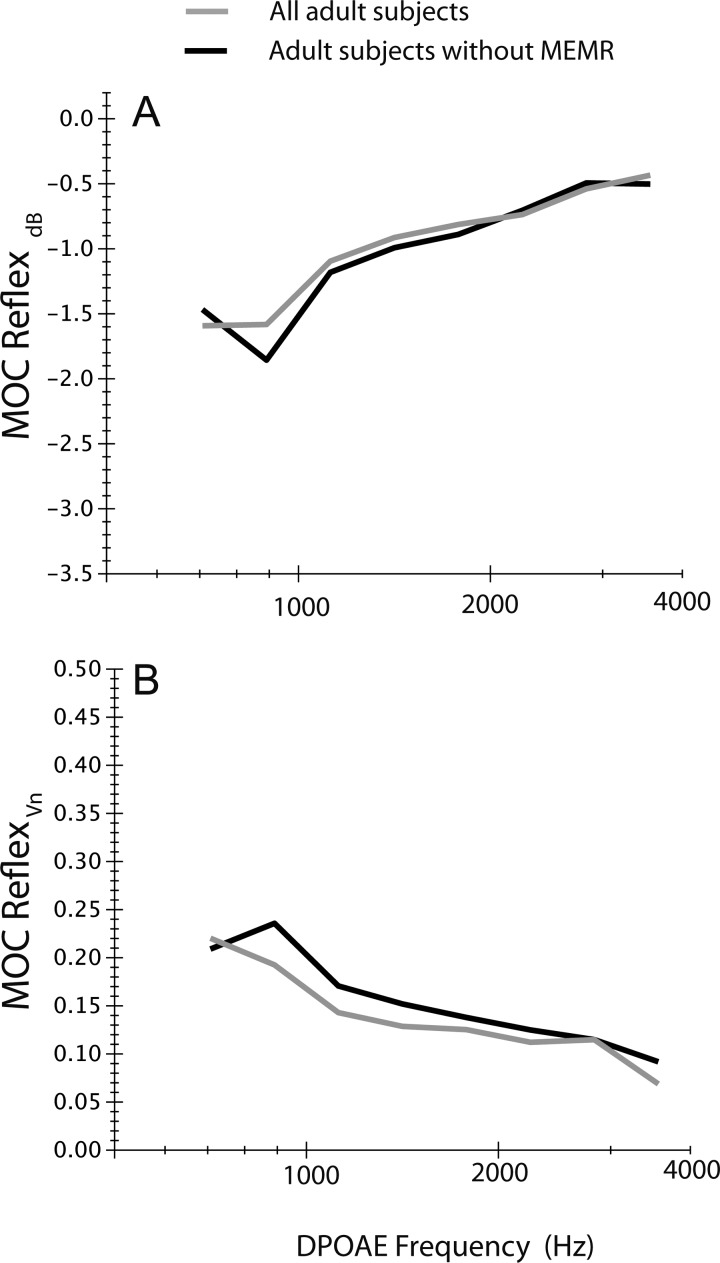
Figure 8 displays mean MOCRdB and MOCRVn for all adult subjects (gray) and for a subset of adult subjects excluding eight individuals determined to have a CAS-elicited MEMR (black). Even in cases where a MEMR appears to have been evoked by CAS, the impact of this contraction did not change the configuration of the MOC effect greatly or produce substantial changes in reflex magnitude.
Figure 8.
(A) Mean MOCRdB and (B) MOCRVn including all adult subjects (gray) and in a subset of adult subjects (black); this subset excluded eight subjects in which middle ear muscle reflexes evoked by CAS had been detected.
DISCUSSION
Maturation of the MOC reflex
DPOAE-based indices of the fast, inhibitory MOC effect were generally comparable across age. The MOC reflex in dB averaged between 0.5 and 2 dB across ages consistent with past findings. This result suggests that the strength of the contralaterally evoked MOC reflex is adultlike by late in the third fetal trimester. It is possible that premature newborns tested at younger post-conceptional ages (<34 weeks) show non-adultlike MOC reflexes as some studies have suggested (Chabert et al., 2006).
The DPOAE measured in the ear canal includes contributions from two sources: the site of primary tone overlap nearest f2, and from the 2f1 – f2 place, each source arising from distinct generation mechanisms (Talmadge et al., 1998; Shera and Guinan, 1999). The MOC reflex acts upon these components differentially (Abdala et al., 2009; Deeter et al., 2009; Henin et al., 2011). Once separated, we observed an age effect on the reflection component only; premature neonates were found to have a reduced MOCRR. However, this effect was incongruous for several reasons. First, the age effect was only present in the high-frequency interval (>1.5 kHz), a frequency range in which the MOC reflex is weakest. Second, as noted in Fig. 4B, premature infants showed an abrupt reversal of this trend in the low-frequency interval, manifesting stronger MOC reflexes than the other age groups (though this age difference does not reach statistical significance). Third, prematurely born neonates did not show reduced reflection-component MOC reflexes when compared to 6-month-old infants, only compared to term newborns and adults. A genuine immaturity in the MOC reflex would be expected to differentiate prematurely born neonates from 6-month-old infants as well.
What emerges from the MOC indices shown in Figs. 234 is that premature newborns exhibit inhibitory MOC reflexes, i.e., defined as reductions in emission level, which cannot be distinguished from adult reflexes in almost all analyses conducted. One could argue that the smaller number of subjects in the premature age group (n = 15) may have adversely affected the statistical power to detect differences; however, this is an unlikely explanation as premature newborns not only show adultlike MOC reflex strength, they manifest other adultlike features: maximal effects at low-mid frequencies, a more marked shift in the reflection component (vs distortion) and shallowed reflection-component phase slope.
It is inevitable that middle ear immaturities influenced the results to some extent. Inefficiencies in infant middle ear transmission likely reduced the impact of CAS, potentially reducing the size of the MOC reflex. We could have compensated for this inefficiency by increasing contralateral noise levels in newborns; however, this is only a partial solution. DPOAE primary tones presented to the test ear were also impacted by middle ear inefficiency, lessening stimulation to the cochlea. This reduced stimulation may have optimized the MOC effect in infants since the largest MOC reflex is generally observed for low-level signals. These two middle ear-related factors may have counteracted one another.
The middle ear muscle reflex (MEMR) can also act as a confound when measuring MOC effects; the noise elicitor can evoke bilateral middle ear muscle contractions, which impede effective transmission of either or both, CAS and primary tones. In 25% of adult subjects, MEMRs appear to have been evoked by contralateral noise. Can these contractions explain the adultlike MOC reflexes measured from newborns in the current study? If a similar proportion of adult and infant subject were impacted by CAS-induced MEMRs, their presence would not influence age trends found here. Recent work suggests that MEMR thresholds are indeed adultlike in newborns (Keefe et al., 2010). Additionally, when MEMRs were evoked, data from Fig. 8 suggest that they did not alter the magnitude or pattern of MOC reflex in the frequency range contributing most strongly to the statistical analyses, i.e., frequencies >1 kHz. Therefore, though it is not possible to fully disentangle the influence of the two acoustically evoked reflexes, there is little evidence to suggest that MEMRs would differentially impact adult and infant MOC data.
CAS-induced enhancement
We did observe an atypical MOC effect in premature newborns: the persistent presence of CAS-induced increases in amplitude even when measuring at fine structure peak frequencies only. In adults, enhancement can be mostly eliminated by recording the MOC effect at peak frequencies in fine structure, where DPOAE components are combining constructively (Abdala et al., 2009). In contrast, at dips in fine structure a differential MOC effect on distortion and reflection components often produces a release of cancellation and an associated jump in level.
Others have reported enhancement effects after MOC activation in mice, but these are slow effects persisting hundreds of seconds after shock activation and distinct from cholinergic effects (Maison et al., 2007). We exclusively assayed the fast MOC effects with our paradigm. Still others have reported MOC-related enhancement in the quadratic, f2 − f1, DPOAE and hypothesize a shift in the cochlear amplifier operating point as the source (Abel et al., 2009). This type of shift could alter distortion generation as well as exacerbate component interference by impacting distortion and reflection sources differentially. However, we controlled for this type of interference in the present study. Additionally, evidence exists indicating that the cochlear amplifier is generally adultlike in human newborns (Abdala and Dhar, 2012).
We consider three possible explanations for the higher rates of contralaterally evoked DPOAE enhancement in premature newborns.
Immaturity
One might conjecture that an immaturity exists in medial efferent function during the preterm period and is responsible for atypical CAS-related enhancements of cochlear emissions. Neonatal kittens have shown enhancement of auditory nerve fiber (ANF) spontaneous discharge rate elicited by contralateral noise, leading to the hypothesis that the MOC-OHC synapse might be excitatory for a transient developmental interlude (Jenkins et al., 1993). However, if this type of immaturity exists in the human newborns tested here, it would not be sporadically present as is DPOAE enhancement. Thirteen of fifteen premature subjects showed some CAS-induced level enhancement; the percentage of enhanced peaks ranged from 3.3 to 71%. Also, the distribution of amplitude enhancement across frequency was idiosyncratic. (See Fig. 1, upper left panel for an example of enhancement in one prematurely born subject.) The sporadic presence of this phenomenon and the absence of any frequency-related pattern argues against a true immaturity in fundamental aspects of the medial efferent effect.
Also, if atypical CAS-induced enhancement indicates an excitatory synapse, as has been postulated, and associated increases in cochlear amplifier gain, one would expect related changes in tuning, i.e., sharper cochlear filters. However as noted in Fig. 7, MOC activation produced shallower phase slope in all ages, as predicted by models of the medial efferent effect on cochlear mechanics (Guinan, 1996). In application of filter theory to reflection-type emissions, shallower emission phase slope and accompanying decreases in the delay are consistent with broadened tuning.
Noise floor
As a group, newborn infants manifest an elevated noise floor. Higher measurement noise no doubt stems from our inability to control body movement in this population, probe slippage during test, and generally higher ambient room noise in the hospital (versus a controlled laboratory setting). Additionally, intrinsic biological noise in developing, in flux organisms, such as newborns, may be exacerbated. It could be argued that this elevated noise floor compromises accurate measurement of the MOC effect in this population. However, heightened measurement noise is equally present in term-born neonates. Term newborns showed overall mean noise floors of −12 dB SPL, not dissimilar from premature newborns at −9 dB SPL. Even so, full term neonates did not show atypical rates of DPOAE level enhancement. Older infants showed the least favorable overall noise floor at −8 dB SPL, yet they did not show atypical DPOAE level enhancement; hence, it does not seem that the noise floor predicts or produces episodes of enhancement. In addition to considering the noise floor, we found no systematic association between overall SNR and CAS-induced enhancements. Also, DPOAE level enhancement was not more prevalent at low-frequencies where the noise floor is most elevated in newborns. Though elevated noise may have impacted MOC calculations in individual newborns and at certain fine structure peak frequencies, a higher noise floor and less favorable SNR values in premature newborns were not sufficient to explain increased CAS-evoked DPOAE level enhancement in this age group.
Component interference
We may not have exerted adequate control of component mixing in premature neonates by recording at fine structure peaks, even though this strategy is effective for other age groups. There are differences in DPOAE fine structure and relative component contribution between newborns and young adults. A strong reflection component is evident in newborn DPOAEs, as evidenced by more fine structure oscillations per given frequency interval, and by deeper oscillations. Additionally, in premature newborns, the difference between distortion and reflection component levels (a rough indicator of how much components might interfere with each another) is smaller (Abdala and Dhar, 2010, 2012).
The robust fine structure and large reflection emissions in premature newborns likely result from middle ear immaturities: multiple internal reflections produced by an immature cochlear-middle ear junction during reverse travel, and/or reduced stimulation to the cochlea due to inefficient forward transfer through the middle ear (Abdala and Keefe, 2006). A detailed study of outer/middle ear immaturities in prematurely born neonates has not been conducted; however, a limited glimpse was provided by Keefe et al. (2000), whose data suggest there may be slight age-related changes in middle ear admittance and reflectance between 33 weeks PCA and term birth.
To explore the possibility that more intrusive component interference in premature newborns contributed to enhancement episodes, we looked to the effects of CAS on the separated reflection component at frequencies corresponding to fine structure peaks. If enhancement is simply a consequence of component interference, separation should eliminate or reduce it. If DPOAE level enhancement persists despite component separation, it suggests that source interference cannot fully explain its occurrence.
The prematurely born subject with the strongest enhancement provided the most unambiguous result: when components were separated, nine of ten peaks previously enhanced by contralateral noise showed typical reductions in level or no change in reflection-component level. In this premature newborn, one might conclude that enhancement of DPOAE level occurred because of inadvertent interference between reflection and distortion components. Results from other enhancing subjects were not so clear. One premature newborn with five enhanced peaks continued to show enhancements in reflection emission level at four of the five fine structure peaks. One could argue that this outcome is more consistent with a deficit in the medial efferent effect on cochlear mechanics because component interference does not offer an adequate explanation. We also acknowledge, but do not explore, an alternative interpretation: Component separation via IFFT is not flawless, and imprecision in component separation is inevitable. If we did not capture the individual effect of CAS on each emission source as intended, it could contribute to persistent enhancement. This question is beyond the scope of the current report though it warrants further study.
Though prematurely born neonates in the present study were tested at post-conceptional ages well beyond the period associated with formation of the medial efferent-OHC synapse (Pujol et al., 1998), some of the neonates had quite abbreviated gestational terms and were born between 24 and 28 weeks post-conception. Post-synaptic cisternae and MOC-OHC synapses are thought to be forming during this time period. When testing prematurely born infants, we assume that features measured ex utero in neonates born prior to full gestation, are the same features that would manifest in utero if they had been carried to term. However, it is possible that we are not only probing immaturity but encountering deficits imposed by premature birth. We cannot rule out that an abbreviated gestational period disrupts the appropriate formation of medial efferent-OHC synapses, producing anomalies such as the atypical MOC enhancement effect consistently observed in this age group. We have, in fact, observed an association between gestational age at birth and episodes of CAS-induced enhancement (Abdala et al., 1999).
Walsh et al. (1998) performed deefferentation in neonatal cats prior to normal innervation of the OHC by medial efferent fibers, and found long-lasting effects including reduced sensitivity of ANFs near characteristic frequency and reductions in the sharpness of tuning. Deefferentation in adult cats or kittens beyond the time of synapse formation produced no auditory deficits. Based on these findings, it is not unreasonable to consider that a disruption in medial efferent innervation of OHCs in very premature human infants could produce lasting deficits in the MOC-based modulation of cochlea.
DPOAE-based measures of the MOC reflex
Results suggest that one should not use DPOAE-based indices of the MOC reflex without controlling for component interference. Controlling for this interference can be done by recording at peak frequencies (though this is not always a complete solution, as shown here in premature newborns), separating components and measuring the impact of the MOC effect on each separately, and/or by manipulating stimulus parameters to bias component contribution to the ear canal DPOAE (Knight and Kemp, 2001; Mauermann and Kollmeir, 2004). This work also reiterates that the two components of the DPOAE are differentially affected by MOC activation, supporting the idea that dual sources of the DPOAE do indeed represent distinct, nonredundant cochlear properties.
It is most efficacious to focus on reflection-type emissions to probe the medial efferent effect. While it is possible to do so by extracting the reflection component from the DPOAE as done here, this energy is generally low level and presents challenging signal-to-noise issues. A better means of measuring the MOC reflex might be stimulus frequency OAEs, as they appear to provide the most direct and frequency-specific reflection-type emission to gauge MOC effects (Guinan et al., 2003; Francis and Guinan, 2010).
OAE-based measures of the MOC reflex should consider shifts in both amplitude and phase of the emission. Recording the MOC-induced shift as a vector difference renders it more robust as noted in Fig. 3. Based on filter theory, reflection-type emissions and their corresponding measures of delay are associated with measures of cochlear tuning. This association has been empirically confirmed in several animal models and humans with a specified set of parameters (Shera et al., 2010). Hence, the effects of CAS on reflection-emission phase slope or delay may provide an effective metric of medial efferent effects. Consistent with this idea, we observed shallower reflection component phase slope when the MOC was activated. Likewise, Francis and Guinan (2010) found small but significant reductions in CEOAE latency in human adults upon contralateral activation of the MOC reflex.
ACKNOWLEDGMENTS
Financial support for this work was provided by grant DC003552 from the National Institutes of Health and by the House Research Institute. We thank Abigail Rogers and Sumaya Sidique for data collection, Sumitrajit Dhar and Radha Kalluri for helpful discussion, Mahnaz Ahmadi and Laurel Fisher for data management and Christopher Shera for sharing his loess script.
References
- Abdala, C., and Dhar, S. (2010). “Distortion product otoacoustic emission (DPOAE) phase and component analysis in human newborns,” J. Acoust. Soc. Am. 127, 316–325. 10.1121/1.3268611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala, C., and Dhar, S. (2012). “Maturation and aging of the human cochlea: A view through the DPOAE looking glass,” J. Assoc. Res. Otolaryngol. 13, 403–421. 10.1007/s10162-012-0319-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala, C., and Keefe, D. (2006). “Effects of middle-ear immaturity on distortion product otoacoustic emission suppression tuning in infant ears,” J. Acoust. Soc. Am. 120, 3832–3842. 10.1121/1.2359237 [DOI] [PubMed] [Google Scholar]
- Abdala, C., Ma, E., and Sininger, Y. S. (1999). “Maturation of medial efferent system function in humans,” J. Acoust. Soc. Am. 105, 2392–2402. 10.1121/1.426844 [DOI] [PubMed] [Google Scholar]
- Abdala, C., Mishra, S. K., and Williams, T. L. (2009). “Considering distortion product otoacoustic emission fine structure in measurements of the medial olivocochlear reflex,” J. Acoust. Soc. Am. 125, 1584–1594. 10.1121/1.3068442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel, C., Wittekindt, A., and Kossl, M. (2009). “Contralateral acoustic stimulation modulates low-frequency biasing of DPOAE: Efferent influence on cochlear amplifier operating state?” J. Neurophysiol. 101, 2362–2371. 10.1152/jn.00026.2009 [DOI] [PubMed] [Google Scholar]
- Chabert, R., Guitton, M. J., Amran, D., Uziel, A., Pujol, R., Lallemant, J. G., and Puel, J. L. (2006). “Early maturation of evoked otoacoustic emissions and medial olivocochlear reflex in preterm neonates,” Pediatr. Res. 59, 305–308. 10.1203/01.pdr.0000196739.16060.0a [DOI] [PubMed] [Google Scholar]
- Cleveland, W. S. (1993). Visualizing Data (Hobart Press, Summit, NJ: ), pp. 88–101. [Google Scholar]
- Deeter, R., Abel, R., Calandruccio, L., and Dhar, S. (2009). “Contralateral acoustic stimulation alters the magnitude and phase of distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 126, 2413–2424. 10.1121/1.3224716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney, M. P., Keefe, D. H., and Marryott, L. P. (2003). “Contralateral acoustic reflex thresholds for tonal activators using WBR and admittance measurements,” J. Speech Language Hearing Res. 46, 128–136. 10.1044/1092-4388(2003/010) [DOI] [PubMed] [Google Scholar]
- Francis, N. A., and Guinan, J. J., Jr. (2010). “Acoustic stimulation of human medial olivocochlear efferents reduces stimulus-frequency and click-evoked otoacoustic emission delays: Implications for cochlear filter bandwidths,” Hear. Res. 267, 36–45. 10.1016/j.heares.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkoritsa, E., Korres, S., Segas, I., Xenelis, I., Apostolopoulos, N., and Ferekidis, E. (2007). “Maturation of the auditory system: 2. Transient otoacoustic emission suppression as an index of the medial olivocochlear bundle maturation,” Int. J. Audiol. 46, 277–286. 10.1080/14992020701261405 [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Jr. (1996). “The physiology of olivocochlear efferents,” in The Cochlea, edited by Dallos P. J., Popper A. N., and Fay R. R. (Springer-Verlag, New York), pp. 435–502. [Google Scholar]
- Guinan, J. J., Jr. (2010). “Cochlear efferent innervation and function,” Curr. Opin. Otolaryngol. Head Neck Surg. 18, 447–453. 10.1097/MOO.0b013e32833e05d6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan, J. J., Jr., Backus, B. C., Lilaonitkul, W., and Aharonson, V. (2003). “Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs,” J. Assoc. Res. Otolaryngol. 4, 521–540. 10.1007/s10162-002-3037-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger, A., Ari-Even Roth, D., Muchnik, C., Kuint, J., and Hildesheimer, M. (1998). “Contralateral acoustic effect of transient evoked otoacoustic emissions in neonates,” Int. Tinnitus J. 4, 53–57. [PubMed] [Google Scholar]
- Henin, S., Thompson, S., Abdelrazeq, S., and Long, G. R. (2011). “Changes in amplitude and phase of distortion-product otoacoustic emission fine-structure and separated components during efferent activation,” J. Acoust. Soc. Am. 129, 2068–2079. 10.1121/1.3543945 [DOI] [PubMed] [Google Scholar]
- Jenkins, J. J., McGee, J., and Walsh, E. J. (1993). “Developmental changes of auditory nerve responses to efferent stimulation,” Soc. Neurosci. Abstr. 23, 534. [Google Scholar]
- Kalluri, R., and Shera, C. A. (2001). “Distortion-product source unmixing: A test of the two-mechanism model for DPOAE generation,” J. Acoust. Soc. Am. 109, 622–637. 10.1121/1.1334597 [DOI] [PubMed] [Google Scholar]
- Kalluri, R., and Shera, C. A. (2007). “Near equivalence of human click-evoked and stimulus frequency emissions,” J. Acoust. Soc. Am. 121, 2097–2110. 10.1121/1.2435981 [DOI] [PubMed] [Google Scholar]
- Kawase, T., Delgutte, B., and Liberman, M. C. (1993). “Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones,” J. Neurophysiol. 70, 2533–2549. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., Fitzpatrick, D., Liu, Y. W., Sanford, C. A., and Gorga, M. P. (2010). “Wideband acoustic-reflex test in a test battery to predict middle-ear dysfunction,” Hear Res. 263, 52–65. 10.1016/j.heares.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, D. H., Folsom, R. C., Gorga, M. P., Vohr, B. R., Bulen, J. C., and Norton, S. J. (2000). “Identification of neonatal hearing impairment: Ear-canal measurements of acoustic admittance and reflectance in neonates,” Ear Hear. 21, 443–461. 10.1097/00003446-200010000-00009 [DOI] [PubMed] [Google Scholar]
- Kim, D. O. (1980). “Cochlear mechanics: Implications of electrophysiological and acoustical observations,” Hear. Res. 2, 297–317. 10.1016/0378-5955(80)90064-7 [DOI] [PubMed] [Google Scholar]
- Knight, R. D., and Kemp, D. T. (2001). “Wave and place fixed DPOAE maps of the human ear,” J. Acoust. Soc. Am. 109, 1513–1525. 10.1121/1.1354197 [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard, M., and Pujol, R. (1988). “Hair cell innervation in the fetal human cochlea,” Acta Otolaryngol. 105, 398–402. 10.3109/00016488809119492 [DOI] [PubMed] [Google Scholar]
- Lee, J., Dhar, S., Abel, R., Banakis, R., Grolley, E., Lee, J., Zecker. S., and Siegel, J. (2012). “Behavioral hearing thresholds from 0.125 and 20 kHz using depth-compensated ear simulator calibration,” Ear Hear. 33, 315–329. 10.1097/AUD.0b013e31823d7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, G. R., Talmadge, C. L., and Lee, J. (2008). “Measuring distortion product otoacoustic emissions using continuously sweeping primaries,” J. Acoust. Soc. Am. 124, 1613–1626. 10.1121/1.2949505 [DOI] [PubMed] [Google Scholar]
- Maison, S. F., Luebke, A. E., Liberman, M. C., and Zuo, J. (2002). “Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells,” J. Neurosci. 22, 10838–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison, S. F., Vetter, D. E., and Liberman, M. C. (2007). “A novel effect of cochlear efferents: In vivo response enhancement does not require alpha9 cholinergic receptors,” J. Neurophysiol. 97, 3269–3278. 10.1152/jn.00067.2007 [DOI] [PubMed] [Google Scholar]
- Mauermann, M., and Kollmeir, B. (2004). “Distortion product otoacoustic emission (DPOAE) input/output functions and influence of the second DPOAE source,” J. Acoust. Soc. Am. 116(4), 2199–2212. 10.1121/1.1791719 [DOI] [PubMed] [Google Scholar]
- Moore, J. K., Simmons, D. D., and Guan, Y. (1999). “The human olivocochlear system: Organization and development,” Audiol. Neurootol. 4, 311–325. 10.1159/000013855 [DOI] [PubMed] [Google Scholar]
- Morlet, T., Collet, L., Salle, B., and Morgon, A. (1993). “Functional maturation of cochlear active mechanisms and of the medial olivocochlear system in humans,” Acta Otolaryngol. 113, 271–277. 10.3109/00016489309135808 [DOI] [PubMed] [Google Scholar]
- Morlet, T., Goforth, L., Hood, L. J., Ferber, C., Ducleaux, R., and Berlin, C. I. (1999). “Development of human cochlear active mechanism asymmetry: Involvement of the medial olivocochlear system?” Hear. Res. 134, 153–162. 10.1016/S0378-5955(99)00078-7 [DOI] [PubMed] [Google Scholar]
- Morlet, T., Hamburger, A., Kuint, J., Ari-Even Roth, D., Gartner, M., Muchnik, C., Collet, L., and Hildesheimer, M. (2004). “Assessment of medial olivocochlear system function in pre-term and full-term newborns using a rapid test of transient otoacoustic emissions,” Clin. Otolaryngol. Allied Sci. 29, 183–190. 10.1111/j.0307-7772.2004.00786.x [DOI] [PubMed] [Google Scholar]
- Pujol, R., Lavigne-Rebillard, M., and Lenoir, M. (1998). “Development of sensory and neural structures in the mammalian cochlea,” in Development of the Auditory System, edited by Rubel E., Popper A., and Fay R. (Springer, New York: ), pp. 146–192. [Google Scholar]
- Ryan, S., and Piron, J. P. (1994). “Functional maturation of the medial efferent olivocochlear system in human neonates,” Acta Otolaryngol. 114, 485–489. 10.3109/00016489409126091 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., and Guinan, J. J., Jr. (1999). “Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAE,” J. Acoust. Soc. Am. 105, 782–798. 10.1121/1.426948 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., Guinan, J. J.Jr., and Oxenham, A. J. (2010). “Otoacoustic estimation of cochlear tuning: Validation in the chinchilla,” J. Assoc. Res. Otolaryngol. 11, 343–365. 10.1007/s10162-010-0217-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera, C. A., Talmadge, C. L., and Tubis, A. (2000). “Interrelations among distortion-product phase-gradient delays: Their connection to scaling symmetry and its breaking,” J. Acoust. Soc. Am. 108, 2933–2948. 10.1121/1.1323234 [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Tubis, A., and Long, G. R. (1998). “Modeling otoacoustic emission and hearing threshold fine structure,” J. Acoust. Soc. Am. 104, 1517–1543. 10.1121/1.424364 [DOI] [PubMed] [Google Scholar]
- Walsh, E. J., McGee, J., McFadden, S. L., and Liberman, M. C. (1998). “Long-term effects of sectioning the olivocochlear bundle in neonatal cats,” J. Neurosci. 18, 3859–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig, G., and Shera, C. A. (1995). “The origin of periodicity in the spectrum of evoked otoacoustic emissions”, J. Acoust. Soc. Amer. 98, 2018–2047. 10.1121/1.413320 [DOI] [PubMed] [Google Scholar]