Abstract
Purpose
Compare gesture use in infants with autism to infants with other developmental disabilities (DD) or typical development (TD).
Method
Children with autism (n = 43), other DD (n = 30), and TD (n = 36) were recruited at ages 2 to 7 years. Parents provided home videotapes of children in infancy. Staff compiled video samples for two age intervals (9-12 and 15-18 months), and coded samples for frequency of social interaction (SI), behavior regulation (BR), and joint attention (JA) gestures.
Results
At 9-12 months, infants with autism were less likely to use JA gestures than infants with other DD or TD, and less likely to use BR gestures than infants with TD. At 15-18 months, infants with autism were less likely than infants with other DD to use SI or JA gestures, and less likely than infants with TD to use BR, SI, or JA gestures. Among infants able to use gestures, infants with autism used fewer BR gestures than those with TD at 9-12 months, and fewer JA gestures than infants with other DD or TD at 15-18 months.
Conclusions
Differences in gesture use in infancy have implications for early autism screening, assessment, and intervention.
Introduction
Autism is a neurodevelopmental disorder defined by impairments in social and communication development, as well as stereotyped patterns of behaviors and interests (American Psychiatric Association, 2000). Current understanding of autism indicates that individuals display variable symptoms of autism to differing degrees, which has led to the conceptualization of autism as a spectrum disorder. Recent prevalence estimates from a large, multisite study suggest that as many as 1 in every 88 children in the United States have an autism spectrum disorder (ASD; Centers for Disease Control, 2012). Concerns about autism prevalence have been largely responsible for efforts by the American Academy of Pediatrics to improve early identification of children at risk for autism (Johnson & Myers, 2007), but support for routine screening programs for autism is not unanimous given concerns about psychometric limitations of available instruments (e.g., Al-Qabandi, Gorter & Rosenbaum, 2011). Thus, better evidence related to developmental characteristics of infants later diagnosed with autism is needed not only to increase our foundational knowledge of the emergence of autism, but also to meet the public health goal of improving the accuracy of early autism screening tools. A promising area of investigation is the use of gestures in children with autism compared to children with typical development (TD) or with other developmental disabilities (DD). The study of the development and use of gestures has theoretical and clinical implications for both researchers and practitioners, as gestures are one of the most consistent early indicators of intentionality.
Development of gestures
Gestures emerge as early as 7 to 9 months in infants with TD (Crais, Douglas, & Campbell, 2004; Guidetti & Nicoladis, 2008; Iverson & Goldin-Meadow, 2005), as infants begin to intentionally communicate with their caregivers. For the purposes of this research, gestures are behaviors involving intentional movements interpretable by others as being for the purpose of communicating meaning. Early gestures can be classified into Bruner's (1981) three earliest functions of intentional communication: social interaction (SI), behavior regulation (BR), and joint attention (JA). SI gestures are used to direct another person's attention to oneself, for example, waving or using “so big” gestures, or bouncing up and down to indicate a desire to continue a “horsey” game of being bounced on the parent's knees. These types of gestures play a role in what is sometimes referred to as dyadic interactions, or interactions focusing on mutual attention between two people (Leekam, Lopez, & Moore, 2000). BR gestures are used to control another person's behavior, as seen in gestures such as pointing to request an object that is out of reach, pushing an object away to protest, or shaking one's head to indicate “No.” JA involves directing another person's attention to an event, object, or person solely to share interest and is “triadic” in the coordination of the gaze of communicative partners toward something else (Leekam et al., 2000). Examples of JA gestures are pointing to draw another person's attention to an airplane in the sky or holding up a toy to share interest with another person. These gestural functions (and forms) develop during the early years of life, with the 9 to 18 month age range marking a crucial time for emergence and refinement (Crais et al., 2004; Wetherby, Cain, Yonclas, & Walker, 1988). The focus of the current study is on the development and use of gestures for these three different functions in this age range. Classifying gestures into these categories depends on the perceived communicative purpose of each gesture rather than its form; for example, giving objects and pointing are two gesture forms that might serve different purposes (requesting or joint attention), and their intent must be interpreted within the specific context of each communicative act.
The earliest emerging gestures are ones used for protesting, a type of BR, (Crais et al. 2004; Carpenter, Mastergeorge & Coggins, 1983), and gestures for SI (Crais et al., 2004). For example, Crais et al. reported that pushing objects away and reaching to be picked up (i.e., actions used for behavior regulation purposes) emerged between 6 and 6.5 months in their sample, and waving in context (i.e., a gesture used for social interaction purposes) emerged at a mean age of 8.4 months. The first gestures used for JA functions emerged, on average, slightly later, between 9 and 9.5 months for giving objects and showing objects. However, the forms and functions of gestures interact with one another over the course of development. For example, although giving to share attention emerged at a mean age of just over 9 months, giving objects to request did not emerge until a mean age of almost 12 months among the infants in this study. In general, however, the available literature suggests that some gestures used for each of these three communicative functions are seen in most TD children by 12 months of age, and that increasingly more varied forms of gestures are used to communicate for each function between 12 and 18 months of age. In terms of frequency of communicative acts (not restricted to gestures) to communicate for different functions, Wetherby et al.(1988) found that, among prelinguistic toddlers in the 11 to 14 month age range, JA (“commenting”) acts were most frequent (49% of acts), followed by BR acts (36%) and SI acts (13%).
Gesture use in preschool and older children with autism
Both function and quantity are important considerations when characterizing gesture use in children with and without disabilities. Children with autism not only use fewer gestures than children with other DD or TD (Mundy, Sigman, & Kasari, 1990; Loveland, Landry, Hughes, Hall, & McEvoy, 1988), but also show different patterns of gesture use across the three communicative functions (Carpenter, Pennington, & Rogers, 2002; Wetherby & Prutting, 1984). Specifically, they show relative strength in the use of gestures for BR and weakness in the use of gestures for JA (Curcio, 1978; Loveland & Landry, 1986; Mundy et al., 1990). Additionally, despite social deficits being a central feature of autism, children with autism do not appear to have the same degree of difficulty in their use of gestures for SI (e.g., social routines and games) as they do for JA (McEvoy, Rogers, & Pennington, 1993; Mundy, Sigman, Ungerer, & Sherman, 1986). Thus, a distinct pattern of gesture use is established by preschool age among children with autism, characterized by relatively low frequencies of communicative gestures overall, and a particularly low occurrence of gestures used for the function of JA. The earlier developmental trajectories leading to this pattern of gesture use, however, are not as well understood.
Gesture development in infants and toddlers with autism
The findings on gesture development in infants and toddlers with autism correspond in some ways to findings with older children; however, some inconsistencies in results related to very young children indicate gaps in knowledge about the earliest phases of gesture development. Compared with TD infants, infants with autism have been found to demonstrate deficits in gesture use by the end of the first year of life, including their inventory of gestures (Mitchell et al., 2006), frequency of JA gestures (Osterling & Dawson, 1994), and variety of SI gestures (Colgan et al., 2006).
Most studies of gestures used by infants with autism around the end of the first year have not explicitly categorized gestures into Bruner's (1981) functions of SI, BR, and JA; thus, the results are more difficult to interpret within this framework. For example, Osterling and Dawson (1994) coded behaviors in the categories of social, affective, joint attention, and communication. Gestures other than JA gestures were included in the communication category (along with nongesture behaviors such as babbling and word use). These authors reported that 12-month-olds with autism produced fewer JA gestures such as pointing and showing when compared to children with TD; however, these authors also included “vague pointing,” which they described as “reaching for something in a communicative way,” within JA behaviors. This description could apply to pointing to request as well as pointing to share attention; within Bruner's framework, pointing to request would not be for the function of JA. Either way, no children with autism in the study exhibited pointing. Osterling, Dawson & Munson (2002) included coding categories similar to those in the earlier study; showing and pointing gestures were so infrequent across all groups (autism, other DD, and TD) that they could not be analyzed. BR gestures, as conceptualized in Bruner's taxonomy, were not included in the coding system used by Osterling, Dawson and colleagues. Moreover, the social behaviors they analyzed included looking at people's faces, looking and smiling, imitating an adult, and engaging in a social game such as “peek-a-boo,” but conventional social gestures such as waving were considered within “communicative” behaviors. Thus, their coding system makes it difficult to draw conclusions about the early development of gestures for different functions across groups of infants. In a prospective study of siblings of children with autism, Mitchell et al. (2006) also did not categorize gestures by function; rather, they focused on “early” and “late” gestures as identified on the MacArthur Communicative Development Inventory-Words & Gestures (Fenson et al., 1993). From Bruner's framework, early gestures would include those used for JA (e.g., showing, giving, pointing), BR (e.g., reaching to be picked up), and gestures used in social games (e.g., peekaboo). Late gestures assessed on the MacArthur Communicative Development Inventory include recognitory (e.g., showing functions of objects such as eating with a spoon) and symbolic play (e.g., pretend) behaviors. Mitchell et al. found that the 12-month-old siblings with ASD produced fewer early and late gestures than the non-ASD siblings or controls. Rozga et al. (2011) recently reported on the use of gestures for different functions at 12 months of age for infant siblings of children with autism who themselves were eventually diagnosed with autism (“affected siblings”), compared to unaffected infant siblings and infants of siblings without autism. They found marginally significant results for JA gestures, with affected siblings using fewer than the other two groups, and significant results for BR gestures with the affected siblings again using fewer than the other two groups. This study did not include a comparison group of children with other DD.
Thus, gestural differences exhibited by infants with autism are salient in comparison to infants with TD; however, the extent to which infants with autism differ from infants with other DD in gesture use at the end of the first year is less clear. One retrospective parent report study (Watson et al., 2007) and one retrospective study of home videos of infants later diagnosed with autism or other DD (Osterling et al., 2002), failed to find differences between 12-month-old infants with autism and those with other DD in the use of gestures. Thus, infants with autism and infants with other DD may have differing trajectories of gesture development, such that they are not consistently distinguished from one another in gesture use at 12 months, but differ in gesture use by the time they reach preschool age. On the other hand, these prior studies are limited by a failure to consider an array of possible gestures for the three major functions of BR, SI and JA.
More findings are available on the use of gestures for different functions by infants with and without autism in the second half of the second year of life. One retrospective study of home videos reported no differences between groups of infants with autism, other DD, and TD in the 16- to 19-month range for social gestures, BR gestures, or pointing for JA, but less use of showing objects for JA by infants with autism than infants in either of the comparison groups (Clifford, Young & Williamson, 2007). Another study (Wetherby, Watt, Morgan & Shumway, 2007) examined the rate of intentional communication (which could include gestures, vocalizations, and verbalizations) for different functions by infants with autism, other DD, and TD in the 18- to 24-month age range. Infants with autism communicated less often for all three functions than infants with TD, but only diverged significantly from infants with other DD in communicating less for JA. In a different analysis of the same infants, Shumway and Wetherby (2009) found that infants with autism produced a greater proportion of their communication for SI and BR than infants with TD, and differed from infants with both other DD and TD by showing a smaller proportion of communication for JA. Taken together, these studies suggest that infants with autism diverge more clearly from infants with other DD during the latter half of the second year of life in their use of JA. Moreover, despite the robustness of findings evidencing relative strengths in BR gestures versus weaknesses in JA gestures in children with autism by early preschool age, we know little about the earlier developmental trajectories of the three primary functions of gesture use among infants with autism. Further research specifically examining trajectories of gesture development for different communicative functions from the end of the first year into the second year could clarify the course of early social-communicative development in infants with autism as compared to those with other DD (Saint-Georges et al., 2010) and TD. Clearer understanding of these trajectories has clinical implications for early screening and assessment of infants with autism, and interventions to best facilitate social-communicative development in this population.
Framework and purpose of the current study
Using retrospective video analysis methodology, this study compares early (i.e., 9-12 months, 15-18 months) patterns of gesture use in infants later diagnosed with autism (referred to as “infants with autism”) to patterns in infants with other DD and with TD. Patterns of gesture use across the communicative functions of SI, BR, and JA are examined. Inclusion of infants with other DD is essential to examine whether patterns of early gesture use in infants with autism are specific indicators of risk for an eventual diagnosis of autism, or are merely more generally indicative of risk for developmental problems. No previous study of which we are aware has included a comparison group of infants with other DD for an in-depth analysis of the use of gesture functions by infants with autism across the 9- to 18-month age range. Examining the communicative functions separately rather than collapsing gestures across functional categories also has both conceptual and practical importance. Conceptually, due to the core social-communication deficits associated with autism (and not with other DD), we would expect that gestures founded on social reciprocity and social motivation—that is, SI and JA gestures— would be used less frequently by infants with autism compared to infants in both other groups. In contrast, we hypothesized that although both infants with autism and infants with other DD might show developmental delays compared to the TD group in their use of BR gestures, they would not differ from one another. Our conceptual model also acknowledges the heterogeneity among children with autism; thus, we assume that some infants with autism will not use gestures for different communicative functions in the age ranges studied, whereas other infants with autism will. We hypothesized, however, that even infants with autism who use communicative gestures will show quantitative differences from children in the other two groups in the early use of gestures for the functions of SI and JA.
This study addresses the following questions: (1) Do infants with autism exhibit differences in the quantity and functions of communicative gesture use compared to infants with other DD or TD at 9-12 months and/or 15-18 months? and (2) What are the developmental trajectories for gesture use from 9-12 months of age to 15-18 months of age across the three groups of infants with respect to SI, BR, and JA gesture functions?
Method
Participants
The participants were recruited from two different geographic settings (i.e., Midwest and Southeast) across a 15-year time period. Recruitment criteria included: (1) child age between tw and seven years at the time of recruitment; (2) available home videotapes of the child between birth and two years of age that parents were willing to share; and (3) enough video footage for at least one 5-minute codable segment (see video editing section below) of the child at either 9-12 or 15-18 months of age. Infants with significant physical, visual, or hearing impairments, as wel as infants with known genetic conditions often associated with autism (i.e., fragile X, tuberous sclerosis, and Rett's syndrome) were excluded from the sample.
Recruitment and diagnostic screening
Participants were recruited through various methods, including: information booths at conferences; mailings to childcare centers, developmental evaluation centers, and early intervention programs; advertisements; collaboration with hospital-based clinics; and research registries. Potential participants were screened to include only those meeting confirmed diagnostic criteria for one of three groups: autism, other DD, and TD. Participants in the autism group had a clinical diagnosis of autism made by a licensed psychologist and/or physician, usually as part of a multidisciplinary team evaluation in their local communities. Trained research staff confirmed autism diagnoses using at least one of the following tools: the Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Renner, 1992), the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, &Risi, 1999) and/or the Autism Diagnostic Interview-Revised (ADI-R; Rutter, LeCouteur, &Lord, 2003). The CARS, a caregiver-report rating scale used to confirm autism diagnosis, is a reliable and valid measure that was commonly used before the development of current gold standard diagnostic measures, but in more recent studies has continued to demonstrate good concurrent validity with both clinical judgment and the results of the ADOS and ADI-R (Kleinman et al., 2008; Perry, Condillac, Freeman, Dunn-Geier &Belair, 2005). The ADOS and ADI-R are considered gold standard diagnostic tools, and are based on diagnostic criteria for autism specified by the American Psychiatric Association (2000).
Participants in the other DD group met one of the following criteria: (1) diagnosis of intellectual disability associated with a genetic syndrome (e.g., Down syndrome); (2) intellectual disability of an idiopathic or nonspecific nature as documented by cognitive scores of at least two standard deviations below the mean on a standardized test of cognitive functioning; or (3) a score of at least 1.5 standard deviations below the mean in at least two of these areas of development: expressive language, receptive language, cognitive or visual reception, fine or gross motor, and/or adaptive behavior. In addition, a CARS score below 25 (i.e., five points below the cutoff score for autism) was an inclusion criterion for the other DD group.
Participants in the TD group attained adaptive behavior scores (on the Vineland Adaptive Behavior Scales (VABS); Sparrow, Balla, &Cicchetti, 1984) or cognitive development scores (on the Mullen Scales of Early Learning; Mullen, 1995) of no more than one standard deviation below the mean and had no parental report of history of learning or developmental difficulties. They were also given the CARS to rule out symptoms of autism.
The final sample for this study included 109 participants: 43 in the AU group, 30 in the DD group, and 36 in the TD group. There were 75 boys (AU=38, DD=13, and TD=24) and 34 girls (AU=5, DD=17, TD=12). See Tables 1 and 2 for participant information pertaining to race standard scores on the VABS (Communication Scale and Adaptive Behavior Composite) at the time of recruitment, and parent education. Ethnicity information was not gathered for all participants due to differences in demographic questionnaires used over the 15 years of data collection; however, ethnicity data that was collected indicated four Hispanic participants, two the AU group, one in the DD group, and one in the TD group.
Table 1. Demographics and Vineland Adaptive Behavior Scales (VABS) Scores.
| Group | Race | CA Mean (SD) | VABS Comm Mean (SD) | VABS ABC Mean (SD) | ||||
|---|---|---|---|---|---|---|---|---|
| White | African American | Asian | Multiracial | Other | ||||
| AU n=43 |
36 | 5 | 0 | 1 | 1 | 53.4 (18.4) |
66.4 (21.4) |
62.1 (14.9) |
| DD n=30 |
29 | 0 | 1 | 0 | 0 | 57.7 (31.2) |
75.8 (16.4) |
70.7 (14.2) |
| TD n=36 |
33 | 1 | 1 | 1 | 0 | 45.2 (20.4) |
107.9 (9.5) |
103.9 (9.0) |
Note: CA = Chronological age in months at recruitment; VABS Comm = standard score on the Communication Scale of the VABS; VABS ABC = Adaptive Behavior Composite standard score of the VABS
Table 2. Highest Level of Parent Education.
| Group | Parent Education (M=mother, F=father) | |||||
|---|---|---|---|---|---|---|
| No High School Diploma or equivalent | High School or equivalent | Associate's degree or some college | Bachelor's degree | Beyond Bachelor's degree | Not Reported | |
| AU | M=0 (0%) F=0 (0%) |
M=3 (9%) F=7 (21%) |
M=8 (25%) F=4 (13%) |
M=14 (44%) F=11 (34%) |
M=6 (14%) F=9 (28%) |
M=1 (3%) F=1 (3%) |
| DD | M=0 (0%) F=0 (0%) |
M=0 (0%) F=2 (13%) |
M=1 (7%) F=2 (13%) |
M=6 (40%) F=7 (47%) |
M=8 (53%) F=3 (20%) |
M=0 (0%) F=1 (7%) |
| TD | M=1 (5%) F=0 (0%) |
M=1 (5%) F=1 (5%) |
M=0 (0%) F=1 (5%) |
M=8 (38%) F=6 (32%) |
M=11 (52%) F=11 (58%) |
M=0 (0%) F=0 (0%) |
Note: Parent education data were not collected for 45 subjects in the Midwest sample
Retrospective video analysis
The method used in the current investigation is retrospective video analysis. Retrospective video analysis applied to the study of autism involves examining infant behavior captured in home videos of infants later diagnosed with autism, usually recorded before the parents were aware of the diagnosis. This methodology has been used in previous examinations of development in infants later diagnosed with autism (e.g., Baranek, 1999; Clifford et al., 2007 Osterling &Dawson, 1994). Retrospective video analysis permits the study of infants with autism who are not recruited on the basis of genetic liability for autism (and thus allows study o a different subpopulation than research on infants recruited due to having an older sibling with ASD). For this study, the method also provided an opportunity to study infant gesture use captured in natural environments with familiar caregivers.
Video editing procedures
Participating families were asked to provide all videotapes containing footage of their child at age 24 months or younger. The tapes included footage from family play situations, vacations, outings, special events, and familiar routines (e.g., mealtimes), with individual variation in exact content of each family's tapes, as would be expected in home videotapes. All tapes were copied and originals were returned to participating families.
The 9-12 months age range was chosen as the first time point for analysis because it (a) represents all participants prior to the development of substantial expressive language, and (b) is the age of typical emergence for a number of play and communicative behaviors of interest in our research program. The 15-18 months age range was chosen as the second time point because it (a) is an age at which the play and communicative behaviors emerging at 9-12 months are more consistently and frequently exhibited in typical development, and (b) is just prior to the age at which autism screening tools have been successfully used, allowing us to examine development across an interval of time for which limited developmental information is available, especially for infants with autism who do not have an older sibling diagnosed with autism.
Once videotapes were copied, they were “content coded”. For this process, staff reviewed and logged videos according to the child's chronological age during each scene, the time meter reading demarking the beginning and end of each different scene, the minutes of videotape at that age, a description of the situation recorded in the scene, and codes for number of people, level of structure in social interactions (e.g., amount of physical/verbal directives, adult's proximity to child), and physical restrictions (e.g., being in a high chair). More details on content coding are provided in Table 3. Chronological ages were calculated by full months based on the child's birth date, and specific dates recorded on the tapes or parental confirmation of child age during each scene. [Note: For participants born prematurely, corrected chronological ages were calculated by adjusting age for the amount of prematurity]. Any scenes for which parents were unable to verify the child's age within one month were discarded. (Content coding manual is available from the authors.)
Table 3. Content Coding Definitions and Codes.
| CODING CATEGORY | CODE DEFINITIONS |
|---|---|
| # Persons | Number of people (adults & children) in scene, including person at camera and excluding the focal child |
| Social/Nonsocial/Both |
Social: verbal and/or physical interactions with other persons, including children Nonsocial: Engagement with or availability of nonsocial activity, such as toys or other objects Both: At least minimal verbal or social interactions with a person, and objects/toys/nonsocial activities occurring in same situation |
| Physical Restriction | The amount of physical containment or confinement (lack of freedom to move) the child is placed High = physically contained/confined by equipment or another person in such as way that s/he has little freedom of movement (e.g., car seat, high chair, bundled in blanket) more than 50% of time in scene Medium = being held with some support but still has freedom to move around (e.g., bath tub, play pen, held loosely in lap) more than 50% of the time in scene, or highly restricted 10-50% of time in scene Low = Child has almost complete freedom to move about if s/he chooses to/has the ability to do so (e.g., on floor without support, sitting on someone's lap as though on a chair without being held or confined by person; or higher levels of restriction occur below levels for coding at High or Medium |
| Structure | The amount of social intrusion or behavioral strategies that the adult or older child is using to engage or elicit a response/interaction from the child. High = structuring behaviors are provided with moderate to high intensity and occur at least 50% of the time during the scene (e.g., adult motors child through an interaction, physically tickles child while playing peek-a-boo) Medium = structuring behaviors are provided with at least moderate intensity and more than 25% of time, or with high intensity but between 10 and 50% of the time. Low = structuring behaviors are provided with low intensity, or with moderate intensity < 25% of time or high intensity <10% of the time. |
One challenge in retrospective video analysis is the variability in amount and content of footage as captured by different families. To address these challenges, this study edited video footage provided by families to standardize the amount of footage and allow for group comparisons of content. Research assistants blind to the research questions and groups edited the videotapes. They were instructed to randomly select a cross-section of scenes from the available footage in the designated age ranges, selecting only scenes in which the child was visible and for which the child's age was documented. Further instructions were to select scenes for all available ages within the respective age intervals (9-12 months, 15-18 months), and to avoid editing scenes in chronological order within the age intervals. Finally, the editors were instructed to select a cross-section of the available situational events (e.g., bath time, meal time, active play, family celebration). The targeted length for each edited clip was around one minute, but editors had latitude to adjust the length of the clip in order to meet other editing goals, such as including all ages when the only usable footage at a given age was shorter than a minute, or avoiding footage when the child was not visible. The aim was to assemble two different five-minute compilations of edited clips (a total of 10 minutes) at each age range for each participant; however, a single five-minute segment was compiled in an age range if there was insufficient footage to create two segments. At 9-12 months, data were available for 40 infants in the autism group, 23 in the other DD group, and 35 in the TD group (n=98). At 15-18 months, data were available for 27 infants in the autism group, 16 in the other DD group, and 16 in the TD group (n=59). Two complete 5-minute segments, or a total of 10 minutes of edited video, were available for 88 infants (89.78%) at 9-12 months, and 47 infants (79.6%) at 15-18 months. Fifty infants had data at both time points (autism=25, DD=9, TD=16).
Content coding categories across the 300 seconds per edited video were added and used to calculate an average score per second. We compared the content coding averages for edited video segments across groups and found no differences in participants' mean age per segment at either 9-12 months [F(2,94) = 2.11, p = .13] or 15-18 months [F(2,53) = .25, p = .78]. The mean age (SD) in months for each group in the 9-12 month interval was 10.7 (.72) for infants with autism, 11.0 (.71) for infants with other DD, and 10.7 (.71) for infants with TD. At the 15-18 month interval, the corresponding values were 16.4 (.78) months for infants with autism, 16.2 (.89) months for infants with other DD, and 16.3 (.74) months for infants with TD. No group differences emerged in the mean level of imposed structure used in engaging the child in interaction at 9-12 months [F(2,94) = .16, p = .85], mean number of persons at either 9-12 months [F(2,94) = 2.86, p =.062] or 15-18 months [F(2,53) = .11, p =.89], or mean amount of physical restriction at 15-18 months [F(2,53) = .23, p = .80]. Group differences were found in mean level of imposed structure used in engaging the child in interaction per segment at 15-18 months [F(2,53) = 6.32, p < .01], with the autism group showing significantly less structure (i.e., medium level) imposed on their social interactions than the other two groups (i.e., medium-high level). Additionally, differences were found in mean amount of physical restriction at 9-12 months [F(2,94) = 5.98, p < .01], with the other DD group showing significantly more physical restriction than the TD group. These differences will be discussed in relation to the results.
Dependent variables
The dependent variables for the current study included frequency of infants' gesture use in the three different categories of communicative function. The research team adapted a form developed by Wetherby and Prizant (1993) to create a coding checklist for communicative gestures applicable to videotapes made by caregivers rather than videotapes of gesture behaviors occurring in a standardized context. The adaptation of the checklist involved revising the list of potential gestures to reflect behaviors seen across multiple children in preliminary, open-ended descriptions of gesture behaviors observed in a subset of the home videos used in this study (checklist available from authors upon request). Following the procedures of Wetherby and Prizant, coders used the checklist to make three judgments regarding infants' behaviors: (a) Was the behavior among those listed as potential gestures on the checklist? These included behaviors such as reaching, pointing, nodding or shaking head, clapping, enactive gestures (such as bouncing one's body up and down to enact the movement experienced in a “horsey” game with a parent), and movements specific to conventional games, such as arms up for “so big,” or covering eyes for “peek-a-boo.” (b) Was there evidence that the child was directing the behavior to another person? and (c) Did the behavior serve a communicative function of SI, BR, or JA? An additional coding rule was that gestures could be either initiated by the child or elicited by another person (e.g., “Wave byebye”), but were not counted if parents physically prompted the child to do the gesture movement. If the behavior met criteria for all three questions, the coder recorded the time when the gesture behavior was observed, the type of gesture behavior (e.g., reaching, clapping), the evidence that it was directed to another person (e.g., eye contact, vocalization), and the function served by the gesture.
Video coding procedures
Research assistants blind to both the hypotheses of the study and to the diagnostic group membership of the infants coded the infants' gestures in the videotapes. Coders first completed a manualized training program on coding gesture behaviors, using video segments not included in the analyses for this investigation for practice and reliability. Coders met with a trainer hroughout the process to discuss general coding procedures and disagreements between the coder and the master codes for training tapes.
Two trained coders independently coded all gestures in each segment. Reliability between coders across video segments was estimated with Type A intraclass correlations (ICCs), which use an absolute agreement definition. ICCs for independently coded gestures (average measures) at 9-12 months were .74 (95% CI = .61 -.83) for SI, .79 (95% CI = .68 - .86) for BR, and .78 (95% CI = .67 - .85) for JA; corresponding values at 15-18 months were .95 (95% CI = .91 - .97) for SI, .77 (95% CI = .60 -. .87) for BR, and .82 (95% CI = .68 - .90) for JA (all ps < .001). The ICCs were impacted by the frequencies of occurrence of different gestures, with lower overall frequencies being associated with lower ICCs. Since the ICC estimates were lower than the desired .80 for four of six of the outcomes to be compared, the best possible integrity of the final data was ensured by the use of a consensus process across all videos. Thus, coders met with each other to compare codes and to reach consensus on all cases of disagreement on either the occurrence of a gesture or on the communicative function served by a gesture. The ICCs for data from the two independent coders and the consensus codes (average measures) ranged from .88 to .93 across the three gesture functions and two time points (all ps < .001). Consensus codes were used in all data analyses.
Data analysis
The above coding procedures yielded count data for gesture use in three categories of communicative functions. We first dichotomized the groups based on the observed count data, and used chi-square analyses to compare the proportion of infants in each group with no observed gestures to infants with one or more observed gestures. The chi-square analyses permitted statistical tests of whether the proportions of infants with no observed gestures were significantly different across the groups. Due to the ordinal nature of the two categories (i.e., the “no gestures” category represents a lower value than the “one or more gestures” category), the significance test for linear-by-linear association was used. To fully examine the comparative use of gestures for different communicative functions across groups, however, the chi-square analyses were insufficient and another analysis method was needed.
Using a zero-inflated Poisson (ZIP) regression model (Atkins & Gallop, 2007) permitted us to examine the data more fully. Low probability count data like these gesture data are appropriately modeled as Poisson distributions, a probability distribution used for event occurrences in fixed periods of time. Use of a ZIP model is appropriate when each group is assumed to be made up of two subpopulations, with one subpopulation for which all the values for the variable of interest are zero, and the other subpopulation having a Poisson distribution (non-zero), with count data for the variable collected during a finite period of time.
Our conceptual model and data fit well with the assumptions of the ZIP model. Our data are characterized by a large number of zeros (i.e., no gestures for a certain function observed at a particular time point). We assume some of these zeros occurred because children did not have gestures for a particular function in their current repertoire, and other zeros occurred because we observed for a finite period of time and missed gestures that would have been observed in a longer time sample. The ZIP model used the distribution of the observed count data to provide estimated proportions of the total populations (infants with autism, other DD, or TD) that would be in each subpopulation for each behavior at each time point. Therefore, the non-zero subpopulation is the estimated proportion of infants who are able to use a particular gesture function at a given time point, and thus presumably would be observed to do so given sufficient observation time. The zero subpopulation is the estimated proportion of infants who are unable to use a particular gesture type at a given time point, and thus presumably would not be observed to do so even with additional observation time. In this article, we will refer to the non-zero subpopulation as the “gesturing” subpopulation or the children “able to use gestures,” and the zero subpopulation as the “non-gesturing” subpopulation or the children “unable to use gestures.” The total population, then, is the combination of these two estimated subpopulations. Beyond providing estimates of proportions of infants in the gesturing versus non-gesturing subpopulations, the ZIP model allowed the comparison of the gesturing subpopulations of infants to identify whether quantitative differences distinguish among the subpopulations of infants able to use gestures. The ZIP model examined each of the three groups at each of the two time points. Using a repeated measures analysis procedure provided by the STATA software package, we adjusted the standard errors for multiple observations within subjects. Evidence that ZIP models show limited biases related to small sample size (Schwartz &Giles, 2011) further supported the selection of this approach to analyzing our data.
Results
Analyses for Research Question 1: Group comparisons of the quantity and functions of communicative gestures at 9-12 months and 15-18 months
We first examined chi-square analysis results for each gesture function at each age point, for a total of six omnibus analyses (see Table 1). At 9-12 months, results indicated significantly different distributions across groups of proportions of infants with no observed gestures versus proportions of infants with one or more observed gestures for both BR gestures and JA gestures, but not for SI gestures. Planned contrasts indicated that for BR gestures, infants with autism did not differ from infants with other DD, but were less likely than infants with TD to use one or more gestures. For JA gestures, infants with autism were less likely than infants with other DD or TD to use one or more gestures. At 15-18 months, results indicated significantly different distributions of gesture use across groups for all three functions. Planned contrasts indicated that infants with autism did not differ from infants with other DD in their likelihood of using any BR gestures, but were less likely than infants with TD to use any BR gestures. Infants with autism were less likely than infants with other DD or TD to use any gestures for the functions of SI or JA. Odds ratios are included in Table 4 as a measure of effect size; these represent the probability that an infant in the other DD or TD group will use a gesture for each function relative to the probability that an infant with autism will use such a gesture (e.g., at 9-12 months, an infant with other DD is 1.39 times as likely to use an SI gesture as an infant with autism).
Table 4. Proportions of infants using gestures and chi-square results.
| 9-12 mos. | 15-18 mos. | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Omnibus DF = 2, N = 98 | Contrasts (χ2, p) | % using gesture | Omnibus DF = 2, N = 59 | Contrasts (χ2, p) | % using gesture | |
| SI | χ2 = 3.78 p= .052 |
DD vs. AU OR = 1.39 No contrasts due to non-significance TD vs. AU OR: 1.60 |
AU=37.5% DD=52.2% TD=60.0% |
χ2 = 5.63 p = .018 |
DD vs. AU (4.63, .031) OR=1.84 TD vs. AU (4.63, .031) OR=1.84 |
AU=40.7% DD=75.0% TD=75.0% |
|
|
|
|||||
| BR |
χ2 = 9.36 p = .002 |
DD vs. AU (1.10, .294) OR=1.55 TD vs. AU (9.32, .002) OR=2.54 |
AU=22.5% DD=34.8% TD=57.1% |
χ2 = 5.27 p = .022 |
DD vs. AU (0.19, .667) OR=1.18 TD vs. AU (5.66, .017) OR=2.03 |
AU=37.0% DD=43.8% TD=75.0% |
|
|
|
|||||
| JA |
χ2 = 13.51 p < .001 |
DD vs. AU (4.16, .041) OR=3.04 TD vs. AU (13.59, <.001) OR=4.86 |
AU=10.0% DD=30.4% TD=48.6% |
χ2 = 3.22 p < .001 |
DD vs. AU (6.82, .009) OR=2.25 TD vs. AU (11.57, .001) OR=2.63 |
AU=33.3% DD=75.0% TD=87.5% |
Key: SI = Social Interaction; BR = Behavior Regulation; JA = Joint Attention; OR = odds ratio
Note: The significance threshold was set at p < .05 with Bonferroni-Holm correction employed for multiple comparisons per time point. Significant findings are indicated in boldface type.
In order to test hypothesized group differences among the subpopulations of infants able to use some gestures at each time point, we next turned to the ZIP model. The ZIP model generated estimated proportions of gesturing infants in each group (i.e., autism, other DD, and TD) across each communicative function (i.e., SI, BR, and JA) at both 9-12 months and 15-18 months (see Table 5). The magnitude of adjustments made by modeling the data can be seen by comparing the estimated proportions in each subpopulation (Table 5) with the observed proportions (Table 4). The ZIP model estimates reflect the same patterns seen in the observed data in Table 4, but the estimates of proportions of infants in the gesturing subpopulations are consistently higher across all groups than the observed proportion of infants using gestures, reflecting the utility of the ZIP model in adjusting for sampling errors.
Table 5. Estimated percentages of gesturing (i.e., non-zero) subpopulations from ZIP model.
| 9-12 months | 15-18 months | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Gesture Type | AU | DD | TD | AU | DD | TD |
| SI | 35% | 58% | 66% | 46% | 68% | 75% |
| (n≈14.0) | (n≈13.3) | (n≈23.1) | (n≈12.4) | (n≈10.9) | (n≈12.0) | |
| BR | 38% | 53% | 62% | 52% | 67% | 74% |
| (n≈15.2) | (n≈12.2) | (n≈21.7) | (n≈14.0) | (n≈10.7) | (n≈11.8) | |
| JA | 13% | 43% | 58% | 45% | 81% | 89% |
| (n≈5.2) | (n≈9.9) | (n≈20.3) | (n≈12.2) | (n≈13.0) | (n≈14.2) | |
Key: SI = Social Interaction; BR = Behavior Regulation; JA = Joint Attention
Note: The ZIP model estimates to number of participants in a given sub-population, and therefore the estimates do not translate to actual numbers of participants, but theoretical numbers of participants. The theoretical numbers of participants in the gesturing subpopulations are estimated in the parenthetical statements below the estimated percentages. These are the numbers that were utilized for all analyses conducted with the ZIP model.
The ZIP model also provided estimates of the mean number of gestures in a 5-minute video segment for the gesturing subpopulations (see Table 6). Recall that for most infants, two 5- minute segments were available at each age point. We employed Wald's chi-square test to determine statistically significant differences across groups for the three gesture functions at 9-12 months and 15-18 months of age for the gesturing subpopulations (see Table 7). At 9-12 months, significant group differences were found in the mean number of BR gestures; infants in the autism gesturing subpopulation did not differ from infants in the other DD gesturing subpopulation, but used fewer gestures than infants in the TD gesturing subpopulation. At 15-18 months, significant group differences were found in the mean number of JA gestures; infants in the autism gesturing subpopulation used fewer JA gestures than did the TD and other DD gesturing subpopulations. Thus, the autism gesturing subpopulation was different from both comparison subpopulations (i.e., TD and other DD) in their use of JA gestures at 15-18 months.
Table 6. Mean gesture behaviors per 5-minute segment for gesturing subpopulations (estimated) and total populations (observed).
| Autism | DD | TD | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Type | Pop. | 9-12 mos. | 15-18 mos. | 9-12 mos. | 15-18 mos. | 9-12 mos. | 15-18 mos. |
| SI | Gest. | 2.873 | 2.489 | 3.768 | 5.000 | 2.217 | 2.904 |
| Total | 0.996 | 1.113 | 2.180 | 3.419 | 1.456 | 2.181 | |
| BR | Gest. | 0.464 | 0.865 | 0.874 | 0.627 | 2.361 | 1.639 |
| Total | 0.174 | 0.449 | 0.465 | 0.421 | 1.460 | 1.220 | |
| JA | Gest. | 1.061 | 0.696 | 0.964 | 2.286 | 1.408 | 2.652 |
| Total | 0.135 | 0.313 | 0.418 | 1.853 | 0.821 | 2.352 | |
Note: Pop. = population, Gest. = gesturing subpopulation, Total = total population
Table 7. Wald's chi-square results using ZIP model adjusted for repeated measures.
| 9-12 months | Contrasts (F(1, 108), p) | 15-18 months | Contrasts (F(1, 108), p) | |
|---|---|---|---|---|
| SI |
F(2,107) = .79 (p = .4555) |
No contrasts run due to non-significance |
F(2,107) = 2.46 (p = .0903) |
No contrasts run due to non-significance |
| BR |
F(2,107) = 10.69 (p = .0001) |
AU vs DD (2.15, .1451) AU vs TD (19.49, <.0001) |
F(2,107)= 2.24 (p =. 1115) |
No contrasts run due to non-significance |
| JA |
F(2,107) = .28 (p = .7565) |
No contrasts run due to non-significance |
F(2,107) = 5.54 (p = .0051) |
AU vs DD (5.13, .0256) AU vs TD (11.02, .0012) |
Note: The significance threshold was set at p < .05 with Bonferroni-Holm correction employed for multiple comparisons per time point
Analyses for Research Question 2: Developmental trajectories for gesture use from 9-12 months of age to 15-18 months of age
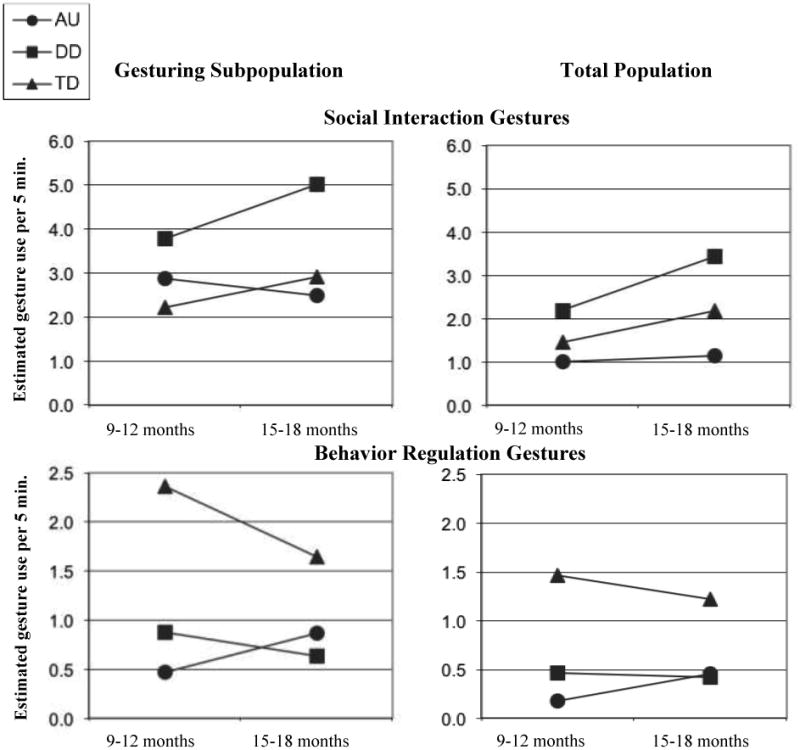
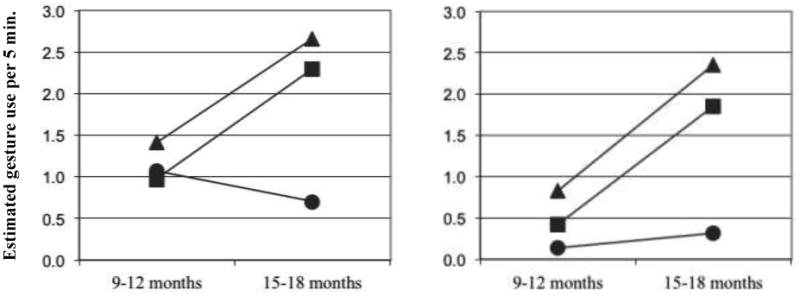
We used general linear regression with a ZIP model (as described above) to look at group-by-time interactions across the three gesture types. Analyses of group-by-time interactions were statistically non-significant for SI (F(2, 107) = .69, p = .51), BR (F(2, 107) = 1.48, p = .23), and JA (F(2, 107) = 1.63, p = .20) gestures. Despite non-significant findings, visual examination of the developmental patterns may inform future research. Therefore, Figure 1 presents trajectories by group, using estimated means for both the gesturing subpopulations and for the total (combined gesturing and non-gesturing) populations. These model-estimated total population means are derived by multiplying the estimated means for the gesturing subpopulation by the probability of being in that subpopulation (see Table 6).
Figure 1. Gesturing subpopulation and total population trajectories using model-estimated means.
Discussion
The results of this study further our understanding of the early development of gestures used for different communicative functions among infants with autism, other DD, and TD, especially in the group comparisons of gesture use at the two time points in the study. Ambiguities in our results, especially in answering the research question we posed about developmental trajectories, also raise interesting possibilities to be addressed in future studies.
Group comparisons of gestures for different communicative functions
Social interaction
The finding in the chi-square analyses of no significant group differences in the proportion of infants using at least one SI gesture at 9-12 months was contrary to our hypotheses (although the trend was in the predicted direction, as shown in Table 4). By 15-18 months, however, infants with autism were significantly less likely to use SI gestures than children in either of the other groups. The ZIP model adds the further unexpected finding that, among the gesturing subpopulations, infants with autism in the current study did not differ from infants with other DD or TD in their use of SI gestures at either 9-12 months or 15-18 months.
We anticipated that dyadic impairments would emerge very early and thus would be reflected in reduced use of SI gestures among infants with autism at 9-12 months, but this was not the case. Other studies have reported reduced dyadic orienting and interaction in infants with autism by 9-12 months compared with infants with other DD and/or infants with TD. For example, in retrospective video analyses, infants with autism showed less response to someone calling their names than infants with other DD or TD (Baranek, 1999, Osterling et al., 2002), and in a prospective study of infant siblings of children with ASD, Ozonoff et al. (2010) reported a declining trajectory in the frequency of gaze to faces, shared smiles, and vocalizations directed to others among infants who went on to get a diagnosis of ASD. The study by Baranek supported the idea that parents compensate (probably unconsciously in many cases) for more limited responsiveness of their infants, for example, by providing more prompts to get an infant to respond to his or her name. The home videos we analyzed for the current study provide many examples of caregivers explicitly teaching various SI gestures (e.g., gestures used in “so big” and “peek-a-boo”), for instance, by physically prompting their child to use the gesture. Also, parents repeatedly cue their children to use many routines that include SI gestures (e.g., “How big is Andrew?” “Bye-bye. Wave bye-bye”). So, similar to their persistence in getting an infant to respond to his or her name, parents may “work” to elicit SI gestures from their child. These types of representational gestures appear to be more influenced than other types of gestures by parental modeling (Crais et al., 2004; Zinober &Martlew, 1985). If parents of infants with autism intensify efforts to teach and elicit SI gestures in order to maintain interactions with their infants, this could result in greater use of SI gestures by infants with autism than might otherwise occur.
Behavior regulation
The patterns of results that emerged from the chi-square analyses and the ZIP model for BR gestures are consistent with our hypothesis that infants with autism would be similar to their counterparts with other DD, but less likely to use BR gestures than infants with TD at both 9-12 and 15-18 months. In fact, only 22.5% of infants with autism were observed to use even one BR gesture at 9-12 months. Further, the gesturing subpopulation of infants with autism used BR gestures less frequently than those with TD at 9-12 months. Thus, our study provides evidence for limited use of BR gestures by infants with autism at 9-12 months. At 15-18 months, however, the gesturing subpopulation of infants with autism was not distinguished from either infants with other DD or TD in terms of frequency of BR gestures, suggesting that by this age some infants with autism show the relative strengths in BR gestures reported for older children with autism (McEvoy et al., 1993; Mundy &Crowson, 1997).
A possible explanation for the early deficit in BR gestures is the varied complexity in BR gestures related to triadic interaction. For example, some BR gestures require coordinating attention between a partner and a desired object, location, or event (e.g., reaching for a toy while alternating gaze between a caregiver and the toy). One possibility is that the complexities of such triadic interactions, even for the function of BR, could explain the lag in the use of BR gestures for infants with autism. This proposal is consistent with the findings of Phillips and colleagues (Phillips, Gómez, Baron-Cohen, Laá & Rivière, 1995) that preschool children with autism were less likely to coordinate eye gaze with other requesting strategies than preschool children with other DD or toddlers with TD.
Joint attention
Our findings related to JA gestures support our hypotheses. In the chi-square analyses, infants with autism were less likely than infants in the other two groups to use JA gestures at both 9-12 and 15-18 months. As seen in Table 4, the odds ratios (effect sizes) were higher for JA gestures than for SI or BR gestures. Results from the ZIP model indicated that although infants with autism, other DD, or TD in the gesturing subpopulations did not differ significantly from one another at 9-12 months, by 15-18 months the infants with autism showed fewer JA gestures than gesturing subpopulations with other DD or TD. Thus, the results are consistent with a wealth of previous research indicating that impairments in JA among children with autism are widespread, and emerge as early as 9-12 months among at least some infants with autism (e.g., Mundy et al, 1990; Osterling et al, 2002). Further, these results add evidence that the use of JA gestures can help differentiate between infants with autism and infants with other DD at 9-12 months. The low overall frequency of occurrence of JA gestures among all infants at this early time point, however, suggests the importance of using assessment strategies that elicit JA acts from children as well as parent report in combination with direct observation.
Video content differences
A potential confounding factor related to differences in gesture use at 15-18 months is that in videos of infants with autism, slightly less structure was imposed by others on the infants when compared to the other two groups. This may represent random differences in scenes, partially accounting for decreased gesture use among infants with autism; however, the difference in video content also could reflect real-life differences across groups whereby the behavior of infants with autism may evoke different levels of structure from others in their environment (e.g., fewer attempts and less success in engaging the infant with autism).
Trajectories of Gesture Development
The ZIP model allowed examination of the group trajectories of gesture use in gesturing subpopulations of infants from 9-12 to 15-18 months of age. Examining these subpopulations, however, resulted in smaller sample sizes that reduced our power to detect differences. We will discuss briefly some tentative interpretations of the different trajectory patterns seen in visual examination of the graphs in Figure 1, because they may fuel later research questions. Interestingly, although the interactions between group and time period were statistically nonsignificant for each gesture function for the gesturing subpopulations, the trajectories of gesture development for each function for infants with autism were in the opposite direction of those for infants with other DD or TD. For JA and SI gestures, the slope for infants with autism was in a negative direction. This pattern may reflect the influences of regression in social-communication skills for some infants with autism across this age range, as reported by Ozonoff et al. (2010). In the graphs for total populations of infants (see the right-hand side of Figure 1), differences in the estimated mean numbers of gestures across groups appear more salient than for the gesturing subpopulations, but a contrast in slope is not as evident. A striking pattern in JA gestures for both the gesturing subpopulations and the total populations is that infants with other DD and TD show sharp increases in the frequency of use, whereas infants with autism do not.
Limitations
There are several limitations to this study. As is true in many research studies, our sample was a sample of convenience that reflected sampling biases, such as relatively high parent education levels. In addition, although the study included a comparison group of children with non-ASD developmental disabilities, this group was very heterogeneous. If a more homogeneous comparison group with other DD were used, their gesture development in infancy could be either more similar to children with autism or more different, depending on the characteristics of the chosen comparison group.
Retrospective video analysis provides ecologically valid samples of daily activity, but has limitations as well (see Palomo, Belinchon &Ozonoff, 2006 for further evaluation of the method). For example, the content of the videos varies; thus, although we used content coding to examine whether groups were well-matched on specific aspects of context, we could not hope to capture the full extent of contextual variations that may affect child communication.
An additional potential limitation in this study is that the edited video samples are relatively short, with 10 minutes of data at each time point for most participants, and only 5 minutes per time point for some participants. Although this amount of footage is comparable to that analyzed in several other retrospective video studies by other research teams (Clifford et al, 2007; Osterling &Dawson, 1994; Osterling et al., 2002), the short time frame may provide a limited snapshot of the gesture use of the participants, despite the inclusion of varied naturally occurring scenes in the edited video segments. This observational limitation is one reason we chose to use the ZIP model for data analysis, because the ZIP model adjusted for the probability that some children who were able to use gestures for different functions were not observed to use gestures in the limited sampling time we analyzed. Technical quality of home videos often limits the possible analyses as well. Both the video and audio quality are variable due to changing quality of recording equipment over the time period in which the home videos were made, the skill of the home videographer, and the context in which the recording was made.
Another potential methodological limitation is that coders used a consensus coding procedure for all data due to challenges in reaching high levels of point-by-point agreement with the coding system as they coded low frequency behaviors. Also, although a relatively large sample was utilized, the sample utilized for analysis in the ZIP regression model was much smaller because only gesturing subpopulations were used, limiting the power of the analyses. Thus, our findings may reflect some Type II errors in not detecting differences between groups. We would be particularly interested in comparing the trajectories of gesture development in a study with more power, given the differences in trend lines in this study.
In addition to considering the above limitations, the results of the present study should be interpreted in the context of several strengths of the methodology. First, the study has high ecological validity due to the naturalistic settings in which the retrospective video data were captured. More than 20 years ago, Wetherby (1986) advocated the study of the communication of children with autism in natural, familiar settings as most appropriate for understanding their communicative abilities. Due to this ecological validity, findings may be especially relevant to parental observations of their infants and toddlers, one of the main sources of information for early screening and clinical decision-making. Second, the study not only provides group comparisons of infants with autism, other DD, and TD at two time points for Bruner's (1981) three functions of communication, but also permits a comparison of developmental trajectories of gesture use across groups. Third, compared to similar studies that did not use statistical analyses addressing the issue of large numbers of zeros in the data, use of the ZIP model in this study provides some unique and important implications for early screening, assessment, and intervention practices for at risk infants with varying developmental profiles.
Implications
Through examination of infants' gestures across groups, communicative functions, and ages, the current study has the potential to inform early screening, assessment, and intervention practices for infants at risk for autism. The striking differences between infants with autism and those with other DD or TD in the likelihood of using JA gestures during the first and second yea of life confirm that the absence of this pivotal social-communication skill is an early and defining feature of autism in many cases. Importantly, even though infants with autism use JA gestures in the 15-18 month age range less frequently than their counterparts with DD or TD, some of them do use JA gestures. Thus, clinicians must be cautious about dismissing the possibility of autism based on the ability of an infant to use JA gestures without a consideration of the frequency of use. Assessing the development of gestures for other, earlier-developing communicative functions also is important if we are to identify infants with a range of developmental profiles. Failing to produce gestures for the earlier-developing function of BR, or the infrequent use of such gestures, are red flags signaling risk for a later diagnosis of autism or other DD, and by 15-18 months, failure to produce SI gestures should raise concerns about specific risk for autism. This study adds to the literature by outlining the developmental profiles of subpopulations of infants with autism able to use gestures across communicative functions.
The results of this study have implications for early screening and surveillance. The research of Baron-Cohen and colleagues (Baron-Cohen et al., 1996) supported the clinical potential of an absence of JA gestures as one item to use in screening 18-month-olds for autism. Importantly, however, JA gestures were not totally lacking among the infants with autism in the present study. Of the 40 infants with autism with footage at 9-12 months, 4 were credited with using at least one JA gesture, and of the 27 with footage at 15-18 months, 9 were credited with using at least one JA gesture. Thus, our findings suggest screening items that tap only the occurrence versus nonoccurrence of JA gestures as a red flag for autism are likely insufficient, and support current efforts to validate screening tools tapping quantitative variability in risk markers for autism, including JA gestures (Allison et al., 2008; Reznick et al., 2007).
Early screening strategies also should consider the presence and frequency of gesture use for SI and BR functions. Failure to use SI gestures by infants in this age range suggests risk for autism, and is especially likely to be a discriminative item for risk at 15-18 months. Including screening items that tap the frequency with which an infant uses BR gestures may also aid in identifying infants at risk for autism in the 9-18 months age range. Although BR gestures did not discriminate between infants with autism and those with other DD, they nevertheless may have utility for identifying infants who warrant more in-depth evaluation for developmental problems. Also, in ongoing surveillance of infant-toddler development, professionals should attend to the developmental trajectories for gestures in the first half of the second year of life. In particular, for JA gestures, the normative expectation for infants with other DD and TD is a dramatic increase in use, so a failure to show this trajectory is a reason for concern.
Results of this study have additional implications for interventions for infants and toddlers with or at risk for autism. Gestures are key behaviors in the development of early social-communication skills. Transactional theories (e.g., Sameroff &Chandler, 1975) highlight the bidirectional influences in development, with child behaviors and characteristics impacting adults' interactions with children and vice versa, and both being influenced by the environmental context. Applying this to communication development, the frequency of child initiations will impact the response of adults in the environment and vice-versa (Warren &Yoder, 1998). Although the differences in estimated mean gesture use in the current study appeared small when examining mean rates in 5-minute samples, the potential cumulative impact of differences in rate is striking when looking across an hour or a day (i.e., 10 waking hours in an infant's day), if the differences in child initiations lead to correspondingly fewer, or different quality, parent-child interactions. Table 8 provides a conversion of model-estimated means to rates per hour and day. Clearly, relatively small differences in rate of gestures found in short video segments in the current study may have large implications for the communicative transactions of children with autism in their daily lives, and moreover, important implications for intervention. This study's findings point to the clinical importance of assessing early gesture development for the purposes of intervention planning, and then supporting its development, especially in infants and toddlers at risk for autism, by providing caregivers with effective strategies for engaging children in communicative interactions even when the children are infrequently initiating such interactions.
Table 8. Total gesture model-estimated means and conversions.
| Group | 5 min. | One hour | 10-hour day | |
|---|---|---|---|---|
| 9-12 mos. | AU | 1.305 | 15.66 | 156.6 |
| DD | 3.063 | 36.756 | 367.56 | |
| TD | 3.737 | 44.844 | 448.44 | |
| 15-18 mos. | AU | 1.892 | 22.704 | 227.04 |
| DD | 5.693 | 68.316 | 683.16 | |
| TD | 5.753 | 69.036 | 690.36 |
Acknowledgments
This research was supported in part by grants from the National Institute for Child Health and Human Development (R01-HD42168) and Cure Autism Now Foundation (now merged with Autism Speaks). We thank the families whose participation made this study possible, the staff who collected data, edited videotapes, and entered data for this project, and the student assistants and volunteers who coded gestures. We also acknowledge the invaluable assistance of John Bulluck in cleaning and verifying the databases prior to analyses, and the conceptual guidance and logistical assistance of Chris Wiesen (from the Odum Institute for Research in Social Science at the University of North Carolina at Chapel Hill) with the statistical analyses of the data.
References
- Al-Qabandi ML, Gorter JW, Rosenbaum P. Early autism detection: Are we ready for routine screening? Pediatrics. 2011;128:e211–e217. doi: 10.1542/peds.2010-1881. [DOI] [PubMed] [Google Scholar]
- Allison C, Baron-Cohen S, Wheelwright S, Charman T, Richler J, Pasco G, et al. The Q-CHAT (Quantitative CHecklist for Autism in Toddlers): A normally distributed quantitative measure of autistic traits at 18–24 months of age: Preliminary report. Journal of Autism and Developmental Disorders. 2008;38(8):1414–1425. doi: 10.1007/s10803-007-0509-7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Publishing, Inc; 2000. text revision; DSM-IV-TR. [Google Scholar]
- Allison DC, Gallop RJ. Rethinking how family researchers model infrequent outcomes: A tutorial on count regression and zero-inflated models. Journal of Family Psychology. 2007;21(4):726–735. doi: 10.1037/0893-3200.21.4.726. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29(3):213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Cox A, Baird G, Swettenham J, Nightingale N, Morgan K, et al. Psychological markers in the detection of autism in infancy in a large population. British Journal of Psychiatry. 1996;168(2):158–163. doi: 10.1192/bjp.168.2.158. [DOI] [PubMed] [Google Scholar]
- Bruner JS. The social context of language acquisition. Language & Communication. 1981;1:155–178. [Google Scholar]
- Carpenter RL, Mastergeorge AM, Coggins TE. The acquisition of communication intentions in infants eight to fifteen months of age. Language and Speech. 1983;26 Part 2:101–116. doi: 10.1177/002383098302600201. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Pennington BE, Rogers SJ. Interrelations among social-cognitive skills in young children with autism. Journal of Autism and Developmental Disorders. 2002;32(2):91–106. doi: 10.1023/a:1014836521114. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorders – autism and developmental disabilities monitoring network, United States 2008. Morbidity and Mortality Weekly Report (MMWR) 2012;61(3):1–19. [PubMed] [Google Scholar]
- Clifford S, Young R, Williamson P. Assessing the early characteristics of autistic disorder using video analysis. Journal of Autism and Developmental Disorders. 2007;37(2):301–313. doi: 10.1007/s10803-006-0160-8. [DOI] [PubMed] [Google Scholar]
- Colgan SE, Lanter E, McComish C, Watson LR, Crais ER, Baranek GT. Analysis of social interaction gestures in infants with autism. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2006;12(4-5):307–319. doi: 10.1080/09297040600701360. [DOI] [PubMed] [Google Scholar]
- Crais E, Douglas D, Campbell C. The intersection of gestures and intentionality. Journal of Speech, Language, and Hearing Research. 2004;47(3):678–694. doi: 10.1044/1092-4388(2004/052). [DOI] [PubMed] [Google Scholar]
- Curcio F. Sensorimotor functioning and communication in mute autistic children. Journal of Autism and Developmental Disorders. 1978;8(3):281–292. doi: 10.1007/BF01539631. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale P, Reznick JS, Thal D, Bates E, Hartung JP, et al. MacArthur Communicative Development Inventories. San Diego: Singular; 1993. [Google Scholar]
- Guidetti M, Nicoladis E. Introduction to Special Issue: Gestures and communicative development. First Language. 2008;28(2):107–115. [Google Scholar]
- Iverson J, Goldin-Meadow S. Gesture paves the way for language development. Psychological Science. 2005;16:368–371. doi: 10.1111/j.0956-7976.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Myers Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- Kleinman JM, Ventola P, Pandey J, Vebalis AD, Barton M, et al. Diagnostic stability in very young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:606–615. doi: 10.1007/s10803-007-0427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, López B, Moore C. Attention and joint attention in preschool children with autism. Developmental Psychology. 2000;26:261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Loveland KA, Landry SH. Joint attention and language in autism and developmental language delay. Journal of Autism and Developmental Disorders. 1986;16(3):335–349. doi: 10.1007/BF01531663. [DOI] [PubMed] [Google Scholar]
- Loveland KA, Landry SH, Hughes SO, Hall SK, McEvoy RE. Speech acts and the pragmatic deficits of autism. Journal of Speech and Hearing Research. 1988;31(4):593–604. doi: 10.1044/jshr.3104.593. [DOI] [PubMed] [Google Scholar]
- McEvoy RE, Rogers SJ, Pennington BF. Executive function and social communication deficits in young autistic children. Journal of Child Psychology and Psychiatry. 1993;34(4):563–578. doi: 10.1111/j.1469-7610.1993.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, et al. Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental and Behavioral Pediatrics. 2006;27(2):S69–78. doi: 10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning (AGS Edition) Los Angeles: Western Psychological Services; 1995. [Google Scholar]
- Mundy P, Crowson M. Joint attention and early social communication: Implications for research on intervention with autism. Journal of Autism and Developmental Disorders. 1997;27:653–675. doi: 10.1023/a:1025802832021. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders. 1990;20(1):115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman MD, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27(5):657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: A study of first birthday home videotapes. Journal of Autism and Developmental Disorders. 1994;24(3):247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology. 2002;14(2):239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio MF, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- Palomo R, Belinchon M, Ozonoff S. Autism and family home movies: A comprehensive review. Journal of Developmental and Behavioral Pediatrics. 2006;27(Suppl 2):S59–S68. doi: 10.1097/00004703-200604002-00003. [DOI] [PubMed] [Google Scholar]
- Perry A, Condillac RA, Freeman NL, Dunn-Geier J, Belair J. Multi-site study of the Childhood Autism Rating Scale in five clinical groups of young children. Journal of Autism and Developmental Disorders. 2005;35:625–634. doi: 10.1007/s10803-005-0006-9. [DOI] [PubMed] [Google Scholar]
- Phillips W, Gómez JC, Baron-Cohen S, Laá V, Rivière A. Treating people as objects, agents or “subjects”: How young children with and without autism make requests. Journal of Child Psychology and Psychiatry. 1995;8:1383–1398. doi: 10.1111/j.1469-7610.1995.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Reznick JS, Baranek GT, Reavis S, Watson LR, Crais ER. A parent-report instrument for identifying one-year-olds at risk for an eventual diagnosis of autism: The First Year Inventory. Journal of Autism and Developmental Disorders. 2007;37(9):1691–1710. doi: 10.1007/s10803-006-0303-y. [DOI] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, Sigman M. Behavioral profiles of affected and unaffected siblings of children with autism: Contributions of measures of mother-infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders. 2011;41:287–301. doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Saint-Georges C, Cassel RS, Cohen D, Chetouani M, Laznik M, Maestro S, Muratori F. What studies of family home movies can teach us about autistic infants: A literature review. Research in Autism Spectrum Disorders. 2010;4:355–366. [Google Scholar]
- Sameroff AJ, Chandler MJ. Reproductive risk and the continuum of caretaker casualty. In: Horowitz FD, editor. Review of child development research. Vol. 4. Chicago: University of Chicago Press; 1975. [Google Scholar]
- Schopler E, Reichler RJ, Rochen Renner B. The Childhood Autism Rating Scale. Los Angeles, CA: Western Psychological Services; 1992. [Google Scholar]
- Schwartz J, Giles DE. Econometrics Working Paper, EWP1102. University of Victoria; Victoria, BC: 2011. Biased-reduced maximum likelihood estimation for the zero-inflated Poisson distribution. [Google Scholar]
- Shumway S, Wetherby AM. Communicative acts of children with autism spectrum disorders in the second year of life. Journal of Speech, Language, and Hearing Research. 2009;52(5):1139–1156. doi: 10.1044/1092-4388(2009/07-0280). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Warren SF, Yoder PJ. Facilitating the transition to intentional communication. In: Wetherby AM, Warren SF, Reichle J, editors. Transitions in prelinguistic communication. Baltimore: Paul H. Brookes; 1998. pp. 365–385. [Google Scholar]
- Watson LR, Baranek GT, Crais ER, Reznick JS, Dykstra J, Perryman T. The first year inventory: Retrospective parent responses to a questionnaire designed to identify one-year-olds at risk for autism. Journal of Autism and Developmental Disorders. 2007;37(1):49–61. doi: 10.1007/s10803-006-0334-4. [DOI] [PubMed] [Google Scholar]
- Wetherby. Ontogeny of communicative functions in autism. Journal of Autism and Developmental Disorders. 1986;16:295–316. doi: 10.1007/BF01531661. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Cain DH, Yonclas DG, Walker VG. Analysis of intentional communication of normal children from the prelinguistic to the multiword stage. Journal of Speech and Hearing Research. 1988;31(2):240–252. doi: 10.1044/jshr.3102.240. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Prizant BM. Profiling communication and symbolic abilities in young children. Communication Disorders Quarterly. 1993;15(1):23–32. [Google Scholar]
- Wetherby AM, Prutting CA. Profiles of communicative and cognitive-social abilities in autistic children. Journal of Speech &Hearing Research. 1984;27(3):364–377. doi: 10.1044/jshr.2703.364. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Watt N, Morgan L, Shumway S. Social communication profiles of children with autism spectrum disorders late in the second year of life. Journal of Autism and Developmental Disorders. 2007;37(5):960–975. doi: 10.1007/s10803-006-0237-4. [DOI] [PubMed] [Google Scholar]
- Zinober B, Martlew M. Developmental changes in four types of gesture in relation to acts and vocalizations from ten to twenty-one months. British Journal of Developmental Psychology. 1985;3:293–306. [Google Scholar]


