Summary
The natural product englerin A (EA) binds to and activates protein kinase C-θ (PKCθ). EA-dependent activation of PKCθ induces an insulin resistant phenotype, limiting the access of tumor cells to glucose. At the same time, EA causes PKCθ-mediated phosphorylation and activation of the transcription factor heat shock factor 1, an inducer of glucose dependence. By promoting glucose addiction while simultaneously starving cells of glucose, EA proves to be synthetically lethal to highly glycolytic tumors.
Keywords: HSF1, englerin A, renal cell cancer, PKCθ, insulin resistance
INTRODUCTION
Many solid tumors are characterized by an altered metabolic program and display increased dependence on glucose. Several signaling pathways and transcription factors are critical for providing sustained intake of glucose by tumor cells and for enforcing their glycolytic dependence, including the insulin signaling pathway (Leto and Saltiel, 2012) and the heat shock transcription factor heat shock factor 1 (HSF1) (Dai et al., 2007). While lack of function of the insulin pathway or HSF1 has been linked to diabetes and aging, hyper-insulinemia and HSF1 activation have been linked to the development of cancer (Whitesell and Lindquist, 2009; Gallagher and Leroith, 2011; Mendillo et al., 2012). Indeed, recent reports suggest that dependence on HSF1 reflects the “non-oncogene addiction” of tumor cells for this transcription factor (Solimini et al., 2007).
Molecular mechanisms underlying HSF1-enforced glucose dependence are not well understood. However, effects of the insulin pathway on glucose uptake and utilization have been well characterized. Insulin and insulin-like growth factors activate the PI3K/AKT pathway to stimulate glucose uptake. Numerous epithelial tumors rely on constitutive activation of this pathway to increase their supply of glucose (Vander Heiden et al., 2009; Leto and Saltiel, 2012). The protein kinase C (PKC) family of kinases exerts both positive and negative effects on this pathway (Nelson et al., 2008). In type II diabetes, activation of some PKCs, including PKCθ, with fatty acids or diacylglycerol can induce insulin resistance via inhibitory phosphorylation of insulin receptor substrate 1 (IRS1) (Griffin et al., 1999; Li et al., 2004). Phosphorylated IRS1 dissociates from the insulin receptor, leading to decreased signaling via PI3K/AKT and reduced glucose uptake (Li et al., 2004; Griffin et al., 1999).
PKC isozymes are divided into 3 groups: conventional PKCs (PKCα, PKCβI, PKCβII, PKCγ), novel PKCs (PKCδ, PKCθ, PKCε, PKCη), and atypical PKCs (PKCζ and PKCι). Although PKCα, δ, and ε are broadly expressed, other isozymes have a more restricted expression. For example, PKCθ is mainly expressed in T lymphocytes and in some tumors (Marsland and Kopf, 2008; Griner and Kazanietz, 2007). Due to the lack of selectivity of available PKC modulators, the role played by each isozyme in tumorigenesis is not well understood (Griner and Kazanietz, 2007).
The epoxyguaiane englerin A (EA) is a natural product that displays selective in vitro cytotoxicity toward kidney cancer cell lines in the NCI-60 cell line panel (Ratnayake et al., 2009). Since EA’s cytotoxicity profile suggests a unique mechanism of action, we sought to identify EA’s molecular target(s) in order to provide potentially novel therapeutic anti-cancer strategies.
RESULTS
EA is selectively cytotoxic for a panel of genetically defined kidney cancer cell lines
Using cell lines derived from three genetically defined kidney cancers (Clear Cell Renal Cell Cancer, 786-0; Hereditary Leiomyomatosis Renal Cell Cancer, UOK262; Birt-Hogg-Dubé Syndrome, UOK257) and their molecularly restored (e.g., stable reexpression of VHL, FH, or Folliculin, respectively; see references in Table 1) non-tumorigenic isogenic counterparts, we assessed EA cytotoxicity by MTT assay and/or manual cell counting (Table 1). While the three genetically defined kidney cancer cell lines displayed an IC50 for EA of 35 – 50 nM, in each case the molecularly restored isogenic counterpart was markedly less sensitive to EA (IC50 > 10 μM). Two non-tumorigenic kidney-derived cell lines, HK2 and HEK293, were similarly insensitive to EA (IC50 > 10 μM). In contrast, the prostate cancer cell line PC3 and the breast cell line SKBr3 displayed intermediate sensitivity to EA (IC50 = 3 – 5 μM). Sensitivity to EA correlated significantly with sensitivity to 2-deoxyglucose (2-DG), an indicator of glucose dependence (Table 1).
Table 1.
EA cytotoxicity correlates with glucose sensitivity in a panel of cell lines.
| Cell line | Origin of the Tissue | EA (IC50) | 2-DG (IC50) |
|---|---|---|---|
| 786-0 | Kidney cancer (VHL−/−) (Williams et al., 1978) | 50 nM | 60 μM |
| 786-0/VHL | VHL-restored cell line (Tong et al., 2011) | >10 μM | >1 mM |
| UOK257 | Kidney cancer (FLCN−/−) (Yang et al., 2008) | 65 nM | 285 μM |
| UOK257WT | FLCN-restored cell line (Hong et al., 2010) | >10 μM | 721 μM |
| UOK262 | Kidney cancer metastasis(FH−/−) (Yang et al., 2010) | 35 nM | 222 μM# |
| UOK262WT | FH-restored cell line (Tong et al., 2011) | >10 μM | >1 mM# |
| HK2 | Normal Kidney (proximal tubule) (Ryan et al., 1994) | >10 μM | >1 mM |
| HEK293 | Embryonic epithelial Kidney cell (Pear et al., 1993) | >10 μM | >1 mM |
| PC3 | Prostate cancer (Kaighn et al., 1979) | 5 μM | 300 μM |
| SKBr3 | Breast cancer (J. Fogh and G. Trempe, 1975) | 3 μM | n/a |
Cells (70% confluence) were treated with a range of EA concentrations (1 nM – 10 μM) in serum-free media. After 48 h, viability was measured by MTT or manual cell counting. For UOK262 and UOK262WT, viability was only assessed by manual cell counting. Molecularly restored kidney cancer cell lines and normal kidney cell lines did not show significant sensitivity towards EA. Glucose dependence of the cells was similarly assessed by determining sensitivity to 2-deoxyglucose (2-DG, 10 nM –100 μM). Cells were cultured in DMEM containing 1g/L glucose and were treated with 2-DG for 72 h (786-0, 786-0/VHL, UOK257, UOK257WT, HK2, HEK293, PC3, and SKBr3),
except for UOK262 and UOK262WT which were treated for 24 h. Viability was assessed by MTT and/or manual cell counting as above. EA sensitivity displays a highly significant positive correlation with glucose dependence, as assessed by determining R2 and the Pearson Correlation Coefficient (R2 = 0.9126; Pearson Correlation Coefficient = 0.962; p = 0.000003). UOK262 data were not included when determining R2 and the Pearson Correlation Coefficient because they were exposed to 2-DG for 24 h and not 72 h like the other cell lines.
EA selectively activates PKCθ
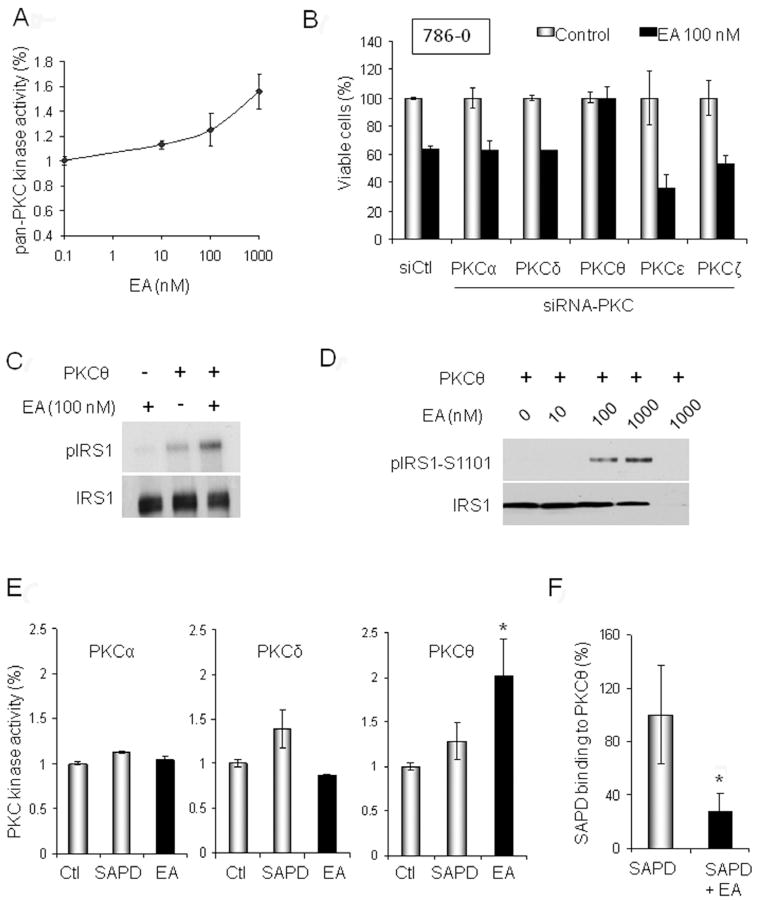
Since nothing is known about EA’s mechanism of action, we predicted potential target(s) by Structure Activity Relationship Analysis (see Experimental Procedures). Fifteen potential molecular targets were identified, half of which were isoforms of protein kinase C (PKC) (Table S1). Therefore, we investigated further the potential effect of EA on PKCs, first using a pan-PKC kinase assay. We found that treatment of whole cell extracts with EA increased pan-PKC activity in a dose-dependent manner (Figure 1A and Figure S1). To identify which PKC isoforms were responsive to EA, we individually silenced expression of PKC-α, -δ, -θ, -η or –ε in 786-0 cells and examined the impact on EA cytotoxicity. Only PKCθ knock-down abrogated EA cytotoxicity (Figure 1B), suggesting that PKCθ may be a target of EA. We confirmed this hypothesis by evaluating the effect of EA on the enzymatic activity of purified PKCθ in vitro. We found that PKCθ-mediated phosphorylation of its substrate IRS1 was dose-dependently enhanced by EA (Figure 1C–D).
Figure 1. EA is a selective PKCθ activator.
A. Pan-PKC kinase activity was assessed in 786-0 cells following EA treatment. Whole cell lysates were incubated for 1 h at 30°C with EA or DMSO in the presence of ATP (10 μM). PKC kinase activity was quantified by spectrophotometry (see Experimental Procedures). B. Viability (determined by MTT assay) after 24-h EA treatment (100 nM) of 786-0 cells silenced for different PKC isoforms by RNA interference. C. Effect of 30 min EA (100 nM) treatment on PKCθ-mediated phosphorylation of IRS1. A radioactive kinase activity assay was performed in the presence of 6 μCi (0.2 μM) of [32P]-ATP and 10 μM non-radioactive ATP using purified PKCθ (50 ng) and its substrate IRS1 (50 ng). D. Non-radioactive dose-dependent effects of EA on PKCθ-mediated phosphorylation of IRS1-S1101 using purified proteins were assessed by immunoblotting (1 h treatment; 10 ng PKCθ, 20 ng IRS1). The last lane omits IRS1 and is a negative control. E. Purified PKCα, PKCδ, or PKCθ (5 ng) were incubated for 1 h with DMSO, SAPD (2 μM), or EA (1 μM) in presence of ATP (10 μM). PKC kinase activity was quantified by spectrophotometry. F. EA competes with the fluorescent phorbol ester SAPD for binding to PKCθ. EA (1 μM) was pre-incubated for 20 min with 5 ng purified PKC protein prior to addition of SAPD (2 μM). SAPD fluorescence shifts from 455 nm to 420 nm when it is bound to PKC. Thus, fluorescence emission was measured at 420 nm (excitation at 355 nm) to assess SAPD binding in presence or absence of EA (*, p<0.05). Data are displayed as mean +/− SD. (see also Fig. S1 and Table S1)
Because PKCθ is structurally very similar to PKCδ, we asked whether EA’s effect on PKC activity is due solely to PKCθ activation. Phorbol esters, including the fluorescent analog sapintoxin D (SAPD), bind to the same pocket in PKCs as does diacylgycerol (DAG) and are well-known PKC activators. Unlike PKCθ and PKCδ, conventional PKCs, including PKCα, require priming with Ca++ in order to bind either DAG or phorbol esters (Luo and Weinstein, 1993; Griner and Kazanietz, 2007). In vitro kinase assay (in the absence of Ca++) using either PKCα,-δ, or –θ proteins confirmed that EA selectively activates PKCθ. In contrast, SAPD activated both PKCδ and PKCθ under the same assay conditions (Figure 1E).
Finally, we took advantage of the fluorescent properties of SAPD to confirm the binding of EA to PKCθ (Figure 1F). Purified PKCθ was incubated for 20 min with EA (1 μM) or DMSO prior to the addition of SAPD (2 μM). We found that pre-mixing PKCθ with EA significantly reduced SAPD binding, supporting the hypothesis that EA interacts with a motif in PKCθ either contiguous with or close to the SAPD/DAG binding domain. Pre-mixing EA with PKCδ had no effect on SAPD binding (data not shown).
PKCθ activation induces an insulin resistant phenotype in tumor cells
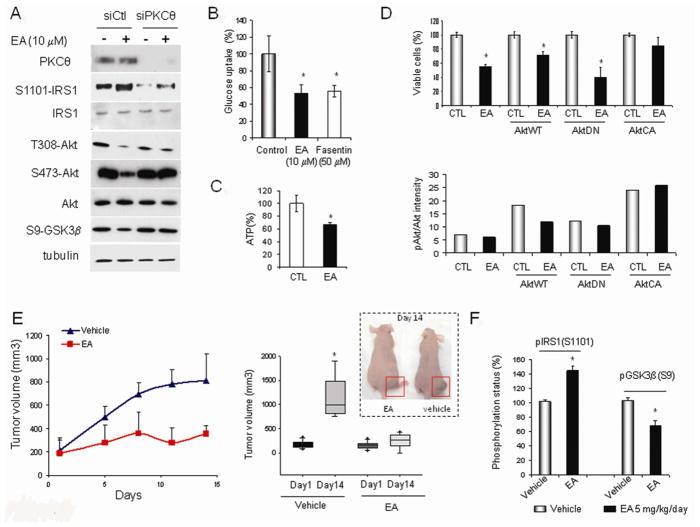
Because we demonstrated that EA enhanced PKCθ-mediated inhibitory phosphorylation of IRS1 (on S1101) in vitro, we hypothesized that EA might induce an insulin resistant phenotype. EA enhanced the inhibitory phosphorylation of IRS1 in 786-0 cells and led to reduced activating phosphorylations of AKT (T308 and S473) and reduced AKT-mediated phosphorylation of GSK3β (S9). These effects are PKCθ-dependent, since they were ameliorated upon siRNA-mediated silencing of PKCθ (Figure 2A). EA also reduced glucose uptake in 786-0 cells, to a similar degree as the Glut1 inhibitor fasentin (Wood et al., 2008) (Figure 2B), and decreased cellular ATP content as well (Figure 2C). However, at the concentration used, fasentin only slightly affected 786-0 cell viability (Figure S2A), while addition of cell permeable pyruvate (methylpyruvate) abrogates EA cytotoxicity (Figure S2B). These data suggest that glucose uptake inhibition contributes to, but does not solely account for EA cytotoxicity.
Figure 2. EA induces insulin resistance in vitro and in vivo.
A. EA-mediated phosphorylation of IRS1 (S1101), AKT (pT308 and pS473), and GSK3β (pS9) in 786-0 cells requires PKCθ expression. EA treatment (10 μM) was for 6 h. B. EA inhibits glucose uptake in 786-0 cells as shown by using the non-degradable fluorescent glucose analog 2-NBDG. The Glut1 inhibitor fasentin (50 μM) is shown as a positive control. EA and fasentin treatments were for 3 h. C. Effect of EA treatment (6 h, 1 μM) on ATP levels in 786-0 cells was measured using ATPlite assay. D. Effect of AKT activation on EA cytotoxicity. 786-0 cells were infected 24 h prior to EA treatment with AKT lentiviral constructs: wild-type AKT (AktWT), dominant negative AKT (AktDN), or constitutively active AKT (AktCA). After treatment with 1 μM EA for an additional 24 h, cell viability was assessed by manual cell counting with trypan blue exclusion (upper panel). Impact of lentiviral infections on AKT activity is shown in lower panel and in Fig. S2D. EA inhibits the activity of wild type, but not of constitutively active AKT. E. Effect of EA treatment (5 mg/kg, daily except Sunday) on xenograft growth of 786-0 cells in athymic mice (vehicle: PBS/DMSO, 1/1). The left panel displays the growth curve of one of two animal experiments, each with 8 animals per group. The right panel represents the averaged mean end point tumor volumes of the two experiments (32 animals in total). F. EA treatment in vivo stimulates inhibitory phosphorylation of IRS1 (pS1101) and reduced phosphorylation of the AKT substrate GSK3β (pS9) in tumor xenografts of treated mice. *, p<0.05. Data are displayed as mean +/− SD. (see also Fig. S2)
Next, we assessed the role played by insulin pathway inhibition in EA-mediated inhibition of AKT. We confirmed that the inhibitory effect of EA on AKT activity was IRS1-dependent, since EA-mediated AKT inhibition was overcome by inclusion of EGF in the culture media (Figure S2C). To confirm the importance of IRS1-dependent AKT inhibition for EA cytotoxicity, we infected 786-0 cells with several AKT viral constructs. As shown in Figure 2D (upper panel), expression of dominant negative AKT (AktDN) enhanced EA cytotoxicity while expression of constitutively active AKT (AktCA) protected cells from EA. These impacts on EA cytotoxicity were consistent with AKT activity status (Figure 2D, lower panel, Figure S2D), and the data clearly implicate IRS1-dependent inhibition of AKT as a necessary component of the cytotoxic response to EA.
To determine whether the in vitro cytotoxicity of EA was obtainable in vivo, we treated athymic mice bearing 786-0 tumor xenografts with EA (5mg/kg i.p., daily except Sunday). EA markedly inhibited tumor growth during the 2-week treatment period (Figure 2E). In agreement with our in vitro data, inhibitory phosphorylation of IRS1 was increased and activity of the PI3K/AKT pathway was decreased in 786-0 tumors excised from mice treated with EA, when compared to tumors from vehicle-treated mice (Figure 2F). Importantly, in a second tumor xenograft model, EA inhibited human prostate tumor growth by up to 60% (Figure S2E), consistent with its ability to stimulate PKCθ in these cells and with its in vitro toxicity profile (Figure S1 and Table 1).
PKCθ induces heat shock-independent activation of HSF1
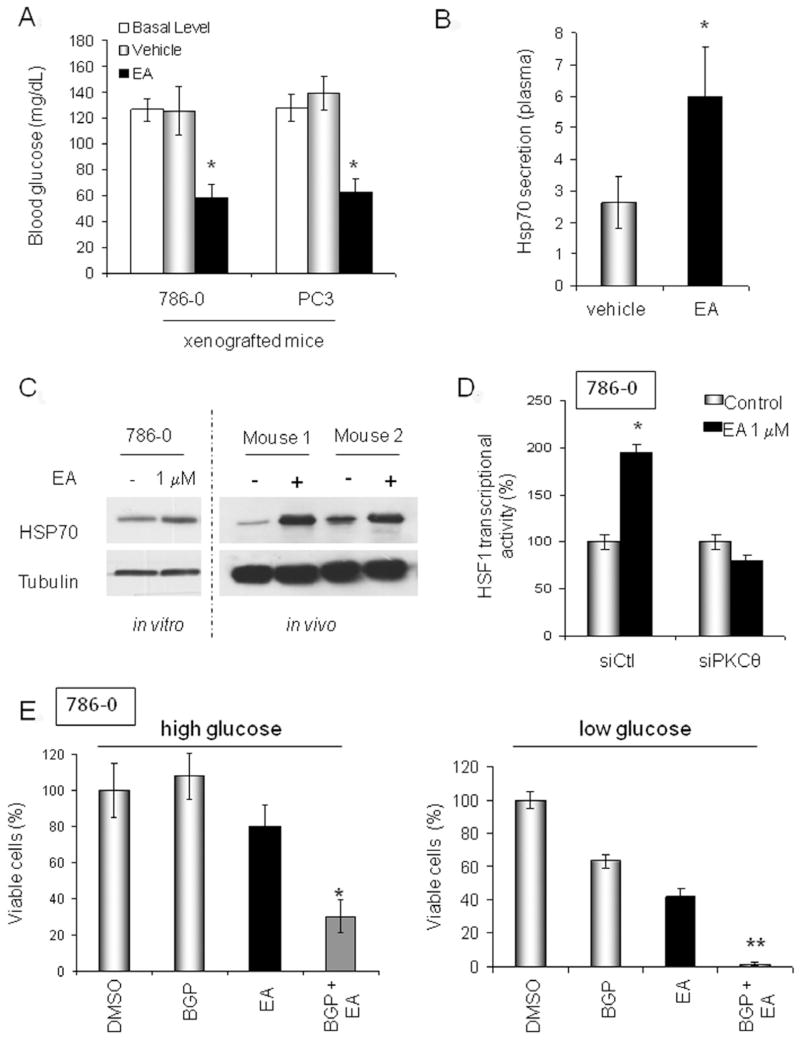
Because the in vivo data support further evaluation of EA as an anti-cancer agent, we asked whether treated animals might develop hyperglycemia due to induction of systemic insulin resistance. We measured blood glucose level in mice harboring either 786-0 or PC3 xenografts before and following a single treatment with either EA or vehicle (PBS/DMSO, 1:1). Surprisingly, mice treated with EA displayed significantly lower blood glucose compared to vehicle-treated mice (Figure 3A).
Figure 3. EA activates HSF1.
A. Effect of EA on blood glucose level in tumor-bearing mice prior to (“basal level”) or five minutes after treatment with EA (EA, 5mg/kg; vehicle: PBS/DMSO, 1/1). B. Quantification of plasma HSP70 level in tumor-bearing mice 4 h following EA treatment (EA: 5 mg/kg; vehicle: PBS/DMSO, 1/1). C. HSP70 protein expression following EA treatment of 786-0 cells in vitro (EA, 1 μM for 8 h) and in vivo (786-0 xenografts; prior to and 8 h after EA, 5mg/kg) was assessed by immunoblotting. D. EA stimulation of HSF1 transcriptional activity requires PKCθ expression. HSF1 activity was measured in cells transiently transfected with a HSP70 HSE-promoter GFP-tagged reporter plasmid 6 h after EA treatment. E. EA cytotoxicity is affected by HSF1 activation and extracellular glucose concentration. BGP-15 prolongs the HSF1 transcriptional response. High glucose medium contains 4.5g/L glucose; low glucose medium contains 1g/L glucose. BGP-15 was used at 50 μM (Chung et al., 2008) and EA was used at 1 μM. Incubation was for 24 h. Viability was assessed by MTT assay and confirmed by manual cell counting of trypan blue-excluding cells using a hemacytometer. *, p<0.05; **, p<0.001. Data are displayed as mean +/− SD. (see also Fig. S3)
Chemically-induced reduction in blood glucose has been reported previously and is thought to be due to increased HSP70, resulting in sensitization of cells to insulin (Chung et al., 2008; Kavanagh et al., 2011). We observed that in mice treated with EA both plasma and tumor HSP70 were elevated compared to vehicle-treated mice (Figure 3B & C). HSP70 is a marker of cell stress and is a transcriptional target of HSF1 (Trepel et al., 2010). Consistent with these data, we observed that EA induced HSF1 nuclear translocation and upregulated its transcriptional activity in a PKCθ-dependent manner (Figure 3D and Figure S3).
Since HSF1 has recently been identified as a contributing factor for tumorigenesis and likely represents a non-oncogene addiction of most tumor cells (Whitesell and Lindquist, 2009; Santagata et al., 2011; Dai et al., 2007; Mendillo et al., 2012; Min et al., 2007), we investigated whether HSF1 activation might compromise EA cytotoxicity, much as it is thought to compromise the cytotoxicity of HSP90 inhibitors (which also induce HSF1 (Zou et al., 1998)). We examined the impact of the HSF1 chemical enhancer BGP-15, currently under clinical evaluation for treating insulin resistance disorders (Chung et al., 2008; Literati-Nagy et al., 2009; Hargitai et al., 2003), on EA-induced cytotoxicity. To our surprise, addition of BGP-15 significantly increased EA cytotoxicity (Figure 3E, left panel). We have shown that PKCθ induced insulin resistance in tumor cells, and Dai et al. demonstrated that HSF1 enforces glucose dependence in tumor cells (Dai et al., 2007). Thus, we hypothesized that the increased cytotoxicity obtained in vitro upon combination of BGP-15 and EA might result from the simultaneous occurrence of these two metabolic events. If this were the case, the cytotoxicity of an EA/BGP-15 drug combination should be augmented in cells exposed to low glucose. Indeed, as shown Figure 3E (right panel), BGP-15 and EA, either administered as individual agents or in combination, displayed greater cytotoxicity when tumor cells were cultured in low glucose media.
EA cytotoxicity requires expression of both PKCθ and HSF1
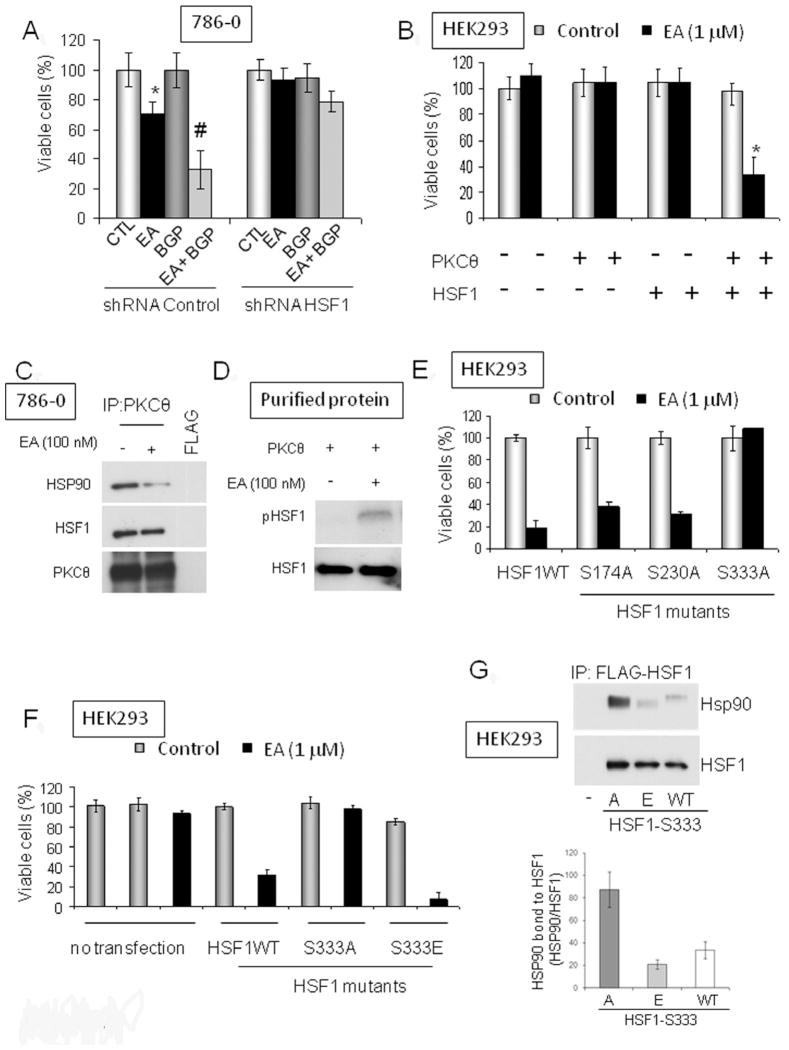
Next, we asked whether HSF1 expression is necessary for EA-induced cytotoxicity. Using shRNA to knock down HSF1 (Figure S4A), we found that HSF1 expression, like PKCθ, is essential for cell sensitivity to EA (Figure 4A). Importantly, the synergistic effect obtained by combining BGP-15 and EA (see Figure 3E) also depended on HSF1 expression. To provide further support for our hypothesis that both PKCθ and HSF1 are necessary for EA cytotoxicity, we made use of the fact that HEK293 cells are insensitive to EA (see Table 1), express undetectable levels of endogenous PKCθ, and do not over-express HSF1 (data not shown). We were able to induce EA sensitivity in HEK293 cells after transfection with both PKCθ and HSF1, but not after transfection with either construct alone (Figure 4B; see Figure S4B for inputs).
Figure 4. PKCθ and HSF1 are both necessary for EA cytotoxicity in tumor cells.
A. EA sensitivity of 786-0 cells to EA and EA/BGP-15 requires HSF1 expression. EA was used at 1 μM and BGP-15 at 50 μM (24 h). HSF1 expression was decreased by transfection of HSF1-specific shRNA 24 h prior to treatment. *, p< 0.05 (compared to control); #, p< 0.05 (compared to EA-treated). B. HEK293 cells are not sensitive to EA. HSF1 and PKCθ over-expression confers EA sensitivity on HEK293 cells (EA treatment was for 24 h at 1 μM, commencing 24 h after transfection). *, p<0.05 (compared to co-transfected control) C. Interaction of endogenous HSF1 and PKCθ in 786-0 cells was visualized by immunoprecipitation of endogenous PKCθ and blotting for associated HSF1. 786-0 cells grown in 6-well plates were lysed in TNESV buffer. The lysates were treated for 1 h with EA (100 nM) or DMSO at 30°C before immunoprecipitation. HSP90 interaction with the PKCθ/HSF1 immunocomplex was reduced by EA treatment (left panel). The inputs for this experiment can be found in Figure S4C. D. EA stimulates PKCθ-mediated phosphorylation of purified HSF1 in vitro (50 ng of purified proteins; treatment for 30 min at 30°C). E. Identification of a putative PKCθ phosphorylation site on HSF1. HEK293 cells were transfected with PKCθ and either wild type or point mutated HSF1 plasmids (see Figure S4E–F) and sensitivity to EA was assessed (treatment for 24 h with 1 μM EA). F. Effect of HSF1 S333 mutants on EA sensitivity of HEK293 cells over-expressing PKCθ. The phosphomimetic mutant HSF1-S333E supports EA cytotoxicity, while the non-phosphorylatable mutant HSF1-S333A does not (treatment for 24 h with 1 μM EA). G. Association of HSF1-S333A, HSF1-S333E, and wild type HSF1 with HSP90. HEK293 cells were transiently transfected with Flag-tagged HSF1 plasmids as indicated. After 24 h, Flag immunoprecipitates were subjected to SDS-PAGE, transferred to PVDF membrane, and immunoblotted for associated endogenous HSP90. Anti-HSF1 antibody was also blotted to monitor the uniformity of HSF1 expression and efficiency of immunoprecipitation. The phosphomimetic mutant HSF1-S333E associates with endogenous HSP90 to a markedly lesser degree than does non-phosphorylatable HSF1-S333A; HSP90 association with wild type HSF1 is shown for comparison. Band optical densities from two separate experiments were obtained by image analysis software and the HSP90 band density in each case was normalized to the respective HSF1 band density (graphical insert). The inputs for this experiment can be found Figure S4H. Data are displayed as mean +/− SD. (see also Fig. S4).
Extending our observation that PKCθ is necessary for HSF1 activation by EA (Figure 3D), we were able to detect the interaction of endogenous PKCθ and HSF1 in 786-0 cells (Figure 4C; see Figure S4C for inputs). In addition, we found that PKCθ phosphorylated HSF1 in vitro in the presence of EA (Figure 4D), and EA-induced serine phosphorylation of endogenous HSF1 in 786-0 cells was PKCθ-dependent (Figure S4D). Further, we observed that HSP90 was also a component of the HSF1/PKCθ complex, but was dissociated after treatment with EA (Figure 4C).
Since dissociation from HSP90 is a prerequisite for HSF1 activation (Anckar and Sistonen, 2011; Zou et al., 1998), these data suggest that PKCθ phosphorylation of HSF1 may promote this process. To identify putative PKCθ phosphorylation site(s) on HSF1, we mutated several predicted PKC consensus phosphorylation sites (see Figure S4E) and we examined the ability of these HSF1 mutants to complement exogenous PKCθ in mediating EA cytotoxicity in HEK293 cells (Figure 4E; see Figure S4F for inputs). EA cytotoxicity was abrogated only when HSF1 serine 333 was mutated to alanine (S333A), implicating S333 as a potential PKCθ phosphorylation site. Supporting this possibility, we found that the phosphomimetic mutant HSF1-S333E, but not the non-phosphorylatable mutant HSF1-S333A, fully complemented PKCθ-dependent EA cytotoxicity (Figure 4F; see Figure S4G for inputs).
Although the domain of HSF1 that interacts with HSP90 is not known, S333 is located within the regulatory domain of the transcription factor, a region rich in posttranslational modification sites and important for the stress inducibility of HSF1 (Anckar and Sistonen, 2011). Therefore, we examined whether S333 mutation to either alanine or glutamic acid affected HSF1 interaction with HSP90. FLAG-tagged HSF1 wild type, S333A, and S333E plasmids were transiently transfected into HEK293 cells and FLAG immunoprecipitates were probed for associated endogenous HSP90. Indeed, we found that HSF1-S333A associated with endogenous HSP90 to a markedly greater extent than did HSF1-S333E (Figure 4G; see Figure S4H for inputs). These data are consistent with the hypothesis that PKCθ-mediated phosphorylation of HSF1 S333 promotes dissociation from HSP90. Supporting this possibility, we found that HSF1-S333E was more efficiently activated (> 2-fold) by heat shock when compared to HSF1-S333A (Figure S4I).
DISCUSSION
Survival of tumor cells depends on their ability to adapt to their environment. Since cellular transformation is associated with an increased dependence on glucose (Vander Heiden et al., 2009), tumor cells have reprogrammed their cellular signaling pathways to allow for increased glucose uptake. Indeed, positron emission tomography with 2-deoxy-2(18F)-fluoro-D-glucose, a non-metabolizable glucose analog, is frequently used to distinguish tumors from adjacent normal tissues (Gambhir, 2002), and targeting glucose uptake and/or metabolism has been explored for its therapeutic potential in treating cancer, including VHL-deficient kidney cancer (Chan et al., 2011; Hamanaka and Chandel, 2012). The insulin pathway and the transcription factor HSF1 are two examples of evolutionarily conserved signaling networks that support and foster the glucose dependence of tumor cells (Barbieri et al., 2003; Pirkkala et al., 2001; Dai et al., 2007).
In this study, we have identified a unique strategy to create metabolic disaster in glucose-dependent tumor cells by selectively activating PKCθ with the natural product EA (schema illustrated in Figure 5). When examined in a panel of kidney cancer-derived cell lines with unique genetic lesions distinct from VHL deficiency, EA cytotoxicity paralleled sensitivity to 2-deoxy-D-glucose (2-DG), itself an indicator of glucose dependence (see Table 1). In each case, correction of the unique genetic lesion in isogenic cell lines abrogated both EA sensitivity and 2-DG cytotoxicity. Non-tumorigenic cell lines derived from normal kidney epithelium were resistant to both EA and 2-DG. Importantly, however, non-tumorigenic HEK293 cells can be made sensitive to EA by exogenous expression of both PKCθ and HSF1.
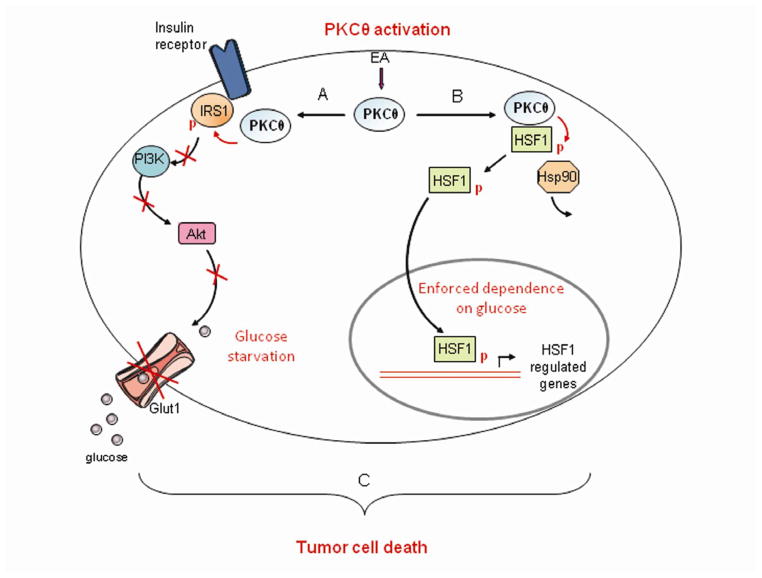
Figure 5. EA proposed mechanism of action: Activation of PKCθ in cells expressing HSF1 leads to simultaneous induction of insulin resistance and glucose dependence, resulting in metabolic catastrophe.
A. EA-dependent activation of PKCθ stimulates an insulin resistant phenotype via inhibitory phosphorylation of IRS1 and inhibition of AKT, limiting access of tumor cells to glucose. B. EA simultaneously stimulates PKCθ-mediated phosphorylation and activation of HSF1, a transcription factor that enforces tumor cell glucose dependence (Dai et al., 2007). C. By simultaneously inducing insulin resistance and glucose dependence, EA is synthetically lethal to glycolytic tumor cells.
Although the crystal structure of EA bound to PKCθ will be necessary to unambiguously identify its binding domain, competition binding experiments with the fluorescent phorbol ester SAPD suggest that EA binds within or adjacent to the C1 domain of PKCθ. Since EA is not able to compete with SAPD binding to PKCδ, and because EA is structurally dissimilar from either phorbol esters or DAG, it is likely that EA has binding requirements that are only met in PKCθ.
Due to the lack of selectivity of most PKC modulators, the unique role of PKCθ in cancer biology has remained unclear. Here, we have identified PKCθ as an important pharmacologic target in glucose-dependent tumor cells. Kim and collaborators first identified a link between PKCθ and insulin resistance when they demonstrated that PKCθ knockout mice were protected from developing fat-induced insulin resistance (Kim et al., 2004). We confirmed the association of PKCθ with insulin resistance in tumor cells by showing that selective activation of PKCθ disrupts insulin signaling to AKT and induces an insulin resistant phenotype reminiscent of that caused in skeletal muscle by a high fat diet and observed in patients with Type 2 diabetes (Samuel and Shulman, 2012).
We suspect that the lack of hyperglycemia in EA-treated mice is due to increased HSP70 levels, since elevated HSP70 has been shown to enhance insulin sensitivity (Chung et al., 2008; Kavanagh et al., 2011). Because EA promoted increased HSP70 expression in tumor xenografts and in tumor cells in vitro, we examined the possible impact of EA on HSF1, a transcriptional regulator of HSP70 and a protein frequently upregulated in cancer (Whitesell and Lindquist, 2009; Santagata et al., 2011). As discussed earlier, HSF1 enhances tumor glucose dependence and the transcription factor, although not transforming on its own, is considered to be a critical contributor to tumor cell survival (Dai et al., 2007; Solimini et al., 2007). Recently, HSF1 has been reported to transcriptionally regulate a number of genes not involved in the heat shock response of normal cells but which are commonly upregulated in cancer cells (Mendillo et al., 2012). Thus, inhibition of HSF1 is predicted to be of therapeutic value in cancer (Whitesell and Lindquist, 2009).
Unexpectedly, although EA stimulates HSF1 transcriptional activity, we found this to be a prerequisite for EA cytotoxicity. Thus, we propose that EA is synthetically lethal for tumor cells that simultaneously express PKCθ and are addicted to HSF1. PKCθ activates HSF1 by phosphorylating serine 333 in the stress responsive regulatory domain. Mutation of this residue to a non-phosphorylatable (S333A) or phosphomimetic (S333E) amino acid markedly affects the interaction of HSF1 with HSP90. Since dissociation of HSF1 from HSP90 is a prerequisite for HSF1 activation and nuclear translocation (Anckar and Sistonen, 2011), these data provide a mechanistic basis to explain PKCθ-dependent activation of HSF1 by EA. Importantly, EA sensitivity is strongly correlated with glucose dependence and is most pronounced when glucose availability is limiting.
In summary, our data show that PKCθ-mediated induction of insulin resistance occurring simultaneously with PKCθ-mediated HSF1 activation is responsible for EA cytotoxicity. PKCθ thus represents a unique molecular target for HSF1-addicted glycolytic tumors, and EA provides a template for designing effective PKCθ-activating drugs.
EXPERIMENTAL PROCEDURES
Cell lines and cell culture
The sporadic VHL-deficient kidney tumor cell line 786-0 (Williams et al., 1978), the prostate cancer cell line PC3 (Kaighn et al., 1979), the breast cancer cell line SKBr3 (J. Fogh and G. Trempe, 1975), the normal kidney cell line HK2 (Ryan et al., 1994), and HEK293T, an embryonic kidney epithelial cell line (Pear et al., 1993), were all purchased from ATCC. UOK262, UOK262wt, UOK257, UOK257-2 (wt), 786/VHL were established within the Urologic Oncology Branch. UOK262 is a kidney cancer cell line derived from a metastasis that is deficient in fumarate hydratase (FH) (Yang et al., 2010). UOK262wt was established by stably transfecting UOK262 with a functional FH gene (Tong et al., 2011) and is therefore considered to be “molecularly restored”. UOK257 is a folliculin (FLCN)-deficient kidney tumor cell line derived from human renal carcinoma of an individual with Birt-Hogg-Dubé (BHD) syndrome and its molecularly restored counterpart UOK257-2 (wt) was established by stably transfecting UOK257 with FLCN (Yang et al., 2008; Hong et al., 2010). The stably VHL-transfected 786-0 (786/VHL) cell line has been described previously (Tong et al., 2011). Cells were cultured in Dulbecco’s modified Eagle’s medium High Glucose without sodium pyruvate (DMEM; Cellgro) or in RPMI-1640 (PC3 only) supplemented with 10% fetal bovine serum (Invitrogen, Grand Island, NY). Viability experiments were performed in serum-free media.
Reagents
EA was generously supplied by R. Akee of the Natural Products Support Group (Developmental Therapeutics Program, National Cancer Institute, Frederick National Laboratory, Frederick, MD). Complete mini-protease inhibitor cocktail tablets were purchased from Roche (Indianapolis, IA). The siRNAs for PKC-α and -ζ were purchased from OriGene (Rockville, MD). The siRNAs for PKC-θ, -δ and -ε were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Purified HSF1, PKC-θ, -α, and -δ were purchased from EnzoLife Sciences (Farmingdale, NY).
Prediction of EA targets
Metadrug (Genego Inc, Carlsbad, CA) is a systems pharmacology platform using QSAR modeling to analyze and compare biological effects of small molecules. We used it to predict potential targets for EA (see a complete list of predicted targets in Table S1).
Non-radioactive PKC kinase assay
PKC kinase activity of cell lysates was measured using the pan-PKC activity assay from EnzoLife Sciences, following the manufacturer’s recommendations. Briefly, cells were lysed in TNESV lysis buffer (50 mM Tris, 1% Nonidet P-40, 2 mM EDTA, 100 mM NaCl and 2 mM Na3VO4). After 15 min of centrifugation (13, 200 rpm, 4°C), clarified supernatant was incubated with 10 μM EA in the kinase buffer provided by the manufacturer (1h at 30°C with 10 μM ATP). The reaction was stopped by emptying the wells prior to measuring the phosphorylation of a PKCsubstrate by spectrophotometry. PKCα, -δ, and θ kinase assays were performed in a similar manner using 5 ng of purified PKC proteins instead of cell lysate (incubation for 1 h at 30°C with 10 μM ATP). Kinase activity was also assessed by incubating purified PKCθ (10 ng) with purified IRS1 (20 ng) in presence of increasing concentrations of EA (incubation for 1 h at 30°C with 10 μM ATP). The reaction was stopped by adding denaturing sample buffer and phosphorylation of IRS1 on S1101 was assessed by immunoblot analysis.
Radioactive in vitro kinase assay
Purified IRS1 (50 ng) or HSF1 (50 ng) was incubated with purified PKCθ (50 ng) in presence or absence of EA (100 nM). Reactions were initiated by the addition of 10 μM nonradioactive ATP and 6 μCi (0.2 μM) of [32P]-ATP and incubated at 30°C for 30 min with periodic mixing. Proteins in the kinase reactions were separated by SDS-PAGE and transferred to PVDF membrane. Phosphorylation of IRS1 or HSF1 was assessed by radiography of PVDF membranes. IRS1 or HSF1 were immunoblotted to ensure equal loading.
Phorbol ester competition binding assay
The fluorescent phorbol ester sapintoxin D (SAPD, 2 μM; Santa Cruz) (Taylor et al., 1981) was incubated with purified PKC proteins (5 ng) after pre-incubation for 20 min with EA (1 μM) or DMSO. Fluorescence of SAPD is shifted from 455 to 420 nm when it is bound to PKC. Therefore, we monitored fluorescence emission at 420 nm to determine SAPD binding to PKC proteins (Das et al., 2004).
Glucose uptake assay
Glucose uptake was measured using a fluorescent non-metabolizable D-glucose analog 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG, Cayman Chemicals, Ann Arbor, MI) as previously described (O’Neil et al., 2005), with the following modifications. Five thousands cells were plated in black-well 96-well plates. After treatment as indicated (3 h), cells were incubated for 20 minutes in KREB buffer containing 1g/L glucose in presence or absence of 20 μM 2-NBDG. Cells were then washed 3 times for 5 minutes with PBS to remove all residual extracellular 2-NBDG. The amount of 2-NBDG imported into the cells was measured by assessing fluorescence at 488 nm. The Glut 1 inhibitor fasentin (50 μM, Sigma-Aldrich) was used as a positive control (Wood et al., 2008).
HSP70 secretion
Plasma HSP70 in tumor-bearing mice was assessed using an ELISA kit purchased from EnzoLife Sciences and following the manufacturer’s protocol. Blood was collected 4 h after EA or vehicle (PBS/DMSO, 1/1) injection.
HSF1 transcriptional activity
Cellular HSF1 transcriptional activity was measured using a GFP-tag HSE promoter reporter (generously provided by Dr Luke Whitesell, Whitehead Institute, Cambridge, MA). Twenty-four hours prior to analysis, 5,000 786-0 cells were plated in 96-well Black-view plates. While still in suspension, cells were transfected with the reporter plasmid (1 μg DNA) using lipofectamine LTX (Invitrogen) and following the manufacturer’s protocol. To avoid potential interference of the phenol red from the media with the GFP reading, phenol red-free DMEM (high glucose and without sodium pyruvate) was used instead of regular DMEM. The following day, 786-0 cells were treated as described with EA 6 h prior to measure the amount of GFP produced using a spectrophotometer (488 nm). For HSF1 silencing experiments, 786-0 cells were transfected with 14 ug of shRNA to HSF1 in 6-well plates using lipofectamine LTX (Invitrogen) 2 days prior to plating into 96-well black-view plates. Nuclear translocation of HSF1 was visualized by immunofluorescence. Three thousands 786-0 cells were plated in 2-well chamber-slides (Nunc., Sigma-Aldrich) and treated for 1 h with EA (1 μM) before fixation with 4% paraformaldehyde. Cells were blocked 1 h with BSA (3%) and permeabilized with Triton (0.5%). HSF1 antibody was incubated overnight at 4°C in a humidified atmosphere. After 3 washes with TBST buffer, slides were incubated 1 h with secondary antibody coupled to Alexa455, washed and mounted. DAPI (Cell Signaling Technology) was used to visualize cell nuclei. Pictures were taken with a confocal microscope (Zeiss NLO510).
Xenograft tumor studies
Animal experiments were carried out following the ethical guidelines of the National Cancer Institute and using an animal protocol approved by the NIH Animal Care Facility. Ten million 786-0 or 1 million PC3 cells were implanted subcutaneously on the left flanks of twenty 7-week old female nude (Nu/Nu) mice (strain code 088; Charles River, Wilmington, MA). After 1 – 4 weeks (depending on the cell line), tumors reached an average volume of 100–150 mm3. Tumor take for both 786-0 and PC3 xenografts was 100%; however, to maintain homogenous group sizes, only 16 mice out of the 20 were used. Mice were then randomly separated in two groups of 8 mice with comparable tumor volumes and treated six times a week (daily except Sunday) intraperitoneally with either EA at 5 mg/kg or vehicle (PBS/DMSO, 1/1). Food and water were available ad libitum. Tumors were measured throughout the duration of the experiment using calipers and tumor volumes were estimated using the formula (LxW2)/2. At the end of the experiment, blood was collected, and tumors were surgicaly excised and frozen for further analysis. Animal experiments were performed twice with 8 animal per group each time.
Statistics
Unless specified, all values are expressed as mean ± standard error. Values were compared using the Student-Newman-Keul’s test. P < 0.05 was considered significant.
Supplementary Material
Significance.
Many epithelial tumors display a glycolytic phenotype characterized by enhanced dependence on glucose. Targeting the abnormal metabolism of such tumors has been a long-term goal of the scientific community. The natural product EA selectively activates PKCθ to induce a metabolic catastrophe in glycolytic tumor cells by promoting insulin resistance and inhibiting glucose uptake while simultaneously activating the heat shock transcription factor HSF1, thereby enforcing glucose dependence. These data identify EA as a mechanistically unique cytotoxic agent.
Highlights.
EA activates PKCθ
PKCθ inhibits the insulin pathway and glucose uptake in tumor cells
PKCθ activates HSF1 to enhance the glucose dependence of tumor cells
EA is synthetically lethal for glycolytic tumor cells expressing PKCθ and HSF1
Acknowledgments
We thank Drs. S. Calderwood (Harvard University, Cambridge, MA) and L. Whitesell (Whitehead Institute, Cambridge, MA) for generously providing reagents. We thank Dr. P. L. Nagy (N-Gene Research Laboratories, Budapest, Hungary) for generously providing BGP-15. We thank Drs N. Kedei and P. Blumberg (National Cancer Institute, Bethesda, MD), and P. Csermely (Semmelweis University, Budapest, Hungary) for helpful discussions. This research was supported with funds provided by the Intramural Research Program of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Addona GH, Sandberg WS, Husain SS, Stehle T, Miller KW. Identification of a general anesthetic binding site in the diacylglycerol-binding domain of protein kinase Cdelta. J Biol Chem. 2004;279:37964–37972. doi: 10.1074/jbc.M405137200. [DOI] [PubMed] [Google Scholar]
- Gallagher EJ, Leroith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546–2551. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. J Exp Med. 2012;209:211–215. doi: 10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargitai J, Lewis H, Boros I, Racz T, Fiser A, Kurucz I, Benjamin I, Vigh L, Penzes Z, Csermely P, Latchman DS. Bimoclomol, a heat shock protein co-inducer, acts by the prolonged activation of heat shock factor-1. Biochem Biophys Res Commun. 2003;307:689–695. doi: 10.1016/s0006-291x(03)01254-3. [DOI] [PubMed] [Google Scholar]
- Hong SB, Oh H, Valera VA, Stull J, Ngo DT, Baba M, Merino MJ, Linehan WM, Schmidt LS. Tumor suppressor FLCN inhibits tumorigenesis of a FLCN-null renal cancer cell line and regulates expression of key molecules in TGF-beta signaling. Mol Cancer. 2010;9:160. doi: 10.1186/1476-4598-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J, Trempe G. J Fogh. Plenum Publishing Corp; 1975. Human Tumor Cells in Vitro; pp. 115–141. Ref Type: Report. [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300:E894–E901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O’Brien WR, Littman DR, Shulman GI. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- Li Y, Soos TJ, Li X, Wu J, Degennaro M, Sun X, Littman DR, Birnbaum MJ, Polakiewicz RD. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101) J Biol Chem. 2004;279:45304–45307. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- Literati-Nagy B, Kulcsar E, Literati-Nagy Z, Buday B, Peterfai E, Horvath T, Tory K, Kolonics A, Fleming A, Mandl J, Koranyi L. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res. 2009;41:374–380. doi: 10.1055/s-0028-1128142. [DOI] [PubMed] [Google Scholar]
- Luo JH, Weinstein IB. Calcium-dependent activation of protein kinase C. The role of the C2 domain in divalent cation selectivity. J Biol Chem. 1993;268:23580–23584. [PubMed] [Google Scholar]
- Marsland BJ, Kopf M. T-cell fate and function: PKC-theta and beyond. Trends Immunol. 2008;29:179–185. doi: 10.1016/j.it.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L, Lindquist S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150:549–562. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JN, Huang L, Zimonjic DB, Moskophidis D, Mivechi NF. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene. 2007;26:5086–5097. doi: 10.1038/sj.onc.1210317. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Sun MK, Hongpaisan J, Alkon DL. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. Eur J Pharmacol. 2008;585:76–87. doi: 10.1016/j.ejphar.2008.01.051. [DOI] [PubMed] [Google Scholar]
- O’Neil RG, Wu L, Mullani N. Uptake of a fluorescent deoxyglucose analog (2-NBDG) in tumor cells. Mol Imaging Biol. 2005;7:388–392. doi: 10.1007/s11307-005-0011-6. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- Ratnayake R, Covell D, Ransom TT, Gustafson KR, Beutler JA. Englerin A, a selective inhibitor of renal cancer cell growth, from Phyllanthus engleri. Org Lett. 2009;11:57–60. doi: 10.1021/ol802339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM, Lindquist S, Ince TA. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci U S A. 2011;108:18378–18383. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007;130:986–988. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Sourbier C, Lindner V, Lang H, Agouni A, Schordan E, Danilin S, Rothhut S, Jacqmin D, Helwig JJ, Massfelder T. The phosphoinositide 3-kinase/Akt pathway: a new target in human renal cell carcinoma therapy. Cancer Res. 2006;66:5130–5142. doi: 10.1158/0008-5472.CAN-05-1469. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gafur MA, Choudhury AK, Evans FJ. Sapintoxin A, a new biologically active nitrogen containing phorbol ester. Experientia. 1981;37:681–682. doi: 10.1007/BF01967918. [DOI] [PubMed] [Google Scholar]
- Tong WH, Sourbier C, Kovtunovych G, Jeong SY, Vira M, Ghosh M, Romero VV, Sougrat R, Vaulont S, Viollet B, Kim YS, Lee S, Trepel J, Srinivasan R, Bratslavsky G, Yang Y, Linehan WM, Rouault TA. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Lindquist S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin Ther Targets. 2009;13:469–478. doi: 10.1517/14728220902832697. [DOI] [PubMed] [Google Scholar]
- Williams RD, Elliott AY, Stein N, Fraley EE. In vitro cultivation of human renal cell cancer. II. Characterization of cell lines. In Vitro. 1978;14:779–786. doi: 10.1007/BF02617972. [DOI] [PubMed] [Google Scholar]
- Yang Y, Padilla-Nash HM, Vira MA, Abu-Asab MS, Val D, Worrell R, Tsokos M, Merino MJ, Pavlovich CP, Ried T, Linehan WM, Vocke CD. The UOK 257 cell line: a novel model for studies of the human Birt-Hogg-Dube gene pathway. Cancer Genet Cytogenet. 2008;180:100–109. doi: 10.1016/j.cancergencyto.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Valera VA, Padilla-Nash HM, Sourbier C, Vocke CD, Vira MA, Abu- Asab MS, Bratslavsky G, Tsokos M, Merino MJ, Pinto PA, Srinivasan R, Ried T, Neckers L, Linehan WM. UOK 262 cell line, fumarate hydratase deficient (FH−/FH−) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet Cytogenet. 2010;196:45–55. doi: 10.1016/j.cancergencyto.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.