SUMMARY
We report a paracrine effect whereby endothelial cells (ECs) promote the cancer stem cell (CSC) phenotype of human colorectal cancer (CRC) cells. We showed that, without direct cell-cell contact, ECs secrete factors that promoted the CSC phenotype in CRC cells via Notch activation. In human CRC specimens, CD133 and Notch intracellular domain-positive cells co-localized with CRC cells in perivascular regions. An EC-derived, soluble form of Jagged-1, via ADAM17 proteolytic activity, led to Notch activation in CRC cells in a paracrine manner; these effects were blocked by immunodepletion of Jagged-1 in EC conditioned medium or blockade of ADAM17 activity. ECs play an active role in promoting Notch signaling and the CSC phenotype by secreting soluble Jagged-1.
INTRODUCTION
Colorectal cancer (CRC) is the second-leading cause of cancer death in the United States, with ~50,000 patients dying each year due to metastatic disease refractory to systemic therapy (ACS, 2010; Davies and Goldberg, 2008; Davies and Goldberg, 2011). Although there are now six Food and Drug Administration approved drugs for the treatment of metastatic CRC, median overall survival remains less than 2 years (Davies and Goldberg, 2011). Targeted therapies, including anti-angiogenic therapies, have not dramatically improved clinical outcomes of patients with metastatic CRC (Saltz et al., 2008). A better understanding of the biology of CRC is imperative for the development of more effective therapeutic approaches that can benefit CRC patients.
There is evidence for the existence of cancer stem cells (CSCs) in CRC (Barker et al., 2009; Du et al., 2008; Huang et al., 2009; O'Brien et al., 2007; Ricci-Vitiani et al., 2007). Because of the intrinsic stem cell-like properties of CSCs, this sub-population of tumor cells is believed to not only initiate and sustain tumor growth but also mediate chemoresistance (Al-Hajj, 2007; Wicha et al., 2006). Notably, a number of studies suggest that CSCs exist in a state of flux and the CSC phenotype can be enhanced by microenvironmental influences (Butler et al., 2010a; Rosen and Jordan, 2009). Therefore, the development of CSC- and/or microenvironment-targeting strategies that could eliminate the CSC population is critical for improving the clinical outcomes of CRC patients.
Several groups have reported that tumor progenitor cells reside in perivascular niches in certain types of cancers (Butler et al., 2010a; Calabrese et al., 2007; Krishnamurthy et al., 2010). Whether perivascular niches of CSCs exist in other solid tumors, including CRCs, is yet unclear. More importantly, how ECs function to establish and maintain tumor-initiating-cell niches remains to be further elucidated. Understanding the mechanisms by which ECs promote the CSC phenotype will provide the foundation for the development of novel and refined CSC-targeting approaches. The purpose of this study was to understand the role of ECs in mediating the CSC phenotype of CRC cells and the mechanism by which this occurs.
RESULTS
Co-culture With ECs Promotes the Cancer Stem Cell Phenotype of CRC Cells
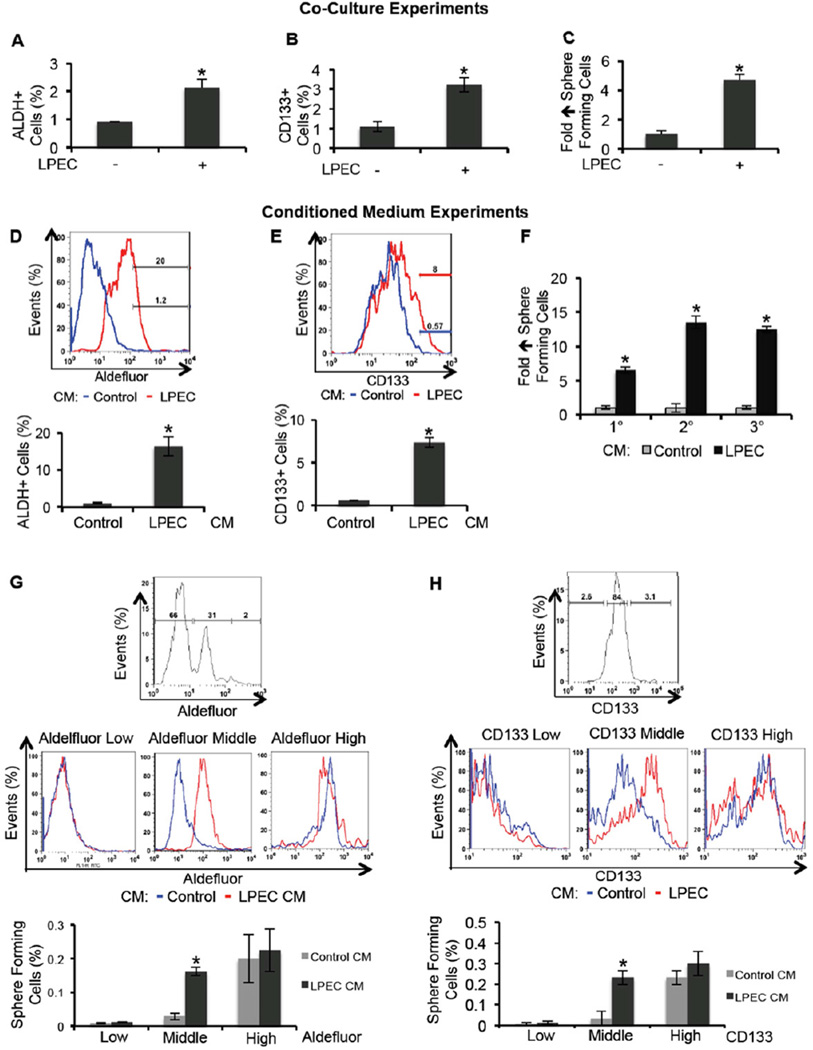
In order to understand tumor-EC cross-talk, and particularly the potential role of ECs in promoting the CSC phenotype of CRC cells, we first conducted a co-culture experiment. We used freshly isolated human CRC cells from surgical specimens after a establishing a first passage xenograft in mice (xhCRC) to expand the number of cells. We also used freshly isolated liver parenchyma ECs (LPECs) labeled with GFP-Luc/mCherry in tissue culture. After co-culture for three days, xhCRC cells were isolated by fluorescence-activated cell sorting (FACS) and analyzed for potential enrichment of the population with CSC characteristics. As shown in Figure 1A, co-culture with LPECs increased the fraction of xhCRC cells that were Aldefluor-positive, a population presumably enriched for CSCs (Huang et al., 2009). Similarly, co-culture of LPECs with CRC cells increased the percent of cells that were CD133 positive (Figure 1B). Co-culture with LPECs also increased the percentage of xhCRC cells with in vitro sphere-forming ability (4-fold, p<0.05, Figure 1C). Collectively, these data suggest that ECs could promote the CSC phenotype of co-cultured CRC cells ex vivo. Similar results were also obtained from in vitro experiments with the established human CRC cell line HCT116 and immortalized human umbilical vein endothelial cell line RF24 (Figure S1A–B).
Figure 1. Endothelial Cells Promote the CSC Phenotype in CRC Cells in vitro.
(A) Freshly isolated xenografted human CRC (xhCRC) cells were co-cultured with freshly isolated human liver parenchyma endothelial cells (LPECs) and the xhCRC Aldefluor-positive cell population was determined.
(B) Freshly isolated xhCRC cells were co-cultured with freshly isolated LPECs and the xhCRC CD133-positive cell population was determined.
(C) Freshly isolated xhCRC cells were co-cultured with freshly isolated LPECs and the xhCRC sphere forming assay was performed.
(D) Freshly isolated human CRC (hCRC) cells were treated with LPEC-derived conditioned medium (CM), and the Aldefluor-positive cell population was determined.
(E) Freshly isolated human CRC (hCRC) cells were treated with LPEC-derived conditioned medium (CM), and the CD133-positive cell population was determined.
(F) Sphere-forming capability in 1°, 2°, and 3° cultures of freshly isolated hCRC cells treated with LPEC CM.
(G) xhCRC cells were FAC-sorted by the Aldefluor assay into high, middle, and low cell populations (upper panel). The Aldefluor-high, -middle, and -low cell populations were treated with LPEC CM in parallel (middle panel); the Aldefluor assay was then repeated to determine which ALDH fraction could be enriched for CSCs by EC CM (lower panel).
(H) xhCRC cells were FAC-sorted for high, middle, and low CD133 expression (upper panel). The CD133-high, -middle, and -low fractions were treated with LPEC CM in parallel (middle panel); the flow cytometry analysis on CD133 was then repeated to determine which CD133 fraction could be enriched for CSCs by EC CM (lower panel).
*p<0.05, mean ± SEM.
See also Figure S1.
Endothelial Cell Conditioned Medium Promotes the Cancer Stem Cell Phenotype of CRC Cells
To determine if the effect observed above required the direct cell-cell contact or if it is mediated by soluble factors secreted by ECs, we cultured freshly isolated human CRC (hCRC) cells in conditioned medium obtained from freshly isolated LPECs, or control medium (MEM containing 1%FBS); after exposure to the CM, hCRC cells were analyzed for the CSC phenotype. Compared to control medium, LPEC CM enriched the Aldefluor-positive hCRC cell population by 16-fold (Figure 1D), and enriched the CD133-positive hCRC cell population by 7-fold (Figure 1E), after 72 of hrs treatment. In addition, LPEC CM treatment also enriched sphere-forming capability of hCRC cells by over 6-fold (Figure 1F). Upon serial passaging, hCRC cells treated with LPEC CM demonstrated significantly greater capability (10–15 fold increase) to form secondary and tertiary tumor spheres (Figure 1F). We were able to obtain similar results from established CRC cell lines (HCT116 and HT29) treated with CM collected from established endothelial cell lines (RF24 and HDMECs), or LPECs (Figure S1C–D). As important controls, CM obtained from freshly isolated human CRC cells, established CRC cell lines (HCT116 and HT29), or fibroblasts were not able to significantly enrich the CSC population in CRC cells (data not shown). Taken together, these data suggest that EC-derived soluble factor(s) can promote the CSC phenotype of CRC cells in a paracrine manner.
We next sought to understand whether ECs promote the colorectal CSC phenotype either by converting a subset of CRC cells into CSCs or by expanding the CSC population. We sorted xhCRC cells by the Aldefluor assay, and treated Aldefluor-high, -middle, and -low fractions with LPEC CM in parallel; the Aldefluor assay was repeated to determine which ALDH population could be enriched for CSCs by EC CM. Interestingly, we found that the CSC population enrichment only occurred in the Aldefluor-middle population, but not in Aldefluor-high (presumably CSCs) or -low populations (presumably terminally differentiated cells) (Figure 1G). Similar results were obtained using another colorectal CSC marker, CD133, as the readout (Figure 1H). These data suggest that the EC CM mediated promotion of the CSC phenotype is primarily achieved in non-stem CRC cells that retain cellular plasticity. We also assessed levels of intestinal differentiation marker expression using quantitative reverse transcription polymerase chain reaction (q-RT-PCR), and found that LPEC CM treatment significantly reduced the expression of Keratin 20 (KRT20) by 8-fold in hCRC cells (Figure S1E); In addition, we tested the expression levels of additional differentiation markers, ANPEP and PRSS7, and found that their expression levels, while easily detectable by qRT-PCR in cells treated with control CM, they were not detectable in the LPEC CM treated group. These findings indicate that LPEC CM promotes the colorectal CSC phenotype by inducing de-differentiation in a subset of non-stem CRC cells.
Endothelial Cell Conditioned Medium Promotes Tumorigenicity and Metastasis of CRC Cells In Vivo
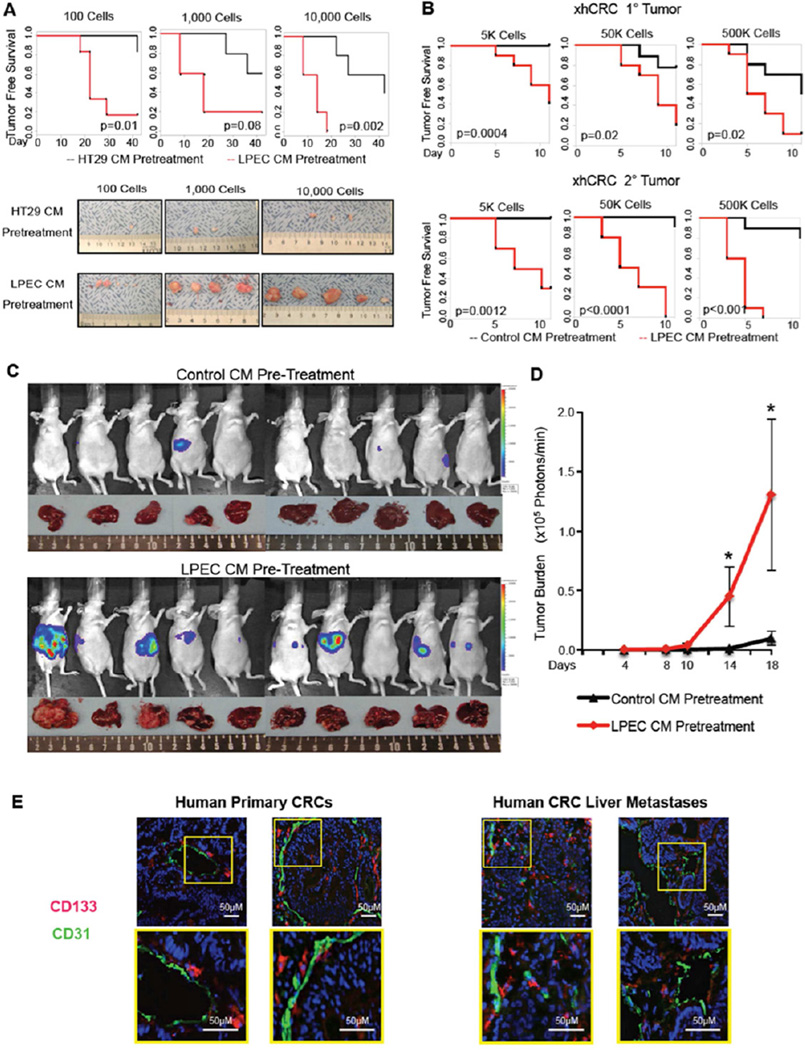
A critical experiment for determining the CSC phenotype is the in vivo tumorigenicity assay by serial dilution (Clarke et al., 2006). Therefore, we sought to validate our in vitro findings by using an in vivo model. To this end, CRC cells pretreated with either EC CM or control CM were serially diluted and injected subcutaneously into mice, and tumorigenicity was determined. LPEC CM pretreated HT29 cells led to earlier tumor formation, as well as greater tumor incidence and growth, compared with control (HT29 CM pretreated) cells (Figure 2A). Similar results were obtained when we compared the effect of RF24 CM on HCT116 tumorigenicity (data not shown). We further extended our study using serial passaging of cells in xenograft experiments with freshly isolated xenografted hCRC (xhCRC) cells, combined with limited dilution, and found that xhCRC cells pretreated with LPEC CM demonstrated significantly increased tumorigenicity compared with those pretreated with control medium, in both primary and secondary xenografts (Figure 2B).
Figure 2. Endothelial Cells Promote the CSC Phenotype of CRC Cells in vivo.
(A) In vivo tumorigenicity assay with limited dilution; HT29 cells pretreated with control CM or LPEC CM.
(B) In vivo tumorigenicity assay with limited dilution combined with serial transplantation using freshly isolated xhCRC cells pretreated with control CM or LPEC CM.
(C/D) Hepatic metastatic incidence and burden of xhCRC cells pre-treated with control CM or LPEC CM in a splenic injection model (*p<0.05, mean ± SEM).
(E) Representative immunofluorescent staining of CD133 and CD31 in human primary CRC surgical specimens (left panel), and human CRC liver metastases (right panel). The highlighted region in the upper panel is enlarged in the lower panel.
See also Figure S2.
We also investigated the effect of EC CM on the metastatic potential of CRC cells. First we employed a tail vein injection, and found that, compared to control (HCT116 CM treated) cells, RF24 CM treated HCT116 CRC cells formed significantly more metastases (Figure S2A–B). We then used a splenic injection model to induce liver metastasis, the most common site of CRC metastasis. Compared with xhCRC cells treated with control medium, xhCRC cells treated with LPEC CM demonstrated an increased incidence of liver metastasis (9 out of 10 in the LPEC CM pretreatment group, compared with 3 out of 10 in the control group. p<0.05) (Figure 2C), and significantly increased tumor burden, demonstrated by an increase in luciferase activity (Figure 2D), and a 57% increase in liver weight (photographs in Figure 2C) (Figure S2C, p<0.05). Collectively, these data demonstrated that EC-derived soluble factor(s) promote the metastatic potential of CRC cells injected in the spleen.
CD133 Positive CRC Cells Are Located Adjacent to ECs in Human Surgical Specimens
Having established that ECs secrete soluble factors to enrich CRC cells for CSC phenotype, we sought to determine whether CRC cells that express the CSC marker CD133 are located in the perivascular regions in human CRC surgical specimens. Immunofluorescent staining for CD133 and CD31 (endothelial cell marker) was carried out in primary colon cancer specimens and chemotherapy-naive human CRC liver metastases. CD133 staining identified CRC cells that were located in proximity to CD31-positive ECs (Figure 2E). Further staining confirmed that these CD133+ cells were not pericytes or stroma cells since CD133 staining did not co-stain with desmin (a pericyte marker) (Figures S2D), nor did they co-stain with smooth muscle actin (a marker of stroma and pericytes), CD3 (a marker of T cells and lymphocytes), CD68 (a marker of monocytes and macrophages), or myeloperoxidase (a marker of myeloid cells) (Figure S2E–H). In addition, we co-stained with antibodies to CD133 and EPCAM (an epithelial marker), and demonstrated co-localization of these markers (Figure S2I). Cumulatively, these immune-staining data show that in clinical specimens, the CD133 positive cells are epithelial in origin and not stromal in origin. These data support our preclinical data suggesting that ECs can mediate the colorectal CSC phenotype in clinical specimens.
Endothelial Cell Conditioned Medium Promotes Chemoresistance of CRC Cells
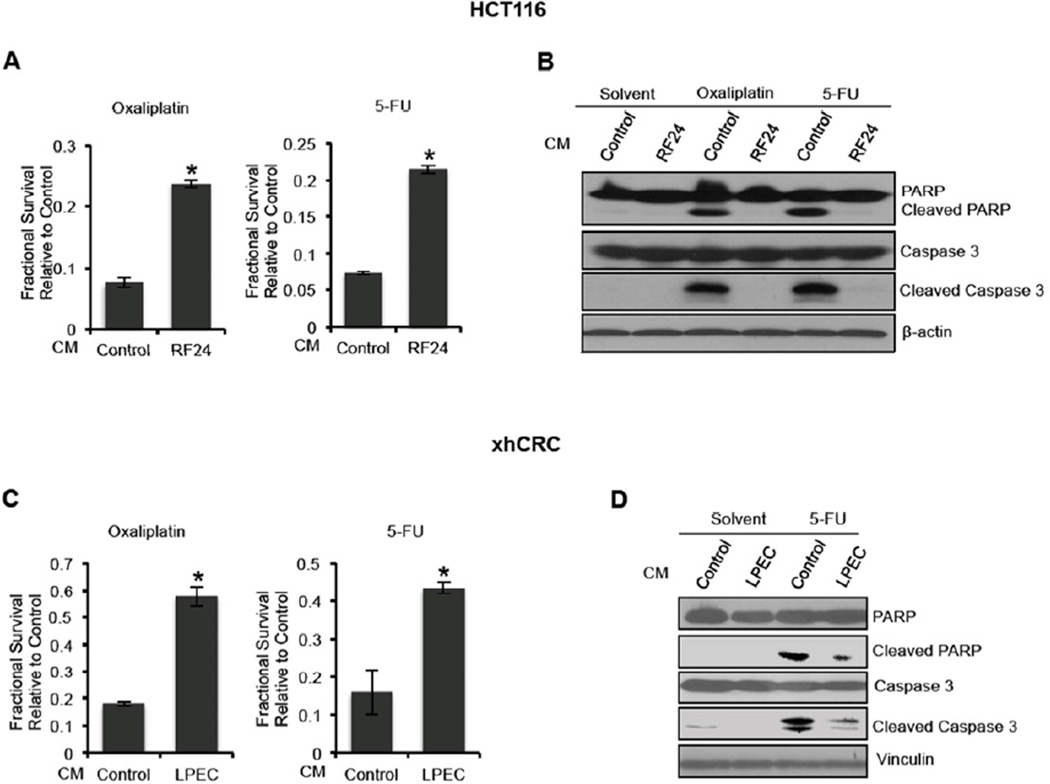
Preclinical studies suggest that CSCs possess intrinsic properties that mediate their resistance to chemotherapy (Al-Hajj, 2007; Dallas et al., 2009; Wicha et al., 2006). We therefore hypothesized that CRC cells pretreated with EC CM would exhibit resistance to chemotherapeutic agents. To test this hypothesis, HCT116 CRC were exposed to oxaliplatin (2µM) or 5-fluorouracil (5-FU, 2µg/ml) in RF24 CM or control medium for 96 hrs; chemosensitivity was determined by MTT assay. Compared with cells that were pretreated with control medium, HCT116 cultured in RF24 CM demonstrated a significantly higher survival rate after oxaliplatin or 5-FU treatment (~3 fold) (Figures 3A). Similarly, LPEC CM treatment for 72 hrs led to chemoresistance of xhCRC cells (Figure 3C). To further validate the chemo-protective effect of EC CM on tumor cells, chemotherapy-induced apoptosis in HCT116 cells was assessed after the cells were treated with chemotherapy while cultured in EC CM or control HCT116 CM for 48 hrs. Annexin V staining demonstrated a significant decrease of apoptotic events in the HCT116 cells treated with oxaliplatin or 5-FU after culture in LPEC CM (28% to 6% with oxaliplatin treatment, and 43% to 15% with 5-FU treatment, respectively, data not shown). Western blot analysis showed lower levels of cleaved poly ADP-ribose polymerase (PARP) and cleaved Caspase 3 in HCT116 cells treated with oxaliplatin or 5-FU while cultured in RF24 CM compared to control CM (Figure 3B). Similar results were observed in xhCRC treated with 5-FU while cultured in LPEC CM (Figure 3D).
Figure 3. Endothelial Cell Conditioned Medium Promotes Chemoresistance of Colorectal Cancer Cells.
(A) HCT116 cells treated with oxaliplatin and 5-FU simultaneous to the addition of control CM or RF24 CM (MTT assay).
(B) Western blot analysis of the expression of pro-apoptotic markers in HCT116 cells treated with oxaliplatin and 5-FU simultaneous to the addition of control CM or RF24 CM.
(C) xhCRC cells treated with oxaliplatin and 5-FU simultaneous to the addition of control CM or LPEC CM (MTT assay).
(D) Western blot analysis of the expression of pro-apoptotic markers in xhCRC cells treated with 5-FU simultaneous to the addition of control CM or LPEC CM.
*p<0.05, mean ± SEM.
Endothelial Cell Conditioned Medium Activates the Notch Pathway in CRC Cells In Vitro
To understand the underlying mechanism by which EC CM promotes the CSC phenotype of CRC cells, we sought to examine the activation of canonical CSC pathways such as Notch (Katoh, 2007), Wnt/beta-catenin (Vermeulen et al., 2010), and Sonic hedgehog (Saif and Chu, 2010) in CRC cells treated with EC CM. To this end, we infected HT29 cells with a lentivirus containing a Hes-1 promoter-driven reporter (Jarriault et al., 1995), TCF reporter, or a Gli reporter (Limgala, 2009), and treated the cells with LPEC CM or control CM. LPEC CM treatment led to significantly increased activity of the Hes-1 reporter but not the Gli or TCF reporter (Figure 4A). Using the identical luciferase reporter assays, we also observed increased Hes-1 promoter activity in xhCRC cells treated with LPEC CM (Figure 4B) and HCT116 cells treated with RF24 CM (Figure S3A). In addition, we found that following EC CM treatment, protein levels of Notch intracellular domain (NICD) and Hes-1 in CRC cells were significantly increased (Figures 4C and S3B–C). These data suggest that EC CM activates Notch signaling in CRC cells.
Figure 4. Endothelial Cells Activate Notch Signaling in Neighboring Colorectal Cancer Cells.
(A) Promoter activity of Hes-1, Gli, and TCF in HT29 cells after treatment with control CM or LPEC CM.
(B) Hes-1 promoter activity in freshly isolated xhCRC cells treated with control CM or LPEC CM (*p<0.05, mean ± SEM).
(C) NICD and Hes-1 expression of freshly isolated hCRC cells treated with control CM or LPEC CM.
(D) Representative immunofluorescent staining for NICD and CD31 in human primary CRCs (left panel) and CRC liver metastases (right panel). The highlighted region in the upper panel is enlarged in the lower panel.
(E) Representative immunofluorescent staining of NICD and CD133 in primary CRCs (left panel) and CRC liver metastases (right panel).
See also Figure S3.
To determine whether Notch activation is indeed responsible for the promotion of the colorectal CSC phenotype by EC CM, gamma secretase inhibitor X (GSI), which inhibits the liberation of NICD, was added to RF24 CM during treatment of the CRC cells. GSI treatment blocked EC CM induced Notch signaling activation and CSC enrichment, as demonstrated by the suppression of both NICD up-regulation and Aldefluor-positive cell population increase (Figures S3D–E).
CRC Cells Adjacent to ECs in Human Specimens Demonstrate Activation of Notch Signaling
Having established that ECs secrete soluble factors to activate Notch signaling, we sought to determine whether CRC cells located in perivascular regions in human tumor samples displayed Notch activation. Immunofluorescent staining for NICD and CD31 was carried out in human CRC surgical specimens. We found that tumor cells expressing NICD were predominantly located in the perivascular region (Figure 4D). These NICD+ cells are not pericytes since NICD staining does not overlap with desmin staining (Figures S3F). Furthermore, co-immunofluorescent staining of CD133 and NICD highlighted the co-localization of CD133-positive and NICD-positive CRC cells in the perivascular niches (Figure 4E).
ECs Secrete a Soluble Form of Jagged-1 to Promote the CSC Phenotype in CRC Cells
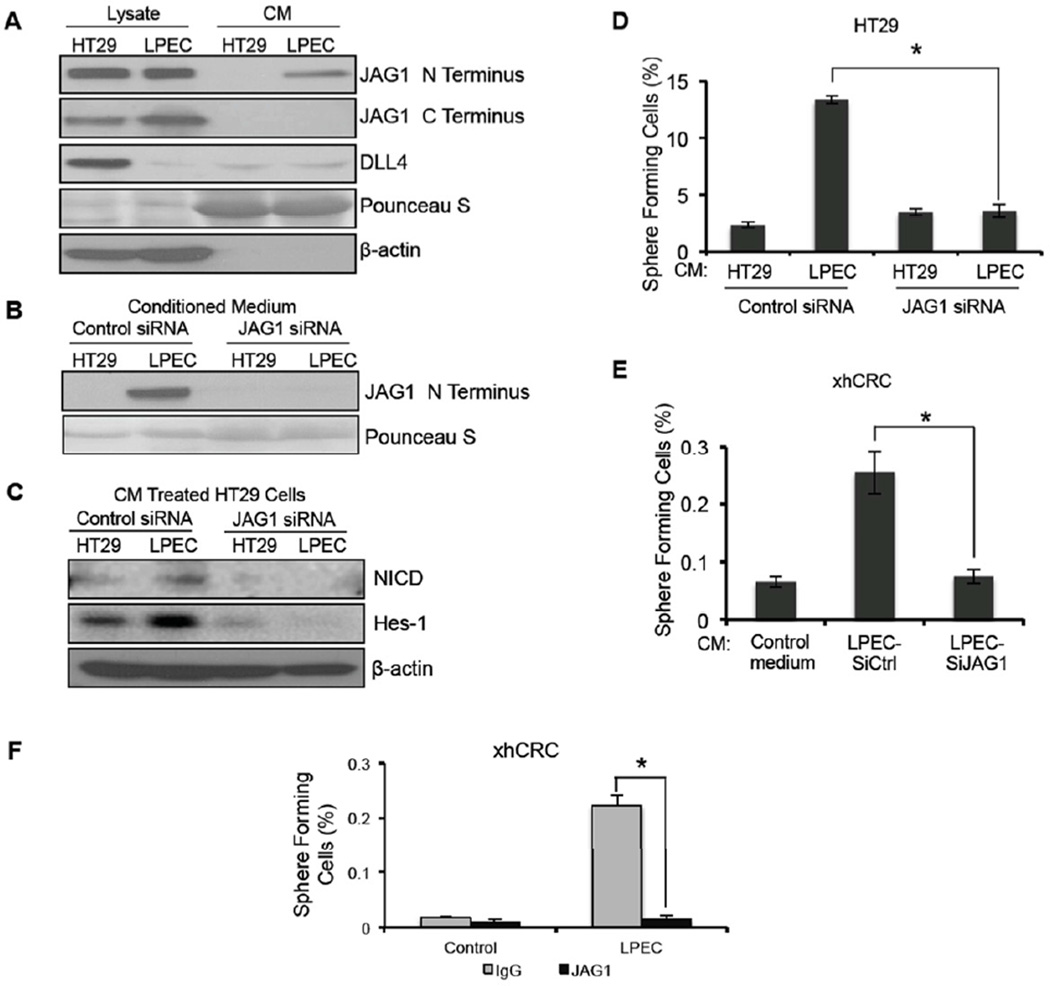
Since the Notch signaling pathway in CRC cells was activated by soluble factors in EC CM, we sought to identify the molecules that activate Notch signaling. Although Notch activation is thought to occur exclusively via a juxtacrine mechanism, some studies reported on the presence of soluble Notch ligands with variable effects on Notch signaling (Hukriede and Fleming, 1997; Nickoloff et al., 2002; Sun and Artavanis-Tsakonas, 1997). Although in contrast to the classic view of Notch activation, our studies suggested the presence of a soluble Notch ligand that was an activator of Notch signaling. Therefore, we examined the presence of the known Notch-1 ligands DLL4 and Jagged-1 in EC CM using western blot analysis. Our results showed that DLL4 was not differentially secreted by CRC cells and ECs, but a significantly greater amount of Jagged-1 (N-terminus antibody) was detected in LPEC and RF24 CM compared to that in HT29 and HCT116 CM (Figure 5A and S4A). Since Notch-activating Jagged-1 is usually membrane bound, we sought to determine if the Jagged-1 in EC CM was associated with microvesicles. Even after depletion of microvesicles, Jagged-1 could still be detected in the RF24 CM supernatant (Figure S4B), suggesting that the Jagged-1 found in EC CM is not anchored on microvesicles.
Figure 5. Endothelial Cells Secrete a Soluble Form of Jagged-1 to Promote the CSC Phenotype in Colorectal Cancer Cells.
(A) Western blot analysis of HT29 and LPEC cell lysates and conditioned medium utilizing antibodies to the N-terminus and C-terminus regions of Jagged-1 (JAG1). Western blotting for DLL4 was also performed.
(B) Detection of soluble Jagged-1 in the conditioned medium from HT29 cells and LPECs after siRNA-mediated Jagged-1 knockdown.
(C) NICD and Hes-1 expression in HT29 cells after treatment with control CM or LPEC CM with decreased Jagged-1 levels by siRNA knockdown.
(D) Sphere forming assay of HT29 cells exposed to control CM or LPEC CM with decreased Jagged-1 levels by siRNA knockdown.
(E) Sphere forming assay of freshly isolated xhCRC cells exposed to control CM or LPEC CM with decreased Jagged-1 levels by siRNA knockdown.
(F) Sphere forming assay of freshly isolated xhCRC cells exposed to the LPEC CM that is Jagged-1 depleted by immunoprecipitation.
See also Figure S4.
To determine if this soluble form of Jagged-1 is responsible for the EC-mediated paracrine or angiocrine activation of Notch signaling in neighboring cancer cells, we first used small interfering RNA (siRNA) to knock down Jagged-1 expression in LPEC cells and decrease secretion of soluble Jagged-1 in LPEC CM (Figure 5B). CM collected from Jagged-1 siRNA- or control (scrambled) siRNA-transfected LPECs were applied to HT29 cells for 72 hrs, and activation of Notch signaling pathway and the CSC phenotypes were then assessed. Depletion of Jagged-1 in LPEC CM by siRNA blocked the EC CM mediated activation of Notch signaling in HT29 cells, as demonstrated by the decreased expression of NICD and Hes-1 compared with that in controls (Figure 5C). Furthermore, depletion of Jagged-1 in LPEC CM also blocked its effect on promotion of the CSC phenotype in HT29 cells and xhCRC cells, as demonstrated by blockade of sphere-forming ability (Figure 5D–E). Similarly, depletion of Jagged-1 in RF24 CM by siRNA (Figure S4C) blocked the activation of Notch signaling (Figure S4D) and blocked enrichment of the Aldefluor-positive cells and sphere forming ability (Figures S4E–F).
We also used an immunoprecipitation-based approach to deplete Jagged-1 in EC CM to determine whether Jagged-1 was responsible for promoting the CSC phenotype. Following depletion of soluble Jagged-1 in EC CM by an antibody recognizing the N-terminus of Jagged-1 (Figure S4G), the EC CM was no longer able to enrich for cells with sphere-forming capability in xhCRC cells and HCT116 cells (Figures 5F and S4H). In a follow-up study, we added a Jagged-1 neutralizing antibody directly to the LPEC CM, and it blocked the enrichment of the sphere forming population of freshly isolated xhCRC cells (data not shown).
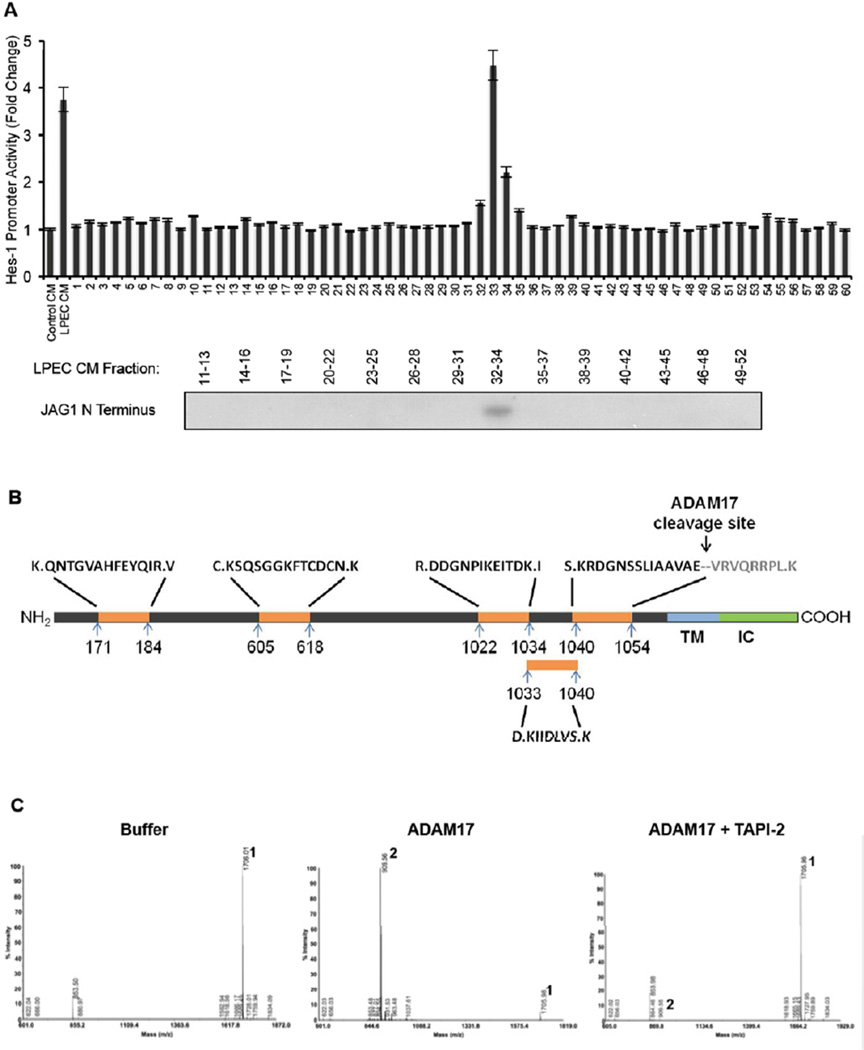
We obtained additional confirmation that the soluble form of Jagged-1 secreted by ECs is indeed the major factor that activates Notch signaling and promotes the CSC phenotype in neighboring CRC cells. First, we fractionated LPEC CM using size exclusion-fast protein liquid chromatography, and determined the fractions for activity that induce Notch signaling using a Hes-1 promoter driven luciferase reporter assay. As is shown in Figure 6A, we identified a single bioactive peak within the combined fractions 32–34 that induced Notch signaling. Simultaneously, we examined the presence of soluble Jagged-1 in the various fractions by Western blotting, and found that the soluble Jagged-1 containing fractions corresponded directly to the fractions (32–34) that activated the Hes-1 reporter (Figure 6A). Second, we immunoprecipitated Jagged-1 from EC CM and ran the IP sample in a polyacrylamide gel electrophoresis (PAGE) gel. After staining with Coomassie blue, we observed a single band representing Jagged-1 (data not shown). Next, we ran both native and denatured LPEC CM protein samples on a non-denaturing PAGE gel, and blotted for Jagged-1 by Western blotting. As shown in Figure S5A, the soluble form of Jagged-1 secreted by ECs is not stably associated with other proteins and is monomeric. These data indicate that the soluble form of Jagged-1 that we identified is the Notch activating molecule in EC CM.
Figure 6. Proteomics Analysis Demonstrate that the EC-secreted Soluble Form of Jagged-1 is C-terminally Truncated by ADAM17.
(A) Concentrated LPEC CM was fractionated by FPLC gel filtration. The fractions were applied to xhCRC cells containing the Hes-1 promoter - luciferase construct, and Hes-1 promoter activity was assessed (upper panel). Western blot detection of soluble Jagged-1 in combined adjacent fractions is shown in the lower panel.
(B) Jagged-1 from LPEC CM was immunoprecipitated using an N-terminal antibody and subjected them to deglycosylation followed by digestion. Mass spectrometric analysis of Jagged-1 proteins was performed with multiple peptides that identified consistent with the N-terminal region of Jagged-1 with a C-terminus at amino acid 1054.
(C) Mass spectrometric analysis of the ADAM17 cleavage site of Jagged-1. A synthetic peptide corresponding to aa1047–1061 of Jagged-1 was incubated with buffer only (left panel). ADAM17 (middle panel), or ADAM17 with its inhibitor TAPI-2 (right panel), and the reaction products were subjected to mass spectrometry. Peak 1 represents the intact substrate SLIAAVAEVRVQRRP, and Peak 2 represents the ADAM17 cleavage product, a 906.56 Da peptide that was determined to be VRVQRRP.
See also S5.
The EC-secreted Soluble Form of Jagged-1 is a C-terminally Truncated Protein
In CRC and EC cell lysates, intact Jagged-1 could be detected by both Jagged-1 N-terminus-specific and C-terminus-specific antibodies. However, soluble Jagged-1 in EC CM could only be detected by the N-terminus-specific antibody (Figures 5A and S4A). After de-glycosylation, the molecular weight of soluble Jagged-1 in CM was ~15–20 kD smaller than that of full length Jagged-1 (Figure S5B). These results suggest that this soluble Jagged-1 is a C-terminally truncated form.
In order to gain insights into whether this C-terminally truncated form of soluble Jagged-1 is relevant in human CRCs, we co-stained human CRC specimens using an antibody against either N-terminal or C-terminal Jagged-1; we simultaneously stained tissue sections with, an antibody against the endothelial cell marker CD31. As shown in Figure S5C, staining with the C-terminal Jagged-1 antibody, which only reacts with full length Jagged-1, largely overlaps with CD31 staining. In contrast, the N-terminal Jagged-1 antibody, which recognizes both full-length and soluble Jagged-1, not only co-stains CD31+ ECs, but also strongly stains areas that are adjacent to ECs. These data support the existence of soluble Jagged-1 in human CRCs.
We then sought to determine the nature of the soluble Jagged-1 protein enriched in the EC CM. First, we sought to determine whether soluble Jagged-1 was a product of alternative splicing. We performed semi-quantitative RT-PCR to detect Jagged-1 splicing variants in CRC cells and ECs, and excluded this possibility as these two type of cells expressed equal levels of the alternatively spliced Jagged-1 mRNA (Figure S5D). We then hypothesized that a post-translational event leads to the generation of this soluble Jagged-1. When we transfected full-length Jagged-1 cDNA into LPEC cells, an increase of soluble Jagged-1 secretion in its CM was observed (data not shown). Compared with the CM of mock-transfected LPECs, CM collected from LPECs with Jagged-1 over-expression could further promote the CSC phenotype of HT29 cells, as demonstrated by a further enrichment of the Aldefluor-positive cell population (data not shown). To further test this possibility, we treated LPECs and RF24 cells with a protease inhibitor cocktail and found that broad spectrum inhibition of protease activity resulted in reduction of the soluble Jagged-1 generation (Figure S5E), further suggesting that EC-secreted soluble Jagged-1 is a product derived from protease cleavage of full-length Jagged-1. Taken together, these results suggest that ECs are capable of post-translational modifications that lead to the production of this soluble form of Jagged-1.
Proteomic Analysis of Soluble Jagged-1
In order to further define the structure of the soluble Jagged-1 protein, we employed a mass spectrometry-based strategy. Briefly, we isolated the Jagged-1 proteins from LPEC CM via immunoprecipitation using an N-terminal antibody and subjected them to deglycosylation followed by digestion. Mass spectrometry identified multiple peptides consistent with the Jagged-1 amino acid sequence (Figure 6B), confirming the identity of soluble Jagged-1 protein in the EC CM. In addition, we identified a peptide “KRDGNSSLIAAVAE” bearing only one Lys-N site, indicating that the possible C-terminus of soluble Jagged-1 was at amino acid 1054. The identical proposed C-terminal peptide was also found in protein samples isolated by immunoprecipitation of N-terminal Jagged-1 from CM of a second endothelial cell line, RF24, indicating that this EC-secreted soluble form of Jagged-1 might be truncated at amino acid 1054 (Figure 6B).
ADAM17 Cleaves Jagged-1 on ECs to Produce the Soluble Form Jagged-1
A recent study reported that the protease ADAM17 could mediate Jagged-1 shedding (Parr-Sturgess et al., 2010); however, the investigators did not report on the specific cleavage site or the function of this cleaved product. Interestingly, by analyzing the protein sequences flanking E1054 of Jagged-1 (Figure 6B), we found that VAEV is a plausible recognition motif for the protease ADAM17, which may cleave the protein between E1054 and V1055 (Caescu et al., 2009). Therefore, we hypothesized that ADAM17 is the protease that cleaves Jagged-1 in ECs to generate the soluble form of the protein. To test our hypothesis, we first synthesized a peptide consisting of aa1047–1061 (SLIAAVAEVRVQRRP) of Jagged-1 and subjected it to ADAM17 cleavage followed by mass spectrometry analysis. After ADAM17 cleavage, a 906.56 Da peptide was identified and determined to be VRVQRRP (Figure 6C, middle panel); this cleavage was inhibited by TAPI-2, an inhibitor of ADAM17 (Figure 6C, right panel). These data confirm that ADAM17 specifically cleaves Jagged-1 between residues E1054 and V1055. We next co-transfected full-length human Jagged-1 and ADAM17 expression plasmids into a mouse fibroblast cell line, L cells. As shown in Figure S6A, we found that the soluble form of Jagged-1 was enriched in the conditioned medium (Here we used two different antibodies that recognize the N-terminus of Jagged-1, to rule out any possibility of a none-specific signal with the use of a single antibody); this conditioned medium could promote the CSC phenotype of freshly isolated hCRC cells, as documented by CD133 FACS analysis (Figure S6B) and sphere forming ability (Figure S6C). These data confirm that ADAM17 could cleave full-length Jagged-1, leading to extracellular release of the soluble form of Jagged-1, which is sufficient to promote the colorectal CSC phenotype.
Inhibition of ADAM17 Blocks the Angiocrine Effect of ECs That Promotes the Colorectal CSC Phenotype By Blocking Soluble Jagged-1 Production
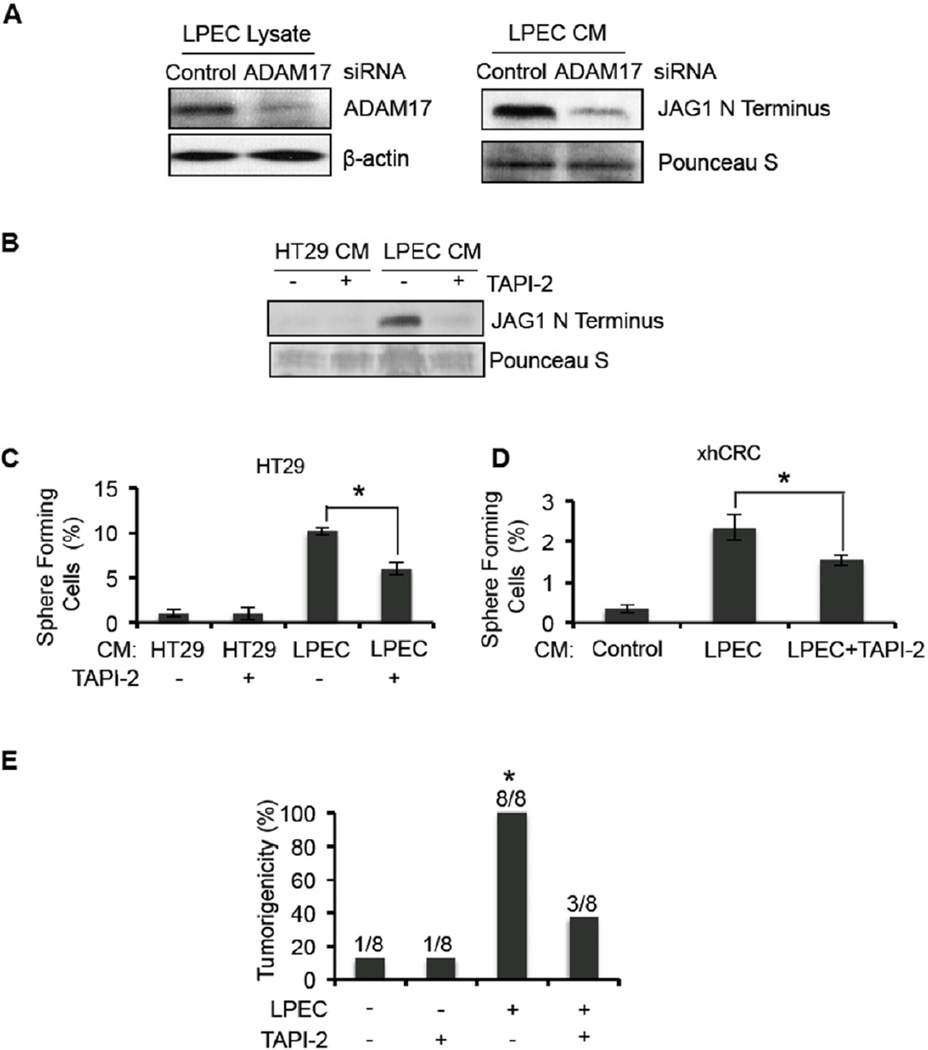
We sought to investigate whether inhibition of ADAM17 could suppress the endothelial cell mediated promotion of colorectal CSC phenotype by blocking Jagged-1 production. We knocked down ADAM17 expression in LPECs by siRNA and found that this resulted in a significant decrease in the production of soluble Jagged-1 in LPEC CM (Figure 7A). We then used the ADAM17 inhibitor TAPI-2 to determine if inhibition of ADAM17 activity would block production of soluble Jagged-1. Treatment of LPECs with TAPI-2 significantly reduced soluble Jagged-1 production in its CM (Figure 7B). Importantly, TAPI-2 also inhibited the angiocrine effect of LPECs that promotes the CSC phenotype of CRC cells, as demonstrated by decreased enrichment of sphere forming populations in HT29 cells and freshly isolated xhCRC cells (Figure 7C–D). These studies confirm that ADAM17 is essential for soluble Jagged-1 production by ECs.
Figure 7. Inhibition of ADAM17 in Endothelial Cells Blocks the Conditioned Medium Promotion of the CSC Phenotype in Human CRC Cells.
(A) Western blot detection of ADAM17 in the cell lysate of LPECs or LPECs with decreased ADAM17 by siRNA knockdown (left panel). The soluble form of Jagged-1 in the CM from the cells shown in the left panel, are shown in the right panel.
(B) Secretion of the soluble form of Jagged-1 into the CM of HT29 cells and LPECs treated with the ADAM17 inhibitor TAPI-2.
(C/D) Sphere forming assays on HT29 cells (Panel C) and freshly isolated xhCRC cells (Panel D) after treatment with CM from LPECs, with or without the ADAM17 inhibitor TAPI-2 (*p<0.05, mean ± SEM).
(E) In vivo tumorigenicity assay (day 10) using freshly isolated xhCRC cells co-injected with LPECs with/without daily TAPI-2 treatment (*p<0.05, LPEC/no TAPI-2 vs all other groups).
See also S6.
Finally we performed an in vivo study using xenograft models to determine if inhibition of ADAM17 can block the angiocrine promotion of tumorigenicity. We co-injected freshly isolated xhCRC cells with LPECs subcutaneously into mice, and treated the animals with daily TAPI-2 by intraperitoneal (IP) injection. We found that TAPI-2 treatment significantly suppressed tumor initiation (Figure 7E). This data suggests that ADAM17 mediated shedding of Jagged-1 from ECs is an important mechanism that mediates the angiocrine effect on tumor initiation.
Taken together, our data suggest that ADAM17 is the protease that cleaves Jagged-1 in ECs, and the resultant soluble Jagged-1 functions in a paracrine manner to activate Notch signaling and promote the CSC phenotype of nearby CRC cells.
DISCUSSION
The concept of targeting the tumor vasculature as an antineoplastic strategy was developed ~40 years ago by Folkman (Folkman, 1971). However, therapeutic agents targeting classic angiogenic mediators, such as vascular endothelial growth factor, have only minimally improved progression-free and overall survival when added to chemotherapy regimens for patients with metastatic CRC (Saltz et al., 2008). Although tumor vascularity correlates with the aggressiveness of CRC, this does not necessarily imply an associated increase in blood flow (Takahashi et al., 1995; Weidner et al., 1991)). It is possible that ECs play a more active role in mediating tumor growth and metastasis than simply providing the physical structure that forms conduits for blood flow. The development of more effective agents targeting the vasculature may require approaches that go beyond targeting the classic angiogenic pathways by current means.
Here, we provide several lines of evidence demonstrating that ECs secrete soluble factors that promote the colorectal CSC phenotype via a paracrine effect: CM derived from ECs can 1) enrich the Aldefluor-positive CRC cell population, 2) enrich CRC cells expressing putative colorectal CSC surface marker CD133, 3) increase CRC cell sphere-forming capability, 4) enhance tumorigenicity, and 5) confers chemoresistance to CRC cells. We also determined that the major EC-secreted factor is an un-reported form of Jagged-1 that activates Notch signaling in neighboring CRC cells to confer the stem cell-like properties.
The identification (or existence) of CSCs remains a point of controversy. Numerous markers have been used to identify CSCs from CRC specimens, including the cell surface marker CD133, functional studies such as the Aldefluor assay and sphere-forming ability, and in vivo studies such as the serial dilution based tumorigenicity assay. In our studies, we assessed all of these markers, and all confirmed that ECs secrete a factor(s) that increase the percentage of CRCs with CSC properties. We believe the tumorigenicity and chemoresistance assays are the most important studies that potentially have clinical implications.
Many studies suggest that CSCs originate from mutations in stem cells that cause malignant transformation (Barker et al., 2009; Dick, 2008; Korkaya and Wicha; Passegue et al., 2003). However, other investigators have noted that CSCs exist in a state of flux and that the CSC population can be enriched by microenvironmental influences (Chaffer et al., 2011; Rosen and Jordan, 2009; Vermeulen et al., 2010). In our studies, EC CM increased the percentage of cells with CSC characteristics, further supporting the idea that the microenvironment can regulate the CSC phenotype. Our findings that EC CM can convert a non-CSC to a cell with CSC properties are in line with recent studies showing that CSCs and non-CSCs do not exist in fixed states (Chaffer et al., 2011; Gupta et al, 2011). More importantly, we demonstrated that Notch signaling activation by a soluble ligand is an important mechanism through which the CSC population is enriched in CRC by the microenvironment.
The observation that CSCs accumulate in perivascular regions has been reported by several groups (Butler et al., 2010a; Calabrese et al., 2007; Krishnamurthy et al., 2010). EC-secreted factors have been shown to promote the self-renewal of CSCs in squamous cell carcinomas, as demonstrated by up-regulated Bmi-1 expression and an increase in sphere formation in vitro (Krishnamurthy et al., 2010). Others have shown that ECs could promote glioblastoma stem-like cell expansion by secreting factors that activate mTOR signaling (Galan-Moya et al., 2011). These studies support the notion that tumor-associated ECs play a direct role in maintaining a population of CSCs. We demonstrated that ECs produce a truncated soluble form of Jagged-1 that activates Notch signaling in CRC cells to promote their CSC phenotype. We also showed that, in both primary and metastatic human CRC specimens, NICD- and CD133-positive cells are primarily located in perivascular areas. Our findings suggest that ECs play an important role in establishing a perivascular niche for colorectal CSCs through inducing Notch signaling in tumor cells by releasing soluble Jagged-1 in a paracrine manner.
Rafii and colleagues reported that EC expression of Jagged-1 and Jagged-2 could stimulate the expansion of Notch-dependent hematopoietic stem cells (Butler et al., 2010b). Similarly, Fan’s group reported that human brain microvascular ECs could activate Notch signaling in adjacent glioblastoma cells in a juxtacrine manner (Zhu et al., 2011). Taketo and colleagues also reported that tumor-associated ECs express Jagged-1 and Dll-4 that activate Notch signaling on CRC cells (Sonoshita et al., 2011). In these studies, direct cell-cell contact (juxtacrine signaling) was required for ECs to maintain hematopoietic/cancer stem cell propagation (Butler et al., 2010b; Kobayashi et al., 2010). In our studies however, we defined a previously unrecognized mechanism through which ECs activate Notch signaling to promote CRC cell stemness, namely, production of a soluble paracrine/angiocrine Notch ligand.
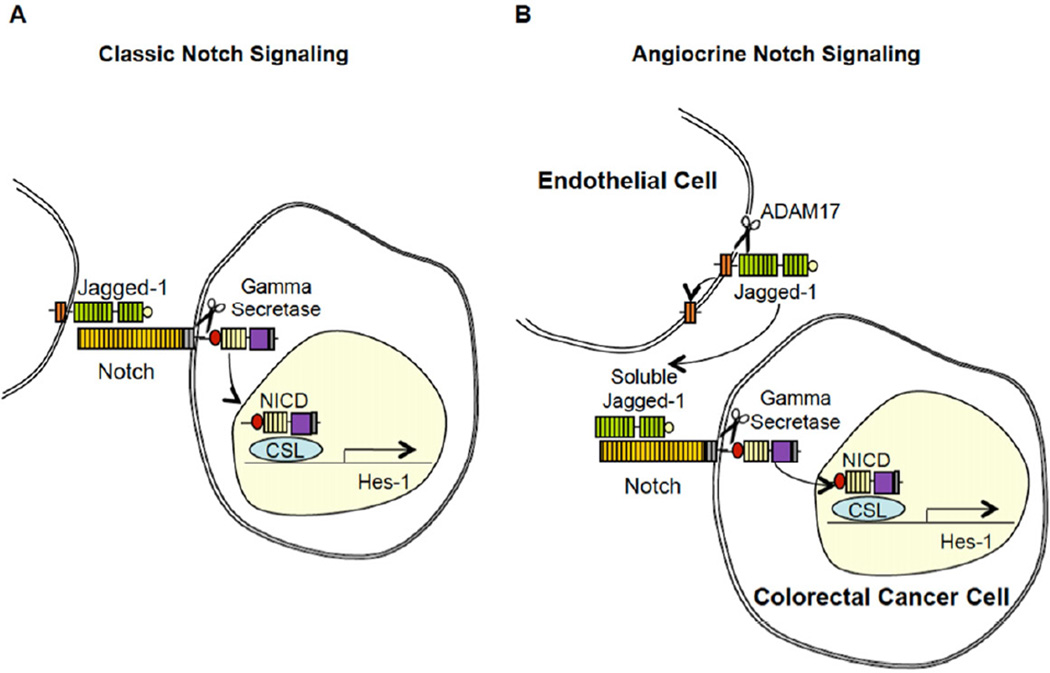
In this study we were able to determine the identity of the soluble form of Jagged-1 secreted by ECs, produced through ADAM17 cleavage. In addition, we determined that this soluble form of Jagged-1 activates Notch signaling and, in turn, mediates the CSC phenotype of CRC cells. In the classic model of Notch signaling (Figure 8A), Notch is activated by cell surface bound ligands, where cell-cell contact is required. We propose a Notch activation model, as shown in Figure 8B, whereby the full-length Jagged-1 is cleaved by ADAM17, which results in the production of a truncated soluble form of Jagged-1 that can activate Notch signaling in nearby cells. This manner of Notch pathway activation does not require cell-cell contact, and therefore, we refer to it as paracrine/angiocrine activation of Notch.
Figure 8. Proposed Model for EC-mediated Paracrine Activation of Notch Signaling in Colorectal Cancer Cells.
(A) The conical model for Notch pathway activation, where membrane bound ligands such as Jagged-1 activates Notch signaling in contacting cells.
(B) Schematic summarizing our proposed model for paracrine activation of the Notch pathway in CRC cells. ADAM17 cleaves membrane bound Jagged-1 on ECs, releasing an N-terminal soluble fragment that binds, and activates, Notch on CRC cells.
Several studies have reported contrasting biologic effects of soluble Notch ligands. Two groups generated soluble mutants of Drosophila Notch ligands Delta and/or Serrate, and found that these forms could function as antagonists of Notch signaling (Hukriede and Fleming, 1997; Sun and Artavanis-Tsakonas, 1997). Similarly soluble forms of human Jagged-1 and DLL-1 composed only of the extracellular domain were reported to inhibit Notch signaling (Small et al., 2001; Trifonova et al., 2004). An engineered soluble Notch ligand, DLL4-Fc, in which the extracellular domain of DLL4 was fused to a human IgG1 Fc fragment, has been shown to inhibit Notch activation (Lobov et al., 2007; Noguera-Troise et al., 2006). In contrast, several groups have shown that soluble Jagged1-derived peptides are able to activate Notch signaling in several cell types, leading to keratinocyte differentiation (Nickoloff et al., 2002), maturation of dendritic cells (Weijzen et al., 2002) apoptosis in B cell leukemia cells (Kannan et al., 2011), and inhibited differentiation of hematopoietic precursor cells (Li et al., 1998). Importantly, a naturally occurring soluble form of Jagged1 was found in human skin, inducing epithelial differentiation, a phenotype consistent with Notch activation (Aho, 2004). We found a naturally occurring soluble Jagged-1 that can activate Notch signaling in CRC cells. The reasons for the apparent discrepancies among different studies are not well understood. A major determinant of whether a soluble ligand activates or inhibits Notch signaling could be the specific structure of the ligand. Thus we speculate that distinct forms of soluble Notch ligands, due to different species, cleavages, post-translational modifications, and/or engineering strategies, may account for their different biologic behaviors.
Although Notch inhibitors are being studied in clinical trials, toxicity has been noted limiting clinical development. Our discovery of truncated soluble form of Jagged-1 that activates Notch signaling in CRC cells has potential clinical implications. Neutralization of Notch activation triggered by soluble Jagged-1 may be a more specific and refined approach to sensitize tumors to anti-neoplastic therapies.
Although we believe that soluble Jagged-1 is the major factor that mediates the angiocrine effect that promotes the colorectal CSC phenotype, we do not exclude the possibility that other signaling events that may also contribute to induction of the CSC phenotype. Given the obvious complexity of tumor microenvironment, we believe other important angiocrine factors remain to be identified, and this will be important in the development and refinement of new therapeutic approaches.
In summary, our study defines a mechanism for the angiocrine function of ECs that leads to Notch activation that, in turn, promotes the CSC phenotype. This study reports the generation of an active, soluble form of Jagged-1 that is the product of ADAM17 protease activity. Our findings, along with other studies in the field of angiocrine signaling, suggest that ECs are more than simply conduits for nutrient and oxygen delivery - they also contribute soluble factors to promote the CSC phenotype and chemoresistance. It is possible that targeting angiocrine signaling, including soluble Jagged-1 and/or ADAM17, may improve the outcomes of therapy for patients with metastatic CRC.
EXPERIMENTAL PROCEDURE
Isolation of Human CRC Cells and Liver Parenchyma ECs
Human CRC (hCRC) cells and LPECs were isolated from CRC or liver parenchyma tissue from surgical specimens, respectively. Xenografted human CRC (hxCRC) cells were isolated from mice after a direct inoculation with freshly isolated human CRC cells derived from patients (See Supplemental Experimental Procedures). The MDACC Institutional Review Board approved human specimen procurement, and the informed consent was obtained.
Tumorigenicity Studies
All mice were housed in the MDACC animal facility, and all experiments were performed in accordance with guidelines approved by the MDACC Institutional Animal Care & Use Committee. LPEC CM pretreated xhCRC cells were injected subcutaneously into nude mice. Primary xenografts were harvested, and treated with LPEC CM for 7 days before secondary xenograft passaging (See Supplemental Experimental Procedures). Tumor growth was monitored by a blinded investigator.
In Vivo Metastasis Studies
Luciferase labeled CRC cells were pre-treated with EC CM for 7 days, and injected intravenously (Saltz et al.) via tail vein, or via the spleen (liver metastasis model). Luciferase activity was used to assess the relative tumor burden (See Supplemental Experimental Procedures).
Statistical Analysis
For all in vitro and ex vivo experiments, statistical analyses were conducted using Student’s t test (Microsoft Excel). For in vivo studies, statistical analyses were performed using the Kaplan Meier test (Software R) (tumorigenicity), Chi-square test (Microsoft Excel) (tumor incidence), and Mann Whitney-U test (number of metastatic sites and tumor burden). All statistical tests were two-sided, and p values ≤0.05 were considered to be significant.
Supplementary Material
HIGHLIGHTS.
ECs secrete soluble Jagged-1 promoting the colon cancer stem cell phenotype.
EC ADAM17 cleaves Jagged-1 to activate Notch in CRC cells via angiocrine signaling.
CD133-positive and NICD-positive CRC cells are located in the perivascular niche.
SIGNIFICANCE.
We found that endothelial cell (EC)-derived soluble factors enhance the cancer stem cell (CSC) phenotype and chemoresistance of colorectal cancer cells (CRCs). This suggests that ECs in the tumor microenvironment are more than simply conduits for blood flow - they also actively secrete factors that promote tumor cell survival and growth. We further defined a soluble, ADAM17-cleaved form of Jagged-1 in endothelial cell conditioned medium that activates Notch signaling and promotes the CSC phenotype in CRC cells. Identification of this mechanism, by which the tumor microenvironment can promote the CSC phenotype, should provide the foundation for more refined targeted therapies for the treatment of both early and late stage malignancies.
ACKNOWLEDGMENTS
This work was supported, in part, by NIH Cancer Center Support Grant CA016672, NIH grant T32CA009599 (S. B., F.T. and E.S.), NIH Grant R01CA157880 (L.M.E.), DOD grant CA100879 (L.M.E.), the William C. Liedtke, Jr., Chair in Cancer Research (L.M.E.), and an R. E. “Bob” Smith Fellowship (S.S.). The authors thank Li Huang and Menashe Bar-Eli, PhD, from the Dept of Cancer Biology, MDACC, for molecular sub-cloning of full-length Jagged-1 and ADAM17. The authors thank John Ladbury, PhD, from The Center of Biomolecular Structure and Function (CBSF), for assistance in identification of the Jagged-1 cleavage site. The authors thank Francesco Stingo, PhD, from Dept of Biostatistics for statistical support. The authors thank Zach Bohannan, Sunita Patterson, and Claire Dawn from the Dept of Scientific Publications, and Rita Hernandez from the Departments of Surgical Oncology and Cancer Biology for editorial assistance. All of the above are from the MDACC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- ACS. Cancer Statistic 2010. 2010 http://wwwcancerorg/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-and-figures-2010. [Google Scholar]
- Aho S. Soluble form of Jagged1: unique product of epithelial keratinocytes and a regulator of keratinocyte differentiation. J Cell Biochem. 2004;92:1271–1281. doi: 10.1002/jcb.20125. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M. Cancer stem cells and oncology therapeutics. Curr Opin Oncol. 2007;19:61–64. doi: 10.1097/CCO.0b013e328011a8d6. [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010a;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010b;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caescu CI, Jeschke GR, Turk BE. Active-site determinants of substrate recognition by the metalloproteinases TACE and ADAM10. The Biochemical journal. 2009;424:79–88. doi: 10.1042/BJ20090549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, 2nd, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JM, Goldberg RM. First-line therapeutic strategies in metastatic colorectal cancer. Oncology (Williston Park) 2008;22:1470–1479. [PubMed] [Google Scholar]
- Davies JM, Goldberg RM. Treatment of metastatic colorectal cancer. Seminars in oncology. 2011;38:552–560. doi: 10.1053/j.seminoncol.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y, Chen Q. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Galan-Moya EM, Le Guelte A, Fernandes EL, Thirant C, Dwyer J, Bidere N, Couraud PO, Scott MG, Junier MP, Chneiweiss H, Gavard J. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO reports. 2011 doi: 10.1038/embor.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukriede NA, Fleming RJ. Beaded of Goldschmidt, an antimorphic allele of Serrate, encodes a protein lacking transmembrane and intracellular domains. Genetics. 1997;145:359–374. doi: 10.1093/genetics/145.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Kannan S, Fang W, Song G, Mullighan CG, Hammitt R, McMurray J, Zweidler-McKay PA. Notch/HES1-mediated PARP1 activation: a cell type-specific mechanism for tumor suppression. Blood. 2011;117:2891–2900. doi: 10.1182/blood-2009-12-253419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. Notch signaling in gastrointestinal tract (review) Int J Oncol. 2007;30:247–251. [PubMed] [Google Scholar]
- Kobayashi H, Butler JM, O'Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, Rafii S. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Wicha MS. Cancer stem cells: nature versus nurture. Nat Cell Biol. 2010;12:419–421. doi: 10.1038/ncb0510-419. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nor JE. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70:9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Milner LA, Deng Y, Iwata M, Banta A, Graf L, Marcovina S, Friedman C, Trask BJ, Hood L, Torok-Storb B. The human homolog of rat Jagged1 expressed by marrow stroma inhibits differentiation of 32D cells through interaction with Notch1. Immunity. 1998;8:43–55. doi: 10.1016/s1074-7613(00)80457-4. [DOI] [PubMed] [Google Scholar]
- Limgala R, Carole Yee, Atsushi Terunuma, Philip Martin, Kathy Kelly, Jonathan Vogel. Isolation of Prostate Cancer Stem Cells using Developmental Signaling Pathway Activities. AACR Meeting Abstracts. 2009 [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9:842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Parr-Sturgess CA, Rushton DJ, Parkin ET. Ectodomain shedding of the Notch ligand Jagged1 is mediated by ADAM17, but is not a lipid-raft-associated event. The Biochemical journal. 2010;432:283–294. doi: 10.1042/BJ20100321. [DOI] [PubMed] [Google Scholar]
- Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11842–11849. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif MW, Chu E. Biology of colorectal cancer. Cancer J. 2010;16:196–201. doi: 10.1097/PPO.0b013e3181e076af. [DOI] [PubMed] [Google Scholar]
- Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- Small D, Kovalenko D, Kacer D, Liaw L, Landriscina M, Di Serio C, Prudovsky I, Maciag T. Soluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotype. J Biol Chem. 2001;276:32022–32030. doi: 10.1074/jbc.M100933200. [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, Hashida H, Takabayashi A, Sasaki M, Robine S, et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell. 2011;19:125–137. doi: 10.1016/j.ccr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Sun X, Artavanis-Tsakonas S. Secreted forms of DELTA and SERRATE define antagonists of Notch signaling in Drosophila. Development. 1997;124:3439–3448. doi: 10.1242/dev.124.17.3439. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- Trifonova R, Small D, Kacer D, Kovalenko D, Kolev V, Mandinova A, Soldi R, Liaw L, Prudovsky I, Maciag T. The non-transmembrane form of Delta1, but not of Jagged1, induces normal migratory behavior accompanied by fibroblast growth factor receptor 1-dependent transformation. The Journal of biological chemistry. 2004;279:13285–13288. doi: 10.1074/jbc.C300564200. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Weijzen S, Velders MP, Elmishad AG, Bacon PE, Panella JR, Nickoloff BJ, Miele L, Kast WM. The Notch ligand Jagged-1 is able to induce maturation of monocyte-derived human dendritic cells. J Immunol. 2002;169:4273–4278. doi: 10.4049/jimmunol.169.8.4273. [DOI] [PubMed] [Google Scholar]
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer research. 2011;71:6061–6072. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.