Abstract
Purpose
Studies have indicated that diabetes is a risk factor for bladder cancer; however, many failed to adjust for confounding variables. An earlier publication from the Iowa Women's Health Study reported a positive association of baseline diabetes with bladder cancer risk between 1986 and 1998, although the number of cases was small (n=112). We re-examined the diabetes–bladder cancer risk association by accounting for 12 more years of follow-up and assessed whether the association varied by diabetes duration, body mass index or waist-to-hip ratio (WHR).
Methods
Proportional hazards regression was used to estimate the hazard ratio (HR) of bladder cancer (n=277) in relation to diabetes (before enrollment and during follow-up) and diabetes duration using a time-dependent approach.
Results
In a multivariate time-dependent analysis, the HR for bladder cancer was 1.69 (95% CI, 1.40-2.41) in relation to diabetes among 37,327 postmenopausal women initially free of cancer. There was an interaction between diabetes and WHR (p =0.01). Bladder cancer HR in diabetic women with WHR>0.9 was 2.5 times higher than expected. There was no dose-response relation of bladder cancer risk with diabetes duration. Compared to no diabetes, HR were 1.77. 2.03, and 1.55 for diabetes durations of ≤5, 6-10, and >10 years, respectively.
Conclusions
We confirmed a positive association between diabetes and bladder cancer risk among white post-menopausal women. We also observed a synergistic interaction between diabetes and high WHR in bladder cancer development that might be explained by increased insulin resistance and inflammation related to abdominal obesity.
Keywords: Bladder cancer, diabetes, prospective study
Introduction
In the United States, 73,510 bladder cancer cases are expected to occur in 2012 and about 14,880 will die from this disease [1]. The established causal risk factors for bladder cancer – age, smoking, occupational exposure to aromatic amines, and infections with schistosomiasis [2-4] do not fully explain bladder cancer incidence. Other risk factors include being male, arsenic in drinking water, and, potentially, diabetes [4, 5]. This study is an effort to further examine whether or not diabetes is a risk factor for bladder cancer.
Several epidemiological studies investigated associations between diabetes and bladder cancer risk, but the results are inconsistent. A meta-analysis of 16 studies reported a relative risk of bladder cancer of 1.24 in adults with diabetes [5]. However, there was a mixture of studies of incident and fatal bladder cancers; many of those studies were small, they did not examine duration of diabetes and did not adjust for important confounding factors such as smoking and obesity that are associated with diabetes and bladder cancer risk.
An earlier analysis in the Iowa Women's Health Study (IWHS) examining risk factors for bladder cancer (1986-1998) reported a hazard ratio HR= 2.5 (95% confidence interval (CI), 1.3-4.5) associated with baseline diabetes [6], but there were only 112 incident bladder cancer cases.
Our main aim for this study was to extend the earlier analysis of bladder cancer risk and diabetes reported at baseline to include new bladder cancer cases ascertained over an additional 12-year follow-up and account for new diabetes cases reported during follow-up. Our other aims were to examine whether or not the association was related to diabetes duration or modified by obesity measures – body mass index (BMI) and waist-to-hip ratio (WHR).
Methods
Study design
The design of the IWHS cohort has been described in detail [6-8]. In brief, in 1986, 41,836 women aged 55-69 years completed a baseline questionnaire about socio-demographic factors, lifestyle behaviors, anthropometric characteristics, and medical history. Prevalent diabetes at baseline was identified from the question, “Have you ever been told by a doctor that you have sugar diabetes (diabetes mellitus)?” or by indicating current use of “insulin” or “pills for sugar diabetes (or to lower blood sugar)”. At baseline, participants also reported age at onset of diabetes. For this study, women with diabetes onset before age 30 years (presumably, type 1 diabetes) were excluded. Diabetes diagnosed after baseline was ascertained at each of the follow-up surveys in 1987, 1989, 1992, 1997, and 2004 (response rates were: 91%, 90%, 83%, 79%, and 69%, respectively) with a question asking participants if they had diabetes diagnosed by a physician since their last survey. No blood testing was performed. For this analysis, 37,327 post-menopausal women, initially free of cancer, were followed from baseline until bladder cancer diagnosis, loss to follow-up, death, or end of follow-up in December 31, 2010. Women with missing covariates were not excluded from the analytical cohort: there were 590 women with missing data on smoking status, no missing values for BMI, and 181 women with missing WHR.
Incident transitional cell bladder cancer cases (n=277) were identified in 1986-2010 through annual linkage to the Iowa Cancer Registry – part of the Surveillance, Epidemiology, and End Results (SEER) Program. We included ICD-O-3 (the International Classification of Diseases of Oncology) topography codes – C670-679 and a transitional cell (urothelial) morphology codes 8120, 8122, 8123, or 8130) [9]. Data from follow-up surveys indicate that the migration rate from Iowa for this cohort is <1% annually, allowing nearly complete cancer follow-up [8]. The IWHS was conducted under a protocol approved for human subjects research by the University of Minnesota Institutional Review Board. The return of baseline and follow-up questionnaires were considered to indicate consent to participate.
Statistical analysis
Age-adjusted cancer incidence rates for bladder cancer were calculated using Poisson regression. Cox proportional hazards regression was used to estimate hazard ratio (HR) of bladder cancer and 95% CI in relation to diabetes (yes, no) before enrollment. The proportional hazards assumption was tested as an interaction of baseline diabetes with time (p=0.22). An extended Cox model accounted for diabetes (yes, no) during follow-up with diabetes as a time-dependent variable. Potential confounders were included if they were associated with bladder cancer and/or diabetes in this or previous IWHS studies [6, 10, 11]. The final model was adjusted for age, BMI, WHR (all – continuous), education (less than high school, high school, and more than high school), smoking status (never, former, current), pack years of smoking (continuous), occupation (never workers or homemakers; those involved in farming or trades/crafts; and professionals or clerical workers), marital status (married, unmarried), physical activity (low, medium, high), and alcohol use (yes, no).
In addition, we adjusted for a three-level categorical variable for diabetes treatment at baseline (no diabetes, diabetes without any treatment, and diabetes treated with either oral medication or insulin) using STRATA statement in PROC PHREG model [12]. Since the results were practically identical to those without adjustment, we did not include this variable in the model. We did not have information about diabetes treatment during follow-up.
Furthermore, we examined potential effect modification of the diabetes–bladder cancer association by age (continuous), smoking status and pack-years (continuous), and BMI and WHR (continuous and categorical). Multiplicative interactions were assessed by including cross-product terms in the models and using the Wald test. An interaction was considered statistically significant when the p-value was <0.05.
Finally, we examined the association of bladder cancer incidence with the duration of diabetes. Diabetes duration was defined as the difference between the end of follow-up and the year of self-reported onset of diabetes diagnosis. The year of onset for diabetes reported at baseline was computed as the sum of birth year and age at diabetes onset, whereas the year of diabetes diagnosis after baseline was calculated as the midpoint of the period between the survey at which a diagnosis of diabetes was first self-reported and the preceding survey. We examined diabetes duration as a time-dependent variable with 3 categories: ≤5, 6-10, and >10 years.
All analyses were performed using SAS 9.2; all p-tests were two-sided.
Results
The study sample for the person-time analysis included 37,327 women with mean age at baseline of 61.7 years. Prevalent diabetes was reported by 2,274 women (6.1%) at baseline; among them 2,011 (94.6 %) women had valid information about treatment, and 1,436 (71.4 %) of those diabetic women reported currently taking oral diabetes medication or insulin. An additional 3,295 women self-reported being diagnosed with diabetes after baseline.
Women with diabetes at baseline were slightly older, heavier, less well-educated and were less likely to be married, be current smokers or alcohol users than those without a history of diabetes (Table 1). Bladder cancer cases tended to smoke more, had a higher mean WHR and lower BMI than did non-cases. HRs for bladder cancer were 0.97 (95%CI, 0.95-1.00) per 1 kg/m2 of BMI and 1.11 (95%CI, 1.00-1.24) per 1 SD of WHR.
Table 1.
Prevalence of baseline characteristics according to self-reported diabetes status at baseline, IWHS, 1986
| Diabetes at baseline |
||
|---|---|---|
| Baseline characteristics | No N= 35,053 (93.9%) | Yes N= 2,274 (6.1%) |
| Mean age at baseline (y) (SD) | 61.7 (4.2) | 62.6 (4.2) |
| Race, White, % | 99.2 | 98.4 |
| Body mass index (SD) (kg/m2) | 26.8 (4.9) | 30.5 (6.3) |
| Waist-to-hip ratio (SD) | 0.83 (0.08) | 0.90 (0.10) |
| Education more than high school (%) | 39.3 | 30.4 |
| Married (yes) (%) | 76.9 | 73.0 |
| Occupation (%) | ||
| Homemaker/never worked | 38.0 | 37.0 |
| Professional/clerical | 40.8 | 34.6 |
| Farmer/crafts | 21.3 | 28.8 |
| Current smoking (%) | 15.1 | 12.1 |
| ≥40 pack-years of smoking (%) | 8.7 | 10.5 |
| Alcohol intake (yes) (%) | 44.9 | 21.3 |
| High or moderate physical activity (yes) (%) | 53.1 | 44.8 |
Age-adjusted incidence rates of bladder cancer were higher in women reporting diabetes at baseline or during follow-up than in those without diabetes (Table 2). In the time-dependent analysis, accounting for incident diabetes during follow-up, the HR for bladder cancer was 1.69 (95% CI, 1.40-2.41) for diabetes compared to no diabetes. The HRs associated with diabetes were similar for women diagnosed with in situ (number of cases N=132), local (N=56), and combined (regional and distant, N=26) stages of bladder cancer: HR=1.88 (95%CI, 1.09-3.23), 1.59 (95%CI, 0.72-3.55), and 2.17 (95%CI, 0.80-5.86), respectively. There was no interaction of diabetes with smoking, age, or BMI, but there was a statistically significant interaction with continuous WHR (p=0.006) and categorical WHR with cut-off >0.9 for abdominal adiposity in older women [13] (p=0.01). The HR for bladder cancer occurrence of 3.01 for high WHR and diabetes jointly was 2.5 times higher than the HR expected from the product of the individual HRs for high WHR (HR=1.13) and diabetes (HR=1.06) (Table 2).
Table 2.
Hazard ratio (HR) of bladder cancer in relation to diabetesa, IWHS (1986-2010).
| No. of casesd (N=277) | Incidence rate per 1,000 person-years, age-adjusted | HR (95%CI) |
|||
|---|---|---|---|---|---|
| Diabetes | Person-yearsd | Age-adjusted | Multivariate-adjustedb | ||
| No | 232 | 664,856 | 0.34 | 1 (reference) | 1 (reference) |
| Yes | 45 | 73,169 | 0.62 | 1.59 (1.16-2.18) | 1.69 (1.19-2.41) |
|
Interaction between diabetes and WHR (p for interaction=0.01) | |||||
| No diabetes and WHRc<0.9 | 188 | 539,824 | 0.35 | 1 (reference) | 1 (reference) |
| Diabetes and WHR<0.9 | 18 | 40,440 | 0.44 | 1.12 ( 0.69-1.82) | 1.06 (0.61-1.84) |
| No diabetes and WHR≥0.9 | 42 | 122,457 | 0.32 | 1.00 (0.71-1.40) | 1.13 (0.79-1.63) |
| Diabetes and WHR≥0.9 | 27 | 32,331 | 0.80 | 2.25 (1.50-3.36) | 3.01 (1.94-4.70) |
Diabetes before enrollment or during follow-up
Adjusted for baseline age, BMI, WHR, education, smoking status, pack years of smoking, occupation, marital status, physical activity, and alcohol use
WHR ≥0.9 is a cut off for abdominal obesity in older women [13]
The numbers of cases and person-years are slightly higher in the analysis of diabetes (yes/no) than in the analysis of interaction with WHR because the former analysis included those with missing WHR.
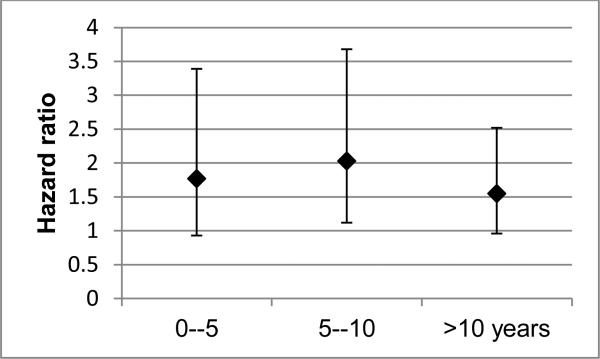
There was no dose-response relation between duration of diabetes and bladder cancer incidence: HRs were 1.77 (95% CI, 0.93-3.39), 2.03 (95% CI, 1.12-3.68), and 1.55 (95% CI, 0.96-2.52) for diabetes duration <5, 6-10, and >10 years, respectively (Figure 1).
Fig. 1.
Hazard ratio of bladder cancer associated with diabetes duration, IWHS, 1986-2010. Reference group is “no diabetes”. Number of cases were 232, 11, 13, and 21 for “no diabetes” and for diabetes durations of ≤5, 6-10, and >10 years. Associations were adjusted for baseline age, BMI, WHR, education, smoking status, pack years of smoking, occupation, marital status, physical activity, and alcohol use
In addition, we extended the earlier IWHS analysis using baseline diabetes alone. For women with, versus without, diabetes, the HR was 1.65 (95% CI, 1.01-2.70) for bladder cancer through 2010, which was similar to the HR estimated using the time-dependent approach.
Finally, diabetic medications such as thiazolidinediones, linked to a modest increased bladder cancer risk in several studies (meta-analysis reported RR~1.2 [15-18]) and metformin, associated with decreased risks of some cancers [19] may have affected the diabetes–bladder cancer association. To test for a potential effect of these medications that came to the US market in the middle to late 1990s, we truncated the follow-up at 1995, and observed increased HRs associated with overall diabetes 2.40 (95% CI, 1.19-4.84) .
Discussion
We have confirmed a statistically significant positive association between diabetes and bladder cancer risk among white post-menopausal women in the IWHS followed through 2010. Using a time-dependent analysis, the HR was significantly increased by 69% for diabetes versus no diabetes, but there was no dose-response relation with diabetes duration. Importantly, the increased bladder cancer risk by 55% was observed even when the diabetes onset preceded bladder cancer diagnosis by >10 years. This supports a hypothesis of diabetes associated with subsequent development of bladder cancer. Furthermore, this study demonstrated a statistically significant synergistic interaction between diabetes and WHR on the bladder cancer development.
In the earlier IWHS analysis (1986-1998), the HR was 2.5 (95% CI, 1.3-4.5) for those with baseline diabetes versus no disease [6]. In the current study, we also conducted a separate analysis for the second half of follow-up using diabetes reported at any visits up to 1998 and follow-up for bladder cancer during 1998-2010: HR=1.3 (95%CI, 0.7-2.5). Several explanations might account for different estimates between these two periods of follow-up. Over the course of follow-up, the criteria for diabetes used in the U.S. became more sensitive. The improved sensitivity for diabetes diagnosis and potentially better diabetes control during the later period could have led to inclusion of less severe diabetes in the later period, and thereby attenuated the HR. In addition, competing risk of death from cardiovascular disease could have selectively removed over time diabetic women with more severe diabetes or who smoked. Furthermore, the baseline diabetes variable may have become increasingly misclassified over the lengthy follow-up (1986-2010), which would tend to attenuate HRs using baseline diabetes only. We do not have enough evidence to conclude which of these (or some other) factors explain the decreased hazard ratios in later follow-up.
The finding of a positive association between diabetes and bladder cancer risk in the IWHS is in agreement with the meta-analysis of 7 case-control (OR=1.37; 95%CI, 1.04-1.80) and 3 cohort studies (RR=1.43; 95%CI, 1.18-1.74) [5]. The findings from studies published after the meta-analysis were mixed: four studies did not find any associations [20-23], whereas one case-control and two cohort studies reported positive associations between diabetes and bladder cancer risk with relative risks ranging from 1.2 to 2.2 [24-26]. The latter findings are in line with our results. The different findings in the studies might be explained by different populations as well as differences in diabetes treatment regimens and levels of control for confounding factors such as smoking and obesity.
In our study, there was no dose-response relation between diabetes duration and bladder cancer risk. Four other studies that examined this association reported inconsistent results. The risk of bladder cancer increased with longer diabetes duration in a US case-control study [24], while in the large Cancer Prevention Study II Nutrition Cohort the risk of invasive bladder cancer was increased only for those with diabetes ≥15 years versus those without diabetes [23]. Two other large cohorts (from the United States [20] and Taiwan [25]) found no trend related to diabetes duration, which is in agreement with our findings.
The positive association between diabetes and bladder cancer could be explained by the mitogenic properties of insulin. Elevated insulin production may lead to increased levels of insulin-like growth factor (IGF)-I, which can stimulate cell proliferation and inhibit apoptosis [27, 28]. Animal and human studies support a role of IGF-I in bladder carcinogenesis [29, 30]. It was also suggested that the diabetes may be associated with bladder cancer risk through urinary tract infections [24, 25], to which diabetic people are more prone [4]. However, the association between urinary tract infections and bladder cancer risk is inconsistent among women [31].
A modifying effect of WHR on the association between diabetes and bladder cancer risk might be explained by the fact that abdominal adiposity increases production of inflammatory mediators, such as tumor necrosis factor-alpha and interleukin-6 [27, 28]. Compared to non-obese diabetic patients, obese diabetic patients have been shown to have higher levels of inflammatory factors, reactive oxygen species and insulin resistance and consequent hyperinsulinemia [14, 32, 33]. It has been suggested that obesity and diabetes act synergistically, exacerbating inflammation, oxidative stress, hyperinsulinemia, and insulin resistance [18, 27], thus contributing to the development of bladder cancer.
Our study has several strengths and limitations. The strength of this large cohort study is its prospective design, almost complete cancer follow-up, and detailed information about potential confounders (smoking status and pack years, BMI, WHR, occupation, physical activity, and alcohol use). A limitation is the reliance on self-reported diabetes, which could lead to misclassification bias. In general, self-reported diabetes is reasonably accurate [34]; however, a small IWHS validation study of 44 self-reported diabetes cases at baseline suggested some over-reporting of diabetes since only 28 (64%) cases were confirmed by physician [35]. On the other hand, misclassification could result from the underdiagnosis of diabetes in the entire population [36]. Bias also might arise from more thorough medical surveillance of women with diabetes. If this bias occurred in this study, one might expect a higher proportion of bladder cancer cases to be diagnosed at earlier stages among women with diabetes than women without diabetes. However, we observed a slightly lower proportion of early stage (in-situ + local) bladder cancer cases in women with, versus without diabetes (82% vs 89%, respectively). Other drawbacks of our study are limited power for subgroup analyses and lack of information about diabetes treatment during follow-up. The later precluded a detailed analysis of the role of anti-diabetic medications in the diabetes–bladder cancer risk association. However, the robustness of the diabetes–bladder cancer association with different times of follow-up suggests that diabetic medications alone are unlikely to explain this association.
In summary, the findings of this study support an increased risk of bladder cancer in relation to diabetes. The increased risk was observed even when the diabetes onset preceded bladder cancer diagnosis by >10 years. A possible interaction between abdominal obesity and diabetes on the bladder cancer risk suggests a role of increased insulin resistance and/or inflammation related to abdominal adiposity in bladder carcinogenesis. Since our cohort included only white post-menopausal women our results may be not generalizable to other populations and should be tested in pooled prospective studies.
Acknowledgments
The authors are thankful to the IWHS staff for consultation and assistance in data preparation.
Grant support. This study was supported by National Cancer Institute grant R01 CA39742. A.E. Prizment was supported as a postdoctoral fellow by the National Cancer Institute (T32CA132670).
Footnotes
Anna Prizment presented this study at the 10th AACR International Conference on Frontiers in Cancer Prevention Research in 2011 and received an AACR-Scholar-in-Training Award.
References
- 1.American Cancer Society . Cancer Facts & Figures 2012. American Cancer Society; Atlanta: 2012. [Google Scholar]
- 2.World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition, Physical activity and the Prevention of Cancer: a Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- 3.Freedman N, Silverman D, Hollenbeck A, Schatzkin A, Abnet C. Association between smoking and risk of bladder cancer among men and women. JAMA (Chicago, Ill.) 2011;306:737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman DT, Devesa SS, Moore LE, Rothman N. Bladder cancer. In: Schottenfeld D, Fraumeni JFJ, editors. Cancer Epidemiology and Prevention. Oxford University Press; New York: 2006. pp. 1101–28. [Google Scholar]
- 5.Larsson S, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia. 2006;49:2819–2823. doi: 10.1007/s00125-006-0468-0. [DOI] [PubMed] [Google Scholar]
- 6.Tripathi A, Folsom A, Anderson K. Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women's Health Study. Cancer. 2002;95:2316–2323. doi: 10.1002/cncr.10975. [DOI] [PubMed] [Google Scholar]
- 7.Folsom AR, Kaye SA, Sellers TA, et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;269:483–487. [PubMed] [Google Scholar]
- 8.Bisgard KM, Folsom AR, Hong CP, Sellers TA. Mortality and cancer rates in nonrespondents to a prospective study of older women: 5-year follow-up. Am J Epidemiol. 1994;139:990–1000. doi: 10.1093/oxfordjournals.aje.a116948. [DOI] [PubMed] [Google Scholar]
- 9.Fritz A, Percy C, Jack A. International Classification of Disease for Oncology (ICD-O) 3rd Ed. WHO; Geneva: 2000. [Google Scholar]
- 10.Anderson KE, Anderson E, Mink PJ, et al. Diabetes and endometrial cancer in the Iowa women's health study. Cancer Epidemiol Biomarkers Prev. 2001;10:611–616. [PubMed] [Google Scholar]
- 11.Limburg P, Anderson K, Johnson T, et al. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2005;14:133–137. [PubMed] [Google Scholar]
- 12.Allison PD. Survival Analysis using SAS: A Practical Guide. SAS Institute; 2010. [Google Scholar]
- 13.ACSM'S Health-Related Physical Fitness Assessment Manual. 2nd edn Lippincott Williams & Wilkins; Philadelphia: 2008. p. 59. [Google Scholar]
- 14.Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 2012;55:1607–1618. doi: 10.1007/s00125-012-2525-1. [DOI] [PubMed] [Google Scholar]
- 15.Colmers I, Bowker S, Majumdar S, Johnson J. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. Can Assoc Med. 2012;184:E675–E683. doi: 10.1503/cmaj.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng C. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012;35:278–280. doi: 10.2337/dc11-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamtani R, Haynes K, Bilker W, et al. Association between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort study. J Natl Cancer Inst. 2012;104:1411–1421. doi: 10.1093/jnci/djs328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onitilo A, Engel J, Glurich I, Stankowski R, Williams G, Doi S. Diabetes and cancer II: role of diabetes medications and influence of shared risk factors. Cancer Causes Control. 2012;23:991–1008. doi: 10.1007/s10552-012-9971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JMM. Metformin and reduced risk of cancer in diabetic patients. Br Med J. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atchison E, Gridley G, Carreon JD, Leitzmann M, McGlynn K. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128:635–643. doi: 10.1002/ijc.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsson S, Andersson S, Johansson J, Wolk A. Diabetes mellitus, body size and bladder cancer risk in a prospective study of Swedish men. Eur J Cancer. 2008;44:2655–2660. doi: 10.1016/j.ejca.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Noto H, Osame K, Sasazuki T, Noda M. Substantially increased risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis of epidemiologic evidence in Japan. J Diabetes Complications. 2010;24:345–353. doi: 10.1016/j.jdiacomp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Newton C, Gapstur S, Campbell P, Jacobs E. Type 2 diabetes mellitus, insulin use, and risk of bladder cancer in a large cohort study. Int J of Cancer. 2012 doi: 10.1002/ijc.27878. doi: 10.1002/ijc.27878. [DOI] [PubMed] [Google Scholar]
- 24.MacKenzie T, Zens M, Ferrara A, Schned A, Karagas M. Diabetes and risk of bladder cancer: evidence from a case-control study in New England. Cancer. 2011;117:1552–1556. doi: 10.1002/cncr.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011;54:2009–2015. doi: 10.1007/s00125-011-2171-z. [DOI] [PubMed] [Google Scholar]
- 26.Woolcott C, Maskarinec G, Haiman C, Henderson B, Kolonel L. Diabetes and urothelial cancer risk: the Multiethnic Cohort study. Cancer Epidemiology. 2011;35:551–554. doi: 10.1016/j.canep.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 28.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 29.Dunn SE, Kari FW, French J, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- 30.Zhao H, Grossman HB, Spitz M, Lerner S, Zhang K, Wu X. Plasma levels of insulin-like growth factor-1 and binding protein-3, and their association with bladder cancer risk. J Urol. 2003;169:714–717. doi: 10.1097/01.ju.0000036380.10325.2a. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Castelao JE, Groshen S, et al. Urinary tract infections and reduced risk of bladder cancer in Los Angeles. Br J Cancer. 2009;100:834–839. doi: 10.1038/sj.bjc.6604889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha MK, Ohannesian JP, Heiman ML, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxena M, Agrawal C, Gautam S, Bid H, Banerjee M. Overt diabetic complications in obese Type 2 diabetes mellitus patients from North India. Archives of Applied Science Research. 2009;1:57–66. [Google Scholar]
- 34.Simpson C, Boyd C, Carlson M, Griswold M, Guralnik J, Fried L. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52:123–127. doi: 10.1111/j.1532-5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- 35.Kaye SA, Folsom AR, Sprafka JM, Prineas RJ, Wallace RB. Increased incidence of diabetes mellitus in relation to abdominal adiposity in older women. J Clin Epidemiol. 1991;44:329–34. doi: 10.1016/0895-4356(91)90044-a. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC) [May 2012];National Diabetes Fact Sheet, 2011. 2011 http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.