Abstract
Advances in imaging technology have allowed optical analysis of Ca2+-permeable ion channel activity. Here, we briefly review novel developments in optical recording of L-type voltage-dependent Ca2+ channel (LTCC) function with high spatial and temporal resolution. Underlying principles supporting the use of total internal reflection fluorescence (TIRF) microscopy for optical measurement of channel activity and new functional characteristics of LTCCs revealed by application of this approach are discussed. Visualization of Ca2+ influx through single LTCCs (“LTCC sparklets”) has demonstrated that channel activity is regionally heterogeneous and that clustered channels are capable of operating in a cooperative, or “coupled” manner. In light of these findings, we describe a current molecular model for the local control of LTCC activity and coupled gating in physiological and pathological contexts.
1. Introduction
Spatial confinement of intracellular second messenger activity has been increasingly recognized as a fundamental means of controlling specificity in divergent signaling processes. As an elemental second messenger that is neither synthesized nor degraded, calcium is a fitting example of this concept. Ca2+ ions are continually moved between extracellular and intracellular environments and among subcellular compartments in an intricate and dynamic interchange that is essential for cellular communication. The complex nature of transient subcellular Ca2+ signals has only recently come into view [1, 2], with pioneering discoveries owing much to key advances in imaging technology. The progression of our ability to visualize micro and nanodomain Ca2+ signaling events in space and time has thus proven essential for fully understanding how cells function and how errant Ca2+ signals can result in disease.
In excitable cells, influx of Ca2+ ions via L-type voltage-dependent Ca2+ channels (LTCCs) controls numerous physiological processes such as excitability, contraction, secretion, neurotransmitter release and synaptic plasticity. LTCCs are composed of pore-forming α1 subunits associated with a combination of accessory, β, γ, and α2δ subunits which influence channel trafficking and gating [3-5]. Alternative splicing of mRNA encoding LTCC subunits confer tissue-selective channels with distinct pharmacological and electrophysiological profiles [6-8]. Channel function is further influenced by interaction with a variety of regulatory and structural proteins, and is subject to modification by several ubiquitous kinases and phosphatases, which serve to finely tune channel function to cellular demand [4, 9, 10]. Importantly, alteration of LTCC expression or function has been linked to multiple human pathological conditions including cardiac arrhythmia, hypertension, immune deficiency and autism [11].
To a large extent, our current understanding of LTCC biophysical properties is the result of experiments employing the patch-clamp technique to isolate and record transmembrane currents. However, patch-clamp applied alone is insufficient in providing information regarding localization and organization of multiple active channels, which is critical for understanding how Ca2+ influx through LTCCs mediates a diversity of signaling pathways and cellular functions (e.g. contraction and transcription). Recent work employing optical methods in combination with patch-clamp electrophysiology have overcome this issue and provided novel information about LTCC organization. These efforts have revealed unique features of channel regulation that may have profound therapeutic implications given the physiological importance of these channels. In this review, we discuss the use of advanced microscopy in combination with Ca2+-sensitive fluorescent indicators to optically record LTCC activity and new aspects of Ca2+ signaling revealed by this approach.
2. Optical recording of Ca2+ channel activity
The physiological importance of Ca2+ ions first became known during the early 1880’s when Sidney Ringer reported the observation that frog hearts would not beat unless a calcium salt was added to the extracellular solution [12]. It is now apparent that signaling in all excitable cells involves control of not only global cytosolic Ca2+, but also fine coordination of short-lived Ca2+ gradients confined to various subcellular compartments. Nonetheless, the study of small amplitude Ca2+ transients in live cells has been hindered by limited microscope and dye technology, until recently. Accordingly, with the emergence of improved fluorescence-based Ca2+-sensitive indicators and advanced microscopy equipment, optical techniques have rapidly evolved as a means to study subcellular Ca2+-signals with unsurpassed spatiotemporal resolution.
The first optical demonstration of dynamic changes in intracellular Ca2+ revealed Ca2+ “waves” in sperm-activated Medaka fish eggs [13]. This early investigation detected emitted light from aequorin, a bioluminescent photoprotein with Ca2+-binding EF-hand motifs isolated from the jellyfish Aequoria victoria. Later, microinjected aequorin was used to simultaneously measure pulsatile Ca2+ increases and corresponding contractions in frog atrial myocytes [14]. However, irreversible consumption of this Ca2+ indicator upon light emission and limited signal amplitudes hindered use of aequorin for analysis of intracellular Ca2+. The subsequent generation of several Ca2+-sensitive fluorescent indicators (e.g. fura-2, fluo-4) triggered a mass of studies to view and characterize subcellular Ca2+ gradients in living cells [15].
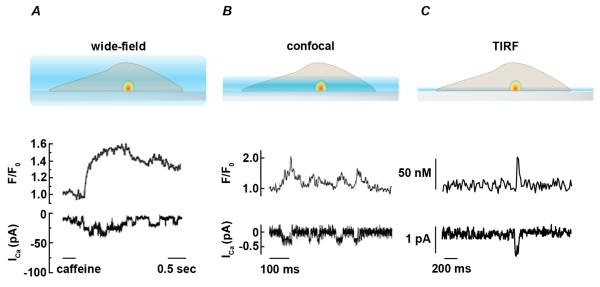
The first two-dimensional analysis of local Ca2+ influx through single plasmalemmal Ca2+-permeable channels was achieved using wide-field microscopy in combination with whole-cell patch-clamp in toad stomach smooth muscle cells (Figure 1A) [16]. In this study, thapsigargin was applied to fluo-3 loaded cells to eliminate Ca2+ release from intracellular stores. Subsequent application of 20 mM caffeine evoked submembrane transient changes in fluo-3 fluorescence with seemingly random distribution. These transients correlated with single channel openings in whole-cell current traces (Figure 1A). With the use of a wide field microscope, fluorescence was detected from a relatively large cytosolic volume, due to collection of light from within and outside the focal plane. Accordingly, changes in fluorescence with this approach reflect total accumulation of regional cytosolic Ca2+ rather than submembrane gradients near the mouth of active channels, which decreases the rate of signal rise and decay with distance from the focal plane. However, the relationship between the total change in fluorescence and degree of Ca2+ influx was later shown to be linear, regardless of whether the transient is in focus, thus allowing accurate optical determination of single Ca2+ channel current from total fluorescence increase or “signal mass” [17].
Figure 1. Relationship between excitation field and temporal resolution of fluorescent signals.
A. Schematic representation (top) showing excitation pattern (blue) for conventional wide-field fluorescent microscope. Fluorescent signal from cytosolic Ca2+ indicator is collected from within and outside of focal plane. Images reflect Ca2+ signals near point source (Ca2+-permeable channel) as well as Ca2+ accumulation in the cytosol. Therefore, rise and decay of the observed fluorescent signal is slower than corresponding single channel current measurements (bottom, redrawn from [16]). B. Cartoon representing excitation profile for confocal microscope with representative single channel current (ICa) and [Ca2+]i (F/FO) traces using line scan confocal microscopy (redrawn from [19]). Excitation field and Ca2+ influx is represented as in A. C. Representation of excitation profile for total internal reflection fluorescence (TIRF) microscope with representative single channel current (ICa) and [Ca2+]i (F/FO) traces (redrawn from [24]). Excitation field and Ca2+ influx is represented as in A.
The development of laser scanning confocal microscopy first permitted “optical sectioning” to examine transient fluorescent signals within close proximity to the point source of Ca2+ influx in excitable cells. Line-scanning confocal imaging of fluo-4 fluorescence led to the detection of spontaneous localized intracellular Ca2+ release (i.e. “Ca2+ sparks”) in quiescent cardiomyocytes resulting from short-lived activation of a cluster of ryanodine receptors residing in the sarcoplasmic reticulum [18]. Subsequent investigation of the elementary plasmalemmal Ca2+ signals evoking these intracellular Ca2+ release events used line-scanning confocal microscopy in combination with the cell-attached patch configuration to demonstrate, for the first time, transient intracellular Ca2+ signals at the intracellular mouth of a single LTCC located within the voltage-clamped patch membrane [19]. In this study, signal amplitudes were enhanced by raising extracellular [Ca2+] in the presence of the LTCC agonist FPL64176, effectively increasing the driving force for Ca2+ and prolonging the duration of channel openings, respectively. In cardiomyocytes, each fluorescent transient produced by opening of a single LTCC, referred to as “LTCC sparklet”, was found to promote Ca2+ spark ignition by triggering activation of four to six ryanodine receptors in closely apposed sarcoplasmic reticulum.
Although confocal microscopy offers the ability to record from a restricted volume (~0.8 μm axial section, Figure. 1B), this point scanning method is associated with limited temporal resolution, unless one-dimensional line scan mode is used. However, significant disadvantages exist with line scanning in that the total number of active sites per cell cannot be obtained and event amplitude and duration may be misrepresented due to outlying Ca2+ signals arising from regions near the scan line. A more suitable approach for recording from large areas of membrane is total internal reflection fluorescence (TIRF) microscopy. In particular, the use of TIRF in combination with ultra-fast high sensitivity electron multiplying charge coupled device (EMCCD) cameras has allowed the direct observation of changes in Ca2+ within a thin (< ~100 nm, Figure. 1C) optical section of the plasmalemmal surface. Indeed, this approach was first successfully implemented in the optical recording of single N-type Ca2+ channel activity in a heterologous expression system [20, 21]. Considering that TIRF allows selective illumination of fluorophore near the surface membrane, this technique resolves Ca2+ influx in regions surrounding Ca2+-permeable channels while avoiding detection of bulk cytoplasmic indicator and therefore has emerged as an advantageous method for optical measurement of Ca2+ channel activity.
3. Use of total internal reflection fluorescence (TIRF) for imaging Ca2+ sparklets
Total internal reflection fluorescence (TIRF) microscopy relies on the physical principle that incident light encountering a boundary with a lower refractive index at the proper angle is completely reflected with the generation of a highly restricted electromagnetic field (evanescent waveform) in the lower-index medium [22]. Whereas the evanescent field carries identical properties as that of incident light (i.e. wavelength, frequency), its intensity decays exponentially with distance, such that illumination extends tens of nanometers, typically one third of the incident wavelength, from the coverslip-cell interface. This precisely restricted illumination limits the harmful effects of laser exposure on live cells and produces a very thin excitation field, thus offering exceptional resolution for imaging targets embedded in or directly adjacent to the plasmalemma. In this section, we briefly describe a general protocol for the use of TIRF in combination with whole-cell patch-clamp electrophysiology to image LTCC sparklets.
First, isolated native or cultured cells are allowed to adhere to a glass coverslip mounted in a recording chamber in physiological saline solution at room temperature. After allowing cell adhesion (~20 min), cells are washed by continuous superfusion. Thapsigargin or cyclopiazonic acid is used to inhibit sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) activity and prevent SR Ca2+ loading. This helps to prevent interference of LTCC-associated fluorescent signals by Ca2+-release events from intracellular stores. Cells are then patch clamped in the whole-cell configuration to allow manipulation of membrane potential as well as introduction of Ca2+ indicator and an excess of EGTA (10 mM). Cytosolic Ca2+ indicator and EGTA act as fluorescent and non-fluorescent buffers that compete for Ca2+ ions. As a result, Ca2+ ions crossing the plasmalemma via Ca2+-permeable channels bind to the lower affinity, but fast binding Ca2+ dye (e.g. fluo 5). Upon release, this Ca2+ is taken up by the more abundant, high-affinity, and non-fluorescent EGTA, thus restricting fluorescence signals to areas near the source of entry. This approach in combination with the thin optical section in TIRF mode increases signal-to-noise and permits the detection of low intensity fluorescent signals. Because the time course of the fluorescence signals closely parallels the time course of current recordings (Figure 1C), analytical methods to quantify Ca2+ sparklet activity are alike that of open probability (PO) analysis for single channel currents. Thus, Ca2+ sparklet activity can be expressed as nPS, which is analogous to nPO, and where n refers to the number of quantal levels and PS is the probability of Ca2+ sparklet occurrence (for details on this analysis see [23]).
Although larger optical sections produced by the two-dimensional (i.e. swept field, spinning-disk) confocal microscope makes observation of small amplitude sparklet events challenging, it should be noted that this method has been used to detect LTCC sparklets and is particularly useful for recording sparklets in situ. For example, loading EGTA-AM to buffer Ca2+ and fluo-4 as a Ca2+ indicator, our laboratory has recently observed localized LTCC-mediated Ca2+ influx events in intact pressurized resistance arteries (Nystoriak and Navedo, unpublished observations). These findings lay the groundwork for future experiments aiming to investigate mechanisms of LTCC sparklet regulation by physiological modulators of vascular tone such as intravascular pressure or endothelial-derived vasoactive factors.
4. Cardiovascular LTCC sparklets
Optical investigation of local Ca2+ influx has revealed unique features regarding regulation of LTCC function in the vasculature, which may apply to channels expressed elsewhere. Considering the strong voltage-dependence of LTCC activation and broad expression of functional LTCCs throughout the plasmalemma of arterial smooth muscle cells, it was long assumed that channel activation is random and that all LTCCs have a similar open probability at a given membrane potential. Yet these assumptions were challenged when the Santana laboratory reported that subpopulations of LTCCs in isolated arterial myocytes operate in a continuously high open probability mode, producing distinct sites of Ca2+ influx [24]. Based on pharmacological, biophysical and molecular properties, the molecular entity underlying these Ca2+ influx events was identified as CaV1.2 channels. For instance, fluorescent Ca2+ influx events produced by opening of single LTCCs (i.e. LTCC sparklets) are concomitant with inward Ca2+ conductance, enhanced by dihydropyridine agonists (i.e. Bay-K 8644), and abolished by dihydropyridine antagonists (i.e. nifedipine) [25]. Consistent with this, heterologous expression of the pore-forming CaV1.2 (i.e. α1C) subunit with accessory CaVβ3 and CaVα2δ1 subunits in tsA-201 cells recapitulates LTCC sparklets with identical pharmacological and biophysical properties as Ca2+ sparklets in native smooth muscle cells [23-25]. Furthermore, smooth muscle cells expressing dihydropyridine-insensitive CaV1.2 channels produced sparklets that are resistant to nifedipine [25]. Surprisingly, LTCC sparklets are observed even at hyperpolarized membrane potentials (−70 mV) and display several activity modes; namely silent, low and high (i.e. persistent) nPS sparklets. A silent LTCC sparklet refers to a typically dormant site, which can be triggered by LTCC agonists, for instance. Low activity sparklets show intermittent, transient openings, whereas high activity (“persistent”) sparklet sites are characterized by prolonged openings and nearly continuous Ca2+ influx. Finally, an amplitude histogram for these events can be fit with a multi-component Gaussian function such that the quantal nature of Ca2+ influx through LTCCs is revealed, with a single quantal unit of Ca2+ influx typically elevating [Ca2+]i in the vicinity of the channel by ~38 nM in the presence of 20 mM external Ca2+ [24].
The spatial distribution of LTCC sparklet sites in arterial myocytes, although varied between cells, differentiated from a Poisson distribution, indicating non-stochastic LTCC activation occurring at defined sites within the myocyte surface membrane [26]. This finding raises a fundamental question: If functional LTCCs are located broadly throughout the plasmalemma of arterial myocytes, what are the mechanistic bases underlying regional heterogeneity of LTCC activity? This conundrum is not yet fully resolved, but considerable mounting evidence suggests that a subpopulation of LTCCs can be differentially regulated by a host of targeted regulatory factors. For example, LTCC sparklet activity is markedly enhanced by protein kinase C (PKC) [24, 27]. Activation of this serine/threonine kinase with phorbol esters (i.e. phorbol 12,13-dibutyrate) activates previously silent LTCC sparklet sites and further increases activity at low nPS sites. Further analysis of this effect demonstrates that spatial heterogeneity of sparklet activity is not random, but results from functional coupling of specific clusters of LTCCs to PKCα by means of the multivalent scaffolding protein, AKAP150 (A-kinase anchoring protein 150; ortholog of human AKAP79) [23, 26, 27]. In this model, AKAP150 binds and targets PKC to specific regions of the surface membrane within close proximity to substrate LTCCs. Consistent with this, genetic ablation of PKCα or AKAP150 abolished persistent Ca2+ sparklet activity [27]. In addition to its role in the targeting of PKC, AKAP150 also binds and targets the cAMP-dependent protein kinase (PKA), and the Ca2+/calmodulin-dependent phosphatase calcineurin to LTCCs. In this model, PKA upregulates LTCC sparklets in a similar fashion as PKC, and calcineurin opposes PKC and PKA activity and thereby inhibits LTCC sparklet activity [28]. Hence, localization of specific kinase and phosphatase activity by the scaffolding protein AKAP150 may represent a mechanism for regional heterogeneity of enhanced Ca2+ influx by LTCCs. Thus far, the molecular determinants of spatially heterogeneous AKAP150 expression in arterial myocyte submembrane compartments are unclear. However, previous findings in neurons suggest that this may involve targeting to membrane regions rich in the acidic phospholipid phosphatidylinositol 4,5 bisphosphate, F-actin or cadherin adhesion proteins via the N-terminal basic domain of AKAP150 [29-31].
In arterial myocytes, localized LTCC-mediated Ca2+ signals influence a variety of physiological phenomena. Persistent LTCC sparklets contribute ~50% of the steady-state Ca2+ entry [32] and directly regulate contractility and vascular tone under physiological conditions. Furthermore, LTCC sparklets can indirectly influence SR function by contributing Ca2+ influx to a cytosolic Ca2+ pool from which the SR can draw to accelerate reloading of intracellular Ca2+ stores [33]. Therefore, by way of elevating SR Ca2+ load, sparklets can presumably increase the frequency of RyR-mediated Ca2+ sparks and activation of nearby large conductance Ca2+-activated K+ (BKCa) channels, ultimately promoting hyperpolarization and decreased LTCC activation. It is conceivable that submembrane Ca2+ gradients produced by LTCC sparklets may also directly influence nearby BKCa channel activity, as this could represent additional feedback regulation to prevent uncontrolled Ca2+ entry in vascular smooth muscle leading to excessive vasoconstriction.
Cardiac and smooth muscle exhibit several similarities and differences with respect to local LTCC signals. In the heart, LTCC expression is concentrated in T-tubules where they are well situated to raise Ca2+ within a cytoplasmic cleft such that Ca2+-induced Ca2+ release occurs via activation of proximal RyRs. These functional “couplons” between individual LTCCs and RyRs form the elementary basis for generation of cell-wide spikes in cytosolic [Ca2+]i, action potential prolongation and contraction [19, 34]. Accordingly, coupling strength between sparklets and sparks, and therefore cardiac contractility is proportional to the amount of Ca2+ flux via LTCCs and local [Ca2+]i [35]. Conversely, a separate subpopulation of LTCCs in cardiomyocytes, restricted to caveolae rather than T-tubules, do not appear to be involved in SR Ca2+ release, but instead participate in NFAT-dependent excitation-transcription events. It is proposed that Ca2+ microdomains generated by caveolae-associated LTCCs contribute to pro-hypertrophic signaling in cardiomyocytes (see below) [36]. The specific elements involved in segregation of LTCC-mediated signals within these specialized microdomains in the heart are yet to be examined.
5. Coupled gating of L-type Ca2+ channels
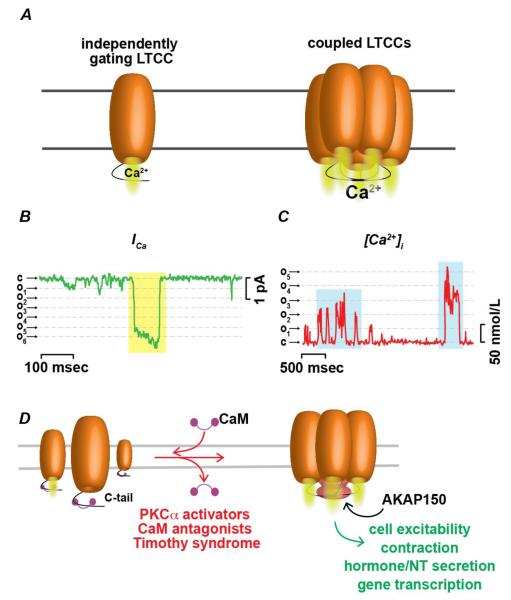
Examination of the amplitude distribution produced by LTCC sparklets led to a striking discovery: the frequency of multi-channel openings far exceeded the probability of observing simultaneous openings of independently gating channels, suggesting that a group of several LTCCs may activate and inactivate in a synchronous manner (e.g. coupled gating) (Figure 2A). Importantly, synchronized opening and closing of multiple channels can be observed by both electrophysiological and optical analytical methods (Figures 2B and C). To explore this further, a Markov chain model has been applied to these recordings to yield the coupling coefficient or strength of “coupled” events (κ) [37]. In this model, values of κ range from 0 for total independent channel gating to 1 for channels exhibiting only synchronized openings. In experiments performed in arterial smooth muscle, neonatal ventricular myocytes and tsA-201 cells expressing CaV1.2 channels, values of κ ranged from 0.1 to 1, with a median κ of 0.2. Under normal conditions, most Ca2+ influx events were found to be the result of independent gating and coupled LTCC gating was relatively weak. However, it remains plausible that physiological stimuli (e.g. increase in intravascular pressure, β-adrenergic agonists) can evoke increases in [Ca2+]i via induction of LTCC coupling.
Figure 2. Mechanisms leading to coupled gating between physically interacting LTCCs.
A Cartoon showing Ca2+ influx via independently gating LTCC (left) and Ca2+ influx via multiple physically coupled channels opening and closing in synchrony. B Representative single and coupled (yellow highlight) LTCC current record during step depolarization from −80mV to −30 mV. Dashed lines show amplitude of quantal levels. C Representative [Ca2+]i trace showing single and coupled (blue highlights) Ca2+ influx events. (B and C redrawn from [37]) D Proposed mechanism of coupled gating between LTCCs. In this model, calmodulin (CaM) binding of LTCC carboxy (COOH) termini prevents interaction between neighboring channels. Dissociation of CaM from LTCCs facilitates physical interaction between C-termini via association with the scaffolding protein AKAP150. Coupling is promoted pharmacologically by CaM antagonists, activators of PKCα, and during pathological conditions of timothy syndrome and hypertension. Coupled gating of LTCCs may represent a ubiquitous physiological and pathological mechanism mediating local amplification of Ca2+ influx across excitable cells potentially contributing to fine control of excitability, contractility, secretory and transcriptional pathways.
Although the precise molecular details of coupled gating in LTCC clusters are not fully understood, spatial proximity between channels is thought to be necessary for coupled gating to occur. Recent work by Dixon et al supports this hypothesis [38]. In this study the authors used a light-activated fusion system consisting of flavin-binding, kelch repeat F box1 (FKF1) and gigantea (GI) proteins of Arabidosis thaliana was used to fuse Cav1.2 channels and determine whether protein-protein interaction among LTCCs enable coupled gating. The C-termini of Cav1.2 channels were tagged with either FKF1 or GI and expressed in tsA-201 cells. Illumination with blue light (473 or 488 nm) induced channel fusion via structural modulation of FKF1, promoting high-affinity binding of GI. Remarkably, fusion of neighboring channels after light stimulation substantially increased the likelihood of coupled events and resulted in a leftward shift in voltage-dependence of activation leading the authors to suggest that physical interaction between C-termini may underlie coupled gating. Yet, how this interaction evokes synchronous gating of several channels is unclear. Notably, light-induced fusion of channels resulted in a shift in voltage-dependent activation towards more hyperpolarized membrane potentials. Thus, one could speculate that interaction between neighboring C-termini may couple pore openings between adjacent channels by equivalent exertion of torque on the voltage-sensors of multiple channels via upstream S6 transmembrane helices, which are thought to impact pore opening in voltage-gated channels [39]. Nonetheless, further experiments are required to fully address the mechanistic basis of coupled gating.
In Dixon et al, physical interactions were induced artificially, being driven by light-sensitive fusion proteins [38]. Hence, another important issue raised by findings of coupled gating of LTCCs is what native factors promote coupling between channels in vivo. One possibility is that a scaffolding protein, such as AKAP150, simultaneously anchors C-termini of multiple channels. Existing evidence suggests that displacement of the ubiquitous Ca2+ sensor calmodulin from the C-terminus of AKAP150-associated LTCC α1 subunits by PKC activators, CaM inhibitors or conformational changes produced by Timothy syndrome is a key step in the induction of LTCC coupling [37] (Figure 2D). Therefore, a potential role for AKAP150 in coupled gating of LTCCs can have broad implications for Ca2+ signaling across multiple organ systems, as this scaffolding protein is ubiquitously expressed and influences LTCC function in a variety of cell types [27, 40-42]. Thus, coupled gating may represent a common mechanism by which LTCCs can enhance local Ca2+ concentrations and potentially influence downstream signaling pathways. For example, one can speculate that the coupling strength between sparklet and sparks in the heart may be substantially increased in couplons with AKAP150-coupled LTCCs than in areas with independently gating channels, as the coordinated opening of multiple channels is more likely to generate the amplification of Ca2+ influx necessary to maximally activate RyRs during EC coupling [43]. In support of this, action potential-evoked [Ca2+]i transients are slightly decreased in cardiac cells from an AKAP150 knockout (AKAP150−/−) mouse [42]. In addition, AKAP150 can behave as an accessory subunit of LTCCs, regulating CaV1.2 kinetics in ventricular myocytes, and is responsible for prolonged cardiomyocytes action potentials during Timothy syndrome. However, considering that not all AKAP150-associated LTCCs consistently display coupling behavior, it is likely that additional factors are also required for cooperative LTCC gating to occur.
6. LTCC-mediated mechanisms of disease
Increased Ca2+ influx via upregulated LTCC expression and/or activity [44-46] in resistance arteries is a major contributor of enhanced arterial tone during several forms of hypertension [47-50]. Consistent with this, LTCC sparklet activity and the frequency of coupled gating events were found to be significantly higher in vascular smooth muscle in response to the potent endogenous vasoconstrictor angiotensin II [45, 46], which is elevated during hypertension. Multiple pathological alterations may collectively contribute to increased LTCC activity in hypertensive animals, including increased expression of CaV1.2 [44], activation of PKCα [46] and possibly PKC α-dependent reactive oxygen species (ROS) signaling [51, 52]. Similar to that observed during hypertensive conditions, persistent LTCC sparklet activity in arterial myocytes is also increased by acute exposure of isolated myocytes to supraphysiological levels of extracellular D-glucose as well as in genetically engineered diabetic (db/db) mice [45]. These recent findings suggest that impaired regulation of LTCC sparklet activity may be a common mechanism of elevated intracellular Ca2+ during vascular dysfunction. However, during acute hyperglycemia and type-II diabetes, increased LTCC sparklet activity and coupled gating is dependent on local activation of PKA, rather than PKC [45]. Interestingly, this study found that anchoring of PKA by an AKAP protein was also critical, as a specific peptide inhibitor of PKA/AKAP interactions (i.e. Ht-31) eliminated the glucose-induced increase in LTCC sparklet activity. Although more experiments are needed to address this issue, selective activation of AKAP-bound PKA may explain this kinase dependent facilitation of LTCC activity, exclusive from parallel potassium channel activation and membrane potential hyperpolarization.
How AKAP-targeted PKA is activated by elevation of extracellular glucose is still unclear. One intriguing possibility involves glucose-induced activation of NADPH oxidase [53] producing discrete sites of ROS generation. Consistent with this hypothesis, localized and direct oxidative kinase activation upon stimulation with hydrogen peroxide has recently been linked to enhanced Ca2+ sparklet activity in arterial myocytes [52]. Whether localized stimulation of adenylyl cyclase and subcellular cAMP production accompanies this discrete PKA-dependent LTCC activation upon stimulation with elevated extracellular glucose has not yet been tested. Additionally, hyperglycemic conditions are known to stimulate extracellular release of uridine triphosphate and adenosine triphosphate in a variety of cell types, including arterial myocytes [54-56]. Binding of these nucleotides to vascular P2Y2 and P2Y6 receptors evokes an increase in cytosolic Ca2+, which is dependent on both Ca2+ influx via LTCCs and intracellular Ca2+ release [57], and ultimately decreases arterial diameter. Further studies are required to address these issues.
A current model for the control of excitation-transcription coupling by LTCCs suggests that a rise in cytosolic Ca2+ triggers activation of the Ca2+/CaM-dependent phosphatase calcineurin, which in turns dephosphorylates members of the NFAT transcription factor family [26, 58, 59]. Removal of phosphate groups at several N-terminal serine and threonine residues within NFAT unmasks nuclear import signals [58, 60], leading to nuclear translocation and activation or deactivation of NFAT-target genes. Importantly, Ca2+-induced NFAT activation is evoked by a variety of stimuli and this signaling pathway is linked to induction of specific genetic programs during the progression of multiple disease states including Alzheimer’s disease, myocardial infarction, heart failure, and hypertension [53, 54, 61, 62]. In arterial myocytes, nuclear translocation of NFATc3, the isoform predominantly expressed in smooth muscle, is a key pathophysiological event responsible for downregulating the expression of voltage-dependent and Ca2+-activated potassium channels [61, 63, 64]. Accordingly, downregulation of these channels may underlie membrane potential depolarization, elevated cytosolic [Ca2+]i and higher arterial tone during hypertension and diabetes. Activation of NFAT in the vasculature during hyperglycemic conditions and diabetes has also been linked to increased production of the matrix cytokine osteopontin [57], a key mediator of vascular inflammation, which is active in the development of atherosclerotic lesions [65]. Thus, specific factors contributing to NFATc3 nuclear translocation represent potentially therapeutic targets for future treatments against vascular disease.
As in the vasculature, compartmentalized Ca2+ signals in cardiomyocytes may also be essential for signaling during pathological stresses contributing to hypertrophic growth and remodeling [66]. For example, calcineurin and NFAT activation during cardiac hypertrophy may be independent of global Ca2+ transients, but rather more linked to local Ca2+ influx via a subgroup of channels residing in membrane subdomains [36, 67]. Nonetheless, the source of “hypertrophic” Ca2+ contributing to calcineurin-NFAT activation in the heart remains controversial. A recent study identified a subpopulation of LTCCs that are not localized to the T-tubules, but are associated with caveolae [36]. Selective blockade of caveolae-associated LTCCs using caveolin-3-targeted Rem proteins nearly abolished Ca2+ influx-induced NFAT nuclear translocation, but did not significantly alter contractility. Additionally, evidence both supports and refutes signaling microdomains housing T-type voltage-dependent Ca2+ channels as important mediators of hypertrophic Ca2+ influx [68, 69]. Finally, inhibition of canonical transient receptor potential (TRP) channels also significantly reduces calcineurin-NFAT activity and was protective in transgenic mice against loss of cardiac functional performance following pressure-overload stimulation [70]. Interestingly, it was recently shown that nuclear translocation of NFATc3 upon overexpression of TRPC3 was completely abolished by the LTCC inhibitor nifedipine [71], suggesting that TRP channels may modulate expression or activity of LTCCs to induce hypertrophic signaling in the heart.
7. The future of fluorescence-based investigation of Ca2+-permeable channels
Ongoing endeavors to investigate functional coupling between Ca2+ transients and signaling pathways at molecular resolution will without question rely on further advances in imaging and indicator technology to isolate fluorescent signals with fewer cytotoxic effects and improved signal-to-noise. A major goal of future analyses of Ca2 signaling pathways will be to visualize localized transient events in more physiologically relevant contexts than that of former studies, which mostly imaged isolated cells loaded with acetoxymethyl ester indicators. Indeed, conventional dyes are severely limited in situ or in vivo due to interfering fluorescence arising from high-intensity signals in adjacent cell types, which may also readily take up indicator. To circumvent this issue, transgenic mice have recently been developed to express genetically encoded Ca2+ indicators, offering the possibility of long-term in situ and in vivo imaging with cell-specific and even microdomain-specific expression profiles [72, 73]. Several variants of GCamP, a circularly permutated enhanced green fluorescent protein (GFP) attached to calmodulin and an N-terminal fragment of myosin light chain kinase (M13) [74, 75], have now been expressed to record Ca2+ signals in neural networks, heart, and vascular endothelial cells [72, 76, 77]. The GFP fluorescent yield of GCamP proteins increases with cooperative binding of calcium ions by calmodulin and M13 fragments over a range of physiological Ca2+ concentrations (KD ≈ 150 nM), making it useful to study intracellular events occurring on millisecond timescales. This indicator can also be selectively targeted to various subcellular compartments. For example, tagging of GCaMP2 with Lck, a Src tyrosine kinase containing tandem myristoylation and palmitoylation domains, increased the local expression of GCamP2 near the membrane in astrocytes of rat hippocampal astrocyte-neuron co-cultures by as much as fourteen fold compared with unmodified GCaMP2 [78]. This strategy lead to the discovery of Ca2+ “microdomains” in fine astrocytic processes that were the result of transmembrane Ca2+ flux, Ca2+ signals that previously went undetected using organic Ca2+ dyes. Although further experiments are required to determine the nature of these astrocytic microdomain signals, this study suggests that Lck-tagged GCaMP provides a powerful strategy to monitor near-membrane signals in small volume cellular compartments.
A new height of target specificity in Ca2+ imaging was reached in a recent study in which a genetically encoded troponin-based Ca2+ indicator, TN-XL, was tethered to the carboxy terminus of CaV2.2 channels heterologously expressed in HEK293 cells [79]. In combination with TIRF microscopy, this approach allows detection of nanodomain changes in fluorescence solely at the pore of the Ca2+-permeable channel of interest, without interfering fluorescence from regions outside the vicinity of the Ca2+ channel. In fact, FRET-based estimates approximate a distance of 55 Å between the sensor and the cytoplasmic mouth of the channel. With stronger buffering of 10 mM EGTA, Ca2+ nanodomains were estimated from TN-XL fluorescence to be just 40 nm in diameter, roughly two-fold larger than estimates of the diameter of a single Ca2+ channel. Importantly, it was shown that fused Cav2.2/TN-XL is resistant to endogenous proteolysis and addition of this sensor to the channel structure did not substantially interfere with channel gating kinetics or pore conductance, although resulting currents were actually slightly enhanced. These innovations provide new insights for the future use of progressively improved genetically encoded indicators fused to Ca2+-permeable channels to isolate nanodomain Ca2+ signals at the pore of single channels.
8. Conclusions
Subcellular compartmentalization of intracellular Ca2+ is essential to the versatility of this ionic second messenger. Optical analysis of local Ca2+ signals arising through single or clusters of Ca2+-permeable channels represents a powerful new approach with the potential for revealing important new information regarding the regulation of Ca2+ channels in excitable cells during health and disease. Used as an adjunct technique to patch clamp electrophysiology, TIRF microscopy has already lead to major improvements in our understanding of functional regulation and spatial characteristics of LTCC-mediated Ca2+ influx in excitable cells. One can anticipate that further development of novel tools to aid in optical investigation of LTCC function will ultimately facilitate a more complete understanding of channel regulation relating to the role these channels play in numerous cellular processes.
Highlights.
Advances in imaging technology (TIRF and confocal) have allowed optical analysis of L-type Ca2+ channel activity with high spatiotemporal resolution in excitable cells.
L-type Ca2+ channel activity varies regionally throughout the plasmalemma of excitable cells.
A subpopulation of L-type Ca2+ channels can operate in a cooperative or coupled manner with profound implications in health and disease.
Emerging approaches such as chemical or genetically encoded Ca2+ indicators are promising developments in our pursuit of understanding nano- and microdomain Ca2+ signals.
Acknowledgements
This work was supported by grants from the American Heart Association – Scientist Development Grant 0735251N and National Institute of Health 1R01HL098200 (to MFN) and T32HL07828 and T32HL086350-05 (to MAN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cheng H, Lederer WJ. Calcium sparks. Physiological reviews. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- [2].Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- [3].Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell alpha2delta-1 subunits are essential for vasoregulation by CaV1.2 channels. Circulation research. 2009;105:948–955. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annual review of cell and developmental biology. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- [5].Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of a membrane-localized beta subunit in the formation and targeting of functional L-type Ca2+ channels. The Journal of biological chemistry. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- [6].Nystoriak MA, Murakami K, Penar PL, Wellman GC. Ca(v)1.2 splice variant with exon 9* is critical for regulation of cerebral artery diameter, American journal of physiology. Heart and circulatory physiology. 2009;297:H1820–1828. doi: 10.1152/ajpheart.00326.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liao P, Yong TF, Liang MC, Yue DT, Soong TW. Splicing for alternative structures of Cav1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovascular research. 2005;68:197–203. doi: 10.1016/j.cardiores.2005.06.024. [DOI] [PubMed] [Google Scholar]
- [8].Bannister JP, Thomas-Gatewood CM, Neeb ZP, Adebiyi A, Cheng X, Jaggar JH. Ca(V)1.2 channel N-terminal splice variants modulate functional surface expression in resistance size artery smooth muscle cells. The Journal of biological chemistry. 2011;286:15058–15066. doi: 10.1074/jbc.M110.182816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca(2+) channels (Ca(V)1.2a,b) by protein kinases, American journal of physiology. Cell physiology. 2001;281:C1743–1756. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- [10].Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- [11].Liao P, Soong TW. CaV1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflugers Archiv : European journal of physiology. 2010;460:353–359. doi: 10.1007/s00424-009-0753-0. [DOI] [PubMed] [Google Scholar]
- [12].Ringer S. A further Contribution regarding the influence of the different Constituents of the Blood on the Contraction of the Heart. The Journal of physiology. 1883;4:29–42. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takahashi SX, Miriyala J, Tay LH, Yue DT, Colecraft HM. A CaVbeta SH3/guanylate kinase domain interaction regulates multiple properties of voltage-gated Ca2+ channels. The Journal of general physiology. 2005;126:365–377. doi: 10.1085/jgp.200509354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rodriguez PFG, Wu Y, Zhao H, Singh H, Lu R, Li M, Zhang Z, Lee S, Toro L, Stefani E. A Custom-Built Fast Scanning STED Microscope with a Large Field of View. Biophys J. 2012;102:141a. [Google Scholar]
- [15].Tsien RY. Fluorescent probes of cell signaling. Annual review of neuroscience. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- [16].Zou H, Lifshitz LM, Tuft RA, Fogarty KE, Singer JJ. Imaging Ca(2+) entering the cytoplasm through a single opening of a plasma membrane cation channel. The Journal of general physiology. 1999;114:575–588. doi: 10.1085/jgp.114.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zou H, Lifshitz LM, Tuft RA, Fogarty KE, Singer JJ. Using total fluorescence increase (signal mass) to determine the Ca2+ current underlying localized Ca2+ events. J Gen Physiol. 2004;124:259–272. doi: 10.1085/jgp.200409066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- [19].Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- [20].Demuro A, Parker I. Imaging the activity and localization of single voltage-gated Ca(2+) channels by total internal reflection fluorescence microscopy. Biophysical journal. 2004;86:3250–3259. doi: 10.1016/S0006-3495(04)74373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Demuro A, Parker I. Optical single-channel recording: imaging Ca2+ flux through individual N-type voltage-gated channels expressed in Xenopus oocytes. Cell calcium. 2003;34:499–509. doi: 10.1016/s0143-4160(03)00154-4. [DOI] [PubMed] [Google Scholar]
- [22].Fish KN. Total internal reflection fluorescence (TIRF) microscopy, Current protocols in cytometry/editorial board. In: Paul Robinson J, editor. Vol. 12. 2009. Unit12 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms Underlying Heterogeneous Ca2+ Sparklet Activity in Arterial Smooth Muscle. Journal of General Physiology. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Navedo MF, Amberg G, Votaw SV, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Navedo MF, Amberg GC, Westenbroek RE, Sinnegger-Brauns MJ, Catterall WA, Striessnig J, Santana LF. Cav1.3 channels produce persistent calcium sparklets, but Cav1.2 channels are responsible for sparklets in mouse arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1359–1370. doi: 10.1152/ajpheart.00450.2007. [DOI] [PubMed] [Google Scholar]
- [26].Santana LF, Navedo MF. Molecular and biophysical mechanisms of Ca2+ sparklets in smooth muscle. J Mol Cell Cardiol. 2009;47:436–444. doi: 10.1016/j.yjmcc.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- [28].Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- [29].Dell’Acqua ML, Faux MC, Thorburn J, Thorburn A, Scott JD. Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4, 5-bisphosphate. Embo J. 1998;17:2246–2260. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gomez LL, Alam S, Smith KE, Horne E, Dell’Acqua ML. Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:7027–7044. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gorski JA, Gomez LL, Scott JD, Dell’Acqua ML. Association of an A-kinase-anchoring protein signaling scaffold with cadherin adhesion molecules in neurons and epithelial cells. Molecular biology of the cell. 2005;16:3574–3590. doi: 10.1091/mbc.E05-02-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Amberg GC, Navedo MF, Nieves-Cintrón M, Molkentin JD, Santana LF. Calcium Sparklets Regulate Local and Global Calcium in Murine Arterial Smooth Muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Takeda Y, Nystoriak MA, Nieves-Cintron M, Santana LF, Navedo MF. Relationship between Ca2+ sparklets and sarcoplasmic reticulum Ca2+ load and release in rat cerebral arterial smooth muscle, American journal of physiology. Heart and circulatory physiology. 2011;301:H2285–2294. doi: 10.1152/ajpheart.00488.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Santana LF, Cheng H, Gomez AM, Cannell MB, Lederer WJ. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996;78:166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- [36].Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, Rizzo V, Molkentin JD, Houser SR. A caveolae-targeted L-type Ca(2)+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circulation research. 2012;110:669–674. doi: 10.1161/CIRCRESAHA.111.264028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circulation research. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dixon RE, Yuan C, Cheng EP, Navedo M, Santana LF. Calcium signaling amplification by oligomerization of L-type Cav1.2 channels. Proc Natl Acad Sci U S A. 2012;109:1749–1754. doi: 10.1073/pnas.1116731109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhao Y, Yarov-Yarovoy V, Scheuer T, Catterall WA. A gating hinge in Na+ channels; a molecular switch for electrical signaling. Neuron. 2004;41:859–865. doi: 10.1016/s0896-6273(04)00116-3. [DOI] [PubMed] [Google Scholar]
- [40].Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hinke SA, Navedo MF, Ulman A, Whiting JL, Nygren PJ, Tian G, Jimenez-Caliani AJ, Langeberg LK, Cirulli V, Tengholm A, Dell’acqua ML, Santana LF, Scott JD. Anchored phosphatases modulate glucose homeostasis. The EMBO journal. 2012 doi: 10.1038/emboj.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cheng EP, Yuan C, Navedo MF, Dixon RE, Nieves-Cintron M, Scott JD, Santana LF. Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein. Circulation research. 2011;109:255–261. doi: 10.1161/CIRCRESAHA.111.248252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Inoue M, Bridge JH. Ca2+ sparks in rabbit ventricular myocytes evoked by action potentials: involvement of clusters of L-type Ca2+ channels. Circulation research. 2003;92:532–538. doi: 10.1161/01.RES.0000064175.70693.EC. [DOI] [PubMed] [Google Scholar]
- [44].Pesic A, Madden JA, Pesic M, Rusch NJ. High Blood Pressure Upregulates Arterial L-Type Ca2+ Channels. Is Membrane Depolarization the Signal? Circ Res. 2004 doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- [45].Navedo MF, Takeda Y, Nieves-Cintron M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells, American journal of physiology. Cell physiology. 2010;298:C211–220. doi: 10.1152/ajpcell.00267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].National High Blood Pressure Education Program Working Group report on hypertension in diabetes Hypertension. 1994;23:145–158. discussion 159-160. [PubMed] [Google Scholar]
- [48].Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16. doi: 10.1159/000115118. [DOI] [PubMed] [Google Scholar]
- [49].Amberg GC, Santana LF. Downregulation of the BK channel β1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- [50].Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- [51].Amberg GC, Earley S, Glapa SA. Local regulation of arterial L-type calcium channels by reactive oxygen species. Circulation research. 2010;107:1002–1010. doi: 10.1161/CIRCRESAHA.110.217018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chaplin NL, Amberg GC. Hydrogen peroxide mediates oxidant-dependent stimulation of arterial smooth muscle L-type calcium channels, American journal of physiology. Cell physiology. 2012;302:C1382–1393. doi: 10.1152/ajpcell.00222.2011. [DOI] [PubMed] [Google Scholar]
- [53].Lunde IG, Kvaloy H, Austbo B, Christensen G, Carlson CR. Angiotensin II and norepinephrine activate specific calcineurin-dependent NFAT transcription factor isoforms in cardiomyocytes. J Appl Physiol. 2011;111:1278–1289. doi: 10.1152/japplphysiol.01383.2010. [DOI] [PubMed] [Google Scholar]
- [54].Hudry E, Wu HY, Arbel-Ornath M, Hashimoto T, Matsouaka R, Fan Z, Spires-Jones TL, Betensky RA, Bacskai BJ, Hyman BT. Inhibition of the NFAT pathway alleviates amyloid beta neurotoxicity in a mouse model of Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3176–3192. doi: 10.1523/JNEUROSCI.6439-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Omar B, Banke E, Guirguis E, Kesson L, Manganiello V, Lyssenko V, Groop L, Gomez MF, Degerman E. Regulation of the pro-inflammatory cytokine osteopontin by GIP in adipocytes - A role for the transcription factor NFAT and phosphodiesterase 3B. Biochemical and biophysical research communications. 2012;425:812–817. doi: 10.1016/j.bbrc.2012.07.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nilsson J, Nilsson LM, Chen YW, Molkentin JD, Erlinge D, Gomez MF. High glucose activates nuclear factor of activated T cells in native vascular smooth muscle. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:794–800. doi: 10.1161/01.ATV.0000209513.00765.13. [DOI] [PubMed] [Google Scholar]
- [57].Nilsson-Berglund LM, Zetterqvist AV, Nilsson-Ohman J, Sigvardsson M, Gonzalez Bosc LV, Smith ML, Salehi A, Agardh E, Fredrikson GN, Agardh CD, Nilsson J, Wamhoff BR, Hultgardh-Nilsson A, Gomez MF. Nuclear factor of activated T cells regulates osteopontin expression in arterial smooth muscle in response to diabetes-induced hyperglycemia. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:218–224. doi: 10.1161/ATVBAHA.109.199299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- [59].Hill-Eubanks DC, Gomez MF, Stevenson AS, Nelson MT. NFAT regulation in smooth muscle. Trends Cardiovasc Med. 2003;13:56–62. doi: 10.1016/s1050-1738(02)00212-8. [DOI] [PubMed] [Google Scholar]
- [60].Luo C, Shaw KT, Raghavan A, Aramburu J, Garcia-Cozar F, Perrino BA, Hogan PG, Rao A. Interaction of calcineurin with a domain of the transcription factor NFAT1 that controls nuclear import. Proc Natl Acad Sci U S A. 1996;93:8907–8912. doi: 10.1073/pnas.93.17.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nieves-Cintrón M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 Down-regulates the β1 Subunit of Large Conductance, Calcium-activated K+ Channels in Arterial Smooth Muscle and Contributes to Hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- [62].Demuro A, Smith M, Parker I. Single-channel Ca(2+) imaging implicates Abeta1-42 amyloid pores in Alzheimer’s disease pathology. The Journal of cell biology. 2011;195:515–524. doi: 10.1083/jcb.201104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 Regulates Kv2.1 Expression in Arterial Smooth Muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- [64].Rossow CF, Minami E, Chase EG, Murry CE, Santana LF. NFATc3-Induced Reductions in Voltage-Gated K+ Currents After Myocardial Infarction. Circ Res. 2004;94:1340–1350. doi: 10.1161/01.RES.0000128406.08418.34. [DOI] [PubMed] [Google Scholar]
- [65].Cho HJ, Cho HJ, Kim HS. Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Current atherosclerosis reports. 2009;11:206–213. doi: 10.1007/s11883-009-0032-8. [DOI] [PubMed] [Google Scholar]
- [66].Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochemical and biophysical research communications. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- [67].Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, Chen CC. The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circulation research. 2009;104:522–530. doi: 10.1161/CIRCRESAHA.108.184051. [DOI] [PubMed] [Google Scholar]
- [69].Jaleel N, Nakayama H, Chen X, Kubo H, MacDonnell S, Zhang H, Berretta R, Robbins J, Cribbs L, Molkentin JD, Houser SR. Ca2+ influx through T- and L-type Ca2+ channels have different effects on myocyte contractility and induce unique cardiac phenotypes. Circulation research. 2008;103:1109–1119. doi: 10.1161/CIRCRESAHA.108.185611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7000–7005. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gao H, Wang F, Wang W, Makarewich CA, Zhang H, Kubo H, Berretta RM, Barr LA, Molkentin JD, Houser SR. Ca(2+) influx through L-type Ca(2+) channels and transient receptor potential channels activates pathological hypertrophy signaling. Journal of molecular and cellular cardiology. 2012 doi: 10.1016/j.yjmcc.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, Sanbe A, Gulick J, Mathai J, Robbins J, Salama G, Nakai J, Kotlikoff MI. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4753–4758. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chen M, Wang Y, Hou T, Zhang H, Qu A, Wang X. Differential mitochondrial calcium responses in different cell types detected with a mitochondrial calcium fluorescent indicator, mito-GCaMP2. Acta biochimica et biophysica Sinica. 2011;43:822–830. doi: 10.1093/abbs/gmr075. [DOI] [PubMed] [Google Scholar]
- [74].Wang Q, Shui B, Kotlikoff MI, Sondermann H. Structural basis for calcium sensing by GCaMP2. Structure. 2008;16:1817–1827. doi: 10.1016/j.str.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Akerboom J, Rivera JD, Guilbe MM, Malave EC, Hernandez HH, Tian L, Hires SA, Marvin JS, Looger LL, Schreiter ER. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. The Journal of biological chemistry. 2009;284:6455–6464. doi: 10.1074/jbc.M807657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mao T, O’Connor DH, Scheuss V, Nakai J, Svoboda K. Characterization and subcellular targeting of GCaMP-type genetically-encoded calcium indicators. PloS one. 2008;3:e1796. doi: 10.1371/journal.pone.0001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nature neuroscience. 2010;13:759–766. doi: 10.1038/nn.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tay LH, Dick IE, Yang W, Mank M, Griesbeck O, Yue DT. Nanodomain Ca(2)(+) of Ca(2)(+) channels detected by a tethered genetically encoded Ca(2)(+) sensor. Nature communications. 2012;3:778. doi: 10.1038/ncomms1777. [DOI] [PMC free article] [PubMed] [Google Scholar]