Abstract
Purpose
To investigate indoor particulate matter (PM) level and various indoor air pollution exposure, and to examine their relationships with risk of lung cancer in an urban Chinese population, with a focus on non-smoking women.
Methods
We conducted a case-control study in Taiyuan, China, consisting of 399 lung cancer cases and 466 controls, of which 164 cases and 218 controls were female non-smokers. Indoor PM concentrations, including PM1, PM2.5, PM7, PM10 and TSP, were measured using a particle mass monitor. Unconditional logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals after adjusting for age, education, annual income and smoking.
Results
Among non-smoking women, lung cancer was strongly associated with multiple sources of indoor air pollution 10 years ago, including heavy exposure to ETS at work (aOR=3.65), high frequency of cooking (aOR=3.30), and solid fuel usage for cooking (aOR=4.08) and heating (aORcoal stove=2.00). Housing characteristics related to poor ventilation, including single-story, less window area, no separate kitchen, no ventilator and rarely having windows open, are associated with lung cancer. Indoor medium PM2.5 concentration was 68ug/m3, and PM10 was 230ug/m3. PM levels in winter are strongly correlated with solid fuel usage for cooking, heating and ventilators. PM1 levels in cases are more than 3-time higher than that in controls. Every 10 ug/m3 increase in PM1 is associated with 45% increased risk of lung cancer.
Conclusions
Indoor air pollution plays an important role in the development of lung cancer among non-smoking Chinese women.
Keywords: Indoor air pollution, lung cancer, particulate matter, Chinese non-smoking female
1. Introduction
Lung cancer is a leading disease with the highest incidence and mortality in the Chinese population. The estimated numbers of new lung cancer cases and of deaths in 2008 were 522,050, and 452,813 in China[1]. Although the etiology of lung cancer has been well-studied and illustrated in Western populations, the reasons for high lung cancer incidence among Chinese remain unclear and needs to be fully studied.
Tobacco smoking, the most critical risk factor for lung cancer in Western nations, seems to play a less important role in the development of lung cancer in Chinese, because Chinese males, with high smoking prevalence (67%), have relatively low lung cancer incidence (53.2/100,000), and Chinese females, with low smoking rates (4%), have relatively high lung cancer incidence (21.1/100,000) in China. Smoking contributes to 75% of male lung cancer and 18% of female lung cancer in China [2]. This suggests that other important risk factors contribute to the disease in this population, particularly among Chinese females.
Indoor air pollution has been suspected to be important in the development of lung cancer. However, exposure assessment of indoor air pollution has been challenging, since various indoor activities and living habits, such as passive smoking, use of solid fuel, and inadequate ventilation systems, produce numerous air pollutants, Air borne particulate matter (PM), is a complex mixture of solid and Aliquid particles of various size and composition. PM is a critical portion of air pollution and has long been known to increase the risk of morbidity and mortality from cancer and other diseases [3–5]. PM is classified according to aerodynamic diameter into coarse (2.5~10µm, PM10), fine (0.1~2.5µm, PM2.5) and ultrafine (≤0.1µm, PM1). Different sizes of PM are from different sources and human activities. PM has been widely used to monitor outdoor air pollution, but not indoor air pollution. Very limited studies have reported indoor PM level in China because of difficulties in monitoring and lack of historical data. Given the complicated nature of indoor air pollution, PM may well represent overall indoor air pollution levels and be worth fully studying.
To better understand the role of indoor air pollution in the development of lung cancer among Chinese females, the current case-control study is designed to explore the relationship between indoor activity, living habits, housing characteristics and the risk of lung cancer among Chinese, with a focus on Chinese female non-smokers. The study also investigated household PM exposure levels and their association with various indoor risk factors.
2. Material and Methods
2.1 Study design and participants
A case-control study was conducted in Taiyuan city, the capital of Shanxi province. Prior to the initiation of the recruitment, IRB approvals were obtained from Fudan University (IRB#04-10-0022) and UCLA (IRB#11-003153), respectively.
Cases recruitment: Eligible cases were lung cancer patients diagnosed in Shanxi Tumor Hospital between 2005 and 2007. The hospital has about 70% of the cancer patients from the city. Prior to the initiation of the study, study staffs set up a fast report system through which any newly diagnosed lung cancer case was reported to the department of medical record in the hospital. Our study staff will be immediately noticed and he/she will ask permission from doctor for contacting patients. The eligible patients must be newly diagnosed, 20 years of age or older, have lived in Taiyuan city for 10 years or more, in stable medical condition and willing to participate. Consent forms were obtained before interview. The study recruited a total of 399 lung cancer patients (response rate was 89%), all of whom completed the study questionnaire. About 33% of female cases and 21% of male cases are adenocarcinoma. About 13% of female cases and 33% of male cases are squamous cell carcinoma. Small cell carcinoma accounts for 16% of female cases and 13% of male cases.
Controls recruitment: We selected thirteen communities that cover the most areas of Taiyuan city. Study personnel randomly selected controls from resident list of each community to match cases according to the distribution of age and gender. Eligible controls were 20 years of age or older, must have lived in Taiyuan city for 10 years or more, and had no history of cancer or any other serious chronic disease. Four hundred sixty-six healthy individuals were willing to participate and completed the questionnaire (response rate was 85%).
2.2 Epidemiologic data collection
All patients were interviewed at the hospital and all controls were interviewed in community health service centers. The questionnaire includes demographic factors, residence and housing history, living habits and indoor activities, dietary and cooking habits, active and passive smoking history, alcohol drinking habits, tea drinking habits, occupational history and related exposure, physical activities and disease history.
2.3 Indoor PM measurement
Participants were asked to have their home PM level measured. After obtaining permission from participants, professional staff from Taiyuan CDC scheduled a home visit to measure their indoor PM levels. Particle mass monitor (Met One® 531 AEROCET Particulate Profiler, Met One Instruments, Inc. Grant Pass, Oregon), was used to measure the concentration of household PM in a subset of cases (75) and controls (337). PM with the following size ranges were measured: PM1, PM2.5, PM7, PM10 and TSP, along with temperature and relative humidity. The measured concentration range is up to 1mg/m3. For each participant, we measured indoor PM levels in twice in the year when they were recruited, one in winter and one in summer. All measurements were conducted in morning between 9 and 12. Each time, two repeated PM measurements were conducted in the living room, bedroom and kitchen, respectively. Each measurement took about 5 minutes. Five minutes break was given between two measurements. All PM measurement data stored in the monitor was transferred to computer at the end of the day.
2.4 Statistical Analysis
All statistical analyses were performed using SAS software (version 9.1). The associations of indoor air pollution exposure with risk of lung cancer were analyzed using unconditional logistic regression models. Odds ratios (ORs) and 95% confidence intervals were estimated for each independent variable. Adjusted ORs (aORs) and 95% CIs were calculated after adjusting potential confounding factors, including age, education level, annual personal income and pack-years of smoking. Wilcoxon rank-sum test was used to examine associations between PM level and indoor risk factors and also to compare PM levels between cases and controls. The indoor PM level was further evaluated as continuous variables in the unconditional logistic regression model after adjusting confounding factors. Results are reported as the changes in risk of lung cancer per 10 µg/m3.
3. Results
3.1 General information of cases and controls
A total of 399 cases (197 females and 202 males) and 466 controls (232 females and 234 males) were recruited in the study. No significant difference in age distribution was found between cases and controls. Female controls had higher education and annual income than female cases. Both male and female controls had higher annual income than cases (Table 1).
Table 1.
General characteristics of cases and controls
| Case |
Control |
Case |
Control |
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Female | Male | |||||||
| Age (years) | ||||||||
| <44 | 42 | 21.3 | 68 | 29.3 | 17 | 8.4 | 15 | 6.4 |
| 45–54 | 57 | 28.9 | 71 | 30.6 | 39 | 19.3 | 68 | 29.1 |
| 55–64 | 57 | 28.9 | 54 | 23.3 | 54 | 26.7 | 62 | 26.5 |
| ≥65 | 41 | 20.8 | 39 | 16.8 | 92 | 45.5 | 89 | 38.0 |
| Total | 197 | 232 | 202 | 234 | ||||
| χ2=4.99, P=0.1729 | χ2=6.27, P=0.0991 | |||||||
| Education | ||||||||
| Illiteracy | 33 | 16.8 | 18 | 7.8 | 10 | 5.0 | 5 | 2.1 |
| Primary school | 60 | 30.5 | 41 | 17. 7 | 46 | 22.8 | 40 | 17.1 |
| Middle school | 57 | 28.9 | 84 | 36.2 | 67 | 33.2 | 91 | 38.9 |
| High school | 29 | 14.7 | 69 | 29.7 | 39 | 19.3 | 51 | 21.8 |
| College & above | 18 | 9.1 | 20 | 8.6 | 40 | 19.8 | 47 | 20.1 |
| Total | 197 | 232 | 202 | 234 | ||||
| χ2=26.91, P<0.0001 | χ2=5.58, P=0.2332 | |||||||
| Annual personal income (RMB) | ||||||||
| <1000 | 60 | 30.5 | 53 | 22.8 | 44 | 21.8 | 53 | 22.7 |
| 1000–2500 | 115 | 58.4 | 114 | 49.1 | 121 | 59.9 | 83 | 35.5 |
| 2500–5000 | 14 | 7.1 | 42 | 18.1 | 23 | 11.4 | 74 | 31.6 |
| ≥5000 | 8 | 4.1 | 23 | 9.9 | 14 | 6.9 | 24 | 10.3 |
| Total | 197 | 232 | 202 | 234 | ||||
| χ2=18.97, P=0.0003 | χ2=35.20, P<0.0001 | |||||||
3.2 Active and passive smoking history of study participants
For comparison purpose, data from male participants are also presented. Seventeen percent of female cases and six percent of female controls were smokers. Heavy female smokers (more than 20 pack-year of smoking) had more than 4-fold increased risk of lung cancer, with an OR of 5.42, 95%CI: (1.48, 19.87) (Table 2). About 30% of non-smoking female cases and 23% of non-smoking female controls were exposed to passive smoking at work. Female non-smokers exposed to heavy passive smoking at work had increased risk of lung cancer, with an OR of 3.65 (95%CI: 1.57–8.48). Heavy passive smoking at work or home was associated with a moderately increased risk, with an adjusted OR of 1.98 (95%CI: 1.12–3.51). Ninety-three percent of male cases and seventy-one percent of controls were smokers. Heavy male smokers (more than 20 pack-year of smoking) had more than 5-fold increased risk of lung cancer, with an OR of 6.35. A significant dose-response relationship between pack-years of smoking and lung cancer risk was observed, p<0.0001. However, no association was found between passive smoking and the disease among male non-smokers.
Table 2.
Active and passive smoking and risk of lung cancer
| Case |
Control |
Cryde OR (95%CI) |
Adjusted OR (95%CI)1 |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Female | ||||||
| Active smoking | ||||||
| No | 164 | 83.3 | 218 | 94.0 | 1 | 1 |
| Yes | 33 | 16.8 | 14 | 6.0 | 3.13 (1.62, 6.04) | 2.52 (1.26, 5.03) |
| Total | 197 | 232 | ||||
| Pack-years | ||||||
| Non smokers | 164 | 83.3 | 218 | 94.0 | 1 | 1 |
| <20 | 18 | 9.1 | 11 | 4.7 | 2.18 (1.00, 4.73) | 1.78 (0.80, 3.97) |
| ≥20 | 15 | 7.6 | 3 | 1.3 | 6.65 (1.89, 23.33) | 5.42 (1.48, 19.87) |
| Total | 197 | 232 | ||||
| P trend | 0.0004 | 0.0040 | ||||
| Female non-smokers | ||||||
| Passive smoking at working place2 | ||||||
| No exposure | 115 | 70.1 | 167 | 76.6 | 1 | 1 |
| Light | 30 | 18.3 | 41 | 18.8 | 1.06 (0.63, 1.80) | 1.35 (0.77, 2.34) |
| Heavy | 19 | 11.6 | 10 | 4.6 | 2.76 (1.24, 6.12) | 3.65 (1.57, 8.48) |
| Total | 164 | 218 | ||||
| P trend | 0.0349 | 0.0033 | ||||
| Passive smoking at home2 | ||||||
| No exposure | 57 | 35.2 | 84 | 38.5 | 1 | 1 |
| Light | 67 | 41.3 | 89 | 41.2 | 1.11(0.70, 1.76) | 1.21(0.75,1.96) |
| Heavy | 38 | 23.5 | 43 | 19.9 | 1.30(0.76, 2.26) | 1.25(0.71, 2.20) |
| Total | 162 | 216 | ||||
| P trend | 0.3523 | 0.4030 | ||||
| Passive smoking at working place or home2 | ||||||
| No exposure | 38 | 23.8 | 67 | 30.7 | 1 | 1 |
| Light | 71 | 43.6 | 99 | 45.8 | 1.26 (0.77, 2.09) | 1.41 (0.84,2.39) |
| Heavy | 54 | 33.1 | 50 | 23.2 | 1.90 (1.10, 3.31) | 1.98 (1.12,3.51) |
| Total | 164 | 218 | ||||
| P trend | 0.0223 | 0.0188 | ||||
| Male | ||||||
| Active smoking | ||||||
| No | 15 | 7.4 | 67 | 28.6 | 1 | 1 |
| Yes | 187 | 92.6 | 167 | 71.4 | 5.00(2.75,9.09) | 5.12(2.80,9.36) |
| Total | 202 | 234 | ||||
| Pack-years | ||||||
| Non smokers | 15 | 7.4 | 67 | 28.6 | 1 | 1 |
| <20 | 21 | 10.4 | 51 | 21.8 | 1.84 (0.86,3.92) | 1.97(0.91,4.25) |
| ≥20 | 166 | 82.2 | 116 | 49.6 | 6.39 (3.48,11.74) | 6.35 (3.44,11.71) |
| Total | 202 | 234 | ||||
| P trend | <.0001 | <.0001 | ||||
| Male non-smokers | ||||||
| Passive smoking at working place2 | ||||||
| No exposure | 4 | 26.7 | 31 | 46.3 | 1 | 1 |
| Light | 8 | 53.3 | 22 | 32.8 | 2.82 (0.75,10.54) | 2.11 (0.51,8.76) |
| Heavy | 3 | 20.0 | 14 | 20.9 | 1.66 (0.33,8.43) | 1.76 (0.29,10.74) |
| Total | 15 | 67 | ||||
| P trend | 0.3947 | 0.4417 | ||||
| Passive smoking at home2 | ||||||
| No exposure | 14 | 93.3 | 46 | 68.7 | 1 | 1 |
| Light | 1 | 6.7 | 21 | 31.3 | 0.16 (0.02,1.27) | 0.21 (0.02,1.76) |
| Total | 15 | 67 | ||||
| Passive smoking at working place or home2 | ||||||
| No exposure | 4 | 26.7 | 24 | 35.8 | 1 | 1 |
| Light | 8 | 53.3 | 29 | 47.8 | 1.66 (0.44,6.17) | 1.16 (0.28,4.89) |
| Heavy | 3 | 20.0 | 14 | 16.4 | 1.29 (0.25,6.60) | 1.26 (0.20,7.89) |
| Total | 15 | 67 | ||||
| P trend | 0.6912 | 0.7934 | ||||
Odds Ratios were adjusted for age, education level and annual personal income.
Only among nonsmokers;
3.3 Indoor air pollution exposure and risk of lung cancer among female non-smokers
Individuals who lived in multi-story houses had about 67% lower risk of developing lung cancer compared to women living in single story houses (Table 3). Less window area and rarely having windows open were associated with increased risk: aORs were 2.18 (95CI: 1.30–3.68) and 2.50 (1.28–4.88) respectively. Those individuals who did not have a separate kitchen also experienced increased risk of lung cancer. About 24% of cases and 38% of controls had a ventilator installed in their home. No ventilator at home is associated with an aOR of 1.78 (95%CI: 1.09, 2.90). Individuals who cooked more than twice a day had 3-fold risk when compared to those did not cook regularly, with an adjusted OR of 3.3 (95%CI: 1.32–8.22). A strong relationship was found between solid fuel usage for cooking and heating. Compared to those who used clean fuel for cooking, subjects who only used solid fuel for cooking (coal, honeycomb, and wood) had a 4-fold risk of lung cancer. Solid fuel for heating, including coal furnaces, coke stoves and heatable brick beds, was also associated with higher risk of lung cancer. OR for heatable brick bed exposure was 8.13 (95%CI: 2.20–30.08). In order to analyze the association between overall indoor air pollution exposure and risk of lung cancer, an indoor air pollution (IAP) index was created by summarizing individual’s exposure to the five major risk factors including passive smoking at home, solid fuel use, heating methods, frequency of opening windows and ventilator in kitchen. If an individual was exposed to all 5 of these factors, an IAP index of five was assigned to that individual, and so on until an IAP index of zero was assigned for those individuals with exposure to none of the 5 risk factors. A dose-response relationship was found between IAP index and lung cancer risk. High IAP is associated with an adjusted OR of 3.09 (95%CI: 1.39, 6.86). Potential interactions between smoking and cooking fuel, smoking and heating fuel exposure were also examined. However, no significant interaction was identified. (Data not shown)
Table 3.
Indoor air pollution and risks of lung cancer among female non-smokers
| Case |
Control |
Cryde OR (95%CI) |
Adjusted OR (95%CI)a |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Housing | ||||||
| Type of housing | ||||||
| Single-story house | 124 | 76.5 | 108 | 49.5 | 1 | 1 |
| Multi-story house | 38 | 23.5 | 110 | 50.5 | 0.30 (0.19,0.47) | 0.33(0.20, 0.54) |
| Total | 162 | 218 | ||||
| Number of windows/ m2 | ||||||
| High (>0.077) | 43 | 27.0 | 75 | 35.9 | 1 | 1 |
| Medium (0.05 ~ 0.077) | 39 | 24.5 | 72 | 34.5 | 0.95 (0.55,1.62) | 0.95 (0.54,1.66) |
| Low ( 0.05) | 77 | 48.4 | 62 | 29.7 | 2.17 (1.31,3.58) | 2.18 (1.30,3.66) |
| Total | 159 | 209 | ||||
| P trend | 0.0018 | 0.0026 | ||||
| Open windows frequently | ||||||
| Yes | 127 | 81.4 | 198 | 92.5 | 1 | 1 |
| No | 29 | 18.6 | 16 | 7.5 | 2.83 (1.48,5.41) | 2.50 (1.28,4.88) |
| Total | 156 | 214 | ||||
| Cooking | ||||||
| Cooking place | ||||||
| Outdoor kitchen/ never cooked | 38 | 28.2 | 60 | 29.1 | 1 | 1 |
| Indoor Kitchen | 41 | 30.4 | 109 | 52.9 | 0.59 (0.35,1.02) | 0.64 (0.37,1.13) |
| Living room | 39 | 28.9 | 27 | 13.1 | 2.28 (1.21,4.31) | 2.62 (1.35,5.08) |
| Bed room | 17 | 12.6 | 10 | 4.9 | 2.68 (1.11,6.47) | 2.29 (0.93,5.63) |
| Total | 135 | 206 | ||||
| Ventilator in kitchen | ||||||
| Yes | 37 | 24.2 | 83 | 38.4 | 1 | 1 |
| No | 116 | 75.8 | 133 | 61.6 | 1.96 (1.23,3.10) | 1.78 (1.09,2.90) |
| Total | 153 | 216 | ||||
| Current frequency of cooking | ||||||
| Never, <7/week | 13 | 9.0 | 32 | 15.2 | 1 | 1 |
| 7/week | 35 | 24.1 | 43 | 20.5 | 2.00 (0.92,4.39) | 1.44 (0.64,3.27) |
| 8–14/week | 67 | 46.2 | 118 | 56.2 | 1.40 (0.69,2.85) | 1.11 (0.53,2.34) |
| 15–21/week | 30 | 20.7 | 17 | 8.1 | 4.34 (1.81,10.44) | 3.30 (1.32,8.22) |
| Total | 145 | 210 | ||||
| P trend | 0.0219 | 0.0733 | ||||
| Cooking fuels | ||||||
| onlyb | ||||||
| Clean fuels ≥ 15 yrs | 28 | 17.5 | 64 | 30.3 | 1 | 1 |
| Clean fuels <15 yrs | 41 | 25.6 | 92 | 43.6 | 1.02(0.57, 1.81) | 1.14 (0.63,2.06) |
| Solid fuel only | 91 | 56.9 | 55 | 26.1 | 3.78(2.17, 6.59) | 4.08 (2.17,7.67) |
| Total | 160 | 211 | ||||
| Heating | ||||||
| Clean/no heatingc | 56 | 34.8 | 124 | 59.1 | 1 | 1 |
| Coal furnace | 70 | 43.5 | 71 | 33.8 | 2.18 (1.38,3.45) | 2.00 (1.24,3.23) |
| Coke oven | 20 | 12.4 | 12 | 5.7 | 3.69 (1.69,8.07) | 3.20 (1.40,7.32) |
| Heatable brick bed | 15 | 9.3 | 3 | 1.4 | 11.07 (3.08,39.78) | 8.13 (2.20, 30.08) |
| Total | 161 | 210 | ||||
| Indoor Air Pollution Indexd | ||||||
| 0 | 12 | 8.0 | 28 | 13.2 | 1 | 1 |
| 1–2 | 43 | 28.5 | 113 | 53.0 | 0.89(0.41, 1.90) | 0.97(0.44, 2.11) |
| 3–5 | 96 | 63.5 | 72 | 33.8 | 3.11(1.48, 6.53) | 3.09(1.39, 6.86) |
| Total | 151 | 213 | ||||
| P trend | <.0001 | <.0001 | ||||
Odds Ratios were adjusted for age, education level, annual personal income and pack-year.
Using natural gas, liquid gas or electric stove,
Cleaning heating: Air conditioning / heater/ electrics/
IAP index calculated by characterizing exposure to passive smoking, solid fuel for heating, solid fuels for cooking, no ventilator in kitchen and low frequency of opening windows as (5) significant risk factors for lung cancer. An IAP index of 5 signified exposure to all 5 risk factors, with 0 being exposure to none of the risk factors listed.
3.4 Indoor PM levels and risk of lung cancer
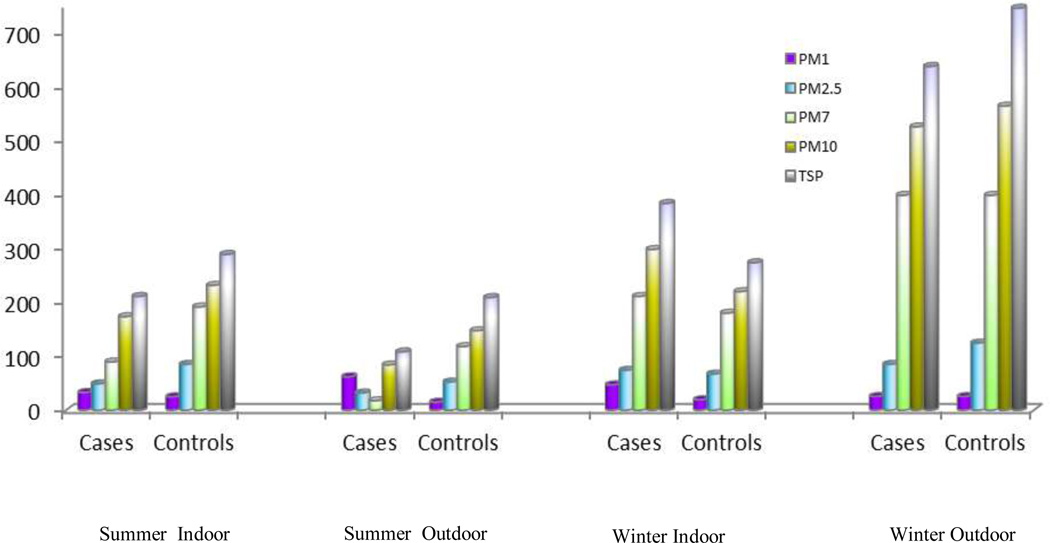
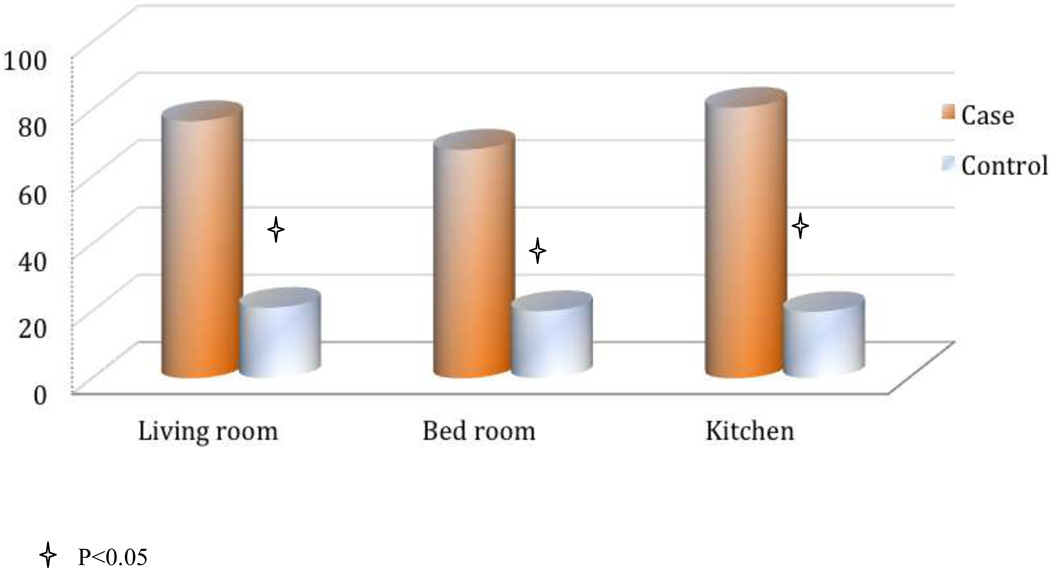
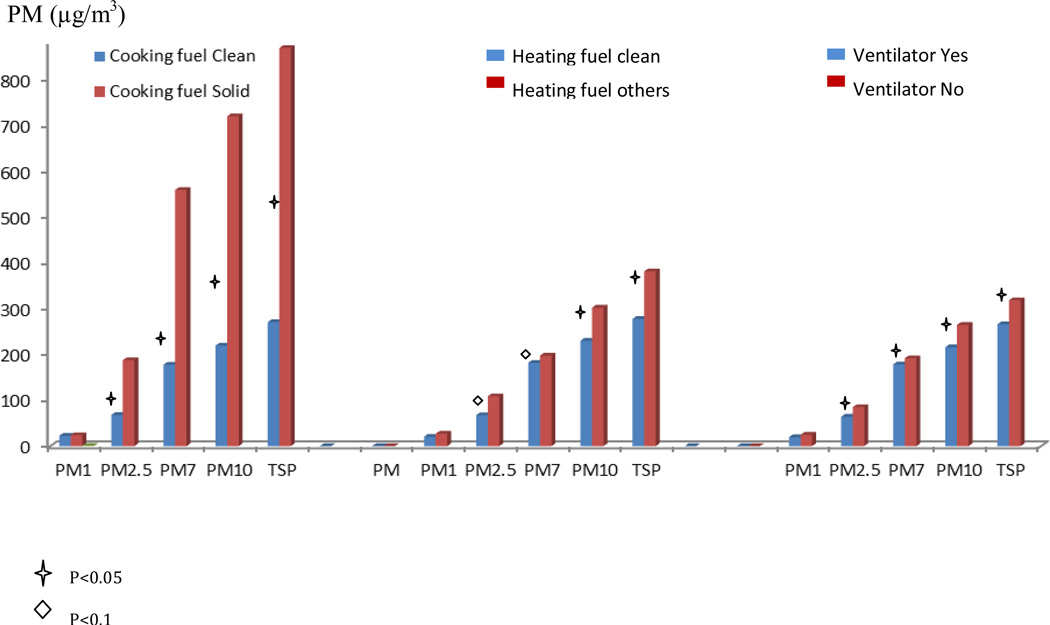
Measurements of PM air pollution shows that indoor levels of large PMs (PM7, PM10, TSP) were higher than outdoor levels during summer, but lower than outdoor levels during winter (Figure1). Indoor medium PM concentration during winter was 21 ug/m3, 68 ug/m3, 180ug/m3, 230ug/m3 and 282ug/m3 for PM1, PM2.5, PM7, PM10 and TSP, respectively. When we compare PM levels between cases and controls, we found that during winter, cases had much higher PM levels than controls, but a significant difference was only observed at the PM1 level (Figure 2). In the further analyses using continuous PM measurements, every 10 ug/m3 increase in PM1 is associated with 45% increased risk of lung cancer (p<0.01). We further explored the association between indoor risk factors and PM level in female. Solid fuel for cooking, ventilator usage in kitchen, solid fuel for heating were significantly associated with PM levels in the bedroom and kitchen during winter, but not in summer (Figure 3).
Figure 1.
Indoor and outdoor PM Levels during summer and winter in non-smoking women. X-axis: Indoor or outdoor, by summer and winter. Y-axis: PM concentration (µg/m3). The colors were based on different size of PM.
Figure 2.
PM1 levels among female non-smoking cases and controls in winter. The comparison of PM1 levels in different rooms among cases and controls are showed in the figure. X-axis: cases and controls by different rooms. Y-axis: PM1 concentration (µg/m3). “Star” represent p value <0.05
Figure 3.
Associations between PM levels and indoor exposures among females. The figure shows whether indoor air pollution exposure is correlated with PM concentration in different size. X-axis: PM in different size by indoor exposures. Y-axis: PM1 concentration (µg/m3). “Star” represent p value <0.05 “Diamond” represent p value<0.1
4. Discussion
4.1 Active and passive smoking and risk of lung cancer
China is one of countries with the highest tobacco consumption in the world. One in three cigarettes smoked in the world today are smoked in China. China has 300 million male smokers and 20 million female smokers[6]. In this study population, smoking rate is 6% in female controls and 16.8% in female lung cancer cases, which are much lower than that in Western females (30%). This study found a moderate association between smoking and lung cancer risk in females (aOR = 2.52), which is lower than that found in males (aOR = 5.01). When we further analyzed pack-years of smoking, for those who smoked more than 20 pack-years, the association with the disease in females is strong (aOR=5.42) and close to that in males (aOR=6.32). Our results are similar to other studies in Asian populations [7], but ORs are smaller than what was found in Western populations (OR: 15–30) [8]. The potential reasons include the fact that half of Chinese female smokers start to smoke later than 32 years old, half of male smokers start to smoker older than 20 years old, while most US smokers start their regular smoking between 11 and 14 years of age[9]. Those who start to smoke at earlier ages tend to have greater likelihood of becoming a heavier smoker and remaining a smoker for longer years [8, 10]. Duration of smoking has a much stronger association with lung cancer risk than daily consumption [11]. Second, in the Chinese population, a smoker is likely to be a tea drinker. In the current study, about 63% of male smokers were tea drinkers, which is much higher than in male non-smokers (45%). Tea drinking became an important confounding factor in the analysis of smoking and lung cancer because tea drinking was found to be protective against various cancers[12–16], which was verified in this study, with an aOR of 0.5 in non-smokers and 0.45 in smokers. Thus the confounding effects might have led to the underestimation of the association between smoking and lung cancer. Third, inherited susceptibility to lung cancer in the Chinese population might be different from Western populations, especially in those genes involved in smoking-related metabolic enzymes and the DNA repair pathway. Hence, unlike the United States where active smoking is responsible for 90% of lung cancers [8], contribution of smoking in Chinese population, in particular Chinese females, is relatively low. Smoking only can explain 17% of female lung cancer in this study and it is strongly related to squamous cell carcinoma. But in Chinese females, adenocarcinoma is the major histological type and has been increasing in the last decades[17]. This indicates that most female lung cancer cases are caused by exposures other than active smoking.
Smoking-related public education in China is far behind that of Western nations. Smoking-control policy has not been effectively implemented in the country. The smoking rate in males remains high (71.4% in healthy controls), which exposes the majority of women to environmental tobacco smoke. In non-smoking females exposed to heavy ETS at work or home, risk of lung cancer increased to 1.98 (95%CI: 1.12–3.51). Interestingly, the study shows that female non-smokers who were exposed to heavy passive smoking at work had 2.7-fold increased risk of lung cancer, although only 12% of cases were exposed to it. The results support findings from most previous studies in Chinese populations [18]. ETS exposure at work has been a worldwide concern[19]. In China, it is very common that many people share a small office or a working space. An indoor smoking ban is rarely set up or applied. Smokers also tend to smoke together as an indoor social activity without considering non-smokers. Work places are often small, crowded and poorly ventilated. Although more than 62% of women are married to smoking husbands, our study did not find that exposure to smoking at home significantly increased lung cancer risk. Our results are consistent with a recent meta-analysis using 16 case-control studies [20] and the only cohort study in the Chinese population [21], but different from results observed in some other previous studies, which found 15–30% increased risk associated with marriage to a smoker [22–26]. First, compared to the time spent at work, the time that most Chinese males spent at home, excluding sleeping hours, might be much less. Second, males might smoke less frequently at home than at work, where they have greater social interaction. Third, the current small sample size could have limited our ability to identify significant associations since the adjusted OR for heavy ETS exposure at home is 1.25, but the 95% confidence interval (0.71–2.20) includes 1.0.
Smoking is one of the major sources of indoor air pollution. Passive smokers are not only exposed to exhaled mainstream-smoke, but also sidestream-smoke, and sidestream smoke can emit at least 17 higher-level carcinogens than mainstream smoke [27]. Passive smoking contributes significantly to total level of PAHs and benzo(a)pyrene diol epoxide, both of which are directly associated with lung cancer [28, 29]. Worldwide, 40% of children, 33% of male non-smokers, and 35% of female non-smokers were exposed to second-hand smoke in 2004. This exposure was estimated to have caused 603,000 deaths, about 1.0% of worldwide mortality, among which 21,400 deaths are from lung cancer [30].
4.2 Indoor air pollution exposure and lung cancer among female non-smokers
Solid fuel includes biomass, coke, coal, and other coal-related products. The current study found that solid fuel for cooking or heating is associated with higher risk of lung cancer. The results are similar to those of previous studies in Chinese populations [31–40]. Individuals who have burned solid fuel (mainly coal, honeycomb coal and wood) for cooking throughout their life have 4 times risk of lung cancer as compared to those who have used clean energy. Coal furnace, a very popular mean of domestic heating, is related to two times greater risk of lung cancer vs clean energy. Also, a relatively large percentage of people use coke stoves or heatable brick beds, and their usage is related to even greater risk of lung cancer than coal furnaces. Heatable brick bed exposure is associated with a 8-fold greater risk of lung cancer, vs clean energy for heating or no heating. Combustion for heating the bed occurs in the bedroom where people are exposed to it every night during the winter. Since no windows are open during winter nights, all pollutants emitted from combustion accumulate in the bedroom to high levels. Thus, this heating method, in particular, should be abolished.
In general, solid fuel combustion in the household is often incomplete because simple stoves influence fuel’s mixing with air. About 10% of coal is not fully combusted. The incomplete combustion results in high emission of various gaseous and particle carcinogens, including sulfur dioxides, carbon monoxide, carbon dioxide, nitrogen oxides, PAH, formaldehyde, heavy metals, and particulate matter[41, 42]. These air pollutants have been found associated with morbidity and mortality from various diseases, especially respiratory diseases, including lung cancer [43, 44]. Both gas-phase and particle phase pollutants contain compounds that are carcinogenic (PAH, benzene, formaldehyde), probably carcinogenic (1,3-butadiene), and possibly carcinogenic to humans [35]. In particular, PAHs and PAH derivatives found in coal smoke exhibit strong mutagenicity [35].
The current study was conducted in Taiyuan city. The city is built on heavy industry, in particular, the coal industry. Because of the large quantity of coal produced, coal has been the dominant source of energy in this area, and using coal for both cooking and heating has been very common. Local coal in Taiyuan has a high concentration of sulfur, which often leads to high emission of sulfur dioxide when it is combusted. The average ambient SO2 in 5 monitor stations varied from 177 to 231 µg/m3 in Taiyuan city in 2000 [45], and sulfur-rich particles account for 10.7% of total particles in Taiyuan [46]. SO2 is also associated with higher lung cancer incidence [47]. Coal consumption accounts for 65% share of the primary energy consumption in 2002 in China, and it has been predicted that if smoking and solid fuel use remain at current levels between 2003 and 2033, 74% of lung cancer deaths will be attributable to the combined effects of smoking and solid-fuel use[48].
Other than pollutants produced by fuel combustion, Chinese cooking also generates carcinogenic air pollutants. In current study, we found that those women who cook more than twice a day have more than 2 times increased risk for lung cancer. Our findings are in line with previous observations[49–55]. Chinese dish cooking often involves stir, frying and deep-frying, which all tend to heat oil to very high temperature. The heated oil produces various volatile compounds including carcinogens such as benzo[α]pyrene and 2, 4-decadienal. IARC has classified high-temperature frying as Group 2 carcinogen [56]. A study also found that women who engaged in regular home cooking had significantly higher levels of mercapturic acids of acrolein, crotonaldehyde and benzene, which are multiorgan carcinogen[57]. Another study observed higher levels of DNA damage biomarkers, urinary 1-hydroxypyrene (1-OHP), urinary 8-hydroxy-2'-deoxyguanosine (8-OHdG), in kitchen staff than that in service staff in Chinese restaurant[58].
Our results also suggested that house layout and ventilation-related characteristics play important roles in the risk of lung cancer. Females who lived in larger and better-ventilated houses, such as multi-story houses and houses with more windows, separate kitchens, installed ventilators, and having windows open more frequently, experienced lower risk than those who lived in small and poor-ventilated houses. Since females stay home longer time and cook more frequently, housing related exposure has much stronger influence on them than on males.
4.3 Indoor PM levels, their relationships with indoor exposure and risk of lung cancer
In the last few decades, standards for ambient air pollution have been established in most countries, and their regulation has significantly improved. However, not much has been done for indoor air pollution, such as monitoring indoor exposure, establishing standard guidelines, and regulating pollution levels. As the current study has demonstrated, indoor air pollution exposure is complicated due to multiple sources, various pollutants and pollution levels. Assessing indoor air pollution level is critical, but also challenging.
Ambient air borne PM has been widely studied. PM level was found to be strongly associated with other pollutants, including SO2, CO2, O3, and NO2. It has become one of the most important indices of ambient outdoor air pollution, but less is known about PM in indoor air. The current study measured indoor and immediate outdoor PM levels of various sizes, including TSP, PM10, PM7, PM2.5 and PM1. First, PM levels in the study area are very high. Comparing to WHO ambient air quality guidelines for 24-hour means of PM2.5 (25µg/m3) and PM10 (50µg/m3), indoor PM2.5 (68µg/m3) and PM10 (230 µg/m3) levels in this study are about 2.5 and 5 times higher, respectively. During the summer, indoor PM levels were higher than outdoor since cooking related indoor activity increased indoor pollution levels. During winter, because of domestic heating emissions to ambient air, most outdoor PM levels are higher than indoor levels. PM1 is the only particulate class that is higher indoors than outdoors in cases during winter, which may due to the heavy combustion of heating and cooking. We also observed that indoor PM levels are correlated with indoor air pollution exposure. The current study found that using solid fuel for cooking, solid fuel for heating, and inadequate ventilation in the kitchen can significantly increase PM levels of all sizes during winter. In particular, solid fuel for cooking can increase PM to three-time noncooking levels. Another study reported that coal-burning particles account for 16.3% of the total number of particles, reflecting the intensity of coal consumption in Taiyuan area[46]. However, no significant association between solid fuel for cooking and PM levels was observed during summer, although there were slight non-significant increases in PM levels among those without ventilators installed in their kitchens. The results verified our findings that house ventilation-related characteristics, including more windows in the house, having windows open more often, and having a ventilator in kitchen, play an important role in reducing indoor air pollution level. A third observation from the current study is that smaller PM is more important than bigger PM. Our results show that indoor PM1 levels are much higher in the case group than in the control group. Every 10 ug/m3 increase in PM1 is associated with 45% increased risk of lung cancer. PM1 is one of the major bioactive fractions of PM pollution, and these very small particles can stay in air for a longer time than larger particles. Unlike PM10 deposited predominantly in the nose and throat and cleared by exhalation or mucociliary clearance and swallowing, PM1 can penetrate into lower airways and alveoli and translocate to the circulation [59–62]. PM contains various compounds that have both oxidative and carcinogenic properties. PM-induced oxidative stress has been hypothesized as a mechanism through which inflammation and oxidative damage are associated with the increased risk of cancers [63–65]. Smaller PMs that translocate into the circulation not only induce airway inflammation, but also systemic inflammation and oxidative damage. Although outdoor PM exposure has been found significantly associated with lung cancer risk and mortality in previous studies, less research has demonstrated indoor PM level and lung cancer risk since lacking of indoor PM monitoring.
4.4 study limitations
The study has limitations in several aspects. First, Our conclusions are largely based on interview data since indoor PM levels we measured are current exposure. The fact significantly limits the study’s ability of suggesting causal association between measured PM level and lung cancer risk. However, Indoor PM levels are closely related to residents’ lifestyle and indoor activities, which are relatively consistent over years in each household. The observed association between PM levels and indoor pollutants exposure in the study supported the assumption. Under the assumption, if residents didn’t change their indoor activity significantly, the measured PM levels might partially represent past indoor air pollution level. On the other hand, because indoor air quality in Taiyuan has been improved over the last decade, the association based on currently measured PM data may be underestimated. Thus, we would like to take precautions before making any conclusion based on the current measurement of PM levels. Second, the sample size of the study is relatively small for conducting stratified analyses. The small sample size also limits our possibility to evaluate the potential association between indoor air pollution and lung cancer risk among non-smoking males. Results from this study will need to be confirmed by larger studies. Third, as with most case-control studies, recall bias might occur since patients may tend to report their exposure more than controls do, and this may lead to misclassification.
Conclusion
In a Chinese population, indoor air pollution played a critical role in the development of lung cancer among non-smoking females. Indoor PM levels were associated with pollutants exposure and might serve as a good measurement of indoor air quality.
Acknowledgments
This work was supported in part by the National Nature Science Foundation of China grant award to Dr. Lina Mu (NSFC-30500417). The work is also partially supported by NIH grants (R01ES018846, R21ES017826, ES06718, CA09142, DA11386) and the Alper Research Center for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center.
Abbreviations
- PM
Particulate Matter
- ETS
Environmental Tobacco Smoke
- OR
Odds Ratio
- aOR
adjusted OR
- 95%CI
95% confidence interval
- IAP
Indoor air pollution
- PAH
Polycyclic Aromatic Hydrocarbons
Footnotes
Conflict of Interest
We have no financial relationship with the organizations that sponsored the research.
References
- 1.IARC. Cancer Incidence, Mortality and Prevalence Worldwide in 2008. Globocan 2008. 2008 [Google Scholar]
- 2.Wang JB, Jiang Y, Wei WQ, Yang GH, Qiao YL, Boffetta P. Estimation of cancer incidence and mortality attributable to smoking in China. Cancer Causes Control. 2010;21(6):959–965. doi: 10.1007/s10552-010-9523-8. [DOI] [PubMed] [Google Scholar]
- 3.Franchini M, Mannucci PM. Short-term effects of air pollution on cardiovascular diseases: outcomes and mechanisms. J Thromb Haemost. 2007;5(11):2169–2174. doi: 10.1111/j.1538-7836.2007.02750.x. [DOI] [PubMed] [Google Scholar]
- 4.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 5.Boffetta P. Human cancer from environmental pollutants: the epidemiological evidence. Mutat Res. 2006;608(2):157–162. doi: 10.1016/j.mrgentox.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, Taylor CE, Becker K, Xu J. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA. 1999;282(13):1247–1253. doi: 10.1001/jama.282.13.1247. [DOI] [PubMed] [Google Scholar]
- 7.Wakai K, Inoue M, Mizoue T, Tanaka K, Tsuji I, Nagata C, Tsugane S. Tobacco smoking and lung cancer risk: an evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol. 2006;36(5):309–324. doi: 10.1093/jjco/hyl025. [DOI] [PubMed] [Google Scholar]
- 8.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidencebased clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 9.Ellickson PL, Orlando M, Tucker JS, Klein DJ. From adolescence to young adulthood: racial/ethnic disparities in smoking. Am J Public Health. 2004;94(2):293–299. doi: 10.2105/ajph.94.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Services UDoHaH. Smoking and health: a national status report. Washington,DC: US Government Printing Office; 1987. [Google Scholar]
- 11.Peto R. Control of tobacco-related disease. Ciba Found Symp. 1985;110:126–142. doi: 10.1002/9780470720912.ch9. [DOI] [PubMed] [Google Scholar]
- 12.Tang N, Wu Y, Zhou B, Wang B, Yu R. Green tea, black tea consumption and risk of lung cancer: a meta-analysis. Lung Cancer. 2009;65(3):274–283. doi: 10.1016/j.lungcan.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Milazzo S, Horneber M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2009;(3):CD005004. doi: 10.1002/14651858.CD005004.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Chang SC, Goldstein BY, Scheider WL, Cai L, You NC, Tarleton HP, Ding B, Zhao J, Wu M, et al. Green tea consumption, inflammation and the risk of primary hepatocellular carcinoma in a Chinese population. Cancer Epidemiol. 2011;35(4):362–368. doi: 10.1016/j.canep.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu M, Liu AM, Kampman E, Zhang ZF, Van't Veer P, Wu DL, Wang PH, Yang J, Qin Y, Mu LN, et al. Green tea drinking, high tea temperature and esophageal cancer in high- and low-risk areas of Jiangsu Province, China: a population-based casecontrol study. Int J Cancer. 2009;124(8):1907–1913. doi: 10.1002/ijc.24142. [DOI] [PubMed] [Google Scholar]
- 16.Mu LN, Lu QY, Yu SZ, Jiang QW, Cao W, You NC, Setiawan VW, Zhou XF, Ding BG, Wang RH, et al. Green tea drinking and multigenetic index on the risk of stomach cancer in a Chinese population. Int J Cancer. 2005;116(6):972–983. doi: 10.1002/ijc.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N, Chen WQ, Zhu WX, Xing XM, Lu AP, Yang L. Incidence trends and pathological characteristics of lung cancer in urban Beijing during period of 1998–2007. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45(3):249–254. [PubMed] [Google Scholar]
- 18.Wang X, Qin Y, Gu J, Wang F, Jia P, Wang H, Yao Q, Zhu S. Systematic review of studies of workplace exposure to environmental tobacco smoke and lung cancer risk. Zhongguo Fei Ai Za Zhi. 2011;14(4):345–350. doi: 10.3779/j.issn.1009-3419.2011.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisner MD. Secondhand smoke at work. Curr Opin Allergy Clin Immunol. 2010;10(2):121–126. doi: 10.1097/ACI.0b013e32833649b3. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Gu J, Xu H, Yang B, Han Y, Li L, Liu S, Yao H. Meta-analysis of the relationship between passive smoking population in China and lung cancer. Zhongguo Fei Ai Za Zhi. 2010;13(6):617–623. doi: 10.3779/j.issn.1009-3419.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen W, Shu XO, Gao YT, Yang G, Li Q, Li H, Zheng W. Environmental tobacco smoke and mortality in Chinese women who have never smoked: prospective cohort study. BMJ. 2006;333(7564):376. doi: 10.1136/bmj.38834.522894.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trichopoulos D, Kalandidi A, Sparros L, MacMahon B. Lung cancer and passive smoking. Int J Cancer. 1981;27(1):1–4. doi: 10.1002/ijc.2910270102. [DOI] [PubMed] [Google Scholar]
- 23.Boffetta P. Involuntary smoking and lung cancer. Scand J Work Environ Health. 2002;28(Suppl 2):30–40. [PubMed] [Google Scholar]
- 24.Boffetta P, Agudo A, Ahrens W, Benhamou E, Benhamou S, Darby SC, Ferro G, Fortes C, Gonzalez CA, Jockel KH, et al. Multicenter case-control study of exposure to environmental tobacco smoke and lung cancer in Europe. J Natl Cancer Inst. 1998;90(19):1440–1450. doi: 10.1093/jnci/90.19.1440. [DOI] [PubMed] [Google Scholar]
- 25.Zhong L, Goldberg MS, Parent ME, Hanley JA. Exposure to environmental tobacco smoke and the risk of lung cancer: a meta-analysis. Lung Cancer. 2000;27(1):3–18. doi: 10.1016/s0169-5002(99)00093-8. [DOI] [PubMed] [Google Scholar]
- 26.Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol. 2007;36(5):1048–1059. doi: 10.1093/ije/dym158. [DOI] [PubMed] [Google Scholar]
- 27.Mohtashamipur E, Mohtashamipur A, Germann PG, Ernst H, Norpoth K, Mohr U. Comparative carcinogenicity of cigarette mainstream and sidestream smoke condensates on the mouse skin. J Cancer Res Clin Oncol. 1990;116(6):604–608. doi: 10.1007/BF01637081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274(5286):430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 29.Hoh E, Hunt RN, Quintana PJ, Zakarian JM, Chatfield D, Wittry BC, Rodriguez E, Matt GE. Environmental Tobacco Smoke as a Source of Polycyclic Aromatic Hydrocarbons in Settled Household Dust. Environ Sci Technol. 2012 doi: 10.1021/es300267g. [DOI] [PubMed] [Google Scholar]
- 30.Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377(9760):139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 31.Hosgood HD, 3rd, Boffetta P, Greenland S, Lee YC, McLaughlin J, Seow A, Duell EJ, Andrew AS, Zaridze D, Szeszenia-Dabrowska N, et al. In-home coal and wood use and lung cancer risk: a pooled analysis of the International Lung Cancer Consortium. Environ Health Perspect. 2010;118(12):1743–1747. doi: 10.1289/ehp.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galeone C, Pelucchi C, La Vecchia C, Negri E, Bosetti C, Hu J. Indoor air pollution from solid fuel use, chronic lung diseases and lung cancer in Harbin, Northeast China. Eur J Cancer Prev. 2008;17(5):473–478. doi: 10.1097/CEJ.0b013e328305a0b9. [DOI] [PubMed] [Google Scholar]
- 33.Lan Q, He X, Costa DJ, Tian L, Rothman N, Hu G, Mumford JL. Indoor coal combustion emissions, GSTM1 and GSTT1 genotypes, and lung cancer risk: a case-control study in Xuan Wei, China. Cancer Epidemiol Biomarkers Prev. 2000;9(6):605–608. [PubMed] [Google Scholar]
- 34.Xu ZY, Blot WJ, Li G, Fraumeni JF, Jr, Zhao DZ, Stone BJ, Yin Q, Wu A, Henderson BE, Guan BP. Environmental determinants of lung cancer in Shenyang, China. IARC Sci Publ. 1991;(105):460–465. [PubMed] [Google Scholar]
- 35.Zhang JJ, Smith KR. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environ Health Perspect. 2007;115(6):848–855. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai XD, Lin CY, Sun XW, Shi YB, Lin YJ. The etiology of lung cancer in nonsmoking females in Harbin, China. Lung Cancer. 1996;14(Suppl 1):S85–S91. doi: 10.1016/s0169-5002(96)90213-5. [DOI] [PubMed] [Google Scholar]
- 37.Luo RX, Wu B, Yi YN, Huang ZW, Lin RT. Indoor burning coal air pollution and lung cancer--a case-control study in Fuzhou, China. Lung Cancer. 1996;14(Suppl 1):S113–S119. doi: 10.1016/s0169-5002(96)90217-2. [DOI] [PubMed] [Google Scholar]
- 38.Sun X, Dai X, Shi Y, Lin Y. A case-control study on the relationship among indoor air pollution,depression and oncogenesis of lung cancer. Zhongguo Fei Ai Za Zhi. 2002;5(2):101–103. doi: 10.3779/j.issn.1009-3419.2002.02.07. [DOI] [PubMed] [Google Scholar]
- 39.Kleinerman RA, Wang Z, Wang L, Metayer C, Zhang S, Brenner AV, Xia Y, Shang B, Lubin JH. Lung cancer and indoor exposure to coal and biomass in rural China. J Occup Environ Med. 2002;44(4):338–344. doi: 10.1097/00043764-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, Wang S, Aunan K, Seip HM, Hao J. Air pollution and lung cancer risks in China--a meta-analysis. Sci Total Environ. 2006;366(2–3):500–513. doi: 10.1016/j.scitotenv.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Smith KR, Ma Y, Ye S, Jiang F, Qi W, Liu P, Khalil MAK, Rasmussen RA, Thorneloe SA. Greenhouse gases and other airborne pollutants from household stoves in China: a database for emission factors. Atmospheric Environment. 2000;34(26):4537–4549. [Google Scholar]
- 42.Zhang J, Smith KR. Hydrocarbon emissions and health risks from cookstoves in developing countries. J Expo Anal Environ Epidemiol. 1996;6(2):147–161. [PubMed] [Google Scholar]
- 43.Chen H, Goldberg MS, Villeneuve PJ. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Reviews on environmental health. 2008;23(4):243–297. doi: 10.1515/reveh.2008.23.4.243. [DOI] [PubMed] [Google Scholar]
- 44.Turner MC, Krewski D, Pope CA, 3rd, Chen Y, Gapstur SM, Thun MJ. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. American journal of respiratory and critical care medicine. 2011;184(12):1374–1381. doi: 10.1164/rccm.201106-1011OC. [DOI] [PubMed] [Google Scholar]
- 45.Heidi Elizabeth Satff Medtl JF. CICERO Report. 2003. Air quality estimate in Taiyuan, Shanxi Province, China. [Google Scholar]
- 46.Xie RK, Seip HM, Liu L, Zhang DS. Characterization of individual airborne particles in Taiyuan City, China. Air Qual Atmos Health. 2009;2(3):123–131. doi: 10.1007/s11869-009-0039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tseng CY, Huang YC, Su SY, Huang JY, Lai CH, Lung CC, Ho CC, Liaw YP. Cell type specificity of female lung cancer associated with sulfur dioxide from air pollutants in Taiwan: An ecological study. BMC public health. 2012;12:4. doi: 10.1186/1471-2458-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin HH, Murray M, Cohen T, Colijn C, Ezzati M. Effects of smoking and solid-fuel use on COPD, lung cancer, and tuberculosis in China: a time-based, multiple risk factor, modelling study. Lancet. 2008;372(9648):1473–1483. doi: 10.1016/S0140-6736(08)61345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang XR, Chiu YL, Qiu H, Au JS, Yu IT. The roles of smoking and cooking emissions in lung cancer risk among Chinese women in Hong Kong. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20(4):746–751. doi: 10.1093/annonc/mdn699. [DOI] [PubMed] [Google Scholar]
- 50.Lee CH, Yang SF, Peng CY, Li RN, Chen YC, Chan TF, Tsai EM, Kuo FC, Huang JJ, Tsai HT, et al. The precancerous effect of emitted cooking oil fumes on precursor lesions of cervical cancer. International journal of cancer Journal international du cancer. 2010;127(4):932–941. doi: 10.1002/ijc.25108. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Yin Z, Guan P, Li X, Cui Z, Zhang J, Bai W, He Q, Zhou B. XRCC1 polymorphisms, cooking oil fume and lung cancer in Chinese women nonsmokers. Lung cancer. 2008;62(2):145–151. doi: 10.1016/j.lungcan.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Yu IT, Chiu YL, Au JS, Wong TW, Tang JL. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer research. 2006;66(9):4961–4967. doi: 10.1158/0008-5472.CAN-05-2932. [DOI] [PubMed] [Google Scholar]
- 53.Metayer C, Wang Z, Kleinerman RA, Wang L, Brenner AV, Cui H, Cao J, Lubin JH. Cooking oil fumes and risk of lung cancer in women in rural Gansu, China. Lung cancer. 2002;35(2):111–117. doi: 10.1016/s0169-5002(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 54.Ko YC, Cheng LS, Lee CH, Huang JJ, Huang MS, Kao EL, Wang HZ, Lin HJ. Chinese food cooking and lung cancer in women nonsmokers. American journal of epidemiology. 2000;151(2):140–147. doi: 10.1093/oxfordjournals.aje.a010181. [DOI] [PubMed] [Google Scholar]
- 55.Zhong L, Goldberg MS, Parent ME, Hanley JA. Risk of developing lung cancer in relation to exposure to fumes from Chinese-style cooking. Scandinavian journal of work, environment & health. 1999;25(4):309–316. doi: 10.5271/sjweh.440. [DOI] [PubMed] [Google Scholar]
- 56.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of household solid fuel combustion and of high-temperature frying. Lancet Oncol. 2006;7(12):977–978. doi: 10.1016/s1470-2045(06)70969-x. [DOI] [PubMed] [Google Scholar]
- 57.Hecht SS, Seow A, Wang M, Wang R, Meng L, Koh WP, Carmella SG, Chen M, Han S, Yu MC, et al. Elevated levels of volatile organic carcinogen and toxicant biomarkers in Chinese women who regularly cook at home. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(5):1185–1192. doi: 10.1158/1055-9965.EPI-09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan CH, Chan CC, Wu KY. Effects on Chinese restaurant workers of exposure to cooking oil fumes: a cautionary note on urinary 8-hydroxy-2'-deoxyguanosine. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(12):3351–3357. doi: 10.1158/1055-9965.EPI-08-0075. [DOI] [PubMed] [Google Scholar]
- 59.Tao F, Gonzalez-Flecha B, Kobzik L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic Biol Med. 2003;35(4):327–340. doi: 10.1016/s0891-5849(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 60.Brauer M, Avila-Casado C, Fortoul TI, Vedal S, Stevens B, Churg A. Air pollution and retained particles in the lung. Environ Health Perspect. 2001;109(10):1039–1043. doi: 10.1289/ehp.011091039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lippmann M, Yeates DB, Albert RE. Deposition, retention, and clearance of inhaled particles. Br J Ind Med. 1980;37(4):337–362. doi: 10.1136/oem.37.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Squadrito GL, Cueto R, Dellinger B, Pryor WA. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic Biol Med. 2001;31(9):1132–1138. doi: 10.1016/s0891-5849(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 63.Moller P, Folkmann JK, Forchhammer L, Brauner EV, Danielsen PH, Risom L, Loft S. Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett. 2008;266(1):84–97. doi: 10.1016/j.canlet.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 64.Scapellato ML, Lotti M. Short-term effects of particulate matter: an inflammatory mechanism? Crit Rev Toxicol. 2007;37(6):461–487. doi: 10.1080/10408440701385622. [DOI] [PubMed] [Google Scholar]
- 65.Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat Res. 2007;636(1–3):95–133. doi: 10.1016/j.mrrev.2007.08.003. Epub 2007 Aug 2017. [DOI] [PubMed] [Google Scholar]