Abstract
Identification of neural stem and progenitor cells (NPCs) in vitro and in vivo is essential to the use of developmental and disease models of neurogenesis. The dog is a valuable large animal model for multiple neurodegenerative diseases and is more closely matched to humans than rodents with respect to brain organization and complexity. It is therefore important to determine whether immunohistochemical markers associated with NPCs in humans and rodents are also appropriate for the dog. The NPC markers CD15, CD133, nestin, GFAP and phosphacan (DSD-1) were evaluated in situ in the canine rostral telencephalon, hippocampal dentate gyrus, and cerebellum at different postnatal time-points. Positive staining results were interpreted in the context of region and cellular morphology. Our results showed that neurospheres and cells within the rostral subventricular zone (SVZ), dentate gyrus subgranular zone (SGZ), and white matter tracts of the cerebellum were immunopositive for CD15, nestin and GFAP. Neurospheres and the cerebellum were immunonegative for CD133, whereas CD133 staining was present in the postnatal rostral SVZ. Anti-phosphacan antibody staining delineated the neurogenic niches of the rostral lateral ventricle SVZ and the hippocampal SGZ. Positive staining for phosphacan was also noted in white matter tracts of the cerebellum and within the Purkinje layer. Our results showed that in the dog these markers were associated with regions shown to be neurogenic in rodents and primates.
Keywords: Dog, Neural precursor cells, Postnatal neurogenesis, Subventricular Zone, Subgranular Zone, Cerebellum
Introduction
Much of the impetus to isolate and characterize neural stem and progenitor cells from different species arises from the existence of animal disease models that can be studied to assess therapeutic modalities and mechanisms of disease. The dog, in particular, is a valuable model for human neurologic diseases because, in contrast to rodents, the canine brain is more similar in physical organization to the human brain. There are many well-defined neurological diseases in dogs, including genetic diseases, which are excellent analogues of human diseases (Alroy et al. 1985; Fischer et al. 1998; Griffiths et al. 1981; Haskins et al. 1984; Hemsley and Hopwood; Koppang 1988; Wenger et al. 1999). This study was designed to assess the usefulness of neural stem/progenitor cell markers in the dog and to characterize the distribution of NPCs in the three major postnatal neurogenic regions, the rostral subventricular zone (SVZ), hippocampus and cerebellum.
True NSCs are multipotent and capable of self-renewal, whereas neural progenitor cells have more restricted potential. Because postnatal neurogenic regions contain both stem and progenitor cells, in this study the term ‘neural precursor cell’ (NPC) will be used to include both neural stem and progenitor cell populations unless a population is known to conform to the definition of a neural stem cell (NSC). Sources of postnatal multipotent NSCs include regions of the brain where postnatal neurogenesis occurs: the dentate gyrus of the hippocampus, the olfactory bulb, and the SVZ, especially the SVZ of the frontal horn lateral ventricles (Gritti et al. 2002; Liu and Martin 2003; Pagano et al. 2000; Palmer et al. 1997; Reynolds and Weiss 1992; Seri et al. 2004; Snyder et al. 1992). Postnatal neurogenesis is also seen in the cerebellum. However, depending upon species and location, postnatal cerebellar neurogenesis is limited to weeks to a year (Gage et al. 1995).
Non-neurogenic regions of the postnatal brain containing glial NPCs have yielded multipotent NPCs when cultured in the presence of EGF and/or bFGF (Alcock and Sottile 2009; Kondo and Raff 2000; Nunes et al. 2003; Palmer et al. 1995). This suggests that the environment in which they reside determines the potency of NPCs. Thus, the composition of the NPC niche may endow at least one stem cell quality (multipotency) on what are normally progenitor cells. Although in one study, postnatal NG2 proteoglycan progenitors showed an immediate multipotency, i.e., one that is not dependent upon long-term growth factor exposure, which argues for an intrinsic ability rather than reprogramming in vitro (Belachew et al. 2003).
NPCs have been retrospectively identified in vitro by proliferation in response to growth factors (Kondo and Raff 2000; Nunes et al. 2003; Palmer et al. 1995; Palmer et al. 1997; Reynolds and Weiss 1992; Roy et al. 2000). In vivo, markers of proliferation such as tritiated thymidine and bromodeoxyuridine have been used to identify progenitor cells (Altman 1962; Altman and Das 1965; Eriksson et al. 1998; Lois and Alvarez-Buylla 1994; Morshead et al. 1994). However, proliferative markers alone do not definitively identify NPCs because they incorporate into the DNA and are present in progeny as well as parent cells. Intracellular filament proteins such as nestin and glial fibrillary acidic protein (GFAP) have proven useful in identifying NPCs, although location is an essential context (Doetsch et al. 1999; Hockfield and McKay 1985; Lendahl et al. 1990; Seri et al. 2001). The subsequent use of nestin and GFAP promoters for targeted ablation or reporter gene expression has greatly aided in the identification and localization of NPCs in situ (Garcia et al. 2004; Imura et al. 2003; Morshead et al. 2003; Roy et al. 2000; Sawamoto et al. 2001). Cell surface markers for human and rodent NPC selection include Lewis X antigen (CD15), CD133/prominin and phosphacan (the chondroitin sulfate proteoglycan (PG), DSD-1-PG) (Campos et al. 2004; Capela and Temple 2002; Johansson et al. 1999; Klassen et al. 2001; Lee et al. 2005; Uchida et al. 2000; von Holst et al. 2006). Although none of these proteins alone is a definitive NSC marker, labeling for combinations of markers in conjunction with cellular morphology and location may serve to accurately identify true NSCs.
Materials and Methods
Experimental animals
Mixed breed dogs were raised in the animal colony at the University of Pennsylvania School of Veterinary Medicine according to NIH and USDA guidelines for the use of animals in research and procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Neural progenitor cell culture and expansion
Mixed breed dogs 19 days of age (n=3) were humanely euthanized by intravenous injection of a barbituate solution. The brain was removed, placed into a balanced salt solution, and then dissected grossly. The cerebellum and rostral one-third of the brain located just caudal to the olfactory bulbs and rostral to the hippocampus were separated from the brain and from each other. The rostral one-third of the brain was further dissected to leave primarily the tissue surrounding the lateral ventricles (including parts of the corpus callosum, caudate putamen, and septae) for 2 dogs. Half of the vermis and half of a lobule were removed from the cerebellum of 1 dog. The resultant tissues from the striatal SVZ and cerebellum were minced separately, then digested in 0.25% trypsin (Worthington) in a 37°C water bath for 45 minutes to 1 hour. The enzymatic digestion was stopped with addition of fetal bovine serum (FBS; Hyclone). The tissues were then incubated with DNAse I (Sigma) for fifteen minutes in a 37°C water bath and triturated to a single cell suspension with successively smaller diameter pipettes, ending at a flame-polished Pasteur pipette.
The NPCs were cultured as described previously (Walton and Wolfe 2007). Briefly, NPCs were initially plated into medium consisting of DMEM:F12 (1:1 ratio; GibcoBRL) supplemented with 10% FBS, 1% N2 supplement (GibcoBRL), 1% antibiotic-antimycotic (PSF)(100 U/mL penicillin; 100 μg/mL streptomycin; 0.25 μg/mL amphotericin B; GibcoBRL), 1% L-glutamine (2mM; GibcoBRL) and a standard combination of growth factors consisting of 20 ng/mL epidermal growth factor (EGF) (recombinant murine; Roche), 20 ng/mL basic fibroblast growth factor (bFGF) (recombinant human; Promega), and heparin (5 μg/mL; Sigma). After 24–48 hours, the medium was changed to a serum-free feeding medium consisting of DMEM:F12 supplemented with 1% N2 supplement, 1% PSF, 1% L-glutamine and standard growth factor combination.
The NPC cultures were maintained at 37 C in humidified 5% CO2 tissue culture incubators. Cultures were fed every 3–5 days by changing half of the medium and adding fresh growth factors. Canine NPCs were plated into 25 cm2 tissue culture flasks (Corning) coated with 10 μg/mL PDL (Sigma). Cultures were passaged at approximately 90% confluence by trypsinizing (0.05% trypsin-EDTA; GibcoBRL) and replating at a concentration of 4 × 104/cm2.
Fluorescence activated cell sorting
Single cell suspensions isolated from three 19-day old dogs were sorted using flow cytometry. Two separate isolations of acutely dissociated striatal subventricular zone tissue and a single isolation of dissociated cerebellar tissue were used. Aliquots of 5×106 cells were rinsed in PBS after centrifugation at 700 rpm for 8 minutes at 4°C. The cells were then incubated in a 1:50 dilution of anti-human CD15 (Table 1) in 2% bovine serum albumin (BSA; GibcoBRL) for 25 minutes at 4°C. The cells were washed twice in 2% BSA and resuspended in a 1:500 dilution of goat anti-mouse IgM Alexa fluor 488 (Molecular Probes) for 20 minutes at 4°C. Control aliquots were incubated only with the secondary antibody. After a final two washes, cells were resuspended in 2% BSA for flow cytometric analysis. Alexa fluor 488+ cells were sorted using a FACSVantage SE cell sorter (BD Biosciences) at a rate of 5000 events/second. The cells were collected into N2 medium supplemented with 10% FBS and plated at a density of 4×104/cm2 into polystyrene wells (BD Falcon) coated with poly-D-lysine (PDL) (10 μg/mL; Sigma).
Table 1.
Primary Antibodies Used in the Characterization of the Postnatal Canine SVZ, SGZ and Cerebellum
| Antigen | Immunogen | Manufacturer, species, type, cat. no., dilution | Characterization | Results |
|---|---|---|---|---|
| Nestin | Fusion protein containing COOH- terminal region of rat nestin | R. McKay (NIH, NIDDS, Bethesda, MD), rabbit, polyclonal, antiserum 130,1:60 | Detects a 200 kDa band on immunoblots of embryonic rat brain (Lendahl et al., 1990) | Stains neurospheres and canine multipotential NPCs (Walton and Wolfe, 2007) |
| GFAP | Purified bovine GFAP | Chemicon (Temecula, CA), rabbit, polyclonal, #AB5804, 1:200 | Recognizes 51kDa band on immunoblot of rat spinal cord | Stains neurospheres and multipotential NPCs (Walton and Wolfe, 2007); stains cells with astrocyte morphology in canine brain tissue |
| GFAP | Gel excised bovine glial filament protein | VM-Y. Lee (Univ of Penn, Philadelphia, PA), rat, IgG2a clone 2.2B10, 1:1 | Recognizes 51kDa band on immunoblot of rat spinal cord | Stains neurospheres and multipotential NPCs (Walton and Wolfe, 2007); stains cells with astrocyte morphology in canine brain tissue |
| CD15 | U-937 histiocytic cell line | BD Pharmingen (San Diego, CA), mouse, IgM clone MMA, #340850, 1:50 | Recognizes lacto-N- fucopentose III on human myelomonocytic cells | Stains neurospheres and NPC regions in brain tissue as reported in Capela et al. (2006) |
| DSD-1 | “Rest-L2” glycoprotein fraction from adult mouse brain | Chemicon (Temecula, CA), rat, IgM clone 473HD, #MAB5790, 1:500 | Recognizes the DSD-1 glycosaminoglycan structure (mouse phosphacan homologue) | Stains NPC regions in canine brain tissue as reported for mice in Gates et al. (1995) |
| CD133/1 | Not specified | Miltenyi Biotec (Auburn, CA), mouse, IgG1 clone AC133, #130-090-422, 1:100 | Detects epitope 1 on a 5- transmembrane cell surface antigen with a molecular weight of 117 kD | Stains ependymal cell surface and cell processes extending to ventricle as described in Pfenninger et al. (2007) |
| CD133/2 | Not specified | Miltenyi Biotec (Auburn, CA), mouse, IgG2b clone 293C3, #130-090-851, 1:100 | Detects epitope 2 on a 5- transmembrane cell surface antigen with a molecular weight of 117 kD | Stains ependymal cell surface and cell processes extending to ventricle as described in Pfenninger et al. (2007) |
| Ki67 | Human recombinant peptide from a 1002 bp Ki-67 cDNA fragment | DakoCytomation (Carpinteria, CA), mouse, IgG1 clone MIB-1, #M7240, 1:200 | Nuclear staining pattern; preferentially expressed during G1, S, G2, and M, but absent in G0 | Stains nucleus of cells in canine SVZ where dividing cells are expected to be |
| β-tubulin III | Synthetic peptide (ESESQGPK) of human class III β-tubulin C terminus | Chemicon (Temecula, CA), mouse, IgG1 clone TU20, #MAB1637, 1:300 | Detects a single band at 50 kDa corresponding to class III beta-tubulin on Western blots of mouse brain lysates | Stains cells with appropriate neuronal morphology in canine brain sections and tissue culture |
| Antigen | Immunogen | Manufacturer, species, type, cat. no., dilution | Characterization | Controls |
|---|---|---|---|---|
| Map2ab | Bovine brain microtubule protein | Chemicon (Temecula, CA), mouse, IgG1 clone AP20, #MAB3418, 1:300 | Migrates as a closely associated doublet having a molecular weight of approximately 280–300 kD (MAP-2A and -2B isoforms) on Western blots of rat brain lysate | Stains cells with appropriate neuronal morphology in canine brain sections and tissue culture |
| R-Mab | Synaptic plasma membranes from bovine hippocampi | J. Grinspan (Children’s Hosp of Phila, Philadelphia, PA), mouse, IgG hybridoma supernatant, 1:1 | Recognizes galactocerebroside, monogalactosyl-diglyceride, sulfatide, seminolipid, and psychosine (Bansal et al., 1989) | Stains oligodendrocytes in canine brain sections and NPC differentiation cultures (Walton and Wolfe, 2008) |
| O4 | Hybridoma clone O4 | J. Grinspan (Children’s Hosp of Phila, Philadelphia, PA), mouse, IgM hybridoma supernatant, 1:1 | Recognizes sulfatide, seminolipid and an unidentified antigen on the surface of oligodendrocyte progenitors prior to expression of galactocerebroside (Bansal et al., 1989) | Stains oligodendrocytes in canine brain sections and NPC differentiation cultures (Walton and Wolfe, 2008) |
Abbreviations: GFAP, glial fibrillary acidic protein; IHC, immunohistochemistry; NPCs, neural precursor cells; SGZ, subgranular zone; SVZ, subventricular zone
Immunocytochemistry
Undifferentiated NPCs were plated at a concentration of 2 × 104 cells/well onto PDL-coated 8-well glass slides (Cel-Line; Erie Scientific) and allowed to attach overnight in feeding medium containing growth factors. Undifferentiated NPC cultures were processed for immunocytochemistry after 24 hours.
For all intracellular markers, cells were rinsed in Tris-buffered saline (TBS) (50mM Tris-base, 0.15M NaCl; pH 7.6), fixed for 10 min in 4% paraformaldehyde (Sigma), rinsed three times with TBS, blocked in 5% goat serum (GibcoBRL) with 0.1% Triton X-100 (Sigma) for 40 minutes, and then incubated with primary antibody in 1% goat serum with 0.02% Triton X-100 for 1 hour at room temperature or overnight at 4 C. After three TBS washes, the secondary antibody was applied for 1 hour at room temperature or overnight at 4 C. Cell surface marker staining was performed on live cells. The cells were rinsed in TBS, incubated with primary antibody diluted in TBS for 30 minutes, rinsed briefly with TBS, incubated with secondary antibody for 40 minutes, rinsed with TBS, and then fixed for 10 minutes in 4% paraformaldehyde. All slides were washed three times with TBS before mounting in Vectashield containing 4′,6 diamidino-2-phenylindole (DAPI; Vector Laboratories). Negative controls consisted of cells incubated with only secondary antibody (primary antibody omitted).
Antibodies
The polyclonal primary antibodies used were rabbit anti-glial fibrillary acidic protein (GFAP) and rabbit anti-nestin (Table 1). Primary monoclonal antibodies consisted of rat anti-GFAP, mouse anti-human CD15, mouse anti-human Ki67, mouse anti-CD133 (AC133 and 293C antibodies) and rat anti-mouse phosphacan (Table 1). Secondary fluorescent antibodies used were goat anti-mouse IgG/IgM FITC, 1:300 dilution (Chemicon); goat anti-rabbit IgG 594 Alexa fluor, 1:300 dilution (Molecular Probes); goat anti-rabbit IgG 488 Alexa fluor, 1:300 dilution (Molecular Probes); goat anti-rat IgM 594 Alexa fluor, 1:100 (Molecular Probes); and goat anti-mouse IgM 488 Alexa fluor, 1:300 dilution (Molecular Probes).
Preparation of brains for immunohistochemistry
Seven dogs aged 3-days, 5-days, 21-days, 51-days (n=2), and 150-days (n=2) were humanely euthanized with an intravenous injection of a barbituate solution. Immediately before death, the dogs were anesthetized and given intravenous heparin (1000U/mL). After death, intracardiac perfusion with cold 0.9% saline followed by 4% paraformaldehyde solution was performed. The brains were removed and fixed in 4% paraformaldehyde for 24 hours prior to dehydration in 30% sucrose solution.
For five of the dogs, the rostral brain was transversely sectioned caudal to the olfactory bulbs and rostral to the hippocampus, and the cerebellum was separated from the brainstem. These sections were then frozen in optimal cutting temperature (OCT) embedding medium and stored at −80°C for cryosectioning in a transverse plane. The brains of the 51-day old dogs were divided into right and left hemispheres. The hemispheres were sectioned at the rostral and caudal borders of the lateral ventricle and placed in 30% sucrose solution for 24–48 hours. The sections were then frozen in OCT for cryosectioning (20 μm slices) in a sagittal plane.
An embryonic day 28 pup (n=1) was obtained from the mother via Caesarian section and humanely euthanized with an intraperitoneal injection of a barbituate solution. The head was removed and fixed in 4% paraformaldehyde for 24 hours prior to dehydration in 30% sucrose solution. The head was then frozen in OCT embedding medium and stored at −80°C for cryosectioning in a transverse plane (20 μm slices).
Immunohistochemistry
Tissue sections were thawed, then blocked for 1 hour in 10% goat serum (GibcoBRL) with 0.2% Triton X-100 (Sigma) in PBS. Sections labeled with antibodies against cell surface antigens did not receive a blocking step. Primary antibody incubation was performed for 2 hours at room temperature or overnight at 4°C in PBS with 2% goat serum and 0.2% Triton X-100 (Triton X-100 was not used for cell surface marker labeling). The sections were washed in PBS and then incubated with secondary antibody in PBS for 1 hour at room temperature or overnight at 4°C. After PBS washes, the slides were mounted in Vectashield containing 4′,6 diamidino-2-phenylindole (DAPI; Vector Laboratories). Negative controls consisted of adjacent tissue sections in which only the secondary antibody was used during incubation (primary antibody was omitted).
Neurosphere preparation
Canine neurospheres derived from NPC cultures from the postnatal day 1 lateral ventricular SVZ (n=1) were washed in PBS, suspended in 4% paraformaldehyde for 10 minutes, dehydrated in 30% sucrose solution for 4 hours, and frozen in OCT. Cryosections of neurospheres (20 μm) were then subjected to immunofluorescent staining as above.
Confocal laser scanning microscopic analysis
Immunolabeled sections were scanned with a Leica DM IRE2 HC fluo TCS 1-B-UV microscope coupled to a Leica TCS SP2 spectral confocal system/UV (Leica, Bannockburn, IL). The 3 fluorochromes were then sequentially scanned. The step-size of the sequences was 0.04 μm.
Results
Canine neurospheres are CD15+, GFAP+, and Nestin+
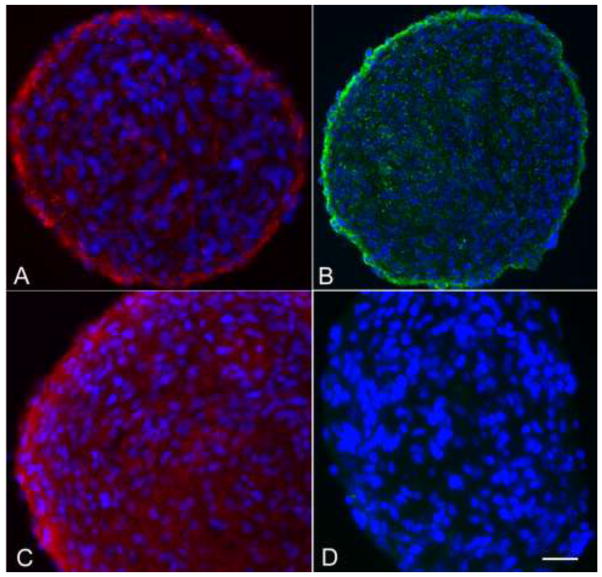
We evaluated cryosections of postnatal canine neurospheres cultured with EGF and bFGF/heparin by immunophenotyping with NPC markers nestin, GFAP, CD133, and CD15 (Figure 1). The spheres stained positively for nestin, glial fibrillary acidic protein (GFAP), and CD15; the neurospheres showed no staining for CD133. There was no staining for neuronal markers β-tubulin III and Map2ab (Figure 1). Positive GFAP-staining with polyclonal GFAP antiserum was confirmed with a monoclonal anti-GFAP antibody to exclude the possibility of cross-reactivity to proteins other than GFAP reported with polyclonal GFAP antiserum (Dolman et al. 2004; Zhang 2001).
Fig. 1.
Canine subventricular zone-generated neurospheres immunolabeled with antibodies against (A) GFAP; (B) CD15; (C) nestin and (D) Map2ab. Staining for Map 2ab and β-tubulin III (not shown) was negative. Bar = 50 μm.
Immunolabeling of the canine neuroepithelium and subventricular zone (SVZ)
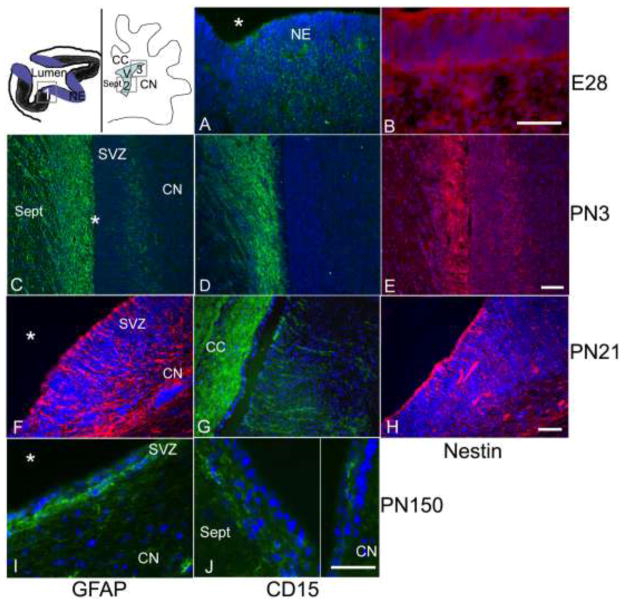
In order to determine whether markers associated with human and murine NPCs would be appropriate for canine NPCs in situ, we evaluated a known region of postnatal neurogenesis, the striatal SVZ (Lois and Alvarez-Buylla 1994; Morshead et al. 1994; Sanai et al. 2004), and its embryonic precursor, the ventricular neuroepithelium. Cryosections of the head of an embryonic day 28 (E28) dog, and the striatal SVZ of postnatal (PN) day 3 and PN day 21 dogs were examined. Sections were assayed for GFAP, nestin and CD15 immunoreactivity (Figure 2). Positive staining for nestin and CD15 was observed in the neuroepithelial cells of the E28 ventricular zone (Figure 2A, B). No staining for GFAP was seen in the E28 ventricular zone or the surrounding area (data not shown). The SVZ of PN day 3 and PN day 21 dogs stained positively for nestin, GFAP, and CD15 (Figure 2C–G). In the PN day 3 dogs, CD15 staining was restricted to the septal SVZ and medial corpus callosum, whereas by PN day 21, CD15 staining was evident in both the striatal and septal SVZs, as well as in the corpus callosum. By PN day 150, CD15 staining was again strongest in the dorsal septum and corpus callosum, and sparse in the striatal SVZ (Figure 2I–J).
Fig. 2.
Evaluation of the canine embryonic (E) day 28 neuroepithelium (A–B) and the SVZ from postnatal (PN) day 3 (C–E), PN day 21 (F–H), and PN day 150 (I–J). The neuroepithelium stained positively for CD15 (A) and nestin (B), but was negative for GFAP staining (not shown). The SVZ at PN day 3 stained positively for GFAP (C), CD15 (D) and nestin (E); CD15 staining was restricted to the septal SVZ (D). At PN day 21, GFAP (F) and CD15 (G) immunostaining was present in the septal SVZ (not shown), corpus callosum and in the dorsal striatal SVZ, similar to that of nestin (H). The SVZ was much thinner at PN day 150 (I–J). GFAP staining was strong in the striatal SVZ, with processes running parallel to and extending up towards the ventricle (I). CD15-positive cells were most abundant in the septal SVZ and rare within the striatal SVZ (J). Asterisks denote the location of the lateral ventricle at all time-points. The cartoon in the upper left corner shows the location of A–B (box 1), C–E (box 2) and F–H (box 3). (A–B) Bar = 50 μm; (C–E) Bar = 100 μm; (F–H) Bar = 50 μm; (I–J) Bar = 50 μm. CC, corpus callosum; CN, caudate nucleus; V, ventricle; Sept, septal nucleus; SVZ, subventricular zone.
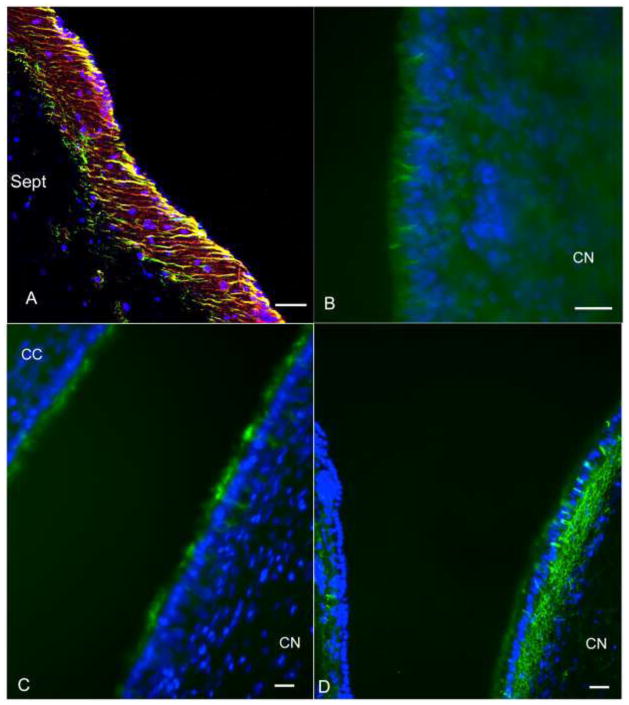
The nestin, GFAP and CD15 staining appeared to co-localize in some areas. To confirm double staining of nestin-positive and GFAP-positive cells, sections from the PN day 3 and PN day 21 dogs were analyzed using confocal microscopy. Small numbers of cells within the subependyma of the septal and striatal regions had a nestin and GFAP double-positive process that extended between ependymal cells (Figure 3A). Similar processes extending between ependymal cells to the ventricle were also noted in the septal and striatal SVZ from PN day 21 dogs when stained for CD15 and CD133, respectively (Figure 3B–C). The CD133 staining was strongest at the apical surface of the ependyma, but intermittently, short CD133+ processes extend from the SVZ located immediately adjacent to the ependymal layer up to the ventricular surface (Figure 3C). There was no CD133-positive staining detected in E28 neuroepithelium (data not shown). GFAP-positive processes extending to the ventricle were also noted in the PN day 150 SVZ (Figure 3D).
Fig. 3.
Subventricular zone (SVZ) immunostaining, canine postnatal rostral SVZ. (A) Confocal z-stack image of nestin (red) and GFAP (green) double-positive processes (yellow) extended from the SVZ to the ventricular surface. Similar processes extending from the SVZ to the ventricular surface were CD15-positive (B), CD133-positive (C), or GFAP-positive (D). Bars = 50 μm. Sept, septum; CN, caudate nucleus; CC, corpus callosum.
Isolation of CD15+ cells from the canine striatal SVZ
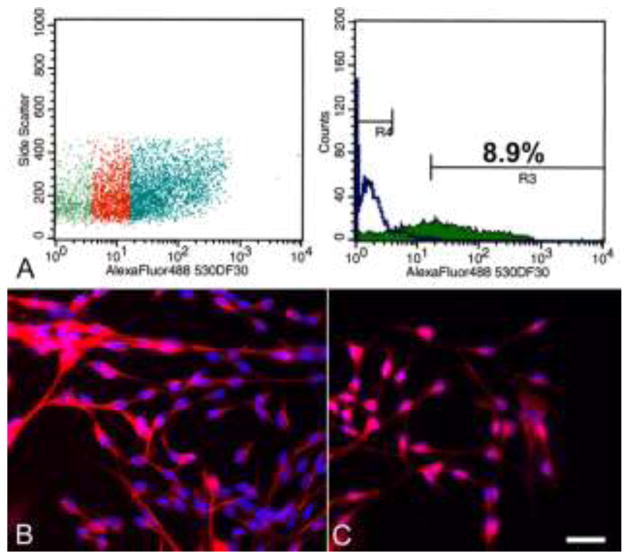
The presence of CD15-positive cells in regions in which neural progenitor cells are known to reside supported the use of CD15 as a potential marker for NPC enrichment. Consequently, acute dissociations of the striatal SVZ were performed for 19-day old dogs. Single cell suspensions were labeled with anti-CD15 antibodies and sorted for immunoreactivity using flow cytometry. The mean proportion of SVZ-derived CD15-positive cells from three independent isolations was 6.6 ± 2.2% (4.4%, 6.6% and 8.9%, respectively; Figure 4). The CD15-positive cell populations were plated into NPC medium with growth factors. Two of the three independently isolated CD15-positive populations could be expanded over ten passages (3 months), growing both as adherent cells and neurospheres in the same culture flask. Although passage ten was the endpoint of this study, the cells were still proliferating at the end of the experiment.
Fig. 4.
Canine CD15-positive cell population isolated from the rostral SVZ via flow cytometry. (A) The proportion of cells in the left panel that showed no overlap with the negative control (green region in left panel) are indicated in blue and comprise 8.9% of all gated cells (R3; right panel). The negative control cells are represented by R4 (right panel). Immunostaining of the CD15+ cells after one week in culture medium containing growth factors showed the cells are positive for nestin (B) and GFAP (C). Note the immature morphology of the cells and the diffuse, non-filamentous GFAP staining pattern. Bar = 25 μm.
The CD15-positive NPCs cultured in medium containing growth factors were immunophenotyped after 7–10 days using a panel of markers for mature and undifferentiated neural cells. The NPCs were nestin-positive and GFAP-positive (Figure 4). The majority of the GFAP-positive cells had an immature, undifferentiated morphology characterized by small cells with either a unipolar or bipolar, fusiform shape. In addition, there was a diffuse, non-filamentous GFAP staining pattern (Figure 4C). This non-filamentous staining was observed with both polyclonal GFAP antiserum and with monoclonal anti-GFAP antibody and has been noted in previous canine NPC cultures (Walton and Wolfe 2007, 2008). Staining for neuronal markers (β-tubulin III and Map2ab) and oligodendrocytic markers (O4 and galactocerebroside) was absent (data not shown).
The subgranular zone (SGZ) of the hippocampal dentate gyrus
The SGZ of the dentate gyrus contains both neural stem and progenitor cells in rodents and humans (Eriksson et al. 1998; Palmer et al. 1997; Seri et al. 2001). Cells with appropriate radial morphology that stained positively for CD15 and GFAP were detected in the SGZ of 5-month old dogs (Figure 5A). Doublecortin-positive cells corresponding to neuroblasts were located in the interface between the SGZ and granular cell layer (GCL) and extended up into the GCL (Figure 5B), whereas mature neurons (Map2ab+) were present in the GCL (Figure 5C).
Fig. 5.
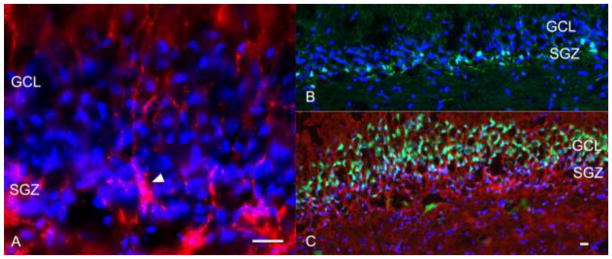
Canine adult hippocampal dentate gyrus. CD15-positive cells with characteristic radial glia morphology were present within the SGZ (arrowhead; A). Doublecortin-positive neuroblasts were located within the SGZ and extended into the GCL (B), whereas Map2ab-positive neurons were present in the GCL (C). Bars = 50 μm. SGZ, subgranular zone; GCL, granule cell layer.
Immunolabeling of the canine cerebellum
In the dog, the external granular layer (EGL) of the cerebellum is composed of actively proliferating cells and is maintained until around 70 days of age (Phemister and Young 1968). Cerebella from 3-day and 21-day old dogs were evaluated for CD15, GFAP and nestin immunoreactivity. We anticipated that in the younger dogs the EGL, a known location of restricted neuronal progenitors, would be the primary region to stain positively for CD15. However, in 3-day old dogs, CD15 immunoreactivity was primarily detected in the white matter and molecular layer of cerebellar folia, and only occasionally in the EGL (Figure 6A inset). Similarly, the staining patterns for both nestin and GFAP were strongest in the white matter tracts and molecular and Purkinje layers, although GFAP staining appeared stronger in the Purkinje layer than in the molecular layer (Figure 6B–C). Staining in the molecular layer was characterized by nestin-positive and GFAP-positive processes extending through the layer and into the EGL.
Fig. 6.
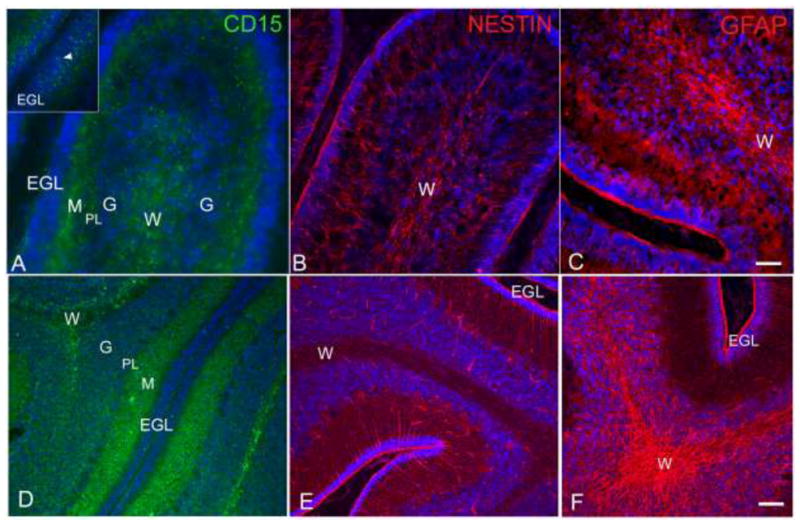
Canine postnatal (PN) day 3, cerebellum (A-C). Immunostaining with three NPC markers revealed strong positive staining in the white matter for CD15 (A), nestin (B) and GFAP (C). There was also strong positive staining of cell bodies in the molecular/Purkinje layer. CD15 positivity was noted within the EGL (A inset), whereas GFAP-positive and nestin-positive processes extended from the molecular layer/Purkinje layer into the EGL. Canine PN day 21, cerebellum. CD15 staining remained strongest in the white matter and molecular layer (D). Nestin (E) and GFAP (F) staining were similar, however, there was increased staining within the granular layer compared to the PN day 3. Nestin-positive and GFAP-positive processes extended through the molecular layer up into the EGL. Bar = 50 μm (C); Bar = 100 μm (F). EGL, external granular layer; G, granular layer; M, molecular layer; PL, Purkinje layer; W, white matter.
Similar to postnatal day 3, the PN day 21 cerebellum showed strong CD15 staining mainly in the white matter tracts of cerebellar folia as well as the molecular layer (Figure 6D). There were no CD15-positive cells observed in the EGL although the surface of the EGL was positive for nestin, CD15 and GFAP. We were interested to learn whether nestin-positive cells would also be found in the white matter tracts of the PN day 21 cerebellum. Serial sections from the same dog revealed the presence of nestin-positive cells within the white matter, as well as nestin-positive processes extending from the Purkinje layer through the EGL (Figure 6E). The GFAP staining pattern was similar to that of nestin, but more GFAP-positive cells were present in the granular layer (Figure 6F).
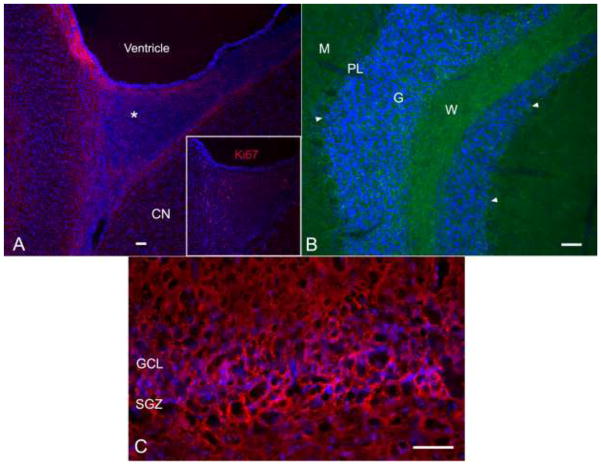
Phosphacan immunolabeling in the postnatal SVZ, SGZ and cerebellar white matter tracts
The NPC-associated chondroitin sulfate proteoglycan phosphacan recognized by the antibody 473HD was evaluated in the postnatal SVZ, SGZ and cerebellum (Figure 7). Staining was predominantly associated with the SVZ around the lateral ventricles (Figure 7A). In addition, there was strong staining in the SVZ rostral horn on sagittal sections and rostral angle on transverse sections. This region represents the beginning of the rostral migratory stream and large numbers of dividing Ki67+ precursor cells were present (Figure 7A inset). Phosphacan staining in the canine cerebellum at PN day 150 was restricted to the white matter tracts, to cells within the granular layer and up into the Purkinje layer, though not the Purkinje cells themselves (Figure 7B). In the dentate gyrus of the hippocampus, there was strong staining along the SGZ (Figure 7C).
Fig. 7.
Canine lateral ventricle and rostral horn postnatal (PN) day 150 (A), cerebellum PN day 51 (B), and dentate gyrus PN day 150 (C). Sections were stained for the NPC-associated chondroitin-sulfate proteoglycan phosphacan. Strong phosphacan staining outlined the SVZ of the lateral ventricle, including the rostral horn (asterisk) (A). The rostral horn, the entrance to the RMS in mice and primates, contained many dividing NPCs, identified by Ki67 staining (inset A). In the cerebellum, there was strong phosphacan staining in the white matter tracts and cells within the granular and Purkinje layers (arrowheads) (B). Strong staining for phosphacan was present within and below the SGZ of the dentate gyrus (C). Bars = 100 μm. CN, caudate nucleus; SVZ, subventricular zone; NPCs, neural precursor cells; SGZ, subgranular zone.
Discussion
The dog is an animal model for a number of human neurodegenerative diseases (Alroy et al. 1985; Fischer et al. 1998; Griffiths et al. 1981; Haskins et al. 1984; Koppang 1988; Wenger et al. 1999). While rodents remain invaluable as animal models of disease, the brain of a human is architecturally more complex and organized differently than that of rodents, whereas the dog brain possesses more of the architectural complexities and organization of the human brain. Canine NPCs have been characterized in vitro, but data in situ are lacking (Milward et al. 1997; Walton and Wolfe 2007, 2008). The validation of neural stem and progenitor cell (NPC) markers for the dog will prove valuable in studies of NPCs and the neural stem cell niche in this species.
Lewis X antigen (CD15) and CD133 (prominin) are cell surface moieties that have shown to greatly enrich for NPC activity as assessed by neurosphere formation in the mouse and in humans (Capela and Temple 2002; Corbeil et al. 2001; Lee et al. 2005; Muramatsu and Muramatsu 2004; Uchida et al. 2000). Our assessment of these two markers in the dog was initiated with growth factor-maintained canine neurospheres. While CD15 staining was present on the surface of cells within and at the margins of the spheres, no CD133 staining was observed. We then examined the neuroepithelium of the ventricular zone and the subventricular zone (SVZ) of the caudate nucleus in embryonic and postnatal dogs, respectively. The embryonic ventricular zone and postnatal SVZ are known to be sites in which multipotent neural progenitor cells reside in rodents and humans (Lois and Alvarez-Buylla 1994; Morshead et al. 1994; Sanai et al. 2004; Tramontin et al. 2003). CD15 staining was observed in the neuroepithelium of the embryonic canine ventricular zone as has been reported for the neuroepithelium of the embryonic human and mouse ventricular zones (Ashwell and Mai 1997; Forutan et al. 2001). CD15 immunostaining was also observed in the postnatal canine SVZ, however, there was a difference in the distribution of immunoreactivity between postnatal (PN) day 3 and PN day 21 dogs. The CD15 staining was restricted to the septal SVZ in PN day 3 dogs, was present in both the striatal and septal SVZ in PN day 21 dogs, and then was seen primarily in the septal SVZ and rarely in the striatal SVZ in PN day 150 dogs. In the hippocampal dentate gyrus, CD15+ cells with radial processes were detected in the SGZ, as reported for the mouse (Capela and Temple 2002).
In the postnatal SVZ, the mean proportion of CD15-positive cells from three independent isolations was 6.6 ± 2.2%. This is similar to the proportion reported for the adult mouse striatal SVZ (4.3 ± 0.2%) (Capela and Temple 2002). The CD15-positive cells could be expanded up to three months (10 passages). Furthermore, these cells were phenotypically consistent with an NPC phenotype (GFAP-positive and nestin-positive) and CD15 staining in vivo was present in known neurogenic regions, i.e., the SVZ and SGZ, as well as in cerebellar white matter tracts, where neural progenitors have been localized (Lee et al. 2005; Zhang and Goldman 1996b).
While no immunoreactivity to CD133 was detected in the postnatal neurospheres or in the neuroepithelium of day 28 embryonic dog, CD133-positive cells were present in the striatal SVZ of PN day 21 dogs. Prominin/CD133 immunoreactivity has been reported for embryonic human NPCs and murine neuroepithelium (Sawamoto et al. 2001; Uchida et al. 2000), as well as for adult human hematopoietic stem cells and postnatal mouse cerebellar NPCs (Corbeil et al. 2001; Lee et al. 2005). It is uncertain why CD133 immunoreactivity was absent with canine neurospheres and embryonic neuroepithelium, but present in postnatal day 21 striatal SVZ. The anti-human CD133 antibodies AC133 and 293C recognize epitopes that are glycosylated and AC133 is detected only in certain stages of cellular differentiation (Corbeil et al. 2000; Mak et al. 2011; Osmond et al. 2010). In addition, the least amount of conservation between mouse prominin and human CD133 (48–57%) is in the extracellular domain that is recognized by antibodies (Corbeil et al. 2001), thus it seems likely that species differences in the antigenicity of a putative canine prominin/CD133 ligand account for the lack of immunoreactivity with the anti-human CD133 antibodies at earlier developmental stages. The nature of the staining (ependymal apical surface and subependymal processes extending to the ventricle) and the region in which staining was detected are consistent with previous reports of CD133 immunoreactivity in the mouse (Coskun et al. 2008; Pfenninger et al. 2007), although the degree of immunoreactivity in the dog is considerably less.
We also evaluated two intracellular markers associated with NPCs, nestin and GFAP. The majority of the cells in the postnatal neurospheres and SVZ were immunoreactive for both GFAP and nestin. The embryonic canine neuroepithelium showed strong nestin immunoreactivity, but no GFAP staining was observed. Similarly, in mice strong nestin staining is concentrated in the embryonic neuroepithelium (Sawamoto et al. 2001), but GFAP expression is acquired during development. Rare to no NPCs isolated from embryonic day (E) 12.5 mouse brain are GFAP+, whereas by birth all are GFAP+ (Imura et al. 2003). We were able to co-localize GFAP and nestin staining in small numbers of SVZ cells. These findings are similar to the mouse SVZ in which GFAP/nestin double-positive cells represent the astrocytic neural stem cell (Doetsch et al. 1997).
The postnatal cerebellum, like the SVZ of the lateral ventricles, retains neurogenic potential, however for a limited period only. While overt neurogenesis has not been observed in the adult cerebellum, cortical interneurons, astrocytes, and oligodendrocytes are generated from NPCs that migrate from the ventricular zone into white matter tracts after birth. The neurogenic potential of these NPCs appears limited to the first two postnatal weeks in rats (Zhang and Goldman 1996a), however, NPC potential was demonstrated in a subpopulation of cells isolated from the adult mouse cerebellum in general (Klein et al. 2005) and, in particular, Sox1-positive Bergmann glial cells (Alcock and Sottile 2009). Bergmann glia are also CD15 and GFAP positive (Baboval et al. 2000; Bartsch and Mai 1991; Ganat et al. 2006) and reside within the Purkinje layer of the cerebellum. In the postnatal dog cerebellum, the presence of CD15-positive, nestin-positive, and GFAP-positive cells in the white matter tracts and in non-Purkinje cells of the Purkinje layer indicates that canine NPCs are also present within the postnatal cerebellum. In previous work, we isolated and propagated postnatal canine cerebellar NPCs in bFGF/heparin and EGF that were capable of differentiating into cells with the morphology and immunophenotype of neurons, oligodendrocytes and astrocytes (Walton and Wolfe 2007). Because bFGF-responsive cells in the mouse cerebellum are not derived from the external granular layer (EGL) (Lee et al. 2005), it is possible that canine cerebellar cells expanded in bFGF/heparin are also derived from the white matter rather than the EGL.
The 473HD antibody recognizes a GAG epitope representing phosphacan, a glial-derived proteoglycan that is a splice variant of the receptor protein tyrosine phosphatase (RPTP)-beta that is selectively expressed on radial glial cells and NPCs (Faissner et al. 2006). Brain-specific chondroitin sulfate proteoglycans (CSPGs) such as phosphacan are expressed by NPCs in vitro and are also associated with the NPC niche in vivo (Ida et al. 2006; Meyer-Puttlitz et al. 1996). As in rodents, anti-phosphacan antibody stained positively adjacent to the SVZ of the lateral ventricles, SGZ of the dentate gyrus, and within cerebellar white matter tracts. There was staining of cells in the external granular layer, as well as cells immediately below and adjacent to Purkinje cells that correspond to Bergmann glia. In adult rats, phosphacan staining is associated with Bergmann glia (Meyer-Puttlitz et al. 1996).
The results of this study demonstrate that antibodies associated with NPCs in rodents and humans identify cells in the dog that are located in known neurogenic regions and have cellular morphology appropriate to NPCs. Cells in the postnatal dog lateral ventricular SVZ, dentate gyrus SGZ, and cerebellar white matter tracts are immunopositive for both intracellular and extracellular NPC markers, facilitating studies of neural progenitor cells and the neural stem cell niche in this large animal model.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (NS056243), National Center for Research Resources (RR007063), and Merial Ltd.
Literature Cited
- Alcock J, Sottile V. Dynamic distribution and stem cell characteristics of Sox1-expressing cells in the cerebellar cortex. Cell Res. 2009;19:1324–1333. doi: 10.1038/cr.2009.119. [DOI] [PubMed] [Google Scholar]
- Alroy J, Orgad U, Ucci AA, Schelling SH, Schunk KL, Warren CD, Raghavan SS, Kolodny EH. Neurovisceral and skeletal GM1-gangliosidosis in dogs with beta-galactosidase deficiency. Science. 1985;229:470–472. doi: 10.1126/science.3925555. [DOI] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ashwell KW, Mai JK. Developmental expression of the CD15 epitope in the hippocampus of the mouse. Cell Tissue Res. 1997;289:17–23. doi: 10.1007/s004410050848. [DOI] [PubMed] [Google Scholar]
- Baboval T, Crandall JE, Kinnally E, Chou DK, Smith FI. Restriction of high CD15 expression to a subset of rat cerebellar astroglial cells can be overcome by transduction with adenoviral vectors expressing the rat alpha 1,3-fucosyltransferase IV gene. Glia. 2000;31:144–154. doi: 10.1002/1098-1136(200008)31:2<144::aid-glia60>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Mai JK. Distribution of the 3-fucosyl-N-acetyl-lactosamine (FAL) epitope in the adult mouse brain. Cell Tissue Res. 1991;263:353–366. doi: 10.1007/BF00318777. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- Corbeil D, Roper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. The Journal of biological chemistry. 2000;275:5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr, Fan G, de Vellis J, Sun YE. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolman DE, Lawrenson JG, Abbott NJ. Cross reactivity of polyclonal GFAP antiserum: implications for the in-vitro characterisation of brain endothelium. Brain Res. 2004;1012:185–186. doi: 10.1016/j.brainres.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Faissner A, Heck N, Dobbertin A, Garwood J. DSD-1-Proteoglycan/Phosphacan and receptor protein tyrosine phosphatase-beta isoforms during development and regeneration of neural tissues. Adv Exp Med Biol. 2006;557:25–53. doi: 10.1007/0-387-30128-3_3. [DOI] [PubMed] [Google Scholar]
- Fischer A, Carmichael KP, Munnell JF, Jhabvala P, Thompson JN, Matalon R, Jezyk PF, Wang P, Giger U. Sulfamidase deficiency in a family of Dachshunds: a canine model of mucopolysaccharidosis IIIA (Sanfilippo A) Pediatr Res. 1998;44:74–82. doi: 10.1203/00006450-199807000-00012. [DOI] [PubMed] [Google Scholar]
- Forutan F, Mai JK, Ashwell KW, Lensing-Hohn S, Nohr D, Voss T, Bohl J, Andressen C. Organisation and maturation of the human thalamus as revealed by CD15. J Comp Neurol. 2001;437:476–495. doi: 10.1002/cne.1296. [DOI] [PubMed] [Google Scholar]
- Gage F, Ray J, Fisher L. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Griffiths IR, Duncan ID, McCulloch M, Harvey MJ. Shaking pups: a disorder of central myelination in the Spaniel dog. Part 1. Clinical, genetic and light-microscopical observations. J Neurol Sci. 1981;50:423–433. doi: 10.1016/0022-510x(81)90154-4. [DOI] [PubMed] [Google Scholar]
- Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, Galli R, Verdugo JM, Herrera DG, Vescovi AL. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437–445. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins ME, Desnick RJ, DiFerrante N, Jezyk PF, Patterson DF. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984;18:980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- Hemsley KM, Hopwood JJ. Lessons learnt from animal models: pathophysiology of neuropathic lysosomal storage disorders. Journal of inherited metabolic disease. 2010;33:363–371. doi: 10.1007/s10545-010-9078-6. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida M, Shuo T, Hirano K, Tokita Y, Nakanishi K, Matsui F, Aono S, Fujita H, Fujiwara Y, Kaji T, Oohira A. Identification and functions of chondroitin sulfate in the milieu of neural stem cells. The Journal of biological chemistry. 2006;281:5982–5991. doi: 10.1074/jbc.M507130200. [DOI] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Klassen H, Schwartz MR, Bailey AH, Young MJ. Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci Lett. 2001;312:180–182. doi: 10.1016/s0304-3940(01)02215-7. [DOI] [PubMed] [Google Scholar]
- Klein C, Butt SJ, Machold RP, Johnson JE, Fishell G. Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development. 2005;132:4497–4508. doi: 10.1242/dev.02037. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Koppang N. The English setter with ceroid-lipofuscinosis: a suitable model for the juvenile type of ceroid-lipofuscinosis in humans. Am J Med Genet Suppl. 1988;5:117–125. doi: 10.1002/ajmg.1320310616. [DOI] [PubMed] [Google Scholar]
- Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Martin LJ. Olfactory bulb core is a rich source of neural progenitor and stem cells in adult rodent and human. J Comp Neurol. 2003;459:368–391. doi: 10.1002/cne.10664. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Mak AB, Blakely KM, Williams RA, Penttila PA, Shukalyuk AI, Osman KT, Kasimer D, Ketela T, Moffat J. CD133 protein N-glycosylation processing contributes to cell surface recognition of the primitive cell marker AC133 epitope. The Journal of biological chemistry. 2011;286:41046–41056. doi: 10.1074/jbc.M111.261545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Puttlitz B, Junker E, Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans in the developing central nervous system. II. Immunocytochemical localization of neurocan and phosphacan. J Comp Neurol. 1996;366:44–54. doi: 10.1002/(SICI)1096-9861(19960226)366:1<44::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Milward EA, Lundberg CG, Ge B, Lipsitz D, Zhao M, Duncan ID. Isolation and transplantation of multipotential populations of epidermal growth factor-responsive, neural progenitor cells from the canine brain. J Neurosci Res. 1997;50:862–871. doi: 10.1002/(SICI)1097-4547(19971201)50:5<862::AID-JNR22>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Garcia AD, Sofroniew MV, van Der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Muramatsu H. Carbohydrate antigens expressed on stem cells and early embryonic cells. Glycoconj J. 2004;21:41–45. doi: 10.1023/B:GLYC.0000043746.77504.28. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Osmond TL, Broadley KW, McConnell MJ. Glioblastoma cells negative for the anti-CD133 antibody AC133 express a truncated variant of the CD133 protein. International journal of molecular medicine. 2010;25:883–888. doi: 10.3892/ijmm_00000418. [DOI] [PubMed] [Google Scholar]
- Pagano SF, Impagnatiello F, Girelli M, Cova L, Grioni E, Onofri M, Cavallaro M, Etteri S, Vitello F, Giombini S, Solero CL, Parati EA. Isolation and characterization of neural stem cells from the adult human olfactory bulb. Stem Cells. 2000;18:295–300. doi: 10.1634/stemcells.18-4-295. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Pfenninger CV, Roschupkina T, Hertwig F, Kottwitz D, Englund E, Bengzon J, Jacobsen SE, Nuber UA. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67:5727–5736. doi: 10.1158/0008-5472.CAN-07-0183. [DOI] [PubMed] [Google Scholar]
- Phemister RD, Young S. The postnatal development of the canine cerebellar cortex. J Comp Neurol. 1968;134:243–254. doi: 10.1002/cne.901340209. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, Nedergaard M, Goldman SA. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kakishita K, Ogawa Y, Toyama Y, Yamamoto A, Yamaguchi M, Mori K, Goldman SA, Itakura T, Okano H. Generation of dopaminergic neurons in the adult brain from mesencephalic precursor cells labeled with a nestin-GFP transgene. J Neurosci. 2001;21:3895–3903. doi: 10.1523/JNEUROSCI.21-11-03895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He DP, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holst A, Sirko S, Faissner A. The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci. 2006;26:4082–4094. doi: 10.1523/JNEUROSCI.0422-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton RM, Wolfe JH. Abnormalities in neural progenitor cells in a dog model of lysosomal storage disease. J Neuropathol Exp Neurol. 2007;66:760–769. doi: 10.1097/nen.0b013e31812571c8. [DOI] [PubMed] [Google Scholar]
- Walton RM, Wolfe JH. In vitro growth and differentiation of canine olfactory bulb-derived neural progenitor cells under variable culture conditions. J Neurosci Methods. 2008;169:158–167. doi: 10.1016/j.jneumeth.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger DA, Victoria T, Rafi MA, Luzi P, Vanier MT, Vite C, Patterson DF, Haskins MH. Globoid cell leukodystrophy in cairn and West Highland white terriers. J Hered. 1999;90:138–142. doi: 10.1093/jhered/90.1.138. [DOI] [PubMed] [Google Scholar]
- Zhang L, Goldman JE. Developmental fates and migratory pathways of dividing progenitors in the postnatal rat cerebellum. J Comp Neurol. 1996a;370:536–550. doi: 10.1002/(SICI)1096-9861(19960708)370:4<536::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Goldman JE. Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron. 1996b;16:47–54. doi: 10.1016/s0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]
- Zhang SC. Defining glial cells during CNS development. Nat Rev Neurosci. 2001;2:840–843. doi: 10.1038/35097593. [DOI] [PubMed] [Google Scholar]