Summary
Deletion of one or more synapsin genes in mice results in a spontaneous epilepsy. In these animals, seizures can be evoked by opening or moving the cage. Aim of the present study was to characterize the evolution of the epileptic phenotype by neurophysiological examination and behavioral observation in synapsin triple knock-out (Syn-TKO) mice. Syn-TKO mice were studied from 20 postnatal days (PND) up to 6 months of age by video-EEG recording and behavioral observation. Background EEG spectral analysis was performed and data were compared to WT animals. Syn-TKO revealed rare spontaneous seizures and increased susceptibility to evoked seizures in mice from 60 to 100 PND. Spontaneous and evoked seizures presented similar duration and morphology. At times, seizures were followed by a post-ictal phase characterized by a 4 Hz rhythmic activity and immobility of the animal. Spectral analysis of background EEG evidenced a slowing of the theta-alpha peak in Syn-TKO mice compared to WT mice within the period from PND 40 to 100. These data indicate that Syn-TKO mice do not exhibit a linear progression of the epileptic phenotype, with the period corresponding to a higher susceptibility to evoked seizures characterized by background EEG slowing. This aspect might be connected to brain dysfunction often associated to epilepsy in the interictal period.
Abbreviations: Syn-TKO, synapsin triple knock-out; PND, postnatal day; DSA, density spectral array; EEG, electroencephalography; EMG, electromyography; MDF, mean dominant frequency; FFT, fast Fourier transform
Keywords: Synapsins, Epilepsy, Electroencephalography (EEG), Spectral analysis, C57BL/6 mice
Introduction
Communication in the brain is mainly achieved through the exocytotic release of chemical neurotransmitters. The process of neurotransmitter release is highly modulated, and involves a set of regulatory proteins whose function is regulated by post-translational modifications, among which phosphorylation plays a prominent role (Greengard et al., 1993; Südhof, 2004). The synapsins (Syns) are a family of synaptic-vesicle associated phosphoproteins involved in synaptic development, function and plasticity (Cesca et al., 2010; Valtorta et al., 2011). In mammals, the synapsins are encoded by three genes (SYN1, SYN2 and SYN3). Mice in which the genes encoding for Syn I, Syn II, Syn I/II or Syn I/II/III have been knocked-out display an epileptic phenotype that tends to aggravate with age and that is accompanied by behavioral alterations and increased loss of neurons in aged animals (Rosahl et al., 1995; Ferreira et al., 1998; Etholm et al., 2011; Gitler et al., 2004; Corradi et al., 2008). Recently, mutations in the genes encoding for human Syns I and II have been found to be associated with epilepsy, autism, aggressive behavior or a combination thereof (Garcia et al., 2004, Cavalleri et al., 2007; Lakhan et al., 2010; Fassio et al., 2011a). The Syn-triple knock-out (Syn-TKO) mouse has been widely characterized by ex vivo studies, showing an impairment in inhibitory neurotransmission (Gitler et al., 2004), cortico-hippocampal hyperexcitability (Boido et al., 2010), resulting in an unbalance between excitatory and inhibitory transmission upon the onset of seizures (Ketzef et al., 2011). When compared to wild type (WT) mice, Syn-TKO mice display decreased levels of synaptic proteins such as synaptobrevin 2, synaptotagmin I or synaptophysin I, but not of the number of synapses (Gitler et al., 2004). Syn-TKO mice display several behavioral abnormalities, such as a deficiency in spatial learning and memory (Gitler et al., 2004) and the tendency to exhibit transient seizures after sensory stimulation (Boido et al., 2010; Ketzef et al., 2011), that has been observed also in single KO for Syn I or Syn II, as well as in the Syn I/II double KO model (Corradi et al., 2008; Etholm et al., 2011). Moreover, Syn-TKO mice exhibit mild abnormalities in coordination and postural reflexes and delayed reflexive behavior (Gitler et al., 2004).
The use of video-EEG is highly useful for a more comprehensive characterization of behavioral and electrophysiological aspects of mice models of epilepsy (Etholm and Heggelund, 2009; Etholm et al., 2011). Moreover, the study of EEG rhythms in mice is becoming of great importance due to the increased availability of genetically modified models. Power spectra analysis of EEG rhythms in mice has been performed under both physiological (Palchykova et al., 2010) and pathological (Baumann et al., 2006) conditions, to detect gross brain network alterations.
Here, we have characterized the behavioral and neurophysiological features of Syn-TKO mice, by observing the development and progress of seizures from PND 20 to 180 and by repeated video-EEG recordings during spontaneous/evoked seizures and in interictal phases for the analysis of background activity.
Methods
Animals
Homozygous Syn-TKO mice (Gitler et al., 2004) were kindly provided by Drs. H.T. Kao (Brown University, Providence, RI, USA) and Paul Greengard (The Rockefeller University, NY, USA).
Syn-TKO mice were rederived on a C57BL/6J background (Charles River, Calco, Italy) obtaining single and multiple Syn-KO strains up to Syn-TKO and matching WT mice. For genotyping, tail DNA was extracted and analyzed by PCR using the previously described primers (Gitler et al., 2004). Mice were housed under controlled temperature on a 12 h light/dark cycle (lights on at 6.30 AM) with free access to chow pellets and tap water (5 animals/cage). Seizures were provoked by cage opening after placing it on a bench (Ketzef et al., 2011; Boido et al., 2010). After cage opening, mice were visually inspected for seizure detection and animal identification. This procedure was routinely performed between 9 and 10 AM three times a week. WT and Syn-TKO mice from PND 20 to 180 were used in this study. Different animals were monitored at different times, ranging from a total of 88 to 134 animals/time slot. In fact, we followed several generations of mice and a higher number of animals was tested in the first 80 PND to better define the age of onset of seizure susceptibility. The number of mice displaying at least one seizure within time slots of 20 days was recorded. For all experiments, male and female mice were tested in the same proportion.
Video-EEG recording
A random subgroup of Syn-TKO (n = 11) and WT (n = 16) mice underwent video-EEG recording. As already described (Magri et al., 2011), epidural stainless steel screw electrodes (0.9 mm diameter, 2 mm length) were surgically implanted under sevoflurane anesthesia (Sevorane™, Abbott S.p.a. Campoverde, Italy) and secured using cyanoancrylate and dental cement (Ketac Cem, ESPE Dental AG, Seefeld, Germany). Two active electrodes were placed on the right and left parietal areas (2 mm lateral to midline, 1 mm posterior to bregma) and one over cerebellum (1 mm posterior to lambda) as a common reference. In addition, a wire electrode was inserted in neck muscles and connected to a pin for electromyographic (EMG) recordings (3 WT and 3 Syn-TKO mice). After a recovery of at least 3 days, unrestrained mice were monitored by video-EEG in recording sessions of 6–24 h in a Faraday cage. For EEG recording, electrodes were connected via a flexible cable to an amplifier and EEG data were recorded and digitally saved using a System Plus device (Micromed, Mogliano Veneto, Italy). EEG traces were filtered between 0.53 and 60 Hz and sampled at 256 Hz. Simultaneous video data were acquired with a Canon MV550I camera connected via FireWire to the EEG recorder. Video-EEG recordings were visually inspected for detection of spontaneous seizures. Seizures were defined as high-amplitude (at least 2 times the baseline) rhythmic discharges (repetitive spikes, spike-and-wave discharges and/or slow waves) lasting at least 5 s. To have electrophysiological correlate of evoked seizures, the same provocative procedure used in the behavioral monitoring was tested in implanted mice (4 Syn-TKO and 3 WT), by lifting the animal by the tail, placing it in the Faraday cage used for video-EEG, and recording the EEG for 5 min. This provocation procedure was performed in the time period of highest susceptibility to evoked seizures, based on observations made in unimplanted Syn-TKO mice (26 trials between P40 and P100). Due to the limitations of the screw implant, which could not be maintained for longer than 30–60 days, different numbers of animals were recorded for each time slot: 4 Syn-TKO vs 4 WT (PND 20-40), 5 Syn-TKO vs 4 WT (PND 40-60), 5 Syn-TKO vs 4 WT (PND 60-80), 5 Syn-TKO vs 4 WT (PND 80-100), 4 Syn-TKO vs 4 WT (PND 100-120), 4 Syn-TKO vs 4 WT (PND 120-140), 4 Syn-TKO vs 4 WT (PND 140-160), 5 Syn-TKO vs 4 WT (PND 160-180).
We made all efforts to minimize the number of animals used and their suffering. This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH) and approved by the San Raffaele Institutional Animal Care and Use Committee (IACUC) and the Italian Ministry of Health.
Background EEG power spectra analysis
EEG power spectra were calculated by Fast Fourier Transform (FFT) using the Welch method (Welch, 1967). These analysis have been performed in smaller groups of WT and Syn-TKO mice (4–5 animals/time slot) during the awake state (exploratory behavior, recognized by video recording inspection). For each recording, a period of 3–4 min of artifact-free background EEG was selected, at least 1 h after the start of recording. EEG sessions including spontaneous or evoked seizures at any time were not considered. FFT was performed on epochs of 2 s within the selected period, tapered with a Hanning window, obtaining spectra for each epoch with a frequency resolution of 0.5 Hz. The mean power spectrum was then estimated by averaging the single epoch spectra and the two channels. Absolute power values were then normalized to the total absolute power to obtain relative values comparable among the different animals.
Mean dominant frequency (MDF), defined as the “center of mass” of a frequency band, was extracted from power spectra within delta (1–4 Hz) and theta-alpha (6–10 Hz) bands, as follows:
where Pi is the power at frequency fi and i is the index sweeping the whole frequency band samples.
Time-frequency analysis was also performed and results were represented by density spectral arrays (DSA). DSA were calculated by applying FFT on consecutive 2 s Hanning windowed EEG epochs, with 1 s partial overlap and displayed in pseudo 3D graphics, where horizontal and vertical axes represented time and frequency, respectively, while the third dimension, reflecting power values, was coded by a color scale. All spectral analyses were performed using the EEG Analyzer function included in the software Micromed System PLUS.
Statistical analysis
To compare relative power spectra and MDF values between Syn-TKO and control mice (WT) the non-parametric Mann–Whitney U-test was used: relative power values were compared for individual spectral samples within delta and theta-alpha bands. No correction for multiple comparison was applied (Perneger, 1998).
Results
Provoked seizures in synapsin I/II/III knock-out mice
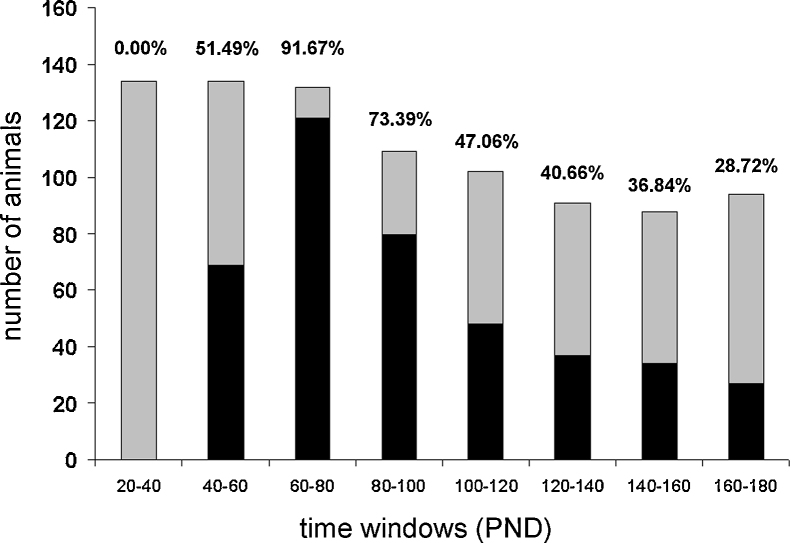
Syn-TKO mice were systematically observed from 20 to 180 PND. None of the mice displayed evoked seizures before PND 44, with an overall average of onset at PND 61 (median = PND 60). By the age of 89 PND, all Syn-TKO mice had exhibited at least one provoked seizure at cage opening, except for one mouse with a first event at PND 110. Between 40 and 60 PND, 65 out of 134 Syn-TKO mice, displayed an epileptic phenotype, with the highest susceptibility to seizures in the 60–80 PND time slot (121 out of 132). After 80 PND, the frequency of evoked seizures gradually decreased, reaching the lowest rate between 160 and 180 PND (Fig. 1). No animal died during the observation period.
Figure 1.
Temporal evolution of evoked seizure susceptibility in Syn-TKO mice. A seizure-provoking maneuver (opening of the cage) was performed three times a week and the mice were observed for 30 min following the maneuver. In gray, the number of observed Syn-TKO mice in the various time slots; in black, the number of animals showing at least one seizure in the same time slots. PND, postnatal days. Note the absence of seizures in the 20–40 PND time slot. The number of animals displaying seizures increased to more than 90% between PND 40 and 80 and then gradually decreased toward low rates (28.72%) at six months of age.
Video-electroencephalographic recordings of spontaneous and evoked seizures
Electroencephalographic recordings of Syn-TKO mice confirmed the low frequency of spontaneous seizures in this model, as already described (Ketzef et al., 2011). During more than 1600 h of EEG recording, a total number of 16 spontaneous seizures were recorded (in 6 out of 11 monitored animals). On average, one spontaneous ictal event was recorded over about 100 h of EEG monitoring if considering only the 6 animals with seizures. During EEG monitoring, evoked seizures were obtained in 2 out of 4 tested Syn-TKO implanted mice, in the time period from PND 40 to 100. Out of 26 trials, 7 seizures were recorded (26.9% success). No seizures or epileptic-like behavior was ever observed in WT animals during or after the same procedure for evoking seizures in Syn-TKO.
All the recorded seizures were related to a behavioral phenotype and the vast majority had a reproducible behavioral expression, which could be subdivided into 4 main phases. A video example of seizure is available as Supplementary material. Generally, seizures started by a sudden blocking of mouse activity immediately followed by head and forelimb jerks (indicated as BACK in the supplementary video) pushing backward the animal, with its hind legs extended, possibly to stabilize posture. The mouse then lost stability falling sideways with a tonic flexion of the truncus, sometimes accompanied by myoclonic jerks of the fore and hind limbs (T-FLEX). After a few seconds, a tonic extension of truncus, neck and head occurred (T-EXT); occasionally the mouse stood up on his hind limbs. At times, during this tonic phase, the Straub tail sign was also observed. Seizure evolved into a stage characterized by rhythmical jerks in muscles of forelimbs, neck or head (H-MOV). The most common observed activity consisted in rhythmical up-down or left-right movement of the head. Usually seizures finished after this phase, but in few cases, a final short stage characterized by fast runs and jumps was seen. At the end of the seizure, animals either tended to return to the normal activity or switched to a post-ictal phase characterized by periods of complete immobility, interrupted by short intervals of movement. This observed phenotype is very similar to what reported in the synapsin I/II double KO model (Etholm and Heggelund, 2009). Only in 4 cases, seizures did not distinctly show all the behavioral manifestations described above, but only part of them in a less clear sequence. In addition, no significant differences were found in EEG seizure length (spontaneous: 32.8 ± 15.0 s; evoked: 29.6 ± 8.4 s; mean ± SD; P = 0.58, t-test).
Fig. 2 presents the EEG recordings of a spontaneous and an evoked seizure with descriptions of the corresponding behavioral manifestations. The initial phase (BACK), corresponding to forelimb jerks and backward movements, is characterized by spikes or sharp waves superimposed to the background EEG; subsequently, tonic truncus flexion and the following truncus extension can be distinguished by main frequency components around 3–4 Hz (T-FLEX) and 7–8 Hz (T-EXT), respectively. Sequences of sharp waves with a mean duration of 200 ms are typical of the final phase corresponding to rhythmic head movements (H-MOV) or other kinds of myoclonies.
Figure 2.
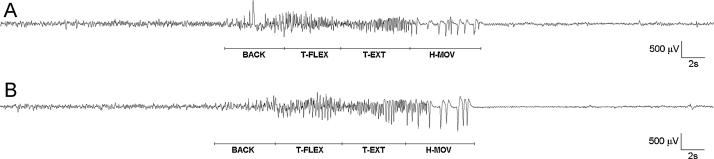
Comparison between evoked and spontaneous seizures in Syn-TKO mice. (A) Evoked and (B) spontaneous seizure in Syn-TKO mice. A single channel is shown for both examples. Behavioral correlates corresponding to different EEG phases are reported. BACK: forelimb jerks causing the mouse to move backward; T-FLEX: truncus flexion activity, sometimes accompanied by myoclonies of fore and hind legs; T-EXT: tonic extension of truncus, neck and head; H-MOV: rhythmical movements of head and/or forelimbs.
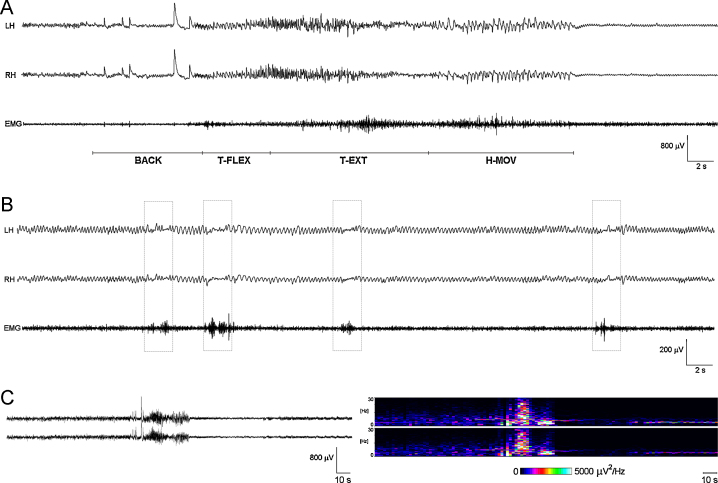
An EEG/EMG recording of a spontaneous seizure is displayed in Fig. 3A; a weak muscular activity of the neck during T-FLEX phase and a stronger one during T-EXT and H-MOV phases are evident. In about half of the recorded seizures, a post-ictal phase characterized by a harmonic oscillatory activity within the 4–7 Hz frequency band and a moderate tonic activity of the neck muscles was observed (n = 11; 8/16 spontaneous and 3/7 evoked seizures), corresponding to a complete immobility of the animal. This activity lasted for a mean period of 107 s (range: 30–210 s) and was interrupted only in correspondence of brief movements of the head, barely detectable in the video recording and corresponding to short-duration, low-amplitude EMG bursts recorded from neck muscles, as evidenced by EMG traces (Fig. 3B).
Figure 3.
EEG–EMG recordings and spectrogram analysis of a typical spontaneous seizure in a Syn-TKO mouse. (A) Epidural two-channel recording of a spontaneous seizure with the simultaneous recording of muscle electrical activity (EMG). In the post-ictal phase, tracings (B) are characterized by a typical EEG pattern with an harmonic oscillatory activity within the 4–7 Hz frequency, corresponding to a complete immobility of the animal, that is interrupted by desynchronized activity, with fast activity also in the muscles (dotted boxes). (C) The same seizure with the corresponding time frequency analysis (right panel), demonstrates differences in the activity preceding and following ictal event. LH, left and RH, right hemisphere; EMG, electromyography.
DSA showed the time–frequency changes related to seizures: EEG spectral components were distributed within the 2–9 Hz frequency range before seizure; a slow frequency power increase determined the beginning of the seizure (BACK) followed by a gradual power increase in the faster frequencies (T-FLEX and T-EXT phases) and terminating with a period of high-power low-frequency activity (H-MOV); the post-ictal phase was characterized by stable power values around 4 Hz (Fig. 3C).
Interictal background EEG
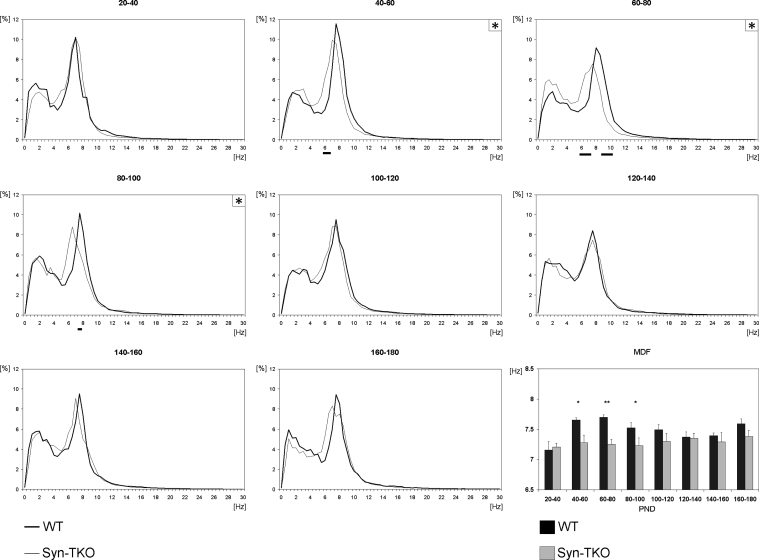
In WT mice, EEG spectra during the awake phase were characterized by a dominant peak within the theta-alpha band, peaking at around 7–8 Hz and by a less represented slower frequency with a broader peak, with a maximum around 2–3 Hz (Fig. 4). Compared with wild type, Syn-TKO mice recorded between 40 and 100 PND had a significant slowing of the theta-alpha peak, both in terms of mean dominant frequency (40–60 PND: WT 7.6 ± 0.03, Syn-TKO 7.2 ± 0.12, p < 0.05; 60–80 PND: WT 7.7 ± 0.03 Syn-TKO 7.2 ± 0.08, p < 0.01; 80–100 PND: WT 7.5 ± 0.08, Syn-TKO 7.2 ± 0.12, p < 0.05), and power: (6–7 Hz, p < 0.05), 60–80 PND (6–7.5 and 8.5–10 Hz, p < 0.05) and 80–100 PND (7.5–8 Hz, p < 0.05). No significant differences within the delta band (1–4 Hz) were found concerning power or MDF.
Figure 4.
Relative power spectra analysis of interictal background EEG in Syn-TKO and WT mice at different time slots. Spectra were calculated for 20 day-time slots from 20 to 180 PND. Only within periods from PND 40 to 60, PND 60 to 80 and PND 80 to 100 significant differences were observed in the higher frequency peaks (panels are marked with asterisk). Spectra were normalized and plotted as percentages of total power across all frequencies. Black lines under the X-axis indicate a significant difference with p < 0.05 (Mann–Whitney U-test; 4–5 mice per group). Bottom-right panel: MDF values (means ± SEM) are displayed for the same 20 day-time slots. Asterisks indicate significant differences (*p < 0.05; **p < 0.01; Mann–Whitney U-test; 4–5 mice per group). Thick lines/black bins, WT; thin lines/gray bins, Syn-TKO.
Discussion
In the present paper we report the behavioral and electroencephalographic characterization of the epileptic phenotype of mice deficient for synapsin I, II, III, observed from the period preceding the onset of seizures to 6 months of age. Due to the paucity of spontaneous seizures in this animal model, we adopted a simple and well documented method to evoke seizures, namely transferring the cage onto a bench and opening it (Boido et al., 2010; Ketzef et al., 2011). Based on a large number of observations, we found that the earliest onset of evoked seizures was after the first 6 weeks of age, consistently with previous reports in Syn-TKO (Ketzef et al., 2011) and in single or double KO for syn I and syn II (Rosahl et al., 1995). Susceptibility to seizures increased up to 60–80 PND, when more than 90% of animals exhibited at least one seizure, and then decreased. This bell-shaped behavior in the frequency of evoked seizures could be due to an initial evolution of the disease, that is somehow counterbalanced along with its chronicization. Synaptic mechanisms differentially affecting excitatory and inhibitory neurons could be involved in this process, as suggested by several authors (Baldelli et al., 2007; Chiappalone et al., 2009; Gitler et al., 2004; Boido et al., 2010; Fassio et al., 2011b). This E/I imbalance is likely to become apparent after the increase in the expression of synapsin I and II that occurs postnatally, and may then attenuate with age, consistent with the known age-dependent decrease in cortical excitability.
Video-EEG observation revealed that the epileptic phenotype in Syn-TKO mice did not differ from the seizure pattern reported in Syn I/II double KO mice by Etholm et al. (2011). Moreover, Syn I/II double KO exhibit a seizure behavior similar to single Syn II KO but not to Syn I single KO (Etholm et al., 2012), while Syn III single KO mice are the only synapsin deficient model not exhibiting epilepsy (Cesca et al., 2010; Valtorta et al., 2011). Although the correct expression of Syn III is crucial in the early stages of life (Ferreira et al., 2000; Valtorta et al., 2011), its absence in Syn-TKO mice does not seem to influence the age of onset of evoked seizures. Syn I/II double KO mice develop handling-induced epileptic seizures at the age of about 2 months (Etholm et al., 2011), the same observed in the present study in Syn-TKO. In addition, our data suggest that the lack of Syn III may play a minor role in the epileptic expression of Syn-TKO compared to Syn I/II double KO mice. The post-ictal phase, characterized by immobility and a stable oscillatory EEG rhythm in the theta frequency, was observed as a consequence of both evoked or spontaneous seizures and was already reported in the literature for synapsin I/II double KO mice (Etholm and Heggelund, 2009).
The availability of long-term EEG monitoring appears quite important for detecting spontaneous seizures, since they seldom occur, consistently with what reported (Ketzef et al., 2011) and unlikely to be found only at routine behavioral observation. The use of simultaneous video recording during EEG monitoring has proved to bring useful information about the behavioral correlates of seizures. Furthermore, EMG was of great utility in detecting the small, brief movements associated with the post-ictal phase, difficult to identify at video inspection only.
Within the same period of increased susceptibility to seizures, we found a significant slowing of EEG interictal activity. In rodents, background EEG slowing and reduced theta-alpha power are non specific findings, usually associated with physiological or drug-induced vigilance deficit. Those are generally considered as a landmark of learning impairment after administration of anticholinergic drugs (Vanderwolf, 1991), aging (Poschel et al., 1985) or pathological conditions, such as traumatic brain injury (Fedor et al., 2010). In the present study, the lack of continuous monitoring does not allow to determine whether EEG background slowing precedes or follows the first ever seizure in these mice. Therefore, whether such slowing indicates an initial involvement of brain networks leading to seizure development, or whether it is a direct consequence of previous seizures, remains an open question. In support of the latter hypothesis, we noticed that the background activity did not differ in Syn-TKO as compared to WT mice outside the age period characterized by expression of the epileptic phenotype. Repeated seizures are known to be associated with neuronal structural and functional alterations (Nakasu et al., 1995; Gitler et al., 2004; Corradi et al., 2008), these contributing to neurocognitive deficits in epilepsy, such as behavioral, memory and learning impairment (Rensing et al., 2005; Elger et al., 2004). On the other hand, the presence of brain bioelectrical dysfunction already before seizure occurrence in Syn-TKO is suggested by in vitro observations indicating an important role of synapsins in the regulation of network excitability, even in the prenatal stage (Chiappalone et al., 2009). Further investigations with continuous recordings starting before the age period with epileptic phenotype are necessary, in order to better define the temporal relationship between seizures and EEG background slowing. Although the association between the finding of EEG slowing and behavioral or learning abnormalities was beyond the scope of the present study, we may not exclude a link between these two features. The cognitive involvement reported in Syn-TKO mice refer to an age period following the appearance of seizures (Gitler et al., 2004; Ketzef et al., 2011). Moreover, the learning abnormalities observed in single or double KO for syn I and syn II were related to aging (Corradi et al., 2008). If the brain dysfunction revealed by background EEG slowing is linked with cognitive or learning abnormalities, we should hypothesize that also the latter may be less evident in the period preceding seizure onset, as well as during the phase of decreased susceptibility to seizures.
In conclusion, although Syn-TKO mice display an evident epileptic phenotype, the progression of the disease is not linear, being prominent between 2 and 3 months of life. Since the susceptibility to seizures make Syn-TKO a possible model for pharmacological studies, our findings identify an adequate time interval for antiepileptic drug screening and testing. Moreover, as the time of increased susceptibility to seizures corresponds to background interictal EEG slowing, caution should be taken while interpreting cognitive abnormalities found during this period.
Acknowledgments
We are grateful to Dr. Luca Muzio – INSPE Scientific Institute San Raffaele – for helpful suggestions. This work was partly supported by a Telethon Facility grant (GTF09022 to L.L.), a Telethon research grant (GGP09134A to F.B. and F.V.), the Italian Ministry of University and Research (PRIN to F.B. and F.V.), the Compagnia di San Paolo, Torino (to F.V. and F.B.).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eplepsyres.2012.07.012.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Baldelli P., Fassio A., Valtorta F., Benfenati F. Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J. Neurosci. 2007;27(49):13520–13531. doi: 10.1523/JNEUROSCI.3151-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C.R., Kilic E., Petit B., Werth E., Hermann D.M., Tafti M., Bassetti C.L. Sleep EEG changes after middle cerebral artery infarcts in mice: different effects of striatal and cortical lesions. Sleep. 2006;29:1339–1344. doi: 10.1093/sleep/29.10.1339. [DOI] [PubMed] [Google Scholar]
- Boido D., Farisello P., Cesca F., Ferrea E., Valtorta F., Benfenati F., Baldelli P. Cortico-hippocampal hyperexcitability in synapsin I/II/III knockout mice: age-dependency and response to the antiepileptic drug levetiracetam. Neuroscience. 2010;171:268–328. doi: 10.1016/j.neuroscience.2010.08.046. [DOI] [PubMed] [Google Scholar]
- Cavalleri G.L., Weale M.E., Shianna K.V., Singh R., Lynch J.M., Grinton B., Szoeke C., Murphy K., Kinirons P., O’Rourke D., Ge D., Depondt C., Claeys K.G., Pandolfo M., Gumbs C., Walley N., McNamara J., Mulley J.C., Linney K.N., Sheffield L.J., Radtke R.A., Tate S.K., Chissoe S.L., Gibson R.A., Hosford D., Stanton A., Graves T.D., Hanna M.G., Eriksson K., Kantanen A.M., Kalviainen R., O’Brien T.J., Sander J.W., Duncan J.S., Scheffer I.E., Berkovic S.F., Wood N.W., Doherty C.P., Delanty N., Sisodiya S.M., Goldstein D.B. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: a case–control study. Lancet Neurol. 2007;6:970–980. doi: 10.1016/S1474-4422(07)70247-8. [DOI] [PubMed] [Google Scholar]
- Cesca F., Baldelli P., Valtorta F., Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog. Neurobiol. 2010;91:313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chiappalone M., Casagrande S., Tedesco M., Valtorta F., Baldelli P., Martinoia S., Benfenati F. Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin I-deficient cortical networks. Cereb. Cortex. 2009;9(6):1422–1439. doi: 10.1093/cercor/bhn182. [DOI] [PubMed] [Google Scholar]
- Corradi A., Zanardi A., Giacomini C., Onofri F., Valtorta F., Zoli M., Benfenati F. Synapsin-I- and synapsin-II-null mice display an increased age-dependent cognitive impairment. J. Cell Sci. 2008;121:3042–3051. doi: 10.1242/jcs.035063. [DOI] [PubMed] [Google Scholar]
- Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–677. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Etholm L., Heggelund P. Seizure elements and seizure element transitions during tonic–clonic seizure activity in the synapsin I/II double knockout mouse: a neuroethological description. Epilepsy Behav. 2009;14:582–590. doi: 10.1016/j.yebeh.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Etholm L., Linden H., Eken T., Heggelund P. Electroencephalographic characterization of seizure activity in the synapsin I/II double knockout mouse. Brain Res. 2011;1383:270–288. doi: 10.1016/j.brainres.2011.01.070. [DOI] [PubMed] [Google Scholar]
- Etholm L., Bahonjic E., Walaas S.I., Kao H.T., Heggelund P. Neuroethologically delineated differences in the seizure behavior of synapsin 1 and synapsin 2 knock-out mice. Epil. Res. 2012;(January) doi: 10.1016/j.eplepsyres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Fassio A., Patry L., Congia S., Onofri F., Piton A., Gauthier J., Pozzi D., Messa M., Defranchi E., Fadda M., Corradi A., Baldelli P., Lapointe L., St-Onge J., Meloche C., Mottron L., Valtorta F., Khoa Nguyen D., Rouleau G.A., Benfenati F., Cossette P. SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum. Mol. Genet. 2011;20(12):2297–2307. doi: 10.1093/hmg/ddr122. [DOI] [PubMed] [Google Scholar]
- Fassio A., Raimondi A., Lignani G., Benfenati F., Baldelli P. Synapsins: from synapse to network hyperexcitability and epilepsy. Semin. Cell Dev. Biol. 2011;22:408–415. doi: 10.1016/j.semcdb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Chin L.S., Li L., Lanier L.M., Kosik K.S., Greengard P. Distinct roles of synapsin I and synapsin II during neuronal development. Mol. Med. 1998;4:22–28. [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Kao H.T., Feng J., Rapoport M., Greengard P. Synapsin III: developmental expression, subcellular localization, and role in axon formation. J. Neurosci. 2000;20:3736–3744. doi: 10.1523/JNEUROSCI.20-10-03736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor M., Berman R.F., Muizelaar J.P., Lyeth B.G. Hippocampal (dysfunction after lateral fluid percussion injury. J. Neurotrauma. 2010;27:1605–1615. doi: 10.1089/neu.2010.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C.C., Blair H.J., Seager M., Coulthard A., Tennant S., Buddles M., Curtis A., Goodship JA Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J. Med. Genet. 2004;41:183–186. doi: 10.1136/jmg.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D., Takagishi Y., Feng J., Ren Y., Rodriguiz R.M., Wetsel W.C., Greengard P., Augustine G.J. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J. Neurosci. 2004;24:11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Valtorta F., Czernik A.J., Benfenati F Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Ketzef M., Kahn J., Weissberg I., Becker A.J., Friedman A., Gitler D. Compensatory network alterations upon onset of epilepsy in synapsin triple knock-out mice. Neuroscience. 2011;189:108–122. doi: 10.1016/j.neuroscience.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Lakhan R., Kalita J., Misra U.K., Kumari R., Mittal B. Association of intronic polymorphism rs3773364 A>G in synapsin-2 gene with idiopathic epilepsy. Synapse. 2010;64:403–408. doi: 10.1002/syn.20740. [DOI] [PubMed] [Google Scholar]
- Magri L., Cambiaghi M., Cominelli M., Alfaro-Cervello C., Cursi M., Pala M., Bulfone A., Garcìa-Verdugo J.M., Leocani L., Minicucci F., Poliani P.L., Galli R. Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell. 2011;9(5):447–462. doi: 10.1016/j.stem.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Nakasu Y., Nakasu S., Morikawa S., Uemura S., Inubushi T., Handa J. Diffusion-weighted MR in experimental sustained seizures elicited with kainic acid. AJNR Am. J. Neuroradiol. 1995;16:1185–1192. [PMC free article] [PubMed] [Google Scholar]
- Palchykova S., Winsky-Sommerer R., Shen H.Y., Boison D., Gerling A., Tobler I. Manipulation of adenosine kinase affects sleep regulation in mice. J. Neurosci. 2010;30:13157–13165. doi: 10.1523/JNEUROSCI.1359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger T.V. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschel B.P., Ho P.M., Ninteman F.W. Arousal deficit shown in aged rat's quantitative EEG and ameliorative action of pramiracetam compared to piracetam. Experientia. 1985;41:1433–1435. doi: 10.1007/BF01950020. [DOI] [PubMed] [Google Scholar]
- Rensing N., Ouyang Y., Yang X.F., Yamada K.A., Rothman S.M., Wong M. In vivo imaging of dendritic spines during electrographic seizures. Ann. Neurol. 2005;58(6):888–898. doi: 10.1002/ana.20658. [DOI] [PubMed] [Google Scholar]
- Rosahl T.W., Spillane D., Missler M., Herz J., Selig D.K., Wolff J.R., Hammer R.E., Malenka R.C., Sudhof T.C. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;8(375):488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Südhof T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Valtorta F., Pozzi D., Benfenati F., Fornasiero E.F. The synapsins: multitask modulators of neuronal development. Semin. Cell Dev. Biol. 2011;22(4):378–386. doi: 10.1016/j.semcdb.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Vanderwolf C.H. Anti-muscarinic drug effects in a swim-to-platform test: dose–response relations. Behav. Brain Res. 1991;44:217–219. doi: 10.1016/s0166-4328(05)80027-0. [DOI] [PubMed] [Google Scholar]
- Welch P.D. The use of fast fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoustics. 1967;AU–15:70–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.