Abstract
In this report, we explore retinoblastoma diagnostic accuracy and review chemotherapy alternatives for retinoblastoma using intravenous, intra-arterial, periocular, and intravitreal routes. A review of 2775 patients referred for management of retinoblastoma, disclosed 78% with confirmed retinoblastoma and 22% with simulating lesions, termed pseudoretinoblastomas. Children ≤2 years old showed leading pseudoretinoblastomas of persistent fetal vasculature, Coats disease, and vitreous haemorrhage, whereas those >5 years showed simulators of Coats, toxocariasis, and familial exudative vitreoretinopathy. The diagnosis of retinoblastoma should be established before planning therapeutic strategy. Chemotherapy strategy depends on tumour laterality and stage of disease. If bilateral retinoblastoma, intravenous chemotherapy (IVC) is important as first-line therapy for control of intraocular disease, prevention of metastasis, and reduction in prevalence of pinealoblastoma and long-term second malignant neoplasms. Bilateral groups D and E retinoblastoma receive additional subtenon's carboplatin boost for improved local control. If unilateral disease is present, then intra-arterial chemotherapy (IAC) is often considered. IAC can be salvage therapy following chemoreduction failure. Unilateral retinoblastoma of groups D and E are managed with enucleation or globe-conserving IVC and/or IAC. Intravitreal chemotherapy is cautiously reserved for recurrent vitreous seeds following other therapies. In conclusion, the strategy for retinoblastoma management with chemotherapy depends on tumour laterality and stage of disease. Bilateral retinoblastoma is most often managed with IVC and unilateral retinoblastoma with IAC, but if advanced stage, combination IVC plus IAC or enucleation.
Keywords: tumour, retinoblastoma, chemoreduction, intravenous chemotherapy, intra-arterial chemotherapy, vitreous chemotherapy
Introduction
Retinoblastoma is a serious ocular malignancy that manifests covertly with painless leukocoria and threatens survival of the patient.1, 2 This intraocular malignancy, if untreated, can lead to death within 1–2 years. Advanced disease with massive tumour, invasive into surrounding structures, is at greatest risk for metastasis. Worldwide, survival parallels economic development as retinoblastoma survival is approximately 30% in Africa, 60% in Asia, 80% in Latin American, and 95–97% in Europe and North America.3
Management of a child with retinoblastoma involves a balance of patient life with globe salvage and ultimate visual potential.4, 5 Management of retinoblastoma is a practiced art that involves tumour recognition and differentiation from simulating conditions, decision-making regarding appropriate therapeutic approach, and meticulous follow-up for detection of tumour recurrence. There are numerous tools for management of retinoblastoma including enucleation, radiotherapy by teletherapy (external beam) or brachytherapy (plaque radiotherapy), chemotherapy using various delivery routes and chemotherapy protocols, and focal treatments with cryotherapy, transpupillary thermotherapy, and laser photocoagulation.4 Chemotherapy remains the most common method for conservative globe salvage and in this review, we will focus on chemotherapy alternatives.
Clinical features of retinoblastoma
The clinical features of retinoblastoma vary depending on the extent of tumour, often dependent on the degree of delay in diagnosis. In the United States, an evaluation of 1265 patients revealed that the most common presenting signs included leukocoria (56%), strabismus (24%), and poor vision (8%).6 Further study on a cohort of 1196 eyes found median age at presentation of 15 months with 51% male, 49% female and 53% unilateral, 47% bilateral.7 Zhao et al8 from China reviewed 470 eyes and found leukocoria (47%) to be most common. On the other extreme, presentations of retinoblastoma differ. From Sudan9, buphthalmos (56%) and leukocoria (32%) were most common, and from Mali in Africa10, proptosis (55%), leukocoria (38%), strabismus (6%), and buphthalmos (2%) were found. Efforts are underway internationally to educate clinicians, nurses, patients, and entire populations for improvement in retinoblastoma detection.11
Classification of retinoblastoma
There have been several classifications12 proposed for intraocular retinoblastoma, including the Reese Ellsworth Classification13, Essen Classification14, and Philadelphia Classification15. The most commonly used current classification is the International Classification of Retinoblastoma16, 17 designed in Paris in 2003 based predominantly on the presence and extent of subretinal and vitreous tumours seeds, similar to the Philadelphia Classification. The International Classification of Retinoblastoma is practical and specifically applicable for chemotherapy outcomes, as it has been found predictive of treatment success following intravenous chemotherapy (IVC) (Table 1).
Table 1. The International classification of retinoblastoma.
| Group | Philadelphia version | Los Angeles version |
|---|---|---|
| A | Rb ≤3 mm | Rb ≤3 mm, at least 3 mm from the foveola, and 1.5 mm from optic nerve. No seeding |
| B | Rb>3 mm or Macular location or Juxtapapillary location (<1.5 mm to disc) or SRF present | Eyes with no vitreous or subretinal seeding and retinal tumours of any size or location not included in group A. Small cuff of subretinal fluid ≤5 mm from tumour margin |
| C | Rb with SRS ≤3 mm from Rb or VS ≤3 mm from Rb | Eyes with focal vitreous or subretinal seeding and discrete tumour of any size or location. Seeding must be local, fine, and limited so as to be theoretically treatable with a radioactive plaque. Up to one quadrant subretinal fluid may be present. |
| D | Rb with SRS >3 mm from Rb or VS >3 mm from Rb | Eyes with diffuse vitreous or subretinal seeding and/or massive, nondiscrete endophytic or exophytic disease. Seeding more extensive than group C. Retinal detachment >1 quadrant |
| E | Rb with Size >50% of globe or Neovascular glaucoma or Opaque media or Invasion of optic nerve, choroid, sclera, orbit, anterior chamber | Massive Rb with anatomic or functional destruction of the eye with one or more of the following Neovascular glaucoma Massive intraocular haemorrhage Aseptic orbital cellulitis Tumour anterior to anterior vitreous face Tumour touching lens Diffuse infiltrating tumour Phthisis or pre-phthisis |
Abbreviations: Rb, retinoblastoma; SRF, subretinal fluid; SRS, subretinal seeds; VS, vitreous seeds.
Leading simulators of retinoblastoma
The diagnosis of retinoblastoma is based on characteristic clinical features of a yellow-white retinal mass often with surrounding subretinal fluid, subretinal seeding and vitreous seeding. Ancillary testing with fluorescein angiography, ultrasonography, computed tomography, or magnetic resonance imaging can confirm the diagnosis. Fine needle aspiration biopsy or open biopsy of retinoblastoma is not performed owing to risk for local tumour dissemination. The diagnosis is established based on clinical features alone. Despite classic manifestations, retinoblastoma can display a spectrum of unusual features that overlap with other conditions (pseudoretinoblastomas) and can lead to diagnostic confusion.18
Accurate clinical diagnosis is important to avoid mistreatment, particularly with chemotherapy. In our current series, we assessed 2775 eyes referred with possible retinoblastoma and confirmed retinoblastoma in 2171 (78%) eyes, and a simulating lesion (pseudoretinoblastoma) in 604 eyes (22%).18 Overall, the leading pseudoretinoblastoma diagnoses included Coats disease (40%), persistent fetal vasculature (26%), and vitreous haemorrhage (5%). The pseudoretinoblastomas differed based on age at presentation (Table 1). Of patients aged ≤1 year, persistent fetal vasculature (49%) was the most common pseudoretinoblastoma, whereas in children aged >2 years, Coats disease (60%) was most common (Table 2).
Table 2. Lesions simulating retinoblastoma (pseudoretinoblastoma) in 604 patients based on age at presentation.
| Pseudoretinoblastoma diagnosis |
Patient age
Number (% per diagnosis) [% per age group] |
|||||
|---|---|---|---|---|---|---|
| Mean, median [range] in years | 0–1 year n=283 | >1–2 years n=57 | >2–5 years n=89 | >5 years n=175 | All ages n=604 | |
| Coats disease | 6, 4 [0.2–30] | 58 (24) [20] | 33 (14) [59] | 54 (22) [50] | 99 (41) [58] | 244 (100) [40] |
| Persistent fetal vasculature (PFV) | 2, 1 [0.2–24] | 138 (87) [49] | 6 (4) [11] | 6 (4) [7] | 8 (5) [5] | 158 (100) [26] |
| Vitreous haemorrhage | 1, 1 [0.5–8] | 21 (78) [7] | 3 (11) [5] | 1 (4) [1] | 2 (7) [1] | 27 (100) [5] |
| Toxocariasis | 8, 8 [1–18] | 1 (5) [<1] | 0 (0) [0] | 7 (32) [8] | 14 (64) [8] | 22 (100) [4] |
| Familial exudative vitreoretinopathy (FEVR) | 7, 7 [0.6–16] | 5 (28) [2] | 1 (6) [2] | 1 (6) [1] | 11 (61) [6] | 18 (100) [3] |
| Rhegmatogenous retinal detachment | 5, 1 [0.5–24] | 10 (56) [4] | 0 (0) [0] | 3 (17) [3] | 5 (28) [3] | 18 (100) [3] |
| Coloboma | 3, 1 [0.3–11] | 9 (53) [3] | 1 (6) [2] | 3 (18) [3] | 4 (24) [2] | 17 (100) [3] |
| Astrocytic hamartoma | 8, 6 [0.5–28] | 3 (20) [1] | 1 (7) [2] | 3 (20) [3] | 8 (53) [5] | 15 (100) [2] |
| Combined hamartoma | 4, 2 [0.5–16] | 4 (27) [1] | 5 (33) [9] | 1 (7) [1] | 5 (33) [3] | 15 (100) [2] |
| Endogenous endophthalmitis | 5, 5 [0.2–11] | 2 (20) [<1] | 0 (0) [0] | 2 (20) [2] | 6 (60) [3] | 10 (100) [2] |
| Myelinated nerve fibres | 4, 4 [0.5–11] | 3 (33) [1] | 0 (0) [0] | 2 (22) [2] | 4 (44) [2] | 9 (100) [1] |
| Congenital cataract | 3, 1 [0.2–12] | 5 (63) [2] | 1 (13) [2] | 0 (0) [0] | 2 (25) [1] | 8 (100) [1] |
| Peripheral uveoretinitis | 3, 2 [0.5–6] | 3 (43) [1] | 1 (14) [2] | 0 (0) [0] | 3 (43) [2] | 7 (100) [1] |
| Retinopathy of prematurity | 2, 2 [0.8–7] | 3 (43) [1] | 2 (29) [4] | 1 (14) [1] | 1 (14) [<1] | 7 (100) [1] |
| Non-rhegmatogenous retinal detachment | 1, 1 [0.6–4] | 4 (80) [1] | 0 (0) [0] | 1 (20) [1] | 0 (0) [0] | 5 (100) [<1] |
| Medulloepithelioma | 4, 4 [2–5] | 0 (0) [0] | 1 (25) [2] | 3 (75) [3] | 0 (0) [0] | 4 (100) [<1] |
| X-linked retinoschisis | 2, 1 [0.6–7] | 3 (75) [1] | 0 (0) [0] | 0 (0) [0] | 1 (25) [<1] | 4 (100) [<1] |
| Vitreoretinal tuft | 3, 1 [0.6–8] | 2 (67) [<1] | 0 (0) [0] | 0 (0) [0] | 1 (33) [<1] | 3 (100) [<1] |
| Incontinentia pigmenti | 4, 4 [2–6] | 0 (0) [0] | 1 (50) [2] | 0 (0) [0] | 1 (50) [<1] | 2 (100) [<1] |
| Juvenile xanthogranuloma | 1, 1 [0.7–0.8] | 2 (100) [<1] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 2 (100) [<1] |
| Norrie's disease | 1, 1 [0.7–0.8] | 2 (100) [<1] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 2 (100) [<1] |
| Vasoproliferative tumour | 10, 10 [3–17] | 2 (100) [<1] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 2 (100) [<1] |
| Choroidal osteoma | 3 | 0 (0) [0] | 0 (0) [0] | 1 (100) [1] | 0 (0) [0] | 1 (100) [<1] |
| Morning glory disc anomaly | 1 | 1 (100) [<1] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 1 (100) [<1] |
| Retinal capillary hemangioma | 16 | 1 (100) [<1] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 1 (100) [<1] |
| Retrolental fibrosis | 2 | 0 (0) [0] | 1 (100) [2] | 0 (0) [0] | 0 (0) [0] | 1 (100) [<1] |
| Toxoplasmosis | 1 | 1 (100) [<1] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 1 (100) [<1] |
Management of retinoblastoma: general concepts
Management of retinoblastoma has evolved over four decades.19 In the 1970s, enucleation was important for improved life prognosis. Enucleation continues to remain critical for advanced retinoblastoma, particularly in Asia and Africa.3, 10, 11 In the 1980s, external beam radiotherapy (EBRT) was popular, but later-realized risks of radiation-related second cancers have lead to reduction in use of this modality. In the 1990s, systemic IVC was introduced with agents of vincristine, etoposide, and carboplatin (VEC).20, 21, 22, 23, 24, 25, 26 Currently, IVC remains prevalent worldwide for intraocular retinoblastoma control as well as prevention of systemic metastasis. In the 2000s, interest in intra-arterial chemotherapy (IAC) has been explored.27, 28, 29, 30
Chemotherapy strategies
Chemotherapy with various agents and duration, usually combined with consolidation with thermotherapy, cryotherapy, or plaque radiotherapy, has been used for two decades to manage retinoblastoma. In the early 1990s, Kingston et al20 from London recognized that a specific protocol of IVC, classically used for neuroblastoma, was particularly effective for retinoblastoma. If delivered prior to EBRT for advanced group V retinoblastoma, IVC increased tumour control with ocular salvage from 30 to 70%.2 Others observed similar results.21, 22, 23 These landmark observations commenced the IVC era, otherwise termed ‘chemoreduction', and this era strongly continues.
Intravenous chemotherapy
The IVC protocol is used in standard dose VEC based on patient weight (Tables 3 and 4) and escalated to higher dose if there is bilateral groups D and/or E.31 This chemotherapy, usually delivered with consolidation treatment, is used worldwide and is effective for intraocular retinoblastoma control as well as prevention of metastasis, pinealoblastoma, and second cancers.
Table 3. Various chemotherapy protocols for retinoblastoma.
| Chemotherapy drug | Dose | Schedule |
|---|---|---|
| Intravenous chemotherapy | ||
| Carboplatin (C) | 560 mg/M2 in 120 cc/M2 D51/4NS IVSS over 60 min | Day 0 of each cycle (18.6 mg/kg for patients <36 months of age) |
| Etoposide (E) | 150 mg/M2 in 150 cc/M2 D51/4NS IVSS over 60 min | Days 0 and 1 of each course (5 mg/kg for patients <36 months of age) |
| Vincristine (V) | 1.5 mg/M2 IVSS over 15 minutes | Day 0 of each cycle (0.05 mg/kg for patients <36 months of age). Maximum vincristine dose not to exceed 2 mg |
| Antiemetic drug | ||
| Ondansetron | 0.45 mg/kg IVSS (maximum dose 24 mg) prior to therapy | Days 0 and 1 of each cycle, with Dexamethasone 0.25 mg/kg IVSS prior to therapy days 0 and 1 of each cycle |
| Phenergen | 0.5 mg/kg p.o. h.s. on | Day 0 and then every 6 h prn with emesis |
| Diphenhydramine | 1 mg/kg p.o. h.s. | Day 0 and then every 6 h |
| Therapy continues every 4 weeks for a total of six cycles. Prior to institution of each subsequent cycle, the absolute neutrophil count must be >750 cells/μl and platelets must be >75 000 cells/μl | ||
| Intra-arterial chemotherapy | ||
| Melphalan | Slow pulsatile infusion over 30 min | |
| 0–2 years old | 3 mg/30 cc | |
| 2–5 years old | 5 mg/30 cc | |
| >5 years old | 7.5 mg/30 cc | |
| Carboplatin | 30 mg/30 cc | Slow pulsatile infusion over 30 min |
| Topotecan | Slow pulsatile infusion over 30 min | |
| 0–2 years old | 0.5 mg/30 cc | |
| >2 years old | 1.0 mg/30 cc | |
| Subtenon's chemotherapy | ||
| Carboplatin | 20 mg/2 cc | Inject into subtenon's space directly over sclera in area of tumour |
| Intravitreal chemotherapy | ||
| Melphalan | 8–30 μg/0.1 cc | Inject intravitreally through pars plana or clear corneal approach, cryotherapy to injection site, jiggle eye to mix chemotherapy. Deliver monthly |
| Methotrexate | 400-800 μg/0.1 cc | Inject intravitreally through pars plana or clear corneal approach, cryotherapy to injection site. Deliver twice weekly for 1 month, then weekly for 1 month, then monthly for 1 year |
Table 4. Indications for various chemotherapy methods for retinoblastoma.
| Feature | Intravenous chemotherapy | Intra-arterial chemotherapy | Periocular chemotherapy | Intravitreal chemotherapy |
|---|---|---|---|---|
| Primary therapy | ||||
| Bilateral retinoblastoma | +++ | + | + | ∼ |
| Unilateral retinoblastoma | ++ | +++ | + | ∼ |
| Secondary therapy for recurrent/persistent tumour | ||||
| Retinoblastoma | ++ | +++ | + | ∼ |
| Subretinal seeds | ++ | +++ | + | ∼ |
| Vitreous seeds | ++ | ++ | + | +++ |
+++ marked, ++ intermediate, + minimal, ∼ little to none.
Subtenon's chemotherapy as primary therapy is used in conjunction with intravenous chemotherapy for groups D and E.
If intravenous chemotherapy is used as primary and secondary therapy, the regimen is changed to different agents in secondary therapy.
Control of intraocular retinoblastoma
There have been several reports to provide evidence that three-agent IVC for 6–9 consecutive months is remarkably effective for retinoblastoma.20, 21, 22, 23, 26, 31 According to the International Classification of Retinoblastoma in 249 consecutive eyes, globe salvage was achieved in 100% of group A eyes, 93% of group B, 90% of group C, 47% of group D, and 25% of group E eyes26, 32 (Figure 1). Currently, group D eyes show improved control with additional subtenon's carboplatin (20 mg/2 cc) injection and group E eyes show improved control with the addition of low-dose radiotherapy leading to globe salvage in 83% of cases or IAC leading to globe salvage in 63%.26, 32, 33
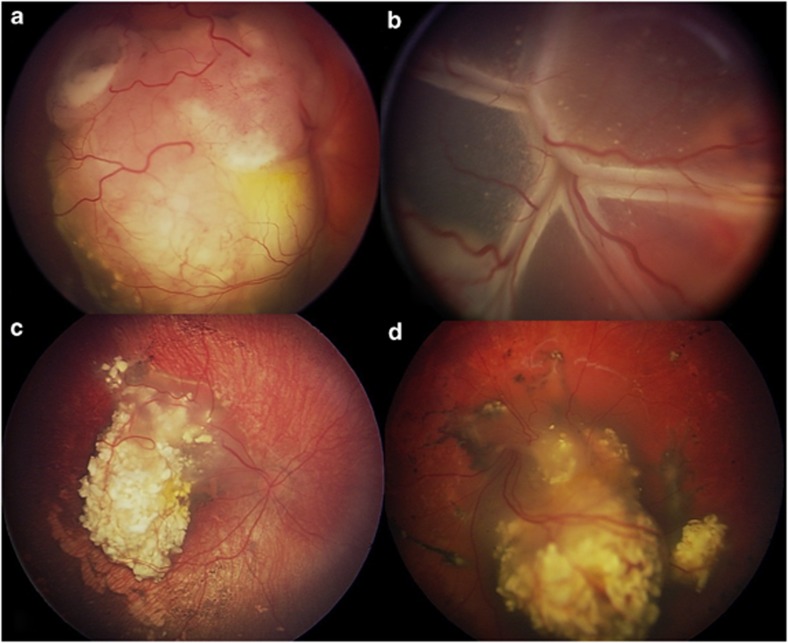
Figure 1.
IVC (chemoreduction) for retinoblastoma. (a, b) Before IVC showing viable retinoblastoma in the right (a) and left (b) eyes. (c, d) After IVC showing complete tumour regression in the right (c) and left (d) eyes.
Long-term systemic toxicity related to IVC is minimal. Transient pancytopenia and fever can be encountered, as with most systemic chemotherapy.34 Long-term hearing and renal toxicity are rare, particularly if medications are prescribed accurately.35, 36 Furthermore, despite inaccurate comments about fertility issues,37 there is no evidence that current VEC regimen causes infertility.36 Our experience in Philadelphia over nearly 20 years, has resulted in satisfactory tumour control with minimal toxicities and no fertility issues. Results from India indicate that VEC has improved survival in patients with retinoblastoma up to 95%, similar to the United States and Europe (Presentation by Honavar S at the 25th Jubilee Anniversary Meeting of LV Prasad Eye Institute, Hyderabad, India, 1 June 2012).
Worldwide, IVC with consolidation treatment continues to remain the primary conservative method for retinoblastoma management. This modality provides excellent intraocular tumour control, particularly for patients with germline mutation, with additional benefits of prevention of pinealoblastoma, reduction in second cancers, minimal systemic toxicities, and no ophthalmic toxicities.
Control of retinal detachment
Using IVC for retinoblastoma with total retinal detachment, complete resolution of subretinal fluid was documented in 76% eyes following therapy.5 The presence of retinal detachment does not preclude use of IVC but caution is advised to withhold consolidation therapy with thermotherapy until the subretinal fluid resolves.
Saving advanced group D or E eyes
Most children with advanced groups D or E retinoblastoma are best managed with enucleation because of the massive tumour, poor visual potential, and risk for metastatic disease. However, if globe salvage is considered, particularly if the opposite eye has been enucleated, then IVC is employed to control the intraocular malignancy as well as prevent systemic metastasis. The chemotherapy protocol for IVC is similar to that used for prevention of metastasis in high-risk retinoblastoma.38 Unfortunately, IVC alone might not completely control advanced retinoblastoma because group D eyes show 53% local intraocular recurrence and group E eyes show 75% local recurrence, necessitating EBRT, IAC, or enucleation.26, 32 In one analysis, globe salvage was achieved using IVC followed by IAC in 67% of group D eyes and 63% of group E eyes33 (Figures 2 and 3).
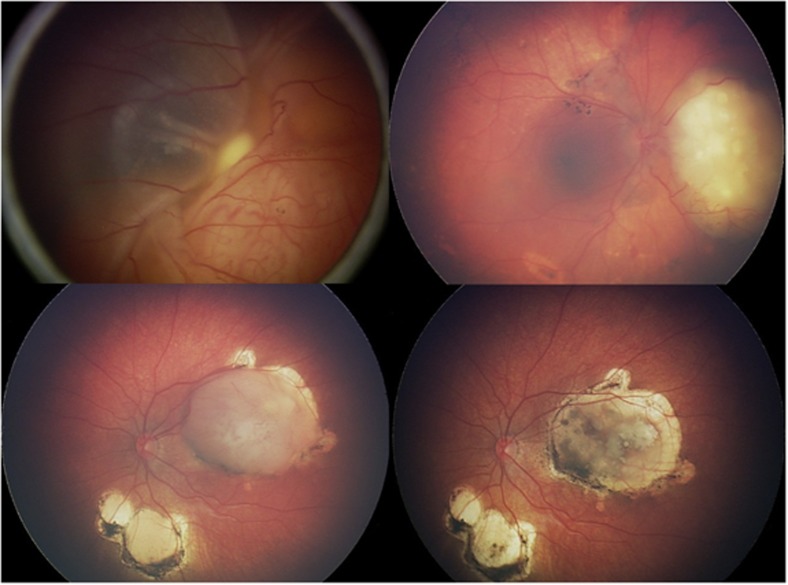
Figure 2.
IAC for retinoblastoma. (a, b) Primary IAC showing before (a) and after (b) three cycles of melphalan. (c, d) Secondary IAC following IVC but with recurrence of macular and inferior tumours (c) that responded completely (d) to three cycles of melphalan.
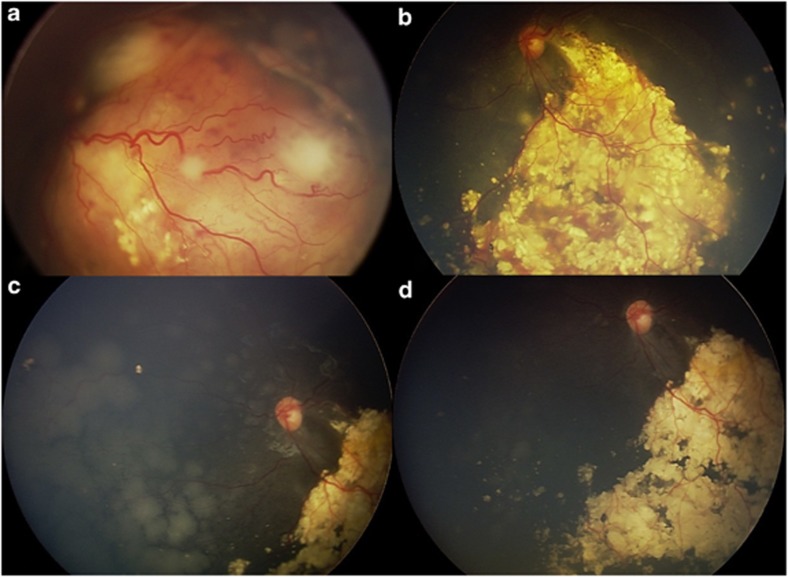
Figure 3.
Combination primary IVC plus secondary IAC for bilateral group D retinoblastoma. (a, b) Active retinoblastoma at first visit (a) that responded to primary IVC with tumour regression (b). (c, d) Recurrent subretinal seeds (c) necessitated further treatment with IAC (d) with ultimate seed control.
Preservation of visual acuity
Demirci et al39 studied long-term visual acuity outcome in eyes treated with IVC. If treatment was successful and enucleation and/or EBRT was avoided, mean 5-year visual outcome was 20/20–20/40 in 50% of patients and 20/200 or better in 67%. The main factor predictive of poor vision was foveal involvement with initial tumour or subretinal fluid. There was no local toxicity of IVC on the eye or visual outcome. Narang et al40 found visual acuity of 20/200 in 71% eyes and 20/40 or better in 37% at 6 years following IVC.
Prevention of pinealoblastoma
In 2000, Shields et al41 noted that the incidence of fatal pinealoblastoma had dropped dramatically in children who received IVC, likely related to the neoadjuvant use of chemotherapy in providing tumour control. Others believed that this finding might be related to the avoidance of EBRT.42 Analysis of 100 consecutive children with germline mutation retinoblastoma on IVC disclosed no case of pinealoblastoma, despite the fact that it was estimated that 8–10% should have manifested pinealoblastoma.41 We continue to observe an extremely low rate of pinealoblastoma in children on IVC.
Prevention of systemic metastasis in high-risk retinoblastoma
Patients at greatest danger for metastasis from retinoblastoma are those that display high-risk retinoblastoma, defined histopathologically as retinoblastoma with tumour invasion into the optic nerve, uvea, or a combination of both.38, 43, 44, 45 High-risk retinoblastoma leads to metastasis in 24% of patients if not treated with systemic chemotherapy compared with 4% of those that receive IVC.44
The International Classification of Retinoblastoma can predict those eyes with high-risk retinoblastoma. It is presumed that groups A, B, and C rarely show high-risk features, but they rarely come to enucleation for histopathological inspection. However, eyes with group D retinoblastoma show high-risk features in 15–17% and group E in 24–50% cases.46, 47 It appears that patients with high-risk retinoblastoma should receive systemic IVC for prevention of metastatic disease as well as for control of the intraocular tumour. Kaliki et al38 found that the standard IVC protocol using VEC resulted in complete tumour control in all (100%) high-risk cases with no evident metastasis.
Reduction in long-term second cancers
One concern with the current IVC protocol was the induction of secondary leukaemia from etoposide, usually within 5 years after exposure. Gombos et al48 identified several cases worldwide, but high and prolonged dosing could have been a factor in those cases. In our extensive experience with nearly 500 patients treated with IVC, there has been no case of leukaemia to develop in any child treated with chemotherapy alone. Furthermore, evidence from the Surveillance, Epidemiology, and End Results database confirmed the lack of secondary leukaemia in this population.49
Children with germline mutation retinoblastoma are at risk for long-term second malignant neoplasms. Turaka et al49 recently reported that fewer-than-expected second cancers were found in children treated with IVC using standard six-cycle chemotherapy. In that report, only 4% of children with germline mutation retinoblastoma treated with IVC as front-line therapy developed second cancers at mean 11-year follow-up and no patient with non-germline mutation showed second cancer.49 Based on those observations, the authors concluded that IVC is efficacious for life and vision preservation in children with retinoblastoma, without additional risk for second cancer.
Intra-arterial chemotherapy
IAC is an exciting new option for management of eyes with retinoblastoma, particularly unilateral cases. The method of IAC has been described by Kaneko et al27 and later refined by Gobin et al50 and Abramson et al.28 Shields and Shields19 published a four-decade perspective of retinoblastoma therapy on various treatments that have been popular and later abandoned, issuing caution with new therapies until proper assessment of limitations are realized. In a 4-year perspective, Gobin et al50 found IAC safe and effective for treatment of retinoblastoma with successful catheterization in 98% of procedures with ocular survival at 2 years in 82% if IAC was primary treatment and 58% if secondary treatment. Shields et al30 found primary therapy with IAC successful in 100% of group C, 100% of group D and 33% of group E eyes (Figure 2). Muen et al51 reported secondary IAC for eyes that failed previous systemic IVC or local therapy and found 80% control (Figure 3). Shields et al52 found ‘minimal exposure IAC', using only one or two doses of IAC remarkably effective for select group C and less-advanced group D eyes. Eyes with retinal detachment from retinoblastoma show complete resolution of detachment following IAC if the detachment is partial and complete reattachment in most of those with total detachment.5
There are few systemic complications of IAC, including haematoma at groin entry site and transient pancytopenia from bone marrow suppression. Brain complications have been rarely encountered with carotid vascular spasm, stroke, and magnetic resonance imaging displaying focal perfusion defects. Local ocular toxicities of IAC relate mostly to vascular compromise of the ophthalmic artery, retinal artery, or choroidal vessels51, 53, 54, 55 (Table 5). Muen et al51 found that 80% of eyes treated with IAC showed ocular side effects of cranial nerve palsy (40%), orbit/eyelid oedema (20%), retinal detachment (7%), vitreous haemorrhage (27%), and retinal pigment epithelial changes (47%). The retinal pigment epithelial changes could be related to previous retinal detachment or choroidal vascular compromise from chemotherapy toxicity. Both Munier et al53 and Shields et al54 observed this finding. Ocular vascular compromise likely leads to poor visual outcome, but long-term assessment of visual acuity in eyes treated with IAC has not yet been analysed. New approaches with delivery of chemotherapy into the ostium of the ophthalmic artery, to avoid wedging of blood flow and intimal trauma has been advised. Additionally, greater facility with technique and short time of surgery can reduce vascular events. Fortunately, in our series, there was no incident of stroke, metastasis, or death.
Table 5. Outcomes of various chemotherapy methods for retinoblastoma.
| Feature | Intravenous chemotherapy | Intra-arterial chemotherapy | Periocular chemotherapy | Intravitreal chemotherapy |
|---|---|---|---|---|
| Tumour control of | ||||
| Retinoblastoma | +++ | +++ | + | ∼ |
| Subretinal seeds | +++ | +++ | ++ | ∼ |
| Vitreous seeds | ++ | ++ | + | +++ |
| Resolution of retinal detachment | +++ | +++ | ∼ | ∼ |
| Prevention of pinealoblastoma | +++ | ∼ | ∼ | ∼ |
| Reduction in long-term second cancers | ++ | ∼ | ∼ | ∼ |
| Complications ocular | ||||
| Ptosis | ∼ | ++ | + | ∼ |
| Eyelid oedema | ∼ | + | ++ | ∼ |
| Forehead redness | ∼ | + | ∼ | ∼ |
| Dysmotility | ∼ | + | + | ∼ |
| Ophthalmic artery obstruction | ∼ | + | ∼ | ∼ |
| Retinal artery obstruction | ∼ | + | ∼ | ∼ |
| Choroidal vascular attenuation | ∼ | ++ | ∼ | ∼ |
| Vitreous haemorrhage | ∼ | + | ∼ | ∼ |
| Retinal vasculitis | ∼ | + | ∼ | ∼ |
| Optic neuropathy | ∼ | + | ∼ | + |
| Phthisis | ∼ | ++ | + | + |
| ∼ | + | ∼ | ++ | |
| Complications brain | ||||
| Carotid spasm | ∼ | + | ∼ | ∼ |
| Stroke | ∼ | + | ∼ | ∼ |
| Brain haemorrhage | ∼ | ∼ | ∼ | ∼ |
| Brain vascular perfusion defects | ∼ | + | ∼ | ∼ |
| Complications systemic | ||||
| Transient pancytopenia | ++ | + | ∼ | ∼ |
| Ototoxicity | + | ∼ | ∼ | ∼ |
| Renal toxicity | + | ∼ | ∼ | ∼ |
| Leukaemia | + | + | ∼ | ∼ |
+++ marked, ++ intermediate, + minimal, ∼ little to none.
Studies of the effects of IAC in animals has been pursued by Wilson et al.56, 57 They showed in a monkey model with real-time imaging that IAC with melphalan caused whitening of the retinal vessels at optic disc, choroidal blanching, retinal arterial narrowing, and retinal oedema in all cases at the time of injection. They additionally showed in vitro that IAC with melphalan could be toxic to the vascular endothelium, leading to factors that might cause endothelial changes and fibrosis.
Periocular chemotherapy
Periocular injection of carboplatin has been used for retinoblastoma control over two decades, often as an adjunct to systemic chemotherapy. Periocular chemotherapy achieves rapid levels within the vitreous in 30 min, achieves doses that are 6–10 times that achieved by intravenous route, and can last for hours.58, 59 The route of delivery has varied as either subconjunctival or subtenon's space location. The method of injection as a plain liquid injection or injection with a vehicle, such as a Lincoff balloon, iontophoresis, long-acting fibrin sealant, or nanoparticles, has been explored.58, 59, 60, 61 Initial reports suggested some success with subtenon's carboplatin as primary therapy.62 Because of later recurrences, however, this therapy was used more often in conjunction with systemic chemotherapy to boost the local dose of chemotherapy in the vitreous.
Complications of periocular chemotherapy include orbital and eyelid oedema and ecchymosis, orbital fat atrophy, muscle fibrosis leading to strabismus, and optic atrophy.63 Long-term observations on complications have not been published.
Intravitreal chemotherapy
Intravitreal chemotherapy for retinoblastoma was initially explored in the 1960s using thiotepa.64 Ocular toxicity was established in rabbit eyes.64 Inomata and Kaneko65 found melphalan to be the most effective chemotherapeutic agent against retinoblastoma based on in vitro testing of 12 agents and a dose of 4 μg/ml achieved complete tumour suppression. In their rabbit model, a concentration of 5.9 μg/ml showed no retinal toxicity and this correlates to human vitreous doses of 20–30 μg, depending on globe size.66 Kaneko66 performed intravitreal injection of 8–30 μg melphalan combined with ocular hyperthermia for vitreous tumour seeding in 41 eyes and unpublished results revealed eye-preservation rate of nearly 51% (Presentation at the International Society of Ocular Oncology, Buenos Aires, Argentina on 16 November 2011). Munier et al67 studied 23 patients with heavily treated retinoblastoma with recurrent vitreous seeds, treated with 20–30 μg melphalan on a weekly basis and noted 83% success with avoidance of enucleation and/or EBRT at 15 months. Kivela et al68 found success with intravitreal methotrexate, but noted numerous injections into the eye of a child over a 1-year period.
Ghassemi and Shields69 evaluated 12 eyes treated with intravitreal melphalan for recurrent vitreous seeds following previous therapies of IVC and IAC. Eyes treated with low-dose melphalan (8–10 μg) showed less control and minimal side effects, whereas those treated with higher doses (30–50 μg) showed excellent control, but the 50-μg dose was toxic with persistent hypotonia and phthisis bulbi. There was no extraocular tumour seeding.
The role of intravitreal chemotherapy is yet to be defined, but it could be important as second-line therapy for recurrent vitreous seeding. In addition, it might be considered with systemic IVC during first-line therapy if vitreous seeding persists while on IVC.
Conclusion
In summary, before embarking on chemotherapy for retinoblastoma, the diagnosis should be unequivocally confirmed by clinical examination and testing. There are several chemotherapy approaches to retinoblastoma. In general, most children with bilateral retinoblastoma receive systemic IVC for ocular tumour control and prevention of metastasis, pinealoblastoma, and long-term second cancers. For unilateral retinoblastoma, IAC provides excellent control with minimal systemic effect. Periocular chemotherapy is used in conjunction with IVC to enhance dose at the eye in advanced cases. Intravitreal chemotherapy is currently reserved for those eyes with recurrent vitreous seeds following incomplete control with other methods.
Acknowledgments
This work was supported by the Lucille Wiedman Fund for Pediatric Eye Cancer, Philadelphia, PA (JAS, CLS), Lift for a Cure, Morrisdale, PA (CLS), and the Carlos G. Bianciotto Retinoblastoma Research Fund c/o the Eye Tumor Research Foundation, Philadelphia, PA (CLS, JAS).
The authors declare no conflict of interest.
Footnotes
This work was presented at the Cambridge Ophthalmological Symposium on Cancer of the Eye, 14 September 2012 at St. John's College, Cambridge, England by CL Shields.
References
- Ramasubramanian A, Shields CL.Epidemiology and magnitude of the problemIn: Ramasubramanian A, Shields CL, (eds). Retinoblastoma Jaypee Brothers Medical Publishers: New Delhi, India; 201210–15. [Google Scholar]
- Shields JA, Shields CL.RetinoblastomaIn: Shields JA, Shields CL, (eds). Intraocular Tumors. An Atlas and Textbook2nd ed.Lippincott Williams Wilkins: Philadelphia, PA; 2008293–365. [Google Scholar]
- Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93:1129–1131. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- Shields CL, Shields JA. Retinoblastoma management: advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy. Curr Opin Ophthalmol. 2010;21:203–212. doi: 10.1097/ICU.0b013e328338676a. [DOI] [PubMed] [Google Scholar]
- Shields CL, Kaliki S, Rojanaporn D, Al-Dahmash S, Bianciotto CG, Shields JA. Intravenous and intra-arterial chemotherapy for retinoblastoma: What have we learned. Curr Opin Ophthalmol. 2012;23:202–209. doi: 10.1097/ICU.0b013e3283524130. [DOI] [PubMed] [Google Scholar]
- Abramson DH, Frank CM, Susman M, Whalen MP, Dunkel IJ, Boyd NW. Presenting signs of retinoblastoma. J Pediatr. 1998;132:505–508. doi: 10.1016/s0022-3476(98)70028-9. [DOI] [PubMed] [Google Scholar]
- Epstein J, Shields CL, Shields JA. Trends in the management of retinoblastoma; Evaluation of 1196 consecutive eyes during 1974–2001. J Pediatr Ophthalmol Strabismus. 2003;40:196–203. doi: 10.3928/0191-3913-20030701-05. [DOI] [PubMed] [Google Scholar]
- Zhao J, Li S, Shi J, Wang N. Clinical presentation and group classification of newly diagnosed intraocular retinoblastoma in China. Br J Ophthalmol. 2011;95:1072–1076. doi: 10.1136/bjo.2010.191130. [DOI] [PubMed] [Google Scholar]
- Ali AA, Elsheikh SM, Elhaj A, Osman N, Abuidris D, Eltayeb EA, et al. Clinical presentation and outcome of retinoblastoma among children treated at the National Cancer Institute (NCI) in Gezira, Sudan: a single institution experience. Ophthalmic Genet. 2011;32:122–125. doi: 10.3109/13816810.2010.546822. [DOI] [PubMed] [Google Scholar]
- Boubacar T, Fatou S, Fousseyni T, Mariam S, Fatourmata DT, Toumeni S, et al. A 30-month prospective study on the treatment of retinoblastoma in the Gabriel Toure Teaching Hospital, Bamako, Mali. Br J Ophthalmol. 2010;94:467–469. doi: 10.1136/bjo.2009.159699. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Galindo C, Wilson MW, Chantada G, Fu L, Qaddoumi I, Antoneli C, et al. Retinoblastoma: one world, one vision. Pediatrics. 2008;122:e763–e770. doi: 10.1542/peds.2008-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubramanian A, Shields CL.Staging and treatment strategiesIn: Ramasubramanian A, Shields CL, (eds). Retinoblastoma Jaypee Brothers Medical Publishers: New Delhi, India; 201270–78. [Google Scholar]
- Reese AB, Ellsworth RM. The evaluation and current concept of retinoblastoma therapy. Trans Am Acad Ophthalmol Otolaryngol. 1963;67:164–172. [PubMed] [Google Scholar]
- Hopping W.The new Essen prognosis classification for conservative sight-saving treatment of retinoblastomaIn: Lommatzsch PK, Blodi FC, (eds). Intraocular Tumors International Symposium Under the Auspices of the European Ophthalmological Society Springer-Verlag: Berlin, Germany; 1983497–505. [Google Scholar]
- Shields CL, Mashayekhi A, Demirci H, Meadows AT, Shields JA. Practical approach to management of retinoblastoma. Arch Ophthalmol. 2004;122:729–735. doi: 10.1001/archopht.122.5.729. [DOI] [PubMed] [Google Scholar]
- Murphree AL. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18:41–53. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Shields CL, Shields JA. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol. 2006;17:228–234. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- Shields CL, Schoenfeld E, Kocher K, Shukla S, Kaliki S, Shields JA.Lesions simulating retinoblastoma (pseudoretinoblastoma) in 604 cases Ophthalmology 2012(in press). [DOI] [PubMed]
- Shields CL, Shields JA. Intra-arterial chemotherapy for retinoblastoma: the beginning of a long journey. Editorial. Clin Exp Ophthalmol. 2010;38:638–643. doi: 10.1111/j.1442-9071.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1339–1343. doi: 10.1001/archopht.1996.01100140539004. [DOI] [PubMed] [Google Scholar]
- Gallie BL, Budning A, DeBoer G, Thiessen JJ, Koren G, Verjee Z, et al. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch Ophthalmol. 1996;114:1321–1328. doi: 10.1001/archopht.1996.01100140521001. [DOI] [PubMed] [Google Scholar]
- Murphree AL, Villablanca JG, Deegan WF, Sato JK, Malogolowkin M, Fisher A, et al. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1348–1356. doi: 10.1001/archopht.1996.01100140548005. [DOI] [PubMed] [Google Scholar]
- Shields CL, DePotter P, Himelstein BP, Shields JA, Meadows AT, Maris JM. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1330–1338. doi: 10.1001/archopht.1996.01100140530002. [DOI] [PubMed] [Google Scholar]
- Shields CL, Honavar SG, Shields JA, Demirci H, Meadows AT, Naduvilath TJ. Factors predictive of recurrence of retinal tumor, vitreous seeds and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol. 2002;120:460–464. [PubMed] [Google Scholar]
- Gombos DS, Kelly A, Coen PG, Kingston JE, Hungerford JL. Retinoblastoma treated with primary chemotherapy alone: The significance of tumor size, location and age. Br J Ophthalmol. 2002;86:80–83. doi: 10.1136/bjo.86.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–2280. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol. 2004;9:69–73. doi: 10.1007/s10147-004-0392-6. [DOI] [PubMed] [Google Scholar]
- Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115:1398–1404. doi: 10.1016/j.ophtha.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Vajzovic LM, Murray TG, Aziz-Sultan MA, Schefler AC, Wolfe SQ, Hess D, et al. Supraselective intra-arterial chemotherapy: evaluation of treatment-related complications in advanced retinoblastoma. Clin Ophthalmol. 2011;5:171–176. doi: 10.2147/OPTH.S12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CL, Bianciotto CG, Ramasubramanian A, Lally SE, Jabbour P, Griffin GC, et al. Intra-arterial chemotherapy for retinoblastoma. Report #1: control of tumor, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129:1399–1406. doi: 10.1001/archophthalmol.2011.150. [DOI] [PubMed] [Google Scholar]
- Leahey AM.Systemic chemotherapy: a pediatric oncology perspectiveIn: Ramasubramanian A, Shields CL, (eds). Retinoblastoma Jaypee Brothers Medical Publishers: New Delhi, India; 201281–85. [Google Scholar]
- Shields CL, Ramasubramanian A, Thangappan A, Hartzell K, Leahey A, Meadows AT, et al. Chemoreduction (CRD) for group E retinoblastoma. Comparison of CRD alone versus CRD plus low dose external radiotherapy in 76 eyes. Ophthalmology. 2009;117:544–551. doi: 10.1016/j.ophtha.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Shields CL, Kaliki S, Al-Dahmash S, Rojanaporn D, Leahey A, Griffin G, et al. Management of advanced retinoblastoma (group D or E) with primary intravenous chemotherapy followed by secondary intra-arterial chemotherapy as an alternative to enucleation 2012(in press). [DOI] [PubMed]
- Friedman DL, Himelstein B, Shields CL, Shields JA, Miller D, Needle M, et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol. 2000;18:12–17. doi: 10.1200/JCO.2000.18.1.12. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Shields CL, Meadows AT. A retrospective review of hearing in children with retinoblastoma treated with carboplatin-based chemotherapy. Pediatr Blood Cancer. 2008;50:223–226. doi: 10.1002/pbc.21155. [DOI] [PubMed] [Google Scholar]
- Leahey A. A cautionary tale: dosing chemotherapy in infants with retinoblastoma. J Clin Oncol. 2012;30:1023–1024. doi: 10.1200/JCO.2011.39.4254. [DOI] [PubMed] [Google Scholar]
- Friedrich MJ. Retinoblastoma therapy delivers power of chemotherapy with surgical precision. JAMA. 2011;305:2276–2278. doi: 10.1001/jama.2011.778. [DOI] [PubMed] [Google Scholar]
- Kaliki S, Shields CL, Shah SU, Eagle RC, Shields JA, Leahey A. Postenucleation adjuvant chemotherapy with vincristine, etoposide and carboplatin for the treatment of high-risk retinoblastoma. Arch Ophthalmol. 2011;129:1422–1427. doi: 10.1001/archophthalmol.2011.289. [DOI] [PubMed] [Google Scholar]
- Demirci H, Shields CL, Meadows AT, Shields JA. Long-term visual outcome following chemoreduction for retinoblastoma. Arch Ophthalmol. 2005;123:1525–1530. doi: 10.1001/archopht.123.11.1525. [DOI] [PubMed] [Google Scholar]
- Narang S, Mashayekhi A, Rudich D, Shields CL.Predictors of long-term visual outcome after chemoreduction for management of intraocular retinoblastoma Clin Experiment Ophthalmol 2012. e-pub ahead of print 2 February 2012; doi: 10.1111/j.1442 9071.2012.02757.x [DOI] [PubMed]
- Shields CL, Meadows AT, Shields JA, Carvalho C, Smith A. Chemoreduction for retinoblastoma may prevent intracranial neuroblastic malignancy (trilateral retinoblastoma) Arch Ophthalmol. 2001;119:1269–1272. doi: 10.1001/archopht.119.9.1269. [DOI] [PubMed] [Google Scholar]
- Moll AC, Imhof SM, Schouten-Van Meeteren AY, Boers M, van Leeuwen F, Hofman P. Chemoreduction for retinoblastoma. Arch Ophthalmol. 2003;121:1513. doi: 10.1001/archopht.121.10.1513. [DOI] [PubMed] [Google Scholar]
- Usitalo MS, Van Quill KR, Scott IU, Matthay KK, Murray TG, O'Brien JM. Evaluation of chemoprophylaxis in patients with unilateral retinoblastoma with high-risk features on histopathologic examination. Arch Ophthalmol. 2001;119:41–48. [PubMed] [Google Scholar]
- Honavar SG, Singh AD, Shields CL, Meadows AM, Demirci H, Cater J, et al. Post-enucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120:923–931. doi: 10.1001/archopht.120.7.923. [DOI] [PubMed] [Google Scholar]
- Eagle RC. High-risk features and tumor differentiation in retinoblastoma: a retrospective histopathologic study. Arch Pathol Lab Med. 2009;133:1203–1209. doi: 10.5858/133.8.1203. [DOI] [PubMed] [Google Scholar]
- Wilson MW, Qaddoumi I, Billups C, Haik BG, Rodriguez-Gallindo C. A clinicopathological correlation of 67 eyes primarily enucleated for advanced intraocular retinoblastoma. Br J Ophthalmol. 2011;95:553–558. doi: 10.1136/bjo.2009.177444. [DOI] [PubMed] [Google Scholar]
- Kaliki S, Shields CL, Rojanaporn D, Al-Dahmash S, McLaughlin J, Shields JA, et al. International classification of retinoblastoma predicts high-risk retinoblastoma: Analysis of 519 enucleated eyes Ophthalmology 2012(in press). [DOI] [PubMed]
- Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma. Is chemotherapy a factor. Ophthalmology. 2007;114:1378–1383. doi: 10.1016/j.ophtha.2007.03.074. [DOI] [PubMed] [Google Scholar]
- Turaka K, Shields CL, Leahey A, Meadows AT. Second malignant neoplasms following chemoreduction for retinoblastoma in 272 patients. Pediatr Blood Cancer. 2012;59:121–125. doi: 10.1002/pbc.23278. [DOI] [PubMed] [Google Scholar]
- Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129:732–737. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- Muen WJ, Kingston JE, Robertson F, Brew S, Sagoo MS, Reddy MA. Efficacy and complications of super-selective intra-ophthalmic artery melphalan for the treatmen of refractory retinoblastoma. Ophthalmology. 2012;119:611–616. doi: 10.1016/j.ophtha.2011.08.045. [DOI] [PubMed] [Google Scholar]
- Shields CL, Kaliki S, Shah SU, Bianciotto CG, Liu D, Jabour P, et al. Minimal exposure (1 or 2 cycles) of intra-arterial chemotherapy in the management of retinoblastoma. Ophthalmology. 2012;119:188–192. doi: 10.1016/j.ophtha.2011.06.036. [DOI] [PubMed] [Google Scholar]
- Munier FL, Beck-Popovic M, Balmer A, Gaillard MC, Bovey E, Binaghi S. Occurrence of sectoral choroidal occlusive vasculopathy and retinal arteriolar embolization after superselective ophthalmic artery chemotherapy for advanced intraocular retinoblastoma. Retina. 2011;31:566–573. doi: 10.1097/IAE.0b013e318203c101. [DOI] [PubMed] [Google Scholar]
- Shields CL, Bianciotto CG, Jabbour P, Griffin GC, Ramasubramanian A, Rosenwasser R, et al. Intra-arterial chemotherapy for retinoblastoma. Report #2: treatment complications. Arch Ophthalmol. 2011;129:1407–1415. doi: 10.1001/archophthalmol.2011.151. [DOI] [PubMed] [Google Scholar]
- Bianciotto CG, Shields CL, Iturralde JC, Sarici A, Jabbour P, Shields JA. Fluorescein angiographic findings after intra-arterial chemotherapy for retinoblastoma. Ophthalmology. 2012;119:843–849. doi: 10.1016/j.ophtha.2011.09.040. [DOI] [PubMed] [Google Scholar]
- Wilson MW, Jackson JS, Phillips BX, Buchanan J, Frase S, Wang F, et al. Real-time ophthalmoscopic findings of superselective intraophthalmic artery chemotherapy in a nonhuman primate model. Arch Ophthalmol. 2011;129:1458–1465. doi: 10.1001/archophthalmol.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MW, Haik BG, Dyer MA. Superselective intraophthalmic artery chemotherapy. What we do not know. Arch Ophthalmol. 2011;129:1490–1491. doi: 10.1001/archophthalmol.2011.361. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Abramson DH, Madden T, Tong W, Tran HT, Dunkel IJ. Intraocular concentrations of chemotherapy following systemic or local administration. Arch Ophthalmol. 1998;116:1209–1212. doi: 10.1001/archopht.116.9.1209. [DOI] [PubMed] [Google Scholar]
- Hayden BC, Jockovich ME, Murray TG, Voigt M, Milne P, Kralinger M, et al. Pharmacokinetics of systemic versus focal Carboplatin chemotherapy in the rabbit eye: possible implication in the treatment of retinoblastoma. Invest Ophthalmol Vis Sci. 2004;45:3644–3649. doi: 10.1167/iovs.04-0228. [DOI] [PubMed] [Google Scholar]
- Martin NE, Kim JW, Abramson DH. Fibrin sealant for retinoblastoma: where are we. J Ocul Pharmacol Ther. 2008;24:433–438. doi: 10.1089/jop.2007.0110. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Durairaj C, Kompella UB, O'Brien JM, Grossniklaus HE. Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch Ophthalmol. 2009;127:1043–1047. doi: 10.1001/archophthalmol.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson DH. Periocular chemotherapy for retinoblastoma: Success with problems. Arch Ophthalmol. 2005;123:128–129. doi: 10.1001/archopht.123.1.128-b. [DOI] [PubMed] [Google Scholar]
- Mulvihill A, Budning A, Jay V, Vandenhoven C, Heon E, Gallie BL, et al. Ocular motility changes after subtenon carboplatin chemotherapy for retinoblastoma. Arch Ophthalmol. 2003;121:1120–1124. doi: 10.1001/archopht.121.8.1120. [DOI] [PubMed] [Google Scholar]
- Ericson LA, Kalberg B, Rosengren BH. Trials of intravitreal injections of chemotherapeutic agents in rabbits. Acta Ophthalmol. 1964;42 (4:721–726. doi: 10.1111/j.1755-3768.1964.tb01723.x. [DOI] [PubMed] [Google Scholar]
- Inomata M, Kaneko A. Chemosensitivity profiles of primary and cultured retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res. 1987;78 (8:858–868. [PubMed] [Google Scholar]
- Ueda M, Tanabe J, Inomata M, Kaneko A, Kimura T. Study on conservative treatment of retinoblastoma—effect of intravitreal injection of melphalan on the rabbit retina. Nippon Ganka Gakkai Zasshi. 1995;99:1230–1235. [PubMed] [Google Scholar]
- Munier F, Gaillard MC, Balmer A, Soliman S, Podlisky G, Moulin AP, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96:1078–1083. doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- Kivelä T, Eskelin S, Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology. 2011;118:1689. doi: 10.1016/j.ophtha.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Ghassemi F, Shields CL.Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma Arch Ophthalmol 2012(in press). [DOI] [PubMed]