Abstract
Ocular lymphomas can be divided into intraocular lymphomas and ocular adnexal lymphomas. The vitreoretinal lymphoma—usually a diffuse large B-cell lymphoma (DLBCL) of high-grade malignancy—is the most common lymphoid malignancy arising in the eye, while the extranodal marginal zone B-cell lymphoma (EMZL), an indolent often recurrent tumour, occurs most frequently in the ocular adnexal tissue. The two lymphoma subtypes differ significantly in their clinical presentation, subsequent course and outcome as well as in their underlying morphological, immunophenotypical and genetic features. The molecular processes involved in DLBCL and EMZL development are complex, and include chromosomal translocations, mutations caused by aberrant somatic hypermutation, sporadic somatic mutations, and copy number alterations, characterized by deletions and amplifications. These lead to alterations in particular signalling pathways, which in turn activate transcription factors, such as nuclear factor NF-κB. This review provides an overview of the histological features of DLBCL and EMZL, and discusses the current insights into the molecular mechanisms underlying the development of these tumours, when they occur systemically and particularly when they arise in ocular tissues.
Keywords: ocular adnexal lymphoma, extranodal marginal zone lymphoma, diffuse large B-cell lymphoma, ABC DLBCL, GCB DLBCL, vitreoretinal lymphoma
Introduction
The lymphomas are malignant neoplasms derived from a monoclonal proliferation of B- or T-lymphocytes and less commonly natural killer (NK) cells. They can be divided into two major groups: Hodgkin's lymphoma (HL) and non-HL (NHL). The NHLs are a large heterogeneous group of neoplasms, which can be further subdivided into those arising from B-lymphocytes or their precursors (80%), T cells (14%) and from NK cells (6%).1 Both HL and NHL can occur in lymph nodes or in extranodal sites, although the latter feature is more common for NHL. The latest WHO Lymphoma classification is used for the subtyping of HL and NHL: it emphasizes an approach whereby the clinical characteristics are correlated with distinct morphological, immunophenotypical, and genotypical features of each neoplasm.1 For each lymphoma entity, a putative cell of origin is postulated.
Ocular lymphomas can be divided into intraocular lymphomas and ocular adnexal lymphomas. Intraocular lymphomas are rare with the most common type being the vitreoretinal lymphoma (VRL; previously called ‘primary intraocular lymphoma'), which is usually a diffuse large B-cell lymphoma (DLBCL) occurring in the vitreous and retina, and with frequent involvement of the central nervous system (CNS).2, 3, 4 Lymphomas of the ocular adnexa are relatively uncommon, accounting for ∼8% of all extranodal malignant lymphomas. Most are primary tumours and are usually NHL of B-cell type: the most common primary lymphoma subtype occurring in the ocular adnexa is the low-grade malignant extranodal marginal zone B-cell lymphoma (EMZL), accounting for approximately two thirds of all ocular adnexal lymphomas.5, 6, 7, 8, 9 Interestingly, the EMZL is also the most common primary lymphoma occurring in the choroid.3
In the following review, the molecular pathologies of only DLBCL and EMZL will be discussed. Concerning the other lymphoma subtypes occurring in the ocular adnexa and in the other intraocular tissues (eg the choroid), the reader is referred to recent reviews. Pivotal to the understanding of the pathogenesis of lymphomas, particularly B-NHL, is the germinal centre (GC), and the so-called ‘germinal centre reaction', which encompasses a number of physiological processes and will be discussed firstly below.
The GC reaction
The GC is a highly specialized microenvironment, where B cells undergo distinct genetic processes to generate high-affinity antibodies (Figures 1 and 2). GCs are formed by proliferating naive B cells in secondary lymphoid tissues—such as lymph nodes mucosa and tonsils—following T-cell-dependent antigen stimulation.
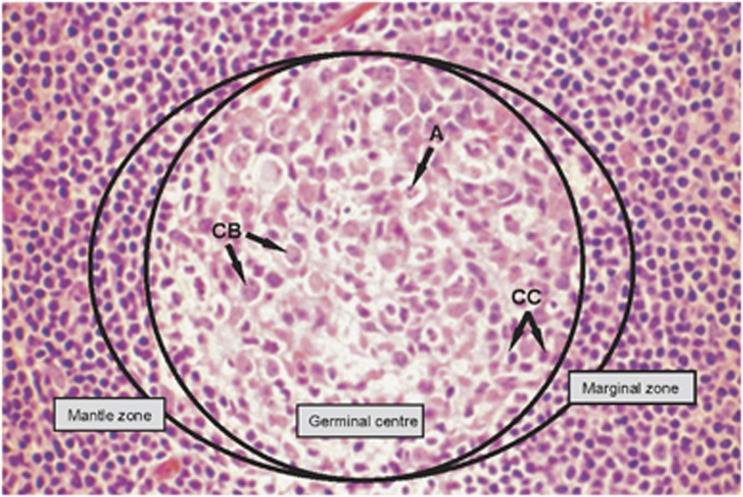
Figure 1.
Haematoxylin and eosin-stained section of a secondary lymphoid follicle, composed of a GC surrounded by a mantle zone and the marginal zone. The normal population of a reactive GC is composed of centroblasts (CB), centrocytes (CC), follicular dendritic cells, T cells and macrophages containing apoptotic bodies (a) within their cytoplasm. (Magnification, × 60 objective).
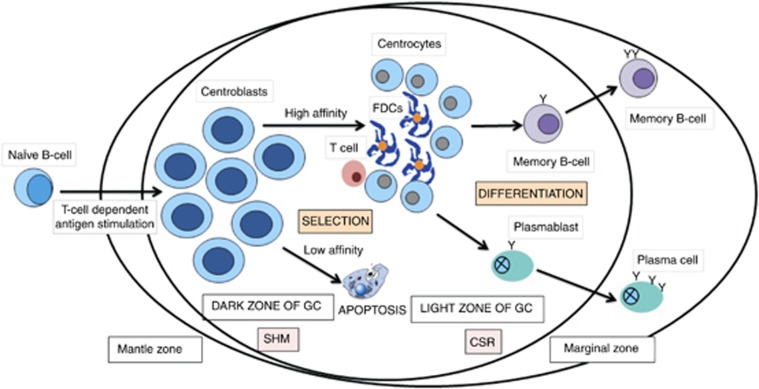
Figure 2.
The GC reaction. Following T-cell-dependent antigen stimulation, naive B cells migrate into secondary lymphoid tissue (eg, tonsils, nodes or mucosa), differentiate into centroblasts, and subsequently proliferate in the dark zone of the GCs. Within the dark zone, the centroblasts undergo SHM, which introduces mostly single base-pair changes into the Ig variable region of the heavy- and light-chain locus, with the aim of increasing their affinity for the antigen. Centroblasts then move to the light zone, where they differentiate into centrocytes and undergo class-switch recombination (CSR). T cells and follicular dendritic cells (FDCs) help to re-challenge the centrocytes with the antigen. Those B cells with a low-affinity Ig-receptor are eliminated by apoptosis (approximately 90% of the B-cell population!), whereas a small subset of centrocytes with high-affinity to the antigen are selected to differentiate further into either memory B cells or plasma cells. The GC reaction is associated with a change in the immunophenotype of the B cells: for example, CBs express BCL6 in the GC; on exiting the GC, this expression is downregulated and the cells are immunoreactive for IRF4 (also called MUM1). Therefore in the physiological state, a B-cell does not normally co-express BCL6 and IRF4/MUM1: this only occurs in the aberrant (ie malignant) state.
These structures can be schematically divided into two morphologically well-recognizable areas, known as the ‘dark' zone and the ‘light' zones (Figure 2). The dark zone of the GC consists of rapidly proliferating centroblasts, characterized by a doubling time of < 12 h. Here, the B cells entering the GC undergo somatic hypermutation (SHM) of the variable region of the immunoglobulin (Ig) gene (IgV).10 SHM produces not only single-nucleotide substitutions, but also deletions and duplications in the IgV heavy- and light-chain genes, resulting in the production of antibodies with high affinity for the antigen.10, 11, 12 SHM can also target a number of non-Ig genes in normal B cells, for example, the 5′-untranslated region of B-cell lymphoma 6 (BCL6).13, 14, 15 SHM occurs via DNA strand breaks and requires activation-induced cytidine deaminase (AID), which initiates the process by converting deoxycytidines to uracils, which are then further processed by DNA repair enzymes.16, 17, 18
The initiation and maintenance of the GC is dependent on BCL6, a transcriptional repressor belonging to the BTB/POZ/zinc finger family of transcription factors. The gene BCL6 is pivotal in the GC reaction, as evidenced by the observation that mice lacking BCL6 cannot form GCs or produce high-affinity antibodies.19, 20 BCL6 is highly expressed in centroblasts, where it directly binds to and represses more than 1200 genes. This was recently identified through an integrated biochemical, functional, and bioinformatics approach, demonstrating that BCL6 target genes are involved in a variety of signalling pathways that are important for the GC reaction, including: (i) DNA-damage response, (ii) apoptosis, (iii) plasma cell differentiation, (iv) B-cell receptor (BCR) signalling, (v) CD40 signalling, (vi) tumour necrosis factor (TNF)-signalling, (vii) interferon signalling, (viii) Toll-like receptor signalling, (ix) WNT signalling, and (x) T-cell-mediated activation.21, 22, 23, 24, 25, 26, 27, 28 All these data indicate that BCL6 is essential for the continuous rapid proliferation of centroblasts, while allowing GC B cells to undergo DNA modifications without inducing an unwanted DNA-damage response. Furthermore, BCL6 inhibits the expression of several transcription factors that are essential for plasma cell differentiation.21, 24, 25, 29, 30
It is thought that centroblasts subsequently progress into the ‘light' zone of the GC (Figure 2), where they differentiate into centrocytes with decrease and ultimate cessation of proliferation. Here they are re-challenged by antigen to allow for the selection for B cells that produce high-affinity antibodies, whereas cells with a low-affinity Ig receptor are eliminated by apoptosis.31 Approximately 90% of the B-cell population is thought to be eliminated by apoptosis at this stage. Furthermore, centrocytes undergo class-switch recombination (CSR), an intrachromosomal DNA recombination event that confers distinct effector functions to the antibodies by changing their Ig class from IgD and IgM to IgG, IgA, or IgE (Figure 2).32 CSR occurs via non-homologous end joining and requires AID.33, 34
Another critical process that is initiated in the light zone of the GCs is the differentiation of B cells with a high-affinity Ig receptor into effector plasma cells or memory B cells. The downregulation of BCL6 is essential to allow for this terminal stage of B-cell differentiation and is accomplished in these cells through at least two distinct mechanisms (ie, activation of CD40 and stimulation of the BCR). CD40 activation via CD40 ligand, expressed on CD4+ T-cells, leads to nuclear factor (NF)-κB-mediated activation of interferon regulatory factor 4 (IRF4; also termed MUM1), and subsequent transcriptional silencing of BCL6.35, 36 The stimulation of the BCR promotes mitogen-activated protein kinase-mediated phosphorylation of BCL6, followed by its ubiquitination and subsequent proteasomal degradation.12, 31, 37 Downregulation of BCL6, in turn, restores DNA-damage responses, arrests proliferation, and allows for the expression of a transcription factor required for plasma cell differentiation, termed ‘positive regulatory domain–containing 1' (PRDM1/BLIMP1).25, 29
This brief and schematic description only partially reflects the complex dynamics of the GC reaction, but is nonetheless useful in the understanding of B-NHL pathogenesis. All B-cell NHLs—with the exception of mantle cell and lymphoblastic lymphoma—derive from either GC cells or B cells that have passed through the GC, as indicated by the fact that these lymphomas carry hypermutated IgV genes.38 The genetic alterations reported in NHLs include chromosomal translocations, mutations caused by aberrant SHM, sporadic somatic mutations, and copy number alterations, denoted by deletions and amplifications. Chromosomal translocations in NHLs represent reciprocal and balanced recombination events, frequently but not exclusively involving the Ig locus, with the breakpoint located either in the switch region or in the target region of SHM.39, 40 With few exceptions, NHL-associated translocations do not lead to gene fusions, but cause dysregulated expression of the target gene. Given its critical function in both CSR and SHM, AID has been suggested to significantly contribute to B-cell lymphomagenesis by facilitating the occurrence of chromosomal translocations and aberrant SHM in the pathological state.
Diffuse large B-cell lymphoma
DLBCL is the most common form of adult NHL, accounting for 30–40% of cases.41 DLBCL is remarkably diverse in both clinical presentation and outcome, likely reflecting both its pathogenetic and biological heterogeneity. DLBCL is the most frequent lymphoma subtype arising in the eye as VRL (see below),3 and the second most common lymphoma subtype occurring in the ocular adnexa.6, 42, 43
Molecular genetic subtyping of DLBCL
Over the past decade, the use of genome-wide expression profiling (GEP) has not only allowed a better understanding of the molecular mechanisms underlying the development of this disease, but also revealed a number of features associated with an unfavourable clinical outcome.44, 45, 46, 47, 48, 49 According to similarities to the putative cell of origin, peripheral DLBCL can be divided into at least three different groups: (i) GC B-cell-like (GCB) DLBCL, which derives from centroblasts, (ii) activated B-cell-like (ABC) DLBCL, which resembles features of plasmablastic B cells committed to terminal B-cell differentiation, and (iii) primary mediastinal large B-cell lymphoma, presumably arising from thymic B cells.44, 47, 50, 51 However, 15–30% of DLBCL cannot be classified into any of the above subgroups.50, 52 The cell-of-origin–based classification has prognostic value because ABC−DLBCL have a poorer overall survival compared with GCB−DLBCL and respond less effectively to current therapeutic regimens, with cure rates of around 40%.46, 53 The genetic alterations associated with the three different types of DLBCL are summarized in Table 1.
Table 1. Genetic lesions associated with different subtypes of DLBCL.
| Gene | Frequency | Gene function/mechanism of transformation |
|---|---|---|
| GCB-DLBCL subtype | ||
| BCL2 translocations | 30–40% | Ectopic BCL2 expression; enhanced resistance to apoptosis |
| MYC translocations | 10% | Enhanced proliferation and growth; DNA replication |
| EZH2 mutations | 22% | H3K27 methyltransferase/epigenetic reprogramming |
| BCL6 mutations in BSE1 | 20% | Enhanced proliferation; impaired DNA-damage responses, block in differentiation |
| MEF2B mutations | 8% | Unclear |
| ABC-DLBCL subtype | ||
| BCL2 amplification | 30% | Enhanced resistance to apoptosis |
| PRDM1 (BLIMP1) mutations/deletions | 25% | Block in terminal B-cell differentiation |
| MYD88 mutations | 29% | Constitutive activation of NF-κB and JAK-STAT signalling |
| TNFAIP3 (A20) mutations/deletions | 20% | Constitutive activation of NF-κB signalling owing to loss of negative regulation |
| CD79A, CD79B mutations | 20% | Constitutive activation of NF-κB and BCR signalling |
| CARD11 mutations | 9% | Constitutive activation of NF-κB signalling |
| PMBCL | ||
| REL amplification | 75% | Constitutive activation of NF-κB signalling |
| JAK2 amplification | 63% | Activation of JAK-STAT pathway |
| JMJD2C amplification | 63% | Histone modification/epigenetic reprogramming |
| PDL1, PDL2 amplifications | 63% | T-cell exhaustion; reduced tumour cell immunogenicity |
| SOCS1 mutations/deletions | 45% | Enhanced JAK2 signalling owing to impaired JAK2 degradation |
| STAT6 mutation | 36% | Possible activation of JAK-STAT pathway |
| CIITA translocations | 38% | Reduced tumour cell immunogenicity; downregulation of HLA class II |
| Shared lesions | ||
| MLL2 mutations | 32% | H3K4 methyltransferase/epigenetic reprogramming |
| CREBBP/EP300 mutations/deletions | 22–40% | Epigenetic reprogramming; impaired p53 activation and BCL6 inactivation |
| BCL6 translocations | 25–40% | Enhanced proliferation; impaired DNA-damage responses, block in differentiation |
| B2M mutations/deletions | 29% | Reduced tumour cell immunogenicity; downregulation of HLA class I |
| CD58 mutations/deletions | 21% | Reduced tumour cell immunogenicity |
Abbreviations: ABC, activated B-cell-like; BSE1, BCL6-binding sites in exon 1; DLBCL, diffuse large B-cell lymphoma; GCB, germinal centre B-cell-like; PMBCL, primary mediastinal B-cell lymphoma.
Surrogate immunohistochemical markers have been demonstrated to aid discrimination between the individual DLBCL genetic subgroups, and several of these algorithms—CD10, BCL6, IRF4/MUM1, B-cell lymphoma 2 (BCL2), and cyclin D2—have been demonstrated to be predictive of survival.51, 54 The ‘Hans classifier' composed of CD10, IRF4/MUM1, and BCL6 protein expression can divide DLBCL in GCB−DLBCL and non-GCB−DLBCL with about 80% concordance with the GEP.51 A combination of five immunohistochemical markers—GCTE1, CD10, BCL6, IRF4/MUM1, and FOXP1—can achieve about 90% concordance with the GEP.55 In addition to the difference in cell of origin, these subgroups are associated with diverse genetic alterations (see below), suggesting that they depend on distinct oncogenic programs.
A separate classification scheme using gene-set enrichment analyses identified three phenotypic subsets characterized by the expression of genes involved in oxidative phosphorylation, BCR signalling, and host inflammatory response.48 Tumours in the latter subset exhibit increased expression of macrophage/dendritic cell markers, T/NK cell receptor and activation pathway components, as well as complement cascade members, and inflammatory mediators, suggesting an increased inflammatory response.48 In host inflammatory response tumours and increased number of infiltrating T cells and dendritic cells was observed. Despite the increased immune response, these tumours do not have a favourable clinical outcome.48
Vitreoretinal lymphoma
As mentioned above, VRL is a high-grade malignancy, which is often associated with cerebral disease. The clinical features and treatment of VRL will not be discussed here: the reader is referred to the accompanying manuscript of Dr Janet Davis in this edition of Eye, as well as to other recent reviews.2, 56, 57
Histologically, VRL can be subtyped in most cases as DLBCL,3 according to the latest WHO lymphoma classification.1 Rare subtypes include T-cell-rich B-cell lymphoma58 and T-cell lymphoma.59, 60, 61, 62 VRL are characterized by a subretinal or perivascular retinal infiltration of pleomorphic medium-to-large sized cells with minimal basophilic cytoplasm, indented or folded nuclei, and prominent, often multiple, nucleoli. These changes can be quite discreet (Figure 3). Occasionally, atypical mitotic figures can be seen. Necrosis and apoptosis, with background scavenging macrophages, are frequent characteristics of these tumours, making the diagnosis of lymphoma difficult on vitreous biopsies.63
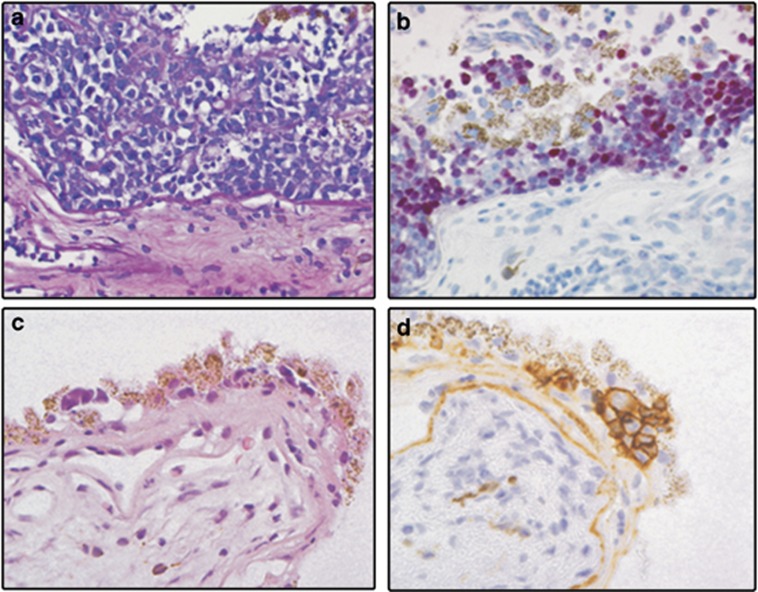
Figure 3.
(a) Haematoxylin and eosin-stained section of a chorioretinal biopsy, which has been infiltrated by medium-to-large sized atypical lymphoid blasts (objective × 40). (b) On immunohistochemistry these cells are immunoreactive for B-cell antigens (not shown), BCL6 (not shown) and for IRF4 (strong nuclear staining). (c) Chorioretinal biopsy with small groups of cells underneath and disrupting the retinal pigment epithelium (Haematoxylin and eosin, objective × 40). (d) Clear membranous positivity of the malignant lymphocytes for the B-cell antigen CD20.
Immunohistochemically, VRL are characterized by the following expression profile: CD79a+, CD20+, PAX-5+, BCL2+, IRF4/MUM1+, OCT2+, BOB.1+, BCL6+/−, CD10−/+, Pu.1−/+ (Figure 3), and are usually monotypical for IgM.64 The co-expression of BCL6 and IRF4/MUM1 by CD20+ B-blasts in a vitreous or chorioretinal biopsy is aberrant and sufficient to suggest that the B cells are malignant (Figure 3). Staining with Ki-67 shows that the tumour cell growth fraction is usually very high (ie, about 90%). Clonality assessment can also be performed on DNA extracted from VRL cells using PCR directed against the Ig heavy and light chains in suspected B-cell lymphomas, and against the T-cell receptor in T-cell lymphomas.65
With regard to molecular genetic alterations in VRL, it has been demonstrated that the tumour cells carry an intermediate to large number of hypermutated IgV genes with no evidence of antigen selection or significant intraclonal heterogeneity.65, 66 Interestingly, a similar high mutation IgV frequency has been reported in primary CNS lymphoma.67, 68 The findings of a large somatic mutation load in the IgV genes of VRL, together with the tumour cell immunophenotype (IRF4/MUM1+, BCL6+/−, CD10+), suggest that it is a DLBCL of ABC type.
To date, the only reported chromosomal translocation in VRL is t(14;18), which involves the BCL2 gene, with rearrangements being reported in up to 67% of cases.69 The presence of this mutation and consequent overexpression of the BCL2 protein in some VRL could, therefore, suggest that some VRL are DLBCL of GCB type. Further studies, particularly GEP investigations, are needed to determine genetic subtypes and putative cell(s) of origin of VRL. If there are indeed differing molecular genetic subtypes of VRL, this may explain the variable clinical courses of VRL patients. However, GEP data from primary CNS lymphoma suggest that the centrally-located DLBCL may not be so easily classified into the molecular subgroups described for the peripheral DLBCL:70 considering the similarities between VRL and primary CNS lymphoma, this may also apply to VRL. The GEP classification of DLBCL according to other genes, for example, those involved in host inflammatory response,48 may also be relevant for VRL when taking into account the immune privileged status of the eye.
Extranodal marginal zone B-cell lymphoma
EMZL are low-grade B-cell lymphomas, and is the most common subtype of NHL occurring in the ocular adnexa, and interestingly when occurring as a primary tumour in the choroid.3, 5, 6, 7, 8, 9 EMZL were first described in the stomach by Isaacson and Wright in 1984.71 They are often termed as mucosa-associated lymphoid tissue (‘MALT') lymphomas when involving an overlying epithelium—for example, the gastric mucosa, conjunctiva or acini of the lacrimal gland. This term is not appropriate, however, for those lesions located deep in the orbit, where no epithelium is present; in such lesions, the overarching ‘EMZL' should be applied.
EMZL typically arise in conditions of chronic antigenic stimulation, as evidenced by the association of Helicobacter pylori, Campylobacter jejuni, Borrelia burgdorferi, and hepatitis C virus with EMZLs that arise in the stomach, small intestine, skin, and spleen, respectively.72 Of note, when EMZL are diagnosed at an early stage, removal of the antigenic stimulus may result in complete regression on the lymphoproliferation. The significance of C. psittaci with respect to the EMZL of the ocular adnexa remains unclear: there appears to be substantial geographic variation in its association.73 A relationship between autoantigens, present in autoimmune diseases, and ocular adnexal EMZL seems likely as evidenced by somatic mutation analyses of these tumours, which suggest an antigen selection process.74, 75, 76
Regardless of the site of origin, EMZL have similar clinical, morphological, and immunohistochemical features.72 With respect to molecular genetic characteristics, however, recent evidence does suggest that the frequency of particular molecular alterations of EMZL vary according to site of origin (Table 2): this is particularly the case with the EMZL occurring in the ocular adnexa (see in detail below).77
Table 2. Chromosomal aberrations of EMZL at different extranodal sites.
| Location | Chromosomal alteration | Frequency (%) |
|---|---|---|
| Ocular adnexa | t(11;18)(q21;q21) | 10 |
| t(14;18)(q32;q21) | 19 | |
| t(3;14)(p14.1;q32) | 14 | |
| A20 inactivation (6q23 deletion) | 20 | |
| Trisomy 3, 18 | ? | |
| 5q (ODZ2) and 9p (JMJD2C) | ? | |
| Stomach | t(11;18)(q21;q21) | 22–24 |
| t(1;14)(p22;q32) | 3 | |
| Trisomy 3, 7, 12, 18 | ||
| Skin | t(14;18)(q32;q21) | 14 |
| t(3;14)(p14.1;q32 | 10 | |
| Trisomy 3, 18 | ||
| 5q (ODZ2) | ||
| Intestine | t(11;18)(q21;q21) | 13 |
| t(1;14)(p22;q32) | 10 | |
| Trisomy 3, 12, 18 | ||
| Lung | t(14;18)(q32;q21) | 38–53 |
| t(1;14)(p22;q32) | 11 | |
| Trisomy 3, 12, 18 | 7 | |
| Salivary gland | t(11;18)(q21;q21) | 1 |
| t(14;18)(q32;q21) | 5 | |
| A20 inactivation | ? | |
| Trisomy 3, 7, 18 | ||
| Thyroid gland | t(3;14)(p14.1;q32) | 50 |
| A20 inactivation | ? | |
| Trisomy 3, 12 | ||
| Breast | Trisomy 3, 18 | Rare |
Morphologically, ocular adnexal EMZL are characterized by an expansion of the marginal zone, which may or may not surround residual reactive GCs. They composed of morphologically heterogeneous small B cells, including centrocyte-like cells, monocytoid cells, small lymphocytes and scattered immunoblasts. Consistent with the indolent nature of these tumours, there are usually few mitoses, with an increased mitotic count only being seen in EMZL that may be transforming to high-grade tumours. The tumour cells may extend into the overlying conjunctival epithelium, creating ‘lymphoepithelial lesions' (Figure 4).
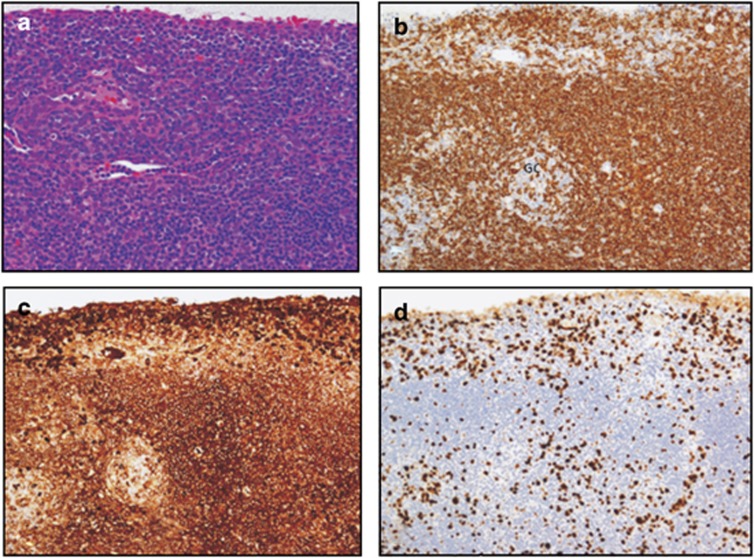
Figure 4.
(a) Haematoxylin and eosin-stained section of a conjunctival biopsy with a dense infiltrate of small lymphocytes with some degree of plasmacellular differentiation, occasional scattered blasts and a few mitotic figures (objective × 20). (b) Immunohistochemical stain for the B-cell antigen CD20, demonstrating a dominance of the B-lymphocytic population. Note that the proliferation of the neoplastic cells is occurring mainly in the marginal zone surrounding a residual reactive GC (objective × 20). (c) The neoplastic B cells show a monotypical expression of the Ig heavy chain IgM (objective × 20). (d) The Ki-67 immunostaining demonstrates a tumour cell growth fraction of ∼15% (objective × 20).
The immunoprofile of EMZL is as follows: CD79a+, CD20+, BCL2+, CD43+/− as well as monotypical expression of an Ig heavy and/or light chains (usually IgM and IgK), depending on the degree of plasmacellular differentiation of the tumour cells.(Figure 4) The neoplastic B cells are usually negative for CD5, CD23, CD10, BCL6, and cyclin D1; therefore, excluding the diagnoses of chronic lymphocytic leukaemia, mantle cell lymphoma and follicular lymphoma. Immunohistochemistry with Ki-67 confirms that the tumour cell growth fraction is usually low (about 5–15%) (Figure 4).
The NK-κB activation pathway and the molecular alterations in ocular adnexal EMZL
A number of recurrent chromosomal aberrations have been reported in EMZL from distinct anatomical locations (Table 2). These abnormalities include five mutually exclusive chromosomal translocations, somatic deletion and/or mutation of A20 (also called TNFα-inducible protein 3 [TNFAIP3]), as well as trisomies of chromosomes 3, 7, 12 and 18 (Table 2). Despite the chromosomal aberrations involving different genes, it is apparent that most changes affect or target genes encoding for regulators of NK-κB.
Therefore, before continuing it is important to mention NF-κB and its activation pathways. Briefly, NF-κB is a master transcription factor critical for a number of biological processes involved in both innate and adaptive immunity. It mediates lymphocyte development, activation and survival for regulated immune responses. It has become clear that aberrant deregulated NF-κB activation is a hallmark of several lymphoid malignancies, particularly EMZL, and is directly linked to advanced disease.77 NF-κB activation occurs through a number of cell surface receptors via either the ‘canonical' or the ‘alternative' pathway. Signalling from antigen receptors such as BCR, T-cell receptor, toll-like receptor, interleukin-1 receptor, TNF-α receptor or lymphocyte co-receptors such as CD30 and CD40, or receptor activator of NF-κB, leads to activation of the canonical NF-κB pathway. In contrast, signalling from the B-cell-activating factor receptor, lymphotoxin-β receptor and CD40 activates the alternative NF-κB pathway, which is particularly important in mature B cells. For further details of these pathways, which are beyond the scope of this article, the reader is referred to recent reviews.77
Most EMZL are genetically characterized by several recurrent, but mutually exclusive, chromosome translocations. Translocations that are consistently seen include t(11;18) (q21;q21); t(1;14)(p22;q32) and variant t(1;2)(p22;p12); t(14;18)(q32;q21); and t(3;14)(p14.1;q32). The frequency of these cytogenetic alterations varies considerably at different sites (Table 2). The oncogenic products of these translocations—API2-MALT1, BCL10-IGH, IGH-MALT1 and FOXP1-IGH, respectively—have been demonstrated to activate the above-mentioned canonical NF-B pathway.77 BCL10 and MALT1 are critical components linking the antigen receptor signalling to the canonical NF-κB activation pathway. Expression of BCL10, MALT1 or API2-MALT1 both in vitro and in vivo causes NK-κB activation.77
The above translocations occur frequently in EMZL of the stomach and lung, but rarely in those of the ocular adnexa, salivary glands and thyroid.77 By genomic profiling of so-called ‘translocation negative' ocular adnexal EMZL, it was demonstrated that the A20 gene,—an essential global NK-κB inhibitor—, was found to be inactivated either by somatic deletion and/or mutation in ocular adnexal EMZL.78, 79 The A20 deletion is most commonly heterozygous, and is mutually exclusive from the above-described MALT1 and IGH translocations. Further, it was shown that the A20 mutation/deletion is significantly associated with an increased expression of NK-κB target genes. These findings appear to be of clinical relevance: complete A20 inactivation is associated with poor lymphoma-free survival, and the patients with A20 mutation/deletion required significantly higher radiation dosages than those without the A20 abnormalities to achieve complete remission.80 Validation of these findings is presently underway in a large and separate cohort of EMZL collated between several centres belonging to the European Ophthalmological Oncology Group (www.oog.eu.com).
Conclusion
In summary, the molecular pathology of lymphomas is complex. The genetic alterations reported in most NHLs include chromosomal translocations, mutations caused by aberrant SHM, sporadic somatic mutations, and copy number alterations, characterized by deletions and amplifications. It is likely that alterations in AID,—whose role is critical in regulating both SHM and CSR in the physiological state within the GC—contribute to the development of lymphomas, particularly DLBCL. In EMZL development, chronic stimulation and ultimately dysregulation of the NF-κB pathway via chromosomal translocations, somatic deletion and/or mutations have central roles. It appears, however, that these alterations are necessary but not sufficient for malignant transformation, and thus they need to cooperate with other factors (eg, such as cell surface and chemokine receptors and factors involved with immune and inflammatory response) in the lymphomagenesis of EMZL. Although great advances have been made recently in the understanding the pathogenesis of peripheral DLBCL and indeed in ocular adnexal EMZL, further efforts are required to comprehend the histiogenesis of VRL, and translate this knowledge to the ‘bedside' in the form of better therapeutic agents to improve the prognosis of these patients. This can only be achieved through international multicentre collaborative studies, currently in progress.
The authors declare no conflict of interest.
References
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues4th ed.IARC Press: Lyon, France; 2008 [Google Scholar]
- Chan CC. Primary intraocular lymphoma: clinical features, diagnosis, and treatment. Clin Lymphoma. 2003;4 (1:30–31. doi: 10.1016/s1526-9655(11)70005-7. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Damato B. Understanding intraocular lymphomas. Clin Experiment Ophthalmol. 2008;36 (6:564–578. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- Davis JL. Diagnosis of intraocular lymphoma. Ocul Immunol Inflamm. 2004;12 (1:7–16. doi: 10.1076/ocii.12.1.7.28072. [DOI] [PubMed] [Google Scholar]
- White WL, Ferry JA, Harris NL, Grove AS. Ocular adnexal lymphoma. A clinicopathologic study with identification of lymphomas of mucosa-associated lymphoid tissue type. Ophthalmology. 1995;102 (12:1994–2006. doi: 10.1016/s0161-6420(95)30764-6. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Krause L, Delecluse HJ, Anagnostopoulos I, Foss HD, Hummel M, et al. Lymphoproliferative lesions of the ocular adnexa. Analysis of 112 cases. Ophthalmology. 1998;105 (8:1430–1441. doi: 10.1016/S0161-6420(98)98024-1. [DOI] [PubMed] [Google Scholar]
- Cho EY, Han JJ, Ree HJ, Ko YH, Kang YK, Ahn HS, et al. Clinicopathologic analysis of ocular adnexal lymphomas: extranodal marginal zone b-cell lymphoma constitutes the vast majority of ocular lymphomas among Koreans and affects younger patients. Am J Hematol. 2003;73 (2:87–96. doi: 10.1002/ajh.10332. [DOI] [PubMed] [Google Scholar]
- Ferry JA, Fung CY, Zukerberg L, Lucarelli MJ, Hasserjian RP, Preffer FI, et al. Lymphoma of the ocular adnexa: A study of 353 cases. Am J Surg Pathol. 2007;31 (2:170–184. doi: 10.1097/01.pas.0000213350.49767.46. [DOI] [PubMed] [Google Scholar]
- Stefanovic A, Lossos IS. Extranodal marginal zone lymphoma of the ocular adnexa. Blood. 2009;114 (3:501–510. doi: 10.1182/blood-2008-12-195453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354 (6352:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67 (6:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381 (6585:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Shen HM, Peters A, Baron B, Zhu X, Storb U. Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science. 1998;280 (5370:1750–1752. doi: 10.1126/science.280.5370.1750. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Migliazza A, Fracchiolla N, William C, Neri A, Baldini L, et al. BCL-6 mutations in normal germinal center B cells: evidence of somatic hypermutation acting outside Ig loci. Proc Natl Acad Sci USA. 1998;95 (20:11816–11821. doi: 10.1073/pnas.95.20.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliazza A, Martinotti S, Chen W, Fusco C, Ye BH, Knowles DM, et al. Frequent somatic hypermutation of the 5' noncoding region of the BCL6 gene in B-cell lymphoma. Proc Natl Acad Sci USA. 1995;92 (26:12520–12524. doi: 10.1073/pnas.92.26.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418 (6893:99–103. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci USA. 2003;100 (7:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt FW, et al. deamination by the AID antibody diversification enzyme. Nature. 2003;422 (6933:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997;16 (2:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- Ye BH, Rao PH, Chaganti RS, Dalla-Favera R. Cloning of bcl-6, the locus involved in chromosome translocations affecting band 3q27 in B-cell lymphoma. Cancer Res. 1993;53 (12:2732–2735. [PubMed] [Google Scholar]
- Basso K, Saito M, Sumazin P, Margolin AA, Wang K, Lim WK, et al. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood. 2010;115 (5:975–984. doi: 10.1182/blood-2009-06-227017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432 (7017:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- Saito M, Novak U, Piovan E, Basso K, Sumazin P, Schneider C, et al. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2009;106 (27:11294–11299. doi: 10.1073/pnas.0903854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13 (2:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004;173 (2:1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- Phan RT, Saito M, Basso K, Niu H, Dalla-Favera R. BCL6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat Immunol. 2005;6 (10:1054–1060. doi: 10.1038/ni1245. [DOI] [PubMed] [Google Scholar]
- Ci W, Polo JM, Cerchietti L, Shaknovich R, Wang L, Yang SN, et al. The BCL6 transcriptional program features repression of multiple oncogenes in primary B cells and is deregulated in DLBCL. Blood. 2009;113 (22:5536–5548. doi: 10.1182/blood-2008-12-193037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Cattoretti G, Dalla-Favera R. BCL6 controls the expression of the B7-1/CD80 costimulatory receptor in germinal center B cells. J Exp Med. 2003;198 (2:211–221. doi: 10.1084/jem.20021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Mack DH, Davis MM. Blimp-1,a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77 (2:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7 (7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8 (1:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Arpin C, de Bouteiller O, Guret C, Banchereau J, Martinez-Valdez H, et al. Sequential triggering of apoptosis, somatic mutation and isotype switch during germinal center development. Semin Immunol. 1996;8 (3:169–177. doi: 10.1006/smim.1996.0021. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102 (5:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102 (5:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Allman D, Jain A, Dent A, Maile RR, Selvaggi T, Kehry MR, et al. BCL-6 expression during B-cell activation. Blood. 1996;87 (12:5257–5268. [PubMed] [Google Scholar]
- Saito M, Gao J, Basso K, Kitagawa Y, Smith PM, Bhagat G, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12 (3:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Niu H, Ye BH, Dalla-Favera R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 1998;12 (13:1953–1961. doi: 10.1101/gad.12.13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341 (20:1520–1529. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- Kuppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20 (40:5580–5594. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- Lenz G, Nagel I, Siebert R, Roschke AV, Sanger W, Wright GW, et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J Exp Med. 2007;204 (3:633–643. doi: 10.1084/jem.20062041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2012;117 (19:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen PK, Ralfkiaer E, Prause JU, Sjö LD, Toft PB, Siersma VD, et al. Diffuse large B-cell lymphoma of the ocular adnexal region: A nation-based study Acta Ophthalmole-pub ahead of print 2 May 2012; doi: 10.1111/j.1755-3768.2011.02337.x [DOI] [PubMed]
- Stacy RC, Jakobiec FA, Herwig MC, Schoenfield L, Singh A, Grossniklaus HE. Diffuse large B-cell lymphoma of the orbit: clinicopathologic, immunohistochemical, and prognostic features of 20 cases. Am J Ophthalmol. 2012;154 (1:87–98. doi: 10.1016/j.ajo.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Alizadeh AA, Gentles AJ, Lossos IS, Levy R. Molecular outcome prediction in diffuse large-B-cell lymphoma. N Engl J Med. 2009;360 (26:2794–2795. doi: 10.1056/NEJMc0902616. [DOI] [PubMed] [Google Scholar]
- Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8 (1:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346 (25:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- Savage KJ, Monti S, Kutok JL, Cattoretti G, Neuberg D, De Leval L, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102 (12:3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105 (5:1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198 (6:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403 (6769:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;1103 (1:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2003;100 (17:9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359 (22:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, McClintock S, Cleveland RP, Trzpuc T, Vesole DH, Logan B, et al. Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. Am J Surg Pathol. 2004;28 (4:464–470. doi: 10.1097/00000478-200404000-00005. [DOI] [PubMed] [Google Scholar]
- Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15 (17:5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Rubenstein JL, Coupland SE, Davis JL, Harbour JW, Johnston PB, et al. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2010;16 (11:1589–1599. doi: 10.1634/theoncologist.2011-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic A, Davis J, Murray T, Markoe A, Lossos IS. Treatment of isolated primary intraocular lymphoma with high-dose methotrexate-based chemotherapy and binocular radiation therapy: a single-institution experience. Br J Haematol. 2010;151 (1:103–106. doi: 10.1111/j.1365-2141.2010.08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings TJ, Stenzel TT, Klintworth G, Jaffe GJ. Primary intraocular T-cell-rich large B-cell lymphoma. Arch Pathol Lab Med. 2005;129 (8:1050–1053. doi: 10.5858/2005-129-1050-PITLBL. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Anastassiou G, Bornfeld N, Hummel M, Stein H. Primary intraocular lymphoma of T-cell type: report of a case and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2005;243 (3:189–197. doi: 10.1007/s00417-004-0890-2. [DOI] [PubMed] [Google Scholar]
- Goldey SH, Stern GA, Oblon DJ, Mendenhall NP, Smith LJ, Duque RE. Immunophenotypic characterization of an unusual T-cell lymphoma presenting as anterior uveitis. A clinicopathologic case report. Arch Ophthalmol. 1989;107 (9:1349–1353. doi: 10.1001/archopht.1989.01070020419047. [DOI] [PubMed] [Google Scholar]
- Lobo A, Larkin G, Clark BJ, Towler HM, Lightman S. Pseudo-hypopyon as the presenting feature in B-cell and T-cell intraocular lymphoma. Clin Experiment Ophthalmol. 2003;31 (2:155–158. doi: 10.1046/j.1442-9071.2003.00624.x. [DOI] [PubMed] [Google Scholar]
- Saenz AD, Amador A, Ruiz BM, Davis J, Ruiz P. Cytofluorographic and molecular identification of a CD8-positive, TCR-alpha/beta-negative intraocular T cell lymphoma: a case report and review of the literature. J Med Case Rep. 2007;1:114. doi: 10.1186/1752-1947-1-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland SE. Vitreous biopsy: specimen preparation and interpretation. Monogr Clin Cytol. 2012;21:61–71. doi: 10.1159/000331037. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Loddenkemper C, Smith JR, Braziel RM, Charlotte F, Anagnostopoulos I, et al. Expression of immunoglobulin transcription factors in primary intraocular lymphoma and primary central nervous system lymphoma. Invest Ophthalmol Vis Sci. 2005;46 (11:3957–3964. doi: 10.1167/iovs.05-0318. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Hummel M, Muller HH, Stein H. Molecular analysis of immunoglobulin genes in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2005;46 (10:3507–3514. doi: 10.1167/iovs.05-0401. [DOI] [PubMed] [Google Scholar]
- Malumbres R, Davis J, Ruiz P, Lossos IS. Somatically mutated immunoglobulin IGHV@ genes without intraclonal heterogeneity indicate a postgerminal centre origin of primary intraocular diffuse large B-cell lymphomas. Br J Haematol. 2007;138 (6:749–755. doi: 10.1111/j.1365-2141.2007.06744.x. [DOI] [PubMed] [Google Scholar]
- Montesinos-Rongen M, Kuppers R, Schluter D, Spieker T, Van Roost D, Schaller C, et al. Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the V4-34 gene segment. Am J Pathol. 1999;155 (6:2077–2086. doi: 10.1016/S0002-9440(10)65526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pels H, Montesinos-Rongen M, Schaller C, Schlegel U, Schmidt-Wolf IG, Wiestler OD, et al. VH gene analysis of primary CNS lymphomas. J Neurol Sci. 2005;228 (2:143–147. doi: 10.1016/j.jns.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101:275–292. [PMC free article] [PubMed] [Google Scholar]
- Montesinos-Rongen M, Siebert R, Deckert M. Primary lymphoma of the central nervous system: just DLBCL or not. Blood. 2009;113 (1:7–10. doi: 10.1182/blood-2008-04-149005. [DOI] [PubMed] [Google Scholar]
- Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer. 1984;53 (11:2515–2524. doi: 10.1002/1097-0142(19840601)53:11<2515::aid-cncr2820531125>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Isaacson PG. Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4 (8:644–653. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- Chanudet E, Zhou Y, Bacon CM, Wotherspoon AC, Müller-Hermelink HK, Adam P, et al. Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions. J Pathol. 2006;209 (3:344–351. doi: 10.1002/path.1984. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Foss HD, Anagnostopoulos I, Hummel M, Stein H. Immunoglobulin VH gene expression among extranodal marginal zone B-cell lymphomas of the ocular adnexa. Invest Ophthalmol Vis Sci. 1999;40 (3:555–562. [PubMed] [Google Scholar]
- Hara Y, Nakamura N, Kuze T, Hashimoto Y, Sasaki Y, Shirakawa A, et al. Immunoglobulin heavy chain gene analysis of ocular adnexal extranodal marginal zone B-cell lymphoma. Invest Ophthalmol Vis Sci. 2001;42 (11:2450–2457. [PubMed] [Google Scholar]
- Bahler DW, Szankasi P, Kulkarni S, Tubbs RR, Cook JR, Swerdlow SH. Use of similar immunoglobulin VH gene segments by MALT lymphomas of the ocular adnexa. Mod Pathol. 2009;22 (6:833–838. doi: 10.1038/modpathol.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du MQ. MALT lymphoma: many roads lead to nuclear factor-kappa b activation. Histopathology. 2011;58 (1:26–38. doi: 10.1111/j.1365-2559.2010.03699.x. [DOI] [PubMed] [Google Scholar]
- Chanudet E, Huang Y, Ichimura K, Dong G, Hamoudi RA, Radford J, et al. A20 is targeted by promoter methylation, deletion and inactivating mutation in MALT lymphoma. Leukemia. 2010;24 (2:483–487. doi: 10.1038/leu.2009.234. [DOI] [PubMed] [Google Scholar]
- Kim WS, Honma K, Karnan S, Tagawa H, Kim YD, Oh YL, et al. Genome-wide array-based comparative genomic hybridization of ocular marginal zone B cell lymphoma: comparison with pulmonary and nodal marginal zone B cell lymphoma. Genes Chromosomes Cancer. 2007;46 (8:776–783. doi: 10.1002/gcc.20463. [DOI] [PubMed] [Google Scholar]
- Bi Y, Zeng N, Chanudet E, Huang Y, Hamoudi RA, Liu H, et al. A20 inactivation in ocular adnexal MALT lymphoma. Haematologica. 2012;97 (6:926–930. doi: 10.3324/haematol.2010.036798. [DOI] [PMC free article] [PubMed] [Google Scholar]