Abstract
Most uveal melanomas are treated with radiotherapy. An adequate understanding of the effects of radiation on the tumour and the healthy ocular tissues is necessary. Ionizing radiation damages cell membranes, organelles, and DNA. Irradiated cells are lysed or undergo apoptosis, necrosis, and senescence. These effects occur in tumour cells and vascular endothelial cells, resulting in tumour shrinkage, ischaemia, infarction, exudation, and fibrosis, which can cause exudative maculopathy, serous retinal detachment, rubeosis, and neovascular glaucoma (ie, ‘toxic tumour syndrome'). Such abnormalities must be distinguished from collateral damage to healthy ocular tissues that receive high doses of radiation, and these include radiation-induced retinopathy, optic neuropathy, choroidopathy, cataract, and scleral necrosis. Radiation retinopathy can be treated effectively with photodynamic therapy, anti-angiogenic agents, and intravitreal steroid injections. In some patients, optic neuropathy may improve with intravitreal steroids or anti-angiogenic agents. Neovascular glaucoma resolves with intra-cameral bevacizumab. Exudative retinal detachment can regress with intra-vitreal steroid injections. Cataract is treated in the usual manner. Scleral necrosis, if severe, may require grafting, possibly using a lamellar flap from the same eye. Depending on the bulk of the residual toxic tumour, treatment can consist of intra-vitreal steroids and/or anti-angiogenic agents, transpupillary thermotherapy or photodynamic therapy to the tumour, or surgical removal of the tumour by endo- or exo-resection. Measures aimed at preventing collateral damage include eccentric placement of ruthenium plaques or iodine seeds and delivery of a notched proton beam. The decision to treat a uveal melanoma with radiotherapy requires the ability to manage iatrogenic side effects and complications.
Keywords: radiotherapy, uveal melanoma, eye, treatment, radiation-induced side effects
Introduction
Without timely treatment, uveal melanomas can make the eye blind, inflamed, and painful. Approximately 50% of patients die from metastatic disease, which occurs by haematogenous spread to the liver and other organs. The aims of ocular treatment are to prevent metastatic spread, if possible conserving the eye and useful vision.
Most uveal melanomas are treated by radiotherapy, which can consist of various forms of brachytherapy, proton beam radiotherapy, and stereotactic radiotherapy. The various forms of radiotherapy differ greatly from each other, not only with respect to their therapeutic effects, but also with regards to the ocular morbidity that is induced. Treatment of such iatrogenic side effects demands a good understanding of the biological effects of the various forms of radiotherapy on the tumour and on surrounding healthy tissues.
The aims of this review are to outline the biological effects of radiotherapy on uveal melanomas and on healthy ocular tissues and to summarize current treatment modalities of radiation-related side effects.
Radiation effects on molecules and cells
Ionizing radiation displaces electrons from atoms to produce ion pairs, consisting of positive cations and negative electrons, which damage molecular bonds. Ionizing radiation also interacts with water to produce free radicals, which have unpaired electrons. These result in highly toxic hydroxyl (OH) radicals, either directly, or by forming hydrogen peroxide (H2O2), which then cleaves into OH.
Ionizing radiation damages cells directly, by disrupting chemical bonds in molecules, and indirectly, by the formation of toxic-free radicals. The most important effects occur in DNA, cell membranes, and organelles. DNA damage mostly occurs by indirect ionization, which disrupts the base pairs and or strands. Base pairs can be injured or deleted, depending on whether both bases of the pair are destroyed. Abnormal pairing of the bases results in a ‘cross-linkage injury', altering the DNA conformation. Single-strand breaks can be repaired, but double-strand breaks can result in a variety of chromosomal abnormalities, many of which are lethal to the cell. On average, 1 Gy of radiation induces 40 double-strand DNA breaks but fewer than 1 chromosome aberration per cell.
Radiation doses up to 30 Gy increase membrane permeability. Higher doses rupture membranes, allowing extracellular fluid to enter the cell, causing cell death.
Doses of 5–100 Gy disrupt cytoplasmic lysosomes, releasing enzymes, which digest the cellular structures. Disturbed mitochondrial function interrupts the production of ATP, causing cell death if reserves become depleted.
When irradiated cells lose their ability to divide, they enter a prolonged phase of cell-cycle arrest so that they undergo senescence and eventually die. Alternatively, irradiated cells can enter abnormal and fatal mitosis. Another mechanism of cell death is apoptosis, which occurs when irreparable DNA damage activates the TP53 gene. Unlike necrosis, apoptosis does not damage surrounding cells by the release of harmful substances, which are eliminated by phagocytosis.
Radiation effects on tissues
Tumour
Necrotic tumour cells show cytoplasmic vacuolation, balloon cell degeneration, and nuclear disruption inducing inflammation, with the accumulation of neutrophils, lymphocytes, and plasma cells.1 Macrophages phagocytose apoptotic cells as well as debris from necrotic cells. The tumour blood vessels become depleted of endothelial cells, with thickening of the basement membrane. These changes result in vascular occlusion and leakage. Eventually fibrosis develops.
Tumour regression depends on the type of radiotherapy and the tumour cell-doubling time. Regression is more rapid after ruthenium-106 plaque radiotherapy than after proton beam treatment, probably because of the very high doses of radiation delivered to the tumour base during brachytherapy. Several authors report that more rapid regression of uveal melanoma is associated with higher mortality and this is probably because tumours with high-grade malignancy have shorter cell-doubling times. Immediately after the radiotherapy, there may be a transient increase in tumour size as a result of interstitial oedema. Rarely, an irradiated collar-stud tumour may appear to grow as necrotic tissue herniates through the defect in Bruch's membrane into the sub-retinal space.
Retina
Retinal blood vessels show similar histological changes to irradiated tumour vessels.2 Small vessels develop outpouchings, fusiform dilatation, and microaneurysms. A collateral circulation often develops. Vascular incompetence results in vascular leakage, oedema (Figure 1), and lipid exudates. There is also narrowing of the capillary lumen and localized closure, causing ischaemia and infarction (Figure 2a).
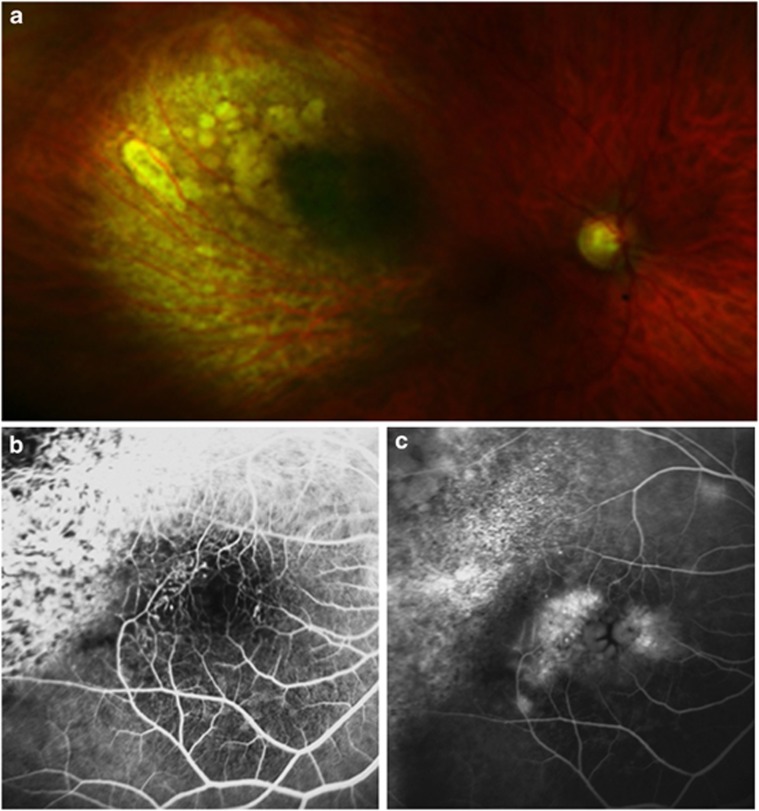
Figure 1.
Colour photograph showing radiation-induced maculopathy 1.5 years after ruthenium-106 plaque brachytherapy of a choroidal melanoma (a). Fluorescein angiogram showing an abnormal perifoveolar capillary network with early views defining leaking microaneurysms (b) and late views demonstrating leakage causing the characteristic petaloid pattern of cystoid macular oedema (c).
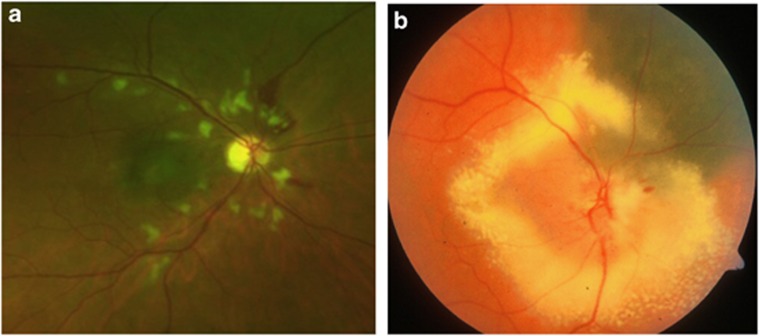
Figure 2.
Colour photographs showing radiation retinopathy with cotton-wool spots and retinal haemorrhages (a) and optic neuropathy, with optic nerve oedema and peripapillary exudates (b). Both photographs were taken 2 years after proton beam radiotherapy for choroidal melanoma.
Exudative retinal detachment (ERD) can occur acutely as a result of increased vascular permeability caused by high-dose radiation (eg, if a ‘hot plaque' is used, with a high-dose rate). This usually resolves spontaneously after a few weeks or months. Persistent detachment can result in irreversible photoreceptor atrophy. ERD can also develop as a result of delayed radiation-induced vasculopathy and can induce rubeosis and neovascular glaucoma (‘toxic tumour syndrome').
Non-replicating retinal cells are radio-resistant, especially receptor cells. Retinal atrophy can occur, however, as a result of prolonged oedema or retinal detachment.
Retinal pigment epithelium
The irradiated retinal pigment epithelium develops atrophy, loss of melanin, accumulation of lipofuscin, and areas of hyperplasia.3 Such atrophy is caused both by direct effects of ionizing radiation on the cells themselves and by ischaemia following closure of the choriocapillaris. Clinically, these RPE changes are manifest as scattered areas of hyper- and hypo-pigmentation.
Choroid
Radiation-induced effects are predominantly vascular, and include beading, telangiectatic-like dilatations, micro aneurysms, sclerosis, closure, and new vessels.4
Optic nerve
Radiation optic neuropathy (RON) is associated with significant visual loss,5 with most eyes having visual acuity of less than counting fingers at 5 years.6 About a third of eyes show significant spontaneous improvement over 5 years.6
Optic neuropathy is most likely to develop if the dose received by optic nerve exceeds 50 Gy. This complication occurs as a result of direct, neuropathic effects and because of radiation-induced vasculopathy (Figure 2b). Glial cells show demyelination and neuronal degeneration, which can precede vascular changes.7 Blood vessels show loss of endothelial cells, perivascular inflammation, hyalinization and fibrosis of vessel walls, causing infarction, and reactive gliosis.8 As a result of these abnormalities, the optic nerve shows areas of necrosis with lymphocytic infiltrates.
Iris
Direct effects of radiation on the iris include atrophy, reduced thickness, and loss of cellularity. There can also be indirect effects such as rubeosis, induced by ischaemia in the posterior segment.9 This rubeosis causes neovascular glaucoma, which is a common reason for enucleation.
Lens
Radiation damages the DNA of the proliferative cells, which become rounded and bladder-like (‘Wedl cells'). Lens fibres become deformed and debris accumulates in the sub-capsular regions, especially posteriorly.10 These abnormalities result in cataract, which generally occurs at doses exceeding 8–10 Gy, especially with limited fractionation. In 2007, the Collaborative Ocular Melanoma Study group reported the incidence of cataract after I-125 brachytherapy. By 5 years, 83% of study eyes had developed cataract and 12% had undergone cataract surgery. In all, 18% of eyes that received a dose of 24 Gy or higher to the lens underwent cataract surgery, whereas only 4% of patients with <12 Gy to the lens required such surgery.11
Sclera
Scleral necrosis is an uncommon complication of radiation therapy for uveal melanoma.12 After iodine-125 plaque radiotherapy of large choroidal melanomas, Shields et al report an incidence of approximately 9% at 10 years. Scleral necrosis has also been reported after proton beam radiotherapy.12
Risk factors include tumour thickness exceeding 6 mm, ciliary body involvement, and increased intraocular pressure.13, 14 Damato has observed perforation of exposed, irradiated sclera when conjunctival closure was inadequate (unpublished data). Proposed physiopathogical mechanisms include ischaemia and inflammation, aggravated by tumour necrosis.12, 13, 15 The severity of this complication ranges from mild scleral translucency to perforation.
Treatment of radiation-induced morbiditiy
Radiation retinopathy
Different treatment modalities have been used in the management of radiation retinopathy. These include laser photocoagulation, photodynamic therapy, corticosteroids, and anti-vascular endothelial growth factor (anti-VEGF) agents.
Retinal laser photocoagulation
Grid laser photocoagulation provides only modest benefit. Kinyoun16 compared visual outcomes in 19 grid-laser-treated patients and 23 untreated patients with radiation-induced macular oedema. They report a significant treatment effect (P=0.003), with treated eyes having a mean final visual acuity of 0.34 logMAR units better than untreated eyes after a mean follow-up of 51 months; however, eyes with persistent macular oedema continued to lose vision despite treatment. After Pd-103 plaque radiotherapy, Finger and Kurli17 administered sector argon laser photocoagulation to 45 eyes with early retinopathy. Regression of retinopathy was observed in 64% of these eyes, but 47% lost three or more lines of vision at final follow-up (mean of 48 months). Hykin et al18 administered grid macular laser photocoagulation to 19 eyes with radiation-induced macular oedema. Six months later, 42% of treated eyes had better visual acuity than observed controls, but by 24 months there was no significant difference between the two groups.
Photodynamic therapy
Bakri and Beer19 reported the effect of PDT in four patients with radiation-induced macular oedema. The PDT was centred on the fovea or on the area of leakage. All four eyes had a marked reduction in hard exudates, with improved vision in three eyes.
Corticosteroids
Intra-vitreal steroids are effective in reducing radiation-induced macular oedema. Their efficacy was first reported by Sutter in 2003.20 Shields et al21 treated 31 patients with symptomatic radiation-induced maculopathy with a single intravitreal triamcinolone acetonide injection (4 mg/0.1 ml). The visual acuity was stable or improved in 91% of patients by 1 month and in 45% by 6 months. Mean foveal thickness by optical coherence tomography was 417 μm at injection, 207 μm at 1 month, and 292 μm 6 months after injection. Complications included transient elevation in intraocular pressure (16%), persistent glaucoma requiring topical medications (10%), and cataract (10%). In a randomized clinical trial, Horgan et al22 administered periocular triamcinolone injections to 108 patients, who had received iodine-125 plaque radiotherapy for uveal melanoma. Injections given at the time of the plaque application and 4 and 8 months later reduced macular oedema for up to 18 months, improving vision without significant rates of glaucoma and cataract. More recently, Russo et al23 reported functional and anatomic improvement 4 weeks after a single intravitreal injection of dexamethasone 0.7 mg (Ozurdex) in a case of radiation-induced macular oedema following ruthenium-106 plaque brachytherapy for a choroidal melanoma.
Anti-VEGF agents
Several studies have reported promising results with the use of anti-VEGF agents in the treatment of radiation-induced macular oedema. Mason et al,24 in a retrospective case series of 10 consecutive patients, evaluated the effect of a single intravitreal injection of bevacizumab. The mean visual acuity improved from 20/100 to 20/86 at 6 weeks and to 20/95 at 4 months. The mean foveal thickness measured by OCT was 482 μm before injection, 284 μm 6 weeks after injection, and 449 μm 4 months after injection. Finger25 reported the results of intravitreal injections of bevacizumab (1.25 mg in 0.05 ml) repeated every 6–12 weeks in 21 patients with radiation retinopathy. They noted reduction in retinal haemorrhage and exudation while visual acuity was maintained in 86% of patients, with 14% regaining two or more lines of visual acuity. Gupta and Muecke26 reported five patients who developed radiation-induced macular oedema after Ru-106 plaque radiotherapy for choroidal melanoma, suggesting that younger patients with shorter duration of macular oedema may benefit the most after intravitreal injections of bevacizumab. A phase 1, open-label, Genentech-sponsored study of five consecutive patients with RR-related macular oedema after Pd-103 plaque radiotherapy for uveal melanoma showed visual acuity improvement in four patients and decreased foveal thickness in all cases after monthly intravitreal ranibizumab (0.5 mg) injections for at least four cycles.27 In summary, most published studies suggest that anti-VEGF agents reduce radiation-induced macular oedema (Figure 3) and retinal neovascularization, although not all studies demonstrate improvement in visual acuity.28 The optimal treatment regime has yet to be defined.
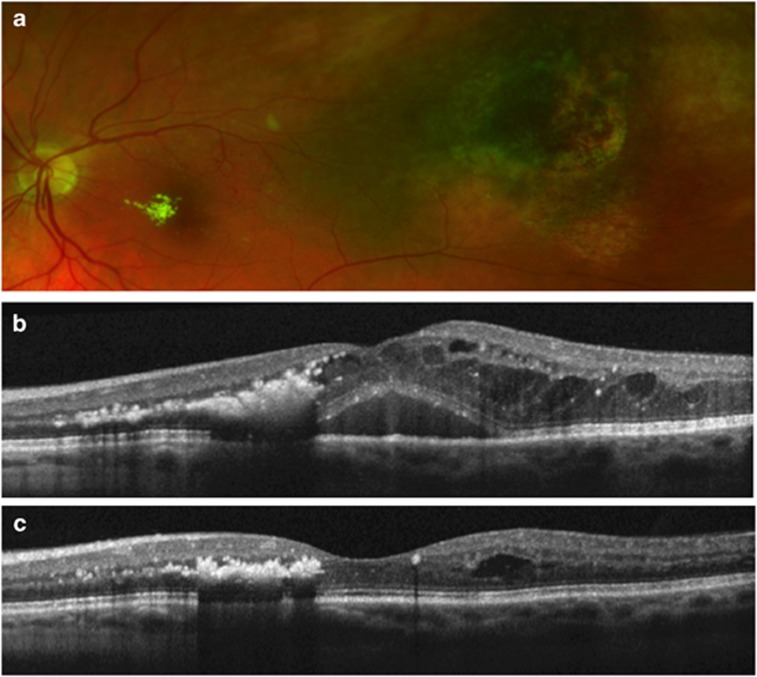
Figure 3.
Fundus photograph showing macular exudates after plaque radiotherapy of a choroidal melanoma in the left eye (a). OCT scan showed marked cystoid macular oedema, which reduced the visual acuity to 6/60 (b). OCT scan 1 month after an intravitreal injection of bevacizumab, showing significant anatomic improvement (c). The visual acuity improved to 6/36.
Other treatment modalities
Other treatment modalities have also been explored. Anatomical improvement after hyperbaric oxygen therapy in a case of Ru-106-induced radiation retinopathy and optic neuropathy has been reported,29 whereas Gupta et al30 reported anatomic and functional improvement after treatment with oral pentoxifylline in a patient with radiation retinopathy following stereotactic radiosurgery.
Radiation optic neuropathy
The optimal treatment of RON has yet to be defined. A few small series have reported isolated cases of visual improvement after hyperbaric oxygen therapy, particularly when this was administered within days of symptom onset.31, 32 Systemic corticosteroids and anticoagulation are ineffective.33, 34, 35 Shields et al36 reported the results of intravitreal triamcinolone acetonide (4 mg/0.1 ml) in nine patients with RON after plaque radiotherapy for choroidal melanoma. They observed rapid resolution of optic disc hyperaemia and oedema with modest improvement of visual acuity.
Encouraging results have been reported with anti-angiogenic agents. Finger and Chin37 evaluated intravitreal administration of bevacizumab in 14 patients with RON related to plaque radiotherapy for choroidal melanoma. They observed reduction in optic disc haemorrhage and oedema in all patients while visual acuity was stable or improved in 9 of the 14 patients.
Preventative strategies of RR and RON
Strategies to minimize the radiation dose to the macula have included: reducing the overall tumour treatment dose, the use of collimating, and ‘custom-designed' plaques and the eccentric placement of plaques.38, 39, 40, 41 Puusaari et al41 evaluated simulated radiation dose distribution with conformal positioning of I-125 seeds and collimating plaque design. They found that clinically significant dose reduction to normal tissues was feasible. Damato et al39, 40, 42 advocate eccentric positioning of ruthenium plaques in an effort to reduce radiation dose to the fovea and optic nerve when treating posteriorly located choroidal melanomas. With this technique, the posterior plaque edge is aligned with the posterior tumour margin, relying on side-scatter radiation to treat any lateral tumour extension (Figure 4a). This approach has improved conservation of vision without any increase in local tumour recurrence rates.
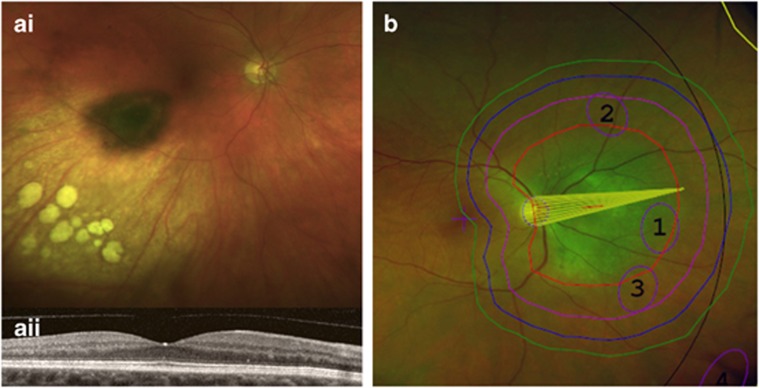
Figure 4.
Preventative strategies of radiation-induced complications. Colour photograph of the right fundus 2 years after brachytherapy of a choroidal melanoma with eccentric ruthenium-106 plaque placement (ai). The visual acuity was 6/6 with no signs of macular oedema on the OCT (aii) Proton beam treatment plan showing a notch to reduce irradiation of the optic nerve (b).
There has also been progress in the avoidance of ‘direct' radiation-induced complications, such as the development of the notched proton beam irradiation for juxtapapillary tumours (Figure 4b).43, 44, 45 Oliver et al46 performed cadaveric ex vivo studies and Monte Carlo simulation demonstrating that intraocular 1000-cSt silicone oil attenuated I-125 plaque radiation, protecting optic disc and macula. This shielding effect was confirmed by Ahuja et al,47 both in vitro and with three patients undergoing I-125 plaque radiotherapy; however, all three patients developed cataract and one required retinal detachment surgery.
Neovascular glaucoma and ERD
Anti-VEGF agents
The literature on the effect of anti-VEGF on ERD is inconsistent. Newman et al48 reported complete resolution of the ERD secondary to choroidal melanoma in two patients after systemic treatment with bevacizumab (10 mg/kg intravenous bevacizumab every 2 weeks for three or four cycles) after plaque radiotherapy. Parrozzani et al49 evaluated the efficacy and safety of prompt intravitreal triamcinolone acetonide injection (4 mg/0.1 ml) vs intravitreal bevacizumab injection (1.25 mg/0.05 ml) vs observation in the management of extensive ERD secondary to posterior uveal melanoma. After a follow-up of approximately 37 months, marked ERD regression was documented in 22 (69%) of eyes treated with intravitreal triamcinolone vs 11 (34%) treated with intravitreal bevacizumab and 9 (28%) untreated eyes. No statistical significance was found between the intravitreal bevacizumab group and the observation group (P=0.45). Vásquez et al50 reported resolution of ERD after intracameral bevacizumab in a case of neovascular glaucoma after brachytherapy for choroidal melanoma. Similarly, Dunavoelgyi et al51 described a case of successful management of ERD and neovascular glaucoma of radiation-induced ERD and neovascular glaucoma with intravitreal ranibizumab.
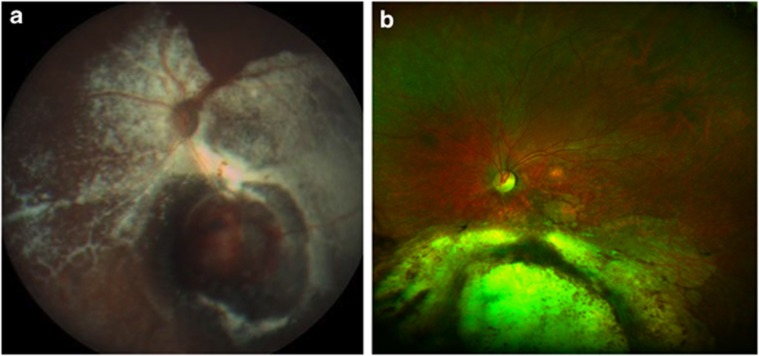
Damato coined the term ‘toxic tumour syndrome' to describe ERD, rubeosis and neovascular glaucoma caused by the persistence of an ischaemic and exudative tumour after radiotherapy. This condition can be treated with intraocular steroids and/or anti-angiogenic agents, transpupillary thermotherapy, photodynamic therapy, endoresection, or transscleral local resection depending on the size of this toxic mass and the severity of the complications.45 Damato et al52, 53, 54 performed secondary exoresection for toxic tumour in 13 patients with encouraging results. Damato52, 55, 56 also advocated that endoresection is an effective treatment for the toxic tumour syndrome (Figure 5) and may be performed if the tumour is too large for transpupillary thermotherapy and too small and posterior for trans-scleral local resection.
Figure 5.
Severe exudation after proton beam radiotherapy of a choroidal melanoma in the left eye (a). The visual acuity was counting fingers. Resolution of the exudation after endoresection of the toxic tumour (b).
Cataract
Cataract is treated in the standard manner. Wachtlin et al57 reported the results of phacoemulsification following radiation treatment of choroidal melanoma. Preoperative problems included rubeosis iridis, secondary glaucoma, and posterior synechiae. Intraoperatively, defects of the posterior capsule occurred in 12.5% of the patients.
Corneoscleral necrosis
Patients with mild scleral necrosis can be managed by observation.12 More severe cases require a scleral patch and/or a conjunctival flap, with some patients coming to enucleation.14 Damato has successfully treated a small number of patients with scleral necrosis using a partial-thickness scleral patch graft obtained from the same eye (unpublished data).
Conclusions
Radiotherapy of uveal melanomas requires not only sterilization of the tumour but also treatment of any radiation-induced ocular morbidity. Proper management of such iatrogenic complications demands an adequate understanding of the biological effects of the radiotherapy on the target tumour as well as on healthy ocular tissues.
The authors declare no conflict of interest.
References
- Avery RB, Diener-West M, Reynolds SM, Grossniklaus HE, Green WR, Albert DM. Histopathologic characteristics of choroidal melanoma in eyes enucleated after iodine 125 brachytherapy in the collaborative ocular melanoma study. Arch Ophthalmol. 2008;126 (2:207–212. doi: 10.1001/archophthalmol.2007.50. [DOI] [PubMed] [Google Scholar]
- Archer DB, Gardiner TA. Ionizing radiation and the retina. Curr Opin Ophthalmol. 1994;5 (3:59–65. doi: 10.1097/00055735-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Egbert PR, Fajardo LF, Donaldson SS, Moazed K. Posterior ocular abnormalities after irradiation for retinoblastoma: a histopathological study. Br J Ophthalmol. 1980;64 (9:660–665. doi: 10.1136/bjo.64.9.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoaku WM, Lafaut B, Sallet G, De Laey JJ. Radiation choroidal vasculopathy: an indocyanine green angiography study. Eye (London, England) 1995;9 (Part 6:738–744. doi: 10.1038/eye.1995.187. [DOI] [PubMed] [Google Scholar]
- Danesh-Meyer HV. Radiation-induced optic neuropathy. J Clin Neurosci. 2008;15 (2:95–100. doi: 10.1016/j.jocn.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Kim IK, Lane AM, Egan KM, Munzenrider J, Gragoudas ES. Natural history of radiation papillopathy after proton beam irradiation of parapapillary melanoma. Ophthalmology. 2010;117 (8:1617–1622. doi: 10.1016/j.ophtha.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Fike JR.GGT. Central nervous system radiation injury in large animal modelsIn: Gutin SAL PH, Sheline GE, (eds).In Radiation Injury to the Nervous System. Raven Press: New York; 1991113–135. [Google Scholar]
- Levin LA, Gragoudas ES, Lessell S. Endothelial cell loss in irradiated optic nerves. Ophthalmology. 2000;107 (2:370–374. doi: 10.1016/s0161-6420(99)00054-8. [DOI] [PubMed] [Google Scholar]
- Cappin JM. Malignant melanoma and rubeosis iridis. Histopathological and statistical study. Br J Ophthalmol. 1973;57 (11:815–824. doi: 10.1136/bjo.57.11.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam GR, Worgul BV. Experimental radiation cataract--its clinical relevance. Bull N Y Acad Med. 1983;59 (4:372–392. [PMC free article] [PubMed] [Google Scholar]
- Collaborative-Ocular-Melanoma-Study Incidence of cataract and outcomes after cataract surgery in the first 5 years after iodine 125 brachytherapy in the Collaborative Ocular Melanoma Study: COMS Report No. 27. Ophthalmology. 2007;114 (7:1363–1371. doi: 10.1016/j.ophtha.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Radin PP, Lumbroso-Le Rouic L, Levy-Gabriel C, Dendale R, Sastre X, Desjardins L. Scleral necrosis after radiation therapy for uveal melanomas: report of 23 cases. Graefe's Archive for Clinical And Experimental Ophthalmology. 2008;246 (12:1731–1736. doi: 10.1007/s00417-008-0920-6. [DOI] [PubMed] [Google Scholar]
- Gunduz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Plaque radiotherapy of uveal melanoma with predominant ciliary body involvement. Arch Ophthalmol. 1999;117 (2:170–177. doi: 10.1001/archopht.117.2.170. [DOI] [PubMed] [Google Scholar]
- Chaudhry IA, Liu M, Shamsi FA, Arat YO, Shetlar DJ, Boniuk M. Corneoscleral necrosis after episcleral Au-198 brachytherapy of uveal melanoma. Retina (Philadelphia, PA) 2009;29 (1:73–79. doi: 10.1097/IAE.0b013e3181863f7c. [DOI] [PubMed] [Google Scholar]
- Correa ZM, Augsburger JJ, Freire J, Eagle RC. Early-onset scleral necrosis after iodine I 125 plaque radiotherapy for ciliochoroidal melanoma. Arch Ophthalmol. 1999;117 (2:259–261. doi: 10.1001/archopht.117.2.259. [DOI] [PubMed] [Google Scholar]
- Kinyoun JL. Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:325–335. [PMC free article] [PubMed] [Google Scholar]
- Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol. 2005;89 (6:730–738. doi: 10.1136/bjo.2004.052159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hykin PG, Shields CL, Shields JA, Arevalo JF. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. 1998;105 (8:1425–1429. doi: 10.1016/S0161-6420(98)98023-X. [DOI] [PubMed] [Google Scholar]
- Bakri SJ, Beer PM. Photodynamic therapy for maculopathy due to radiation retinopathy. Eye (London, England) 2005;19 (7:795–799. doi: 10.1038/sj.eye.6701637. [DOI] [PubMed] [Google Scholar]
- Sutter FK, Gillies MC. Intravitreal triamcinolone for radiation-induced macular edema. Arch Ophthalmol. 2003;121 (10:1491–1493. doi: 10.1001/archopht.121.10.1491. [DOI] [PubMed] [Google Scholar]
- Shields CL, Demirci H, Dai V, Marr BP, Mashayekhi A, Materin MA, et al. Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina (Philadelphia, PA) 2005;25 (7:868–874. doi: 10.1097/00006982-200510000-00009. [DOI] [PubMed] [Google Scholar]
- Horgan N, Shields CL, Mashayekhi A, Salazar PF, Materin MA, O'Regan M, et al. Periocular triamcinolone for prevention of macular edema after plaque radiotherapy of uveal melanoma: a randomized controlled trial. Ophthalmology. 2009;116 (7:1383–1390. doi: 10.1016/j.ophtha.2009.01.051. [DOI] [PubMed] [Google Scholar]
- Russo A, Avitabile T, Uva M, Faro S, Franco L, Sanfilippo M, et al. Radiation macular edema after Ru-106 plaque brachytherapy for choroidal melanoma resolved by an intravitreal dexamethasone 0.7-mg implant. Case Reports in Ophthalmology. 2012;3 (1:71–76. doi: 10.1159/000337144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JO, Albert MA, Persaud TO, Vail RS. Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina (Philadelphia, PA) 2007;27 (7:903–907. doi: 10.1097/IAE.0b013e31806e6042. [DOI] [PubMed] [Google Scholar]
- Finger PT. Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin) Int J Radiat Oncol Biol Phys. 2008;70 (4:974–977. doi: 10.1016/j.ijrobp.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin) Retina (Philadelphia, PA) 2008;28 (7:964–968. doi: 10.1097/IAE.0b013e3181706302. [DOI] [PubMed] [Google Scholar]
- Finger PT, Chin KJ. Intravitreous ranibizumab (lucentis) for radiation maculopathy. Arch Ophthalmol. 2010;128 (2:249–252. doi: 10.1001/archophthalmol.2009.376. [DOI] [PubMed] [Google Scholar]
- Giuliari GP, Sadaka A, Hinkle DM, Simpson ER. Current treatments for radiation retinopathy. Acta Oncologica (Stockholm, Sweden) 2011;50 (1:6–13. doi: 10.3109/0284186X.2010.500299. [DOI] [PubMed] [Google Scholar]
- Gall N, Leiba H, Handzel R, Pe'er J. Severe radiation retinopathy and optic neuropathy after brachytherapy for choroidal melanoma, treated by hyperbaric oxygen. Eye (London, England) 2007;21 (7:1010–1012. doi: 10.1038/sj.eye.6702820. [DOI] [PubMed] [Google Scholar]
- Gupta P, Meisenberg B, Amin P, Pomeranz HD. Radiation retinopathy: the role of pentoxifylline. Retina (Philadelphia, PA) 2001;21 (5:545–547. doi: 10.1097/00006982-200110000-00026. [DOI] [PubMed] [Google Scholar]
- Borruat FX, Schatz NJ, Glaser JS, Feun LG, Matos L. Visual recovery from radiation-induced optic neuropathy. The role of hyperbaric oxygen therapy. J Clin Neuroophthalmol. 1993;13 (2:98–101. [PubMed] [Google Scholar]
- Guy J, Schatz NJ. Hyperbaric oxygen in the treatment of radiation-induced optic neuropathy. Ophthalmology. 1986;93 (8:1083–1088. doi: 10.1016/s0161-6420(86)33617-0. [DOI] [PubMed] [Google Scholar]
- Barbosa AP, Carvalho D, Marques L, Monteiro M, Castro Neves A, Machado Carvalho A, et al. Inefficiency of the anticoagulant therapy in the regression of the radiation-induced optic neuropathy in Cushing's disease. J Endocrinol Invest. 1999;22 (4:301–305. doi: 10.1007/BF03343560. [DOI] [PubMed] [Google Scholar]
- Danesh-Meyer HV, Savino PJ, Sergott RC. Visual loss despite anticoagulation in radiation-induced optic neuropathy. Clin Experiment Ophthalmol. 2004;32 (3:333–335. doi: 10.1111/j.1442-9071.2004.00825.x. [DOI] [PubMed] [Google Scholar]
- Girkin CA, Comey CH, Lunsford LD, Goodman ML, Kline LB. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104 (10:1634–1643. doi: 10.1016/s0161-6420(97)30084-0. [DOI] [PubMed] [Google Scholar]
- Shields CL, Demirci H, Marr BP, Mashayekhi A, Dai VV, Materin MA, et al. Intravitreal triamcinolone acetonide for acute radiation papillopathy. Retina (Philadelphia, PA) 2006;26 (5:537–544. doi: 10.1097/00006982-200605000-00007. [DOI] [PubMed] [Google Scholar]
- Finger PT, Chin KJ. Antivascular endothelial growth factor bevacizumab for radiation optic neuropathy: secondary to plaque radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82 (2:789–798. doi: 10.1016/j.ijrobp.2010.11.075. [DOI] [PubMed] [Google Scholar]
- Horgan N, Shields CL, Mashayekhi A, Shields JA. Classification and treatment of radiation maculopathy. Curr Opin Ophthalmol. 2010;21 (3:233–238. doi: 10.1097/ICU.0b013e3283386687. [DOI] [PubMed] [Google Scholar]
- Russo A, Laguardia M, Damato B. Eccentric ruthenium plaque radiotherapy of posterior choroidal melanoma. Graefe's Archive for Clinical And Experimental Ophthalmology. 2012;250 (10:1533–1540. doi: 10.1007/s00417-012-1962-3. [DOI] [PubMed] [Google Scholar]
- Damato B, Patel I, Campbell IR, Mayles HM, Errington RD. Visual acuity after Ruthenium(106) brachytherapy of choroidal melanomas. Int J Radiat Oncol Biol Phys. 2005;63 (2:392–400. doi: 10.1016/j.ijrobp.2005.02.059. [DOI] [PubMed] [Google Scholar]
- Puusaari I, Heikkonen J, Kivelä T. Effect of radiation dose on ocular complications after iodine brachytherapy for large uveal melanoma: empirical data and simulation of collimating plaques. Invest Ophthalmol Vis Sci. 2004;45 (10:3425–3434. doi: 10.1167/iovs.04-0066. [DOI] [PubMed] [Google Scholar]
- Damato B, Patel I, Campbell IR, Mayles HM, Errington RD. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol Phys. 2005;63 (2:385–391. doi: 10.1016/j.ijrobp.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Damato B, Kacperek A, Chopra M, Campbell IR, Errington RD. Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005;62 (5:1405–1411. doi: 10.1016/j.ijrobp.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Damato BE. Treatment selection for uveal melanoma. Dev Ophthalmol. 2012;49:16–26. doi: 10.1159/000328251. [DOI] [PubMed] [Google Scholar]
- Damato B. Progress in the management of patients with uveal melanoma. The 2012 Ashton Lecture. Eye (London, England) 2012;26 (9:1157–1172. doi: 10.1038/eye.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SC, Leu MY, DeMarco JJ, Chow PE, Lee SP, McCannel TA. Attenuation of iodine 125 radiation with vitreous substitutes in the treatment of uveal melanoma. Arch Ophthalmol. 2010;128 (7:888–893. doi: 10.1001/archophthalmol.2010.117. [DOI] [PubMed] [Google Scholar]
- Ahuja Y, Kapoor KG, Thomson RM, Furutani KM, Shultz RW, Stafford SL, et al. The effects of intraocular silicone oil placement prior to iodine 125 brachytherapy for uveal melanoma: a clinical case series. Eye (London, England) 2012;26 (11:1487–1489. doi: 10.1038/eye.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman H, Finger PT, Chin KJ, Pavlick AC. Systemic bevacizumab (Avastin) for exudative retinal detachment secondary to choroidal melanoma. Eur J Ophthalmol. 2011;21 (6:796–801. doi: 10.5301/EJO.2011.6477. [DOI] [PubMed] [Google Scholar]
- Parrozzani R, Pilotto E, Dario A, Miglionico G, Midena E.Intravitreal triamcinolone vs intravitreal bevacizumab in the treatment of exudative retinal detachment secondary to posterior uveal melanoma Am J Ophthalmole-pub ahead of print 18 September 2012; doi: 10.1016/j.ajo.2012.06.026 [DOI] [PubMed]
- Vasquez LM, Somani S, Altomare F, Simpson ER. Intracameral bevacizumab in the treatment of neovascular glaucoma and exudative retinal detachment after brachytherapy in choroidal melanoma. Canadian J Ophthalmol. 2009;44 (1:106–107. doi: 10.3129/i08-171. [DOI] [PubMed] [Google Scholar]
- Dunavoelgyi R, Zehetmayer M, Simader C, Schmidt-Erfurth U. Rapid improvement of radiation-induced neovascular glaucoma and exudative retinal detachment after a single intravitreal ranibizumab injection. Clin Experiment Ophthalmol. 2007;35 (9:878–880. doi: 10.1111/j.1442-9071.2007.01632.x. [DOI] [PubMed] [Google Scholar]
- Damato BE. Local resection of uveal melanoma. Dev Ophthalmol. 2012;49:66–80. doi: 10.1159/000328261. [DOI] [PubMed] [Google Scholar]
- Schalenbourg A, Coupland S, Kacperek A, Damato B. Iridocyclectomy for neovascular glaucoma caused by proton-beam radiotherapy of pigmented ciliary adenocarcinoma. Graefe's Archive for Clinical and Experimental Ophthalmology. 2008;246 (10:1499–1501. doi: 10.1007/s00417-008-0852-1. [DOI] [PubMed] [Google Scholar]
- Damato BFW.Surgical resection of choroidal melanomaIn: Schachat-Ryan, (eds).Retina (Philadelphia, PA) Mosby: St Louis; 2006769–778. [Google Scholar]
- Damato B.Vasculopathy after treatment of choroidal melanomaIn: Joussen AM, Gardner TW, Kirchhof B, Ryan SJ, (eds).Retinal Vascular Disease Springer: Berlin, Germany; 2007582–591. [Google Scholar]
- Damato B, Groenewald C, McGalliard J, Wong D. Endoresection of choroidal melanoma. Br J Ophthalmol. 1998;82 (3:213–218. doi: 10.1136/bjo.82.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtlin J, Bechrakis NE, Schueler AO, Helbig H, Bornfeld N, Foerster MH. Phacoemulsification following treatment of choroidal melanoma. Graefe's Archive for Clinical and Experimental Ophthalmology. 2000;238 (12:942–948. doi: 10.1007/s004170000208. [DOI] [PubMed] [Google Scholar]