Abstract
Purpose
Although ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) has been established as the standard of care in patients with advanced Hodgkin lymphoma, newer regimens have been investigated, which have appeared superior in early phase II studies. Our aim was to determine if failure-free survival was superior in patients treated with the Stanford V regimen compared with ABVD.
Patients and Methods
The Eastern Cooperative Oncology Group, along with the Cancer and Leukemia Group B, the Southwest Oncology Group, and the Canadian NCIC Clinical Trials Group, conducted this randomized phase III trial in patients with advanced Hodgkin lymphoma. Stratification factors included extent of disease (localized v extensive) and International Prognostic Factors Project Score (0 to 2 v 3 to 7). The primary end point was failure-free survival (FFS), defined as the time from random assignment to progression, relapse, or death, whichever occurred first. Overall survival, a secondary end point, was measured from random assignment to death as a result of any cause. This design provided 87% power to detect a 33% reduction in FFS hazard rate, or a difference in 5-year FFS of 64% versus 74% at two-sided .05 significance level.
Results
There was no significant difference in the overall response rate between the two arms, with complete remission and clinical complete remission rates of 73% for ABVD and 69% for Stanford V. At a median follow-up of 6.4 years, there was no difference in FFS: 74% for ABVD and 71% for Stanford V at 5 years (P = .32).
Conclusion
ABVD remains the standard of care for patients with advanced Hodgkin lymphoma.
INTRODUCTION
Several studies have established doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as the standard of care in patients with advanced Hodgkin lymphoma (HL).1–6 Over the past two decades, new regimens have been developed to either improve efficacy or reduce toxicity. Horning et al7 published single-institution data reporting 5-year failure-free (FFS) and overall survival (OS) of 89% and 95%, respectively, using Stanford V, a combined-modality approach for patients with advanced HL. This regimen was designed to minimize both short- and long-term toxicity. Treatment was shortened to 12 weeks by delivering potentially non–cross-resistant chemotherapy at weekly intervals and reducing exposure to the cardiotoxic drug doxorubicin and the pulmonary-toxic drug bleomycin. Importantly, patients with lymph nodes < 5 cm at diagnosis were treated with 36-Gy irradiation of those sites after chemotherapy. These data were validated in a cooperative group setting8 in 47 patients with a reported freedom from progression of 85% at 5 years. To address the question of superiority of Stanford V over ABVD in advanced HL, in April 1999, the Eastern Cooperative Oncology Group, along with the Cancer and Leukemia Group B and the Southwest Oncology Group, initiated an intergroup trial, joined in 2000 by the Canadian NCIC Clinical Trials Group. The trial closed to accrual in June 2006.
PATIENTS AND METHODS
Patients were eligible if they had previously untreated, histologically proven classical HL and advanced (stage III or IV) or locally extensive disease with bulky mediastinal adenopathy, as defined by a mass > one third of the maximum intrathoracic diameter on standing posteroanterior chest x-ray. Histology was determined using central review when available, then local pathologic review. Concordance rate was assessed in patients with both central and local pathologic review.
Statistics
FFS was defined as the time from random assignment to progression, relapse, or death, whichever occurred first. OS was measured from random assignment to death as a result of any cause. This study planned to accrue 850 patients (10% ineligibility rate assumed) over 4 1/3 years, with an additional 3 years of follow-up, to reach full information for 240 failures with 756 eligible patients. This design provided 87% power to detect a 33% reduction in FFS hazard rate, which corresponds to a difference in 5-year FFS of 64% versus 74%, at a two-sided .05 significance level. Two interim analyses were planned at approximately 33% and 67% of the anticipated number of failures.
Comparisons between treatment groups were conducted according to the intent-to-treat principle among eligible patients with a stratified log-rank test. Two stratification factors were employed, including extent of disease (localized v extensive) and the number of international prognostic risk factors, as defined by the International Prognostic Factors Project Score (IPS; 0 to 2 v 3 to 7).9 Treatment groups were used as a stratification factor in planned subgroup analysis. The Kaplan-Meier method and Cox proportional regression model were used to estimate failure rates, hazard ratios (HRs), and 95% CIs.10,11 Time-varying covariate was used in Cox proportional regression to test the interaction between treatment groups and time and determine the inflection point in the HR. Toxicity was evaluated in all patients regardless of eligibility. Fisher's exact and Wilcoxon rank sum tests were used to compare proportions and medians, respectively.
Treatment
The randomization schema is shown in Figure 1. ABVD was administered for six to eight cycles (Appendix Table A1, online only), depending on response as determined by computed tomography (CT) scan, and Stanford V was administered for 12 weeks (Appendix Table A2, online only). Patients administered Stanford V received prophylactic antibiotics, which included oral trimethoprim/sulfamethoxazole and ketoconazole, whereas those administered ABVD did not. Radiation therapy (RT) was administered to all patients, with bulky mediastinal adenopathy beginning 2 to 3 weeks after completion of chemotherapy. Radiation fields included the mediastinum, bilateral hilar, and bilateral supraclavicular areas, treated to a dose of 36 Gy. Additionally, and only for patients treated with Stanford V, 36 Gy was delivered to any pretreatment site > 5 cm and for macroscopic splenic disease detected by CT. Radiation fields for all patients in both arms receiving RT were retrospectively reviewed for quality control, and rates of compliance were similar, with 67% and 66% with no or minor variation for ABVD and Stanford V, respectively.
Fig 1.
CONSORT diagram and randomization schema of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) versus Stanford V. CT, computed tomography; IFRT, involved-field radiation therapy.
RESULTS
Between April 1999 and June 2006, 854 patients were enrolled (ABVD, 428; Stanford V, 426), and 794 were determined eligible and randomly assigned to receive ABVD (n = 395) or Stanford V (n = 399). Of the 60 ineligible patients, 39 were ineligible for reasons other than pathologic exclusions, as follows: lack of baseline measurements within 4 weeks (n = eight), stage I to II without a large mediastinal mass (n = 27), no baseline record (n = two), WBC < 4,000 (n = one), and unknown (n = one). There were 626 patients evaluated by central pathologic review, balanced by treatment arm, and 21 were found to be ineligible because of incorrect pathology, as follows: lymphocyte-predominant HL (n = 16), anaplastic lymphoma kinase–positive large-cell lymphoma (n = one), unclassified B-cell lymphoma (n = one), primary mediastinal large-cell lymphoma (n = one), diffuse large B-cell lymphoma (n = two). Pathologic exclusions were balanced between the two arms, with 11 in the ABVD arm and 10 in the Stanford V arm.
Patient Characteristics
Patient characteristics are listed in Table 1. Baseline patient characteristics were balanced between two treatment arms for age, performance status, WHO subtype, disease stage, extranodal involvement, and IPS risk factors. The median age in the two regimen arms was 33 years, with even distribution among men and women. The majority of patients had performance status of 0 to 1, and approximately 64% of patients had IPS of 0 to 2 (Table 2). Most patients (ABVD, 93.5%; Stanford V, 96.3%) had stage II, III, or IV disease. Lung involvement was the most frequent extranodal site of disease (ABVD, 14.7%; Stanford V, 13.5%). Bone marrow involvement was 8% and 9.1% in the Stanford V and ABVD arms, respectively.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | ABVD Arm(n = 395) |
Stanford V Arm(n = 399) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 33 | 33 | ||
| Range | 18-83 | 16-83 | ||
| Sex | ||||
| Male | 208 | 52.7 | 217 | 54.4 |
| Female | 187 | 47.3 | 182 | 45.6 |
| ECOG performance status | ||||
| 0 | 227 | 57.5 | 221 | 55.4 |
| 1 | 151 | 38.2 | 162 | 40.6 |
| 2 | 8 | 2.0 | 15 | 3.0 |
| Missing/unknown | 9 | 2.3 | 1 | 0.3 |
| B symptoms | ||||
| Yes | 232 | 58.7 | 227 | 56.9 |
| Missing/unknown | 8 | 2.0 | 1 | 0.3 |
| Cell type | ||||
| Lymphocyte rich | 8 | 2.0 | 6 | 1.5 |
| Nodular sclerosis | 274 | 69.4 | 288 | 72.2 |
| Mixed cellularity | 47 | 11.9 | 40 | 10.0 |
| Lymphocyte depleted | 0 | 0.0 | 2 | 0.5 |
| Unclassified | 46 | 11.6 | 54 | 13.5 |
| Missing/unknown | 20 | 5.1 | 9 | 2.3 |
| Lymphocyte predominance | 0 | 0.0 | 0 | 0.0 |
| Disease stage | ||||
| I | 18 | 4.6 | 14 | 3.5 |
| II | 104 | 26.3 | 105 | 26.3 |
| IIE | 15 | 3.8 | 25 | 6.3 |
| III | 129 | 32.7 | 140 | 35.1 |
| IIIE | 16 | 4.1 | 16 | 4.0 |
| IV | 105 | 26.4 | 98 | 24.6 |
| Missing/unknown | 8 | 2.0 | 1 | 0.3 |
| Extranodal involvement | ||||
| Lung | 58 | 14.7 | 54 | 13.5 |
| Liver | 28 | 7.1 | 29 | 7.3 |
| Bone | 28 | 7.1 | 35 | 8.8 |
| Bone marrow | 36 | 9.1 | 32 | 8.0 |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; ECOG, Eastern Cooperative Oncology Group.
Table 2.
Risk Factors by IPS
| IPS | ABVD Arm(n = 395) |
Stanford V Arm(n = 399) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Missing/unknown | 8 | 2.0 | 4 | 1.0 |
| 0 | 33 | 8.4 | 27 | 6.8 |
| 1 | 98 | 24.8 | 94 | 23.6 |
| 2 | 123 | 31.1 | 137 | 34.3 |
| 3 | 76 | 19.2 | 75 | 18.8 |
| 4 | 37 | 9.4 | 34 | 8.5 |
| 4 | 18 | 4.6 | 21 | 5.3 |
| 6 | 2 | 0.5 | 7 | 1.8 |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; IPS, International Prognostic Factors Project Score.
Risk Factors
Risk factors by IPS are listed in Table 2 and were balanced between the two groups.
Number of Chemotherapy Cycles and RT
In the ABVD arm, 52.2% of patients received six cycles (24 weeks), 54.2% received six to seven cycles (28 weeks), and 88.6% received six to eight cycles (32 weeks). In the Stanford V arm, 95.2% of patients were administered the specified 12 weeks of treatment (Appendix Fig A1, online only). The median duration of chemotherapy (excluding RT) for patients receiving ABVD was 24 weeks (range, 4 to 56 weeks), whereas for those receiving Stanford V, it was 12 weeks (range, 4 to 20 weeks). The decision to administer six versus eight cycles of ABVD was specified in the protocol by response based on CT scan. The duration of treatment in the Stanford V arm was not response based, and 95.2% of patients received the full 12 weeks of chemotherapy. Seventy-five percent of patients received RT in the Stanford V arm, whereas in the ABVD arm, 41% received RT for bulky mediastinal disease only. Early treatment discontinuation occurred because of progressive disease, loss to follow-up, patient choice, or physician discretion.
Response
Response rates are listed in Table 3. There was no difference in the overall response rate between the two arms, with complete remission (CR) and clinical CR rates of 72.7% (95% CI, 68% to 77%) for ABVD and 68.7% (95% CI, 64% to 73%) for Stanford V.
Table 3.
Response Rates
| Response (%) | ABVD Arm (n = 394) | Stanford V Arm (n = 399) |
|---|---|---|
| CR and CCR | 72.7 | 68.7 |
| PR | 7.6 | 7.5 |
| SD | 8.4 | 10.5 |
| Progression | 0.3 | 2.0 |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; CCR, clinical complete remission; CR, complete remission; PR, partial response; SD, stable disease.
FFS and OS
At a median follow-up of 6.4 years, there was no difference in FFS: 74% for ABVD and 71% for Stanford V at 5 years (P = .32; Fig 2A). The FFS curves crossed at approximately 5.5 years, and significant interaction between the treatment groups and time (P = .02) was observed. Extended Cox regression with time-varying covariates revealed a stratified HR for ABVD over Stanford V of 1.22 (95% CI, 0.89, 1.67) before 2 years and 0.93 (95% CI, 0.51, 1.70) after 2 years. Neither time interval difference was significant (P = .21 and P = .82, respectively). There were 50 deaths in the ABVD group and 50 in the Stanford V group, with no difference in OS (Fig 2B; ABVD, 88%; Stanford V, 88% at 5 years; P = .86; HR, 0.97; 95% CI, 0.65 to 1.43).
Fig 2.

(A) Failure-free (P = .32) and (B) overall survival (P = .86) are shown for all patients, showing no difference between the two arms. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine.
Subgroup Analysis
Locally advanced versus extensive.
The planned subgroup analysis comparing FFS (Fig 3A) and OS (Fig 3B) in patients with stages III and IV disease (n = 525) with those with locally advanced disease (n = 268) stratified by treatment arm indicated that patients with locally advanced disease did significantly better than patients with stages III and IV disease, with 3-year FFS of 82% versus 71% and 5-year FFS of 82% versus 67%, respectively (P = .001). Similar results were seen for OS, with 5-year OS of 94% versus 85%, respectively (Fig 3B; P < .001).
Fig 3.
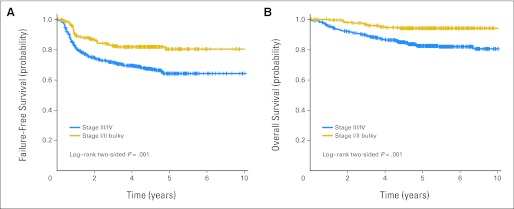
Patients with locally extensive disease (stage I to II bulky) were compared with patients with advanced disease (stage III to IV); patients with locally advanced disease had better (A) failure-free survival (FFS; P = .001) and (B) overall survival (OS; P = .002), but there were no differences in FFS or OS between ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and Stanford V (data not shown).
Risk factors by IPS.
Stratification in the protocol was by the IPS risk factors of 0 to 2 versus 3 to 7 (Figs 4A to 4D). In the low-risk group (n = 512), there was no difference between the ABVD and Stanford V arms in FFS (ABVD, 77%; Stanford V, 78% at 5 years; HR, 0.91; 95% CI, 0.62 to 1.33; P = .62; Fig 4A) or OS (ABVD, 91%; Stanford V, 93% at 5 years; HR, 0.59; 95% CI, 0.32 to 1.07; P = .08; Fig 4B). By contrast, as shown in Figure 4C, for the high-risk group by IPS (n = 270), there was a significant difference in FFS in favor of ABVD versus Stanford V (67% v 57% at 5 years), with an HR of 1.6 (95% CI, 1.06 to 2.47; P = .02). There was no significant difference in OS (Fig 4D) for the high-risk group (ABVD, 84%; Stanford V, 77% at 5 years; HR, 1.5; 95% CI, 0.86 to 2.56; P = .15).
Fig 4.
In patients with low-risk disease (International Prognostic Factors Project Score [IPS], 0 to 2), there were no differences between ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and Stanford V in terms of (A) failure-free survival (FFS; P = .62) or (B) overall survival (OS; P = .08). In patients with high-risk disease (IPS, 3 to 7), there was (C) significant improvement in FFS with ABVD versus Stanford V (P = .02), but (D) no significant difference in OS (P = .15).
Toxicity
Toxicity was assessed in all patients, and data are available for 835 patients. Toxicity was similar between the two arms, including grades 3 and 4 neutropenia. However, in the Stanford V arm, compared with the ABVD arm, there were more instances of grade 3 lymphopenia (78% v 42%; P < .001), grade 3 or 4 leukocytopenia (36% [grade 3] and 19% [grade 4] v 28% [grade 3] and 5% [grade 4]; P < .001), grades 3 and 4 sensory neuropathy (9% [grade 3] and 1% [grade 4] v 2% [grade 3] and 1% [grade 4]; P < .001), and grade 3 or 4 motor neuropathy (5% [grade 3] and 1% [grade 4] v < 1% [grade 3] and < 1% [grade 4]; P = .006). Grade 5 toxicity was < 1% in both arms. There was no difference in reported pulmonary toxicity between the two arms, as measured by required diffusing capacity of carbon monoxide, forced expired volume in 1 second, and reported symptoms of cough and dyspnea. With a median follow-up of 6.4 years, the risk of second cancers was equal in the two groups, with 15 instances in the ABVD arm and 19 in the Stanford V arm. Second primary cancers among those receiving ABVD included: non-HL (n = two), myelodysplastic syndrome (MDS; n = one), cervical cancer (n = one), non–small-cell lung cancer (n = one), renal cell cancer (n = one), colon cancer (n = one), anal cancer (n = one), nonmelanoma skin cancer (n = three), sarcoma (n = one), breast cancer (n = one), and unknown or other (n = two). In the Stanford V arm, these included: non-HL (n = three), AML or MDS (n = three), breast cancer (n = four), gastric cancer (n = one), prostate cancer (n = one), colon cancer (n = two), lung cancer (n = one), sarcoma (n = one), brain (n = one), and unknown (n = two). There was no apparent relation to radiation field.
DISCUSSION
In this large, randomized phase III trial of ABVD versus Stanford V, with 794 eligible patients with advanced HL, there were no significant differences in FFS or OS between the two treatment groups. The FFS and OS data for the ABVD arm were similar to what has been reported with contemporary ABVD,12 but in our trial, for Stanford V, the 5-year FFS and OS rates of 71% and 88%, respectively, were lower than those originally reported for Stanford V in phase II nonrandomized studies.7,8 We found no differences in outcome among patients with stage III or IV disease; however, with high-risk disease (IPS ≥ 3), ABVD had a better 3-year FFS (ABVD, 72% v Stanford V, 62%; HR, 1.6; 95% CI, 1.06 to 2.47; log-rank P = .02).
Toxicity was similar between the two groups, with a higher incidence of lymphopenia and neuropathy in the Stanford V arm, likely attributable to the vincristine and prednisone in the Stanford V regimen. This did not translate into a higher infection rate, possibly because patients administered Stanford V received prophylactic trimethoprim-sulfamethoxazole and ketoconazole, whereas those on ABVD did not. There was no significant difference in second cancers, whether leukemia or MDS, or solid tumors among patients treated with ABVD versus Stanford V.
In the British randomized trial of ABVD versus Stanford V involving 520 randomly assigned patients, Hoskin et al1 reported similar results, with no difference between the two arms in FFS or OS. Initially, radiation was used in both arms of the trial, according to the Stanford V regimen, but it was subsequently restricted in the ABVD arm to only those patients with bulky mediastinal disease. In the Stanford V arm, 73% of patients received RT, compared with 53% in the ABVD arm. The overall response rate was 91% for Stanford V and 92% for ABVD. With a median follow-up of 4.3 years, the projected 5-year FFS and OS were 76% and 90% for ABVD and 74% and 92% for Stanford V, respectively. The Gruppo Italiano Studio Linfomi reported inferior progression-free survival with a modified version of Stanford V compared with ABVD, with no difference in OS.13,14 In this trial, the study design deviated from the published Stanford V regimen in that RT was limited to patients with initial bulky disease defined as > 6 cm in size with two sites of disease and/or partial response to chemotherapy. The data from this trial were recently updated,15 and with median follow-up of 86 months, they confirm no advantage with Stanford V over ABVD or MEC (meclorethamine, CCNU, vindesine, alkeran, prednisone, epidoxorubicin, vincristine, procarbazine, vinblastine, bleomycin) chemotherapy. Overall, our results and those of others demonstrate that Stanford V is not significantly superior to ABVD. Of interest, in the United Kingdom trial and in our trial, results with ABVD were significantly better than those reported in older studies.
In a recent study reported by Viviani et al6 from Italy, 331 patients with advanced HL were randomly assigned to treatment with ABVD or BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; escalated 4× followed by baseline 4×), with planned radiation (25.2 Gy to initial sites of bulky disease and 30.6 Gy to sites of residual disease). For those patients not achieving CR or who relapsed, state-of-the-art salvage therapy (multiple courses of an ifosphamide-containing regimen followed by autologous stem-cell transplantation with BEAM [carmustine, etoposide, cytarabine, and melphalan] conditioning) was planned. The investigators found that there was no difference in OS among patients treated with ABVD versus BEACOPP.
The Stanford V and ABVD regimens differed not only by the drugs in the regimen and the drug schedule, but also because in the Stanford V regimen, radiation was administered to all sites > 5 cm, whereas with ABVD, only patients with bulky mediastinal disease were treated with RT. The United Kingdom Lymphoma Group analyzed the outcomes of nonrandomized consolidative involved-field radiation after chemotherapy with ABVD or multidrug regimens in the LY09 study. At least 30 Gy was delivered to residual masses or sites of original bulky disease (mediastinal mass ratio > one third) at presentation. The 5-year outcomes were superior for patients who received radiation (progression-free survival, 86% v 71%; P = .001).16
In a large European Organisation for Research and Treatment of Cancer trial,17,18 RT for nonbulky disease was of no benefit compared with observation when administered after CR induction by a MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) –ABV regimen, but there was suggestion of benefit in patients with partial response. In long-term follow-up, however, 15% of patients in the RT group developed second cancers, compared with 6% in the non-RT group. Of the 15% who developed second cancers in the RT group, 53% had AML/MDS, whereas in the non-RT group, 17% of the 6% had AML/MDS. Three of the 172 patients in the RT arm developed solid tumors within the radiation field, and two did so outside the radiation field; four patients in the non-RT group developed second tumors. The risk of second cancer after RT for HL seems to be increasing,19 and recent studies have reviewed late effects of combined-modality therapy,20 including significant cardiac toxicity,21 especially when RT and doxorubicin are part of the regimen.22 Additional studies have demonstrated no significant advantage with adjuvant radiation in advanced HL for patients who achieve a complete response to conventional chemotherapy.23 In early-stage disease, combined-modality therapy remains the standard of care,24,25 but the role of radiation remains a controversial issue.26,27 Here, there was no significant difference in second cancers among patients treated with ABVD or Stanford V.
One approach to the resolution of the controversy of combined-modality therapy rests on our ability to extrapolate results that were largely based on CT criteria to current [18F]fluorodeoxyglucose– positron emission tomography (PET) –based criteria to define CR.28 Data in the HD15 trial suggest that patients with a PET-negative residual mass at completion of chemotherapy do not require consolidation RT, but this concept is still under investigation in the German Hodgkin Study Group HD18 trial.
Toxicity was similar between the two regimens. Prednisone likely accounted for the lymphopenia and vincristine for the increased peripheral neuropathy seen with Stanford V. Thus far, there are no apparent differences in cardiac and pulmonary toxicity, but follow-up has been short.
Patients with locally extensive disease did better than patients with advanced disease, but there was no difference between the two regimens in either of these subsets. Duration of treatment was significantly shorter for patients treated in the Stanford V arm, but there was more grade 3 to 4 toxicity and no improvement in FFS or OS, with the potential for long-term risks of radiation. However, for patients with three to seven IPS risk factors, ABVD resulted in superior FFS but no significant difference in OS. The question of whether there are still subsets of patients who might benefit from more intensive, escalated BEACOPP is being evaluated in a North American Intergroup trial using interim [18F]fluorodeoxyglucose-PET.
In conclusion, our data, derived from the largest randomized trial of chemotherapy in advanced HL ever performed in North America to our knowledge, found no significant difference in response rate, FFS, OS, or 5-year toxicity for ABVD (plus RT for bulky mediastinal disease) compared with Stanford V (with RT for nodal sites > 5 cm). ABVD, with consolidation RT to sites of pretreatment bulky disease, remains the standard of care for advanced and locally extensive HL in North America.
Acknowledgment
Presented at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 4-7, 2010.
Appendix
Fig A1.

Number of chemotherapy cycles and number of weeks of treatment for (A) ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and (B) Stanford V. In the ABVD arm, 52% of patients received six cycles (24 weeks), 54% received six to seven cycles (28 weeks), and 89% received six to eight cycles (32 weeks). In the Stanford V arm, 95% of patients received the specified 12 weeks of treatment.
Table A1.
ABVD Regimen
| Drug | Dosage |
|---|---|
| Adriamycin | 25 mg/m2 IV, days 1 and 15 |
| Bleomycin | 10 u/m2 IV, days 1 and 15 |
| Vinblastine | 6 mg/m2 IV, days 1 and 15 |
| Dacarbazine | 375 mg/m2 IV, days 1 and 15 |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; IV, intravenous.
Table A2.
Stanford V Regimen
| Drug | Dose | Week |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Doxorubicin | 25 mg/m2 | X | X | X | X | X | X | ||||||
| Vinblastine | 6 mg/m2 | X | X | X | X | X | X | ||||||
| Nitrogen mustard | 6 mg/m2 | X | X | X | |||||||||
| Etoposide | 60 mg/m2 × 2 | X | X | X | |||||||||
| Vincristine | 1.4 mg/m2 | X | X | X | X | X | X | ||||||
| Bleomycin | 5 U/m2 | X | X | X | X | X | X | ||||||
| Prednisone | 40 mg/m2 once every other day (taper weeks 10 to 12) | X | X | X | X | X | X | X | X | X | X | X | X |
NOTE. X indicates treatment was administered.
Footnotes
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00003389.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Richard I. Fisher, Gilead Sciences (C), Allos Therapeutics (C), Algeta (C), Millennium Pharmaceuticals (C), Genentech (C), Chesapeake Biotech (C), Roche Australia (C), Roche Canada (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Leo I. Gordon, Fangxin Hong, Bruce D. Cheson, Richard T. Hoppe, Sandra J. Horning
Provision of study materials or patients: Richard I. Fisher, Patrick J. Stiff, Richard T. Hoppe
Collection and assembly of data: Leo I. Gordon, Fangxin Hong, Randy D. Gascoyne, Henry Wagner, Ranjana Advani, Brad S. Kahl, Richard T. Hoppe, Sandra J. Horning
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported in part by Public Health Service Grants No. CA21115, CA23318, CA66636, CA17145, CA77440, CA11083, CA32102, CA46441, CA46282, CA38926, CA77202, CA21076, CA31946, and CA13650 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
REFERENCES
- 1.Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the Stanford V regimen and ABVD in the treatment of advanced Hodgkin's lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390–5396. doi: 10.1200/JCO.2009.23.3239. [DOI] [PubMed] [Google Scholar]
- 2.Bonadonna G, Bonfante V, Viviani S, et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin's disease: Long-term results. J Clin Oncol. 2004;22:2835–2841. doi: 10.1200/JCO.2004.12.170. [DOI] [PubMed] [Google Scholar]
- 3.Bonadonna G, Uslenghi C, Zucali R. Recent trends in the medical treatment of Hodgkin's disease. Eur J Cancer. 1975;11:251–266. doi: 10.1016/0014-2964(75)90006-7. [DOI] [PubMed] [Google Scholar]
- 4.Bonadonna G, Zucali R, Monfardini S, et al. Combination chemotherapy of Hodgkin's disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36:252–259. doi: 10.1002/1097-0142(197507)36:1<252::aid-cncr2820360128>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478–1484. doi: 10.1056/NEJM199211193272102. [DOI] [PubMed] [Google Scholar]
- 6.Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 7.Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin's disease: Mature results of a prospective clinical trial. J Clin Oncol. 2002;20:630–637. doi: 10.1200/JCO.2002.20.3.630. [DOI] [PubMed] [Google Scholar]
- 8.Horning SJ, Williams J, Bartlett NL, et al. Assessment of the Stanford V regimen and consolidative radiotherapy for bulky and advanced Hodgkin's disease: Eastern Cooperative Oncology Group pilot study E1492. J Clin Oncol. 2000;18:972–980. doi: 10.1200/JCO.2000.18.5.972. [DOI] [PubMed] [Google Scholar]
- 9.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease: International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 10.Cox D. Regression models and life-tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 12.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: Report of an intergroup trial. J Clin Oncol. 2003;21:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 13.Federico M, Luminari S, Iannitto E, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: Results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27:805–811. doi: 10.1200/JCO.2008.17.0910. [DOI] [PubMed] [Google Scholar]
- 14.Gobbi PG, Levis A, Chisesi T, et al. ABVD versus modified Stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate- and advanced-stage Hodgkin's lymphoma: Final results of a multicenter randomized trial by the Intergruppo Italiano Linfomi. J Clin Oncol. 2005;23:9198–9207. doi: 10.1200/JCO.2005.02.907. [DOI] [PubMed] [Google Scholar]
- 15.Chisesi T, Bellei M, Luminari S, et al. Long-term follow-up analysis of HD9601 trial comparing ABVD versus Stanford V versus MOPP/EBV/CAD in patients with newly diagnosed advanced-stage Hodgkin's lymphoma: A study from the Intergruppo Italiano Linfomi. J Clin Oncol. 2011;29:4227–4233. doi: 10.1200/JCO.2010.30.9799. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PW, Sydes MR, Hancock BW, et al. Consolidation radiotherapy in patients with advanced Hodgkin's lymphoma: Survival data from the UKLG LY09 randomized controlled trial (ISRCTN97144519) J Clin Oncol. 2010;28:3352–3359. doi: 10.1200/JCO.2009.26.0323. [DOI] [PubMed] [Google Scholar]
- 17.Aleman BM, de Bruin ML, Dorresteijn LD, et al. Re: Late effects from radiation therapy: The hits just keep on coming. J Natl Cancer Inst. 2010;102:576–577. doi: 10.1093/jnci/djq069. author reply 577-578. [DOI] [PubMed] [Google Scholar]
- 18.Aleman BM, Raemaekers JM, Tirelli U, et al. Involved-field radiotherapy for advanced Hodgkin's lymphoma. N Engl J Med. 2003;348:2396–2406. doi: 10.1056/NEJMoa022628. [DOI] [PubMed] [Google Scholar]
- 19.van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18:487–497. doi: 10.1200/JCO.2000.18.3.487. [DOI] [PubMed] [Google Scholar]
- 20.Ng AK, Mauch PM. Late effects of Hodgkin's disease and its treatment. Cancer J. 2009;15:164–168. doi: 10.1097/PPO.0b013e31819e30d7. [DOI] [PubMed] [Google Scholar]
- 21.Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117:412–418. doi: 10.1182/blood-2010-06-291328. [DOI] [PubMed] [Google Scholar]
- 22.Myrehaug S, Pintilie M, Tsang R, et al. Cardiac morbidity following modern treatment for Hodgkin lymphoma: Supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma. 2008;49:1486–1493. doi: 10.1080/10428190802140873. [DOI] [PubMed] [Google Scholar]
- 23.Ferme C, Sebban C, Hennequin C, et al. Comparison of chemotherapy to radiotherapy as consolidation of complete or good partial response after six cycles of chemotherapy for patients with advanced Hodgkin's disease: Results of the Groupe d'Études des Lymphomes de l'Adulte H89 trial. Blood. 2000;95:2246–2252. [PubMed] [Google Scholar]
- 24.Diehl V. Chemotherapy or combined modality treatment: The optimal treatment for Hodgkin's disease. J Clin Oncol. 2004;22:15–18. doi: 10.1200/JCO.2004.10.910. [DOI] [PubMed] [Google Scholar]
- 25.Yahalom J, Ryu J, Straus DJ, et al. Impact of adjuvant radiation on the patterns and rate of relapse in advanced-stage Hodgkin's disease treated with alternating chemotherapy combinations. J Clin Oncol. 1991;9:2193–2201. doi: 10.1200/JCO.1991.9.12.2193. [DOI] [PubMed] [Google Scholar]
- 26.Canellos GP. Chemotherapy alone for early Hodgkin's lymphoma: An emerging option. J Clin Oncol. 2005;23:4574–4576. doi: 10.1200/JCO.2005.01.911. [DOI] [PubMed] [Google Scholar]
- 27.Longo DL. Radiation therapy in Hodgkin disease: Why risk a Pyrrhic victory? J Natl Cancer Inst. 2005;97:1394–1395. doi: 10.1093/jnci/dji310. [DOI] [PubMed] [Google Scholar]
- 28.Gallamini A. Positron emission tomography scanning: A new paradigm for the management of Hodgkin's lymphoma. Haematologica. 2010;95:1046–1048. doi: 10.3324/haematol.2010.024885. [DOI] [PMC free article] [PubMed] [Google Scholar]




