Abstract
Purpose
Although gemcitabine and carboplatin (GCa) is a standard option for patients with advanced urothelial cancer (UC) who are ineligible for cisplatin, outcomes remain poor. This trial evaluated the efficacy and safety of bevacizumab with GCa in advanced UC.
Patients and Methods
Patients with Karnofsky performance status of 60% to 70%, creatinine clearance less than 60 mL/min, visceral metastasis, or solitary kidney were eligible and received a lead-in dose of bevacizumab 10 mg/kg followed 2 weeks later by gemcitabine 1,000 mg/m2 on days 1 and 8 and carboplatin at area under the [concentration-time] curve (AUC) 5.0 or 4.5 and bevacizumab 15 mg/kg on day 1 every 21 days for six cycles. Patients achieving at least stable disease (SD) continued bevacizumab 15 mg/kg every 21 days for 18 additional cycles. The study was powered to detect a 50% improvement in median progression-free survival (PFS) over a historical control.
Results
Fifty-one patients, median age 67 years (range, 42 to 83 years), were enrolled onto the study and were evaluable for toxicity. Twenty (39%) experienced grade 3 to 4 toxicity, and 10 (20%) had thromboembolic events (deep venous thrombosis or pulmonary embolism). Four received one or fewer cycles leaving 47 evaluable for outcomes. Twenty-three (49%) achieved response (three complete; 20 partial), and 11 had SD. Median PFS was 6.5 months (95% CI, 4.7 to 7.8 months); PFS was greater in the carboplatin AUC 5.0 group (P = .04). Median overall survival (OS) was 13.9 months.
Conclusion
The 95% one-sided lower confidence bound of 4.77 months for median PFS did not meet the predesignated PFS of more than 4.8 months considered sufficient for further study. Median OS was greater than expected. An ongoing phase III trial in patients who are eligible for therapy with cisplatin will define the role of bevacizumab in UC.
INTRODUCTION
Metastatic urothelial cancer (UC) is a malignancy sensitive to cisplatin-based chemotherapy. Yet nearly 50% of patients are ineligible for cisplatin because of advanced age, renal insufficiency, and/or medical comorbidities.1,2 Cisplatin-ineligible patients are a heterogeneous population common in both community and academic centers. Thus, there has been a recent effort to identify these patients for clinical trials, culminating in a consensus definition of cisplatin ineligibility.1
Recent trials3–8 have explored alternative therapies for patients ineligible for cisplatin, but outcomes are consistently inferior to those observed with cisplatin-based regimens. European Organisation for Research and Treatment of Cancer (EORTC) 30986 recently defined gemcitabine and carboplatin as a standard therapy in patients who are not eligible for cisplatin-based therapy compared with the older regimen of methotrexate, carboplatin, and vinblastine.9 Patients treated with gemcitabine plus carboplatin had a response rate (RR) of 41%, a median time to progression (TTP) of 5.8 months, and overall survival (OS) of 9.3 months. This study established Level I evidence for this doublet in patients who were ineligible for cisplatin, providing a comparator for future investigational therapies.
Multiple lines of preclinical and clinical evidence support targeting angiogenesis in UC.10–20 Bevacizumab, a recombinant humanized monoclonal antibody that binds human vascular endothelial growth factor (VEGF), has improved outcomes when added to chemotherapy in advanced non–small-cell lung cancer and colon cancer.21,22 Recently, a phase II trial of gemcitabine, cisplatin, and bevacizumab in metastatic UC demonstrated a 72% RR and a 19.1-month median OS which compares favorably with gemcitabine and cisplatin alone.23,24 In this phase II trial, begun in June 2006, we evaluated the addition of bevacizumab to gemcitabine and carboplatin in patients with advanced UC.
PATIENTS AND METHODS
Patients
This study was performed through a protocol approved by the institutional review board, and all patients signed informed consent. Eligible patients included those with previously untreated unresectable or metastatic UC of the bladder, urethra, ureter, or renal pelvis histologically confirmed at Memorial Sloan-Kettering Cancer Center (MSKCC). All patients were ineligible for and/or incurable by cisplatin as defined by calculated creatinine clearance (CrCl) 30 to 59 mL/min (Jelliffe equation), solitary kidney, Karnofsky performance status (KPS) 60% to 70%, or visceral metastases (bone, liver, lung). Additional inclusion criteria included KPS ≥ 60%, absolute neutrophil count ≥ 1.2 × 109/L, platelets ≥ 100 × 109/L, bilirubin ≤ 1.5 × the upper limit of normal (ULN), AST and ALT ≤ 3.0 × ULN (≤ 5.0 × ULN if liver had tumor involvement), serum creatinine less than 2.0 mL/min, or calculated CrCl ≥ 30 mL/min.
Prior treatment with systemic chemotherapy was not allowed, although prior intravesical therapy was permitted. A bleeding diathesis, coagulopathy, presence of CNS metastases, urinary albumin more than 1.0 g/24 hours, gastrointestinal fistula/perforation or intra-abdominal abscess (contraindications to bevacizumab therapy according to US Food and Drug Administration product labeling) within 6 months of initiating treatment, or persistent gross hematuria were also protocol exclusions.
Methods
Patients initially received bevacizumab 10 mg/kg intravenously (IV) followed 2 weeks later with combination therapy. The rationale for this 2-week lead-in treatment was based on the vascular normalization hypothesis induced by anti-VEGF therapy putatively allowing for improved chemotherapy penetration in tumors.25–27 Gemcitabine 1,000 mg/m2 on days 1 and 8 and carboplatin IV at area under the [concentration-time] curve (AUC) 5.0 on day 1 were administered every 21 days. Bevacizumab 15 mg/kg IV was administered on day 1 of each 21-day cycle. This dose and schedule were based on a phase II study28 in advanced non–small-cell lung cancer showing the regimen to be well tolerated. The first 13 patients enrolled were treated with carboplatin AUC 5.0; a subsequent protocol amendment reduced the dose to AUC 4.5 when data from a similar study showed increased hematologic toxicity with carboplatin AUC 5.0.4 Patients received a total of six cycles of combination therapy unless disease progression or unacceptable toxicity occurred. Patients who developed unacceptable toxicity attributable to bevacizumab during combination treatment continued gemcitabine and carboplatin on study. Patients who achieved stable disease (SD), a partial response (PR), or a complete response (CR) after six cycles continued maintenance bevacizumab for a maximum of 18 doses at 3-week intervals until progression or unacceptable toxicity.
Patient Evaluation
Baseline evaluation included a complete history; physical examination, including vital signs and performance status; CBC; comprehensive metabolic panel, including serum electrolytes, blood urea nitrogen, creatinine, AST, ALT, alkaline phosphatase, total protein, albumin, and total bilirubin; a coagulation profile; urinalysis and spot urine protein:creatinine ratio; ECG; computed tomography scan of chest, abdomen, and pelvis; and a bone scan and/or magnetic resonance imaging scan when indicated. A CBC and comprehensive metabolic panel were performed every cycle. A spot urine protein:creatinine ratio was performed on alternating cycles. Radiographic evaluations were performed after every three cycles (at approximately 9-week intervals) for all patients while on treatment. Tumor measurements were assessed at baseline and every three cycles until progression or treatment discontinuation. Response was assessed by using Response Evaluation Criteria in Solid Tumors (RECIST) v1.0 by a reference radiologist (S.M.). After completion or discontinuation of treatment, patients were observed with evaluations and imaging every 3 months for the first 18 months, every 6 months for the subsequent 18 months, then yearly until documented progression. Safety assessments were performed before every dose of any of the three agents.
Biostatistics
The study's primary end point was progression-free survival (PFS) with a planned enrollment of 47 patients to estimate the median PFS and test whether this regimen provided a 50% improvement over gemcitabine and carboplatin alone, which had a reported median TTP (defined as time from treatment initiation until disease progression, treatment discontinuation for any reason, or death) of 4.8 months.3 PFS in this study was defined as the time from the start of treatment until progression, excessive toxicity requiring discontinuation of protocol therapy, or death and was analyzed by using the Kaplan-Meier method. Duration of response was measured from the time of best response by RECIST until documented progression or therapy discontinuation. Patients alive without documented progression at the time of analysis were censored on the date of last follow-up. Assuming a one-sided test, a type I error of 5%, and 18% censoring at the time of analysis, the study was designed to have 80% power to show a 50% improvement in median PFS. The regimen warranted further study if the lower one-sided 95% confidence bound was more than 4.8 months. A secondary end point was RR (percentage of PR and CR) and was estimated by using binomial proportions. The frequency and grading of toxicity was tabulated according to the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) v3.0.
RESULTS
Patient Characteristics
Fifty-one patients (37 men; 14 women) were enrolled between June 15, 2006, and June 16, 2010 (Table 1). Four patients discontinued therapy before completing one full cycle because of major accidental trauma (n = 1), pulmonary embolism (n = 1), need for palliative radiotherapy to bone (n = 1), or acute gemcitabine pulmonary toxicity (n = 1). All 51 patients enrolled were evaluable for toxicity, and the 47 patients completing at least one cycle of therapy were evaluable for outcomes. Primary tumor sites included bladder (31), renal pelvis (18), and ureter (two). Thirty-five patients (69%) had visceral disease, including lung (22), liver (13), bone (nine), and adrenal gland (two). Sixteen patients (31%) had metastatic disease confined to lymph nodes only. Regarding the protocol definition of cisplatin ineligibility and/or lack of cisplatin utility, four patients (8%) had KPS 60% to 70%, 15 (29%) had a solitary kidney, 39 (76%) had a calculated CrCl 30 to 59 mL/min, and 27 (53%) had visceral metastatic disease.
Table 1.
Baseline Clinical Characteristics (N = 51)
| Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 67 | |
| Range | 42-83 | |
| Sex | ||
| Male | 37 | 72.5 |
| Female | 14 | 27.5 |
| KPS | ||
| 70 | 6 | 12 |
| 80 | 24 | 47 |
| 90 | 21 | 41 |
| Primary tumor site | ||
| Bladder | 31 | 61 |
| Upper urinary tract | 20 | 39 |
| Prior curative surgery | 30 | 59 |
| No. of metastatic sites | ||
| Median | 2 | |
| Range | 1-4 | |
| Metastatic disease sites | ||
| Lung | 22 | 43 |
| Liver | 13 | 25 |
| Bone | 9 | 18 |
| Adrenal gland | 2 | 4 |
| Lymph node only | 16 | 31 |
| MSKCC risk group | ||
| 0 | 14 | 27 |
| 1 | 33 | 65 |
| 2 | 4 | 8 |
| Reason for cisplatin ineligibility | ||
| Poor performance status | 4 | 8 |
| Solitary kidney | 15 | 29 |
| Impaired renal function | 39 | 76 |
| Visceral metastatic disease | 27 | 53 |
Abbreviations: KPS, Karnofsky performance status; MSKCC, Memorial Sloan-Kettering Cancer Center.
Treatment Administration and Toxicity
A median of six cycles of gemcitabine, carboplatin, and bevacizumab was administered; 28 patients (60%) achieved at least SD without excessive toxicity after completion of combination therapy and received maintenance bevacizumab. A median of 5.5 cycles of maintenance bevacizumab was administered, and seven patients received more than nine cycles of maintenance. Four patients who continued to derive benefit completed the entire planned protocol treatment. Twenty-eight (60%) of 47 patients discontinued treatment because of progressive disease, and 15 (29%) of 51 discontinued treatment because of toxicity. Dose reductions for myelosuppression were required in 23 patients (49%; 12 with gemcitabine, seven with carboplatin, and four with both). Four of seven carboplatin dose reductions occurred in the 13 patients treated with carboplatin AUC 5.0.
Treatment-related grade 3 or 4 toxicity occurred in 39% of patients; the most common was neutropenia in 16 patients (31%; Table 2). Grade 3 or 4 vascular thrombotic events (VTEs) occurred in 10 patients (20%; two with deep venous thrombosis and eight with pulmonary embolism). Six of eight pulmonary emboli were diagnosed in the setting of symptoms and two were diagnosed incidentally on computed tomography restaging. Eight of 10 VTEs occurred during combination chemotherapy.
Table 2.
Treatment-Related Toxicity According to CTCAE v3.0 (N = 51)
| AE | Grade 1 or 2 |
Grade 3 or 4 |
||
|---|---|---|---|---|
| No. | Frequency (%) | No. | Frequency (%) | |
| Nonhematologic | ||||
| Venous thromboembolism | 0 | 0 | 10 | 20 |
| Infection | 1 | 2 | 6 | 12 |
| Fatigue | 49 | 96 | 5 | 10 |
| Increased LFTs | 35 | 69 | 5 | 10 |
| Bleeding | 24 | 47 | 4 | 8 |
| Constipation | 39 | 76 | 3 | 6 |
| Nausea | 27 | 53 | 3 | 6 |
| Pain | 11 | 22 | 3 | 6 |
| Renal impairment | 37 | 73 | 2 | 4 |
| Neuropathy | 17 | 33 | 1 | 2 |
| Weight loss | 14 | 27 | 1 | 2 |
| Hypertension | 2 | 4 | 1 | 2 |
| Duodenal ulcer | 1 | 2 | 1 | 2 |
| Cardiac ischemia | 0 | 0 | 1 | 2 |
| Pneumonitis | 0 | 0 | 1 | 2 |
| Proteinuria | 0 | 0 | 1 | 2 |
| Increased amylase/lipase | 1 | 2 | 1 | 2 |
| Dyspnea | 17 | 33 | 0 | 0 |
| Diarrhea | 16 | 31 | 0 | 0 |
| Skin toxicity | 15 | 29 | 0 | 0 |
| Mucositis | 12 | 24 | 0 | 0 |
| Edema | 11 | 22 | 0 | 0 |
| Muscle weakness | 7 | 14 | 0 | 0 |
| GI obstruction | 1 | 2 | 0 | 0 |
| Fever | 1 | 2 | 0 | 0 |
| Hematologic | ||||
| Febrile neutropenia | 0 | 0 | 1 | 2 |
| Neutropenia | 32 | 63 | 16 | 31 |
| Leukopenia | 44 | 86 | 12 | 24 |
| Thrombocytopenia | 38 | 75 | 11 | 22 |
| Anemia | 50 | 98 | 10 | 20 |
| Lymphopenia | 0 | 0 | 8 | 16 |
Abbreviations: AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; LFT, liver function test.
Outcomes
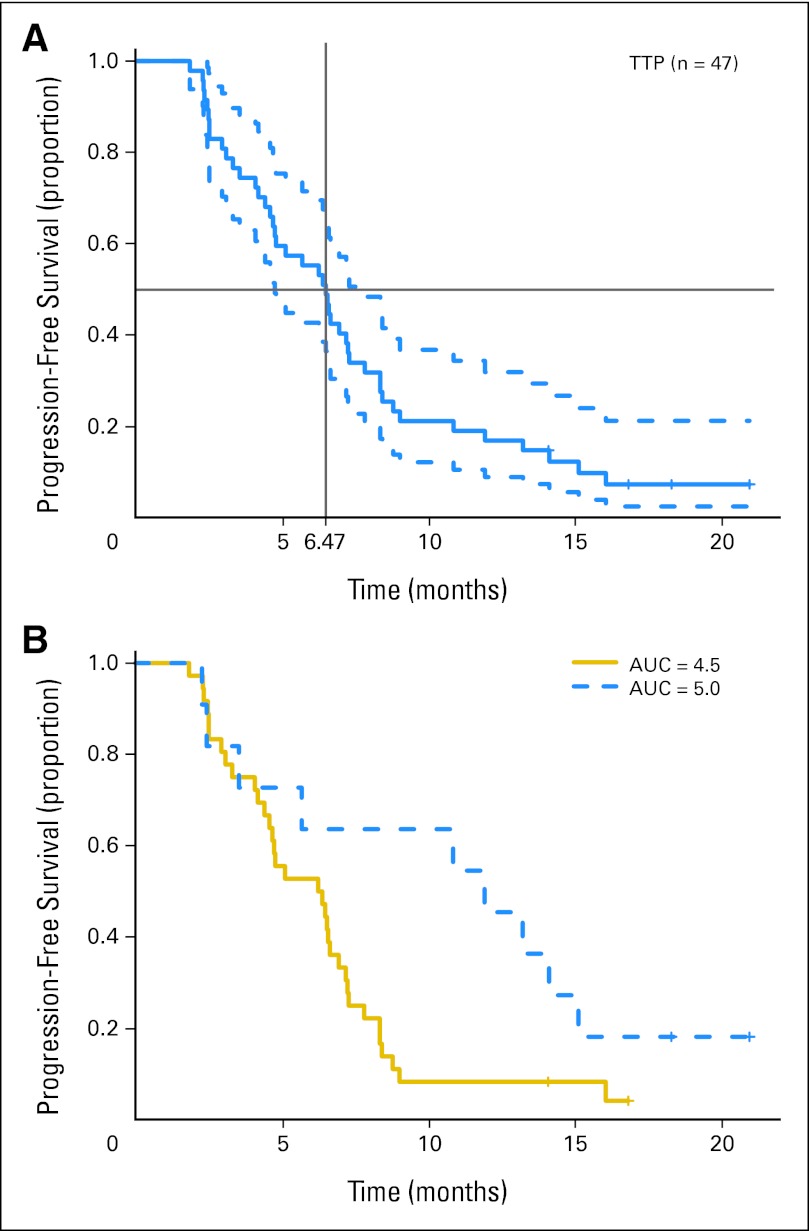
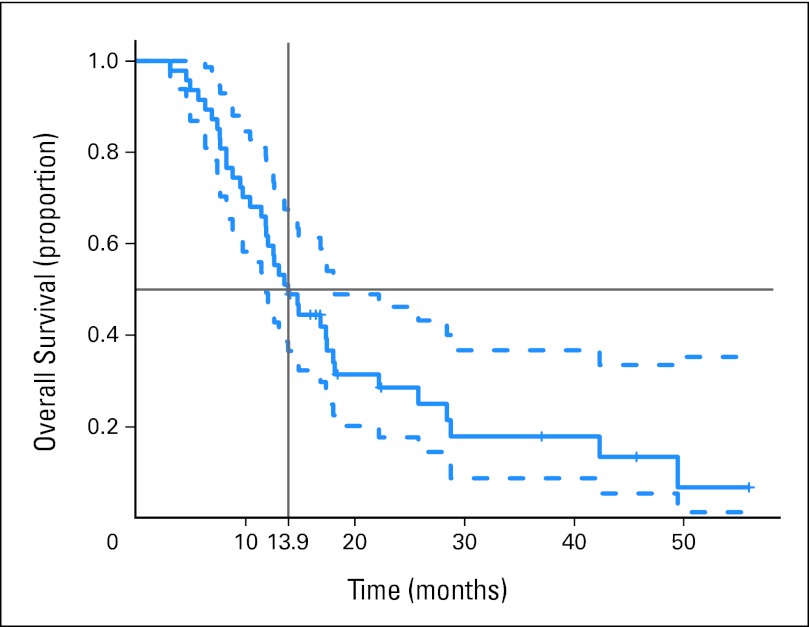
Twenty patients (43%) achieved a PR, and three (6%) achieved a CR by RECIST v1.0 for a 49% overall RR. Eleven patients (23%) achieved SD lasting a median of 4.8 months (range, 0 to 12.7 months). Median PFS was 6.5 months (95% CI, 4.7 to 7.8 months; 43 events; four patients censored; Fig 1A). The 95% one-sided lower bound for PFS was 4.77 months, just short of meeting the primary end point of more than 4.8 months. Median OS was 13.9 months (95% CI, 11.9 to 18.1 months; 37 deaths, 10 patients censored; Fig 2). One of 47 evaluable patients who discontinued treatment for toxicity subsequently died in a motor vehicle accident.
Fig 1.
Kaplan-Meier plot of progression-free survival (A) with median and 95% CIs and (B) by initial carboplatin dose. AUC, area under the [concentration-time curve]; TTP, time to progression.
Fig 2.
Kaplan-Meier plot of overall survival with median and 95% CIs.
Thirty-nine (77%) of 47 evaluable patients met EORTC criteria4,9 for cisplatin ineligibility (35 with CrCl < 60 mL/min, two with poor KPS, and two with both); they had a median PFS of 6.6 months (95% CI, 5.1 to 8.3 months) and median OS of 13.9 months (95% CI, 11.9 to 22.2 months). Post hoc subgroup analysis of PFS by initial carboplatin dose demonstrated an 11.9-month median PFS for the 11 evaluable patients treated at AUC 5.0, significantly greater than the 6.3-month median PFS for the 36 evaluable patients treated at AUC 4.5 (P = .04; Fig 1B; Table 3).
Table 3.
Clinical Outcomes and Analysis by Carboplatin Dose
| Variable | No. | % | 95% CI | No. | % | 95% CI |
|---|---|---|---|---|---|---|
| Median PFS, months (n = 47) | 6.5 | 4.7 to 7.8 | ||||
| Median OS, months (n = 47) | 13.9 | 11.9 to 18.1 | ||||
| Response outcomes (n = 47) | ||||||
| CR and PR | 23 | 49 | ||||
| SD | 11 | 23 | ||||
| AUC 5.0(n = 11) | AUC 4.5(n = 36) | |||||
|---|---|---|---|---|---|---|
| Analysis by initial carboplatin AUC dose | ||||||
| Age, years | ||||||
| Median | 67 | 68 | ||||
| Range | 53-82 | 42-83 | ||||
| Sex | ||||||
| Male | 8 | 73 | 28 | 78 | ||
| Female | 3 | 27 | 8 | 22 | ||
| KPS | ||||||
| 70 | 1 | 9 | 5 | 14 | ||
| 80 | 8 | 73 | 14 | 39 | ||
| 90 | 2 | 18 | 17 | 47 | ||
| MSKCC risk group | ||||||
| 0 | 3 | 27 | 9 | 25 | ||
| 1 | 8 | 73 | 23 | 64 | ||
| 2 | 0 | 0 | 4 | 11 | ||
| Median PFS, months | 11.9 | 5.7 to N/A | 6.3 | 4.6 to 7.2 | ||
| Response outcomes | ||||||
| CR and PR | 7 | 64 | 16 | 44 | ||
| SD | 2 | 18 | 9 | 25 | ||
Abbreviations: AUC, area under the [concentration-time] curve; CR, complete response; KPS, Karnofsky performance status; MSKCC, Memorial Sloan-Kettering Cancer Center; PFS, progression-free survival; PR, partial response; N/A, not assessable; OS, overall survival; SD, stable disease.
Identification and Analysis of Contemporary Controls
We sought to identify an MSKCC contemporary control population to place the protocol population in context (Table 4). A retrospective chart review was conducted to identify all chemotherapy-naive patients with advanced UC who were similarly treated with the doublet regimen of gemcitabine and carboplatin (without bevacizumab) at MSKCC between June 2006 and November 2010. Excluded were those who received prior systemic chemotherapy, had a concurrent active malignancy, or received treatment on this protocol. Ninety patients (60 males; 30 females) were identified, with a median age of 77 years (range, 44 to 85 years), and they were treated with a median of five chemotherapy cycles (range, one to 11.5 cycles). Primary sites of disease included bladder (n = 66), renal pelvis (n = 16), ureter, (n = 3), urethra (n = 1), or multifocal primary disease (n = 4). The MSKCC prognostic score distributions were 0 (n = 32), 1 (n = 45), and 2 (n = 13). Median survival was 10.3 months (95% CI, 8.2 to 14.5 months). When data from trial patients and control patients were combined, age, sex, and primary disease site were not significantly associated with survival on univariate analysis, but MSKCC prognostic score was. In a multivariate model for survival that included MSKCC prognostic score, the addition of bevacizumab demonstrated an adjusted hazard ratio of 0.73 (95% CI, 0.49 to 1.09; P = .12).
Table 4.
Phase II Trial Patients Versus MSKCC Contemporary Patients Treated With GCa Alone
| Characteristic | GCa + B (n = 47) |
GCa Alone (n = 90) |
||||
|---|---|---|---|---|---|---|
| No. of Patients | % | 95% CI | No. of Patients | % | 95% CI | |
| Age, years | ||||||
| Median | 67 | 77 | ||||
| Range | 42-83 | 44-85 | ||||
| Sex | ||||||
| Male | 36 | 77 | 60 | 67 | ||
| Female | 11 | 23 | 30 | 33 | ||
| KPS | ||||||
| ≤ 70 | 6 | 13 | 21 | 23 | ||
| 80 | 22 | 47 | 46 | 51 | ||
| 90 | 19 | 40 | 23 | 26 | ||
| MSKCC risk group | ||||||
| 0 | 12 | 26 | 32 | 36 | ||
| 1 | 31 | 66 | 45 | 50 | ||
| 2 | 4 | 9 | 13 | 14 | ||
| Median OS, months | 13.9 | 11.9 to 18.1 | 10.3 | 8.2 to 14.5 | ||
Abbreviations: B, bevacizumab; GCa, gemcitabine and carboplatin; KPS, Karnofsky performance status; MSKCC, Memorial Sloan-Kettering Cancer Center; OS, overall survival.
DISCUSSION
Effective and well-tolerated combination chemotherapy for patients with advanced UC ineligible to receive or unlikely to benefit from cisplatin-based chemotherapy is desperately needed. Bevacizumab, which neutralizes circulating VEGF, was a logical choice to study.21,22,29–32 This phase II study sought to test the hypothesis that bevacizumab increased PFS over that previously observed with gemcitabine and carboplatin alone. In fact, the median PFS of 6.5 months observed in this trial with the addition of bevacizumab was a 35% improvement over the historical 4.8-month TTP in the reference study. Further, the lower bound of the one-sided 95% CI was just short of reaching the primary end point of more than 4.8 months. Therefore, although the primary end point was technically not met, this combination may still be promising.
The most critical end point in phase II and III trial development is survival, which may in fact be independent of PFS. The median OS of 13.9 months was encouraging, and it was longer than that observed with gemcitabine and carboplatin alone in the prior phase II trial cited in the study's statistical design (7.2 months) and the EORTC phase III study (9.3 months).3,9 Juxtaposed with these reports, the survival observed in this study suggests a benefit with the addition of bevacizumab. However, any such conclusion would be flawed because of the nature of the comparisons and the potential imbalance of prognostic factors, including the treatment center. Our contemporary institutional experience provides perspective; 90 patients treated with first-line gemcitabine and carboplatin at MSKCC during the enrollment period for this protocol had a median OS of 10.3 months. Unfortunately, this nonprotocol population differs from the patients enrolled onto this trial in terms of MSKCC prognostic factors and potentially other variables, eliminating direct comparison. Even this prognostic score may be insufficient for comparison. A recent study33 suggests that other pretreatment features such as albumin and hemoglobin may have an impact on survival in addition to performance status and sites of metastases. Hence, only a phase III trial, balanced for known factors, can address whether bevacizumab improves survival.
Overall, this regimen was reasonably well tolerated. Major complications previously reported with bevacizumab such as wound dehiscence, bowel perforation, and fistula were not observed in this trial. At first glance, the 20% rate of VTE observed in this study implicated bevacizumab because carboplatin had not been viewed as thrombogenic. However, our group reported a 17% VTE rate in patients treated with gemcitabine and carboplatin alone, similar to the rates observed in both cisplatin-treated patients and our protocol population.34 Furthermore, eight of the 10 VTEs occurred during combination therapy when carboplatin was co-administered. This observation is potentially confounded by the increased number of VTEs diagnosed incidentally because of improved imaging techniques in both our study and the Hoosier Oncology Group study.23 The contribution of bevacizumab to the development of VTEs is somewhat controversial.35,36 Advanced cancer is a significant risk factor for thrombosis, which is often enhanced by cytotoxic chemotherapy. A retrospective study of 932 patients treated with cisplatin for a variety of solid tumors at MSKCC demonstrated an 18.1% rate of thromboembolic events.37 On multivariate analysis, a variety of host factors, including age, performance status, and Khorana Risk Score (a model incorporating cancer site, platelet count, hemoglobin, leukocyte count, and body mass index validated to predict chemotherapy-associated VTE) significantly predicted for thromboembolic events. Advanced age and poor performance status are commonly coincident with advanced UC, which itself arises from a high-risk cancer site.38 Interestingly, the Hoosier Oncology Group study23 had a markedly high VTE rate (39%) observed in the initial cohort that was attributed to higher gemcitabine dose, which necessitated a protocol amendment for dose reduction. The VTE rate in the dose-reduced cohort was significantly lower (8%).
A post hoc analysis revealed a potential relationship between carboplatin dose and PFS, adding to the controversy of dose-density and dose-intensity of platinum in UC. The carboplatin AUC dose was decreased to 4.5 because of safety concerns raised by a study by Bellmunt et al,4 providing an opportunity for further investigation. Although the significantly longer median PFS observed with AUC 5.0 may be a consequence of chance or small numbers, increased chemotherapy dose-intensity has been associated with improved RR and PFS in patients eligible for cisplatin.39 Recent phase II studies and the EORTC 30986 study evaluating gemcitabine and carboplatin investigated carboplatin at AUC 4.5 only, so a potential carboplatin dose-response relationship has not been fully explored.3–5,9 However, the investigation of dose-intense carboplatin therapy in a frail patient population would be associated with increased hematologic toxicity and potential morbidity as was evidenced in the initial dose-finding study of gemcitabine and carboplatin.4 Although provocative, the potential for improvement in response and PFS may warrant revisiting this approach in a selected population.
Criteria used in this trial to define cisplatin ineligibility, as with all published studies to date, are not uniform, thus making cross-trial comparison difficult. Conceivably, inclusion of certain patient subsets such as those with a solitary kidney or visceral disease may have affected the outcomes in this study. To the best of our knowledge, no adequately powered trials have evaluated the renal safety or survival benefit of cisplatin in patients with a solitary kidney; thus, these patients can be considered for carboplatin-based therapy. In this study, 14 of 15 patients with a solitary kidney had an estimated CrCl of less than 60 mL/min (Jelliffe equation). Although patients with extensive visceral disease are not ineligible for cisplatin based solely on renal function, prior prognostic studies from our center suggest that these patients have poor survival, which provides a basis for inclusion.40 The long-term follow-up of patients on phase II and III studies of cisplatin-based therapy41,42 add to the body of literature indicating a low likelihood of curability of patients with visceral metastases (ie, those who cannot be surgically cured after initial response to chemotherapy).43 Less toxic carboplatin therapy is a consideration in these typically frail patients.
Ultimately, predictive biomarkers that can identify patients appropriate for antiangiogenic therapy and other novel therapies are needed. VEGFA genotypes have been shown to predict median PFS and OS in patients with breast and ovarian cancer treated with regimens containing bevacizumab.44,45 In our study, nine of 28 patients who received maintenance bevacizumab experienced continued tumor regression, suggesting that a subset of patients may benefit from single-agent bevacizumab. Response may be attributable to germline DNA variation. A recent report of an association between single nucleotide polymorphisms in genes implicated in pazopanib metabolism and/or angiogenesis and response to pazopanib, another VEGF axis inhibitor, suggests a potential mechanism.46 To date, no association between inherited genetic variation and outcome to antiangiogenic therapy has been independently validated, and thus, its potential use in clinical practice is early in development.
In conclusion, results from this study and from the study of cisplatin-eligible patients by Hahn et al23 provide a compelling argument for the investigation of bevacizumab in UC. Cancer and Leukemia Group B (CALGB) 90601, an ongoing phase III study of gemcitabine and cisplatin with or without bevacizumab in advanced UC, will ultimately define the clinical benefit of bevacizumab added to a platinum-based doublet. This trial addresses an important question and is worthy of vigorous support from the oncology community.
Footnotes
See accompanying article on page 670
Supported in part by Genentech and by Grant No. NIH T32 CA009207 (D.F.B.).
Presented at the 2011 Genitourinary Cancers Symposium, Orlando, FL, February 17-19, 2011, and the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00588666.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Employment or Leadership Position: None Consultant or Advisory Role: David J. Gallagher, Roche (C); Dean F. Bajorin, Bristol-Myers Squibb (C), Dendreon (C), Eli Lilly (C), Novartis (C), Pfizer (C) Stock Ownership: None Honoraria: Dean F. Bajorin, Eli Lilly, Pfizer Research Funding: Dean F. Bajorin, Amgen, Dendreon, Genentech, Genta, Novartis, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Arjun V. Balar, Matthew I. Milowsky,Dean F. Bajorin
Administrative support: Ilana R. Garcia-Grossman
Collection and assembly of data: Arjun V. Balar, Andrea B. Apolo, Alisa Trout, Ashley M. Regazzi, Ilana R. Garcia-Grossman, Matthew I. Milowsky, Dean F. Bajorin
Data analysis and interpretation: Arjun V. Balar, Andrea B. Apolo, Irina Ostrovnaya, Svetlana Mironov, Alexia Iasonos, Ashley M. Regazzi, David J. Gallagher, Matthew I. Milowsky, Dean F. Bajorin
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–2438. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- 2.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 3.Linardou H, Aravantinos G, Efstathiou E, et al. Gemcitabine and carboplatin combination as first-line treatment in elderly patients and those unfit for cisplatin-based chemotherapy with advanced bladder carcinoma: Phase II study of the Hellenic Co-operative Oncology Group. Urology. 2004;64:479–484. doi: 10.1016/j.urology.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Bellmunt J, de Wit R, Albanell J, et al. A feasibility study of carboplatin with fixed dose of gemcitabine in “unfit” patients with advanced bladder cancer. Eur J Cancer. 2001;37:2212–2215. doi: 10.1016/s0959-8049(01)00295-7. [DOI] [PubMed] [Google Scholar]
- 5.Carles J, Nogué M, Domènech M, et al. Carboplatin-gemcitabine treatment of patients with transitional cell carcinoma of the bladder and impaired renal function. Oncology. 2000;59:24–27. doi: 10.1159/000012132. [DOI] [PubMed] [Google Scholar]
- 6.Nogué-Aliguer M, Carles J, Arrivi A, et al. Gemcitabine and carboplatin in advanced transitional cell carcinoma of the urinary tract: An alternative therapy. Cancer. 2003;97:2180–2186. doi: 10.1002/cncr.10990. [DOI] [PubMed] [Google Scholar]
- 7.Shannon C, Crombie C, Brooks A, et al. Carboplatin and gemcitabine in metastatic transitional cell carcinoma of the urothelium: Effective treatment of patients with poor prognostic features. Ann Oncol. 2001;12:947–952. doi: 10.1023/a:1011186104428. [DOI] [PubMed] [Google Scholar]
- 8.Vaughn DJ, Manola J, Dreicer R, et al. Phase II study of paclitaxel plus carboplatin in patients with advanced carcinoma of the urothelium and renal dysfunction (E2896): A trial of the Eastern Cooperative Oncology Group. Cancer. 2002;95:1022–1027. doi: 10.1002/cncr.10782. [DOI] [PubMed] [Google Scholar]
- 9.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC Study 30986. J Clin Oncol. 2012;30:191–199. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chodak GW, Scheiner CJ, Zetter BR. Urine from patients with transitional-cell carcinoma stimulates migration of capillary endothelial cells. N Engl J Med. 1981;305:869–874. doi: 10.1056/NEJM198110083051506. [DOI] [PubMed] [Google Scholar]
- 11.Jaeger TM, Weidner N, Chew K, et al. Tumor angiogenesis correlates with lymph node metastases in invasive bladder cancer. J Urol. 1995;154:69–71. [PubMed] [Google Scholar]
- 12.Bochner BH, Cote RJ, Weidner N, et al. Angiogenesis in bladder cancer: Relationship between microvessel density and tumor prognosis. J Natl Cancer Inst. 1995;87:1603–1612. doi: 10.1093/jnci/87.21.1603. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson AJ, Fox SB, Persad RA, et al. Quantification of angiogenesis as an independent predictor of prognosis in invasive bladder carcinomas. Br J Urol. 1994;74:762–766. doi: 10.1111/j.1464-410x.1994.tb07122.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown LF, Berse B, Jackman RW, et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993;143:1255–1262. [PMC free article] [PubMed] [Google Scholar]
- 15.Chopin DK, Caruelle JP, Colombel M, et al. Increased immunodetection of acidic fibroblast growth factor in bladder cancer, detectable in urine. J Urol. 1993;150:1126–1130. doi: 10.1016/s0022-5347(17)35705-1. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen M, Watanabe H, Budson AE, et al. Elevated levels of the angiogenic peptide basic fibroblast growth factor in urine of bladder cancer patients. J Natl Cancer Inst. 1993;85:241–242. doi: 10.1093/jnci/85.3.241. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Slaton JW, Davis DW, et al. Treatment of human metastatic transitional cell carcinoma of the bladder in a murine model with the anti-vascular endothelial growth factor receptor monoclonal antibody DC101 and paclitaxel. Clin Cancer Res. 2000;6:2635–2643. [PubMed] [Google Scholar]
- 18.Inoue K, Chikazawa M, Fukata S, et al. Docetaxel enhances the therapeutic effect of the angiogenesis inhibitor TNP-470 (AGM-1470) in metastatic human transitional cell carcinoma. Clin Cancer Res. 2003;9:886–899. [PubMed] [Google Scholar]
- 19.Wu W, Shu X, Hovsepyan H, et al. VEGF receptor expression and signaling in human bladder tumors. Oncogene. 2003;22:3361–3370. doi: 10.1038/sj.onc.1206285. [DOI] [PubMed] [Google Scholar]
- 20.Shu X, Wu W, Mosteller RD, et al. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol. 2011;29:1525–1530. doi: 10.1200/JCO.2010.31.6067. [DOI] [PubMed] [Google Scholar]
- 24.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 25.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 26.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 27.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraut MJ. Phase II study of gemcitabine and carboplatin plus bevacizumab for stage III/IV non-small cell lung cancer: Preliminary safety data. J Clin Oncol. 2006;24(suppl; abstr 17091):674s. [Google Scholar]
- 29.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 30.Burger RA, Brady MF, Bookman MA, et al. Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), or fallopian tube cancer (FTC): A Gynecologic Oncology Group study. J Clin Oncol. 2010;28(suppl; abstr LBA1):5s. doi: 10.1016/j.ygyno.2013.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajorin DF, Ostrovnaya I, Iasonos A, et al. A nomogram predicting survival of patients (pts) with metastatic or unresectable urothelial cancer (UC) treated with cisplatin-based chemotherapy. J Clin Oncol. 2007;25(suppl; abstr 5055):248s. [Google Scholar]
- 34.Apolo AB, Regazzi AM, Milowsky MI, et al. Vascular thromboembolic events in patients (pts) with advanced urothelial cancer (UC) treated with carboplatin/gemcitabine alone or in combination with bevacizumab. J Clin Oncol. 2009;27(suppl; abstr 5074):252s. [Google Scholar]
- 35.Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 36.Hurwitz HI, Saltz LB, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: A pooled analysis of patients in randomized phase II and III studies. J Clin Oncol. 2011;29:1757–1764. doi: 10.1200/JCO.2010.32.3220. [DOI] [PubMed] [Google Scholar]
- 37.Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J Clin Oncol. 2011;29:3466–3473. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50–54. doi: 10.1016/j.ejca.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 40.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 41.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 42.Stadler WM, Hayden A, von der Maase H, et al. Long-term survival in phase II trials of gemcitabine plus cisplatin for advanced transitional cell cancer. Urol Oncol. 2002;7:153–157. doi: 10.1016/s1078-1439(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 43.Dodd PM, McCaffrey JA, Herr H, et al. Outcome of postchemotherapy surgery after treatment with methotrexate, vinblastine, doxorubicin, and cisplatin in patients with unresectable or metastatic transitional cell carcinoma. J Clin Oncol. 1999;17:2546–2552. doi: 10.1200/JCO.1999.17.8.2546. [DOI] [PubMed] [Google Scholar]
- 44.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultheis AM, Lurje G, Rhodes KE, et al. Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin Cancer Res. 2008;14:7554–7563. doi: 10.1158/1078-0432.CCR-08-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu CF, Bing NX, Ball HA, et al. Pazopanib efficacy in renal cell carcinoma: Evidence for predictive genetic markers in angiogenesis-related and exposure-related genes. J Clin Oncol. 2011;29:2557–2564. doi: 10.1200/JCO.2010.32.9110. [DOI] [PubMed] [Google Scholar]