Abstract
Purpose
To determine the prevalence of pulmonary hypertension, a late effect of cancer therapy not previously identified in aging survivors of childhood cancer, and associations with chest-directed radiation therapy (RT) and measures of current cardiac function, lung function, and exercise capacity.
Patients and Methods
Cross-sectional evaluation of 498 survivors at a median age of 38.0 years (range, 20.0 to 59.0 years) and a median of 27.3 years (range, 12.2 to 46.0 years) from primary cancer diagnosis was performed. Abnormal tricuspid regurgitant jet velocity (TRV) was defined as more than 2.8 m/s by Doppler echocardiography.
Results
Increased TRV was identified in 25.2% of survivors who received chest-directed RT and 30.8% of those who received more than 30 Gy. In multivariable models, increased TRV was associated with increasing dose of RT (1 to 19.9 Gy: odds ratio [OR], 2.09; 95% CI, 0.63 to 6.96; 20 to 29.9 Gy: OR, 3.46; 95% CI, 1.59 to 7.54; ≥ 30 Gy: OR, 4.54; 95% CI, 1.77 to 11.64 compared with no RT; P for trend < .001), body mass index more than 40 kg/m2 (OR, 3.89; 95% CI, 1.46 to 10.39), and aortic valve regurgitation (OR, 5.85; 95% CI, 2.05 to 16.74). Survivors with a TRV more than 2.8 m/s had increased odds (OR, 5.20; 95% CI, 2.5 to 11.0) of severe functional limitation on a 6-minute walk compared with survivors with a TRV ≤ 2.8 m/s.
Conclusion
A substantial number of adult survivors of childhood cancer who received chest-directed RT have an increased TRV and may have pulmonary hypertension as a result of both direct lung injury and cardiac dysfunction. Longitudinal follow-up and confirmation by cardiac catheterization are warranted.
INTRODUCTION
Treatment of children with cancer has become increasingly successful, with more than 80% of patients achieving 5-year survival1 and the majority surviving into adulthood.2 Treatment may include the use of chemotherapeutic agents such as anthracyclines and/or chest-directed radiation therapy (RT) that may adversely impact cardiac or pulmonary function. Impaired left ventricular systolic and diastolic function,3–5 pericardial thickening, premature coronary artery disease, cardiac valve abnormalities,6 decreased lung volumes,7–9 and impaired pulmonary gas exchange7,10 have all been described in survivors of childhood cancer. However, pulmonary hypertension has been described in only a few case reports from the immediate post-treatment period.11–17
Pulmonary hypertension is a progressive condition characterized by increased pulmonary artery pressure that may lead to right ventricular failure, which is associated with increased mortality without treatment.18 Direct measurement of pulmonary artery pressure by right-heart catheterization is the gold standard for diagnosis of pulmonary hypertension. However, Doppler echocardiography is widely available, noninvasive, and relatively inexpensive, and thus is frequently used to screen for pulmonary hypertension in susceptible or at-risk populations by estimating pulmonary artery pressure via measurement of the tricuspid regurgitant jet velocity (TRV).19–22 We undertook this study to estimate the prevalence of and risk factors for an increased TRV by echocardiography in adult long-term survivors of childhood cancer who received cancer-related treatment that could adversely affect long-term cardiac or pulmonary function.
PATIENTS AND METHODS
Patients
Patients treated for cancer at St Jude Children's Research Hospital (SJCRH) who were 18 years of age or older and 10 or more years from diagnosis were eligible for the St Jude Lifetime Cohort Study (SJLIFE). Details of eligibility, recruitment methods, and study design have been previously published.23 Eligible survivors were randomly selected for recruitment to SJLIFE in consecutive blocks of 50 patients. This analysis includes participants recruited to the first 19 blocks (931 eligible; Fig 1). Participation involved completion of questionnaires and risk-based medical screening according to the Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers24 developed by the Children's Oncology Group (COG Guidelines). This investigation was approved by the institutional review board at SJCRH.
Fig 1.
Diagram of recruitment of eligible St Jude Lifetime Cohort Study (SJLIFE) cohort participants for evaluation of pulmonary hypertension. RT, radiation therapy; SJCRH, St Jude Children's Research Hospital.
This analysis was limited to patients who underwent a screening echocardiogram according to the COG Guidelines because of their prior exposure to cardiotoxic therapy (ie, anthracyclines, chest-directed RT). Of the first 931 survivors recruited to SJLIFE, 737 were exposed to cardiotoxic therapy and were eligible for echocardiography (Fig 1). One patient with congenital heart disease was excluded from evaluation of TRV. Five hundred five patients (68.5% of eligible) completed echocardiography. Participants were similar to nonparticipants in all categories except for sex (P = .02). Ultimately, 498 patients undergoing echocardiography had an evaluable TRV, and seven were inevaluable for TRV.
Outcome Measures
Echocardiograms were performed by using a VIVID-7 cardiovascular ultrasound system (n = 435; General Electric, Milwaukee, WI) or an iE33 echocardiography system (n = 70; Phillips, Andover, MA). Complete two-dimensional echocardiograms with Doppler ultrasound were performed following the American Society of Echocardiography (ASE) guidelines.25 Diastolic assessment was limited to peak mitral flow velocity of the early rapid filling wave (E), early diastolic velocity of the mitral annulus (E′) and their ratio (E/E′).26 Increased TRV was defined on the basis of Evian criteria as a TRV more than 2.8 m/s on Doppler ultrasound.27 Moderate or severe valvular regurgitation was determined according to ASE Guidelines.28 Patients who received therapies associated with lung injury, per COG Guidelines, also underwent pulmonary function testing (PFTs). Subsequently, study procedures were changed to expand screening beyond COG Guideline recommendations to include PFTs in survivors treated with spinal-field RT. PFTs were performed on 187 survivors in a single laboratory according to the guidelines of the American Thoracic Society/European Respiratory Society task force.29 PFTs included spirometry, lung volume measurements, and single-breath carbon monoxide diffusing capacity (DLCO). Spirometry was performed with a pneumotachograph, and lung volumes were determined with a plethysmograph (Medical Graphics Platinum Elite, St Paul, MN). DLCO was measured by using the single-breath technique and was corrected for hemoglobin concentration to yield the DLCO-corr. The observed values of each patient for forced vital capacity (FVC), forced expiratory volume in one second (FEV1), total lung capacity (TLC), and DLCO-corr were reported as a percentage of predicted values based on the patient's age, race, sex, and height by using reference equations.30,31 The 6-minute walk was performed indoors, according to the guidelines established by the American Thoracic Society.32 Participants who walked less than 300 meters were categorized as having a severe functional limitation.33
Demographic and Exposure Variables
Exposures to chemotherapy (anthracycline, carmustine, lomustine, bleomycin, busulfan, and cyclophosphamide) and chest-directed RT (maximum dose to the chest) were abstracted from each patient's medical record. Additional variables included age at diagnosis, age at evaluation, sex, race/ethnicity, education, primary cancer diagnosis, body mass index (BMI), and splenectomy (Table 1). Hypertension was defined as blood pressure of more than 140/90 mmHg in participants seated upright in a chair with both feet on the floor for 5 minutes. Coronary artery disease was defined as a history of myocardial infarction (n = 9) or detection of coronary disease on research-based screening evaluation (n = 12).
Table 1.
Characteristics of Patients Who Were Eligible for Echocardiographic Assessment and Had Echocardiography With an Evaluable TRV
| Characteristic* | Eligible for Evaluation (n = 737) |
Evaluable for TRV (n = 498) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Sex | ||||
| Male | 369 | 50.1 | 232 | 46.6 |
| Female | 368 | 49.9 | 266 | 53.4 |
| Race/ethnicity | ||||
| Non-Hispanic white | 661 | 89.7 | 444 | 89.2 |
| Others | 76 | 10.3 | 54 | 10.8 |
| Age at diagnosis, years | ||||
| 0-4 | 261 | 35.5 | 170 | 34.1 |
| 5-9 | 164 | 22.3 | 112 | 22.5 |
| 10-14 | 151 | 20.5 | 99 | 19.9 |
| 15-19 | 152 | 20.7 | 111 | 22.3 |
| 20-24 | 8 | 1.1 | 6 | 1.2 |
| Diagnosis | ||||
| Acute lymphoblastic leukemia | 288 | 39.1 | 196 | 39.4 |
| Acute myelogenous leukemia | 31 | 4.2 | 19 | 3.8 |
| Chronic myelogenous leukemia | 2 | 0.3 | 2 | 0.4 |
| Carcinoma | 2 | 0.3 | 1 | 0.2 |
| CNS tumor | 6 | 0.8 | 3 | 0.6 |
| Ewing sarcoma family of tumors | 49 | 6.7 | 32 | 6.4 |
| Germ cell tumor | 3 | 0.4 | 3 | 0.6 |
| Hodgkin lymphoma | 198 | 26.9 | 134 | 26.9 |
| Non-Hodgkin lymphoma | 24 | 3.3 | 18 | 3.6 |
| Neuroblastoma | 36 | 4.9 | 22 | 4.4 |
| Osteosarcoma | 58 | 7.9 | 40 | 8.0 |
| Rhabdomyosarcoma | 18 | 2.4 | 11 | 2.2 |
| Soft tissue sarcoma | 3 | 0.4 | 2 | 0.4 |
| Wilms tumor | 19 | 2.6 | 15 | 3.0 |
| Chest-directed RT dose, Gy | ||||
| None | 388 | 52.7 | 264 | 53.0 |
| > 0 to < 20 | 59 | 8.1 | 38 | 7.6 |
| 20 to 29.9 | 127 | 17.2 | 89 | 17.9 |
| ≥ 30 | 163 | 22.1 | 107 | 21.5 |
| Anthracycline exposure | ||||
| Yes | 558 | 75.7 | 377 | 75.7 |
| No | 179 | 24.3 | 121 | 24.3 |
| Anthracycline cumulative dose, mg/m2 | ||||
| > 350 | 156 | 21.5 | 108 | 21.8 |
| > 150 to 350 | 137 | 18.8 | 98 | 19.8 |
| 0 to 150 | 255 | 35.1 | 169 | 34.1 |
| None | 179 | 24.6 | 121 | 24.4 |
| Bleomycin, mg/m2 | ||||
| Yes | 63 | 8.6 | 43 | 8.6 |
| No | 674 | 91.5 | 455 | 91.4 |
| Median | 109.5 | 116 | ||
| Range | 32-237 | 34-237 | ||
| Education level | ||||
| 0: No high school | — | — | 54 | 11.1 |
| 1: High school or some college | — | — | 253 | 52.0 |
| 2: Bachelor's degree or higher | — | — | 180 | 37.0 |
| Age at questionnaire, years | ||||
| 16-25 | — | — | 71 | 14.3 |
| 26-35 | — | — | 137 | 27.6 |
| 36-45 | — | — | 220 | 44.3 |
| 46-55 | — | — | 66 | 13.3 |
| > 55 | — | — | 3 | 0.6 |
| Body mass index, kg/m2 | ||||
| Normal or underweight (< 25) | — | — | 175 | 35.1 |
| Overweight (25–29) | — | — | 148 | 29.7 |
| Obese I (30–34) | — | — | 96 | 19.3 |
| Obese II (35–39) | — | — | 44 | 8.8 |
| Obese III (≥ 40) | — | — | 35 | 7.0 |
| Splenectomy | ||||
| Yes | — | — | 87 | 17.5 |
| No | — | — | 411 | 82.5 |
| Hypertension (BP > 140/90 mmHg) | ||||
| Yes | — | — | 115 | 23.1 |
| No | — | — | 383 | 76.9 |
| Systolic function (EF), % | ||||
| ≥ 50 | — | — | 433 | 90.4 |
| < 50 | — | — | 47 | 9.6 |
| Aortic valve regurgitation† | ||||
| Yes | — | — | 19 | 3.8 |
| No | — | — | 479 | 96.2 |
| Coronary artery disease | ||||
| Yes | — | — | 21 | 4.2 |
| No | — | — | 477 | 95.8 |
| Pulmonary function | ||||
| Normal (≥ 60% predicted) | — | — | 126 | 25.3 |
| Abnormal (< 60% predicted) | — | — | 61 | 12.3 |
| Not screened | — | — | 311 | 62.5 |
| Left atrial pressure (E/E′) | ||||
| < 9 | — | — | 339 | 70.2 |
| ≥ 9 to < 13 | — | — | 94 | 19.5 |
| ≥ 13 | — | — | 50 | 10.4 |
Abbreviations: BP, blood pressure; E/E′, ratio of early rapid filling wave and early diastolic velocity of the mitral annulus; EF, ejection fraction; RT, radiotherapy; TRV, tricuspid regurgitant jet velocity.
Percentages provided for total number of participants for whom data were available for a given characteristic.
Aortic valve regurgitation limited to moderate and severe regurgitation as defined by the American Society of Echocardiography Guidelines.
Statistical Methods
Univariate logistic regression was used to evaluate the relationship between increased TRV and demographic and treatment variables. Covariates with P values less than .1 in the univariate analysis were included in the multiple logistic regression models. The multivariable model used for evaluating the association between increased TRV and prior chest-directed RT and anthracycline exposure was adjusted for race, sex, BMI, education level, and age at the time of echocardiography. All of these factors were significant in the univariate model except for sex, education level, and current age, which were forced into the model. To further explore the potential pathway between RT exposure and increased TRV, we evaluated the impact of organ injury (cardiac left atrial pressure [E/E′] v pulmonary function, defined as < 60% of predicted function on at least one PFT), systolic dysfunction (ejection fraction [EF] < 50%), and cardiac injury pathways (coronary artery disease, aortic valve regurgitation, hypertension) on increased TRV in multivariable models. All models were adjusted for race, sex, BMI, education level, and age at the time of echocardiography. In addition, associations between increased TRV and individual pulmonary function tests were examined by the Cochran-Armitage test for trend. All analyses were performed by using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Clinical Characteristics
The characteristics of the study population are provided in Table 1. Evaluable participants were most commonly treated for acute lymphoblastic leukemia or Hodgkin lymphoma (HL), had a median current age of 38.0 years (range, 20.0 to 59.0 years), and were a median of 27.3 years (range, 12.2 to 46.0 years) from cancer treatment. Cardiotoxic exposures included anthracycline without chest-directed RT (n = 264; 53.0%), chest-directed RT without anthracyclines (n = 121; 24.3%), and anthracyclines plus chest-directed RT (n = 113; 22.7%). The mean EF was 60.7% (standard deviation, 9.94%).
Prevalence of Increased TRV
The mean TRV was 2.50 m/s ± 0.34 m/s standard deviation, and 75 (15.1%) of 498 survivors had a TRV more than 2.8 m/s. Patients treated for HL had a prevalence of increased TRV of 27.6% (Fig 2A). Among patients who received chest-directed RT, the prevalence was 25.2% (Table 2). Prevalence increased with increasing dose of chest-directed RT (Fig 2B). Lower levels of pulmonary function (decreased FVC, FEV1, TLC, and DLCO-corr) were statistically significantly associated with increasing TRV (P for trend < .01; Appendix Fig A1, online only).
Fig 2.

Distribution of tricuspid regurgitant jet velocity (TRV; median and 95% CIs) by (A) primary cancer diagnosis and (B) dose of chest-directed radiation therapy received. Horizontal line at 2.8 m/s represents the cutoff for an abnormal TRV.
Table 2.
Distribution of TRVs
| TRV (m/s) | No. of Patients | % | Median TRV (m/s)* | Range (m/s) |
|---|---|---|---|---|
| Total population (N = 498) | ||||
| ≤ 2.8 | 423 | 85.0 | 2.4 | 0.9-2.8 |
| > 2.8-3.4 | 69 | 13.9 | 3.0 | 2.8-3.4 |
| > 3.4 | 6 | 1.2 | 3.6 | 3.4-4.0 |
| Survivors with any chest-directed RT (n = 234) | ||||
| ≤ 2.8 | 175 | 74.8 | 2.5 | 1.8-2.8 |
| > 2.8-3.4 | 54 | 23.1 | 3.0 | 2.8-3.4 |
| > 3.4 | 5 | 2.1 | 3.5 | 3.4-4.0 |
| Survivors with chest-directed RT ≥ 30 Gy (n = 107) | ||||
| ≤ 2.8 | 74 | 69.2 | 2.5 | 1.8-2.8 |
| > 2.8-3.4 | 28 | 26.2 | 3.0 | 2.8-3.4 |
| > 3.4 | 5 | 4.7 | 3.5 | 3.4-4.0 |
Abbreviations: RT, radiotherapy; TRV, tricuspid regurgitant jet velocity.
Median and range do not include patients for whom Doppler echocardiography was fully evaluable but TRV was not measurable.
Diagnosis and Treatment-Related Risk Factors
In univariate analysis, diagnosis of HL (OR, 4.20; 95% CI, 2.16 to 8.16), but not acute lymphoblastic leukemia (OR, 1.53; 95% CI, 0.77 to 3.07), compared with all other cancer diagnoses was associated with development of a TRV more than 2.8 m/s. Treatment with any dose of chest-directed RT (1 to 19.9 Gy: OR, 3.00; 95% CI, 1.09 to 8.24; 20 to 29.9 Gy: OR, 4.43; 95% CI, 2.18 to 9.00; ≥ 30 Gy: OR, 6.91; 95% CI, 3.60 to 13.25, compared with no RT), BMI ≥ 40 kg/m2 (OR, 2.40; 95% CI, 1.03 to 5.60, compared with BMI < 25 kg/m2), splenectomy (OR, 3.15; 95% CI, 1.82 to 5.44), older current age (OR, 1.07; 95% CI, 1.04 to 1.11), left atrial pressure (E/E′ ≥ 13 v < 9: OR, 9.27; 95% CI, 4.79 to 17.95), systolic cardiac dysfunction (EF < 50% v ≥ 50%: OR, 2.11; 95% CI, 1.04 to 4.28), hypertension (OR, 1.85; 95% CI, 1.09 to 3.15), aortic valve regurgitation (OR, 7.08; 95% CI, 2.77 to 18.08), coronary artery disease (OR, 3.01; 95% CI, 1.17 to 7.72), and pulmonary dysfunction defined as pulmonary function less than 60% of predicted value (FVC: OR, 3.97; 95% CI, 1.89 to 8.36; FEV1: OR, 2.95; 95% CI, 1.42 to 6.13; TLC: OR, 3.35; 95% CI, 1.36 to 8.25; DLCO-corr: OR, 2.29; 95% CI, 1.07 to 4.86) were significantly associated with an increased risk for a TRV more than 2.8 m/s. Anthracycline exposure (yes/no) was associated with a significantly reduced risk (OR, 0.25; 95% CI, 0.15 to 0.42).
Multivariable regression identified chest-directed RT as an independent risk factor for increased TRV in a dose-dependent fashion (P for trend < .001; Table 3). BMI more than 40 kg/m2 remained significantly associated with increased TRV in the multivariable analysis, but anthracycline exposure was no longer identified as a statistically significant protective factor (P = .50).
Table 3.
ORs in Multivariable Models for Association Between Increased TRV and Primary Treatment Exposures, Cardiac and Lung Injury, Systolic Function, and Mechanisms of Cardiac Injury*
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Primary treatment exposure model | |||
| Race/ethnicity | |||
| Other | 2.52 | 1.21 to 5.23 | .01 |
| Non-Hispanic white | 1.00 | ||
| Body mass index, kg/m2 | |||
| Obese III (≥ 40) | 3.89 | 1.46 to 10.39 | < .01 |
| Obese II (35–39) | 1.14 | 0.38 to 3.42 | .81 |
| Obese I (30–34) | 1.19 | 0.53 to 2.65 | .67 |
| Overweight (25–29) | 0.91 | 0.46 to 1.82 | .79 |
| Normal (< 25) | 1.00 | ||
| Chest-directed RT dose, Gy | |||
| ≥ 30 | 4.54 | 1.77 to 11.64 | < .01 |
| 20-29.9 | 3.46 | 1.59 to 7.54 | < .01 |
| 1-19.9 | 2.09 | 0.63 to 6.96 | .23 |
| None | 1.00 | ||
| Anthracycline | |||
| Yes | 0.77 | 0.35 to 1.65 | .50 |
| No | 1.00 | ||
| Organ injury model | |||
| Pulmonary function | |||
| Abnormal (< 60% predicted) | 1.73 | 0.76 to 3.92 | .19 |
| Not screened | 0.59 | 0.29 to 1.21 | .15 |
| Normal (≥ 60% predicted) | 1.00 | ||
| Left atrial pressure (E/E′) | |||
| Moderate/severe dysfunction (≥ 13) | 4.55 | 2.04 to 10.13 | < .01 |
| Mild dysfunction (9–13) | 0.95 | 0.44 to 2.05 | .90 |
| Normal (< 9) | 1.00 | ||
| Cardiac function model | |||
| EF, % | |||
| < 50 | 2.05 | 0.94 to 4.45 | .07 |
| ≥ 50 | 1.00 | ||
| Cardiac injury model | |||
| Hypertension | |||
| Yes | 0.97 | 0.51 to 1.85 | .94 |
| No | 1.00 | ||
| Aortic valve regurgitation | |||
| Yes | 5.85 | 2.05 to 16.74 | < .01 |
| No | 1.00 | ||
| Coronary artery disease | |||
| Yes | 2.38 | 0.80 to 7.07 | .12 |
| No | 1.00 |
Abbreviations: E/E′, ratio of early rapid filling wave and early diastolic velocity of the mitral annulus; EF, ejection fraction; OR, odds ratio; TRV, tricuspid regurgitant jet velocity.
All models adjusted for sex, race/ethnicity, education level, current age, and body mass index.
Because both abnormal pulmonary and cardiac function were significant in univariate analysis, we hypothesized that, although the primary inciting event in the causal pathway for increased TRV was radiation exposure, the causal pathway for increased TRV could be mediated through either a pulmonary or cardiac injury pathway or both (Fig 3). To clarify the causal pathway, additional multivariable models that excluded treatment factors (chest-directed RT, anthracycline) demonstrated that increased left atrial pressure and aortic valve regurgitation were independently and statistically significantly associated with the development of a TRV more than 2.8 m/s (Table 3) and were associated with chest-directed RT (Appendix Table A1, online only).
Fig 3.
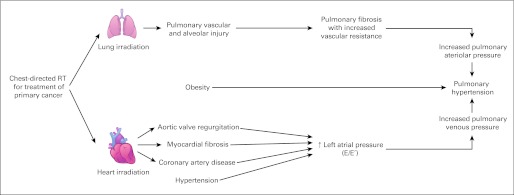
Model of radiation therapy (RT) –induced lung and cardiac injury and resultant pulmonary hypertension.
Functional Assessment
Of the 498 survivors with an evaluable TRV, 485 (98%) completed the 6-minute walk evaluation. Of 73 survivors with a TRV value more than 2.8 m/s who completed the 6-minute walk, 14 (19.4%) walked less than 300 meters, 12 of whom could not complete the first 50 meters of the test because of dyspnea, tachycardia, or increased blood pressure requiring early termination of the assessment. None of these participants had either a lower extremity amputation or a limb-sparing surgical procedure for their primary cancer that would interfere with walking performance. Six survivors had a BMI more than 30 kg/m2. By comparison, only 18 survivors (4.4%) in the group with a TRV ≤ 2.8 m/s walked less than 300 m on the 6-minute walk test. Adjusting for height, weight, and age, the mean difference between the two groups on the 6-minute walk test was 63.1 m (SE 13.2 m; P = .02). Increased TRV was associated with an increased risk for functional limitation on the 6-minute walk (OR, 5.20; 95% CI, 2.5 to 11.0).
DISCUSSION
Adult survivors of childhood cancer are at risk for several cardiopulmonary late effects due to cancer and its treatment. Pulmonary hypertension has not previously been investigated or reported in this population. Through the unique resource of the SJLIFE population, we identified a TRV more than 2.8 m/s by Doppler echocardiography in 15.1% of adult survivors (75 of 498) exposed to cardiotoxic therapy and in 25.2% of those who received chest-directed RT. This risk was associated with chest-directed RT in a dose-dependent manner, reaching a prevalence of 30.8% among those who received more than 30 Gy. Although previous echocardiographic evaluations of cardiac structure and function in survivors have been reported, these studies, largely evaluating younger populations of survivors, have not commented on pulmonary hypertension as defined by an increased TRV.3–6,34,35 Thus, to the best of our knowledge, this is the first report addressing the risk of pulmonary hypertension as a late sequela of chest-directed RT among long-term survivors of childhood cancer.
A definitive diagnosis of pulmonary hypertension should be based on right-heart catheterization.27 However, Doppler echocardiography is often used to screen for possible pulmonary hypertension since it is widely available, noninvasive, and relatively inexpensive.19–22 Although Doppler echocardiography parameters for pulmonary hypertension are established,27,36 and echocardiographic detection of pulmonary hypertension (TRV > 2.8 m/s) is associated with a higher risk for mortality in other noncancer populations,22,37 echocardiography may result in both over- and underestimation of the pulmonary artery pressure measured at catheterization.38 A recent meta-analysis of 29 studies demonstrated that estimations of systolic pulmonary artery pressure based on TRV have modest diagnostic accuracy (sensitivity 83%, specificity 72%) and modest correlation (r = 0.70) with pulmonary artery pressures from catheterization.39 Thus, Doppler echocardiography as an initial screening tool is warranted, but definitive diagnosis of pulmonary hypertension and follow-up of hemodynamic assessments should be based on right-heart catheterization.40
In this study, increased left atrial pressure was associated with an increased risk for increased TRV on multivariable analysis. Cardiac injury is a well-established complication of chest-directed RT that may be manifested as valvular incompetence or stenosis, pericarditis, and/or early onset coronary artery disease. In addition, both RT and anthracycline exposure can cause cardiomyopathy resulting in systolic dysfunction.4,41 Our findings suggest that RT-related aortic valve regurgitation, coronary artery disease, and subsequent increased left atrial pressure may ultimately lead to an increase in pulmonary venous pressure (Fig 3). Furthermore, systemic hypertension, although not significantly associated with TRV on multivariate analysis, remains an important potential etiology of pulmonary hypertension, because it is a modifiable risk factor and a potential target for risk reduction.42 In the general population, pulmonary hypertension resulting from left-heart failure can lead to right-heart failure, which is associated with poor survival; however, the natural history of pulmonary hypertension after chest-directed RT is unknown.
Chest-directed RT can also result in pulmonary injury characterized by pulmonary fibrosis.43 Chest-directed RT can secondarily affect lung function through impairment of chest wall and respiratory muscle growth. However, the failure of pulmonary function testing to achieve statistical significance in multivariable models suggests that RT-related cardiac injury may play a greater role in the development of pulmonary hypertension than direct lung injury does. Furthermore, in this analysis, obesity was associated with increased TRV in survivors. Although this might be expected, because obesity is an established risk factor for pulmonary hypertension in the general population,44 female survivors who received cranial RT have increased rates of obesity and represent a population at increased risk for obstructive sleep apnea.45 Future evaluations for pulmonary hypertension should include assessment for sleep-disordered breathing in this high-risk population.
There are several limitations that must be considered when interpreting our findings. First, although TRV was increased in many survivors, most fell within a range of only modest increase (> 2.8 to 3.4 m/s). However, on functional assessment, survivors with an increased TRV demonstrated significantly reduced exercise capacity compared with those with normal TRV, strengthening the suggestion that underlying pulmonary hypertension may exist. Future evaluations should include stress echocardiography, formal assessment of maximal aerobic exercise capacity, and catheterization for a definitive diagnosis of pulmonary hypertension.46 Second, the cross-sectional nature of this investigation did not allow us to determine whether the natural history of increased TRV after chest-directed RT is progressive. Third, because the COG guidelines prescribe screening echocardiography only for survivors exposed to cardiotoxic therapies, we did not have a control population of nonexposed survivors. Similarly, only patients exposed to pulmonary-toxic therapies received screening pulmonary function testing. Fortunately, however, survivors with no such exposure (and thus not undergoing formal pulmonary function assessment) were less likely to develop increased TRV when compared with survivors with normal pulmonary function who were exposed to pulmonary-toxic agents. Finally, future assessment of this population may benefit from echocardiographic evaluation that includes comprehensive diastolic assessment, based on recent guidelines from the ASE.26 In addition to E/E′, assessment of left atrial volume may more adequately reflect chronically increased left atrial pressure. Likewise, more precise cardiac dosimetry may allow for more specificity in the dose-response relationship between cardiac RT exposure and increased TRV.
In conclusion, we identified an increased prevalence of TRV more than 2.8 m/s, which may indicate pulmonary hypertension, in a large, well-characterized population of childhood cancer survivors. Increased risk was associated with chest-directed RT exposure, potentially mediated through both cardiac and pulmonary dysfunction. Current guidelines for screening and early detection of cardiomyopathy, developed by the COG, recommend transthoracic two-dimensional echocardiography for evaluation of systolic function for all survivors exposed to cardiotoxic therapy.24 Although subsequent studies should validate the prevalence of pulmonary hypertension by catheterization, our current findings suggest that inclusion of TRV in screening echocardiography should be considered. Fortunately, the design of the SJLIFE study will allow for continued investigation of these survivors. Future investigation of this population will include longitudinal follow-up by echocardiography with recommended confirmation of pulmonary hypertension by right-heart catheterization in survivors with functional deficits. Other mechanisms, such as sleep-disordered breathing among obese survivors, will be explored as a possible etiology. Finally, on the basis of the considerable prevalence of this finding among survivors exposed to chest-directed RT and the known progressive nature of pulmonary hypertension, compliance with the COG screening guidelines for echocardiography after chest-directed RT should be emphasized. Evaluation of TRV should be part of the routine screening echocardiogram, although ongoing studies seek to further define the significance and natural history of an increased TRV in this population.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Gregory T. Armstrong, Vijaya M. Joshi, Deokumar Srivastava, Kirsten K. Ness, Leslie L. Robison, Melissa M. Hudson, Daniel M. Green
Collection and assembly of data: Gregory T. Armstrong, Vijaya M. Joshi, Kirsten K. Ness, James A. Fowler, Leslie L. Robison, Melissa M. Hudson, Daniel M. Green
Data analysis and interpretation: Gregory T. Armstrong, Vijaya M. Joshi, Liang Zhu, Deokumar Srivastava, Nan Zhang, Kirsten K. Ness, Dennis C. Stokes, Matthew T. Krasin, Leslie L. Robison, Melissa M. Hudson, Daniel M. Green
Manuscript writing: All authors
Final approval of manuscript: All authors
Appendix
Fig A1.

Distribution of tricuspid regurgitant jet velocity by increasing level of pulmonary dysfunction including (A) forced vital capacity (FVC), (B) forced expiratory volume in one second (FEV1), (C) total lung capacity (TLC), and (D) carbon monoxide diffusing capacity (DLCO-corr). (*) Denotes the P value of trend test.
Table A1.
Frequencies of Cardiopulmonary Outcomes, Stratified by Chest-Directed RT Exposure
| Outcome* | Chest-Directed RT (n = 234) |
No Chest-Directed RT (n = 264) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Left atrial pressure (E/E′) | |||||
| < 9 | 127 | 56.7 | 212 | 81.9 | < .001 |
| ≥ 9 to < 13 | 55 | 24.6 | 39 | 15.1 | |
| ≥ 13 | 42 | 18.8 | 8 | 3.1 | |
| Aortic valve regurgitation | |||||
| Yes | 19 | 8.1 | 0 | 0 | < .001 |
| No | 215 | 91.9 | 264 | 100 | |
| Coronary artery disease | |||||
| Yes | 19 | 8.1 | 2 | 0.8 | < .001 |
| No | 215 | 91.9 | 262 | 99.2 | |
| Pulmonary function | |||||
| Normal (≥ 60% predicted) | 114 | 48.7 | 12 | 4.6 | < .001 |
| Abnormal (< 60% predicted) | 58 | 24.8 | 3 | 1.1 | |
| Not screened | 62 | 26.5 | 249 | 94.3 | |
| Systolic function (EF), % | |||||
| ≥ 50 | 206 | 89.2 | 237 | 91.5 | .38 |
| < 50 | 25 | 10.8 | 22 | 8.5 | |
Abbreviations: E/E′, ratio of early rapid filling wave and early diastolic velocity of the mitral annulus; EF, ejection fraction; RT, radiotherapy.
Percentages provided for total number of participants for whom data were available for a given characteristic.
Footnotes
Supported by the American Lebanese Syrian Associated Charities and National Institutes of Health Cancer Center Support (CORE) Grant No. P30 CA 21765.
Presented at the 42nd Congress of the International Society of Pediatric Oncology, Boston, MA, October 21-24, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975-2008. based on November 2010 SEER data submission, posted to the SEER web site, 2011. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 2.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Hancock SL, Vagelos RH, et al. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977–982. doi: 10.1016/j.ahj.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Hudson MM, Rai SN, Nunez C, et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25:3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 6.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 7.Weiner DJ, Maity A, Carlson CA, et al. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer. 2006;46:222–227. doi: 10.1002/pbc.20457. [DOI] [PubMed] [Google Scholar]
- 8.O'Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med. 1990;323:378–382. doi: 10.1056/NEJM199008093230604. [DOI] [PubMed] [Google Scholar]
- 9.Mann JR, Pearson D, Barrett A, et al. Results of the United Kingdom Children's Cancer Study Group's malignant germ cell tumor studies. Cancer. 1989;63:1657–1667. doi: 10.1002/1097-0142(19900501)63:9<1657::aid-cncr2820630902>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Marina NM, Greenwald CA, Fairclough DL, et al. Serial pulmonary function studies in children treated for newly diagnosed Hodgkin's disease with mantle radiotherapy plus cycles of cyclophosphamide, vincristine, and procarbazine alternating with cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine. Cancer. 1995;75:1706–1711. doi: 10.1002/1097-0142(19950401)75:7<1706::aid-cncr2820750723>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Seguchi M, Hirabayashi N, Fujii Y, et al. Pulmonary hypertension associated with pulmonary occlusive vasculopathy after allogeneic bone marrow transplantation. Transplantation. 2000;69:177–179. doi: 10.1097/00007890-200001150-00030. [DOI] [PubMed] [Google Scholar]
- 12.Limsuwan A, Pakakasama S, Rochanawutanon M, et al. Pulmonary arterial hypertension after childhood cancer therapy and bone marrow transplantation. Cardiology. 2006;105:188–194. doi: 10.1159/000091638. [DOI] [PubMed] [Google Scholar]
- 13.Morales IJ, Anderson PM, Tazelaar HD, et al. Pulmonary cytolytic thrombi: Unusual complication of hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2003;25:89–92. doi: 10.1097/00043426-200301000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Qasim W, Gerritsen B, Veys P. Anticardiolipin antibodies and thromboembolism after BMT. Bone Marrow Transplant. 1998;21:845–847. doi: 10.1038/sj.bmt.1701184. [DOI] [PubMed] [Google Scholar]
- 15.Bentur L, Cullinane C, Wilson P, et al. Fatal pulmonary arterial occlusive vascular disease following chemotherapy in a 9-month-old infant. Hum Pathol. 1991;22:1295–1298. doi: 10.1016/0046-8177(91)90116-7. [DOI] [PubMed] [Google Scholar]
- 16.Shankar S, Choi JK, Dermody TS, et al. Pulmonary hypertension complicating bone marrow transplantation for idiopathic myelofibrosis. J Pediatr Hematol Oncol. 2004;26:393–397. doi: 10.1097/00043426-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Vaksmann G, Nelken B, Deshildre A, et al. Pulmonary arterial occlusive disease following chemotherapy and bone marrow transplantation for leukaemia. Eur J Pediatr. 2002;161:247–249. doi: 10.1007/s00431-002-0961-5. [DOI] [PubMed] [Google Scholar]
- 18.Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:1527–1538. doi: 10.1016/j.jacc.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin V, Humbert M, Coghlan G, et al. Pulmonary arterial hypertension: The most devastating vascular complication of systemic sclerosis. Rheumatology (Oxford) 2009;48:iii25–iii31. doi: 10.1093/rheumatology/kep107. [DOI] [PubMed] [Google Scholar]
- 20.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 21.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 22.Morris CR, Kim HY, Trachtenberg F, et al. Risk factors and mortality associated with an elevated tricuspid regurgitant jet velocity measured by Doppler-echocardiography in thalassemia: A Thalassemia Clinical Research Network report. Blood. 2011;118:3794–3802. doi: 10.1182/blood-2010-11-319152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 29.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 30.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 31.Morris AH, Kanner RE, Crapo RO, et al. Clinical Pulmonary Function Testing: A Manual of Uniform Laboratory Procedures (ed 2) Salt Lake City, UT: Intermountain Thoracic Society; 1984. [Google Scholar]
- 32.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 33.Rostagno C, Olivo G, Comeglio M, et al. Prognostic value of 6-minute walk corridor test in patients with mild to moderate heart failure: Comparison with other methods of functional evaluation. Eur J Heart Fail. 2003;5:247–252. doi: 10.1016/s1388-9842(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 34.Heidenreich PA, Hancock SL, Lee BK, et al. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42:743–749. doi: 10.1016/s0735-1097(03)00759-9. [DOI] [PubMed] [Google Scholar]
- 35.Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin's disease in children and adolescents. J Clin Oncol. 1993;11:1208–1215. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- 36.Galiè N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension: The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25:2243–2278. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359:2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 38.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: A systematic review and meta-analysis. Heart. 2011;97:612–622. doi: 10.1136/hrt.2010.212084. [DOI] [PubMed] [Google Scholar]
- 40.Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: Implications for clinical practice. Chest. 2011;139:988–993. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 41.Lipshultz SE, Vlach SA, Lipsitz SR, et al. Cardiac changes associated with growth hormone therapy among children treated with anthracyclines. Pediatrics. 2005;115:1613–1622. doi: 10.1542/peds.2004-1004. [DOI] [PubMed] [Google Scholar]
- 42.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang TT, Hudson MM, Stokes DC, et al. Pulmonary outcomes in survivors of childhood cancer: A systematic review. Chest. 2011;140:881–901. doi: 10.1378/chest.10-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McQuillan BM, Picard MH, Leavitt M, et al. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–2802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 45.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 46.Pellikka PA, Nagueh SF, Elhendy AA, et al. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]



