Case Report
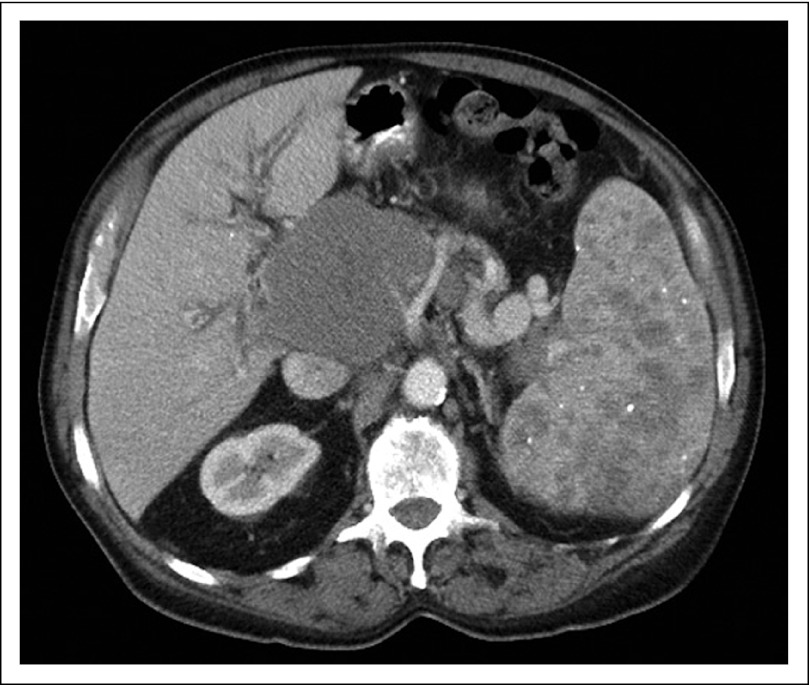
A 74-year-old Greek man with a previous medical history that was significant for hypertension, glaucoma, erosive gastritis, and prostate cancer was admitted with symptoms of increasing abdominal pain, nausea, vomiting, lightheadedness, and constipation. He was afebrile and normotensive on presentation with physical examination findings that were notable for temporal wasting, distended but nontender abdomen with splenomegaly, 2+ pitting edema of lower extremities, and bilateral palpable axillary lymphadenopathy. Presenting CBC and chemistries included hemoglobin, 8.4 g/dL; WBC count, 7.6 K/μL (68% segmented neutrophils, 12% lymphocytes, 20% monocytes); platelets, 210 K/μL; sodium, 131 mEq/L; potassium, 4.3 mEq/L; chloride, 96 mEq/L; bicarbonate, 27 mEq/L; blood urea nitrogen, 24 mg/dL; creatinine, 1.0 mg/dL; calcium, 9.3 mg/dL; albumin, 3.3 g/dL; AST, 99 U/L; ALT, 41 U/L; alkaline phosphatase, 444 U/L; total bilirubin, 2.6 mg/dL; prothrombin time, 17.1 seconds; activated partial thromboplastin time, 48.0 seconds, and international normalized ratio of 1.4. A computed tomography scan of his chest, abdomen, and pelvis revealed an 8.2 × 8.3-cm mass anterior to the head of the pancreas with complete compression and thrombosis of right, left, and main portal veins (Fig 1). In addition, multiple lymph nodes were enlarged in bilateral axillae, the mediastinum, and the retroperitoneum. The spleen was not enlarged but contained multiple hypodensities throughout that were suggestive of lymphomatous involvement (Fig 1). The abdominal mass was first biopsied via endoscopic ultrasound with fine needle aspirate, but was nondiagnostic. The patient subsequently underwent excisional biopsy of a right axillary lymph node.
Fig 1.
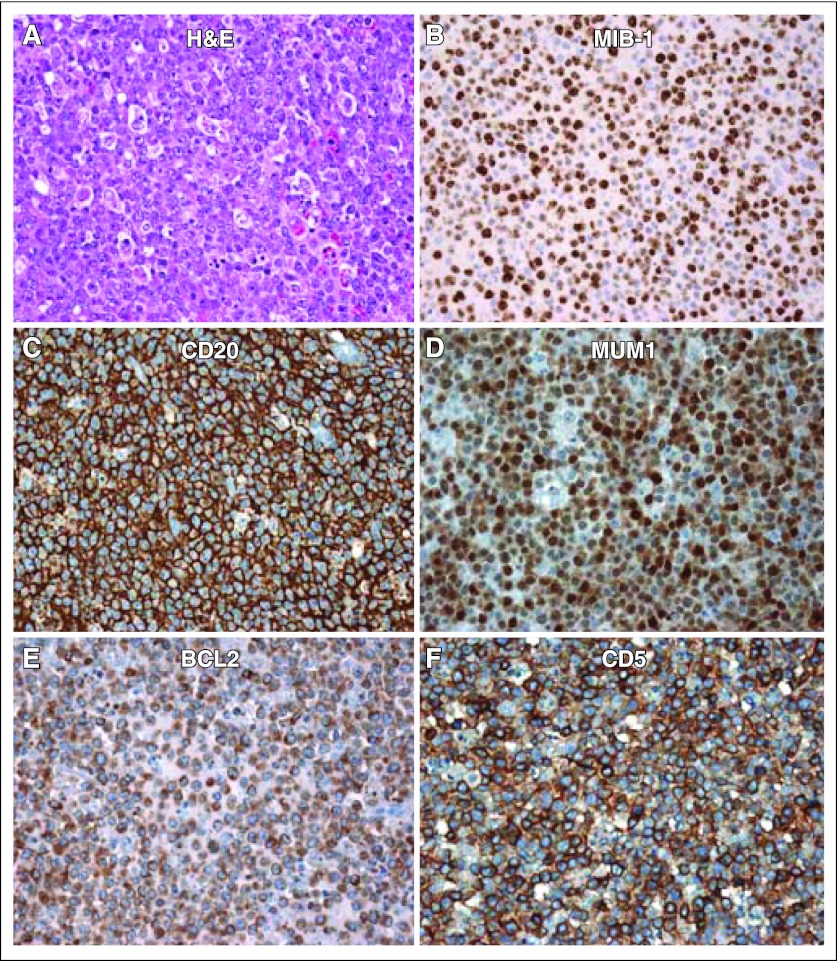
Pathologic evaluation of the axillary lymph node revealed diffuse effacement of the nodal architecture with a proliferation of medium to large sized atypical lymphoid cells (Fig 2A, H&E, hematoxylin and eosin). Large areas of necrosis were present, and there was extension of the atypical cells through the lymph node capsule with preservation of the subcapsular sinus. The proliferation rate as measured by Ki-67 staining was estimated at 75% to 80% (Fig 2B). Epstein-Barr virus in situ hybridization was negative. Immunohistochemical staining patterns demonstrated atypical lymphoid cells to express CD20 (Fig 2C), PAX5, MUM1 (Fig 2D), BCL2 (Fig 2E), CD5 (Fig 2F), and BCL6, but were negative for expression of CD10, CD3, CD23, and cyclin D1 (not shown). Notably, the CD5 expression was biphasic with more intense staining of the T lymphocyte (CD3) and less intense positive expression of the neoplastic B cells (CD20); no additional markers were tested. Flow cytometry for further cellular characterization could not be performed because of poor viability. The diagnosis was consistent with CD5+ diffuse large B-cell lymphoma (DLBCL; Fig 2).
Fig 2.
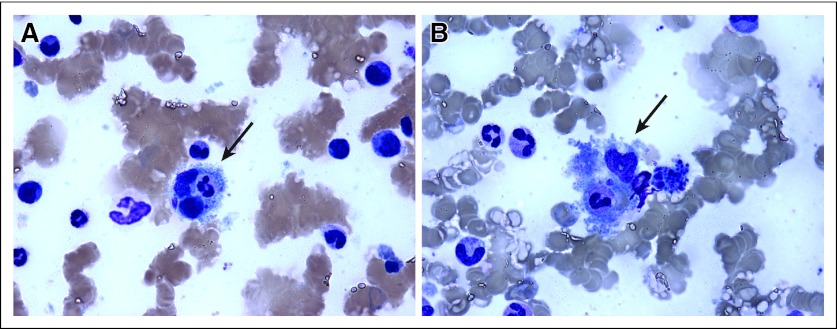
The patient's clinical condition quickly worsened and he became encephalopathic, with an ammonia level of 173 mcg/dL, prothrombin time of 22.7 seconds, and activated partial thromboplastin time of 57.6 seconds; however, there was no evidence of bleeding, but such derangements precluded invasive investigations to his encephalopathy. Other abnormal laboratory studies included hemoglobin of 7.2 g/dL with reticulocyte count of 2.7%. Evaluation of the peripheral blood film demonstrated evidence of multiple schistocytes that were consistent with consumptive microangiopathic hemolytic anemia. Fibrinogen levels were elevated at 456 mg/dL, and serum ferritin (2,365 ng/ml) and lactate dehydrogenase (2,365 U/L) were markedly elevated. These findings were most consistent with a hyperinflammatory state such as hemophagocytosis, which was subsequently demonstrated on bone marrow biopsy (Fig 3, arrows). Other laboratory derangements included platelet count (111 K/μL), albumin (2.9 g/dL), alkaline phosphatase (778 U/L), AST (151 U/L), ALT (64 U/L), and total bilirubin (6.4 mg/dL). Uric acid and triglycerides were within normal limits and there was no serologic evidence of acute cytomegalovirus, hepatitis, HIV, or Epstein-Barr virus infection.
Fig 3.
Given the patient's rapid clinical deterioration, emergent therapy was planned with dose-adjusted etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone with rituximab; however, because of his poor performance status and tenuous liver reserve he was first treated with high-dose corticosteroids with rituximab, in response to which he had an immediate decrease in his hepatic enzymes. The following day, cyclophosphamide was administered, but despite these interventions, the patient continued to have rising ammonia levels with increasing abdominal pain and progressively worsening lactic acidosis. He was transferred to intensive care and was found to have an ischemic bowel. Surgery was declined and the patient subsequently died. An autopsy was not performed. Posthumous bone marrow biopsy results demonstrated megakaryocyte hyperplasia, moderate normocytic anemia, mild thrombocytopenia, and hemophagocytosis (Fig 3, arrows).
Given the lack of clinical history of chronic lymphocytic leukemia (CLL)/small lymphocytic leukemia (SLL) in our patient, the absence of morphologic features suggestive of transformation out of a CLL/SLL background, and CD23 negativity, Richter transformation was not favored. Furthermore, the lack of cyclin D1 expression did not favor mantle-cell lymphoma (MCL). Therefore, de novo CD5+ DLBCL was the favored diagnosis with the notable presence of coexisting hemophagocytosis.
Discussion
DLBCL is the most common B-cell non-Hodgkin lymphoma, with an estimated incidence of approximately 40,000 cases to be diagnosed in 2012.1 Recent molecular characterization of DLCBL has allowed it to be further subdivided into cell-of-origin phenotypes on the basis of gene expression profiling.2 The different subgroups of DLCBL not only differ in overall prognosis, but they also exhibit differential response to therapy such as bortezomib.3 Recently, it has been recognized that a subset of patients with DLBCL express surface T-cell antigen CD5 and have a particularly poor prognosis.
CD5 expression in B-cell malignancies is typically associated with CLL, SLL, and MCL. CD5+ DLBCL is most commonly reported as the result of Richter transformation.4,5 However, de novo CD5+ DLBCL is increasingly recognized as a distinct pathologic phenomenon with a specific clinical picture.
Several case series of de novo CD5+ DLBCL that have been reported from Japan6,7 reveal distinct phenotypic differences between patients who express CD5 and those who do not. Specifically, these case series revealed that patients with CD5+ DLBCL were older, had poorer performance status, and were more likely to be female. They presented with higher lactate dehydrogenase levels, advanced stage (III/IV), more than one extranodal site, higher International Prognostic Index scores, and commonly had B symptoms.7
CD5, a member of a highly conserved superfamily of protein receptors named SRCR (scavenger receptor cysteine-rich), is highly expressed in T cells and is therefore used as a surface marker to identify these cells. However, CD5 can also be found in other subtypes of lymphocytes at different stages of development and maturation.9 CD5+ B cells predominate in the human fetal spleen and cord blood10; however, they also represent approximately 10% of healthy adult peripheral blood B cells.10 They have also been associated with autoimmune disorders11 and hematologic malignancies. In hematologic malignancies, CD5 is expressed in most cases of CLL, MCL, marginal zone B-cell lymphoma,12 and rarely in DLBCL.13 A recent clinicopathologic analysis showed that in comparison to CD5- DLBCL and MCL, CD5+ DLBCL has a more aggressive phenotype and an inferior response to chemotherapy.7 The mere presence of CD5 on an aggressive subtype of DLBCL, namely activated B-cell–type DLBCL, showed a poorer response to treatment and hence prognosis than its CD5- counterpart.14,15 Postulations for the aggressive phenotype of CD5+ DLBCL may be related to altered regulation of specific genes. For instance, the CD5 genetic signature in DLBCL revealed downregulation of a particular set of genes that are associated with the extracellular matrix such as POSTN, SPARC, COL1A1, COL3A1, CTSK, MMP9, and LAMB3 as well as specific gene clusters associated with immune response in T cells, macrophages, and follicular dendritic cells.14 Furthermore, other studies have shown that immunophenotypically, CD5+ DLBCL is associated with expression of BCL2.15 Upregulation of antiapoptotic signals in aggressive lymphomas leads to a more chemoresistant phenotype.16–18 Notably, CD5 has also been shown to affect chemoresistance in patients with CLL via upregulation of Mcl-1, a member of the BCL2 antiapoptotic family, and expression of interleukin-10.19,20 Interleukin-10 has been shown to enhance B-cell survival, in part by upregulating the expression of BCL2.21 In part, this could explain why CD5 expression in a DLBCL cell inherently gives it a worse prognosis in response to chemotherapy; expression of CD5 in an aggressive lymphoma may induce chemoresistance through a process of upregulating antiapoptotic signals.
Another unusual component of our patient's case, in addition to CD5 positivity, is the presence of hemophagocytosis. Hemophagocytic syndrome (HPS) is a clinical syndrome that is characterized by a severe hyperinflammatory condition resulting in increased production of cytokines, impaired cytotoxic T-cell and natural killer cell production, and stimulation of mononuclear cells.22–25 Core signs of HPS include prolonged fever, hepatosplenomegaly with lab derangements of cytopenias, hypertriglyceridemia, low fibrinogen, and elevated ferritin. HPS may be categorized as a primary or secondary disorder and either condition may occur at any age, although primary HPS typically occurs in childhood, has a genetic or familial component, and is associated with immunodeficiency.24,26 Secondary or acquired HPS typically occurs in adults and is associated with infections, autoimmune disorders, and malignancy.23,27
The majority of HPS-associated malignancies are T-cell and natural killer–cell lymphomas. B-cell lymphoma-associated HPSs (B-LAHSs) have mainly been reported in the Asian population,27–29 but rarely has B-LAHS been reported in the Western literature.30 Diagnostic criteria for adult LAHS were proposed in the Japanese literature (Table 1).27 B-LAHS has been regarded as a variant of intravascular lymphoma,29 which we cannot fully exclude in our patient without autopsy.
Table 1.
Diagnostic Criteria for Adult LAHS27
| Criteria |
|---|
| 1. High fever for more than 1 week (peak ≥ 38°C) |
| 2. Anemia (Hb 9 g/dL) or thrombocytopenia (plt < 1,000,000/μL) |
3. At least two of four subitems:
|
| 4. Hemophagocytosis in bone marrow, spleen, or liver |
| 5. No evidence of infection |
| 6. Histopathologically confirmed malignant lymphoma |
NOTE. A diagnosis of LAHS requires all of the items listed in this table to be fulfilled. When items 1 through 5 are present for 2 weeks and glucocorticoids or γ-globulin therapy are not effective, a diagnosis of probable LAHS can be made and chemotherapy against malignant lymphoma can be started.
Abbreviations: CT, computed tomography; FDP, fibrin degradation products; Hb, hemoglobin; LAHS, lymphoma-associated hemophagocytic syndrome; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; plt, platelets; US, ultrasonography.
In summary, we present a patient with a rare, aggressive variant of DLBCL (CD5+ DLBCL) associated with HPS, a constellation of clinical features not frequently reported in patients of Western origin. CD5+ may confer a phenotype for DLBCL that may result in chemoresistance of neoplastic cells via upregulating antiapoptotic signals, abnormal associations with the extracellular environment, and affecting cellular immunity, thereby culminating in an aggressive malignancy further evidenced by hemophagocytosis.
ACKNOWLEDGMENT
We thank Stefania Pittaluga, MD, PhD, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, for her help reviewing and assembling histologic images and for her critical review of the manuscript.
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or US government. We certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the writing of the document, and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgments in the document; and that each takes public responsibility for it.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.National Cancer Institute: Surveillance, Epidemiology, and End Results. http://seer.cancer.gov. [Google Scholar]
- 2.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362:1417–1429. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matolcsy A, Chadburn A, Knowles DM. De novo CD5-positive and Richter's syndrome-associated diffuse large B cell lymphomas are genotypically distinct. Am J Pathol. 1995;147:207–216. [PMC free article] [PubMed] [Google Scholar]
- 5.Ohno T, Oka K, Yamaguchi M, et al. Frequent expression of shared idiotypes in mantle cell lymphoma and extranodal small lymphocytic/non-mantle cell diffuse small cleaved lymphoma. Leukemia. 1995;9:1935–1939. [PubMed] [Google Scholar]
- 6.Yamaguchi M, Nakamura N, Suzuki R, et al. De novo CD5+ diffuse large B-cell lymphoma: Results of a detailed clinicopathological review in 120 patients. Haematologica. 2008;93:1195–1202. doi: 10.3324/haematol.12810. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi M, Seto M, Okamoto M, et al. De novo CD5+ diffuse large B-cell lymphoma: A clinicopathologic study of 109 patients. Blood. 2002;99:815–821. doi: 10.1182/blood.v99.3.815. [DOI] [PubMed] [Google Scholar]
- 8.Soldevila G, Raman C, Lozano F. The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Curr Opin Immunol. 2011;23:310–318. doi: 10.1016/j.coi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalloul A. CD5: A safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev. 2009;8:349–353. doi: 10.1016/j.autrev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Antin JH, Emerson SG, Martin P, et al. Leu-1+ (CD5+) B cells: A major lymphoid subpopulation in human fetal spleen—Phenotypic and functional studies. J Immunol. 1986;136:505–510. [PubMed] [Google Scholar]
- 11.Youinou P, Renaudineau Y. CD5 expression in B cells from patients with systemic lupus erythematosus. Crit Rev Immunol. 2011;31:31–42. doi: 10.1615/critrevimmunol.v31.i1.30. [DOI] [PubMed] [Google Scholar]
- 12.Ferry JA, Yang WI, Zukerberg LR, et al. CD5+ extranodal marginal zone B-cell (MALT) lymphoma: A low grade neoplasm with a propensity for bone marrow involvement and relapse. Am J Clin Pathol. 1996;105:31–37. doi: 10.1093/ajcp/105.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Burns BF, Warnke RA, Doggett RS, et al. Expression of a T-cell antigen (Leu-1) by B-cell lymphomas. Am J Pathol. 1983;113:165–171. [PMC free article] [PubMed] [Google Scholar]
- 14.Suguro M, Tagawa H, Kagami Y, et al. Expression profiling analysis of the CD5+ diffuse large B-cell lymphoma subgroup: Development of a CD5 signature. Cancer Sci. 2006;97:868–874. doi: 10.1111/j.1349-7006.2006.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niitsu N, Okamoto M, Tamaru JI, et al. Clinicopathologic characteristics and treatment outcome of the addition of rituximab to chemotherapy for CD5-positive in comparison with CD5-negative diffuse large B-cell lymphoma. Ann Oncol. 2010;21:2069–2074. doi: 10.1093/annonc/mdq057. [DOI] [PubMed] [Google Scholar]
- 16.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal J, Meyer PN, Smith LM, et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res. 2011;17:7785–7795. doi: 10.1158/1078-0432.CCR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomita N. BCL2 and MYC dual-hit lymphoma/leukemia. J Clin Exp Hematop. 2011;51:7–12. doi: 10.3960/jslrt.51.7. [DOI] [PubMed] [Google Scholar]
- 19.Kitada S, Andersen J, Akar S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: Correlations with In vitro and In vivo chemoresponses. Blood. 1998;91:3379–3389. [PubMed] [Google Scholar]
- 20.Perez-Chacon G, Vargas JA, Jorda J, et al. CD5 provides viability signals to B cells from a subset of B-CLL patients by a mechanism that involves PKC. Leuk Res. 2007;31:183–193. doi: 10.1016/j.leukres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245–253. doi: 10.1016/j.blre.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Shabbir M, Lucas J, Lazarchick J, et al. Secondary hemophagocytic syndrome in adults: A case series of 18 patients in a single institution and a review of literature. Hematol Oncol. 2011;29:100–106. doi: 10.1002/hon.960. [DOI] [PubMed] [Google Scholar]
- 24.Szyper-Kravitz M. The hemophagocytic syndrome/macrophage activation syndrome: A final common pathway of a cytokine storm. Isr Med Assoc J. 2009;11:633–634. [PubMed] [Google Scholar]
- 25.Verbsky JW, Grossman WJ. Hemophagocytic lymphohistiocytosis: Diagnosis, pathophysiology, treatment, and future perspectives. Ann Med. 2006;38:20–31. doi: 10.1080/07853890500465189. [DOI] [PubMed] [Google Scholar]
- 26.Shimazaki C, Inaba T, Nakagawa M. B-cell lymphoma-associated hemophagocytic syndrome. Leuk Lymphoma. 2000;38:121–130. doi: 10.3109/10428190009060325. [DOI] [PubMed] [Google Scholar]
- 27.Jordan MB, Allen CE, Weitzman S, et al. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyahara M, Sano M, Shibata K, et al. B-cell lymphoma-associated hemophagocytic syndrome: Clinicopathological characteristics. Ann Hematol. 2000;79:378–388. doi: 10.1007/s002770000155. [DOI] [PubMed] [Google Scholar]
- 29.Shimazaki C, Inaba T, Okano A, et al. Clinical characteristics of B-cell lymphoma-associated hemophagocytic syndrome (B-LAHS): Comparison of CD5+ with CD5- B-LAHS. Intern Med. 2001;40:878–882. doi: 10.2169/internalmedicine.40.878. [DOI] [PubMed] [Google Scholar]
- 30.Wilson MS, Weiss LM, Gatter KC, et al. Malignant histiocytosis: A reassessment of cases previously reported in 1975 based on paraffin section immunophenotyping studies. Cancer. 1990;66:530–536. doi: 10.1002/1097-0142(19900801)66:3<530::aid-cncr2820660321>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]