Abstract
The mechanism of release and the role of l-aspartate as a central neurotransmitter are controversial. A vesicular release mechanism for l-aspartate has been difficult to prove, as no vesicular l-aspartate transporter was identified until it was found that sialin could transport l-aspartate and l-glutamate when reconstituted into liposomes. We sought to clarify the release mechanism of l-aspartate and the role of sialin in this process by combining l-aspartate uptake studies in isolated synaptic vesicles with immunocyotchemical investigations of hippocampal slices. We found that radiolabeled l-aspartate was taken up into synaptic vesicles. The vesicular l-aspartate uptake, relative to the l-glutamate uptake, was twice as high in the hippocampus as in the whole brain, the striatum, and the entorhinal and frontal cortices and was not inhibited by l-glutamate. We further show that sialin is not essential for exocytosis of l-aspartate, as there was no difference in ATP-dependent l-aspartate uptake in synaptic vesicles from sialin-knockout and wild-type mice. In addition, expression of sialin in PC12 cells did not result in significant vesicle uptake of l-aspartate, and depolarization-induced depletion of l-aspartate from hippocampal nerve terminals was similar in hippocampal slices from sialin-knockout and wild-type mice. Further, there was no evidence for nonvesicular release of l-aspartate via volume-regulated anion channels or plasma membrane excitatory amino acid transporters. This suggests that l-aspartate is exocytotically released from nerve terminals after vesicular accumulation by a transporter other than sialin.—Morland, C., Nordengen, K., Larsson, M., Prolo, L. M., Farzampour, Z., Reimer, R. J., Gundersen, V. Vesicular uptake and exocytosis of l-aspartate is independent of sialin.
Keywords: synaptic transmission, VRAC, excitatory amino acid transporters
Besides serving a role as a precursor of Krebs' cycle intermediates and as a building block of proteins, l-aspartate has been proposed to act as a transmitter in the brain. This was first suggested in the hippocampus (1), and later in the cerebellum (2). In favor of aspartergic neurotransmission is the evidence for exocytotic release of l-aspartate from nerve terminals (3–5) and that l-aspartate stimulates the N-methyl d-aspartate (NMDA) type of l-glutamate receptors (6), producing NMDA-receptor-dependent postsynaptic responses (7, 8). A major argument against aspartergic neurotransmission is the conflicting evidence on uptake of l-aspartate into synaptic vesicles. While there seems to be consensus that l-aspartate does not inhibit the vesicular uptake of l-glutamate (9–11), only a few studies have directly measured the uptake of l-aspartate into synaptic vesicles. Using isolated cortical synaptic vesicles, Naito and Ueda (12) found a significant l-aspartate uptake. Other studies show negligible vesicular accumulation of l-aspartate (11, 13).
Recently sialin, which is known to transport sialic acid out of lysosomes (14), was reported to also carry l-aspartate (and l-glutamate) into reconstituted proteoliposomes (15). However, whether sialin has a role in the release of l-aspartate from nerve terminals in intact brain tissue has not been tested. To clarify these ambiguities in aspartergic neurotransmission, we here used brain tissue from the hippocampus, the brain region showing the most robust evidence for synaptic release of l-aspartate (3–5). We first measured the ATP-dependent uptake of l-aspartate in synaptic vesicles isolated from the hippocampus and compared it to the uptake in vesicles isolated from the whole brain and 3 other forebrain regions. We then used sialin-knockout mice to analyze the sialin-dependent l-aspartate vesicle uptake. As a second control for sialin dependency, we measured l-aspartate uptake in vesicles isolated from native PC12 cells and PC12 cells overexpressing sialin. By immunogold cytochemistry of hippocampal slices from sialin-knockout and wild-type mice, we then determined whether sialin was necessary for release of l-aspartate in intact brain tissue. Our results show that l-aspartate is exocytotically released from nerve terminals after vesicular accumulation by a sialin-independent mechanism.
MATERIALS AND METHODS
Animals
All animals were treated in strict accordance with the guidelines of the Norwegian Committees on Animal Experimentation (Norwegian Animal Welfare Act and European Communities Council Directive of 24 November 1986-86/609/EEC) and the Stanford Institutional Animal Care and Use Committee. Male Wistar rats were from Scanbur (Sollentuna, Sweden). Male heterozygous sialin-knockout mice (B6; 129S5–Slc17a5tm1Lex) were obtained from the Mutant Mouse Regional Resource Centre (http://www.mmrrc.org) and bred to homozygosity (16). Mouse genotypes were determined by PCR (16).
Preparation of synaptic vesicles
Synaptic vesicles from rat whole brain, hippocampus, striatum, entorhinal cortex, and frontal cortex, as well as from the whole brain of sialin-knockout and wild-type mice, were isolated as described by Erickson et al. (17). For isolation of synaptic vesicles from the forebrain subregions, the dissected tissue was kept on ice until all brain regions from 10 Wistar rats (∼200 g body weight) had been dissected out (<30 min). Brain tissues were homogenized in 0.32 M sucrose (5%, w/v) and centrifuged for 10 min at 1000 g and 4°C. The supernatants were collected and centrifuged for 30 min at 21,000 g and 4°C. The resulting synaptosomal pellets (P2 fractions), were osmotically shocked by resuspension in ice-cold purified water (7.5%, w/v) and centrifuged for 30 min at 21.000 g to remove the crude synaptosomal membranes. Osmolarity was restored with 0.1 M potassium tartrate and 25 mM Hepes (pH 7.4), before the mixtures were centrifuged for 1 h at 100,000 g and 4°C. The pellets (LP2 fractions), containing crude synaptic vesicles, were gently resuspended in 0.32 M sucrose, frozen in liquid N2, and stored at −80°C or used for uptake assay immediately.
Vesicular uptake of l-aspartate and l-glutamate
Vesicular uptake of l-glutamate and l-aspartate was determined as described by Fykse et al. (18) with slight modifications. Synaptic vesicles from ∼35 mg brain tissue of the whole brain, the hippocampus, the striatum, the frontal cortex, and the entorhinal cortex were preincubated at 30°C for 15 min in an incubation mixture containing 10 mM K-HEPES (pH 7.4), 4 mM KCl, 4 mM MgSO4, 0.25 M sucrose, and 1 mM reduced glutathione. For experiments with Rose Bengal, preincubation time was 25 min in the presence or absence of Rose Bengal, 5 μM. The uptake was started when a substrate solution containing (final concentrations) 2 mM ATP and 1 mM l-[3,4-3H]glutamic acid or 1 mM l-[2,3-3H]aspartic acid (1 μCi; both from PerkinElmer Life and Analytical Sciences, Boston, MA, USA) was added. To determine whether the vesicular uptake of l-aspartate could be blocked by l-glutamate, in some experiments the vesicles were exposed to l-glutamate (1 mM) both during preincubation and incubation. In these experiments, ATP was included also in the preincubation step, to allow complete filling of the vesicles with l-glutamate prior to incubation with [3H]l-aspartate. The uptake of [3H]l-glutamate and [3H]l-aspartate was stopped after 5 min by addition of 7 ml of ice-cold 0.15 M KCl to the 300 μl samples. Blanks were treated as described above, except that ATP was omitted from the incubation buffer. As a negative control, some samples were incubated in ice-cold buffer without ATP. The samples were immediately filtered (Skatron filterMAT 11731; Skatron Instruments, Newmarket, UK). The filters were washed with 3 × 7 ml of 0.15 M KCl, dissolved in Filter-Count (PerkinElmer) and counted for retained radioactivity in a Packard Tri-Carb 300 (Packard Instrument Co., Meriden, CT, USA). The radioactivity in the blanks (incubated at 30°C without ATP) was 20% higher than the values in samples incubated on ice without ATP. ATP-dependent uptake was defined as the radioactivity in the samples (incubated at 30°C with ATP) minus the radioactivity in the blanks and presented as means ± se, n = 3–5 individual experiments for each observation. In each experiment, ATP-dependent uptake was measured in triplicates. Due to small volumes of the subregional vesicular fractions, the protein concentrations of these were not measured, and thus most of the data are given as percentage of the l-glutamate uptake in the same region. However, in one experiment, the protein content in the samples was analyzed (BC assay; Interchem, Montfuçon, France) and the absolute uptake values (pmol/min/mg protein) were calculated. Whether the uptake values in the hippocampus were significantly different from the values in the whole brain and the other subregions was tested by 1-way ANOVA post hoc test for multiple comparisons (SPSS, Chicago, IL, USA). Uptake in sialin-knockout and wild-type vesicles was statistically evaluated by Student's t test, 2 tails (SPSS).
PC12 cell vesicle preparation
The rat vesicular glutamate transporter 1 (VGLUT1) expression plasmid was derived as described previously (19). The rat sialin cDNA (20) was subcloned into the plasmid expression vector pcDNA3-Amp (Invitrogen, Carlsbad, CA, USA). Subsequently, a cDNA encoding the red fluorescent protein (RFP) monomeric Cherry (mCherry) was subcloned at the 5′ end of the sialin sequence to generate a chimeric protein. The plasmids were introduced into PC12 cells using Lipofectamine 2000 (Invitrogen). The cells were then selected in 800 mg/ml G418 (effective concentration; Life Technologies, Gaithersburg, MD, USA), and the resulting clones were screened by direct fluorescence microscopy for sialin-expressing cells and immunofluorescence microscopy for VGLUT1-expressing cells to identify those with the highest level of expression. Cells were maintained in DMEM (Life Technologies) supplemented with 10% equine serum (HyClone, South Logan, UT, USA), 5% calf serum (HyClone), and penicillin-streptomycin (Life Technologies). Vesicle membranes were prepared from PC12 as follows: cells were harvested by trituration washed once in PBS, and cells from each 15-cm plate were resuspended in 1 ml of 0.3 M sucrose and 10 mM HEPES-KOH, pH 7.4 (SH buffer), containing 0.2 mM diisopropylfluorophosphate (DFP), 1 mg/ml pepstatin, 2 mg/ml aprotinin, 2 mg/ml leupeptin, 1 mg/ml E64, and 1.25 mM MgEGTA. The cells were then disrupted by homogenization at 4°C through a ball-bearing device with a clearance of 10 μm (Isobiotec, Heidelberg, Germany). The nuclear debris was sedimented at 1000 g for 5 min, and heavier membranes were eliminated by centrifugation at 20,000 g for 20 min (P2). The remaining light membrane vesicles (crude synaptic-like microvesicles) were sedimented at 100,000 g for 1 h, and the resulting pellet (P3) was resuspended in SH buffer containing the same protease inhibitors, at a final protein concentration of 10 μg/μl.
Transport assay for PC12 vesicles
To initiate the reaction, a 10-μl aliquot of membranes was added to 190 μl SH buffer containing 4 mM MgCl2, 4 mM KCl, 4 mM ATP, 100 μM unlabeled substrate, and 2 μCi 3H-substrate (American Radiolabeled Chemicals, St. Louis, MO, USA). Incubation was performed at 29°C for 5 min, and the reaction was terminated by rapid filtration (DAWP, 0.65 μm; Millipore, Billerica, MA, USA), followed by immediate washing with 6 ml cold SH buffer. Background uptake was determined by parallel assays done in the presence of nigericin (5 μM) and valinomycin (20 μM) to dissipate the proton electrochemical gradient. The trapped radioactivity was measured by scintillation counting in 2.5 ml Cytoscint (ICN Biomedicals, Inc., Aurora, OH, USA). Transport measurements were repeated ≥3 times using ≥2 different membrane preparations.
For Western blotting, P2 and P3 fractions from homogenized PC12 cells (30 μg of protein/lane) were separated by SDS-PAGE and subsequently electrotransferred to polyvinylidene fluoride membranes. Blots were blocked with Blotto (PBS with 5% nonfat dry milk and 0.1% Tween-20) for 1 h and then incubated with an antibody directed against sialin (Alpha Diagnostics, Owings Mills, MD, USA) at a 1:500 dilution or against RFP at 1:5000 overnight at 4°C. Blots were washed, incubated with anti-rabbit horseradish peroxidase-coupled secondary antibody (1:10,000; Pierce, Rockford, IL, USA) in Blotto for 1 h. Proteins were visualized using an ECL Western blotting detection system (Amersham Biosciences, Piscataway, NJ, USA) and exposure of the blot to autoradiography film (MidSci, St. Louis, MO, USA).
Preparation and incubation of hippocampal slices
Hippocampal slice experiments were performed as described previously (21–23). Wistar rats were anesthetized with halotane or isoflurane and decapitated. The brains were removed and the hippocampi dissected out on ice and chopped into 300 μm slices. The slices were preincubated in oxygenated Krebs' solution (126 mM NaCl; 3 mM KCl; 10 mM sodium phosphate buffer, pH 7.4; 1.2 mM CaCl2; 1.2 mM MgSO4; and 10 mM glucose) for 45 min at 30°C. Then the slices were incubated for 45 min at 30°C in Krebs' solution containing physiological (3 mM) or depolarizing (55 mM, Na+ reduced to 80 mM) concentrations of K+. To ensure that l-aspartate was not released through excitatory amino acid transporters (EAATs), all incubations were done in the presence of the EAAT inhibitor (3S)-3-[[3-[[4-(trifluoromethyl)benzoyl]amino]phenyl]methoxy]-l-aspartic acid (TFB-TBOA; 5 μM; Tocris Bioscience, Ellisville, MO, USA), except in one experiment where we incubated the slices without TFB-TBOA at basal conditions (3 mM K+) and with and without TFB-TBOA at depolarizing conditions (55 mM K+). Some slices were exposed to 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB, 100 μM; Sigma, St Louis, MO, USA), which inhibits volume-regulated anion channels, or KB-R7943 (50 μM; Tocris), which inhibits Ca2+ influx via the reversed Na+-Ca2+ exchanger (NCX(rev)), NMDA receptors, and transient receptor potential cation (TRPC)-Ca2+ channels. All inhibitors were present for the last 10 min of preincubation and for the whole incubation period. Some slices were incubated at 55 mM K+ with 0.1 mM Ca2 + (Mg2+ increased to 10 mM).
Slice experiments using sialin-knockout and wild-type mice (3.5 wk of age) were performed essentially as described above, but with the following modifications: 300-μm-thick frontal slices were vibrosliced into oxygenated carbonate-buffered Krebs' solution (126 mM NaCl, 26 mM NaHCO3, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM MgCl2, 2 mM CaCl2, and 10 mM glucose). Then the slices were incubated for 1 h at 32°C in the same buffer at 3 or 55 mM K+, without TFB-TBOA.
After incubation, the slices were fixed in 2.5% glutaraldehyde and 1% formaldehyde for 1 h at room temperature (∼20°C). The slices were stored in fixative diluted 1:10 at 4°C until embedding and sectioning.
Electron microscopic immunocytochemistry
The slices were embedded in Lowicryl HM20 (mouse slices; EMS, Hatfield, PA, USA) or Durcupan (rat slices; EMS) as described previously (24). After the embedding, ultrathin sections (80–100 nm) were cut and labeled with the 435 l-aspartate (diluted 1:300), 607 l-glutamate (diluted 1:40,000 for single labeling and 1:5,000 for double labeling), and 990 GABA (diluted 1:300) antisera. The 435 l-aspartate, 607 l-glutamate, and 990 GABA antisera have been characterized (22, 23, 25–27). To avoid possible cross-reactivities, the l-glutamate, l-aspartate, and GABA antisera were used with the addition of 0.2 mM complexes of glutaraldehyde/formaldehyde-treated l-aspartate, asparagine plus glutamine and asparagine, l-glutamate plus GABA, and l-glutamate plus β-alanine, respectively. As a specificity test, ultrathin test sections containing conjugates of amino acids fixed to brain proteins by formaldehyde and glutaraldehyde (28) were run along with the immunogold experiments. This test system showed that the primary antibodies labeled only the conjugate containing the amino acid against which the antibodies were raised (data not shown).
To visualize aspartate/glutamate and aspartate/GABA in the same nerve terminals, we performed double-labeling experiments, in which the ultrathin sections were first treated with the l-aspartate antibodies and then with the l-glutamate or GABA antibodies. Between the first and the second step, the sections were exposed to formaldehyde vapor (80°C, 1 h) to prevent interference between the sequential incubations (29). Secondary antibodies coupled to colloidal 10- and 15-nm gold particles (British Biocell International, Cardiff, UK) were used in the first and second step, respectively.
The sections were studied in a Tecnai 12 electron microscope (FEI, Hillsboro, OR, USA). Electron micrographs were randomly taken from the CA1 stratum radiatum, granule, or pyramidal cell layer. Excitatory terminals were defined as those making asymmetric synapses with dendritic spines. Inhibitory terminals were defined as those making symmetric synapses with stem dendrites or granule cell bodies and containing GABA immunogold particles. The densities (gold particles/μm2) of l-aspartate and l-glutamate gold particles in excitatory terminals and of l-aspartate gold particles in inhibitory nerve terminals, as well as the spatial relation between gold particles signaling l-aspartate and l-glutamate and synaptic vesicles in excitatory terminals in sialin-knockout and wild-type slices incubated at 3 mM K+, were calculated (30). A plug-in for ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/) was used to outline the plasma membrane of nerve terminals, and to record the distance between the center of a gold particle or points randomly placed over the excitatory terminals (the number of random points were approximately the same as the number of gold particles in the terminals) and the center of the closest synaptic vesicle. Recorded coordinates were submitted to a program written in Python (http://www.python.org) for computation of particle density (gold particles/μm2; ref. 31). The source code of the ImageJ plug-in and the Python program are available online (http://www.neuro.ki.se/broman/maxl/software.html). The gold particle-synaptic vesicle distances were sorted into bins of 5 nm, and the frequencies of the intercenter distances for each bin were calculated (Excel; Microsoft, Redmond, WA, USA). The quantitative results were statistically evaluated by χ2 test, 1-way ANOVA post hoc test for multiple comparisons (SPSS), and Student's t test, 2 tails (SPSS).
RESULTS
l-Aspartate is taken up into synaptic vesicles
We compared the ATP-dependent uptake of l-aspartate in isolated synaptic vesicles from the hippocampus with the uptake in vesicles from the whole brain and different other brain regions. The ATP-dependent vesicular l-aspartate uptake was assessed relative to the ATP-dependent vesicular l-glutamate uptake in the same vesicle fraction. In hippocampal vesicles, the ATP-dependent l-aspartate uptake was 18.1% of the l-glutamate uptake (Fig. 1A). In comparison, vesicles isolated from the whole brain showed an ATP-dependent l-aspartate uptake of 9.1% of the l-glutamate uptake. In the entorhinal cortex, the frontal cortex, and the striatum, ATP-dependent l-aspartate uptake was 9.6, 10.1, and 11.9% (relative to the ATP-dependent l-glutamate uptake), respectively (Fig. 1A). The vesicular uptake of l-aspartate was not inhibited by l-glutamate, as addition of 1 mM l-glutamate during the preincubation and incubation steps did not alter the uptake of radiolabeled l-aspartate in hippocampal vesicles (Fig. 1B). The l-aspartate and l-glutamate uptake values reported were obtained from frozen vesicles; however, to validate the vitality of these vesicles, a control experiment (n = 6–9 replicates from 2–3 experiments) was performed where we compared l-aspartate and l-glutamate uptake into frozen and fresh hippocampal vesicles. The uptake into frozen vesicles (59.2±1.8 pmol/min/mg protein for l-glutamate and 7.8±1.3 pmol/min/mg protein for l-aspartate) were only slightly lower that the uptake in freshly prepared vesicles (67.3±7.0 pmol/min/mg protein for l-glutamate and 9.8±1.9 pmol/min/mg protein for l-aspartate, means±sd). l-Glutamate uptake, but not l-aspartate uptake, could be almost completely blocked by the vesicular glutamate uptake blocker Rose Bengal (5 μM, data not shown; for characterization of Rose Bengal; see ref. 32), supporting the notion that l-aspartate is accumulated into synaptic vesicles by another transporter than the VGLUTs (33).
Figure 1.
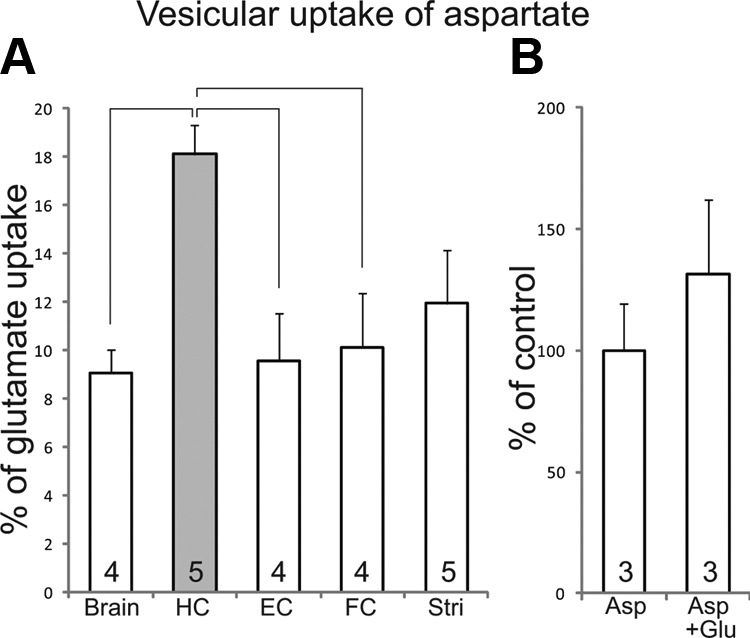
l-Aspartate is taken up into synaptic vesicles. A) ATP-dependent uptake of l-aspartate relative to the l-glutamate uptake (average±sem, number of experiments indicated at bottom of each bar) in isolated rat synaptic vesicles from the whole brain (brain), hippocampus (HC), entorhinal cortex (EC), frontal cortex (FC), and striatum (Stri). In each experiment, the ATP-dependent uptake was analyzed in triplicate. Brackets indicate that the value in the hippocampus is significantly different from the values in the whole brain, entorhinal cortex. and frontal cortex (P<0.05, 1-way ANOVA post hoc; SPSS). B) Vesicular uptake of l-aspartate without (Asp) and with l-glutamate (Asp+Glu) (average±sem, number of experiments indicated at bottom of each bar). The individual aspartate uptake values of each experiment without and with l-glutamate were normalized to the average aspartate uptake without l-glutamate (0.0113 pmol/min/mg tissue, n=3 experiments). sem was calculated based on the normalized values for the individual experiments. There was no significant difference between uptake values in Asp and Asp+Glu (P>0.05, Student's 2-tailed t test; SPSS).
Vesicular accumulation of l-aspartate is sialin-independent
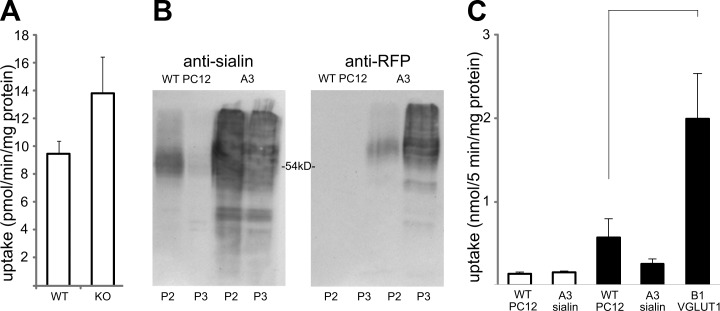
Previous studies have demonstrated that sialin, when reconstituted into proteoliposomes, can catalyze transmembrane l-aspartate transport. This activity was competitively inhibited by l-glutamate. To determine whether sialin is an important vesicular transporter for l-aspartate, we measured ATP-dependent accumulation of l-aspartate in synaptic vesicles from sialin-knockout and wild-type whole brain. We found that the lack of sialin did not reduce the ability of the vesicles to accumulate l-aspartate (Fig. 2A) or l-glutamate (data not shown). This was further confirmed in another test system, in which we overexpressed sialin (tagged with the RFP mCherry) in PC12 cells and isolated synaptic like microvesicles (P3 fraction) from wild-type cells and cells overexpressing sialin. Western blotting with antibodies against sialin showed that wild-type cells contained endoegenous sialin in the crude synaptosome fraction (containing lysosomes), while sialin was not detected in synaptic-like microvesicles (P3 fraction; Fig. 2B). In PC12 cells overexpressing sialin, antibodies against sialin, as well as against RFP, recognized recombinant sialin in both the P2 and the P3 fraction (Fig. 2B). There was essentially no l-aspartate uptake in P3 vesicles from wild-type or sialin-overexpressing PC12 cells (Fig. 2C), while vesicles from cells expressing the vesicular l-glutamate transporter VGLUT1 displayed robust uptake of l-glutamate (Fig. 2C). Together these findings strongly suggest that sialin is not capable of mediating l-aspartate transport in native vesicles.
Figure 2.
Vesicular uptake of l-aspartate is independent of sialin. A) Measurement of ATP-dependent vesicular accumulation of 3H-l-aspartate in whole-brain synaptic vesicles from sialin-knockout (KO) and wild-type (WT) mice. ATP-dependent uptake of l-aspartate was 9.5 ± 1.8 pmol/min/mg protein in the WT and 13.8 ± 5.2 pmol/min/mg protein in the KO (means±se, n=4 experiments, P>0.05, Student's t test; SPSS). B) Western blots of vesicles derived from WT (WT PC12) and PC12 cells expressing recombinant sialin (A3) with an antibody directed against sialin (left panel) and RFP (right panel) demonstrate marked overexpression of sialin in the stable cell line. The mCherry tag (RFP) on the recombinant protein causes the primary band in the recombinant cells to migrate more slowly than the band in the WT cells. C) Measurement of vesicular accumulation (means±sd; nmol/mg/5 min, n=2 experiments) of 3H-l-aspartate (open bars) and 3H-l-glutamate (solid bars) in crude synaptic-like microvesicles derived from wild-type PC12 cells (WT PC12) and those expressing recombinant sialin (A3 sialin). There is a >3-fold increase in accumulation of l-glutamate in synaptic-like microvesicles from VGLUT1 (B1 VGLUT1)-expressing cells compared to WT. Brackets indicate that the glutamate uptake was significantly higher in B1 VGLUT1 than in WT (P<0.05, Student's t test).
Depletion of l-aspartate from hippocampal terminals is independent of sialin
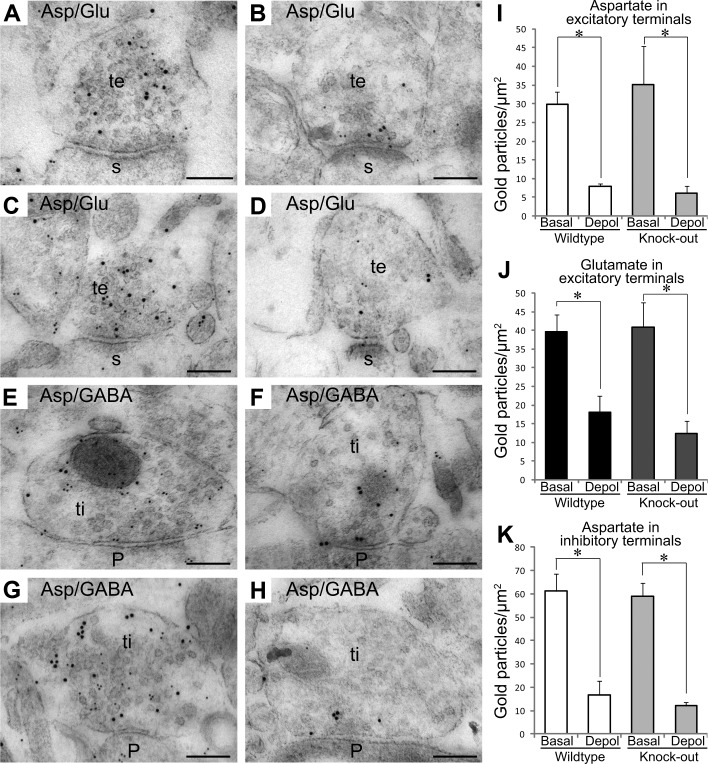
To investigate whether sialin is required for exocytotic release of l-aspartate in intact brain tissue, we exposed hippocampal slices from sialin-knockout and wild-type mice to physiological (3 mM) or depolarizing (55 mM) concentrations of K+. As observed previously in rats (22, 34, 35), in mouse slices incubated at 3 mM K+, l-aspartate immunogold particles colocalized with l-glutamate immunogold particles in excitatory terminals in the CA1 stratum radiatum (Fig. 3A, C) and with GABA immunogold particles in inhibitory terminals in the granule and pyramidal cell layers (Fig. 3E, G). On depolarization with 55 mM K+, gold particles signaling l-aspartate were depleted from both types of terminals (Fig. 3A vs. B; E vs. F). If sialin carries l-aspartate into synaptic vesicles before release, we would expect that the absence of sialin would greatly reduce vesicular release of l-aspartate from nerve terminals. However, there was no difference in l-aspartate labeling of excitatory terminals between sialin knockouts (Fig. 3C, D) and wild types (Fig. 3A, B), either in the basal condition (Fig. 3A, C) or after depolarization (Fig. 3B, D). Quantitative analysis showed that excitatory nerve terminals in wild-type and knockout slices displayed a similar reduction in the density of gold particles representing l-aspartate (73 and 83%, respectively) in response to depolarization (Fig. 3I). Correspondingly, sialin was not necessary for the depolarization-induced depletion of l-glutamate from excitatory nerve terminals (Fig. 3A–D, J). Likewise, in inhibitory terminals, there was no difference in l-aspartate labeling between sialin-knockout (Fig. 3G, H) and wild-type slices (Fig. 2E, F), either in the basal condition (Fig. 3E, G) or after depolarization (Fig. 3F, H). Quantifications showed that the depolarization-induced decrease in l-aspartate labeling was similar in wild-type and knockout slices (73 and 80%, respectively; Fig. 2K).
Figure 3.
Depletion of l-aspartate from nerve endings is independent of sialin. Electron micrographs from wild-type (A, B, E, F) and sialin-knockout (C, D, G, H) slices incubated at 3 mM K+ (A, C, E, G) and 55 mM K+ (B, D, F, H). A–D) Labeling for l-aspartate (small gold particles) and l-glutamate (large gold particles) is shown in excitatory terminals (te) making asymmetric synapses with dendritic spines (s) in CA1 stratum radiatum. E–H) Labeling for l-aspartate (small gold particles)/GABA (large gold particles) is shown in inhibitory terminals (ti) making symmetric synapses with pyramidal cell bodies (P). I–K) Bar charts show the density (average±sem; gold particles/μm2) of immunogold particles for l-aspartate (I) and l-glutamate (J) in excitatory terminals and for l-aspartate in inhibitory terminals (K) in wild-type and sialin-knockout slices incubated at 3 mM K+ (basal) and 55 K+ (depol). Asterisk indicate that the values at 3 mM K+ are significantly different from the values at 55 mM K+ (P<0.05, Student's 2-tailed t test; SPSS). Quantifications were done in 3 slices from wild-type basal slices (total of 84 excitatory and 71 inhibitory terminals), 3 wild-type depol slices (total of 88 excitatory and 70 inhibitory terminals), 3 knockout basal slices (total of 77 excitatory and 67 inhibitory terminals), and 3 knockout depol slices (total of 79 excitatory and 63 inhibitory terminals).
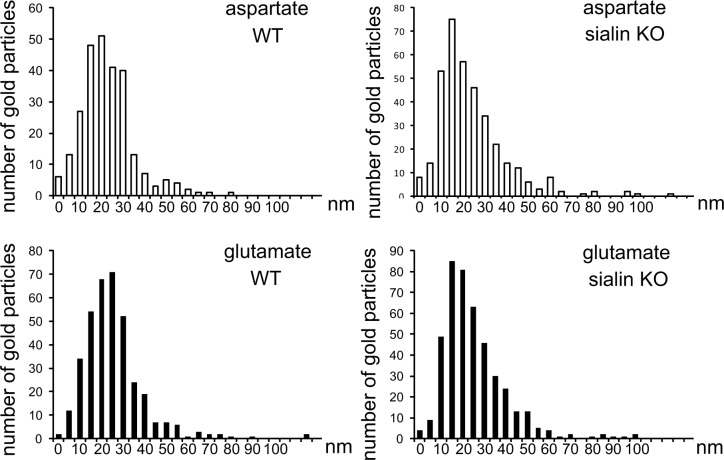
To confirm that l-aspartate is contained within synaptic vesicles in sialin-knockout hippocampus, we measured the distance between the center of gold particles signaling l-aspartate and the center of the closest synaptic vesicle in excitatory terminals in wild-type and sialin-knockout slices. The l-aspartate intercenter distances were compared to those produced by the l-glutamate antibodies. The quantifications showed that most l-aspartate and l-glutamate gold particles were situated within a distance of 30 nm (which is similar to the immunogold lateral resolution, i.e., the distance that separates a gold particle and an epitope; ref. 24) from the vesicle center and that this relation was similar in hippocampus from wild-type and sialin-knockout mice (Fig. 4). In wild-type hippocampus, 85 and 60% of l-aspartate and l-glutamate gold particles were closer to the nearest synaptic vesicle than 30 nm, while in sialin-knockout slices, these values were 80 and 77% (Fig. 4). Thus, both in wild-type and sialin-knockout hippocampus, the large majority of l-aspartate and l-glutamate gold particles represent synaptic vesicle epitopes.
Figure 4.
l-Aspartate and l-glutamate are present in synaptic vesicles lacking sialin. Histograms show the frequency distribution of the distances separating l-aspartate and l-glutamate gold particles and synaptic vesicles in excitatory terminals in wild-type (WT) and sialin-knockout (KO) slices. Distances are put into bins of 5 nm (bars, x axis), and the number of gold particles in each bin is given along the y axis. Because of the lateral resolution of the immunogold method (∼30 nm), gold particles could be separated by a distance of up to ∼30 nm and still signal epitopes in the vesicle. Distributions of l-aspartate and l-glutamate intercenter distances in WT slices were not significantly different from the distributions in sialin-KO slices (P>0.05, χ2 test). These quantifications were done in 60 nerve terminals positive for aspartate/glutamate in both WT and sialin KO CA1 radiatum.
l-Aspartate release from nerve terminals is Ca2+ dependent
As we could not find any evidence of sialin-dependent release of l-aspartate from nerve terminals, we wanted to clarify the Ca2+ dependency of l-aspartate depletion from hippocampal nerve terminals (21, 22). During ischemic conditions, the release of excitatory amino acids can occur through reversal of EAATs (36–41). As this release can be blocked by inhibiting the EAATs (36–38, 40, 42–44), we incubated the slices in the presence of the nontransportable EAAT blocker TFB-TBOA (45) to determine whether EAATs contribute to the release of l-aspartate from nerve terminals during depolarization. As shown previously (23), addition of TBOA per se at high K+ did not significantly reduce the depletion of gold particles signaling l-aspartate from excitatory nerve terminals (Fig. 5A–D), suggesting that l-aspartate is not released via EAATs during membrane depolarization.
Figure 5.
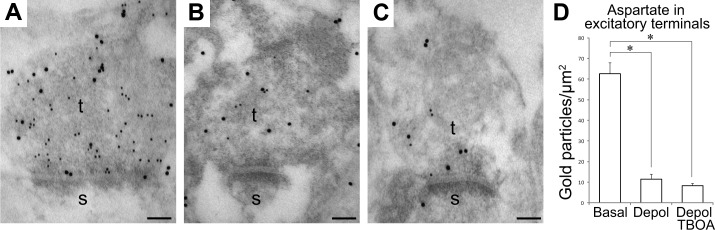
l-Aspartate is not depleted from nerve terminals through excitatory amino acid transporters. A–C) Electron micrographs show gold particles representing l-aspartate (small gold particles) and l-glutamate (large gold particles) in excitatory nerve terminals (t) making asymmetric synapses with dendritic spines (s) in the CA1 stratum radiatum. Slices were incubated at 3 mM K+ (A), 55 mM K+ (B), or 55 mM K+ with TFB-TBOA (C). D) Quantifications of gold particles representing l-aspartate in excitatory nerve terminals in slices incubated at 3 mM K+ (basal), at 55 mM K+ (depol), and at 55 mM K+ with TFB-TBOA (depol TBOA). Bar charts indicate particle density (average±sem; gold particles/μm2). Quantification was performed in 4 basal slices (total of 53 terminals), 4 depol slices (total of 123 terminals), and 6 depol TBOA slices (total of 74 terminals). *P < 0.05; 1-way ANOVA, Dunnett post hoc test.
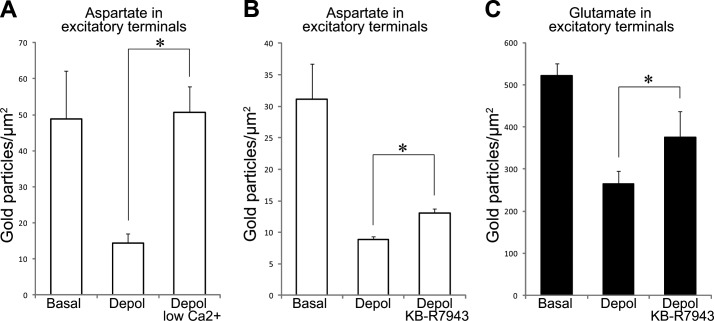
To clarify whether omission of extracellular Ca2+ could inhibit l-aspartate release when the EAATs were blocked, we exposed the slices to low Ca2+/high Mg2+ during depolarization in the presence of TFB-TBOA. At 3 mM K+, there was strong l-aspartate labeling in excitatory nerve terminals, which was robustly reduced on depolarization with 55 mM K+ (Fig. 6A, B). When external Ca2+ was replaced by Mg2+ at 55 mM K+, the depletion of l-aspartate immunogold particles from the terminals was blocked (Fig. 6A). Further highlighting the calcium dependency of l-aspartate release from nerve terminals, we found that KB-R7943, a blocker of calcium entrance through, e.g., NCX(rev) (46), NMDA receptors, (47), and TRPC-Ca2+ channels (48, 49), weakly inhibited depletion of both l-aspartate (Fig. 6B) and l-glutamate (Fig. 6C) from nerve terminals (by 21 and 41%, respectively). Taken together, these data show that l-aspartate is released in a strictly Ca2+-dependent manner even when the EAATs were blocked, supporting the idea of exocytotic release of l-aspartate.
Figure 6.
Depletion of l-aspartate from nerve endings is Ca2+ dependent also when excitatory amino acid transporters are blocked. Quantification of gold particles representing l-aspartate (A, B) and l-glutamate (C) in excitatory nerve terminals in CA1 stratum radiatum in slices incubated at 3 mMK+ (basal) and at 55 mM K+ (depol), and under depolarizing conditions where exocytosis was inhibited by low Ca2+/high Mg2+ (depol low Ca2+) or by KB-R7943 (depol KB-R7943). All slices were incubated in the presence of TFB-TBOA. Bar charts indicate particle density (average±sem; gold particles/μm2). Quantification in A was performed in 3 basal slices (total of 59 terminals), 3 depol slices (total of 65 terminals), and 4 depol low Ca2+ slices (total of 176 terminals). Quantification in B and C was performed in 3 basal slices (total of 56 and 91 terminals, respectively), 3 and 5 depol slices (total of 62 and 149 terminals) and 4 depol KB-R7943 slices (total of 101 and 133 terminals). *P < 0.05, Student's 2-tailed t test (SPSS).
l-Aspartate release from nerve terminals is independent of volume-regulated anion channels (VRACs)
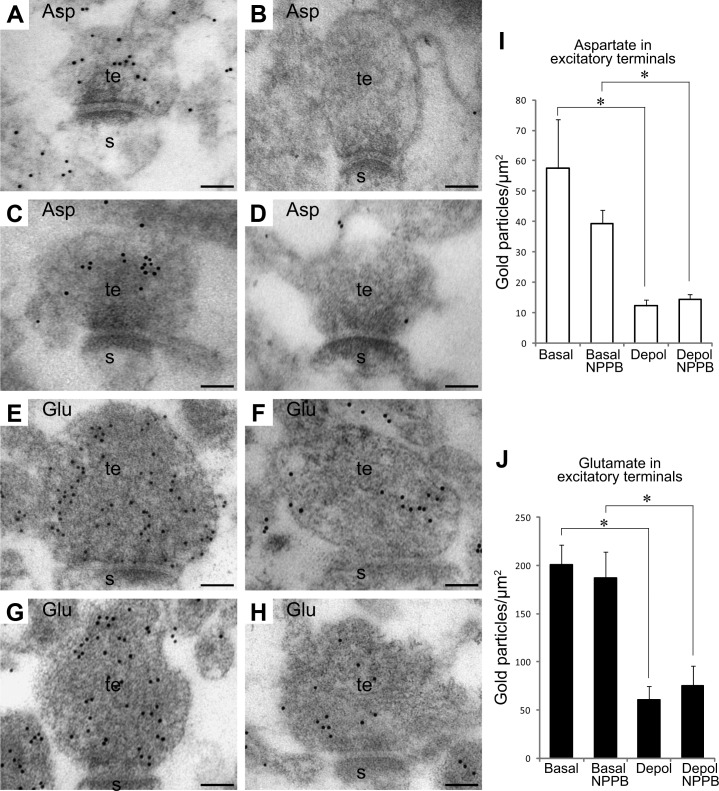
As VRACs are proposed to release excitatory amino acids (50, 51), and the activity of these channels is thought to be Ca2+ dependent (52), we investigated whether VRACs could account for the release of l-aspartate from hippocampal nerve terminals. Hippocampal slices were exposed to physiological and depolarizing concentrations of K+ in the presence or absence of the VRAC inhibitor NPPB. All incubations were done in the presence of TFB-TBOA. NPPB did not affect the density of l-aspartate immunogold particles in nerve terminals at either basal or depolarizing conditions (Fig. 7A–D, I). The same was true for l-glutamate immunogold particles (Fig. 7E–H, J).
Figure 7.
l-Aspartate is not depleted from nerve terminals through volume-regulated anion channels. A–H) Electron micrographs show gold particles representing l-aspartate (A–D) or l-glutamate (E–H) in excitatory nerve terminals (t) making asymmetric synapses with dendritic spines (s) in the CA1 stratum radiatum. A, E) Terminals incubated at 3 mM K+. C, G) Terminals incubated at 3 mM K+ with NPPB. B, F) Terminals incubated at 55 mM K+. D, H) Terminals incubated at 55 mM K+ with NPPB. I, J) Quantification of gold particles representing l-aspartate (I) and l-glutamate (J) in excitatory nerve terminals in slices incubated at 3 mM K+ (basal), at 3 mM K+ with NPPB (basal NPPB) at 55 mM K+ (depol), and at 55 mM K+ with NPPB (depol NPPB). Bar charts indicate particle density (average±sem; gold particles/μm2). Quantifications in I and J were performed in 3 and 4 basal slices (total of 68 and 138 terminals, respectively), 3 basal NPPB slices (total of 132 and 91 terminals), 3–4 depol slices (total of 62 and 97 terminals), and 3–4 depol NPPB slices (total of 110 and 158 terminals). *P < 0.05; Student's 2-tailed t test (SPSS).
DISCUSSION
Here we show that l-aspartate is transported into synaptic vesicles in a sialin-independent manner. The highest vesicular uptake of l-aspartate (relative to the uptake of l-glutamate) was found in hippocampal vesicles, which is consistent with slice data from the present and previous studies (21–23) showing that l-aspartate is released by exocytosis from hippocampal nerve terminals. There was no difference in the ATP-dependent uptake of l-aspartate in vesicles from sialin-knockout compared to wild-type vesicles, and synaptic-like microvesicles from PC12 cells transfected with sialin were not capable of l-aspartate uptake. Consistent with this, our results from sialin-knockout mice show that the vesicular l-aspartate release in the hippocampus is independent of sialin, indicating that l-aspartate is packed into vesicles via a hitherto unknown transporter.
Our l-aspartate uptake data are in agreement with the results of Naito and Ueda (12), who reported l-aspartate uptake to be 7.5% of the vesicular l-glutamate uptake, but at odds with the studies of Fykse et al. (11) and Maycox et al. (13), who did not find evidence for uptake of l-aspartate in synaptic vesicles. The reason for this discrepancy is not clear, but in order to obtain near-saturation of the vesicles with transmitter (12), we used 5 min incubation time, while the other studies used 1.5 and 2 min. In addition, Maycox et al. (13) did not measure vesicular l-aspartate uptake directly, but used acidification of the vesicles as an indirect measure of uptake. Relative to l-glutamate, the highest l-aspartate uptake was found in the hippocampus. Because vesicular l-glutamate uptake is similar in the hippocampus and the whole brain (53), this means that the l-aspartate uptake is about twice as high in hippocampal as in whole-brain vesicles. The fact that the uptake ratio of l-aspartate to l-glutamate varied between forebrain regions suggests that l-aspartate and l-glutamate are taken up into different pools of vesicles, or into partially overlapping vesicle pools, in which some vesicles take up both l-aspartate and l-glutamate, while others take up only l-aspartate or l-glutamate. Supporting incongruent vesicle pools for l-aspartate and l-glutamate is our finding that l-glutamate does not inhibit vesicular l-aspartate uptake, and previous findings showing that l-aspartate does not inhibit l-glutamate uptake (11, 54). Also suggesting that l-aspartate is released from a different vesicular pool than l-glutamate is the fact that the vesicular release of l-aspartate can be regulated differently from that of l-glutamate (7, 8). For instance, NMDA enhances l-aspartate, but not l-glutamate, release from hippocampal slices (55), and reduced levels of 5-lipoxygenase metabolites selectively reduced the release of l-aspartate (56). Along the same line, we recently showed that valproate reduces the nerve terminal content of aspartate, but not that of glutamate (35).
The lysosomal H+-coupled sialic acid transporter, sialin, was recently suggested to transport l-aspartate and l-glutamate into hippocampal synaptic vesicles (15). Here we show that synaptic vesicles from sialin-knockout and wild-type mice do not differ in their ability to take up l-aspartate. In line with the vesicular uptake data are our immunogold results indicating that l-aspartate is present in synaptic vesicles from both wild-type and sialin-knockout brains (Fig. 5). Further supporting the idea that vesicular uptake of l-aspartate is independent of sialin is that when we overexpresses sialin in the vesicles of PC12 cells, they were still unable to accumulate l-aspartate. Finally, l-aspartate release from hippocampal nerve terminals occurs in the absence of sialin. As sialin transports l-aspartate and l-glutamate with similar affinities (15), and the intracellular concentration of l-glutamate is about 5 times the l-aspartate concentration (57), sialin should contribute to at least a part of the total vesicular uptake of l-glutamate. Thus, at least a part of the vesicular l-glutamate uptake should be inhibited by adding external l-aspartate to isolated vesicles, which, however, was not found to be the case (9–11, 54). Taken together, our findings strongly suggest that sialin is neither sufficient for vesicular storage nor necessary for release of l-aspartate in intact brain preparations. This indicates that either sialin is present in insufficient amounts in the vesicular membrane, or sialin does not transport l-aspartate into synaptic vesicles under physiological conditions.
In Miyaji et al. (15), the researchers report to have detected sialin and measured vesicular l-aspartate uptake in the hippocampal P2 fraction. According to standard nomenclature, the P2 fraction refers to a crude synaptosomal fraction (see, e.g., refs. 18, 58, 59), which is likely to contain nonvesicular membranes, and presumably also lysosomal membranes. Thus, the localization of sialin in synaptic vesicles could be questioned. The notion that sialin is not an important constituent of synaptic vesicles, or present in low amounts, is supported by a proteomic study that did not detect sialin among the synaptic vesicle proteins (60).
In the course of this study we set out to localize sialin at the subcellular level using immunogold cytochemistry. However, during antibody testing, the sialin antibodies (commercially available from Alpha Diagnostics and from R.R.) produced several bands on Western blots of brain tissue (unpublished results), hampering the use of these antibodies in immunolocalization studies. Two previous studies have reported sialin immunolocalization in the brain (61, 62). However, none of these give ultrastructural data, and thus whether sialin is present in synaptic vesicles could not be inferred from these studies.
VRACs could form an alternative release mechanism for l-aspartate and l-glutamate, as these channels have been shown to be responsible for release of excitatory amino acids from cultured astrocytes and from pathological brain tissue (44, 50, 51, 63, 64). Because the opening of these channels is reported to be Ca2+ sensitive (52) it was essential to determine whether l-aspartate could escape from nerve terminals through VRACs. Using the VRAC blocker NPPB, we found no evidence for reduced l-aspartate or l-glutamate release from nerve terminals during membrane depolarization. Thus, VRACs do not appear to contribute to the nerve terminal release of excitatory amino acids. This is a novel finding, as previous work on VRAC-mediated release of excitatory amino acids from intact brain tissue has not distinguished between astrocytic and neuronal release. Similarly, as EAATs are present on hippocampal nerve terminals (25, 65) and reported to mediate l-aspartate and l-glutamate release in pathological states (41, 43, 44, 66), we investigated whether l-aspartate could be released from nerve terminals via these transporters. In this respect, it should be noted that blocking the EAATs effectively inhibits reversed transport of excitatory amino acids (23, 34, 36–38, 40, 41, 67) showing that TBOA blocks both the forward and reverse aspartate/glutamate transport across terminal membranes. We found that the EAAT blocker TBOA did not inhibit the release of l-aspartate (or l-glutamate). This is in line with data showing that, under conditions of partial ischemia (51), as well as under physiological conditions (23, 68, 69), the EAATs are not responsible for any significant release of l-aspartate and l-glutamate. Hence, our results on inhibition of EAATs and VRAC strongly suggest that nonvesicular mechanisms do not play a role in the nerve terminal release of l-aspartate.
These results show that nerve terminals have an l-aspartate-containing pool of synaptic vesicles, which undergo exocytosis during neuronal stimulation. The sensitivity of l-aspartate release to Ca2+ (present study and refs. 8, 18, 21, 22, 55, 70–77) and tetanus toxin (22, 69, 78, 79) reported in previous studies further support a vesicular release mechanism. Low micromolar concentrations of KB-R7943 have previously been shown to selectively inhibit aspartate release from synaptosomes (74) and organotypic cultures (7). In our study, KB-R7943, used at a concentration (50 μM) that probably inhibits several receptors and channels that mediate Ca2+ influx (47–49, 80), reduced the nerve terminal depletion of both l-aspartate and l-glutamate, highlighting the calcium-dependency of l-aspartate/l-glutamate release. The higher concentration of KB-R7943 might explain the discrepancy between our results and those of Nadler and coworkers.
We conclude that l-aspartate is taken up into synaptic vesicles by a yet unidentified vesicular transporter and is subjected to regulated exocytotic release.
Acknowledgments
The authors are grateful to Inger-Lise Bogen for advice on the vesicular uptake assay and to Anna Torbjørg Bore for technical support.
The project was supported by grant 06/5289 from the Faculty of Medicine, University of Oslo, to C.M. and K.N., and awards NS050417 and NS045634 to R.J.R. and NS065664 to L.M.P. from the U.S. National Institute of Neurological Disorders and Stroke.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the U.S. National Institutes of Health. L.M.P. is in the Medical Scientist Training Program at Stanford University School of Medicine.
Footnotes
- EAAT
- excitatory amino acid transporter
- mCherry
- monomeric Cherry
- NCX(rev)
- reversed Na+-Ca2+ exchanger
- NMDA
- N-methyl d-aspartate
- NPPB
- 5-nitro-2-(3-phenylpropylamino) benzoic acid
- RFP
- red fluorescent protein
- TFB-TBOA
- (3S)-3-[[3-[[4-(trifluoromethyl)benzoyl]amino]phenyl]methoxy]-l-aspartic acid
- TRPC
- transient receptor potential cation
- VGLUT1
- vesicular glutamate transporter 1
- VRAC
- volume-regulated anion channel
REFERENCES
- 1. Nadler J. V., Vaca K. W., White W. F., Lynch G. S., Cotman C. W. (1976) Aspartate and glutamate as possible transmitters of excitatory hippocampal afferents. Nature 260, 538–540 [DOI] [PubMed] [Google Scholar]
- 2. Wiklund L., Toggenburger G., Cuenod M. (1982) Aspartate: possible neurotransmitter in cerebellar climbing fibers. Science 216, 78–80 [DOI] [PubMed] [Google Scholar]
- 3. Nadler J. V. (2011) Aspartate release and signalling in the hippocampus. Neurochem. Res. 36, 668–676 [DOI] [PubMed] [Google Scholar]
- 4. Gundersen V. (2008) Co-localization of excitatory and inhibitory transmitters in the brain. Acta Neurol. Scand. 188(Suppl.), 29–33 [DOI] [PubMed] [Google Scholar]
- 5. Gundersen V., Storm-Mathisen J. (2000) Aspartate-neurochemical evidence for a transmitter role. In Handbook of Chemical Neuroanatomy (Ottersen O. P., Storm-Mathisen J., eds) pp. 45–62, Elsevier, Amsterdam [Google Scholar]
- 6. Curras M. C., Dingledine R. (1992) Selectivity of amino acid transmitters acting at N-methyl-D-aspartate and amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors. Mol. Pharmacol. 41, 520–526 [PubMed] [Google Scholar]
- 7. Zhang X., Nadler J. V. (2009) Postsynaptic response to stimulation of the Schaffer collaterals with properties similar to those of synaptosomal aspartate release. Brain Res. 1295, 13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fleck M. W., Henze D. A., Barrionuevo G., Palmer A. M. (1993) Aspartate and glutamate mediate excitatory synaptic transmission in area CA1 of the hippocampus. J. Neurosci. 13, 3944–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christensen H., Fykse E. M., Fonnum F. (1991) Inhibition of gamma-aminobutyrate and glycine uptake into synaptic vesicles. Eur. J. Pharmacol. 207, 73–79 [DOI] [PubMed] [Google Scholar]
- 10. Christensen H., Fykse E. M., Fonnum F. (1990) Uptake of glycine into synaptic vesicles isolated from rat spinal cord. J. Neurochem. 54, 1142–1147 [DOI] [PubMed] [Google Scholar]
- 11. Fykse E. M., Iversen E. G., Fonnum F. (1992) Inhibition of L-glutamate uptake into synaptic vesicles. Neurosci. Lett. 135, 125–128 [DOI] [PubMed] [Google Scholar]
- 12. Naito S., Ueda T. (1983) Adenosine triphosphate-dependent uptake of glutamate into protein I-associated synaptic vesicles. J. Biol. Chem. 258, 696–699 [PubMed] [Google Scholar]
- 13. Maycox P. R., Deckwerth T., Hell J. W., Jahn R. (1988) Glutamate uptake by brain synaptic vesicles: energy dependence of transport and functional reconstitution in proteoliposomes. J. Biol. Chem. 263, 15423–15428 [PubMed] [Google Scholar]
- 14. Verheijen F. W., Verbeek E., Aula N., Beerens C. E., Havelaar A. C., Joosse M., Peltonen L., Aula P., Galjaard H., van der Spek P. J., Mancini G. M. (1999) A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nat. Genet. 23, 462–465 [DOI] [PubMed] [Google Scholar]
- 15. Miyaji T., Echigo N., Hiasa M., Senoh S., Omote H., Moriyama Y. (2008) Identification of a vesicular aspartate transporter. Proc. Natl. Acad. Sci. U. S. A. 105, 11720–11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prolo L. M., Vogel H., Reimer R. J. (2009) The lysosomal sialic acid transporter sialin is required for normal CNS myelination. J. Neurosci. 29, 15355–15365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erickson J. D., Masserano J. M., Barnes E. M., Ruth J. A., Weiner N. (1990) Chloride ion increases [3H]dopamine accumulation by synaptic vesicles purified from rat striatum: inhibition by thiocyanate ion. Brain Res. 516, 155–160 [DOI] [PubMed] [Google Scholar]
- 18. Fykse E. M., Christensen H., Fonnum F. (1989) Comparison of the properties of gamma-aminobutyric acid and L-glutamate uptake into synaptic vesicles isolated from rat brain. J. Neurochem. 52, 946–951 [DOI] [PubMed] [Google Scholar]
- 19. Bellocchio E. E., Reimer R. J., Fremeau R. T., Jr., Edwards R. H. (2000) Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 289, 957–960 [DOI] [PubMed] [Google Scholar]
- 20. Wreden C. C., Wlizla M., Reimer R. J. (2005) Varied mechanisms underlie the free sialic acid storage disorders. J. Biol. Chem. 280, 1408–1416 [DOI] [PubMed] [Google Scholar]
- 21. Gundersen V., Ottersen O. P., Storm-Mathisen J. (1991) Aspartate- and glutamate-like immunoreactivities in rat hippocampal slices: depolarization-induced redistribution and effects of precursors. Eur. J. Neurosci. 3, 1281–1299 [DOI] [PubMed] [Google Scholar]
- 22. Gundersen V., Chaudhry F. A., Bjaalie J. G., Fonnum F., Ottersen O. P., Storm-Mathisen J. (1998) Synaptic vesicular localization and exocytosis of L-aspartate in excitatory nerve terminals: a quantitative immunogold analysis in rat hippocampus. J. Neurosci. 18, 6059–6070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holten A. T., Morland C., Nordengen K., Gundersen V. (2008) Vesicular release of l- and d-aspartate from hippocampal nerve terminals: immunogold evidence. Open Neurosci. J. 2, 51–58 [Google Scholar]
- 24. Bergersen L. H., Storm-Mathisen J., Gundersen V. (2008) Immunogold quantification of amino acids and proteins in complex subcellular compartments. Nat. Protoc. 3, 144–152 [DOI] [PubMed] [Google Scholar]
- 25. Gundersen V., Danbolt N. C., Ottersen O. P., Storm-Mathisen J. (1993) Demonstration of glutamate/aspartate uptake activity in nerve endings by use of antibodies recognizing exogenous d-aspartate. Neuroscience 57, 97–111 [DOI] [PubMed] [Google Scholar]
- 26. Gundersen V., Fonnum F., Ottersen O. P., Storm-Mathisen J. (2001) Redistribution of neuroactive amino acids in hippocampus and striatum during hypoglycemia: a quantitative immunogold study. J. Cereb. Blood Flow Metab 21, 41–51 [DOI] [PubMed] [Google Scholar]
- 27. Gundersen V., Holten A. T., Storm-Mathisen J. (2004) GABAergic synapses in hippocampus exocytose aspartate on to NMDA receptors: quantitative immunogold evidence for co-transmission. Mol. Cell Neurosci. 26, 156–165 [DOI] [PubMed] [Google Scholar]
- 28. Ottersen O. P. (1987) Postembedding light- and electron microscopic immunocytochemistry of amino acids: description of a new model system allowing identical conditions for specificity testing and tissue processing. Exp. Brain Res. 69, 167–174 [DOI] [PubMed] [Google Scholar]
- 29. Ottersen O. P., Zhang N., Walberg F. (1992) Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience 46, 519–534 [DOI] [PubMed] [Google Scholar]
- 30. Larsson M., Sawada K., Morland C., Hiasa M., Ormel L., Moriyama Y., Gundersen V. (2012) Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb. Cortex 22, 1203–1214 [DOI] [PubMed] [Google Scholar]
- 31. Larsson M., Broman J. (2005) Different basal levels of CaMKII phosphorylated at Thr286/287 at nociceptive and low-threshold primary afferent synapses. Eur. J. Neurosci. 21, 2445–2458 [DOI] [PubMed] [Google Scholar]
- 32. Ogita K., Hirata K., Bole D. G., Yoshida S., Tamura Y., Leckenby A. M., Ueda T. (2001) Inhibition of vesicular glutamate storage and exocytotic release by Rose Bengal. J. Neurochem. 77, 34–42 [DOI] [PubMed] [Google Scholar]
- 33. Fremeau R. T., Jr., Voglmaier S., Seal R. P., Edwards R. H. (2004) VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 27, 98–103 [DOI] [PubMed] [Google Scholar]
- 34. Holten A. T., Gundersen V. (2008) Glutamine as a precursor for transmitter glutamate, aspartate and GABA in the cerebellum: a role for phosphate-activated glutaminase. J. Neurochem. 104, 1032–1042 [DOI] [PubMed] [Google Scholar]
- 35. Morland C., Nordengen K., Gundersen V. (2012) Valproate causes reduction of the excitatory amino acid aspartate in nerve terminals. Neurosci. Lett. 527, 100–104 [DOI] [PubMed] [Google Scholar]
- 36. Longuemare M. C., Swanson R. A. (1995) Excitatory amino acid release from astrocytes during energy failure by reversal of sodium-dependent uptake. J. Neurosci. Res. 40, 379–386 [DOI] [PubMed] [Google Scholar]
- 37. Roettger V., Lipton P. (1996) Mechanism of glutamate release from rat hippocampal slices during in vitro ischemia. Neuroscience 75, 677–685 [DOI] [PubMed] [Google Scholar]
- 38. Jabaudon D., Scanziani M., Gahwiler B. H., Gerber U. (2000) Acute decrease in net glutamate uptake during energy deprivation. Proc. Natl. Acad. Sci. U. S. A. 97, 5610–5615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Phillis J. W., O'Regan M. H. (2000) Hyperglycemia and extracellular glutamate in the ischemic brain. Stroke 31, 1462–1463 [DOI] [PubMed] [Google Scholar]
- 40. Marcaggi P., Hirji N., Attwell D. (2005) Release of l-aspartate by reversal of glutamate transporters. Neuropharmacology 49, 843–849 [DOI] [PubMed] [Google Scholar]
- 41. Phillis J. W., Smith-Barbour M., Perkins L. M., O'Regan M. H. (1994) Characterization of glutamate, aspartate, and GABA release from ischemic rat cerebral cortex. Brain Res. Bull. 34, 457–466 [DOI] [PubMed] [Google Scholar]
- 42. Phillis J. W., Ren J., O'Regan M. H. (2000) Inhibition of Na(+)/H(+) exchange by 5-(N-ethyl-N-isopropyl)-amiloride reduces free fatty acid efflux from the ischemic reperfused rat cerebral cortex. Brain Res. 884, 155–162 [DOI] [PubMed] [Google Scholar]
- 43. Rossi D. J., Oshima T., Attwell D. (2000) Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403, 316–321 [DOI] [PubMed] [Google Scholar]
- 44. Seki Y., Feustel P. J., Keller R. W., Jr., Tranmer B. I., Kimelberg H. K. (1999) Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke 30, 433–440 [DOI] [PubMed] [Google Scholar]
- 45. Shimamoto K., Sakai R., Takaoka K., Yumoto N., Nakajima T., Amara S. G., Shigeri Y. (2004) Characterization of novel l-threo-beta-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters. Mol. Pharmacol. 65, 1008–1015 [DOI] [PubMed] [Google Scholar]
- 46. Omelchenko A., Bouchard R., Le H. D., Choptiany P., Visen N., Hnatowich M., Hryshko L. V. (2003) Inhibition of canine (NCX1.1) and Drosophila (CALX1.1) Na(+)-Ca(2+) exchangers by 7-chloro-3,5-dihydro-5-phenyl-1H-4,1-benzothiazepine-2-one (CGP-37157). J. Pharmacol. Exp. Ther. 306, 1050–1057 [DOI] [PubMed] [Google Scholar]
- 47. Sobolevsky A. I., Khodorov B. I. (1999) Blockade of NMDA channels in acutely isolated rat hippocampal neurons by the Na+/Ca2+ exchange inhibitor KB-R7943. Neuropharmacology 38, 1235–1242 [DOI] [PubMed] [Google Scholar]
- 48. Kraft R. (2007) The Na+/Ca2+ exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem. Biophys. Res. Commun. 361, 230–236 [DOI] [PubMed] [Google Scholar]
- 49. Pezier A., Bobkov Y. V., Ache B. W. (2009) The Na+/Ca2+ exchanger inhibitor, KB-R7943, blocks a nonselective cation channel implicated in chemosensory transduction. J. Neurophysiol. 101, 1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y., Zhang H., Feustel P. J., Kimelberg H. K. (2008) DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra. Exp. Neurol. 210, 514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feustel P. J., Jin Y., Kimelberg H. K. (2004) Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke 35, 1164–1168 [DOI] [PubMed] [Google Scholar]
- 52. Mongin A. A., Kimelberg H. K. (2005) ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am. J. Physiol. Cell Physiol. 288, C204–C213 [DOI] [PubMed] [Google Scholar]
- 53. Lewis S. M., Lee F. S., Todorova M., Seyfried T. N., Ueda T. (1997) Synaptic vesicle glutamate uptake in epileptic (EL) mice. Neurochem. Int. 31, 581–585 [DOI] [PubMed] [Google Scholar]
- 54. Naito S., Ueda T. (1985) Characterization of glutamate uptake into synaptic vesicles. J. Neurochem. 44, 99–109 [DOI] [PubMed] [Google Scholar]
- 55. Zhou M., Peterson C. L., Lu Y. B., Nadler J. V. (1995) Release of glutamate and aspartate from CA1 synaptosomes: selective modulation of aspartate release by ionotropic glutamate receptor ligands. J. Neurochem. 64, 1556–1566 [DOI] [PubMed] [Google Scholar]
- 56. Peterson C. L., Thompson M. A., Martin D., Nadler J. V. (1995) Modulation of glutamate and aspartate release from slices of hippocampal area CA1 by inhibitors of arachidonic acid metabolism. J. Neurochem. 64, 1152–1160 [DOI] [PubMed] [Google Scholar]
- 57. Nadler J. V., White W. F., Vaca K. W., Perry B. W., Cotman C. W. (1978) Biochemical correlates of transmission mediated by glutamate and aspartate. J. Neurochem. 31, 147–155 [DOI] [PubMed] [Google Scholar]
- 58. Kadota K., Kadota T. (1973) Isolation of coated vesicles, plain synaptic vesicles, and flocculent material from a crude synaptosome fraction of guinea pig whole brain. J. Cell Biol. 58, 135–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huttner W. B., Schiebler W., Greengard P., De C. P. (1983) Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 96, 1374–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Takamori S., Holt M., Stenius K., Lemke E. A., Gronborg M., Riedel D., Urlaub H., Schenck S., Brugger B., Ringler P., Muller S. A., Rammner B., Grater F., Hub J. S., De Groot B. L., Mieskes G., Moriyama Y., Klingauf J., Grubmuller H., Heuser J., Wieland F., Jahn R. (2006) Molecular anatomy of a trafficking organelle. Cell 127, 831–846 [DOI] [PubMed] [Google Scholar]
- 61. Aula N., Kopra O., Jalanko A., Peltonen L. (2004) Sialin expression in the CNS implicates extralysosomal function in neurons. Neurobiol. Dis. 15, 251–261 [DOI] [PubMed] [Google Scholar]
- 62. Yarovaya N., Schot R., Fodero L., McMahon M., Mahoney A., Williams R., Verbeek E., de B. A., Hampson M., van der S. P., Stubbs A., Masters C. L., Verheijen F. W., Mancini G. M., Venter D. J. (2005) Sialin, an anion transporter defective in sialic acid storage diseases, shows highly variable expression in adult mouse brain, and is developmentally regulated. Neurobiol. Dis. 19, 351–365 [DOI] [PubMed] [Google Scholar]
- 63. Phillis J. W., Song D., O'Regan M. H. (1997) Inhibition by anion channel blockers of ischemia-evoked release of excitotoxic and other amino acids from rat cerebral cortex. Brain Res. 758, 9–16 [DOI] [PubMed] [Google Scholar]
- 64. Phillis J. W., Song D., O'Regan M. H. (1998) Tamoxifen, a chloride channel blocker, reduces glutamate and aspartate release from the ischemic cerebral cortex. Brain Res. 780, 352–355 [DOI] [PubMed] [Google Scholar]
- 65. Furness D. N., Dehnes Y., Akhtar A. Q., Rossi D. J., Hamann M., Grutle N. J., Gundersen V., Holmseth S., Lehre K. P., Ullensvang K., Wojewodzic M., Zhou Y., Attwell D., Danbolt N. C. (2008) A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2). Neuroscience 157, 80–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Szatkowski M., Barbour B., Attwell D. (1990) Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature 348, 443–446 [DOI] [PubMed] [Google Scholar]
- 67. Phillis J. W., Ren J., O'Regan M. H. (2000) Transporter reversal as a mechanism of glutamate release from the ischemic rat cerebral cortex: studies with DL-threo-beta-benzyloxyaspartate. Brain Res. 868, 105–112 [DOI] [PubMed] [Google Scholar]
- 68. Raiteri L., Zappettini S., Milanese M., Fedele E., Raiteri M., Bonanno G. (2007) Mechanisms of glutamate release elicited in rat cerebrocortical nerve endings by ‘pathologically’ elevated extraterminal K+ concentrations. J. Neurochem. 103, 952–961 [DOI] [PubMed] [Google Scholar]
- 69. Cavallero A., Marte A., Fedele E. (2009) L-aspartate as an amino acid neurotransmitter: mechanisms of the depolarization-induced release from cerebrocortical synaptosomes. J. Neurochem. 110, 924–934 [DOI] [PubMed] [Google Scholar]
- 70. Nadler J. V., Martin D., Bustos G. A., Burke S. P., Bowe M. A. (1990) Regulation of glutamate and aspartate release from the Schaffer collaterals and other projections of CA3 hippocampal pyramidal cells. Prog. Brain Res. 83, 115–130 [DOI] [PubMed] [Google Scholar]
- 71. Burke S. P., Nadler J. V. (1988) Regulation of glutamate and aspartate release from slices of the hippocampal CA1 area: effects of adenosine and baclofen. J. Neurochem. 51, 1541–1551 [DOI] [PubMed] [Google Scholar]
- 72. Szerb J. C. (1988) Changes in the relative amounts of aspartate and glutamate released and retained in hippocampal slices during stimulation. J. Neurochem. 50, 219–224 [DOI] [PubMed] [Google Scholar]
- 73. Roisin M. P., Brassart J. L., Charton G., Crepel V., Ben A. Y. (1991) A new method for the measurement of endogenous transmitter release in localized regions of hippocampal slices. J. Neurosci. Methods 37, 183–189 [DOI] [PubMed] [Google Scholar]
- 74. Bradford S. E., Nadler J. V. (2004) Aspartate release from rat hippocampal synaptosomes. Neuroscience 128, 751–765 [DOI] [PubMed] [Google Scholar]
- 75. Girault J. A., Barbeito L., Spampinato U., Gozlan H., Glowinski J., Besson M. J. (1986) In vivo release of endogenous amino acids from the rat striatum: further evidence for a role of glutamate and aspartate in corticostriatal neurotransmission. J. Neurochem. 47, 98–106 [DOI] [PubMed] [Google Scholar]
- 76. Paulsen R. E., Fonnum F. (1989) Role of glial cells for the basal and Ca2+-dependent K+-evoked release of transmitter amino acids investigated by microdialysis. J. Neurochem. 52, 1823–1829 [DOI] [PubMed] [Google Scholar]
- 77. Zappettini S., Grilli M., Salamone A., Fedele E., Marchi M. (2010) Pre-synaptic nicotinic receptors evoke endogenous glutamate and aspartate release from hippocampal synaptosomes by way of distinct coupling mechanisms. Br. J. Pharmacol. 161, 1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. McMahon H. T., Foran P., Dolly J. O., Verhage M., Wiegant V. M., Nicholls D. G. (1992) Tetanus toxin and botulinum toxins type A and B inhibit glutamate, gamma-aminobutyric acid, aspartate, and met-enkephalin release from synaptosomes: clues to the locus of action. J. Biol. Chem. 267, 21338–21343 [PubMed] [Google Scholar]
- 79. Wang L., Nadler J. V. (2007) Reduced aspartate release from rat hippocampal synaptosomes loaded with Clostridial toxin light chain by electroporation: evidence for an exocytotic mechanism. Neurosci. Lett. 412, 239–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brustovetsky T., Brittain M. K., Sheets P. L., Cummins T. R., Pinelis V., Brustovetsky N. (2011) KB-R7943, an inhibitor of the reverse Na+/Ca2+ exchanger, blocks N-methyl-d-aspartate receptor and inhibits mitochondrial complex I. Br. J. Pharmacol. 162, 255–270 [DOI] [PMC free article] [PubMed] [Google Scholar]