Abstract
Germline transgenesis is an important procedure for functional investigation of biological pathways, as well as for animal biotechnology. We have established a simple, nonviral protocol in three important biomedical model organisms frequently used in physiological studies. The protocol is based on the hyperactive Sleeping Beauty transposon system, SB100X, which reproducibly promoted generation of transgenic founders at frequencies of 50–64, 14–72, and 15% in mice, rats, and rabbits, respectively. The SB100X-mediated transgene integrations are less prone to genetic mosaicism and gene silencing as compared to either the classical pronuclear injection or to lentivirus-mediated transgenesis. The method was successfully applied to a variety of transgenes and animal models, and can be used to generate founders with single-copy integrations. The transposon vector also allows the generation of transgenic lines with tissue-specific expression patterns specified by promoter elements of choice, exemplified by a rat reporter strain useful for tracking serotonergic neurons. As a proof of principle, we rescued an inborn genetic defect in the fawn-hooded hypertensive rat by SB100X transgenesis. A side-by-side comparison of the SB100X- and piggyBac-based protocols revealed that the two systems are complementary, offering new opportunities in genome manipulation.—Katter, K., Geurts, A. M., Hoffmann, O., Mátés, L., Landa,V., Hiripi, L., Moreno, C., Lazar, J., Bashir, S., Zidek, V., Popova, E., Jerchow, B., Becker, K., Devaraj, A., Walter, I., Grzybowksi, M., Corbett, M., Rangel Filho, A., Hodges, M. R., Bader, M., Ivics, Z., Jacob, H. J., Pravenec, M., Bősze, Z., Rülicke, T., Izsvák, Z. Transposon-mediated transgenesis, transgenic rescue, and tissue-specific gene expression in rodents and rabbits.
Keywords: SB100X, Sleeping Beauty, mosaicism, piggyPac, silencing
There is considerable interest in simple, efficient, transgenic tools, which can be applied to a variety of model systems. Genome manipulation techniques were primarily developed for the mouse and have revolutionized the field of functional genomics in the past 2 decades. Still, the laboratory rat has been the preferred animal model for physiology, toxicology, nutrition, behavior, and neoplasia for >150 yr. Although >500 inbred rat strains have been developed for a wide range of physiological phenotypes and different disease models, connecting physiology to genomics is still a challenge. Similarly, rabbits are valuable alternative models in instances when the causative mutations resulting in human diseases do not give rise to the expected pathological symptoms or cannot be investigated in mice. Transgenic rabbits are capable of modeling human diseases, such as atherosclerosis, hypertrophic cardiomyopathy, and lipoprotein metabolism defects (1). At present, the most commonly accepted method to produce transgenic animals remains the pronuclear microinjection of linear DNA transgenes into fertilized oocytes, first developed by Gordon et al. (2) >30 yr ago. The main limitation of this approach is the low yield of transgenic animals, especially in species other than the mouse, with frequencies typically ranging from 1 to 5% of injected zygotes (5–25% of founders; refs. 3–5). Higher transgenic rates were reported by injecting recombinant lentiviral vectors into the perivitelline space of zygotes in various animal models, including rodents and certain livestock species (6, 7). However, there are still a number of species, e.g., rabbits (8), in which neither the classical nor the lentiviral strategy produces transgenics at satisfying rates. In addition, when using lentiviral vectors to create transgenic animals, several problems remain with regard to limited cargo capacity or vector-related toxicity. Furthermore, both classical microinjection and lentiviral gene transfer are accompanied by the problem of genetic mosaicism and gene silencing. Pronuclear microinjection results in concatemeric, multicopy transgene integrations that can promote heterochromatin formation and gene silencing (9, 10). Although retroviruses insert the transgene as a defined integration cassette, epigenetic changes triggered by viral integration frequently result in transcriptional silencing (7, 11). Thus, methods with high germline transmission rates, predictable transgene expression patterns, technical ease, and safe implementation would significantly improve transgenic studies in multiple mammalian models.
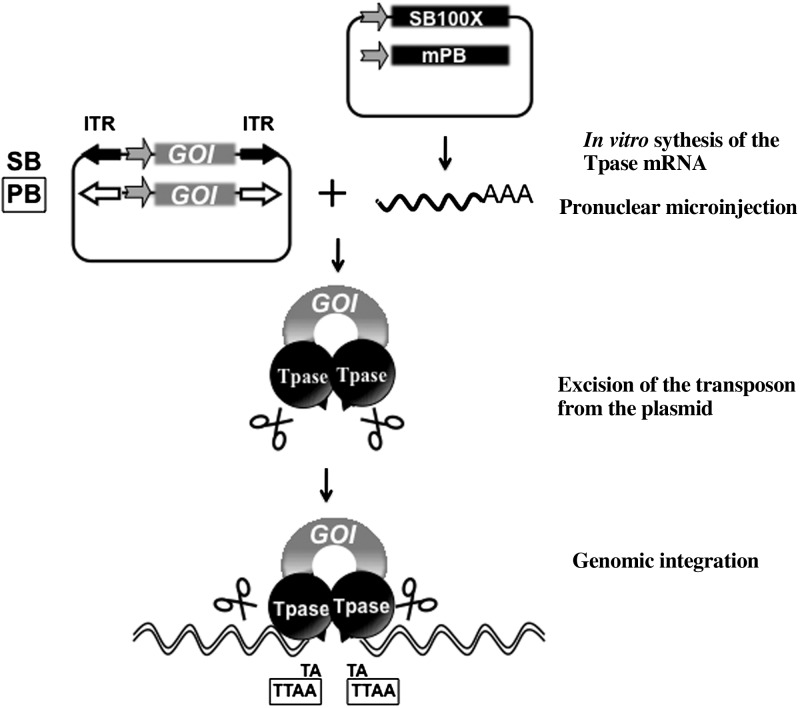
As an emerging alternative, plasmid-based DNA transposons are noninfectious, integrating gene delivery vehicles. Similarly to retroviruses, DNA transposons are mobile DNA elements, which can integrate into the chromosomes of the host cells, a feature that forms the basis of their use as gene transfer vectors. Under laboratory conditions, these systems are applied as binary tools consisting of a transposon vector and a source of the transposase enzyme. The transpositional mechanism involves the excision of a transgene cassette of interest flanked by transposon-inverted repeat sequences from a plasmid vector followed by genomic integration (Fig. 1).Both the excision and integration events are catalyzed by the transposase, supplied as plasmid DNA or synthetic mRNA. Molecular reconstruction of the Sleeping Beauty (SB) transposon represents a milestone in applying transposition-mediated gene delivery to vertebrate species in which genome manipulation was hitherto not possible (12). Indeed, the SB system has been developed into a technology platform for vertebrate genetics with applications including gene therapy (13), somatic mutagenesis (cancer research; ref. 14), and germline mutagenesis (15). In recent studies, SB was even demonstrated to be functional in spermatogonial stem cells (15, 16). A recently developed hyperactive SB transposase variant, SB100X (17), has been able to address the bottleneck problem of nonviral gene delivery, namely efficacy. The SB100X system is an appealing tool for transgenesis because of its ability to incorporate a transgene into genomic DNA with high efficiency (17), including cells that are not actively dividing, and to yield low silencing frequencies in transgenic mammalian cells (18). To date, the SB (19, 20), piggyBac (PB; ref. 21), and Tol2 (22) transposon systems have been applied to rodent transgenesis, mostly in mice. The SB and the PB systems are similar in many respects. However, while SB transposon vectors have been demonstrated to have benign transcription activity and integrate in a fairly random manner (23, 24), PB transposon transgene integration tends to target transcriptionally active regions in the genome (25–27).
Figure 1.
The plasmid-based SB and PB transposon systems. The SB and PB systems are binary transposition platforms comprising a transposon construct with the gene of interest flanked by inverted terminal repeats (ITR) and the transposase (Tpase) helper protein. The Tpase is introduced as in vitro synthesized mRNA and translated by the cellular machinery. Following delivery of the components to the cells, the Tpase protein is translated (black ball) and binds the ITRs flanking the gene of interest (GOI) and catalyzes the excision and subsequent genomic integration of the transposon. Integrations occur at TA or TTAA, sequence motifs using the SB or the PB systems, respectively. These motifs are duplicated to flank the transposon at the site of insertion.
Here, we provide an extensive evaluation of an SB100X-mediated protocol for transgenesis in the three most frequently used mammalian experimental models: laboratory mice, rats, and rabbits. We have analyzed various parameters of the method, including efficacy, toxicity, mosaicism, germline transmission, copy number, genomic integration profile, and gene silencing across multiple generations of breeding. To promote early integration and avoid concatemerization of the transgene, we delivered the SB100X transposase as mRNA along with the transposon transgene in the form of a circular plasmid. Reproducibility of the SB100X-mediated transgenesis was tested in a number of different rat strains, using transgenes ranging from 3.6 to 10.2 kb in size. The expression of the transgene was monitored in transgenic animals, where transgene expression was driven from either ubiquitous or tissue-specific promoters. To generate single-copy transgenics, an approach of controlling the number of transgene integration per genome was applied. To demonstrate genetic rescue, a congenital genetic phenotype was challenged by expressing the cDNA of Rab38 from the SB transposon vector in fawn-hooded hypertensive (FHH) rats. Finally, to further expand the toolkit and options for routine genome manipulation, we demonstrate that the transposon plasmid vector/transposase mRNA coinjection method can be adapted to the alternative PB transposon system.
MATERIALS AND METHODS
Transgenesis
In all transgenic experiments, the microinjection cocktail of a circular plasmid carrying a transposon and mRNA coding for the transposase was prepared as described previously (17). The exact conditions are summarized in Table 1.
Table 1.
Transposon-mediated transgenic efficiencies
| Model | Transgene | Tpase mRNA | Transferred zygotes | F0 borna | Foundersb | F0 expressing transgene |
|---|---|---|---|---|---|---|
| Mouse | ||||||
| B6;D2 | T2/Venusc | SB100Xd | 157 | 53 (33) | 34 (64, 21) | 34/34 |
| B6;D2 | T2/Venusc | SB100Xe | 110 | 42 (38) | 21 (50, 19) | 19/21 |
| B6;D2 | T2/Venusf | SB100Xd | 74 | 31 (42) | 16 (52, 21) | 16/16 |
| B6;D2 | pBac/Venusg | mPBh | 275 | 76 (27) | 14 (18, 5) | 13/14 |
| B6;D2 | pBac/SB100Xg | mPBh | 82 | 53 (65) | 8 (15,10) | 1/3 |
| B6;D2 | pBac/SB LacZg | mPBh | 36 | 29 (81) | 6 (21, 17) | 1/4 |
| Rat | ||||||
| SHR | T2/Venusc | SB100Xd | 165 | 51 (31) | 11 (22, 7) | 11/11 |
| ACI | pBac/SB100Xg | mPBh | 47 | 20 (43) | 5 (25,11) | ND |
| SS | T2/FH1g | SB100Xd | 158 | 27 (17) | 8 (29,5) | 2/2 |
| SS | T2/eGFPg | SB100Xd | 141 | 15 (11) | 5 (33,4) | 5/5 |
| SS and SS.BN13 con. | T2/P67phoxg | SB100Xd | 133 | 31 (23) | 14 (42,11) | 1/1 |
| SS | T2/Cyp4a1g | SB100Xd | 50 | 9 (18) | 2 (22,4) | 2/2 |
| SS | T2/ePet-eGFPg | SB100Xd | 80 | 18 (23) | 13 (72,16) | 2/2 |
| FHH | T2/Rab38g | SB100Xd | 86 | 14 (16) | 2 (14,2) | 2/2 |
| SS and FHH | T2/iCRE-2a-GFPg | SB100Xd | 107 | 8 (7) | 3 (38,3) | 0/3 |
| SD | T2/Neph-Hmox1g | SB100Xd | 46 | 6 (13) | 6 (67,9) | ND |
| Rabbit | ||||||
| Hycole | T2/Venusc | SB100Xd | 472 | 46 (10) | 7 (15, 1) | 7/7 |
ACI, August Copenhagen Irish (ACI/SegHSD); con, congenic; FHH, fawn-hooded hypertensive (FHH/EurMcwi); ND, not detected; SD, Sprague-Dawley (SD/Crl); SHR, spontaneously hypersensitive rat; SS, Dahl salt-sensitive (SS/JrHsdMcwi).
Values in parentheses indicate percentage of transferred zygotes.
Values in parentheses indicate percentage of F0 born, percentage of transferred zygotes.
0.4 ng/μl transgene in transposon vector.
5 ng/μl transposase mRNA.
1.25 ng/μl transposase mRNA.
0.1 ng/μl transgene in transposon vector.
2 ng/μl transgene in transposon vector.
15 ng/μl transposase mRNA.
Mouse transgenesis
Generation of transgenic mice was performed as described earlier (28). Briefly, zygote donors were housed under standard laboratory conditions with a light cycle from 6:00 AM to 6:00 PM. B6D2F1 hybrid females were superovulated at an age of ∼8 wk by i.p. injection of 5.0 IU eCG (Folligon; Intervet, Boxmeer, The Netherlands) at 10:00 A.M., and 47.5 h later of 5.0 IU hCG (Chorulon; Intervet) and subsequently mated with B6D2F1 males. The microinjection cocktail was injected into the male pronucleus of fertilized oocytes. Injected embryos were implanted into pseudopregnant ICR surrogate mothers. The institutional ethics committee of the Veterinärmedizinische Universität (Vetmeduni; Vienna, Austria) approved the study, and an animal experiment license was granted by the Austrian Federal Ministry of Science and Research under BMWF-68.205/0084-II/10b/2008.
Rat transgenesis
Collection and microinjection of rat zygotes at the Medical College of Wisconsin (MCW; Mcwi; Milwaukee, WI, USA) were performed as described previously (29). Briefly, superovulated females from the Dahl salt-sensitive (SS)/JrHsdMcwi, SS.brown Norway (BN)-(D13Rat61-D13Rat32)/Mcwi, FHH/EurMcwi inbred strains or Sprague-Dawley (SD; Crl:SD) outbred stock (Charles River Laboratories, Frederick, MD, USA) were mated with stud males, and embryos were collected at ∼9 h postfertilization. The microinjection cocktail was injected into the male pronucleus. Surviving embryos were transferred to Crl:SD pseudopregnant females to be carried to parturition. Founder generation animals and their offspring were screened with transposon transgene-specific primers by PCR. At the Czech Academy of Sciences (Prague, Czech Republic), transgenic spontaneously hypersensitive rat (SHR)/Ola rats were similarly derived by microinjection of zygotes obtained from superovulated females. These animal studies were performed under protocols approved either by the Institutional Animal Care and Use Committee of MCW or by the Ethics Committee of the Institute of Physiology, Czech Academy of Sciences, in agreement with the Animal Protection Law of the Czech Republic.
The Pet-1 enhancer (ePet) promoter was reconstructed by synthesis (Geneart AG, Regensberg, Germany), based on published descriptions (30) to include the mouse Pet-1 enhancer region from NCBI37/mm9 chromosome 1:74932055-74933901 and cloned into the pT2/enhanced green fluorescent protein (eGFP) transpsoson vector, replacing the ubiquitous CAG promoter. The pT2/Rab38 transposon vector was constructed by amplifying the Rab38 cDNA sequence from BN/NHsdMcwi bone marrow cDNA and adding a Kozak consensus sequence (underscored) using the primers 5′-GCCACCATGCAGACACCGCACAAGGAG-3′ and 5′-CTAGGATTTGGCACAGCCAGAG-3′. The PCR product was cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA, USA) and verified by sequencing before cloning the Rab38 cDNA into the pT2/eGFP transposon using EcoRI, replacing eGFP. The transgenes and phenotypes of other transgenic projects will be described elsewhere.
Rabbit transgenesis
Collection of rabbit zygotes and transfer of injected embryos to recipient does were performed as described earlier (31). All experiments were approved by the Animal Care and Ethics Committee of the Agricultural Biotechnology Center (Gödöllő, Hungary) and complied with the Hungarian Code of Practice for the Care and Use of Animals for Scientific Purposes, including conditions for animal welfare and handling prior to sacrifice.
Estimating genetic mosaicism
Deviation from the mendelian inheritance ratio was estimated by the Fisher's exact test (P≤ 0.05), as it is designed for small sample sizes. Multiple integrations were assumed to be genetically unlinked and independently transmitted. The expected mendelian values of germline transmissions for 1, 2, 3, and 4 independently transmitted integration events are 50, 75, 88, and 94%, respectively. If the number of integrations was not determined, the expected mendelian value was set as 50%. It should be noted that because of the small size of F1 populations originated from the individual founders, the estimation is only indicative.
Tail bleeding
Conscious FHH (n=3) or FHH-T2/Rab38-A (n=3) rats were placed in a restrainer, and a transverse incision was made with a sterile surgical blade over the lateral tail vein. Following the incision, the blood was allowed to flow into a 50-ml conical tube of sterile saline at 37°C. Time was measured from the initiation of the cut until bleeding stopped to a maximum of 10 min, when bleeding was stopped with styptic powder. Tail bleeding times in FHH-T2/Rab38-A transgenic animals were compared to control FHH/EurMcwi animals using the unpaired Student's t test.
Histology
Sections of 2 founder mice and 8 F1 offspring (4 female and 4 male) were compared for gross interindividual variations in Venus expression. The tissue samples were fixed in 4% phosphate-buffered formalin for 24 h at room temperature. Paraffin embedding was performed overnight using the Shandon Excelsior Tissue Processor (Thermo Fisher Scientific, Waltham, MA, USA). Sections of 3 μm were made and put on coated slides. 4′,6-Diamidino-2-phenylindole (DAPI) nuclear-stained sections were examined with a confocal laser-scanning microscope (LSM 510 METAMK4; Carl Zeiss, Vienna, Austria). Rabbit tissues were fixed in 4% paraformaldehyde (PFA), and then kept in 30% sucrose for dehydration. Afterward, they were embedded in cryostat-embedding compound (Tissue-Tek, Torrance, CA, USA) and cut into 10-μm-thick sections on a cryostat (Microm, Heidelberg, Germany). Sections were coverslipped with DAPI mounting medium (Vector, Burlingame, CA) and analyzed with a Zeiss Axio/Apotome microscope.
Immunohistochemistry
Two transgenic T2/ePet-eGFP rats were deeply anesthetized with 20% isoflurane in propylene glycol (v/v) prior to transcardial perfusion with 40 ml of PBS, followed by 30 ml of cold 4% paraformaldehyde in PBS. The hindbrain was extracted, immersed in 30% sucrose, and frozen at −20°C until frozen-sectioned (25 μm) from caudal (obex) to rostral (midbrain), and sections were adhered to charged slides (Fisher Superfrost Plus; Thermo Fisher Scientific). Tissue sections were then dried at room temperature (1 h), washed in PBS (1 h), blocked with horse serum (5%) in 0.1% Triton X-100/PBS (1 h), incubated with a mouse monoclonal primary antibody (1:1000) targeting tryptophan hydroxylase (TPH; T0678, clone WH-3; Sigma, St. Louis, MO, USA), or a rabbit polyclonal antibody (1:1000) targeting GFP (A11122; Invitrogen, Carlsbad, CA, USA) in 2.5% normal horse serum, 0.1% Triton X-100/PBS over 2 nights at 4°C. After a 30-min PBS wash, the tissue was incubated (1 h) with either FITC- or Texas red-conjugated secondary antibodies against mouse IgG (1:500; F-8771; Sigma) or rabbit IgG (1:500, TI-1000; Vector Laboratories, Burlingame, CA, USA), respectively, washed in PBS, and coverslipped with Vectashield (Vector Laboratories) containing DAPI. Images (16 bit) were obtained using a Nikon E600 upright microscope (Nikon, Tokyo, Japan) with ×20 Plan Fluo phase NA 0.5 objective, xenon arc lamp, and Lambda 10-2 optical filter changer (Sutter Instruments, Novato, CA, USA) controlling excitation filters (420, 480, and 560 nm), bandpass emission filters (460, 535, and 605 nm), and a cooled CCD camera (Princeton Instruments MicroMax) for image acquisition in MetaMorph 6.3 (Universal Imaging Co., Downingtown, PA, USA). Images were imported into ImageJ (http://rsbweb.nih.gov/ij/), pseudocolored, and autooptimized for brightness and contrast, and the individual images were merged to render a composite.
Copy number determination and integration site analysis
All the copy number determination assays were established and performed in the same laboratory. For Southern blot analysis, 20 μg of NdeI-digested genomic DNA was separated on a 1% agarose gel, transferred to a nylon membrane (Hybond-XL; Amersham; Little Chalfont, UK), and hybridized with a 32P-labeled probe (Decalabel DNA labeling kit; Fermentas; Thermo Fisher Scientific) corresponding to the Venus region of the construct. qPCR was performed as described previously (32); the protocol for ligation-mediated PCR (LMPCR) is outlined in Supplemental Figure S3 and was essentially performed as described previously (16). Transposase-mediated integrations were verified by concatemer-specific PCR, using primers T2OncPub_F (TGTGCTGCAAGGCGATTA) and ITR-L nested (GACTTGTGTCATGCACAAAGTAGATGTCC) that amplifies the joint between the backbone and the 5′ ITR (263 bp). The original pT2-Venus plasmid was used as a positive control, and the Col14a1 primers WT1F (GGGGAAATGTCACCTTCAAA) and WT 1R (TGGGAGGATGGCTGTGTA) were included as amplification controls (678 bp).
RESULTS
Analysis of SB-mediated transgenesis in mice
A pilot study using the SB100X gene transfer system demonstrated the potential of this transposon-based strategy in mouse transgenesis (17). In the present, comprehensive analysis, an optimized cocktail of a circular plasmid carrying a Venus-tagged SB transposon (pT2/Venus; 0.4 ng/μl) and mRNA coding for the SB100X transposase (5 ng/μl) (17) was microinjected into the pronucleus of fertilized oocytes (28). Genotyping PCR revealed that 64% (34/53) of F0 generation animals were transgenically modified by this procedure (Table 1); 33 of 34 transgenic animals expressed the Venus transgene uniformly over the body surface, indicating that no significant transgene silencing occurred in the F0 generation. To determine the efficiency of germline transmission and to monitor transgene silencing in the F1 generation, all 34 transgenic founder mice were mated. Notably, all founders transmitted the transgenes to the next generation (Supplemental Table S1) and germline transmission from 94% (32/34) of the transgenic founders was near or above the expected mendelian rate, indicating the presence of multiple, independently integrated transposon copies in the genome. Indeed, the average number of transgene integrations was 2.9 among 12 randomly selected founders, with a quarter of the animals carrying a single copy of the transgene (Supplemental Table S1). Transgene silencing was only observed in 2 of the 297 F1 animals analyzed in total (Supplemental Table S1). These two F1 animals derived from founders with multiple integrations. The presence of plasmid vector DNA flanking the transposon cassette was not detected by backbone-specific PCR analysis in any of the 53 (0/53) F0 animals (not shown), indicating that all transgene integrations were transposase mediated. Thus, the use of circular plasmid DNA substrate for the SB100X transposase favored the transposition reaction as opposed to a random, transposase-independent integration of the pT2/Venus plasmid.
Controlling transgene copy number
Multiple integrations of a transgene construct per genome might require multiple rounds of breeding to separate individual transgenic loci. The copy number was shown to be dependent on and, hence, controllable by careful titration of the transposon components in cultured cells (18). In a similar manner, a 4-fold reduction of either the SB100X transposase mRNA (from 5 to 1.25 ng/μl) or the circular transposon plasmid DNA (from 0.4 to 0.1 ng/μl) in the microinjection mixture reduced transgene copy number by 35–40%, without significantly compromising the efficacy of transgenesis (Table 1, Supplemental Table S2, and Supplemental Fig. S3). Thus, by using these reduced nucleic acid concentrations in the microinjection mixture, one can control and reduce the number of transposon integrations in the transgenic founders.
SB-mediated transgenesis in laboratory rats and rabbits
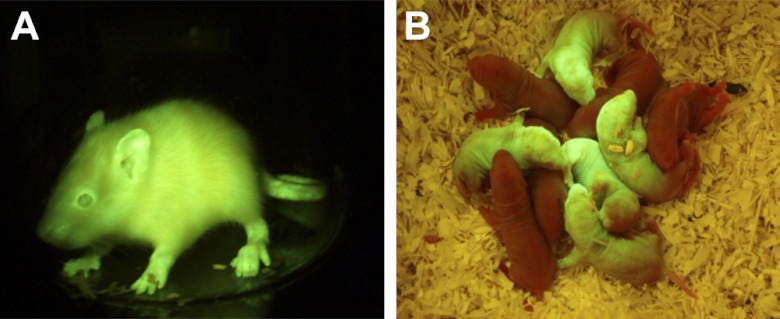
Because the SB system is applicable to a wide range of vertebrate species (33), we hypothesized that SB-mediated transgenesis may be extended to other valuable model species. Using the above conditions, we microinjected embryos of the SHR rat strain with the pT2/Venus transgene and found that 22% (11/51) of the F0 generation were transgenic founders (Table 1); 10 of 11 transgenic founders showed ubiquitous Venus fluorescence (Fig. 2), and no transgene silencing was detectable in the F1 generation. Increasing the concentration of the transposon (from 0.4 to 2 ng/μl) enhanced the average transgenic frequency to ∼41% (53/128), across several transgene constructs and genetic backgrounds. The average copy number also increased from 2 to 3.43 (Supplemental Table S3).
Figure 2.
Detection of the Venus fluorescent protein expression in SHR transgenic rats. A) T2/Venus founder F, carrying a single transgene integration on Chr13. B) Mendelian F1 litter of T2/Venus founder A, carrying a single transgene integration on Chr11. Venus fluorescence was detected using blue light illumination (FSH/LS-1B) with a barrier filter cutoff below 500 nm with a GFSP-5 headset (BLS, Hungary).
Altogether, we applied 9 different transgenes (Supplemental Fig. S1) to embryos from 5 different inbred and outbred rat strains, including the SHR, SS, FHH, SD, and an SS-BN congenic model (Table 1) with an average transgenic frequency of 36% (64/179) among the F0 animals born. Germline transmission was verified in 87% (27/31) of rat founders, with little evidence of mosaicism (Supplemental Table S3).
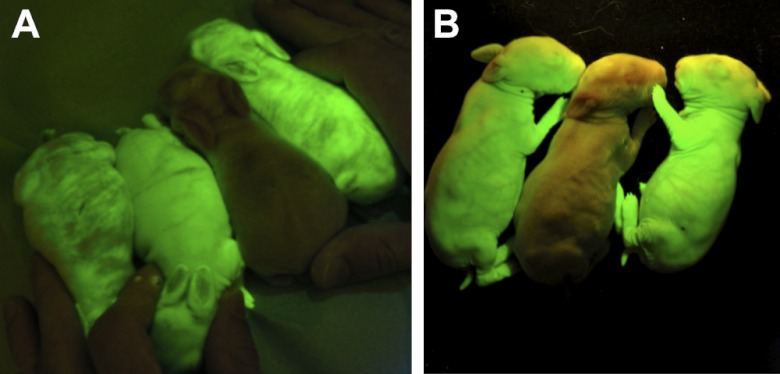
The potential of the SB100X-mediated transgenesis was also evaluated in rabbits. Injected embryos (n=472) were transferred into 25 pseudopregnant females. Altogether, 46 rabbits were born from 10 does (Table 1). Litter size varied from 2 to 11, which on average was higher than 2-4 in our earlier pronuclear microinjection experiments (34, 35). Of the 7 transgenic founders (7/46, 15%) 2 were stillborn, and 1 died before sexual maturity. Young hairless transgenic F0 pups displayed a mosaic pattern of Venus fluorescence expression, as shown in Fig. 3A. However, all 4 rabbit founders were germline transgenics, as 35 of the 80 F1 rabbits (44%) inherited the transgene, and all F1 transgenic rabbits expressed the Venus protein ubiquitously (Supplemental Table S4 and Fig. 3B). Thus, SB100X transgenesis is applicable in rabbits, and at a 15% transgenic founder rate with high germline transmission, it is significantly more efficient than other published methods (5, 8).
Figure 3.
Detection of the Venus fluorescent protein expression in transgenic rabbits. A) T2/Venus founder rabbits with a nontransgenic littermate. B) F1 litter of T2/Venus founder 1, carrying a single transgene integration. Founder rabbits exhibit variable Venus expression, while ubiquitous and strong expression was detected in F1 generation. Venus fluorescence was detected as in Fig. 2.
Molecular analysis of the integration sites
The number of integrated transgenes was determined by various methods, including Southern blot analysis, qPCR specifically established to quantify SB integration sites (32), and LMPCR (16). All of these methods gave comparable results. In total, 169 integration sites were identified (99, 63 and 7, from mice, rats, and rabbits, respectively; Supplemental Table S6). Single-copy integration events were readily identified in all three species (Supplemental Tables S1–S4 and Supplemental Fig. S3). Following LMPCR, cloning and sequencing of the flanking genomic sequences of 75 selected integrations, 70 sequence tags could be unambiguously mapped to the corresponding reference genomes. As expected, the transgenes integrated into a TA target site, which was duplicated and flanked the integrated transposon, apart from a single case where the integration was into a CA dinucleotide instead (data not shown). Comparison of the respective genomes revealed that 40% (28/70) of these inserted into introns of genes, and one in an exon of a putative protein-coding gene (Supplemental Table S6). These data are in agreement with the relatively random integration profile of SB (27, 36, 37), suggesting that the distribution of integration sites is similar regardless of whether integration occurs in the early embryo or adult somatic cell. Thus, when compared to alternative integrating gene delivery vectors, the SB system is expected to cause the least interference with host genes and regulatory sequences when used either for therapeutic applications (13) or in transgenesis (38). The majority of transgenic founders, including those that apparently transmitted a single copy of the transgene, were maintained for multiple generations (≥4) without any sign of epigenetic silencing of transgene expression.
SB100X-mediated transgenesis using either ubiquitous or tissue-specific promoters
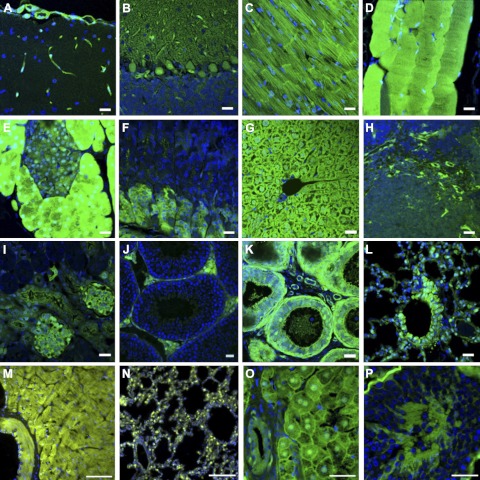
The flexible transposon vector system allows the investigator to use alternative promoter/enhancer elements to drive expression of transgenes. Among CAG-promoter (39)-driven Venus transgenic mice and rabbits produced with the SB100X method, reporter gene expression was observed in all 12 analyzed internal organs of transgenic F1 generation mice of both sexes and a transgenic male rabbit harboring a single-copy transgene by detailed fluorescence and histological analyses on a microscopic scale (Fig. 4). Thus, even a single copy of the transposon transgene utilizing the strong CAG promoter is sufficient for visible reporter gene expression in a variety of tissues.
Figure 4.
Histological sections of organs and analysis of CAG-Venus expression. Characteristic pictures are shown from a transgenic male F1 mouse (1, single-copy transgenic) cerebrum (A), cerebellum (B), myocardium (C), skeletal muscle (D), pancreas (E), stomach (F), liver (G), spleen (H), kidney (I), testis (J), epididymis (K), and lung (L), and a male F1 rabbit (1, single-copy transgenic) heart (M), lung (N), liver (O), and testis (P). Venus expression could be detected in sections of all analyzed organs in both species. In the mouse, fluorescence was seen in the capillaries of the brain, spleen, and kidney (glomerula). In the kidney, the epithelium of the distal tubule was fluorescent, including the region of the macula densa. Muscle tissues (heart muscle, smooth muscle of tunica muscularis, smooth muscle cells in the tunica media of blood vessels, skeletal muscle) were positive for Venus expression. In contrast to the photographed F1 male, several animals exhibited an irregular pattern in the liver and in the epithelium of the ductus epididymis. In cerebellum, Purkinje cells showed Venus fluorescence. In testis, Venus was expressed in Leydig cells. In the pancreas, all exocrine glandular cells showed strong Venus expression, extending to the nuclei, whereas in endocrine cells, the cytoplasm was weakly fluorescent. In the digestive tract, positive cells have formed a mosaic pattern in the intestinal epithelium and in the main cells of the fundus region of the stomach. In the lung, smooth muscle cells, capillaries, and parts of the bronchiolar epithelium expressed Venus. Venus expression patterns were similar in rabbits, except that there was less variability in rabbit hepatocytes, and expression in the testis was not confined to Leydig cells. Scale bars = 20 μm.
The benign transcription activity of the SB vector and the flexibility of transposon vector design allowed us to test tissue-specific promoters in transgenic models. Using the neuron-specific ePet promoter region (ref. 30 and Fig. 5A) to drive the expression of the eGFP marker, 13 transgenic founder rats were produced (Table 1). Five transgenic founders were subjected to further analysis. Figure 5B shows serotonergic neuron-specific eGFP expression in expected regions of the brain in one sacrificed founder rat at 12 d of age, confirmed by comparative immunostaining for TPH. In contrast, no expression was seen in the nonserotonergic facial motor nucleus. Four additional founders (T2/ePet-eGFP-A–D) were backcrossed to generate F1 animals (Supplemental Table S3), from which the brain stems of transgenic offspring were vibratome sectioned, and living tissue slices were examined by IR-DIC and gross fluorescence microscopy. F1 pups from T2/ePet-eGFP founders B–D demonstrated nonspecific expression in the brain tissue or lower levels (fewer cells) of specific eGFP expression and were not studied further (data not shown). However, founder T2/ePet-eGFP-A yielded offspring with serotonergic neuron-specific expression in live tissue at 28 d of age (Fig. 5C). From a litter of 10 F1 pups, 3 additional animals were analyzed further. Tissue-specific expression was correlated with 1 of 3 LMPCR-mapped integrations (T2/ePet-eGFP-A1), mapping to rat chromosome 7 by expression analysis and genotyping (Fig. 5D). After backcrossing and intercrossing an animal with this transgene integration, heterozygous and homozygous animals showed similar patterns of eGFP fluorescence (not shown). PCR analysis was consistent with only single-copy transgenes integrated by transposition in this transgenic line and no concatemers (Supplemental Figure S3). In summary, 2 of 5 transgenic founders either directly displayed (Fig. 5B) or transmitted (Fig. 5C and Table 2) serotonergic neuron-specific expression of eGFP, while the remaining three founders transmitted nonspecific transgene expression.
Figure 5.
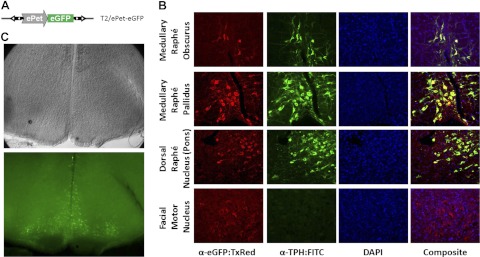
Tissue-specific reporter gene expression. A) The T2/ePet-eGFP transposon transgene harbors the eGFP protein under the control of a chimeric promoter consisting of the mouse Pet-1 enhancer and human β-globin minimal promoter fragment (ePet). B) Transgenic expression in a representative founder animal showing convergent expression of eGFP using a Texas-red-conjugated antibody with TPH labeled with a FITC-conjugated antibody by immunohistochemistry in the medullary raphé obscurus and pallidus, the lateral wings of the pontine dorsal raphé, and absence from motor neurons in the facial motor nucleus. DAPI was used to stain all nuclei. C) Live tissue sectioning of a T2/ePet-eGFP transgenic F1 animal shows neuron-specific expression in the medullary raphé. Table 1 shows correlation of tissue-specific expression to specific transgene integration ePet-eGFP-A1 on rat chromosome 7 in 3 examined animals.
Table 2.
Tissue-specific expression correlates to specific transgene integration ePet-eGFP-A1 on rat chromosome 7 in 3 examined animals
| Parameter | Pup analysis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Serotonergic-specific expression | ND | ND | + | + | ND | + | ND | ND | ND | ND |
| Insert ID and position | ||||||||||
| ePet-eGFP-A1; Chr. 7: ∼123.8 Mbp | + | − | + | + | + | + | + | + | − | − |
| ePet-eGFP-A2; Chr. X: ∼85.4 Mbp | − | − | − | − | + | − | − | − | − | − |
| ePet-eGFP-A3; Chr. 2: ∼92.2 Mbp | + | − | − | + | − | − | − | + | + | + |
See Fig. 5. Pup 7 was backcrossed to establish a breeding line for integration ePet-eGFP-A1. ND, not determined.
Transgenic rescue of an inborn genetic disorder using SB-mediated transgenesis
To demonstrate the applicability of the SB100X-mediated transgenesis in functional genomics, we applied this method to correct a genetic defect in the inbred FHH rat, which is characterized by a distinctive coat pattern and a bleeding disorder (40). Using classical genome mapping, a platelet storage pool defect had been previously mapped to a ∼1-cm region in the FHH rat strain (40). Within this interval, the search for a causative relationship pointed to the Rab38 gene, whose expression was abolished by a natural null mutation in the FHH strain (41). Rab38 cDNA amplified from the BN rat strain was placed under control of the CAG promoter (Fig. 6A) in the transposon vector (pT2/Rab38). Using SB100X-mediated transgenesis, we injected this construct into FHH-strain zygotes, resulting in 2 transgenic founders among 12 pups (Fig. 6B). Although some mosaicism was observed in one transgenic founder by coat color (Fig. 6B, top panels), only ubiquitous expression was seen in the N2 generation from both founders (lines A and B). A single transposon integration was cloned from the offspring of each transgenic founder (Supplemental Table S3). Following inbreeding, analysis of F9 generation homozygous transgenic offspring showed no evidence of concatemer transgene insertions as determined by PCR (Supplemental Figure S3), demonstrating that a single-copy integration of the transgene by transposition was sufficient to rescue the coat-color phenotype. Notably, SB100X-mediated transgenic rescue of RAB38 deficiency also restored the tail bleeding time of FHH-T2/Rab38-A animals (Fig. 6C), confirming that RAB38 expression is required for normal pigmentation (42) and preventing the storage pool defect in the FHH strain. We have bred these transgenic rats for 9 generations with no evidence of transgene silencing. Thus, SB100X-mediated transgenesis is applicable for efficient, stable genetic rescue of mutant phenotypes by transgenic complementation in an in vivo animal setting.
Figure 6.
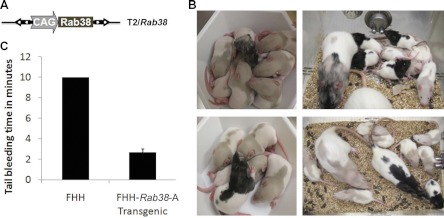
Transgenic rescue. A) The T2/Rab38 transposon transgene harbors the BN allele of the Rab38 cDNA controlled by the ubiquitous CAG promoter (39). B) Two transgenic founder pups (F0) demonstrate mosaic rescue of the fawn coat color defect of FHH rats, evidenced by black fur, which was transmitted to transgenic pups of the F1 generation. C) Platelet storage pool defect of FHH rats is also rescued by the T2/Rab38 transgene, FHH-T2/Rab38-A transgenic animals (n=3) show reduced bleeding time compared to FHH control animals (n=3), which do not stop bleeding after 10 min (P<0.003; Student's unpaired t test).
Comparison of the SB and PB transposon systems
Finally, we directly compared the SB and PB transposon systems for routine laboratory rodent transgenesis. Structurally identical SB and PB constructs were generated for comparison (see Fig. 1). As for the SB system (17), the optimal ratio of the circular pBac/Venus transposon donor plasmid and the mouse codon-optimized PB transposase (mPB; ref. 43) mRNA was determined using an in vitro embryo culture assay (Supplemental Fig. S2). Our criteria to select the optimal condition for the transfer of injected zygotes into pseudo-pregnant foster mothers were a high survival rate of the embryos (∼90%), efficient transgenesis, and the lack of obvious mosaic transgene expression. The in vitro optimized conditions were used to generate transgenic mice and compared side-by-side to SB100X-mediated transgenesis. In comparison to the SB system (0.4 ng/μl circular pT2/Venus plasmid and 5 ng/μl SB100X mRNA), the best condition for PB was 2 ng/μl pBac/Venus plasmid coinjected with 15 ng/μl mPB mRNA, resulting in 44% transgenic embryos in vitro (Supplemental Fig. S2). Overall, both transposon systems were useful for generating transgenic mouse models, but the SB system outperformed PB, as the germline-transgenic frequencies in the F0 generation for the SB and the PB systems were 64 vs. 18%, respectively (Table 1 and Supplemental Table S5).
DISCUSSION
Here, we provide a simple, reproducible, transposon-mediated transgenic protocol in three important biomedical models. The reported transgenic rates, 50–64, 14–72, and 15% of live founders for mice, rats, and rabbits, respectively (Table 1), represent a significant improvement as compared to the classical pronuclear injection of linear DNA fragments (44–46). In our protocol, in vitro synthesized mRNA encoding the hyperactive SB100X transposase was coinjected with circular plasmid DNA carrying the transposon. On translation of the transposase mRNA, enzyme-mediated excision of the transgene cassettes from the plasmids followed by permanent genomic integration produced stable transgenic animal lines. Fine-tuning the composition of the injection cocktail decreased the number of integration sites without significantly reducing the frequency of transgenesis (Table 1 and Supplemental Table S2). Despite physiological differences, the protocol worked well in all three species, including various inbred strains of laboratory rats, avoiding strain-specific limitations of transgenesis reported using alternative strategies (7). As a proof of principle, SB100X-mediated transgenesis demonstrated transgenic rescue by expressing an endogenous gene in a mutant genetic background (Fig. 6).
Since the transposase is injected in the form of mRNA, it only requires translation before it can catalyze the excision and genomic integration of the transgenic DNA via a cut-and-paste mechanism (17). Early transgene integration by the transposase mRNA increases the probability of transgene incorporation into the founder germline (47, 48). In contrast, following administration of a lentivirus, the vector requires several steps of processing, including reverse transcription, thus delaying transgene integration (reviewed in ref. 7). Notably, using SB100X-mediated transgenesis, germline transmission in founder mice and rats was at or frequently over mendelian rates, indicating that transgene integration often occurred prior to first cell division. We should note here that because of the relatively long half-life of the transposase (49), integration could also occur at later time points and, as in alternative transgenic strategies, could contribute to genetic mosaicism. However, it is important to point out that stable integration occurred early enough to facilitate germline transgenesis in nearly all F0 animals generated for all three species. Furthermore, we have demonstrated that the SB100X-mediated transgenesis can be optimized to generate transgenic founders with single-copy integrations. Finally, single-copy SB transposon transgenes driven from either a ubiquitous (Figs. 3 and 6) or a tissue-specific promoter (Fig. 5) maintained transgene expression across multiple generations without significant loss of expression.
In contrast to mice and rats, all rabbit founder pups showed some degree of mosaic fluorescence in the skin (Fig. 3A). We propose that this phenomenon is associated with the characteristically fast early embryonic development of the rabbit. In contrast to the development of mouse or rat embryos that reach 2- to 4-cell stage in 32–48 h postcoitus, rabbit zygotes undergo a rapid series of cell divisions after fertilization, and reach the same developmental stage within 24 h of the preimplantation period (50, 51). Therefore, compared to rodents, the time window for transgenesis in the pronuclear stage of rabbit embryos is significantly narrower, resulting in elevated genomic mosaicism. In rabbits, the integration of a transgene occurs more frequently after the first cell division (8), manifested by a mosaic Venus expression pattern in the skin. Mosaicism is frequently observed with transgenesis via lentiviral vectors that need to go through additional steps of processing and integrate mostly after the first cell division in rabbits, resulting in poor germline transmission rates and high percentage of genetic mosaics (8). Thus, in contrast to rodents and some livestock species, lentiviral transduction is not a suitable method for large-scale germline transgenesis in rabbits. In summary, when compared to lentiviral transduction (8) or traditional pronuclear microinjection in rabbits (5), SB100X-mediated transgenesis is one or two orders of magnitude more effective, respectively. Thus, SB100X-mediated transgenesis is far more efficient than any other method previously applied in rabbits.
The SB100X-mediated protocol also seems to be able to address another general problem of transgenesis, namely expression mosaicism (position effect variegation). Expression mosaicism is the variegated expression and unreliable tissue specificity following integration and is generally thought to be the result of triggering epigenetic silencing (reviewed in ref. 7). Repeated DNA and viral sequences including lentiviral vectors were shown to alert this host-specific defense mechanism. In principle, the combined ∼450 bp of ITR sequences of fish origin within an SB vector (12) is not expected to be actively recognized in phylogenetically distant genomes, such as rodents or rabbits. On the other hand, in the absence of the transposase, the transposon vector is not immune to concatemerization and epigenetic silencing (18). Thus, the SB transposon can be considered as a neutral piece of DNA that neither triggers nor insulates DNA from epigenetic transgene silencing. Notably, across multiple SB transposon transgenes and animals made at three different research institutes, we generally observed low rates of expression mosaicism. We propose that the reliable expression of SB transposon-based transgenes is associated with the integration of the transgene as an individual, well-defined transgene unit from a circular molecule (Fig. 1), the preferred substrate of SB transposition (52). Therefore, concatemerization of the transgene, as well as repeat-induced heterochromatin formation resulting in transgene silencing, is not expected to present an issue in transposition-mediated transgenesis. As was demonstrated in all of the three model organisms, a single-copy transgene expressed from the transposon vector is capable of supporting significant gene expression for multiple generations (Figs. 3–6). Furthermore, the transcriptionally inert SB transposon is not expected to override tissue-specific expression patterns specified by promoter elements in the transgene cassette, which is particularly valuable for driving tissue-specific expression. Indeed, depending on the promoter, the SB vector supported both ubiquitous (Figs. 2 and 6) and tissue-specific transgene expression (Fig. 5).
Our protocol worked reproducibly in various transgenic facilities, using a variety of promoters and transgenes of different sizes (ranging from 3.2 to 10.2 kb; Supplemental Fig. S1) with two alternative transposon vector systems and applied to three different animal models. Unlike retroviruses, which have a maximum packaging capacity, transposon vectors do not possess strict limitations in transgene size. Indeed, although transgenes > 7 kb integrate at a reduced rate (33), transposase-mediated transgenesis was shown to deliver bacterial artificial chromosome (BAC) transgenes at low frequencies with the Tol2 (53), PB, and SB100X transposon systems (54, 55).
The comparative study between the SB and PB vectors was performed in the same animal facility, using transgenic vectors of identical structure and following individual optimization. The transposase mRNA coinjection method, as well as the use of improved versions of transposases (17, 43), significantly improved transgenic rates for both SB and PB.
In summary, transposition-mediated gene delivery can provide reproducibly high transgenic and germline transmission rates and sustained transgene expression for functional genomic approaches in the mouse, rat, and rabbit animal models. The quality of transgenic animals was markedly superior with the transposon-mediated approach when compared to either the pronuclear (47) or the lentiviral approaches (6–8). Thus, transposition-mediated transgenesis compares favorably in terms of both efficiency and reliable transgene expression to the nonviral, pronuclear microinjection strategy, and offers comparable efficacy to lentiviral approaches. In addition, transposon-mediated transgenesis can address problems of using retroviral lentiviral vectors, including packaging limitations, toxicity, and biosafety concerns (7). Notably, the SB100X-mediated transgene integrations were less prone to genetic mosaicism and gene silencing as compared to either the classical pronuclear injection or to lentivirus-mediated transgenesis. Transposition-mediated gene delivery is a feasible, plasmid-based method that can easily be implemented by any laboratory. Indeed, by using this strategy, genetic manipulation of a number of mammalian species is possible, including large animals such as the pig (56) or those where pronuclear or retro/lentiviral strategies do not produce satisfactory results, e.g., in rhesus macaques (7) and where no molecular tools are available for genomic manipulation. This method could advance animal transgenesis by providing investigators with an alternative method to genetically modify animals at a more cost-effective manner for biomedical, as well as agricultural and pharmaceutical research.
Supplementary Material
Acknowledgments
The authors thank Drs. Mingyu Liang, Allen Cowley, Jr., Duann Pu, Elias Lianos, and Richard Roman for allowing us to share our experiences with their transgenic rat projects. Many thanks also to Tien Yin Yau, Lill Andersen and Dieter Fink for technical assistance and fruitful discussions.
This work was supported by the U.S. National Institutes of Health, National Heart, Lung and Blood Institute, grants P30HL101353 (A.G.), R01HL069321 (H.J.), and P301/10/0290 (M.P); the Grant Agency of the Czech Republic, grants LH12061 (V.L.) and LL1204 (within the ERC CZ program; M.P.); the Ministry of Education, Youth, and Sports of the Czech Republic, grants OM-00118/2008 (Z.B.), DFG IZ52/1-2 (Z.Iz.), and HEALTH-F4-2010-241504 (Z.Iz., H.J., M.P., and M.B.); and the Austrian Genome Research Programme, GEN-AU II and III (Austromouse; T.R.).
Author contributions: L.M., A.G., Z.Iz., Z.B., M.P., M.B., and T.R designed research, K.K., T.R., I.W., L.M., V.L., L.H., C.M., J.L., I.H., S.B., V.Z., L.P., K.B., A.D., M.H., E.P., M.G., M.C., and A.R.F. performed research; B.J., Z.Iz., M.P., M.B., H.J., Z.B., and T.R. analyzed data; Z.Iz., A.G., Z.Iv., L.M., T.R., and Z.B. wrote the paper.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BN
- brown Norway
- DAPI
- 4′,6-diamidino-2-phenylindole
- eGFP
- enhanced green fluorescent protein
- ePet
- Pet-1 enhancer
- FHH
- fawn-hooded hypertensive
- LMPCR
- ligation-mediated PCR
- Mcwi
- Medical College of Wisconsin
- PB
- piggyBac
- SB
- Sleeping Beauty
- SB100X
- hyperactive Sleeping Beauty transposase
- SD
- Sprague-Dawley
- SHR
- spontaneously hypersensitive rat
- SS
- Dahl salt sensitive
- TPH
- tryptophan hydroxylase
REFERENCES
- 1. Bosze Z., Houdebine L. (2006) Application of rabbits in biomedical research. World Rabbit Sci. 14, 1–14 [Google Scholar]
- 2. Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. (1980) Genetic transformation of mouse embryos by microinjection of purified DNA. Proc. Natl. Acad. Sci. U. S. A. 77, 7380–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rulicke T., Hubscher U. (2000) Germ line transformation of mammals by pronuclear microinjection. Exp. Physiol. 85, 589–601 [PubMed] [Google Scholar]
- 4. Filipiak W. E., Saunders T. L. (2006) Advances in transgenic rat production. Transgenic Res. 15, 673–686 [DOI] [PubMed] [Google Scholar]
- 5. Hirabayashi M., Takahashi R., Ito K., Kashiwazaki N., Hirao M., Hirasawa K., Hochi S., Ueda M. (2001) A comparative study on the integration of exogenous DNA into mouse, rat, rabbit, and pig genomes. Exp. Anim. 50, 125–131 [DOI] [PubMed] [Google Scholar]
- 6. Lois C., Hong E. J., Pease S., Brown E. J., Baltimore D. (2002) Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872 [DOI] [PubMed] [Google Scholar]
- 7. Park F. (2007) Lentiviral vectors: are they the future of animal transgenesis? Physiol. Genomics 31, 159–173 [DOI] [PubMed] [Google Scholar]
- 8. Hiripi L., Negre D., Cosset F. L., Kvell K., C, zömpöly T., Baranyi M., Gócza E., Hoffmann O., Bender B., Bosze Z. (2010) Transgenic rabbit production with simian immunodeficiency virus-derived lentiviral vector. Transgenic Res. 19, 799–808 [DOI] [PubMed] [Google Scholar]
- 9. Garrick D., Fiering S., Martin D. I., Whitelaw E. (1998) Repeat-induced gene silencing in mammals. Nat. Genet. 18, 56–59 [DOI] [PubMed] [Google Scholar]
- 10. McHenry J. Z., Leon A., Matthaei K. I., Cohen D. R. (1998) Overexpression of fra-2 in transgenic mice perturbs normal eye development. Oncogene 17, 1131–1140 [DOI] [PubMed] [Google Scholar]
- 11. Ellis J., Yao S. (2005) Retrovirus silencing and vector design: relevance to normal and cancer stem cells? Curr. Gene Ther. 5, 367–373 [DOI] [PubMed] [Google Scholar]
- 12. Ivics Z., Hackett P. B., Plasterk R. H., Izsvak Z. (1997) Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91, 501–510 [DOI] [PubMed] [Google Scholar]
- 13. Izsvak Z., Hackett P. B., Cooper L. J., Ivics Z. (2010) Translating Sleeping Beauty transposition into cellular therapies: victories and challenges. Bioessays 32, 756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collier L. S., Carlson C. M., Ravimohan S., Dupuy A. J., Largaespada D. A. (2005) Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature 436, 272–276 [DOI] [PubMed] [Google Scholar]
- 15. Izsvak Z., Fröhlich J., Grabundzija I., Shirley J. R., Powell H. M., Chapman K. M., Ivics Z., Hamra F. K. (2010) Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat. Methods 7, 443–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ivics Z., Izsvak Z., Chapman K. M., Hamra F. K. (2011) Sleeping Beauty transposon mutagenesis of the rat genome in spermatogonial stem cells. Methods 53, 356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mates L., Chuah, M K., Belay E., Jerchow B., Manoj N., Acosta-Sanchez A., Grzela D. P., Schmitt A., Becker K., Matrai J., Ma L., Samara-Kuko E., Gysemans C., Pryputniewicz D., Miskey C., Fletcher B., VandenDriessche T., Ivics Z., Izsvák Z. (2009) Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 41, 753–761 [DOI] [PubMed] [Google Scholar]
- 18. Grabundzija I., Irgang M., Mátés L., Belay E., Matrai J., Gogol-, Döring A., Kawakami K., Chen W., Ruiz P., Chuah M. K., VandenDriessche T., Izsvák Z., Ivics Z. (2010) Comparative analysis of transposable element vector systems in human cells. Mol. Ther. 18, 1200–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dupuy A. J., Clark K., Carlson C. M., Fritz S., Davidson A. E., Markley K. M., Finley K., Fletcher C. F.., Ekker S. C., Hackett P. B., Horn S., Largaespada DA. (2002) Mammalian germ-line transgenesis by transposition. Proc. Natl. Acad. Sci. U. S. A. 99, 4495–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carlson D. F., Geurts A. M., Garbe J. R., Park C. W., Rangel-Filho A., O'Grady S. M., Jacob H. J., Steer C. J., Largaespada D. A., Fahrenkrug S. C. (2011) Efficient mammalian germline transgenesis by cis-enhanced Sleeping Beauty transposition. Transgenic Res. 20, 29–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ding S., Wu X., Li G., Han M., Zhuang Y., Xu T. (2005) Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122, 473–483 [DOI] [PubMed] [Google Scholar]
- 22. Sumiyama K., Kawakami K., Yagita K. (2010) A simple and highly efficient transgenesis method in mice with the Tol2 transposon system and cytoplasmic microinjection. Genomics 95, 306–311 [DOI] [PubMed] [Google Scholar]
- 23. Walisko O., Schorn A., Rolfs F., Devaraj A., Miskey C., Izsvák Z., Ivics Z. (2008) Transcriptional activities of the Sleeping Beauty transposon and shielding its genetic cargo with insulators. Mol. Ther. 16, 359–369 [DOI] [PubMed] [Google Scholar]
- 24. Moldt B., Yant S. R., Andersen P. R., Kay M. A., Mikkelsen J. G. (2007) Cis-acting gene regulatory activities in the terminal regions of sleeping beauty DNA transposon-based vectors. Hum. Gene Ther. 18, 1193–1204 [DOI] [PubMed] [Google Scholar]
- 25. Wilson M. H., Coates C. J., George A. L., Jr. (2007) PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther. 15, 139–145 [DOI] [PubMed] [Google Scholar]
- 26. Galvan D. L., Nakazawa Y., Kaja A., Kettlun C., Cooper L. J., Rooney C. M., Wilson M. H. (2009) Genome-wide mapping of PiggyBac transposon integrations in primary human T cells. J. Immunother. 32, 837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ammar I., Gogol-, Döring A., Miskey C., Chen W., Cathomen T., Izsvák Z., Ivics Z. (2012) Retargeting transposon insertions by the adeno-associated virus Rep protein. Nucleic Acids Res. 40, 6693–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rulicke T. (2004) Pronuclear microinjection of mouse zygotes. Methods Mol. Biol. 254, 165–194 [DOI] [PubMed] [Google Scholar]
- 29. Geurts A. M., Cost G. J., Rémy S., Cui X., Tesson L., Usal C., Ménoret S., Jacob H. J., Anegon I., Buelow R. (2010) Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol. Biol. 597, 211–225 [DOI] [PubMed] [Google Scholar]
- 30. Scott M. M., Krueger K. C., Deneris E. S. (2005) A differentially autoregulated Pet-1 enhancer region is a critical target of the transcriptional cascade that governs serotonin neuron development. J. Neurosci. 25, 2628–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Besenfelder U., Brem G. (1993) Laparoscopic embryo transfer in rabbits. J. Reprod. Fertil. 99, 53–56 [DOI] [PubMed] [Google Scholar]
- 32. Kolacsek O., Gogol-, Döring A., Miskey C., Chen W., Cathomen T., Izsvák Z., Ivics Z. (2011) Reliable transgene-independent method for determining Sleeping Beauty transposon copy numbers. Mob. DNA 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yant S. R., Meuse L., Chiu W., Ivics Z., Izsvak Z., Kay M. A. (2000) Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat. Genet. 25, 35–41 [DOI] [PubMed] [Google Scholar]
- 34. Bodrogi L., Brands R., Raaben W., Seinen W., Baranyi M., Fiechter D., Bosze Z. (2006) High level expression of tissue-nonspecific alkaline phosphatase in the milk of transgenic rabbits. Transgenic Res. 15, 627–636 [DOI] [PubMed] [Google Scholar]
- 35. Hiripi L., Makovics F., Halter R., Baranyi M., Paul D., Carnwath J. W., Bösze Z., Niemann H. (2003) Expression of active human blood clotting factor VIII in mammary gland of transgenic rabbits. DNA Cell Biol. 22, 41–45 [DOI] [PubMed] [Google Scholar]
- 36. Vigdal T. J., Kaufman C. D., Izsvak Z., Voytas D. F., Ivics Z. (2002) Common physical properties of DNA affecting target site selection of sleeping beauty and other Tc1/mariner transposable elements. J. Mol. Biol. 323, 441–452 [DOI] [PubMed] [Google Scholar]
- 37. Yant S. R., Wu X., Huang Y., Garrison B., Burgess S. M., Kay M. A. (2005) High-resolution genome-wide mapping of transposon integration in mammals. Mol. Cell. Biol. 25, 2085–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sauvain M. O., Dorr A. P., Stevenson B., Quazzola A., Naef F., Wiznerowicz M., Schütz F., Jongeneel V., Duboule D., Spitz F., Trono D. (2008) Genotypic features of lentivirus tansgenic mice. J. Virol. 82, 7111–7119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Niwa H., Yamamura K., Miyazaki J. (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 [DOI] [PubMed] [Google Scholar]
- 40. Datta Y. H., Wu F. C., Dumas P. C., Rangel-Filho A., Datta M. W., Ning G., Cooley B. C., Majewski R. R., Provoost A. P., Jacob H. J. (2003) Genetic mapping and characterization of the bleeding disorder in the fawn-hooded hypertensive rat. Thromb. Haemost. 89, 1031–1042 [PubMed] [Google Scholar]
- 41. Ninkovic I., White J. G., Rangel-Filho A., Datta Y. H. (2008) The role of Rab38 in platelet dense granule defects. J. Thromb. Haemost. 6, 2143–2151 [DOI] [PubMed] [Google Scholar]
- 42. Marks M. S. (2006) Darkness descends with two Rabs. J. Cell Biol. 175, 199–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cadinanos J., Bradley A. (2007) Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res. 35, e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. (1985) Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc. Natl. Acad. Sci. U. S. A. 82, 4438–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Charreau B., Tesson L., Soulillou J. P., Pourcel C., Anegon I. (1996) Transgenesis in rats: technical aspects and models. Transgenic Res. 5, 223–234 [DOI] [PubMed] [Google Scholar]
- 46. Wall R. J. (2001) Pronuclear microinjection. Cloning Stem Cells 3, 209–220 [DOI] [PubMed] [Google Scholar]
- 47. Whitelaw C. B., Springbett A. J., Webster J., Clark J. (1993) The majority of G0 transgenic mice are derived from mosaic embryos. Transgenic Res. 2, 29–32 [DOI] [PubMed] [Google Scholar]
- 48. Davidson A. E., Balciunas D., Mohn D., Shaffer J., Hermanson S., Sivasubbu S., Cliff M. P., Hackett P. B., Ekker S. C. (2003) Efficient gene delivery and gene expression in zebrafish using the Sleeping Beauty transposon. Dev. Biol. 263, 191–202 [DOI] [PubMed] [Google Scholar]
- 49. Geurts A. M., Yang Y., Clark K. J., Liu G., Cui Z., Dupuy A. J., Bell J. B., Largaespada D. A., Hackett P. B. (2003) Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol. Ther. 8, 108–117 [DOI] [PubMed] [Google Scholar]
- 50. Witschi E. (1956) Development of Vertebrates. W. B. Saunders, Philadelphia [Google Scholar]
- 51. Sultana F., Hatori M., Shimozawa N., Ebisawa T., Sankai T. (2009) Continuous observation of rabbit preimplantation embryos in vitro by using a culture device connected to a microscope. J. Am. Assoc. Lab. Anim. Sci. 48, 52–56 [PMC free article] [PubMed] [Google Scholar]
- 52. Yant S. R., Ehrhardt A., Mikkelsen J. G., Meuse L., Pham T., Kay M. A. (2002) Transposition from a gutless adeno-transposon vector stabilizes transgene expression in vivo. Nat. Biotechnol. 20, 999–1005 [DOI] [PubMed] [Google Scholar]
- 53. Suster M. L., Abe G., Schouw A., Kawakami K. (2011) Transposon-mediated BAC transgenesis in zebrafish. Nat. Protoc. 6, 1998–2021 [DOI] [PubMed] [Google Scholar]
- 54. Li M. A., Turner D. J., Ning Z., Yusa K., Liang Q., Eckert S., Rad L., Fitzgerald T. W., Craig N. L., Bradley A. (2011) Mobilization of giant piggyBac transposons in the mouse genome. Nucleic Acids Res. 39, e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rostovskaya M., Fu J., Obst M., Baer I., Weidlich S., Wang H., Smith A. J., Anastassiadis K., Stewart A. F. (2012) Transposon-mediated BAC transgenesis in human ES cells. Nucleic Acids Res. 40, e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Garrels W., Mátés L., Holler S., Dalda A., Taylor U., Petersen B., Niemann H., Izsvák Z., Ivics Z., Kues W. A. (2011) Germline transgenic pigs by Sleeping Beauty transposition in porcine zygotes and targeted integration in the pig genome. PLoS One 6, e23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.