Abstract
Malignant hyperthermia (MH) susceptibility has been attributed to a leaky sarcoplasmic reticulum (SR) caused by missense mutations in RYR1 or CACNA1S, and the MH crisis has been attributed solely to massive self-sustaining release of Ca2+ from SR stores elicited by triggering agents. Here, we show in muscle cells from MH-RyR1R163C knock-in mice that increased passive SR Ca2+ leak causes an enlarged basal influx of sarcolemmal Ca2+ that results in chronically elevated myoplasmic free Ca2+ concentration ([Ca2+]i) at rest. We discovered that Gd+3 and GsMTx-4 were more effective than BTP2 or expression of the dominant-negative Orai1E190Q in reducing both Ca2+ entry and [Ca2+]i, implicating a non-STIM1/Orai1 SOCE pathway in resetting resting [Ca2+]i. Indeed, two nonselective cationic channels, TRPC3 and TRPC6, are overexpressed, and [Na]i is chronically elevated in MH-RyR1R163C muscle cells. [Ca2+]i and [Na+]i are persistently elevated in vivo and further increased by halothane in MH-RyR1R163C/WT muscle. These increases are markedly attenuated by local perfusion of Gd+3 or GsMTx-4 and completely suppressed by dantrolene. These results contribute a new paradigm for understanding MH pathophysiology by demonstrating that nonselective sarcolemmal cation channel activity plays a critical role in causing myoplasmic Ca2+ and Na+ overload both at rest and during the MH crisis.—Eltit, J. M., Ding, X., Pessah, I. N., Allen, P. D., Lopez, J. R. Nonspecific sarcolemmal cation channels are critical for the pathogenesis of malignant hyperthermia.
Keywords: skeletal muscle, dantrolene
Malignant hyperthermia (MH) is an autosomal dominant hypermetabolic condition triggered by volatile anesthetics, such as halothane, and depolarizing neuromuscular blockers, such as succinylcholine (1). The MH crisis is characterized by hypermetabolism, hypercapnia, hyperthermia, tachycardia, hypoxemia, muscle rigidity, and respiratory and metabolic acidosis. If left untreated, the mortality is >70%, but improved understanding, better monitoring, and availability of the skeletal muscle relaxant dantrolene has reduced the mortality to <8% (1, 2).
Molecular genetic studies have established the type 1 ryanodine receptor (RYR1) gene encoding the skeletal muscle sarcoplasmic reticulum (SR) Ca2+ release channel as the primary locus for MH susceptibility (3–6). More than 180 RYR1 mutations have been associated with MH and/or central core disease (6). Another locus implicated for susceptibility is CACNA1S, encoding the pore-forming subunit of the skeletal muscle L-type voltage-dependent Ca2+ channel (CaV1.1; refs. 7–9). The molecular mechanisms by which both RyR1 and CaV1.1 mutations confer MH susceptibility are unknown. A common characteristic of muscle expressing MH-RyR1 and MH-CaV1.1 mutations is an increased resting intracellular Ca2+ concentration ([Ca2+]i) in susceptible patients (10) and experimental models (11–14) compared to wild-type (WT) muscle. Exposure to triggering agents (i.e., halothane at clinically relevant concentrations) accentuates this difference causing [Ca2+]i to rise severalfold in MH muscle, whereas exposure to the same concentration of halothane is without effect in WT muscle (13, 15, 16). Until now, the mechanism for the MH crisis has been attributed solely to massive self-sustaining release of Ca2+ from the SR on exposure to a triggering agent. Counter to this view, our results introduce a new paradigm that implicates nonspecific sarcolemmal cation entry channels as both the predominant source of acutely elevated Ca2+ during fulminant MH and a significant contributor to chronically elevated [Ca2+]i in quiescent MH-susceptible muscles. Furthermore, prevention of nonspecific sarcolemmal cation entry represents one of the pharmacological mechanisms by which dantrolene can prevent or abort the MH crisis.
MATERIALS AND METHODS
Myotube culture
Primary myoblasts were generated from the hindlimb and forelimb muscles of neonatal homozygous F2 R163C C57BL6 (MH-RyR1R163C) mice and their WT littermates (17, 18). Myoblasts were differentiated into myotubes by withdrawal of growth factors, as described previously (19). Animals were cared for and euthanized according to the standards set by the Harvard Medical School Institutional Animal Care and Use Committee.
Mn2+ quench
Differentiated WT and MH-RyR1R163C primary myotubes were loaded with 5 μM Fura-2-AM (Invitrogen, Eugene, OR, USA) for 30 min at 37°C. The myotubes were then washed 3 times with mammalian Ringer solution (see Solutions) and transferred to the stage of a Nikon TE2000 inverted microscope (Nikon, Tokyo, Japan) and illuminated at the isosbestic wavelength for Fura-2 (360 nm). Fluorescence emission was captured at 512 nm from regions of interest within each myotube from 3–10 individual cells at 5 frames/s using a ×40, 1.3-NA objective. To measure the specific rate of dye quench by Mn2+ entry, the Ringer solution under constant perfusion was switched to Mn2+-containing solution (see Solutions) at 23°C. The basal signal in Ringer solution and in Mn2+-containing solution was fitted to a linear regression (y=a+bx) independently. The specific rate of Fura-2 quenching induced by Mn2+ entry was calculated subtracting the basal slope to the slope during the Mn2+ solution application and expressed as fluorescence arbitrary units (f.a.u.) per second.
Ca2+-selective microelectrodes
Double-barreled Ca2+-selective microelectrodes were prepared as described previously (12, 20). Pulled microelectrodes were backfilled first with the neutral carrier ETH 129 (21193; Fluka-Sigma-Aldrich, Buchs, Switzerland) and then with pCa 7 solution. Each Ca2+-selective microelectrode was individually calibrated as described previously (21), and only those with a linear relationship between pCa 3 and 7 (nernstian response, 29.5 and 30.5 mV/pCa unit at 23°C and 37°C, respectively) were used experimentally. To better mimic the intracellular ionic conditions, all calibration solutions were supplemented with 1 mM Mg2+ and 8 mM Na+. The 0.75-mm OD barrel was backfilled with 3 M KCl just before its use. Interference from Na+ and Mg2+ on the calibration of these electrodes was negligible (e.g., 20 mM Na+ and 3 mM Mg2+ did not alter the response at pCa7). After making measurements of [Ca2+]i, all electrodes were recalibrated, and if the two calibration curves did not agree within 3 mV, data from that microelectrode were discarded. Before starting the studies, we determined by direct calibration that the Ca2+ sensitivity of Ca2+ microelectrodes was not affected between pCa 6 and 7 by any of the drugs used in the present study.
Na+-selective microelectrodes
Double-barreled Na+-selective microelectrodes were prepared in a manner similar to preparation of Ca2+-selective electrodes described above. The electrodes were backfilled first with the Na+-sensitive ion cocktail (Sodium Ionophore I Cocktail A; Fluka Sigma-Aldrich) based on the neutral ligand ETH-227 and 24 h later with a solution containing 8 mM NaCl. Microelectrodes were calibrated 24 h later in solutions containing different [Na+] and 1 mM MgCl2. The microelectrodes gave virtually nernstian responses at free [Na+] between 100 and 10 mM. However, at concentrations between 10 and 1 mM [Na+], the electrodes had a subnernstian response (40–45 mV), but their response was stable and of a sufficient amplitude to be able to measure intracellular Na+ concentration ([Na+]i). After making measurements of resting [Na+]i, all electrodes were recalibrated, and if the two calibration curves did not agree within 3 mV, data from that microelectrode were discarded.
Ca2+ and Na+ microelectrode recordings
Microelectrode recordings were performed as described previously (12). Single myotubes or vastus lateralis muscle fibers were impaled either with double-barreled Ca2+- or double-barreled Na+-selective microelectrodes, and their potentials were recorded via high-impedance amplifier (WPI Duo 773 electrometer; WPI, Sarasota, FL, USA). The potential from the 3 M KCl microelectrode (Vm) was subtracted electronically from either the potential of the Ca2+ electrode (VCaE), or the Na+ electrode (VNaE) to produce a differential Ca2+-specific potential (VCa) or Na+-specific potential (VNa) that represents the [Ca2+]i or [Na+]i, respectively. Vm, VCa, and VNa were filtered (30–50 kHz) to improve the signal-to-noise ratio and stored in a computer for further analysis.
Permanent overexpression of Orai1WT and Orai1E190Q
The Orai1WT and Orai1E190Q cDNAs were cloned into a retroviral expression vector with a separate eGFP expression cassette (plasmids 12199 and 21662, respectively; Addgene, Cambridge, MA, USA). These plasmids were kindly provided by Dr. Anjana Rao (Harvard Medical School and Immune Disease Institute, Boston, MA, USA). We verified that the correct sequence of both inserts and retroviral particles was packaged in HEK 293 helper cells, and myoblasts were infected at an MOI of 5, allowed to recover for 12 h, and then selected with puromycin (0.5 μg/ml) for 1 wk. After the selection period, all remaining cells on the plate showed expression of the EGFP marker. Uninfected cells did not survive to the selection protocol.
Western blot analysis
The determination of the expression levels of transient receptor potential cation (TRPC) proteins TRPC1, TRPC3, and TRPC6 was done as described previously (22).
SR loading capacity
Differentiated WT and MH-RyR1R163C primary myotubes were loaded with 5 μM Fluo-4-AM for 20 min at 37°C. The myotubes were placed on the stage of an epifluorescence microscope (Nikon TE2000) coupled to a digital acquisition system (Stanford Photonics, Stanford, CA, USA). The filter set used was excitation, 480/30 nm, T495LP dichroic and emission, 535/40 nm. The emission signal was acquired at a frequency of 30 frames/s. The amount of SR Ca2+ was estimated by taking the combined area under the curve of 3 sequential exposures to 20 mM caffeine in Ca2+-free medium to prevent Ca2+ entry to get the total area under the curve to assess the total amount of Ca2+ released. To estimate the total intracellular Ca2+ store, Fluo-4-AM-loaded myotubes were exposed to the Ca2+ ionophore 4Br-A23187 in Ca2+-free medium. The area of the released Ca2+ was computed for each myotube type studied.
[Ca2+]i and [Na+]i determination in mice in vivo
The measurements were done as described previously (23) with the following modifications. Heterozygous F12 C57BL6 (MH-RyR1R163C/WT) knock-in mice and their WT littermates were anesthetized (100 mg/kg ketamine and 5 mg/kg xylazine), intubated and ventilated with air using a mouse ventilator. A rectal temperature probe was placed, to measure core temperature and to provide a feedback signal to the heating pad to keep the mice euthermic. A small incision was made in the skin in the upper lateral part of both hind legs, the vastus lateralis muscle was identified, and its aponeurosis was partially removed. The superficial fibers were exposed and perfused with mammalian Ringer. The determination of [Ca2+]i or [Na+]i was carried out in both legs simultaneously using ion-selective microelectrodes described above (Supplemental Fig. S2). The exposed fibers were perfused with Ringer solution (left hind leg, control) or Ringer with the tested drug (right hind leg, test drug) locally. The ventilator was connected to a halothane vaporizer that was switched from 0 to 1.5 vol% in air to administer halothane. Several muscle fibers were impaled to measure ion concentrations for each condition in each leg (control condition, after test-drug application and after halothane exposure).
Solutions
Mn2+-containing solution (quench solution) had the following composition (in mM): 140 NaCl, 5 KCl, 0.5 MnCl2, 5.5 glucose, and 10 HEPES, pH 7.4. The mammalian Ringer solution used in this study had the following composition (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 glucose, and 10 HEPES, pH 7.4. The Ca2+-free solution had the following composition (in mM): 140 NaCl, 5 KCl, 2 MgCl2, 1 EGTA, 5 glucose, and 10 HEPES, pH 7.4. N-{4-[3,5-bis-(trifluoromethyl)-1H-pyrazol-1-yl]phenyl}-4-methyl-1,2,3-thiadiazole-5-carboxamide (BTP2; CAS 223499-30-7), Gd3+, 1-oleoyl-2-acetyl-sn-glycerol (OAG; CAS 86390-77-4), GsMTx-4, and dantrolene solutions were made by adding the desired concentration of the reagent to the physiological solution.
Statistics
All values are expressed as means ± se. Statistical analysis was performed using 1-way ANOVA and Tukey's t test for multiple measurements to determine significance (P<0.05).
RESULTS
Basal store-operated calcium entry (SOCE) is higher in resting homozygous MH-RyR1R163C myotubes
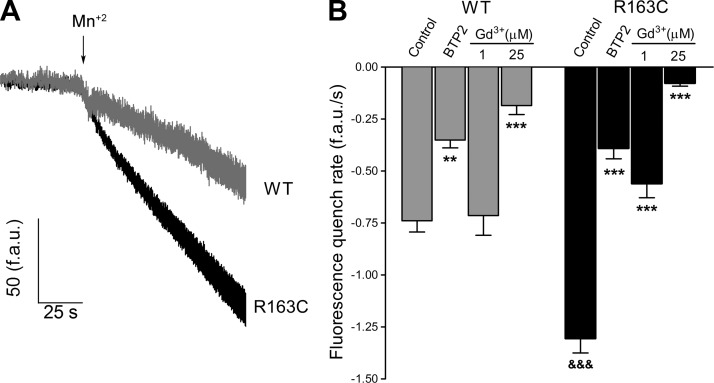
We employed Mn2+ quench of Fura-2 fluorescence to quantify unidirectional divalent cation flux into intact cultured skeletal muscle cells at rest. This technique allows the estimation of Ca2+ permeation from the extracellular space into the intact cell (24). Mn2+-quench experiments show that resting entry is 77% greater in MH-RyR1R163C myotubes than in WT cells (−1.30±0.07 f.a.u./s, n=160, and −0.74±0.05 f.a.u./s, n=107, respectively; P<0.001; Fig. 1). The Ca2+ release-activated Ca2+ current (CRAC) and TRPC channel blocker BTP2 (25) decreased Mn2+ entry 53% in WT cells and by 70% in MH-RyR1R163C cells. The stretch-activated channel blocker Gd3+ (26) at a concentration of 1 μM caused a 57% reduction in Mn2+ quench in MH-RyR1R163C cells but had no effect in WT cells. Increasing the concentration of Gd3+ to 25 μM reduced Mn2+ quench by 74% in WT cells and by 94%, almost completely blocking quench, in MH-RyR1R163C cells (Fig. 1).
Figure 1.
Resting sarcolemmal divalent cation permeability is greater in MH-RyR1R163C than WT cultured muscle cells. Sarcolemmal permeability to Ca2+ was estimated using the rate with which external Mn2+ quenches cytoplasmic Fura-2 fluorescence, as described in Materials and Methods. A) Change in the slope of the Fura-2 fluorescence (f.a.u./s) after switching to Mn2+ containing external solution (Mn2+) accounts for the rate of Mn2+ entry into the cells; traces showing the Mn2+ entry in quiescent WT and MH-RyR1R163C cells are depicted. B) Rates of Fura-2 fluorescence quenching in the presence of BTP2 (5 μM), Gd3+ (1 μM), and Gd3+ (25 μM) were computed and plotted. Means ± se (n≥50 myotubes) for each condition are shown. **P < 0.01, ***P < 0.001 vs. control; &&&P < 0.001 vs. corresponding WT.
Elevated [Ca2+]i in homozygous MH-RyR1R163C myotubes is partially normalized by modulation of Orai1
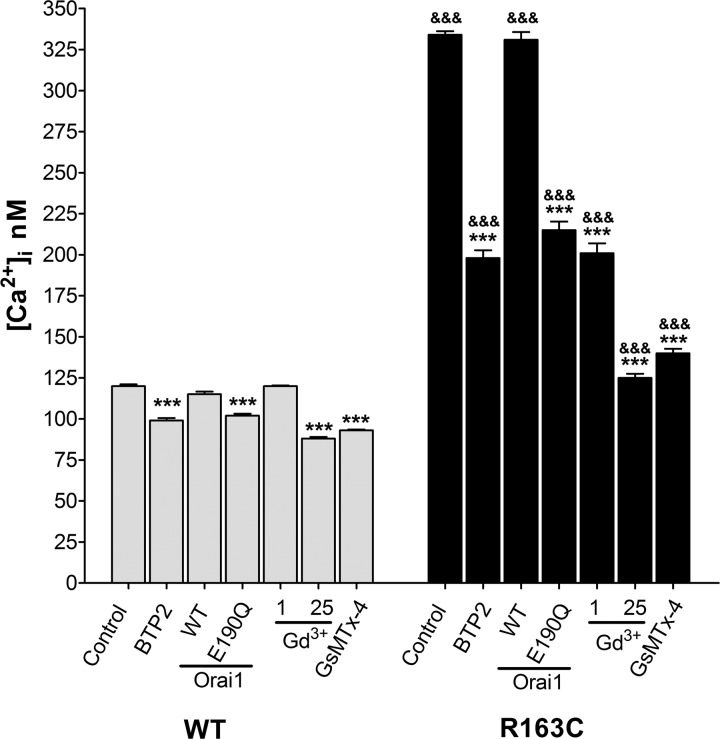
Resting membrane potentials and [Ca2+]i were measured in quiescent WT and MH-RyR1R163C myotubes with double-barreled ion-specific microelectrodes. [Ca2+]i was 2.8-fold higher in MH-RyR1R163C cells than that observed in WT cells (334±2 nM, n=25, vs. 120±1 nM; n=25, respectively; P<0.001; Fig. 2). There were no differences in membrane potential between WT and MH-RyR1R163C cells.
Figure 2.
Gd3+ and GsMTx-4 are more effective than BTP2 or overexpression of Orai1E190Q in reducing [Ca2+]i at rest in MH-RyR1R163C cultured muscle cells. [Ca2+]i at rest was measured in cells exposed to BTP2 (5 μM), stably transduced to overexpress Orai1WT or Orai1E190Q, exposed to Gd3+ (1 and 25 μM) or exposed to GsMTx-4 (5 μM). Means ± se (n≥10 myotubes) for each condition are shown. ***P < 0.001 vs. control; &&&P < 0.001 vs. corresponding WT.
Incubation of WT and MH-RyR1R163C cells with BTP2 for 5 min reduced [Ca2+]i in both groups, but the effect was greater in MH-RyR1R163C (40% decrement) than in WT (17% decrement, Fig. 2). A similar reduction in [Ca2+]i was observed when the dominant-negative form of Orai1E190Q was overexpressed in MH-RyR1R163C and WT cells. Overexpression of Orai1WT did not affect [Ca2+]i in either group (Fig. 2). Gd3+ (1 μM) decreased [Ca2+]i by 40% in MH-RyR1R163C myotubes but, as was the case for Mn2+ quench experiments above, had no effect on WT cells. On the other hand, 25 μM Gd3+ decreased [Ca2+]i in both WT and MH myotubes, with the decrement being significantly greater in MH-RyR1R163C myotubes (27 vs. 63%, respectively). GsMTx-4 (5 μM), a cationic hydrophobic polypeptide that blocks mechanosensitive (stretch-activated) ion channels that conduct Ca2+ and Na+ (27–29), also decreased [Ca2+]i in both WT and MH-RyR1R163C myotubes (20 and 58%, respectively; Fig. 2).
Differential expression of TRPC1, TRPC3, and TRPC6 in WT and homozygous MH-RyR1R163C myotubes
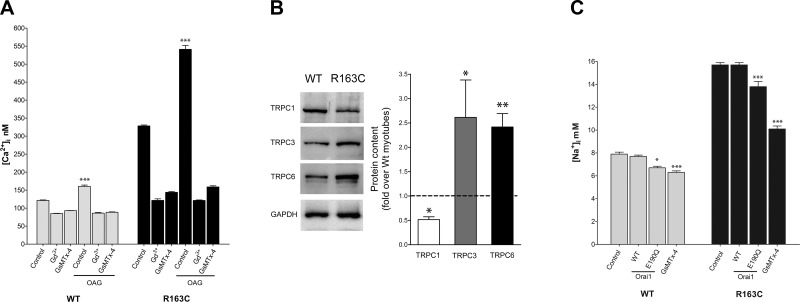
Myotubes were exposed to OAG, a membrane-permeable diacylglycerol analog that is a known activator of TRPC3/6 channels (30, 31). After 5 min of OAG (30 μM) application, [Ca2+]i increased by only 32% (from 122±1.4 nM, n=20, to 161±3.7 nM, n=10; P<0.001) in WT myotubes, whereas it increased 64% (from 329±2.6 nM, n=20, to 541±10.6 nM, n=10; P<0.001) in MH-RyR1R163C cells. The effect of OAG could be completely blocked with addition of either 25 μM Gd3+ or 5 μM GsMTx-4 to the assay medium of either genotype (Fig. 3A). Western blot analysis showed that TRPC1 was significantly decreased in MH-RyR1R163C myotubes compared to WT (0.5±0.06-fold, n=4), whereas the expression of both TRPC3 and TRPC6 was significantly increased (2.6±0.8-fold, n=4, and 2.4±0.3-fold, n=4, respectively) compared to WT (Fig. 3B).
Figure 3.
Expression of TRPC1, TRPC3, and TRPC6 in WT and MH-RyR1R163C cultured muscle cells differ. A) [Ca2+]i was measured in WT and MH-RyR1R163C cultured muscle cells. Effect of 5-min exposure to OAG (30 μM, DAG analog) whether alone or in the presence of Gd3+ (25 μM) or GsMTx-4 (5 μM) is plotted. Means ± se (n≥10 myotubes) for each condition are shown. B) Representative Western blot analysis shows the expression of TRPC1, TRPC3, and TRPC6 in WT and MH-RyR1R163C cells; GAPDH was also analyzed as loading control. Densitometric analysis of 4 independent Western blots for each TRPC protein is shown and expressed relative to the WT expression. Means ± se for each TRPC protein are shown. C) [Na+]i was measured in WT and MH-RyR1R163C cultured muscle cells. Effects of Orai1WT or Orai1E190Q overexpression and pretreatment with GsMTx-4 (5 μM) are shown. Means ± se (n≥10 myotubes) for each condition are shown. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
Resting [Na+]i is increased in homozygous MH-RyR1R163C myotubes
Because the permeability of TRPCs is not Ca2+ specific, their overactivity could also possibly allow significant Na+ entry, which could increase resting [Na+]i. In this regard, [Na+]i was 2-fold higher in MH-RyR1R163C myotubes than in WT (15.7±0.20 mM, n=23, vs. 7.9±0.17 mM, n=26, respectively; P<0.001; Fig. 3C). Overexpression of Orai1WT did not change [Na+]i in either WT or MH cells. Unexpectedly, the expression of the dominant-negative Orai1E190Q, which is unable to conduct calcium current, caused a 12-13% decrease in [Na+]i in both WT cells and MH cells (Fig. 3C). Application of GsMTx-4 (5 μM), which blocks Na+ current through stretch-activated channels (28), significantly reduced [Na+]i in both groups of cells but caused a 36% decrease in MH and only an 18% decrease in WT cells (Fig. 3C).
Blocking sarcolemmal Ca2+ and Na+ entry attenuates halothane-induced increases in [Ca2+]i and [Na+]i in heterozygous MH-RyR1R163C/WT mice in vivo
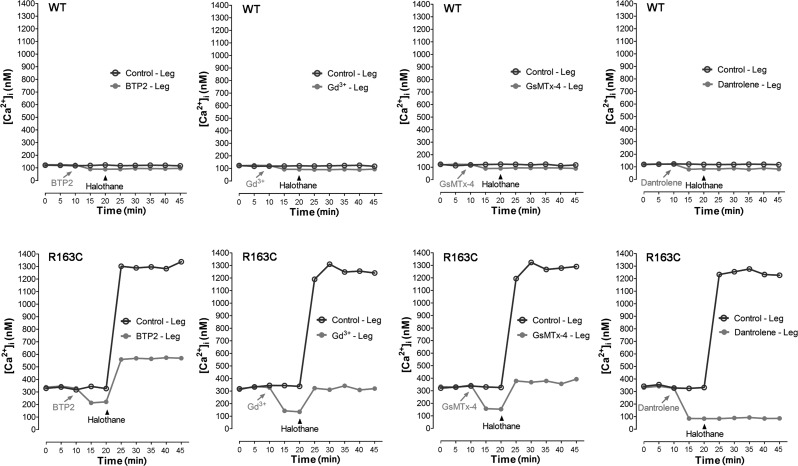
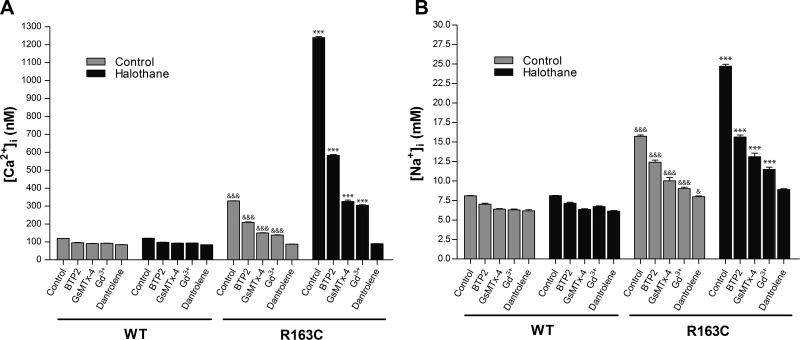
[Ca2+]i or [Na+]i was measured simultaneously in the right and left vastus lateralis muscles in heterozygous MH-RyR1R163C/WT and WT mice before and after exposure to 1.5% halothane vapor in their inspired gas. Representative experiments for in vivo measurements of [Ca2+]i for each treatment used are shown in Fig. 4. Mean changes in [Ca2+]i or [Na+]i before and after halothane for each treatment are summarized in Fig. 5.
Figure 4.
Determination of the intracellular Ca2+ concentrations in the vastus lateralis muscle in vivo. [Ca2+]i was monitored using Ca2+-selective microelectrodes in the vastus lateralis muscle. Measurements were done in the right and left thigh simultaneously both in MH-RyR1R163C/WT and WT mice. Exposed fibers were perfused with Ringer solution alone (left hind leg, control, solid line) or Ringer solution plus the tested drug (right hind leg, test drug, shaded line). Prior to the experiment, the animals were anesthetized using ketamine, intubated, and actively ventilated. The ventilator was connected to a halothane dispenser, which was switched from 0 to 1.5% where indicated. Drugs used in the experiments were dantrolene (40 μM), BTP2 (5 μM), Gd3+ (25 μM), or GsMTx-4 (5 μM). Each graph represents consecutive measurements done in a representative single mouse.
Figure 5.
Ca2+ and Na+ concentrations before and during halothane exposure in the vastus lateralis muscle in vivo. Intracellular Ca2+ determinations (A) or Na+ determinations (B) in vastus lateralis muscle were done using ion-selective microelectrodes and performed as described in Materials and Methods. The intracellular Ca2+ and Na+ concentrations in the absence (shaded columns) or in the presence (solid columns) of 1.5% halothane are shown. Experiments were done in the presence of BTP2 (5 μM), GsMTx-4 (5 μM), Gd3+ (25 μM), or dantrolene (40 μM). Means ± se for each condition are shown. Measurements were done in ≥5 mice/group***P < 0.001 vs. corresponding control treatment group; &P < 0.05, &&&P < 0.001 vs. corresponding WT group.
In MH-RyR1R163C/WT mice, [Ca2+]i measured in vivo was 328 ± 1.2 nM (n=76) and increased during halothane administration to 1240 ± 4.9 nM (n=76). In WT mice, [Ca2+]i was 120 ± 0.3 nM (n=60) and did not increase with halothane exposure. Local pretreatment of MH-RyR1R163C/WT muscle fibers with 5 μM BTP2 decreased [Ca2+]i in MH-RyR1R163C/WT mice to 209 ± 2.0 nM (n=13), and although it was able to attenuate the increase in [Ca2+]i during halothane exposure to 583 ± 4.1 nM (n=22), it was unable to completely abrogate the response. Local pretreatment of MH-RyR1R163C/WT muscle fibers with either GsMTx-4 or Gd3+ (5 and 25 μM, respectively) decreased [Ca2+]i to 149 ± 2.1 nM (n=9) and 138 ± 1.9 nM (n=23), respectively, and both decreased the rise in [Ca2+]i during halothane exposure by 81% (325±8.2 nM, n=23, and 303.7±3.70 nM, n=25, respectively) compared to the contralateral muscle pretreated with vehicle alone (Figs. 4 and 5). Local treatment of WT vastus lateralis fibers with any of the three drugs caused a small decrement in [Ca2+]i similar to their influence on WT myotubes. Exposure to halothane had no effect on [Ca2+]i in WT muscle with or without drug pretreatment. Local pretreatment of muscle fibers with 40 μM dantrolene decreased [Ca2+]i to similar levels in both WT and MH-RyR1R163C/WT muscle (84±0.8 nM, n=13, and 87±0.9 nM, n=11, respectively). Furthermore, the increase in [Ca2+]i seen during halothane exposure was completely ablated in dantrolene-pretreated MH-RyR1R163C/WT muscles.
To test the hypothesis that the increase in [Ca2+]i during exposure to halothane during the MH crisis could be the result of an increased sarcolemmal Ca2+ entry through a nonspecific sarcolemmal cation channel, as was suggested from our results in MH-susceptible myotubes at rest, we measured [Na+]i in vivo under the same conditions as above for [Ca2+]i (Fig. 5B). Similar to our data from myotubes, [Na+]i was elevated at rest in vastus lateralis of MH-RyR1R163C/WT mice compared to WT (15.7±0.16 vs. 8.2±0.06 mM, n=38). [Na+]i rose by 57% during halothane exposure (24.7±0.25 mM, n=51) in MH-RyR1R163C/WT fibers but remained unchanged in WT. Pretreatment with either BTP2, GsMTx-4 (5 μM), or 25 μM Gd3+ significantly lowered [Na+]i in MH-RyR1R163C/WT muscle fibers (P<0.001 vs. untreated) but did not restore it to WT levels. Furthermore, all three blockers reduced (by 62, 66, and 72%, respectively; P<0.001 vs. untreated) but could not ablate the elevation in [Na+]i seen in the contralateral leg during exposure to halothane. Similar to what we observed in measuring [Ca2+]i, dantrolene pretreatment reduced [Na+]i to WT levels in MH-RyR1R163C/WT fibers at rest and prevented any increase in [Na+]i in the treated leg during exposure to halothane.
DISCUSSION
Skeletal muscles in individuals and animals that are susceptible to MH have altered Ca2+ homeostasis at rest (10, 11). The most striking alteration is the increase of [Ca2+]i and [Na+]i both at rest and during a fulminant MH episode compared to the normal muscle (Figs. 2 and 4). Since RyR1 mutations have been described as the most common cause of this syndrome, there has been speculation that a persistent leak of Ca2+ ions from the SR should fully account for the increased [Ca2+]i. This hypothesis was supported by several studies that described an increased passive SR Ca2+leak in muscle cells and heterologous cells expressing RyR1s with MH mutations (12, 32, 33).
In the present study, we provide experimental evidence that a mutation in RyR1 that enhances SR Ca2+ leak (12) in MH susceptible muscles is closely associated with enhanced sarcolemmal Ca2+ and Na+ permeability, thereby satisfying the boundary theorem, which established that steady-state (long-term) changes in Ca2+ set point must be driven by fluxes in the plasma membrane (22, 34–37). Mn2+ quench experiments showed that MH-RyR1R163C muscle cells at rest have ∼80% more sarcolemmal cation permeability than WT, most likely a consequence of accentuated Ca2+ leak through RyR1R163C that chronically partially depletes SR stores at rest (Supplemental Fig. S1), which, in turn, activates a type of SOCE, as demonstrated by an increase in sarcolemmal Mn2+ permeability. It is important to note that unlike the SOCE activated by complete depletion of SR stores with no possibility of reuptake, as is the case with thapsigargin treatment, the resting Ca2+ entry shows a very slow rate compared to those measured as a consequence of complete SR store depletion (22, 38). Thus, although resting rates of entry are significantly higher in MH-RyR1R163C muscle cells than those observed with WT, they are not sufficient to cause a plateau in the quench of Fura-2 even after 90 s of Mn2+ exposure. To determine which channels were responsible for this increase, we used both pharmacological and molecular blockade of the classical Stim1/Orai1 (CRAC/SOCE) pathway. We found that BTP2 decreases Mn2+ permeability and [Ca2+]i and does so to a greater extent in MH-RyR1R163C than in WT cells. Furthermore, overexpression of the dominant-negative form of Orai1E190Q yielded similar results. These observations support the hypothesis that the classical stromal interaction molecule 1 (STIM1)/Orai1 SOCE pathway is constitutively more active at rest in MH cells. However, although BTP2 exposure and Orai1E190Q overexpression reduced [Ca2+]i in MH-RyR1R163C myotubes, they failed to normalize [Ca2+]i to WT levels (Fig. 2). On the other hand, Gd3+ had a stronger effect in decreasing Mn2+ permeability, and both Gd3+ and GsMTx-4 lowered [Ca2+]i in MH-RyR1R163C myotubes to levels nearly identical to untreated WT cells (∼120 nM; Fig. 2). This, coupled with the fact that [Na+]i is also elevated in MH-RyR1R163C myotubes, strongly suggests that in addition to the classical SOCE pathway, other Ca2+ entry pathways must also be involved. Moreover, these two agents block the increase in [Ca2+]i seen in untreated cells after acute exposure to the TRPC3/6 activator OAG (Fig. 3). Accordingly, expression of TRPC6 and the structurally similar TRPC3 is increased in MH-RyR1R163C myotubes compared to WT. The fact that resting sarcolemmal cation influx is much greater in MH-RyR1R163C than in WT myotubes implies an increased conductance of multiple Ca2+ entry pathways as a consequence of partial SR Ca2+ depletion (Supplemental Fig. S1). One long-term consequence of elevated RyR1-mediated SR Ca2+ leak is the chronic and partial depletion of SR stores, which enhances sarcolemmal Ca2+ and Na+ entry in MH-susceptible muscle cells; the latter is primarily responsible for resetting the [Ca2+]i and [Na]i at rest in MH muscles compared to their WT counterparts (Fig. 6). An important consequence of chronically elevated [Ca2+]i is that it is likely to promote elevated production of mitochondrial ROS and metabolic adaptations observed in MH-susceptible muscle in the absence of triggering agents (39–41).
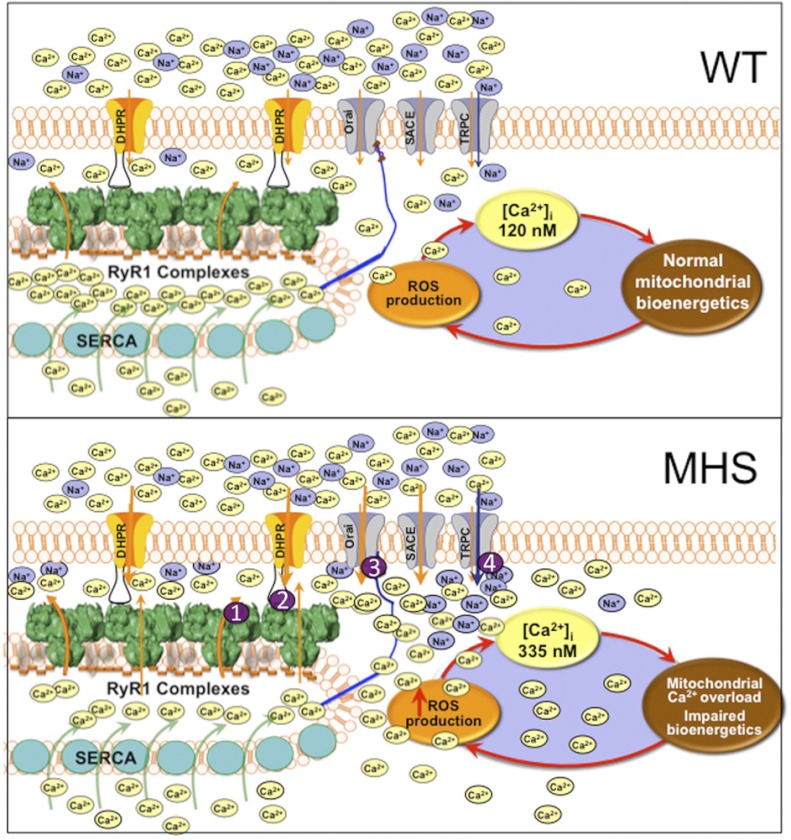
Figure 6.
Scheme showing the differences in sarcolemmal cation flow and intracellular resting [Ca2+] and [Na+] and its effects on mitochondrial ROS production between WT and heterozygous MH-RyR1R163C/WT muscles. Proposed sites for dantrolene's action are indicated with the numbers 1–4.
In addition to an elevated [Ca2+]i, we found that [Na]i is also higher in MH-RyR1R163C than in WT muscle cells. Previous measurements of resting [Na+]i in mammalian muscle vary between 7 and 13 mM, depending on the technique used for its determination (42, 43). Thus, our resting [Na+]i in WT muscle cells (7.9±0.17 mM) is within the published range, and for MH-RyR1R163C/WT, the [Na]i is slightly higher that the value (11.3±1 mM) that we found previously in intact muscle fibers isolated from MH-susceptible swine (Poland China-Pietrain) using the same technique (44). This increase in resting [Na+]i in MH-RyR1R163C muscle cells could arise either by increased influx or decreased efflux of Na+. The fact that the Na+ overload measured in vivo in heterozygous MH-RyR1R163C/WT muscles is substantially attenuated by local application of Gd3+ and GsMTx-4 strongly suggests that the elevation is mediated by an increase in influx rather than a decrease in Na+ efflux (Fig. 5). On the basis of our observation that the expression of TRPC1 is down-regulated and that TRPC3 and TRPC6 are up-regulated in MH-RyR1R163C muscles, the most likely mechanism for this increased influx is an increased conductance through the nonselective TRPC3/6 cation entry channels (Fig. 6 scheme). However, despite the fact that Orai1 has previously been described as absolutely Ca2+ selective (45), based on the fact that overexpression of the dominant-negative Orai1E190Q caused a modest reduction of [Na+]i in both WT and MH-RyR1R163C muscle cells in addition to its effect on [Ca2+]i, it is possible that Orai1 can allow a small amount of Na+ conductance that previously went undetected. Alternatively, overexpression of the mutated Orai1 channel in this environment could have altered either the expression of or conductance of associated TRPC channels (46–48), which then was indirectly responsible for this small decrease in Na+ conductance.
The levels of [Ca2+]i at rest measured in vivo were similar to those measured in myotubes (compare Figs. 2 and 5), with MH-RyR1R163C/WT muscles having almost 3-fold higher resting [Ca2+]i than WT, and resting Vm was identical in WT and MH-RyRR163C/WT mice. (see Table 1). When the animals were exposed to halothane, there was a small (∼4 mV) change in Vm, and [Ca2+]i increased by 4-fold in MH-RyRR163C/WT mice, while there was no change in either parameter in WT mice. In addition to the increase in [Ca2+]i, resting [Na+]i also increased during halothane exposure in MH-RyR1R163C/WT muscles, suggesting that nonspecific sarcolemmal cationic channels are activated during the MH crisis. Pretreatment by local application of BTP2, GsMTx-4, and Gd3+ directly to the muscle reduced both the subsequent response to halothane-induced elevation of [Ca2+]i by 59, 81, and 81%, respectively, and of [Na+]i by 62, 66, and 72%, respectively. In the case of GsMTx-4 and Gd3+, the increase in [Ca2+]i measured in the presence of halothane is not different than the resting [Ca2+]i measured in untreated MH muscle (∼320 nM, Figs. 4 and 5). This inhibition of the halothane-induced increase in [Ca2+]i by Ca2+ entry blockers further supports the hypothesis that sarcolemmal Ca2+ entry and not release from SR stores plays the critical role in maintaining the new steady-state [Ca2+]i that has been observed during an MH crisis (15, 49). This suggestion is especially reinforced in the case of Gd3+ (25 μM), a highly charged membrane impermeant ion, which has previously been shown not to affect electrically induced RyR1-mediated Ca2+ release, as inferred through force production (43). We cannot rule out the possibility that the Ca2+ influx that takes place during halothane exposure may activate RyR1 through a Ca2+-induced-Ca2+release (CICR) mechanism, as previously suggested by others (50, 51). In principle, this possibility would be unlikely, since Mg2+ inhibition and the repression by CaV1.1 keeps the RyR1 silent at rest and tightly controlled by membrane voltage (CaV1.1 control; refs. 20, 52–54). However, it has been suggested that MH mutations affect the regulation of RyR1 by Mg2+ and Ca2+ (55, 56) and alter CaV1.1-mediated RyR1 repression (13), making enhanced CICR a possibility, providing a further means of store depletion, which would then be followed by increased Ca2+ entry via SOCE.
Table 1.
Resting membrane potential determinations in vivo (mV)
| Pretreatment | Treatment |
||||
|---|---|---|---|---|---|
| No drug | BTP2 | Gd3+ | GsMTx-4 | Dantrolene | |
| Control | |||||
| WT | −82 ± 1.4 (n=121) | −83 ± 1.1 (n=13) | −82 ± 1.3 (n=13) | −82 ± 1.7 (n=9) | −82 ± 1.2 (n=14) |
| R163C-MHS | −82 ± 1.3 (n=143) | −83 ± 1 (n=13) | −82 ± 1.4 (n=23) | −82 ± 1.7 (n=9) | −82 ± 1.4 (n=11) |
| Halothane | |||||
| WT | −82 ± 1.2 (n=84) | −83 ± 1.2 (n=22) | −82 ± 1.5 (n=26) | −82 ± 1.4 (n=23) | −82 ± 1.5 (n=21) |
| R163C-MHS | −78 ± 1 (n=90)* | −78 ± 1.1 (n=22)* | −77 ± 1.3 (n=25)* | −78 ± 1 (n=23)* | −81 ± 1 (n=21) |
Resting membrane potentials values are expressed as means ± sd; n represents the number of measurements carried out in muscle fibers from 18 WT and 24 R163C-MHS mice.
P < 0.05 vs. R163C-MHS without halothane (1-way ANOVA, Kruskal-Wallis test, Dunn's multiple-comparison test).
Of particular interest is the action of dantrolene in skeletal muscle. Although dantrolene's primary pharmacological mechanism of action has been related to the inhibition of Ca2+ release (57–60), it attenuates both RyR1-mediated Ca2+ leak (Fig. 6, target 1; ref. 12) and excitation-coupled Ca2+ entry (ECCE) in MH-susceptible myotubes during active stimulation (ref. 38 and Fig. 6, target 2). The mechanisms responsible for how dantrolene modulates cation influx through sarcolemmal pathways not directly mediated by bidirectional signaling between CaV1.1 and RyR1 have been less clear. Remarkably, in addition to lowering resting [Ca2+]i (49, 61) and preventing the elevation induced by exposure to halothane, as previously reported (15, 62), dantrolene pretreatment also completely prevents the increase in [Na+]i caused by halothane exposure in MH-RyR1R163C/WT muscles. This finding supports the previously suggested possibility that one or more of dantrolene's targets reside in the plasma membrane (38, 63) and strongly suggests that the identity of this target is a nonspecific cation entry channel, likely TRPC3, TRPC6, or both (Fig. 6, target 4).
However, because the effect of dantrolene in lowering resting [Ca2+]i is significantly more profound than that of the pure sarcolemmal Ca2+ entry blockers, either the pharmacological agents used are incomplete blockers of their intended sarcolemmal targets or there are additional targets of dantrolene likely to be related to blocking SR Ca2+ leak, such as the leak state of RyR1 (Fig. 6, target 1). Thus, we cannot discount the alternative possibility that the suppression of the halothane-induced increase in [Na+]i and [Ca2+]i by dantrolene is secondary to a direct action on RyR1-mediated Ca2+ leak. This could restore Ca2+ store filling, thereby attenuating Ca2+ permeation through the SOCE pathways. (Fig. 6, targets 3 and 4).
In summary, these results open a new paradigm in the understanding of the MH pathology, demonstrating that nonselective sarcolemmal cation permeability, separate from the classic STIM/Orai pathway, is activated by SR depletion and plays a critical role in the causing cytosolic Ca2+ and Na+ overload both at rest and during the MH crisis. In addition, they suggest the possibility that these channels are critical targets for dantrolene's therapeutic effects.
Supplementary Material
Acknowledgments
This work was supported by grant AR052534 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, U.S. National Institutes of Health, to P.D.A and I.N.P.
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BTP2
- N-{4-[3,5-bis-(trifluoromethyl)-1H-pyrazol-1-yl]phenyl}-4-methyl-1,2,3-thiadiazole-5-carboxamide (CAS 223499-30-7)
- [Ca2+]i
- intracellular Ca+2 concentration
- CaV1.1
- skeletal muscle L-type voltage-dependent Ca2+ channel
- CRAC
- Ca2+ release-activated Ca2+ current
- f.a.u.
- fluorescence arbitrary unit
- MH
- malignant hyperthermia
- [Na+]i
- intracellular Na+ concentration
- OAG
- 1-oleoyl-2-acetyl-sn-glycerol (CAS 86390-77-4)
- RyR1
- type 1 ryanodine receptor
- SOCE
- store-operated calcium entry
- SR
- sarcoplasmic reticulum
- STIM1
- stromal interaction molecule 1
- TRPC
- transient receptor potential cation
- WT
- wild type
REFERENCES
- 1. Nelson T. E. (2002) Malignant hyperthermia: a pharmacogenetic disease of Ca2+ regulating proteins. Curr. Mol. Med. 2, 347–369 [DOI] [PubMed] [Google Scholar]
- 2. Krause T., Gerbershagen M. U., Fiege M., Weisshorn R., Wappler F. (2004) Dantrolene—a review of its pharmacology, therapeutic use and new developments. Anaesthesia 59, 364–373 [DOI] [PubMed] [Google Scholar]
- 3. MacLennan D. H., Duff C., Zorzato F., Fujii J., Phillips M., Korneluk R. G., Frodis W., Britt B. A., Worton R. G. (1990) Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature 343, 559–561 [DOI] [PubMed] [Google Scholar]
- 4. Kausch K., Lehmann-Horn F., Janka M., Wieringa B., Grimm T., Muller C. R. (1991) Evidence for linkage of the central core disease locus to the proximal long arm of human chromosome 19. Genomics 10, 765–769 [DOI] [PubMed] [Google Scholar]
- 5. Jurkat-Rott K., McCarthy T., Lehmann-Horn F. (2000) Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve 23, 4–17 [DOI] [PubMed] [Google Scholar]
- 6. Robinson R., Carpenter D., Shaw M. A., Halsall J., Hopkins P. (2006) Mutations in RYR1 in malignant hyperthermia and central core disease. Hum. Mutat. 27, 977–989 [DOI] [PubMed] [Google Scholar]
- 7. Weiss R. G., O'Connell K. M., Flucher B. E., Allen P. D., Grabner M., Dirksen R. T. (2004) Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am. J. Physiol. Cell. Physiol. 287, C1094–C1102 [DOI] [PubMed] [Google Scholar]
- 8. Pirone A., Schredelseker J., Tuluc P., Gravino E., Fortunato G., Flucher B. E., Carsana A., Salvatore F., Grabner M. (2010) Identification and functional characterization of malignant hyperthermia mutation T1354S in the outer pore of the Cavalpha1S-subunit. Am. J. Physiol. Cell. Physiol. 299, C1345–C1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carpenter D., Ringrose C., Leo V., Morris A., Robinson R. L., Halsall P. J., Hopkins P. M., Shaw M. A. (2009) The role of CACNA1S in predisposition to malignant hyperthermia. BMC Med. Genet. 10, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez J. R., Alamo L., Caputo C., Wikinski J., Ledezma D. (1985) Intracellular ionized calcium concentration in muscles from humans with malignant hyperthermia. Muscle Nerve 8, 355–358 [DOI] [PubMed] [Google Scholar]
- 11. Lopez J. R., Alamo L. A., Jones D. E., Papp L., Allen P. D., Gergely J., Sreter F. A. (1986) [Ca2+]i in muscles of malignant hyperthermia susceptible pigs determined in vivo with Ca2+-selective microelectrodes. Muscle Nerve 9, 85–86 [PubMed] [Google Scholar]
- 12. Yang T., Esteve E., Pessah I. N., Molinski T. F., Allen P. D., Lopez J. R. (2007) Elevated resting [Ca2+]i in myotubes expressing malignant hyperthermia RyR1 cDNAs is partially restored by modulation of passive calcium leak from the SR. Am. J. Physiol. Cell. Physiol. 292, C1591–C1598 [DOI] [PubMed] [Google Scholar]
- 13. Eltit J. M., Bannister R. A., Moua O., Altamirano F., Hopkins P. M., Pessah I. N., Molinski T. F., Lopez J. R., Beam K. G., Allen P. D. (2012) Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc. Natl. Acad. Sci. U. S. A. 109, 7923–7928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng W., Barrientos G. C., Cherednichenko G., Yang T., Padilla I. T., Truong K., Allen P. D., Lopez J. R., Pessah I. N. (2011) Functional and biochemical properties of ryanodine receptor type 1 channels from heterozygous R163C malignant hyperthermia-susceptible mice. Mol. Pharmacol. 79, 420–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopez J. R., Allen P. D., Alamo L., Jones D., Sreter F. A. (1988) Myoplasmic free [Ca2+] during a malignant hyperthermia episode in swine. Muscle Nerve 11, 82–88 [DOI] [PubMed] [Google Scholar]
- 16. Yuen B., Boncompagni S., Feng W., Yang T., Lopez J. R., Matthaei K. I., Goth S. R., Protasi F., Franzini-Armstrong C., Allen P. D., Pessah I. N. (2012) Mice expressing T4826I-RYR1 are viable but exhibit sex- and genotype-dependent susceptibility to malignant hyperthermia and muscle damage. FASEB J. 26, 1311–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rando T. A., Blau H. M. (1994) Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang T., Riehl J., Esteve E., Matthaei K. I., Goth S., Allen P. D., Pessah I. N., Lopez J. R. (2006) Pharmacologic and functional characterization of malignant hyperthermia in the R163C RyR1 knock-in mouse. Anesthesiology 105, 1164–1175 [DOI] [PubMed] [Google Scholar]
- 19. Yang T., Ta T. A., Pessah I. N., Allen P. D. (2003) Functional defects in six ryanodine receptor isoform-1 (RyR1) mutations associated with malignant hyperthermia and their impact on skeletal excitation-contraction coupling. J. Biol. Chem. 278, 25722–25730 [DOI] [PubMed] [Google Scholar]
- 20. Eltit J. M., Li H., Ward C. W., Molinski T., Pessah I. N., Allen P. D., Lopez J. R. (2011) Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak. Proc. Natl. Acad. Sci. U. S. A. 108, 7046–7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopez J. R., Alamo L., Caputo C., DiPolo R., Vergara S. (1983) Determination of ionic calcium in frog skeletal muscle fibers. Biophys. J. 43, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H., Ding X., Lopez J. R., Takeshima H., Ma J., Allen P. D., Eltit J. M. (2010) Impaired Orai1-mediated resting Ca2+ entry reduces the cytosolic [Ca2+] and sarcoplasmic reticulum Ca2+ loading in quiescent junctophilin 1 knock-out myotubes. J. Biol. Chem. 285, 39171–39179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barrientos G. C., Feng W., Truong K., Matthaei K. I., Yang T., Allen P. D., Lopez J. R., Pessah I. N. (2012) Gene dose influences cellular and calcium channel dysregulation in heterozygous and homozygous T4826I-RYR1 malignant hyperthermia-susceptible muscle. J. Biol. Chem. 287, 2863–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merritt J. E., Jacob R., Hallam T. J. (1989) Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J. Biol. Chem. 264, 1522–1527 [PubMed] [Google Scholar]
- 25. Zitt C., Strauss B., Schwarz E. C., Spaeth N., Rast G., Hatzelmann A., Hoth M. (2004) Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J. Biol. Chem. 279, 12427–12437 [DOI] [PubMed] [Google Scholar]
- 26. Allen D. G. (2004) Skeletal muscle function: role of ionic changes in fatigue, damage and disease. Clin. Exp. Pharmacol. Physiol. 31, 485–493 [DOI] [PubMed] [Google Scholar]
- 27. Ostrow K. L., Mammoser A., Suchyna T., Sachs F., Oswald R., Kubo S., Chino N., Gottlieb P. A. (2003) cDNA sequence and in vitro folding of GsMTx4, a specific peptide inhibitor of mechanosensitive channels. Toxicon 42, 263–274 [DOI] [PubMed] [Google Scholar]
- 28. Suchyna T. M., Johnson J. H., Hamer K., Leykam J. F., Gage D. A., Clemo H. F., Baumgarten C. M., Sachs F. (2000) Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J. Gen. Physiol. 115, 583–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whitehead N. P., Streamer M., Lusambili L. I., Sachs F., Allen D. G. (2006) Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul. Disord. 16, 845–854 [DOI] [PubMed] [Google Scholar]
- 30. Hofmann T., Obukhov A. G., Schaefer M., Harteneck C., Gudermann T., Schultz G. (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 [DOI] [PubMed] [Google Scholar]
- 31. Spassova M. A., Hewavitharana T., Xu W., Soboloff J., Gill D. L. (2006) A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. U. S. A. 103, 16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tong J., McCarthy T. V., MacLennan D. H. (1999) Measurement of resting cytosolic Ca2+ concentrations and Ca2+ store size in HEK-293 cells transfected with malignant hyperthermia or central core disease mutant Ca2+ release channels. J. Biol. Chem. 274, 693–702 [DOI] [PubMed] [Google Scholar]
- 33. Brini M., Manni S., Pierobon N., Du G. G., Sharma P., MacLennan D. H., Carafoli E. (2005) Ca2+ signaling in HEK-293 and skeletal muscle cells expressing recombinant ryanodine receptors harboring malignant hyperthermia and central core disease mutations. J. Biol. Chem. 280, 15380–15389 [DOI] [PubMed] [Google Scholar]
- 34. Rios E. (2010) The cell boundary theorem: a simple law of the control of cytosolic calcium concentration. J. Physiol. Sci. 60, 81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eltit J. M., Yang T., Li H., Molinski T. F., Pessah I. N., Allen P. D., Lopez J. R. (2010) RyR1-mediated Ca2+ leak and Ca2+ entry determine resting intracellular Ca2+ in skeletal myotubes. J. Biol. Chem. 285, 13781–13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eltit J. M., Perez C., Pessah I. N., Allen P. D., Lopez J. R. (2010) Reply to Rios: cell boundary theorem and Ca2+ fluxes in skeletal muscle. J. Biol. Chem. 285, le14 [Google Scholar]
- 37. Rios E. (2010) RyR1 expression and the cell boundary theorem. J. Biol. Chem. 285, le13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cherednichenko G., Ward C. W., Feng W., Cabrales E., Michaelson L., Samso M., Lopez J. R., Allen P. D., Pessah I. N. (2008) Enhanced excitation-coupled calcium entry in myotubes expressing malignant hyperthermia mutation R163C is attenuated by dantrolene. Mol. Pharmacol. 73, 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giulivi C., Ross-Inta C., Omanska-Klusek A., Napoli E., Sakaguchi D., Barrientos G., Allen P. D., Pessah I. N. (2011) Basal bioenergetic abnormalities in skeletal muscle from ryanodine receptor malignant hyperthermia-susceptible R163C knock-in mice. J. Biol. Chem. 286, 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Durham W. J., Aracena-Parks P., Long C., Rossi A. E., Goonasekera S. A., Boncompagni S., Galvan D. L., Gilman C. P., Baker M. R., Shirokova N., Protasi F., Dirksen R., Hamilton S. L. (2008) RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 133, 53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chelu M. G., Goonasekera S. A., Durham W. J., Tang W., Lueck J. D., Riehl J., Pessah I. N., Zhang P., Bhattacharjee M. B., Dirksen R. T., Hamilton S. L. (2006) Heat- and anesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse. FASEB J. 20, 329–330 [DOI] [PubMed] [Google Scholar]
- 42. Dunn J. F., Bannister N., Kemp G. J., Publicover S. J. (1993) Sodium is elevated in mdx muscles: ionic interactions in dystrophic cells. J. Neurol. Sci. 114, 76–80 [DOI] [PubMed] [Google Scholar]
- 43. Yeung E. W., Ballard H. J., Bourreau J. P., Allen D. G. (2003) Intracellular sodium in mammalian muscle fibers after eccentric contractions. J. Appl. Physiol. 94, 2475–2482 [DOI] [PubMed] [Google Scholar]
- 44. López J. R., Sanchez V., Dershwitz M., Sreter F. (1993) [K+]i ed [Na+]i in fibre di muscoli suscettibili e no ad ipertermia maligna. Acta Anaesth. Italica 44, 277–280 [Google Scholar]
- 45. Yeromin A. V., Zhang S. L., Jiang W., Yu Y., Safrina O., Cahalan M. D. (2006) Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma H. T., Peng Z., Hiragun T., Iwaki S., Gilfillan A. M., Beaven M. A. (2008) Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J. Immunol. 180, 2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liao Y., Erxleben C., Yildirim E., Abramowitz J., Armstrong D. L., Birnbaumer L. (2007) Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc. Natl. Acad. Sci. U. S. A. 104, 4682–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yuan J. P., Zeng W., Huang G. N., Worley P. F., Muallem S. (2007) STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat. Cell Biol. 9, 636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lopez J. R., Allen P., Alamo L., Ryan J. F., Jones D. E., Sreter F. (1987) Dantrolene prevents the malignant hyperthermic syndrome by reducing free intracellular calcium concentration in skeletal muscle of susceptible swine. Cell Calcium 8, 385–396 [DOI] [PubMed] [Google Scholar]
- 50. Ohta T., Endo M., Nakano T., Morohoshi Y., Wanikawa K., Ohga A. (1989) Ca-induced Ca release in malignant hyperthermia-susceptible pig skeletal muscle. Am. J. Physiol. Cell. Physiol. 256, C358–C367 [DOI] [PubMed] [Google Scholar]
- 51. Nelson T. E. (1983) Abnormality in calcium release from skeletal sarcoplasmic reticulum of pigs susceptible to malignant hyperthermia. J. Clin. Invest. 72, 862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Laver D. R., O'Neill E. R., Lamb G. D. (2004) Luminal Ca2+-regulated Mg2+ inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters. J. Gen. Physiol. 124, 741–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou J., Launikonis B. S., Rios E., Brum G. (2004) Regulation of Ca2+ sparks by Ca2+ and Mg2+ in mammalian and amphibian muscle. An RyR isoform-specific role in excitation-contraction coupling? J. Gen. Physiol. 124, 409–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhou J., Brum G., Gonzalez A., Launikonis B. S., Stern M. D., Rios E. (2005) Concerted vs. sequential. Two activation patterns of vast arrays of intracellular Ca2+ channels in muscle. J. Gen. Physiol. 126, 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Owen V. J., Taske N. L., Lamb G. D. (1997) Reduced Mg2+ inhibition of Ca2+ release in muscle fibers of pigs susceptible to malignant hyperthermia. Am. J. Physiol. Cell. Physiol. 272, C203–C211 [DOI] [PubMed] [Google Scholar]
- 56. Laver D. R., Owen V. J., Junankar P. R., Taske N. L., Dulhunty A. F., Lamb G. D. (1997) Reduced inhibitory effect of Mg2+ on ryanodine receptor-Ca2+ release channels in malignant hyperthermia. Biophys. J. 73, 1913–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Van Winkle W. B. (1976) Calcium release from skeletal muscle sarcoplasmic reticulum: site of action of dantrolene sodium. Science 193, 1130–1131 [DOI] [PubMed] [Google Scholar]
- 58. Pessah I. N., Lynch C., 3rd, Gronert G. A. (1996) Complex pharmacology of malignant hyperthermia. Anesthesiology 84, 1275–1279 [DOI] [PubMed] [Google Scholar]
- 59. Fruen B. R., Mickelson J. R., Louis C. F. (1997) Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J. Biol. Chem. 272, 26965–26971 [DOI] [PubMed] [Google Scholar]
- 60. Zhao F., Li P., Chen S. R., Louis C. F., Fruen B. R. (2001) Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J. Biol. Chem. 276, 13810–13816 [DOI] [PubMed] [Google Scholar]
- 61. Lopez J. R., Gerardi A., Lopez M. J., Allen P. D. (1992) Effects of dantrolene on myoplasmic free [Ca2+] measured in vivo in patients susceptible to malignant hyperthermia. Anesthesiology 76, 711–719 [DOI] [PubMed] [Google Scholar]
- 62. Allen P. D., Lopez J. R., Sanchez V., Ryan J. F., Sreter F. A. (1992) EU 4093 decreases intracellular [Ca2+] in skeletal muscle fibers from control and malignant hyperthermia-susceptible swine. Anesthesiology 76, 132–138 [DOI] [PubMed] [Google Scholar]
- 63. Zhao X., Weisleder N., Han X., Pan Z., Parness J., Brotto M., Ma J. (2006) Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. J. Biol. Chem. 281, 33477–33486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.