Abstract
The Ca2+-sensing stromal interaction molecule (STIM) proteins are crucial Ca2+ signal coordinators. Cre-lox technology was used to generate smooth muscle (sm)-targeted STIM1-, STIM2-, and double STIM1/STIM2-knockout (KO) mouse models, which reveal the essential role of STIM proteins in Ca2+ homeostasis and their crucial role in controlling function, growth, and development of smooth muscle cells (SMCs). Compared to Cre+/− littermates, sm-STIM1-KO mice showed high mortality (50% by 30 d) and reduced bodyweight. While sm-STIM2-KO was without detectable phenotype, the STIM1/STIM double-KO was perinatally lethal, revealing an essential role of STIM1 partially rescued by STIM2. Vascular and intestinal smooth muscle tissues from sm-STIM1-KO mice developed abnormally with distended, thinned morphology. While depolarization-induced aortic contraction was unchanged in sm-STIM1-KO mice, α1-adrenergic-mediated contraction was 26% reduced, and store-dependent contraction almost eliminated. Neointimal formation induced by carotid artery ligation was suppressed by 54%, and in vitro PDGF-induced proliferation was greatly reduced (79%) in sm-STIM1-KO. Notably, the Ca2+ store-refilling rate in STIM1-KO SMCs was substantially reduced, and sustained PDGF-induced Ca2+ entry was abolished. This defective Ca2+ homeostasis prevents PDGF-induced NFAT activation in both contractile and proliferating SMCs. We conclude that STIM1-regulated Ca2+ homeostasis is crucial for NFAT-mediated transcriptional control required for induction of SMC proliferation, development, and growth responses to injury.—Mancarella, S., Potireddy, S., Wang, Y., Gao, H., Gandhirajan, K., Autieri, M., Scalia, R., Cheng, Z., Wang, H., Madesh, M., Houser, S. R., Gill, D. L. Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle.
Keywords: vascular function, gastrointestinal development, Orai channel, transcription factor, mouse knockout
Stromal interaction molecule (STIM) proteins are ubiquitously expressed Ca2+-signal mediators, functioning to coordinate Ca2+ release from intracellular stores with control of Ca2+ entry channels in the plasma membrane (1–5). Expressed mostly in the endoplasmic reticulum (ER) membrane, STIM proteins sense small changes in ER Ca2+ content and translocate into junctions between ER and the plasma membrane, where they tether and activate the highly Ca2+-selective family of Orai channels, which mediate store-operated Ca2+ entry (SOCE) into the cytoplasm (3–5). SOCE signals control important longer-term Ca2+-induced responses, including Ca2+-mediated transcriptional events (3, 5, 6). Thus, locally restricted Ca2+ signals generated through SOCE provide a unique “Ca2+ signature response” that controls gene expression through nuclear factor of activated T cells (NFAT) and other transcription factors (6–10). In addition, SOCE maintains the homeostatic balance of cellular Ca2+, ensuring that ER Ca2+ stores are replenished to protect the functional integrity of ER and to permit the continuous generation of oscillatory Ca2+ signals (4, 5, 11, 12).
In excitable tissues, including smooth, cardiac, and skeletal muscle, voltage-operated Ca2+ channels (VOCCs) are prominent mediators of Ca2+ entry, in particular, for controlling contraction. However, studies revat STIM-mediated SOCE plays distinct roles in mediating prolonged Ca2+ entry signals controlling vital aspects of function and growth (13–19). In skeletal muscle, STIM1 and SOCE appear to be important for the maintenance of stored Ca2+ necessary for contraction and also for generating signals that control muscle growth and development (13, 15). Recent studies on cardiac muscle reveal how STIM1 controls hypeal thertrophy and transcriptional regulation of cardiac remodeling (16, 17). In smooth muscle, SOCE mediated by STIM and Orai is becoming recognized as important in mediating Ca2+ signals that control growth and phenotype change (19–25). Whereas skeletal and cardiac muscle are terminally differentiated, smooth muscle cells are able to undergo phenotypic transition from the differentiated contractile state to a proliferative state. In vascular smooth muscle, this ability to switch phenotype is vital in vasculogenesis and initiating repair in response to vascular injury (19, 26–29). However, the uncontrolled proliferation of vascular SMCs resulting from altered transcriptional control is the basis for major vascular diseases, including atherosclerosis, hypertension, restenosis following angioplasty, and angiogenesis in tumor growth.
Ca2+ signals are known to provide critical control over smooth muscle phenotypic switching (26, 28, 30). The Ca2+/calcineurin-dependent activation of NFAT appears to be a major transcriptional event mediating growth factor-induced transition of smooth muscle phenotype (28, 30–33). Earlier studies had suggested VOCC or receptor-activated TRPC channels might mediate Ca2+ signals controlling vascular smooth muscle cell (VSMC) phenotype change (28–30, 34, 35). However, while Ca2+ entry through VOCCs predominates in contractile VSMCs, the phenotypic transition to the proliferative state is accompanied by an almost complete loss of VOCCs (28, 30–33). Clearly, the underlying trigger for the altered transcriptional control in VSMCs is sustained elevated cytosolic Ca2+, and a growing body of evidence now supports a connection between Orai-mediated Ca2+ entry, NFAT activation, and control of gene transcription in SMCs. Thus, either STIM1 or Orai knockdown induced by short interfering RNA (siRNA) or short hairpin RNA (shRNA) prevented both proliferation and migration of VSMCs (20–23). Moreover, the levels of both STIM1 and Orai1 were shown to be up-regulated in SMCs from media and neointima of injured carotid artery (24, 25). In addition, adenoviral-delivered siRNA or shRNA against STIM1 or Orai1 suppresses neointimal VSMC proliferation following injury (21, 22, 25). Such investigations on the role of STIM1 in smooth muscle have relied on in vitro or in vivo knockdown approaches and/or pharmacological modification of SOCE. Knockdown approaches are only partially effective, and viral RNAi application can suffer from cellular targeting difficulties. Moreover, there are no reliable or specific pharmacological modifiers of STIM or Orai proteins.
Our approach was to examine the function of STIM1 and STIM2 proteins within knockout (KO) mice. Since mouse STIM1 gene KO results in perinatal lethality, and STIM2-KO mice die shortly after birth (36, 37), we used Cre-loxP technology to conditionally target KO of STIM1, STIM2, or STIM1/STIM2 using respective loxP-flanked mouse lines. Floxed mice were crossed with the SM22α-CreKI+ mouse carrying the Cre-recombinase transgene under control of the endogenous SM22α smooth muscle-specific promoter. This mouse model provided the means to systematically evaluate the role of STIM proteins and STIM-dependent Ca2+ signals in the function, growth, and development of SMC, both in vitro and in vivo. We reveal that elimination of STIM protein expression results in major changes in Ca2+ signal generation, altered agonist-induced smooth muscle contractile responses, abnormal development of different smooth muscle tissues, and prevention of growth factor-induced transcriptional control, proliferation, and in vivo phenotype switching in response to injury. These studies, together with the observed perinatal lethality of the smooth muscle-targeted STIM1/STIM2 double KO, reveal the crucial role of STIM proteins in the development, function, growth, and pathophysiology of smooth muscle.
MATERIALS AND METHODS
Generation of smooth muscle-targeted STIM-KO mice
STIM1loxP/loxP, STIM2loxP/loxP, and STIM1loxP/loxP-STIM2loxP/loxP (36) mice were crossed with Cre-recombinase knock-in SM22α transgenic mice (SM22α-CreKI+; 006878; Jackson Laboratory, Bar Harbor, ME, USA; ref. 38) to generate the smooth muscle-specific STIM-KO mice used in this study. Tissue specificity was assessed by crossing SM22α-CreKI+ mice with a double-fluorescent reporter mouse, Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (007576; Jackson Laboratory; ref. 39). Mice were genotyped by polymerase chain reaction (PCR) of DNA extracted from tail clippings. The following primers were used: Stim1 wild type (WT), 5′-CGATGGTCTCACGGTCT-CTAGTTTC; Stim1 KO, 5′-AACGTCTTGCAGTTGCTGTAGGC; and Stim1 antisense (AS), 5′-GGCTCTGCTGACCTGGAACTATAGTG. For STIM2 genotyping, the following primers were used: Stim2 WT, 5′-CATCAGAAGGTAAAACTGTGCAGTGCTC; Stim2 KO, 5′-GCTGAACTGTGTGCTTGACTGTAGC; and Stim2 AS, 5′-GGATGTCCTGGACTCACTCTGTAGACCA. Mice were also genotyped for Cre-recombinase. sm-STIM-WT Cre+/− mice were used as controls. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Temple University. Genotypes were all maintained in the C57BL/6 background for ≥10 generations.
Aortic ring contraction
Vascular reactivity was assessed as described previously (40). Briefly, same-sex 6- to 12-wk-old sm-STIM-WT and sm-STIM1-KO mice were euthanized by cervical dislocation. Thoracic aorta was quickly removed and placed in ice-cold phosphate-buffered saline (PBS). The aorta was cleaned from fat and connective tissue and subsequently cut into four 2.5-mm rings. Rings were denuded of the endothelium and placed in an organ bath filled with Krebs-Henseleit (KH) buffer (mM: 118 NaCl, 4.75 KCl, 1.19 KH2PO4, 1.19 Mg2SO4, 2.54 CaCl2, 12.5 NaHCO3, and 10.0 glucose) and oxygenated with 95% O2 and 5% CO2 at 37°C. Stainless steel wires (40 μm diameter) were threaded through rings and mounted on isometric force transducers connected to a graphic transducer (Radnoti Wire Myograph System; Radnoti LLC, Monrovia, CA, USA). The rings were stretched to a tension of 0.5 g. To test ring viability and quality, KCl (90 mM) was added to the bath. After contractions reached a plateau, rings were washed with KH buffer. KCl treatment was repeated twice. To empty intracellular Ca2+ stores, rings were treated with phenylephrine (PE; 1 μM) and thapsigargin (TG; 10 μM). Verapamil or nifedipine (1-10 μM) was added to inhibit L-type channels. Some aortic rings were cut open, and the lumen was exposed for Ca2+ imaging. Dye loading was performed in KH buffer also containing fluo-4 (20 μM) or Fura-2 (10 μM) at room temperature for 1 h. To improve loading, pluronic F-127 (2% v/v) was also added to the buffer. Each tissue strip was observed >1 h after dye loading. Strips were placed in a perfusion chamber, and the chamber was mounted on an upright confocal microscope (ZeissLSM 710; Carl Zeiss, Oberkochen, Germany); the vessel strip was attached at each end, with the intimal surface facing the microscope objective. The preparation was then equilibrated for ≥30 min before observation. The tissue was continuously perfused with KH solution at a constant rate (1.5 ml/min) at 37°C. Images were acquired every 10 s under a water-immersion objective (×20). Experiments were repeated with Fura-2, measuring the 340/380-nm excitation ratio, and a frame-by-frame analysis to eliminate movement artifacts.
Aortic ligation, morphometric analyses, and nuclear counting
Neointima formation was induced using the shear-stress carotid artery ligation mouse model as previously described (41). STIM1-KO or sm-STIM-WT mice were developed in the membrane-targeted tdTomato (mT)/enhanced green fluorescent protein (EGFP; mG). These mouse models were used to identify Cre-recombinant SMCs. All mice are from a C57 background, which has been shown to develop robust intimal hyperplasia during artery injury (42). Mice were anesthetized, and the left common carotid artery was dissected and ligated near the bifurcation. Right unligated carotid arteries served as controls. After 28 d, mice were euthanized, and tissue was prepared for hematoxylin-and-eosin (H&E) staining and morphological analysis. Digitized images were measured and averaged from ≥5 representative 5-μm-thick stained tissue sections ≥75–100 μm apart, spanning 200–800 μm per carotid artery, and images were analyzed using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA). The circumference of the lumen, the area encircled by the internal elastic lamina (IEL), and the external elastic lamina (EEL) were quantitated. The medial area was calculated by subtracting the area defined by the IEL from the area defined by the EEL, and intimal area was calculated as the difference between the area inside the IEL and the luminal area. Tissue fixation, processing, and staining were performed as described previously (43). Unligated mice, 4/group, underwent whole-body tissue fixation through cardiac puncture, and organs were harvested for morphological evaluation. Nuclear density was assessed by counting nuclei in aortic sections fixed in paraformaldehyde (4% w/v) and stained with H&E. The nuclear counts were quantitated on two aortic segments of equivalent length (0.25 mm) per serial section (≥6 sections/slide). Nuclear density was determined using ImageJ analysis software and expressed as n/μm2 × 10−4, where n = number of nuclei. Data from each animal in each group were averaged to serve as a single value for statistical analysis (44).
SMC culture, proliferation, and migration assays
Primary SMCs were isolated from sm-STIM1-KO or sm-STIM-WT mice as described previously (45). Briefly, aortic SMCs were freshly isolated using collagenase digestion (Worthington type 2, 1 mg/ml; Worthington Biochemical Corp., Lakewood, NJ, USA) and elastase (Worthington, 0.2 mg/ml). Dispersed SMCs were grown in Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS) and penicillin/streptomycin, passaged by trypsinization, and used for experiments until passage 3. For primary SMC proliferation analysis, cells were seeded in a 96-well plate (3×103 cells/well) and incubated for 24 h in DMEM/F12 with 0.1% FBS. Thereafter, FBS was removed, and cells were incubated with either FBS (5%) or medium containing platelet-derived growth factor (PDGF; 10-100 nM) for 48 h at 37°C. SMC proliferation was assessed with CellTiter 96-Aqueous One Solution Reagent (Promega, Madison, WI, USA). Absorbance was measured at 490 nm using a Victor X5 multilabel plate reader (Perkin Elmer, Waltham, MA, USA). Background values were subtracted from each well before determination of cell growth inhibition. Proliferation was also assessed by cell counting using a standard hemocytometer. Cell migration was analyzed in transwell culture chambers with SMCs cultured on the upper surface of polycarbonate membranes (41). Experiments were performed in culture medium containing serum or PDGF placed in the lower chamber for 8 h. Cells that migrated to the lower face of the membrane were quantitated by cresyl violet staining (41). Proliferation and migration experiments were performed in triplicate in 3 independent experiments.
Intracellular Ca2+ measurements
Intracellular Ca2+ measurements in primary SMCs were done as described earlier (40, 46). SMCs were cultured on 25-mm glass coverslips, and at the day of the experiment, cells were incubated with Fura-2 AM for 45 min at room temperature. Cells were washed and incubated for ≥30 min prior to use in experiments, which were conducted at room temperature. The standard extracellular recording solution contained (mM): 130 NaCl, 5 KCl, 0.2 KH2PO4, 1.2 Mg2SO4, 2 CaCl2, 10 HEPES, and 10 d-glucose (pH 7.4). The change in intracellular Ca2+ level was determined from the ratio of Fura-2 emission intensities at 340- and 380-nm excitation, captured using a Leica DMI 6000B fluorescence microscope (Leica Microsystems, Wetzlar, Germany) controlled by Slidebook (Intelligent Imaging Innovations, Denver, CO, USA).
Western blot analysis
Cells were lysed using Nonidet P-40 lysis buffer (1% v/v Nonidet P-40, 150 mM NaCl, and 50 mM Tris-HCl, pH 8.0) containing protease inhibitors (Sigma, St. Louis, MO, USA) and 200 mM phenylmethylsulfonyl fluoride (PMSF). Cells in Nonidet P-40 lysis buffer were homogenized on ice for 30 min and centrifuged to isolate the lysate. Protein concentration was quantified using the DC protein assay (Bio-Rad, Richmond, CA, USA). Protein lysates were resolved on 8% SDS-PAGE gel and transferred to PVDF membranes. The membrane was blocked for 1 h at room temperature with membrane blocking agent (5%; GE Healthcare Life Sciences, New York, NY, USA) and incubated with primary antibodies overnight at 4°C. Membranes were washed twice in Tris-buffered saline-Tween 20 (TBST; 10 mM Tris-Cl, pH 8.0; 150 mM NaCl; and 0.1% Tween 20) and incubated with secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. The peroxidase activity was visualized using ECL (Amersham Biosciences, Piscataway, NJ, USA) with the FluorChem HD2 imaging system from Alpha Innotech (San Leandro, CA, USA). Primary antibodies used were against tubulin (AB15246; Abcam, Cambridge, MA, USA), smooth muscle actin (AB5694; Abcam), calponin (EP798Y; AB46794; Abcam;), STIM1 purified from a polyclonal anti-STIM1 serum, and Cav1.2α1C (AB10515; Millipore, Billerica, MA, USA). Details were as described previously (46, 47).
RNA extraction and real-time PCR
RNA was extracted using the Qiagen RNeasy mini kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. RNA was eluted using 30-50 μl of RNase-free water. RNA was measured using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Barrington, IL, USA). Real-time quantitative reverse transcription–PCR (qRT-PCR) was performed in a 2-step process using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) to synthesize cDNA. The cDNA obtained from reverse transcription was used for performing the qRT-PCR with the TaqMan gene expression system (Applied Biosystems, Foster City, CA, USA) on a 7300 real-time PCR machine (Applied Biosystems, Foster City, CA, USA). qRT-PCR was performed on 15 ng of cDNA at 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. TATA binding protein (TBP) was used to normalize target gene expression. Data were analyzed using the ΔΔCt method. Applied Biosystems primers and probes were as follows: TBP, Mm00446971_m1; smoothelin (SMTN), Mm00449973_m1; versican (VCAN), Mm01283063_m1; collagen type 1 α-1 (COL1A1) chain, Mm00801666_g1; collagen type 3 α-1 (COL3A1) chain, Mm01254476_m1; myosin heavy chain 11 (MYH11), Mm00443013_m1; myosin light-chain kinase (MLCK), Mm00653039; and L-type channel α1C subunit (Cacna1c), Mm01188822_m1.
NFAT nuclear translocation and luciferase reporter activity
Cultured SMCs from adult sm-STIM1-KO and sm-STIM1-WT mice were maintained on 25-mm glass coverslips and exposed to NFATc3-GFP adenovirus for 4 h (Ad-NFAT-GFP), then maintained in FBS-free medium (48). At 48 h postinfection, medium was changed with medium without FBS or containing PDGF (20 ng/ml). Cells were incubated for an additional 6 h before images were taken with an epifluorescence microscope. Nuclei with NFATc3-GFP fluorescence that was 2-fold above cytoplasmic NFAT-GFP fluorescence were deemed to be NFAT positive. For short-term imaging, 48–72 h after infection, SMCs were loaded with Fura-2, and after incubation, coverslips were mounted in the microscope chamber. Cells were continuously perfused with Ringer solution, and PDGF was applied in the perfusate. Fura-2 and NFATc3-GFP were monitored simultaneously, and images were collected every 10 s using the Leica DMI 6000B fluorescence microscope controlled by Slidebook software.
Dual-luciferase assay
SMCs were infected with the NFAT-luciferase and Renilla-luciferase adenoviruses (Vector Biolabs, Burlingame, CA, USA). To determine NFAT-driven transcriptional activity 48 h postinfection, cells were treated with or without PDGF for 6 h. Cell lysates were prepared, and luciferase activity was measured with a Victor X5 multilabel plate reader using the dual-luciferase reporter system (Promega), according to the manufacturer's instructions. The firefly luminescence signals were normalized to the corresponding Renilla luciferase signals acting as an internal control.
Immunofluorescence
To induce and detect NFAT accumulation in smooth muscle nuclei in tissue strips, we employed a protocol previously described (32), with some modification. Ileal sheets (2–4 mm wide, 5–8 mm long) were isolated from mouse intestine and incubated in DMEM at 37°C without FBS for 40 min. Subsequently, tissue strips were treated at room temperature with PDGF (20 ng/ml) or TG (10 μM), for 1 h. After air-drying for 10 min, sheets were fixed with 3.7% formaldehyde in PBS (pH 7.4) for 25 min, permeabilized with 0.25% Triton X-100 in PBS for 30 min, and blocked for 2 h with 5% BSA in PBS. Primary antibody, rabbit anti-NFAT4/c3 (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), was diluted in 5% BSA/PBS, (1:200 dilution) and applied overnight at 4°C. Secondary antibody, Alexa Fluor 647 conjugated-anti-rabbit IgG (1:200 dilution; Life Technologies, Carlsbad, CA, USA) was applied for 2 h at room temperature. Nuclear dye DAPI (1:10,000 dilution) was used for identification of nuclei, and smooth muscle actin was stained with Alexa Fluor 568 phalloidin-conjugate. After washing, ileal sheets were mounted and examined at ×40 using the confocal microscope. For scoring of NFAT4/c3-positive nuclei, multiple fields for each smooth muscle strip were imaged and counted under blinded conditions. For quantification, a cell was considered positive if NFAT (green) was observed in the nucleus (blue) and colocalized (cyan), whereas a cell was considered negative if no colocalization was visualized.
Data and statistical analysis
Results are expressed as means ± se. Differences between groups were evaluated using ANOVA to evaluate differences between individual mean values or by t tests, where appropriate. Differences were considered significant at a level of P < 0.05.
RESULTS
Generation of smooth muscle-targeted STIM-KO mice
The STIM1 KO on the C57BL/6 background is perinatally lethal, and the STIM2-KO mice die 1–4 wk after birth from unknown causes (36, 37). To circumvent the early lethality of the whole-animal KOs and examine the role of STIM proteins in smooth muscle, we used Cre-recombinant technology to generate mice in which STIM1 and/or STIM2 were selectively knocked out in smooth muscle. To achieve this, we crossed STIM1loxP/loxP, STIM2loxP/loxP, or STIM1/2loxP/loxP mice with the well-characterized transgenic knock-in mouse line, SM22α-CreKI+, expressing Cre-recombinase under the smooth muscle-specific SM22α promoter (38). The resulting Cre-heterozygous mice were backcrossed with STIM1loxP/loxP, STIM2loxP/loxP, or STIM1/2loxP/loxP to generate SM22α-CreKI+/−/STIM1loxP/loxP (sm-STIM1-KO), SM22α-CreKI+/−/STIM2loxP/loxP (sm-STIM2-KO), or SM22α-CreKI+/−/STIM1/2loxP/loxP (sm-STIM1/2-DKO), respectively. The SM22α-CreKI+/−/STIMWT (sm-STIM-WT) mice were used as controls.
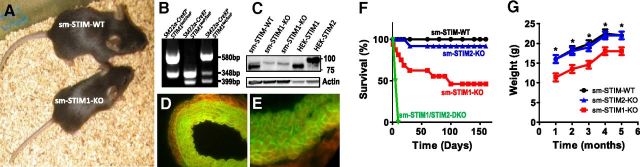
While viable, sm-STIM1-KO mice were visibly distinguishable in size from sm-STIM-WT littermates (Fig. 1A, G). PCR genotyping revealed successful gene recombination resulting in STIM1 deletion (Fig. 1B). Using a STIM1/STIM2 cross-reacting antibody, Western blot analysis of aortas from sm-STIM1-KO and sm-STIM-WT mice revealed a complete absence of STIM1 protein in sm-STIM1-KO mice (Fig. 1C). Notably, STIM2 expression was not visibly altered as a consequence of STIM1 gene deletion. We determined that Cre-recombinase expression closely patterned endogenous SM22α expression by crossing SM22α-CreKI+ mice with a global double-fluorescent (mT/mG) reporter mouse (39). This reporter ubiquitously expresses the floxed mT cassette, conferring red fluorescence on all tissues and cells. SM22α-Cre-mediated excision of the mT cassette activates the mG positioned downstream of mT, allowing distinction between recombinant and nonrecombinant cells. SM22α-CreKI+ Cre-recombinase activity was monitored in several smooth muscle-containing organs from adult mice, including aorta and esophagus (Fig. 1D, E), as well as vascular and ileal smooth muscle during late embryonic development (Supplemental Fig. S1).
Figure 1.
Specificity and phenotype of smooth muscle-targeted STIM1-KO mice. A) Appearance of 6-wk-old sm-STIM-WT and sm-STIM1-KO mouse littermates. B) Southern blot showing PCR genotyping results revealing the WT STIM1 allele (348 bp), recombined allele (580 bp), and floxed allele (399 bp). C) Immunoblot of aortic SMC lysates from sm-STIM-WT and sm-STIM1-KO mice. The STIM1 band is absent in the STIM1-KO tissue, while the STIM 2 band is still visible and unchanged. HEK293 cells overexpressing STIM1 or STIM2 were used as additional positive controls (25 μg total protein/sample). D, E) SMC-specific Cre-mediated EGFP fluorescence expressed in the SM22α-Cre+/−-mT/mG reporter mouse. Cross-sectional images of mouse aorta (D) and esophagus wall (E). Tissue harvested from adult mice was fixed in formalin, paraffin embedded, sectioned, and imaged. EGFP (mG) is visible at 488 nm (green) specifically on SMC membranes, indicating Cre expression, while adventitia expressed mT (red fluorescent protein) visible at 558 nm (red). F) Kaplan-Maier survival curves for WT and STIM-KO mice: sm-STIM-WT, n = 18, black; sm-STIM1-KO, n = 15, red; sm-STIM2-KO, n = 10, blue; sm-STIM1/2-KO, n = 10, green. G) Body weight change with time in sm-STIM1-WT, sm-STIM1-KO, and sm-STIM2-KO mice. Values are expressed as means ± se. *P < 0.005.
Kaplan-Meier survival analysis revealed that sm-STIM1-KO mice have high mortality, ∼40% of sm-STIM1-KO dying during the first month of life (Fig. 1F). Thereafter, the mortality rate decreased such that almost 50% of sm-STIM1-KO mice survived past 150 d. While surviving sm-STIM1-KO mice were fertile during the first 6 mo, few of these mice survived longer than 8 mo. There was no obvious relationship between gender and survival rate. In contrast, the sm-STIM2-KO mice showed no significant change in mortality rate (Fig. 1F). Interestingly, the most severe phenotype was observed in the sm-STIM1/2-DKO mice, which died within a few days after birth, indicating an important, perhaps compensatory, role for STIM2 in the survival of mice in which the function of STIM1 is eliminated.
The size difference between sm-STIM1-KO and sm-STIM-WT mice was clearly discernible over their lifetime. Weight of animals kept on chow diet ad libitum was monitored monthly over an extensive time period. Although the weights of sm-STIM2-KO and sm-STIM-WT were indistinguishable, both male and female sm-STIM1-KO mice were significantly lighter than their sm-STIM-WT littermates (at 2 mo of age sm-STIM-WT weighed 17.2±1.5 g vs. 13.8±1.2 g for sm-STIM1-KO mice; Fig. 1G). Since sm-STIM1/2-DKO mice had a lethal phenotype, and STIM2 KO mice did not exhibit any obvious phenotype, our analyses focused on sm-STIM1-KO mice.
STIM1-deletion leads to smooth muscle aberrations and altered gene expression
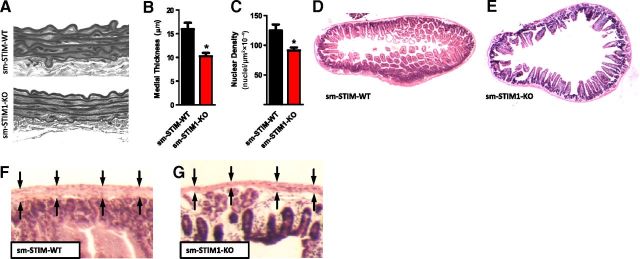
Morphometric analysis of thoracic aorta from sm-STIM1-KO mice revealed a thinner smooth muscle medial layer associated with a mild degradation of the elastic lamina when compared to the aorta from sm-STIM-WT mice (15.92±2.36 vs. 10.24±1.65 μM, n=4, P<0.05; Fig. 2A, B). In addition, aortas from sm-STIM1-KO mice showed a reduced medial nuclear density compared with sm-STIM-WT (124.8±10.2 vs. 91.4±12.14 nuclei/μm2×10−4, n=4 animals/group, P<0.001; Fig. 2A, C), suggesting that the number of SMCs was reduced by almost 30% in STIM1-KO smooth muscle tissue. We examined whether the thinning of the smooth muscle wall observed in the sm-STIM1-KO aorta was also apparent in the smooth muscle of other organs. Macroscopic analysis of the mouse intestine revealed a larger lumen in the sm-STIM1-KO compared to the sm-STIM-WT mice(Fig. 2D, E). This was associated with changes in the height and appearance of intestinal villi, with indications of villus degeneration (Fig. 2D, E). Consistent with our observations on aortic media, high-magnification analysis of the intestine wall showed that smooth muscle layers of the muscularis propria along the length of the intestinal tract were also thinner in sm-STIM1-KO as compared to sm-STIM-WT mice (Fig. 2F, G). The stomach was also highly distended and dilated in sm-STIM1-KO mice (Supplemental Fig. S2). Together, these results suggested that the stretching and thinning of smooth muscle layers was a general consequence of STIM1 loss in smooth muscle.
Figure 2.
Morphological changes in smooth muscle layers in sm-STIM1-KO mice. A) Cross-sectional images of aortic wall from sm-STIM-WT and sm-STIM1-KO mice. Tissue was H&E stained, and images were taken at ×40. B) Measurements of medial aorta thickness in sm-STIM-WT and sm-STIM1-KO mice (means±se). *P < 0.05. C) Aortic medial nuclear density in the same samples (mean±se nuclei/μm2×10−4). *P < 0.05. D, E) Cross-sectional images of H&E-stained ileum: sm-STIM-WT mouse showing normal size and appearance of lumen and normal villi morphology (D) and sm-STIM1-KO mouse showing distended lumen and irregular development of villi (E). F, G) Higher magnification from panels D and E, showing ileal smooth muscle layer (arrows) in both sm-STIM-WT (F) and sm-STIM1-KO (G).
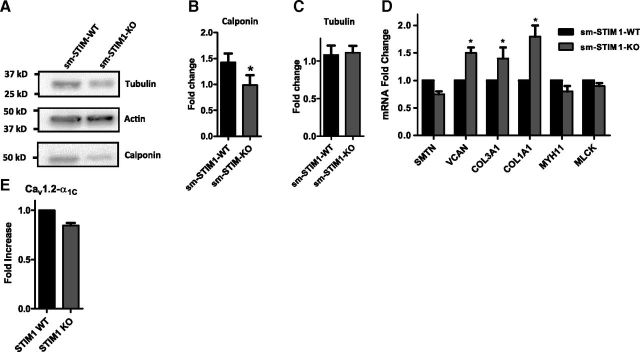
While SMCs are not terminally differentiated, several smooth muscle-specific expression products serve as useful markers of the relative state of differentiation and maturation of SMCs. Hence, we investigated whether the morphological and structural abnormalities were associated with altered gene or protein expression. Western blot analysis of protein extracts from sm-STIM1-KO aortic tissue revealed no difference in actin expression compared with WT tissue (Fig. 3A). Tubulin expression (Fig. 3A, C) was not significantly changed in KO tissue. However, expression of calponin-1 was significantly reduced (1.42±0.21 sm-STIM-WT vs. 0.98±0.25 sm-STIM1-KO, n=4, P<0.05; Fig. 3A, B). Calponin is a calcium-binding regulator of actin-myosin interactions involved in cytoskeletal assembly and control of smooth muscle contraction (49), and its decreased expression is consistent with the altered smooth muscle morphology observed. We also analyzed the message for proteins reflecting the contractile state of SMCs, including MYH11, SMTN, and MLCK, revealing in each case that expression of these genes was modestly down-regulated. Gene expression products that are associated with SMC stress responses, inflammation, and contractile myofilament disarray, such as VCAN, COL1A1, and COL3A1, were all up-regulated in STIM-KO cells (Fig. 3D). These expression data are consistent with the altered maturation and morphological abnormalities observed at the tissue level. Whether such altered expression results from direct STIM1-mediated control or is an indirect compensatory response to STIM1 gene deletion is yet to be determined. Lastly, on the basis of recent evidence that STIM1 can control the function and expression of CaV1.2 channels (46, 50), we examined the expression of the active CaV1.2 α1C pore subunit in aortic tissue from sm-STIM-WT and sm-STIM1-KO mice. While Western blot analysis revealed no difference in protein levels, a small decrease in α1C message level was observed, but this was not statistically significant (Fig. 3E).
Figure 3.
Smooth muscle-targeted STIM1 deletion reduces SMC-specific marker expression. A) Immunoblot for tubulin, actin, and calponin in protein extracts from thoracic aorta from sm-STIM-WT and sm-STIM1-KO mice. B, C) Statistical summary of calponin (B) and tubulin (C) protein expression; data were normalized to actin (n=4). *P < 0.05. D, E) Quantitative analysis of mRNA levels by real-time qRT-PCR. Total RNA was extracted from aorta of sm-STIM-WT and sm-STIM1-KO mice. mRNA expression of L-type calcium channel (Cav1.2, α subunit), SMTN, VCAN, COL3A1, COL1A1, MYH11, and MLCK was evaluated (thoracic aorta n=4). Values are expressed as fold change relative to WT mouse (means±se). *P < 0.05.
Deletion of STIM1 induces changes in vascular reactivity
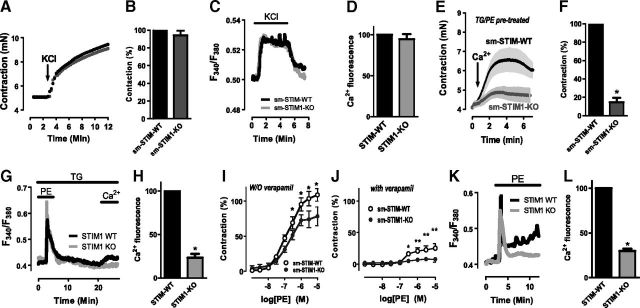
The role of SOCE in smooth muscle contraction and vasoconstriction has remained a long-standing question (18, 40, 46, 51). In addition to controlling store-operated channels, STIM proteins can mediate control over CaV1.2 channels (46, 50), one of the major voltage-operated Ca2+ entry (VOCE) channels in smooth muscle. The sm-STIM1-KO mouse system provides an important means to assess how STIM-mediated control of Ca2+ entry channels contributes to the contractile responses of smooth muscle. To investigate whether STIM1 modulates voltage-operated vascular contractility, we measured aortic ring contraction in response to depolarization using wire myography (Fig. 4). We observed that the maximal force of depolarization-induced contraction was unchanged in sm-STIM1-KO aortic rings (4.26±0.03 mN), as compared to those from sm-STIM-WT (4.30±0.04 mN, n=20 rings/group, P>0.05; Fig. 4A, B). In further studies, we investigated what effects STIM1 deletion might have on voltage-operated Ca2+ influx measured directly in the tissue using either fluo-4- or Fura-2-loaded aortic tissue strips taken from sm-STIM1-KO and sm-STIM-WT mice. We observed similar Ca2+ entry in response to KCl-induced membrane depolarization (Fig. 4C, D and Supplemental Fig. S3), suggesting that VOCE is not affected by elimination of STIM1.
Figure 4.
Contractile and Ca2+ responses measured directly in aortic tissue from sm-STIM-KO and sm-STIM-WT mice. A, B) Representative myograph recordings showing depolarization-induced contraction (90 mM KCl) of thoracic aortic rings from sm-STIM-WT and sm-STIM1-KO mice. C, D) Intracellular Ca2+ responses to depolarization (90 mM KCl) measured in intact aortic tissue loaded with Fura-2. E, F) Myograph recordings showing contraction of aortic rings from either sm-STIM-WT or sm-STIM1-KO mice, pretreated for 5 min with 10 μM PE and 10 μM TG in Ca2+-free extracellular medium to empty stores, followed by a further 15 min with TG alone without PE to allow dissipation of PE-induced second messengers, such as diacylglycerol (this time course is shown in panel G). Ca2+ entry-dependent contractile responses were measured on addition of 2 mM external Ca2+ (arrow) in the continued presence of TG. In sm-STIM1-KO aorta, the Ca2+ response is reduced >80%. G, H) Intracellular Ca2+ responses measured in Fura-2-loaded intact aortic tissue from sm-STIM-WT and sm-STIM1-KO mice. Additions of TG and PE were the same as for E. I) Aortic ring contractile responses to increasing PE levels; sm-STIM1-KO reveals decreased response to higher PE concentrations. J) Contractile responses measured as in I but in presence of 10 μM verapamil. While sm-STIM-WT tissue retains response to higher PE levels, contraction is abolished in sm-STIM1-KO tissue. For I and J, data are expressed as a percentage of the maximal KCl-induced contraction. K, L) Intracellular Ca2+ response to 10 μM PE, measured in Fura-2-loaded intact aortic tissue. All statistical analyses are expressed as means ± se; n ≥ 4. *P < 0.05.
To examine the role of STIM1-mediated SOCE in the contractile responses of intact smooth muscle tissue, we used mouse aortic rings in which we emptied stores with a combination of the SERCA-pump blocker TG and 10 μM PE. In smooth muscle tissue, the leak of Ca2+ from SR induced by TG alone is quite slow, and the coaddition of PE allowed faster and more complete release of stored Ca2+ (40, 52). After such Ca2+ store depletion in the absence of external Ca2+, the addition of Ca2+ in the extracellular medium resulted in a small sustained contraction (Fig. 4E). Compared to responses in the aortic rings from sm-STIM-WT mice, the contraction in the rings from sm-STIM1-KO mice was greatly reduced by 85.2% (Fig. 4E, F). To examine whether the contractile response was mediated by STIM1-dependent Ca2+ entry, we measured the actual changes in cellular Ca2+ within the intact tissue using the same store-emptying protocol (Fig. 4G, H). Thus, the combined action of a pulse of PE and sustained presence of TG ensured Ca2+ store depletion, and the readdition of external Ca2+ could be seen to mediate an increase in Ca2+ in tissue from sm-STIM-WT mice, which was reduced by ∼80% in tissue from sm-STIM1-KO mice. These results provide perhaps the first direct evidence that STIM1 is capable of mediating SOCE-dependent contractile responses in intact, differentiated, smooth muscle tissue.
Whereas SOCE can be observed to mediate a contractile response, complete emptying of Ca2+ stores following pump blockade is not a physiologically relevant condition. Hence, we wished to examine the role of STIM1 in response to agonist-induced contraction. Activation of α1-adrenergic receptors induces phospholipase C-mediated inositol 1,4,5-trisphosphate (InsP3) production and intracellular Ca2+ release. However, the contraction mediated in response to store release involves activation of VOCE channels, possibly through induction of TRPC and/or potassium channel-mediated membrane depolarization (53, 54). Until now, it has not been possible to determine the absolute role of STIM1 in the contractile process in smooth muscle tissue. We examined PE-induced contraction in aortic rings from sm-STIM1-KO mice and revealed a small but highly reproducible component dependent on the presence of STIM1 (Fig. 4I, J). This dependence was more obvious toward the higher PE concentrations, that is, above 30 nM. In response to 100 nM PE, the contractile response in tissue from sm-STIM-WT mice (47.4±4.6) was reduced by ∼38% in sm-STIM1-KO tissue (29.6±4.8); the response to 1 μM PE was reduced by ∼26% in sm-STIM1-KO tissue (Fig. 4I; contractile values are percentage of maximal contraction with KCl). When we undertook the same analysis in the presence of verapamil (10 μM; Fig. 4J), while the contractile responses in sm-STIM1-KO rings were almost completely eliminated, the responses in sm-STIM-WT rings were easily measurable at the higher PE concentrations. Thus, at 1 μM PE, the contractile responses of sm-STIM1-WT and sm-STIM1-KO tissues were 25.4 ± 2.3 and 7.6 ± 2.8, respectively. Similar differential contractile responses were measured in the presence of nifedipine (1 μM). These results reflect a clear STIM1-mediated response to PE. The question of whether this PE contractile response is mediated by SOCE was addressed as above, measuring Ca2+ levels in SMCs in the intact tissue (Fig. 4K, L). PE addition caused a rapid peak due to Ca2+ release, followed by a sustained Ca2+ entry response that was largely eliminated in the sm-STIM1-KO tissue. Overall, the results reveal that STIM-mediated Ca2+ entry can be a significant component of the agonist-induced contraction of vascular smooth muscle tissue.
STIM1 KO has a powerful negative effect on SMC proliferation in vivo and in vitro
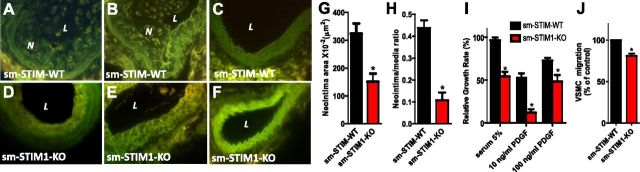
The abnormal appearance of vascular and intestinal smooth muscle tissue in sm-STIM1-KO mice could reflect decreased proliferation and/or possible enhanced apoptosis. Therefore, we examined how STIM1 KO in smooth muscle affected the proliferative responses of SMCs measured in vivo. We measured neointimal growth changes in response to partial carotid artery occlusion, which is widely used to induce SMC proliferation (41, 55). Left carotid arteries were partially occluded near the carotid bifurcation in sm-STIM1-KO and sm-STIM-WT mice. After 28 d of ligation, morphological analysis of the ligated left carotid artery revealed robust neointimal formation in the sm-STIM-WT mice (323.8±22.3 μm2, n=5 mice; Fig. 5A, B, G). In contrast, identical ligation of left carotid arteries in sm-STIM1-KO mice resulted in a highly significant reduction in neointimal formation (150.8±15.4 μm2, n=5 mice; P>0.05; Fig. 5D, E, G). Neointimal/medial ratios were also substantially reduced in the sm-STIM1-KO mice (Fig. 5H). Unligated sham-operated right carotid arteries served as controls (Fig. 5C, F). In addition to the in vivo studies, we examined proliferation and migration of isolated aortic SMCs obtained from sm-STIM1-KO and sm-STIM-WT mice under in vitro culture conditions. Cell proliferation was assessed both colorimetrically and by standard cell counting. Aortic SMCs isolated from adult sm-STIM1-KO mice showed a marked decrease in the proliferative response to 5% serum (Fig. 5I), with a growth rate reduced by 47.5 ± 6.6% (n=4, P<0.05) as compared with the rate of cells from sm-STIM-WT mice. Even more profound was the decrease in proliferative response to 10 ng/ml PDGF, which was reduced by 79.7 ± 10.2% in sm-STIM-KO mouse-derived cells compared to those from sm-STIM1-WT animals (n=4; P<0.001; Fig. 5I). The growth rate in response to a higher PDGF concentrations (100 ng/ml) was reduced somewhat less (33.3±15.0%; n=4, P<0.05; Fig. 5I), perhaps reflecting other pathways for proliferation being turned on by higher PDGF levels. PDGF also induces the migration of aortic SMCs in cultures. We observed that the rate of migration in response to 20 ng/ml PDGF was also reduced in the sm-STIM1-KO cells, but not as profoundly as cell proliferation (19.3±6.2%; n=4; P<0.05; Fig. 5J). Overall, the results reveal the importance of STIM1 in mediating the proliferation and migration responses of VSMCs.
Figure 5.
Smooth muscle-targeted STIM1 deletion suppresses in vivo and in vitro proliferative responses of arterial SMCs. A–F) Representative images of proliferative responses of SMCs in carotid arteries following 28-d ligation injury using sm-STIM1-KO-mT/mG (A–C) or sm-STIM-WT-mT/mG (D–F) reporter mice, showing Cre-specific expression of membrane-bound EGFP in SMCs (×40 objective). A, B) Responses in sm-STIM-WT-mT/mG mice, revealing substantial neointimal formation. D, E) Responses in sm-STIM1-KO-mT/mG mice, revealing small neointimal formation. C, F) Representative nonligated right carotid artery from sm-STIM-WT-mT/mG (C) or sm-STIM-KO mice (F). G, H) Morphological analysis demonstrates a substantial decrease in neointimal area (G) and neointimal/medial ratio (H) in sm-STIM1-KO as opposed to sm-STIM-WT mice. I) Proliferation rate of cultured aortic SMCs from either sm-STIM-WT or sm-STIM1-KO mice, revealing substantially reduced growth rates in sm-STIM1-KO cells in response to both serum (5%) and PDGF (10 or 100 ng/ml). Data are expressed as means ±se from 3 independent experiments. J) Migration of cultured aortic SMCs induced by 10 ng/ml PDGF from either sm-STIM-WT or sm-STIM1-KO mice, revealing significantly reduced migration in sm-STIM1-KO cells. Migrating SMCs were quantitated by counting 4 fields/membrane (means±se from 3 independent experiments). N, neointima; L, lumen. *P < 0.05.
STIM1 is required for store-dependent Ca2+ influx and the refilling of intracellular Ca2+ stores in proliferative SMCs
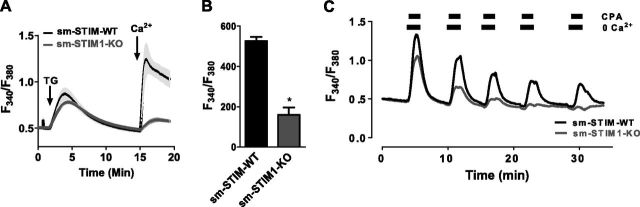
The sm-STIM1-KO mice provided an important system to directly examine how altered Ca2+ signaling and Ca2+ homeostasis are related to the profoundly different growth responses observed in the sm-STIM1-KO cells. Using Fura-2-loaded cultured SMCs derived from sm-STIM1-KO and sm-STIM-WT mice, we initially examined the relative function of stores and the activation of SOCE. As shown in Fig. 6A, application of the irreversible SERCA pump blocker TG to either cell type in the absence of external Ca2+ causes relatively similar release of Ca2+. Although this was not a precise quantitative assessment of store content, it appears that the absence of STIM1 does not preclude stores from filling to similar levels under rest conditions. Indeed, Ca2+ stores can be retained in the complete absence of STIM proteins, as we noted earlier (56). On readdition of external Ca2+, there is prominent SOCE in the sm-STIM-WT cells and very little entry in sm-STIM1-KO cells (Fig. 6A, B). Although knockdown experiments were not conducted, likely this entry is mediated by STIM2. While stores of Ca2+ clearly exist in resting STIM1-KO cells, experiments revealed that the rate of refilling of stores is very different. Thus, as shown in Fig. 6C, cells received pulsed additions of the reversible SERCA pump blocker, cyclopiazonic acid (CPA), in nominally Ca2+-free medium. In between pulses, refilling of stores was permitted in the presence of Ca2+ and absence of CPA. In cells from sm-STIM-WT mice, the stores at least partially refilled during the intervening periods, whereas in cells from sm-STIM1-KO mice, the stores soon became completely empty and were unable to refill (Fig. 6C). This is an interesting and important difference in the STIM1-KO SMCs. In the STIM1 skeletal muscle-specific KO mice, a lack of store refilling gives rise to an important fatigue phenotype (13) in which rapid sequential emptying of stores cannot be sustained. Interestingly, the normal development of skeletal muscle tissue is severely diminished in these mice (15). Thus, in the same way, the diminished development of smooth muscle tissues observed in the smooth muscle-specific STIM1-KO mice may be related to the failure of smooth muscle to be able to maintain longer-term contractile responses.
Figure 6.
STIM1-KO reveals the central role of STIM1 in maintaining Ca2+ store-homeostasis in proliferating SMCs. A, B) Fura-2-loaded aortic SMCs were treated with 2 μM TG (arrow) in Ca2+-free medium, and 2 mM Ca2+ was added later (arrow) to reveal SOCE. smSTIM1-KO cells have greatly reduced SOCE (means ± se; 3 independent measurements, each with >15 cells). *P < 0.01. C) Ca2+ store refilling rates were measured in cultured SMCs from either sm-STIM-WT or sm-STIM1-KO mice. Cells in the presence of 2 mM external Ca2+ were treated repetitively with pulses of cyclopiazonic acid (CPA) to empty stores. At 2 min prior to each CPA pulse, external Ca2+ was removed to avoid Ca2+ entry; Ca2+ was readded simultaneously with CPA removal. In sm-STIM1-KO cells, the refilling rate of stores was substantially reduced.
STIM1 is required for NFAT translocation in response to the PDGF stimulation
Intracellular Ca2+ signals in SMCs play a pivotal role in controlling gene transcription and changes in growth phenotype (27, 28, 30, 57). The change in growth phenotype is crucially regulated by Ca2+ signals, which mediate control over transcription in VSMCs through NFAT activation (28, 30, 31, 33). Sustained increases in Ca2+ activate the calcineurin-mediated translocation of NFAT into nuclei, inducing the proliferative state (28). PDGF is a major activator of VSMC proliferation and phenotype change in response to injury. Earlier studies had suggested that the action of PDGF depended on Ca2+ entry, mediated by the activation of either voltage-operated or receptor-operated channels, perhaps TRPC (28). More recently, Bisaillon et al. (24) revealed the role of Orai channels in mediating the action of PDGF in SMCs. The sm-STIM1-KO mice provide a definitive and stable system in which to determine the role of STIM1 and SOCE in PDGF-induced NFAT activation.
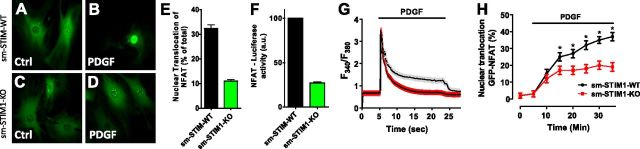
We examined the actions of PDGF on cellular translocation of NFAT in aortic SMCs expressing NFAT-GFP. In SMCs from sm-STIM-WT mice, 6 h treatment with PDGF at 10 ng/ml resulted in robust translocation of NFAT-GFP into nuclei (Fig. 7A, B, E). In contrast, NFAT-GFP nuclear translocation was substantially inhibited in the sm-STIM1-KO cells, such that NFAT appeared in less than one-third of the number of cells (Fig. 7C, D, E). We assessed whether NFAT nuclear translocation was mediating transcriptional control by independently measuring NFAT-induced luciferase activity in response to PDGF in cells from sm-STIM-WT and sm-STIM1-KO mice (Fig. 6F). This revealed exactly the same STIM1 requirement for NFAT-mediated transcriptional activation in the nucleus.
Figure 7.
STIM1 gene deletion prevents nuclear translocation of NFAT-GFP in aortic SMCs. A–D) Representative images depicting aortic SMCs infected with Ad-NFATc3-GFP. Cells were visualized by fluorescence microscopy (488 nm) either before (A, C) or after (B, D) 6 h of stimulation with PDGF (20 ng/ml). E) NFAT-GFP fluorescence was monitored in both nucleus and cytoplasm, and the ratio of nuclear to cytosolic (Fnuc/Fcyt) of each was determined as mean pixel fluorescence. F) Luciferase activity was measured in SMCs adenovirus-infected with NFAT-luciferase and normalized against Renilla luciferase (n=4/group). G, H) Simultaneous measurements of cytoplasmic Ca2+ signals (G) and NFAT-GFP nuclear translocation (H) in Fura-2-loaded aortic SMCs derived from sm-STIM-WT and sm-STIM1-KO mice infected 48 h prior to experiment with NFATc3-GFP. Results are expressed as means ± se; n = 6. *P < 0.05.
We were able to simultaneously measure real-time PDGF-induced Ca2+ signals and translocation of NFAT into the nucleus using Fura-2-loaded SMCs expressing NFAT-GFP (Fig. 7G, H). Application of 10 ng/ml PDGF to SMCs from sm-STIM-WT mice resulted in a rapid spike of intracellular Ca2+, which declined over 10-15 min to become a small sustained increase in intracellular Ca2+ (Fig. 7G). Subsequent removal of PDGF caused the Ca2+ level to rapidly decline in cells, revealing the sustained PDGF-dependent entry of Ca2+ into cells. Using cells from sm-STIM1-KO animals, while the initial spike of Ca2+ was virtually superimposable, the sustained Ca2+ entry phase was almost entirely eliminated (Fig. 7G). In sm-STIM-WT cells, NFAT nuclear translocation could be observed within a few minutes of PDGF addition, and its accumulation continued during the sustained Ca2+ entry phase (Fig. 7H). In the sm-STIM1-KO cells, the accumulation of NFAT-GFP within the nucleus was considerably reduced over the same time period compared with sm-STIM-WT cells (Fig. 7H). The early accumulation was not significantly affected by the absence of STIM1, and likely the initial Ca2+ release peak predominated in the activation of NFAT at these early times. The results reveal that there are both STIM1-dependent and STIM1-independent components of NFAT activation, the latter possibly reflecting release of Ca2+ from stores, or possibly the function of remaining STIM2.
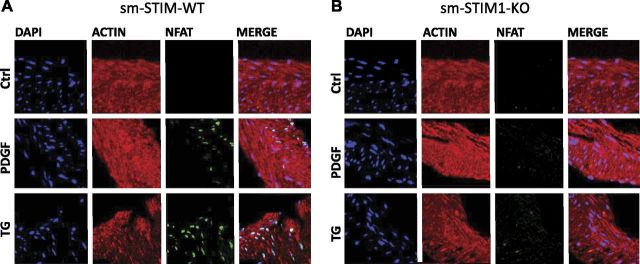
The dependence of NFAT activation on STIM1 could be easily observed in cultured SMCs. However, these cells had already undergone phenotypic change, and we wished to know whether contractile SMCs in situ had similar NFAT responses and whether these were STIM1 dependent or independent. We examined this in ileal smooth muscle, which we revealed had undergone significant developmental phenotypic change in the sm-STIM1-KO mice. Earlier studies revealed that PDGF activates a robust translocation of NFAT in intact ileal smooth muscle, predominantly activating the NFAT3c (also named NFAT4) isoform in this tissue (32). We isolated intact ileal smooth muscle strips from sm-STIM-WT and sm-STIM1-KO mouse intestine and incubated them for 45 min in serum-free medium before treatment with either 20 ng/ml PDGF or 10 μM TG for 1 h. After fixation, tissue was stained and analyzed for NFAT nuclear translocation. We used an antibody against NFAT3c, which accumulates within the nuclei. The tissue was also treated with the nucleic acid stain DAPI and the high-affinity filamentous actin (F-actin) binding bicyclic heptapeptide, phalloidin. We determined the number of nuclei that were positive for both NFAT and DAPI (Fig. 8). In tissue from sm-STIM-WT mice, 1 h of treatment with 20 ng/ml PDGF induced NFAT translocation into 38.5 ± 10.8% (n=10 P<0.01) of DAPI-stained nuclei (Fig. 8A). In tissue from sm-STIM1-KO mice, PDGF-induced translocation into only 7.7 ± 4.3% of nuclei (Fig. 8B). Since PDGF can activate multiple signaling pathways, we also examined the action of TG on NFAT translocation. A 1-h treatment of ileal tissue from sm-STIM-WT and sm-STIM1-KO with 10 μM TG resulted in NFAT translocation into 60.4 ± 8.5 and 5.23 ± 3.7% of nuclei, respectively. Since TG is generally more effective in depleting Ca2+ from stores than PDGF, the results indicate a strong correlation between store depletion and NFAT activation and an almost complete dependence of this action on the presence of STIM1.
Figure 8.
Effects of STIM1 KO on PDGF-induced NFATc3 nuclear translocation in intact ileal smooth muscle. Representative immunofluorescence images of ileal smooth muscle strips costained with DAPI (blue), Alexa Fluor-568 conjugated-phalloidin (red) and NFATc3 (green). NFAT was detected after staining with rabbit anti-NFAT antibody, followed by anti-rabbit antibody labeled with Alexa Fluor-647. Experiments were conducted on ileal strips from either sm-STIM-WT (A) or sm-STIM1-KO (B) animals in response to either PDGF (20 ng/ml), TG (10 μM), or no additions. While nuclear NFAT in nonstimulated tissue was undetectable, nuclear translocation became visible only in WT cells and not STIM1-KO cells. In sm-STIM1-KO cells, NFAT translocation in response to PDGF or TG was almost completely eliminated.
DISCUSSION
The targeted deletion of STIM from smooth muscle defines a number of important phenotypic changes resulting from elimination of STIM proteins and provides an important system for further assessing the roles of STIM expression in smooth muscle. The model system used in these studies, the SM22α-CreKI+ mouse, provides robust Cre expression in SMCs (38), resulting in specific and complete KO in smooth muscle. Our use of SM22αCre+/− controls ensured that the phenotype of the STIM KOs that we observed reflected differences in STIM expression and not differences in Cre or SM22α expression. The high smooth muscle specificity of Cre expression was revealed from our use of the double-fluorescent reporter mouse (mT/mG). SM22α-CreKI+ mice are shown to express Cre recombinase in smooth muscle predominantly late in embryogenesis [little expression is observed before embryonic day 16 (E16) and continues into adulthood; ref. 38]. Expression is observed in all large and small arteries, veins, and visceral smooth muscle, including the bladder and intestinal tract (38). While SM22α-CreKI+ mice also express Cre in cardiac tissue, there is no expression in other tissues, including brain, liver, and skeletal muscle (38, 58). In our studies, the specificity of expression in vascular and intestinal smooth muscle was evident at embryonic age E19 (see Supplemental Fig. S1) and was maintained through adulthood (Fig. 1D, E). The late embryonic expression of Cre is important, and the STIM-KO phenotypes we observe reflect the effect of eliminating STIM genes from already differentiated smooth muscle tissues at a time that the vasculature and other smooth muscle-containing organs are already developed.
Our studies reveal that STIM1 is important for the continued function and growth of smooth muscle, and without the gene, vascular and intestinal tissues undergo significant morphological deterioration, and animals fail to thrive normally. Recent studies have clearly revealed the presence of both STIM and Orai proteins in adult vascular smooth muscle (59), and inwardly rectifying Ca2+-selective CRAC-like currents activated by TG-induced store depletion have been shown in freshly isolated arterial SMCs (60). However, our studies are the first to reveal that STIM1-dependent Ca2+ entry can contribute to receptor-induced contractile responses in adult SMCs. Thus, while STIM-mediated Ca2+ signals are crucial in mediating control over transcription and cell proliferation (5, 6), these Ca2+ signals are not necessarily distinct from those that mediate contractile responses.
Of great significance is the finding that the sm-STIM1/STIM2-DKO animals die perinatally, at which time the genes have been deleted for only a few days. This implies that the STIM2 remaining in sm-STIM1-KO animals can compensate and fulfill some essential function required to continue early postnatal function and/or development of smooth muscle tissues. Surprising, therefore, is the observation that there is no compensatory increase in expression of STIM2 in smooth muscle from sm-STIM1-KO animals. Whole-animal STIM1 KO in C57/BL6 mice is perinatally lethal, while the whole-animal STIM2-KO mice survive a few weeks postnatally (36). The STIM1/STIM2 double KO is undescribed, presumably because it is early embryonically lethal. Conditional KO of both STIM1 and STIM2 has only previously been reported in mouse thymocytes (36, 61), in which the number and function of T cells were severely reduced, and animals developed strong autoimmune-like symptoms. The early lethality of the sm-STIM1/STIM2 DKO and significant defects in the sm-STIM1-KO mice suggest that the function of STIM2 may be quite distinct from STIM1. While STIM2 is more sensitive to Ca2+ store release (5, 11), STIM2 is a poor activator of Orai channels and has dominance over STIM1 (47, 62) functioning likely to limit and control the action of STIM1 (5). Thus, the STIM2 growth phenotype observed in the sm-STIM1-KO mice may reflect an important dominant-negative role of STIM2 in limiting PDGF-induced proliferation of SMCs.
In humans, homozygous nonsense STIM1 mutations lead not only to severe combined immunodeficiency (SCID) but also to skeletal muscle hypotonia and iris smooth muscle hypoplasia (63, 64). Indeed, iris smooth muscle hypoplasia is a prominent dysfunction manifested in rare primary systemic defects in smooth muscle, for example, a smooth muscle α-actin mutation (65). Many patients with SCID have defects in gastrointestinal smooth muscle tone (66) perhaps related to the association between STIM1 gene deletion and gastrointestinal smooth muscle thinning and distension. The hypoplasia that we observe in both aortic and ileal smooth muscle is reminiscent of the diminished growth of skeletal muscle observed after STIM1 knockdown or in skeletal muscle-specific STIM1-KO mice (13, 15). Indeed, the decreased proliferation, reduced nuclear density, and premature death we observe in sm-STIM1-KO mice are phenotypes that are similar to those of the skeletal STIM1-KO mice (15). As noted above, both the smooth muscle STIM1-KO and skeletal muscle STIM1-KO mice have diminished Ca2+ store-refilling, which is likely linked to the attenuated function and/or development of each muscle tissue. We should also consider that different smooth muscle types likely vary in expression of STIM1 vs. STIM2 and that KO of STIM1 may have distinct effects depending on how much residual STIM2 exists. Such differences could result in significant changes in the STIM-KO phenotypes observed in each smooth muscle subtype.
With the morphological abnormalities in smooth muscle seen in the STIM1-KO mice, we would have expected considerable defects in smooth muscle contraction. Yet, despite significant smooth muscle hypoplasia in sm-STIM1-KO mice, the depolarization-induced contractile responses of aortic smooth muscle were little changed (Fig. 4), quite different from the reduced skeletal muscle development that correlated with hypotonia in skeletal STIM1-KO mice (15). Depolarization-induced Ca2+ entry in smooth muscle is mediated by CaV1.2 channels, the function and surface expression of which are inhibited by STIM1 (46, 50). We were unable to detect any difference in expression of the CaV1.2 α1C subunit; thus, the apparently unchanged voltage-activated contraction in smooth muscle of sm-STIM1-KO mice did not appear to reflect a compensatory increase in CaV1.2 channel activity as a result of STIM depletion. The regulatory role STIM1 in controlling the function of CaV1.2 channels in smooth muscle remains to be examined. One particular question is whether the rapid decline in CaV1.2 channel function and expression occurring as SMCs undergo transition to the proliferative phenotype is mediated directly or indirectly by the action of STIM proteins.
Overall, our KO systems reveal important new information on the role of STIM proteins revealing that the relatively small entry of Ca2+ mediated by STIM1 contributes subtly to contraction but has a major role in mediating Ca2+ store refilling, prolonged PDGF-induced Ca2+ entry signals, Ca2+-dependent activation, and translocation of NFAT, growth factor- and injury-induced phenotype transition, and continued proliferation of SMCs. These results agree with recent STIM1 and Orai channel-knockdown studies showing a role for these proteins in proliferation and migration of VSMCs (20–25) but provide great advantages over the incomplete and non-tissue-selective nature of knockdown approaches. The stable knockdown systems of the present study provide defined STIM1/STIM2 protein expression environments with which we reveal the importance of STIM proteins in the development and maintenance of the differentiated state of SMCs, in agreement with in vitro studies showing the role of Ca2+/calcineurin and NFAT signals in maintaining the differentiated SMC phenotype (67).
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
Acknowledgments
This work was supported by U.S. National Institutes of Health grant AI058173 (to D.L.G.), and U.S. National Heart, Blood, and Lung Institute postdoctoral fellowship HL105066 (to S.M.).
The authors thank Dr. Anjana Rao (La Jolla Institute for Allergy and Immunology, La Jolla, CA, USA) and Dr. Masatsugu Oh-hora (Tokyo Medical and Dental University, Tokyo, Japan) for generously providing the floxed STIM mouse lines.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- COL1A1
- collagen type 1 α-1
- COL3A1
- collagen type 3 α-1
- CPA
- cyclopiazonic acid
- DMEM
- Dulbecco's modified Eagle's medium
- EEL
- external elastic lamina
- ER
- endoplasmic reticulum
- FBS
- fetal bovine serum
- GFP
- enhanced green fluorescent protein
- H&E
- hematoxylin and eosin
- IEL
- internal elastic lamina
- KH
- Krebs-Henseleit
- KO
- knockout
- mG
- membrane-targeted enhanced green fluorescent protein
- MLCK
- myosin light-chain kinase
- mT
- membrane-targeted tdTomato
- MYH11
- myosin heavy chain 11
- NFAT
- nuclear factor of activated T cells
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- PDGF
- platelet-derived growth factor
- PE
- phenylephrine
- qRT-PCR
- quantitative reverse transcription–polymerase chain reaction
- shRNA
- short hairpin RNA
- siRNA
- short interfering RNA
- SMC
- smooth muscle cell
- SMTN
- smoothelin
- SOCE
- store-operated Ca2+ entry
- STIM
- stromal interaction molecule
- TG
- thapsigargin
- VCAN
- versican
- VOCC
- voltage-operated Ca2+ channel
- VOCE
- voltage-operated Ca2+ entry
- VSMC
- vascular smooth muscle cell
- WT
- wild type
REFERENCES
- 1.Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Velicelebi G., Stauderman K. A. (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. , 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Meyer T. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. , 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis R. S. (2011) Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harbor Perspect. Biol. a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrasco S., Meyer T. (2011) STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu. Rev. Biochem. , 973–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soboloff J., Rothberg B. S., Madesh M., Gill D. L. (2012) STIM proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. , 549–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekh A. B. (2011) Decoding cytosolic Ca2+ oscillations. Trends Biochem. Sci. , 78–87 [DOI] [PubMed] [Google Scholar]
- 7.Di Capite J., Ng S. W., Parekh A. B. (2009) Decoding of cytoplasmic Ca2+ oscillations through the spatial signature drives gene expression. Curr. Biol. , 853–858 [DOI] [PubMed] [Google Scholar]
- 8.Mancarella S., Wang Y., Gill D. L. (2009) Calcium signals: STIM dynamics mediate spatially unique oscillations. Curr. Biol. , R950–R952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kar P., Bakowski D., Di Capite J., Nelson C., Parekh A. B. (2012) Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc. Natl. Acad. Sci. U. S. A. , 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kar P., Nelson C., Parekh A. B. (2012) CRAC channels drive digital activation and provide analog control and synergy to Ca2+-dependent gene regulation. Curr. Biol. , 242–247 [DOI] [PubMed] [Google Scholar]
- 11.Brandman O., Liou J., Park W. S., Meyer T. (2007) STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell , 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng X., Wang Y., Zhou Y., Soboloff J., Gill D. L. (2009) STIM and Orai-dynamic intermembrane coupling to control cellular calcium signals. J. Biol. Chem. , 22501–22505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stiber J., Hawkins A., Zhang Z. S., Wang S., Burch J., Graham V., Ward C. C., Seth M., Finch E., Malouf N., Williams R. S., Eu J. P., Rosenberg P. (2008) STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat.Cell Biol. , 688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg P. B. (2009) Calcium entry in skeletal muscle. J. Physiol. , 3149–3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li T., Finch E. A., Graham V., Zhang Z. S., Ding J. D., Burch J., Oh-Hora M., Rosenberg P. (2012) STIM1-Ca2+ signaling is required for the hypertrophic growth of skeletal muscle in mice. Mol. Cell. Biol. , 3009–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulot J. S., Fauconnier J., Ramanujam D., Chaanine A., Aubart F., Sassi Y., Merkle S., Cazorla O., Ouille A., Dupuis M., Hadri L., Jeong D., Muhlstedt S., Schmitt J., Braun A., Benard L., Saliba Y., Laggerbauer B., Nieswandt B., Lacampagne A., Hajjar R. J., Lompre A. M., Engelhardt S. (2011) Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation , 796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo X., Hojayev B., Jiang N., Wang Z. V., Tandan S., Rakalin A., Rothermel B. A., Gillette T. G., Hill J. A. (2012) STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. , 136–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Deng X., Hewavitharana T., Soboloff J., Gill D. L. (2008) Stim, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle. Clin. Exp. Pharmacol. Physiol. , 1127–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trebak M. (2012) STIM/Orai signaling complexes in vascular smooth muscle. J. Physiol. , 4201–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potier M., Gonzalez J. C., Motiani R. K., Abdullaev I. F., Bisaillon J. M., Singer H. A., Trebak M. (2009) Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. , 2425–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo R. W., Wang H., Gao P., Li M. Q., Zeng C. Y., Yu Y., Chen J. F., Song M. B., Shi Y. K., Huang L. (2009) An essential role for stromal interaction molecule 1 in neointima formation following arterial injury. Cardiovasc. Res. , 660–668 [DOI] [PubMed] [Google Scholar]
- 22.Aubart F. C., Sassi Y., Coulombe A., Mougenot N., Vrignaud C., Leprince P., Lechat P., Lompre A. M., Hulot J. S. (2009) RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol. Ther. , 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baryshnikov S. G., Pulina M. V., Zulian A., Linde C. I., Golovina V. A. (2009) Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger in proliferating human arterial myocytes. Am. J. Physiol. Cell Physiol , C1103–C1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisaillon J. M., Motiani R. K., Gonzalez-Cobos J. C., Potier M., Halligan K. E., Alzawahra W. F., Barroso M., Singer H. A., Jourd'heuil D., Trebak M. (2010) Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am. J. Physiol. Cell Physiol , C993–C1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W., Halligan K. E., Zhang X., Bisaillon J. M., Gonzalez-Cobos J. C., Motiani R. K., Hu G., Vincent P. A., Zhou J., Barroso M., Singer H. A., Matrougui K., Trebak M. (2011) Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ. Res. , 534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulver R. A., Rose-Curtis P., Roe M. W., Wellman G. C., Lounsbury K. M. (2004) Store-operated Ca2+ entry activates the CREB transcription factor in vascular smooth muscle. Circ. Res. , 1351–1358 [DOI] [PubMed] [Google Scholar]
- 27.Owens G. K., Kumar M. S., Wamhoff B. R. (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. , 767–801 [DOI] [PubMed] [Google Scholar]
- 28.Wamhoff B. R., Bowles D. K., Owens G. K. (2006) Excitation-transcription coupling in arterial smooth muscle. Circ. Res. , 868–878 [DOI] [PubMed] [Google Scholar]
- 29.Beech D. J. (2007) Ion channel switching and activation in smooth-muscle cells of occlusive vascular diseases. Biochem. Soc. Trans. , 890–894 [DOI] [PubMed] [Google Scholar]
- 30.Hill-Eubanks D. C., Gomez M. F., Stevenson A. S., Nelson M. T. (2003) NFAT regulation in smooth muscle. Trends Cardiovasc. Med. , 56–62 [DOI] [PubMed] [Google Scholar]
- 31.Graef I. A., Chen F., Chen L., Kuo A., Crabtree G. R. (2001) Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell , 863–875 [DOI] [PubMed] [Google Scholar]
- 32.Stevenson A. S., Gomez M. F., Hill-Eubanks D. C., Nelson M. T. (2001) NFAT4 movement in native smooth muscle. A role for differential Ca2+ signaling. J. Biol. Chem. , 15018–15024 [DOI] [PubMed] [Google Scholar]
- 33.Wu H., Peisley A., Graef I. A., Crabtree G. R. (2007) NFAT signaling and the invention of vertebrates. Trends Cell Biol. , 251–260 [DOI] [PubMed] [Google Scholar]
- 34.Beech D. J. (2005) Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clin. Exp. Pharmacol. Physiol. , 597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar B., Dreja K., Shah S. S., Cheong A., Xu S. Z., Sukumar P., Naylor J., Forte A., Cipollaro M., McHugh D., Kingston P. A., Heagerty A. M., Munsch C. M., Bergdahl A., Hultgardh-Nilsson A., Gomez M. F., Porter K. E., Hellstrand P., Beech D. J. (2006) Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ. Res. , 557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh-Hora M., Yamashita M., Hogan P. G., Sharma S., Lamperti E., Chung W., Prakriya M., Feske S., Rao A. (2008) Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. , 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varga-Szabo D., Braun A., Kleinschnitz C., Bender M., Pleines I., Pham M., Renne T., Stoll G., Nieswandt B. (2008) The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J. Exp. Med. , 1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J., Zhong W., Cui T., Yang M., Hu X., Xu K., Xie C., Xue C., Gibbons G. H., Liu C., Li L., Chen Y. E. (2006) Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler. Thromb. Vasc. Biol. , e23–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muzumdar M. D., Tasic B., Miyamichi K., Li L., Luo L. (2007) A global double-fluorescent Cre reporter mouse. Genesis , 593–605 [DOI] [PubMed] [Google Scholar]
- 40.Mancarella S., Wang Y., Deng X., Landesberg G., Scalia R., Panettieri R. A., Mallilankaraman K., Tang X. D., Madesh M., Gill D. L. (2011) Hypoxia-induced acidosis uncouples the STIM-Orai calcium signaling complex. J. Biol. Chem. , 44788–44798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sommerville L. J., Kelemen S. E., Autieri M. V. (2008) Increased smooth muscle cell activation and neointima formation in response to injury in AIF-1 transgenic mice. Arterioscler. Thromb. Vasc. Biol. , 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harmon K. J., Couper L. L., Lindner V. (2000) Strain-dependent vascular remodeling phenotypes in inbred mice. Am. J. Pathol. , 1741–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Autieri M. V., Kelemen S., Thomas B. A., Feller E. D., Goldman B. I., Eisen H. J. (2002) Allograft inflammatory factor-1 expression correlates with cardiac rejection and development of cardiac allograft vasculopathy. Circulation , 2218–2223 [DOI] [PubMed] [Google Scholar]
- 44.Ray J. B., Arab S., Deng Y., Liu P., Penn L., Courtman D. W., Ward M. E. (2008) Oxygen regulation of arterial smooth muscle cell proliferation and survival. Am. J. Physiol. Heart Circ. Physiol. , H839–H852 [DOI] [PubMed] [Google Scholar]
- 45.Ray J. L., Leach R., Herbert J. M., Benson M. (2001) Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. , 185–188 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Deng X., Mancarella S., Hendron E., Eguchi S., Soboloff J., Tang X. D., Gill D. L. (2010) The calcium store-sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science , 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Deng X., Zhou Y., Hendron E., Mancarella S., Ritchie M. F., Tang X. D., Baba Y., Kurosaki T., Mori Y., Soboloff J., Gill D. L. (2009) STIM protein coupling in the activation of Orai channels. Proc. Natl. Acad. Sci. U. S. A. , 7391–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X., Nakayama H., Zhang X., Ai X., Harris D. M., Tang M., Zhang H., Szeto C., Stockbower K., Berretta R. M., Eckhart A. D., Koch W. J., Molkentin J. D., Houser S. R. (2011) Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. , 460–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozenblum G. T., Gimona M. (2008) Calponins: adaptable modular regulators of the actin cytoskeleton. Int. J. Biochem. Cell Biol. , 1990–1995 [DOI] [PubMed] [Google Scholar]
- 50.Park C. Y., Shcheglovitov A., Dolmetsch R. (2010) The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science , 101–105 [DOI] [PubMed] [Google Scholar]
- 51.Leung F. P., Yung L. M., Yao X., Laher I., Huang Y. (2008) Store-operated calcium entry in vascular smooth muscle. Br. J. Pharmacol. , 846–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giachini F. R., Chiao C. W., Carneiro F. S., Lima V. V., Carneiro Z. N., Dorrance A. M., Tostes R. C., Webb R. C. (2009) Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: a novel insight into vascular dysfunction. Hypertension , 409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soboloff J., Spassova M. A., Xu W., He L. P., Cuesta N., Gill D. L. (2005) Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J. Biol. Chem. , 39786–39794 [DOI] [PubMed] [Google Scholar]
- 54.Mackie A. R., Byron K. L. (2008) Cardiovascular KCNQ (Kv7) potassium channels: physiological regulators and new targets for therapeutic intervention. Mol. Pharmacol. , 1171–1179 [DOI] [PubMed] [Google Scholar]
- 55.Ruusalepp A., Vaage J., Valen G. (2003) A model of neointima formation in the atherosclerotic carotid artery of mice. Interact. Cardiovasc. Thorac. Surg. , 196–200 [DOI] [PubMed] [Google Scholar]
- 56.Hewavitharana T., Deng X., Wang Y., Ritchie M. F., Girish G. V., Soboloff J., Gill D. L. (2008) Location and function of STIM1 in the activation of Ca2+ entry signals. J. Biol. Chem. , 26252–26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berridge M. J. (2008) Smooth muscle cell calcium activation mechanisms. J. Physiol. , 5047–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshida T., Gan Q., Franke A. S., Ho R., Zhang J., Chen Y. E., Hayashi M., Majesky M. W., Somlyo A. V., Owens G. K. (2010) Smooth and cardiac muscle-selective knock-out of Kruppel-like factor 4 causes postnatal death and growth retardation. J. Biol. Chem. , 21175–21184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S. L., Yeromin A. V., Hu J., Amcheslavsky A., Zheng H., Cahalan M. D. (2011) Mutations in Orai1 transmembrane segment 1 cause STIM1-independent activation of Orai1 channels at glycine 98 and channel closure at arginine 91. Proc. Natl. Acad. Sci. U. S. A. , 17838–17843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brueggemann L. I., Markun D. R., Henderson K. K., Cribbs L. L., Byron K. L. (2006) Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J. Pharmacol. Exp. Ther. , 488–499 [DOI] [PubMed] [Google Scholar]
- 61.Cheng K. T., Alevizos I., Liu X., Swaim W. D., Yin H., Feske S., Oh-Hora M., Ambudkar I. S. (2012) STIM1 and STIM2 protein deficiency in T lymphocytes underlies development of the exocrine gland autoimmune disease, Sjogren's syndrome. Proc. Natl. Acad. Sci. U. S. A. , 14544–14549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soboloff J., Spassova M. A., Hewavitharana T., He L. P., Xu W., Johnstone L. S., Dziadek M. A., Gill D. L. (2006) STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr. Biol. , 1465–1470 [DOI] [PubMed] [Google Scholar]
- 63.Picard C., McCarl C. A., Papolos A., Khalil S., Luthy K., Hivroz C., LeDeist F., Rieux-Laucat F., Rechavi G., Rao A., Fischer A., Feske S. (2009) STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N. Engl. J. Med. , 1971–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feske S., Picard C., Fischer A. (2010) Immunodeficiency due to mutations in ORAI1 and STIM1. Clin. Immunol. , 169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moller H. U., Fledelius H. C., Milewicz D. M., Regalado E. S., Ostergaard J. R. (2012) Eye features in three Danish patients with multisystemic smooth muscle dysfunction syndrome. Brit. J. Ophthalmol. , 1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boeck A., Buckley R. H., Schiff R. I. (1997) Gastroesophageal reflux and severe combined immunodeficiency. J. Allergy Clin. Immunol. , 420–424 [DOI] [PubMed] [Google Scholar]
- 67.Larrieu D., Thiebaud P., Duplaa C., Sibon I., Theze N., Lamaziere J. M. (2005) Activation of the Ca2+/calcineurin/NFAT2 pathway controls smooth muscle cell differentiation. Exp. Cell Res. , 166–175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.