Abstract
Sphingosine-1-phosphate (S1P), a ligand for 5 specific receptors, is a potent lipid mediator that plays important roles in lymphocyte trafficking and immune responses. S1P is produced inside cells and therefore must be secreted to exert its effects through these receptors. Spinster 2 (Spns2) is one of the cell surface transporters thought to secrete S1P. We have shown that Spns2 can export endogenous S1P from cells and also dihydro-S1P, which is active at all cell surface S1P receptors. Moreover, Spns2−/− mice have decreased levels of both of these phosphorylated sphingoid bases in blood, accompanied by increases in very long chain ceramide species, and have defective lymphocyte trafficking. Surprisingly, levels of S1P and dihydro-S1P were increased in lymph from Spns2−/− mice as well as in specific tissues, including lymph nodes, and interstitial fluid. Moreover, lymph nodes from Spns2−/− mice have aberrant lymphatic sinus that appeared collapsed, with reduced numbers of lymphocytes. Our data suggest that Spns2 is an S1P transporter in vivo that plays a role in regulation not only of blood S1P but also lymph node and lymph S1P levels and consequently influences lymphocyte trafficking and lymphatic vessel network organization.—Nagahashi, M., Kim, E. Y., Yamada, A., Ramachandran, S., Allegood, J. C., Hait, N. C., Maceyka, M., Milstien, S., Takabe, K., Spiegel, S. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels and the lymphatic network.
Keywords: sphingosine-1-phosphate, lymphocytes
Sphingosine-1-phosphate (S1P) is a potent bioactive signaling molecule ubiquitous in mammals. S1P is now emerging as an important regulator of many physiological and pathophysiological processes, involved in cancer, inflammation, vascular homeostasis, and immune cell trafficking, to name just a few (1, 2). Most of the known actions of S1P are mediated by binding to 5 specific G-protein-coupled receptors (S1PR1-5), although recent studies indicate that it also has important intracellular targets (1).
Signaling through extracellular receptors requires that S1P, which is made in the cytoplasm and the nucleus by two sphingosine kinase (SphK) isoenzymes, SphK1 and SphK2, respectively, be transported out of cells. Several ATP-binding cassette (ABC) family transporters have been reported to export S1P. These include ABCC1 in mast cells (3), ABCA1 in astrocytes (4), and ABCG2 in breast cancer cells (5) and other family members in platelets and erythrocytes (6, 7). Moreover, expression of the cystic fibrosis transmembrane conductance regulator on epithelial cell lines was shown to contribute to S1P uptake (8, 9). However, recently, spinster 2 (Spns2), a member of the major facilitator superfamily of non-ATP-dependent transporters, has also been shown to be a S1P transporter (10–13). Spns2 was identified independently by two groups, both of whom suggested that it is an S1P transporter based on the observations in zebrafish that a mutation in Spns2 mimicked the heart developmental defects of a S1PR2 mutant (14) and that the Spns2 mutation is rescued by exogenous S1P (10). In addition, export of S1P from cells overexpressing SphK1 required Spns2 (10, 11). However, although it was reported that plasma S1P levels in Spns2-knockout mice are lower than those in wild-type mice (12, 13), no significant reduction was observed in another study (15).
In mammals, circulating levels of S1P are much higher than those in tissues (2, 16). This S1P gradient acting via the S1PR1 receptor is thought to regulate trafficking of a variety of immune cells, including T and B lymphocytes, at various times during differentiation and maturation as well as in inflammatory responses (1, 2). Similarly, levels of S1P are lower in lymphoid tissues than in lymph, and disruption of this S1P gradient blocks lymphocyte egress from lymph nodes and Peyer's patches into lymph (16, 17). Although lymphatic endothelial cell SphK1 contributes S1P to lymph (18), little is known how this lymphatic gradient is maintained (2, 19). Here we show that Spns2 transports endogenous S1P and its close relative dihydro-S1P. Further, Spns2-null mice not only have decreased levels of S1P and dihydro-S1P in blood but also, surprisingly, have increased levels of S1P and dihydro-S1P in lymph. Moreover, certain tissues, including lymph nodes, and interstitial fluid have elevated levels of these phosphorylated sphingoid bases that may also explain the greatly reduced lymphocyte egress from lymph nodes. Remarkably, Spns2−/− mice exhibit altered lymphatic endothelial networks that contain fewer lymphocytes and appear collapsed.
MATERIALS AND METHODS
Cell culture and transfection
Human embryonic kidney (HEK) 293 cells were cultured in DMEM supplemented with 10% FBS. Telomerase-immortalized human microvascular endothelial (TIME) cells were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in endothelial cell growth medium EGM-2 MV (Lonza Walkersville, Inc., Walkersville, MD, USA). Spns2 was down-regulated by transfection with 100 nM On-TargetPlus SmartPool small interfering RNA (siRNA) from Dharmacon RNA Technologies (Lafayette, CO, USA) using Oligofectamine (Invitrogen, Carlsbad, CA, USA). Spns2 was overexpressed by transfection of pCMV6-XL5-Spns2 (Origene, Rockville, MD, USA) with Lipofectamine Plus (Invitrogen). Cells were then washed with PBS, and culture medium was replaced with DMEM containing 0.25% fatty acid-free BSA and 1 mM NaF.
Immunoblotting
Cells were washed with ice-cold PBS and scraped into 500 μl of buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 2 mM sodium orthovanadate, 4 mM sodium pyrophosphate, 100 mM NaF, 1:500 protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO, USA). Equal amounts of proteins were separated by SDS-PAGE and transblotted to nitrocellulose, and immunopositive bands were visualized by enhanced chemiluminescence.
Mice
Spns2-knockout (Spns2tm1a(KOMP)Wtsi) mice were generated by the International Knockout Mouse Consortium and obtained from the Wellcome Trust Sanger Institute (Cambridge, UK; http://www.sanger.ac.uk/mouseportal/search?query=spns2). Mice were bred in pathogen-free conditions with normal lighting. Wild-type and knockout mice were from the same litters. All procedures were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee (accredited by the Association for Assessment and Accreditation of Laboratory Animal Care).
Flow cytometry and cell sorting
Cell suspensions of mouse tissues were prepared in RPMI 1640 with 10% FBS. Blood was collected into heparin-coated tubes by cardiac puncture, and erythrocytes were lysed. Cells were preincubated with anti-CD16/CD32 (BD Biosciences, San Jose, CA, USA) to block nonspecific binding and then were stained with different combinations of fluorochrome-coupled antibodies: CD3, CD4, CD8a, B220, Gr-1, CD11b, CD11c, CD62L, and CD69 (Biolegend, San Diego, CA, USA). The data were collected on a FACSCanto II flow cytometer (BD Biosciences) and analyzed using FACSDiva software (version 6.1.3; BD Biosciences).
Lymph collection
Lymphatic fluid was collected as described previously with some modifications (20). In brief, 200 μl of mineral oil was administered by gavage 2 h before the lymph collection. Under a stereomicroscope, the cisterna chyli was punctured with a fine needle, and lymph fluid was collected with a strip of preweighed absorbent paper.
Lymph node interstitial fluid collection
Interstitial fluid was collected by a centrifugation method (21). We used preweighed inserts closed with nylon mesh (20 μm), which were placed in preweighed centrifuge tubes. Lymph nodes were excised, blotted gently, placed in preweighed inserts, and sectioned with scissors. The inserts were then placed into preweighed centrifuge tubes. Tubes were reweighed to determine lymph node weights. The tubes were centrifuged at 106 g for 10 min at 4°C; the interstitial fluid accumulated in the bottom, and volume was determined by weight. Cold PBS containing phosphatase inhibitors (100 μl) was then added to the interstitial fluid. Samples were recentrifuged at low speed to exclude contaminating cells and S1P and dihydro-S1P were extracted and measured by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). Results of Western blotting for the intracellular protein actin were negative, excluding a significant contribution from broken cells.
Isolation of lymphatic endothelial cells
Lymph nodes were minced and digested with collagenase, dispase, and DNase for 30 min to obtain single-cell suspensions as described previously (22). Subsequently, fluorescence-activated cell sorter (FACS) gating was used to separate lymphatic endothelial cells (LECs) from other cells. TER-119 and CD45 were used to gate out red blood cells (RBCs) and leukocytes, respectively. LECs and blood endothelial cells (BECs) were quantified using gp38 (podoplanin), a specific marker for LECs, and CD31, a marker for both BECs and LECs. LECs were identified as CD45−CD31+gp38+, BECs as CD45−CD31+gp38−, and fibroblast reticular cells as CD45−CD31−gp38+ (22).
Analysis of sphingolipids by LC-ESI-MS/MS
Internal standards (0.5 nmol each; Avanti Polar Lipids, Alabaster, AL, USA) were added to samples, lipids were extracted, and sphingolipids were quantified by LC-ESI-MS/MS (ABI 4000 QTrap system; Applied Biosystems, Foster City, CA, USA) as described previously (23).
Immunofluorescence analysis
Frozen sections (10 μm) were prepared from lymph nodes embedded in optimal cutting medium (OCT 4583; Sakura Finetek, Zoeterwoude, The Netherlands). Sections were fixed in 4% paraformaldehyde, blocked with 10% BSA in PBS for 1 h, and then stained with primary antibodies, anti-lymphatic vessel endothelial-hyaluronan receptor 1 (LYVE-1; Abcam, Cambridge, MA, USA) and anti-CD90.2 (eBioscience, San Diego, CA, USA), at 4°C overnight. After 2 washes with PBS, sections were stained with Alexa Fluor 488- and Alexa Fluor 594-conjugated antibodies (Invitrogen) for 30 min. Nuclei were stained with Hoechst stain for 5 min. Sections were examined with a laser-scanning confocal microscope (LSM510; Carl Zeiss GmbH, Jena, Germany) and a confocal laser scanning microscope (TCS-SP2 AOBS; Leica, Wetzlar, Germany).
Statistical analysis
Results were analyzed for statistical significance with a 2-tailed Student's t test, with P < 0.05 considered significant. Experiments were repeated ≥ 3 times in triplicate with consistent results.
RESULTS
Role of Spns2 in export of S1P and dihydro-S1P
It has previously been shown that Spns2 can transport phosphorylated sphingoid bases from inside cells to the extracellular milieu (10, 11). Because this conclusion was based on overexpression of SphK1 or treatment with exogenously added labeled sphingosine as a tracer, we were curious to examine whether Spns2 also exported S1P and dihydro-S1P from endogenous pools. Therefore, their secretion was measured by LC-ESI-MS/MS, which enabled us to quantify their levels in the medium without exogenously added sphingoid base or overexpression of sphingosine kinase. Indeed, HEK 293 cells overexpressing Spns2 secrete both S1P and dihydro-S1P (Fig. 1A, B). Sphingosine, the precursor of S1P, was also increased by Spns2 expression (24). Conversely, when Spns2 was down-regulated, release of both S1P and dihydro-S1P was significantly decreased, whereas sphingosine levels in the media were not significantly altered (Fig. 1C, D). Notably, Western blot analysis showed that endogenous Spns2 appeared as a doublet, and both bands were enhanced and decreased by overexpression and down-regulation, respectively, suggesting that Spns2 might be post-translationally modified (Fig. 1B, D). This result is consistent with the NetNglyc Server prediction that Spns2 is a glycosylated protein (http://www.cbs.dtu.dk/services/NetNGlyc/). Similarly, overexpression of Spns2 in human microvascular endothelium TIME cells increased export of S1P (Fig. 1E, F), whereas its down-regulation decreased it (Fig. 1G, H).
Figure 1.
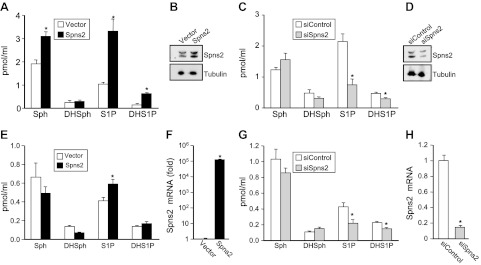
Spns2 transports S1P and dihydro-S1P from cells. A–D) Spns2 was overexpressed (A, B) or down-regulated (C, D) in HEK 293 cells. Then 48 h later, cells were washed, and culture medium was replaced with medium containing a phosphatase inhibitor. After 2 h, medium was collected and sphingosine (Sph), dihydrosphingosine (DHSph), S1P, and dihydro-S1P (DHS1P) were measured by LC-ESI-MS/MS (A, C). Data are means ± sd of triplicate determinations. *P < 0.05. B, D) In duplicate cultures, Spns2 expression was determined with anti-Spns2 antibody (Sigma-Aldrich). Blots were stripped and reprobed with anti-tubulin antibody to ensure equal loading and transfer. E–H) Similar results were found in 2 additional experiments. Spns2 was overexpressed (E, F) or down-regulated (G, H) in TIME cells. Sphingolipids were measured by LC-ESI-MS/MS (E, G), and mRNA levels of Spns2 were determined by quantitative real-time PCR and normalized to glyceraldehyde-3-phosphate dehydrogenase (F, H).
Surprisingly, however, despite the major effect of overexpression of Spns2 on secretion of S1P and dihydro-S1P (Fig. 1A), their intracellular levels were not significantly altered (Fig. 2A). However, sphingosine and dihydrosphingosine (Fig. 2A) and total levels of ceramides (Fig. 2B), monohexosylceramides (Fig. 2C), and sphingomyelins (Fig. 2D) were slightly decreased, although the differences were not statistically significant.
Figure 2.
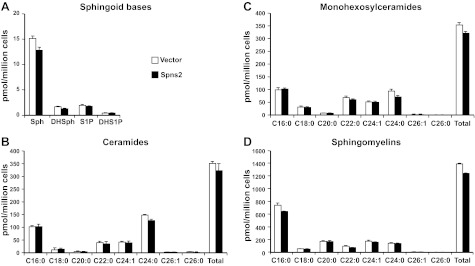
Overexpression of Spns2 does not alter cellular levels of S1P and dihydro-S1P or other sphingolipids. HEK 293 cells transfected with control or Spns2 were washed, and culture medium was replaced with medium containing phosphatase inhibitor. After 2 h, lipids were extracted from cells, and dihydrosphingosine, dihydro-S1P, sphingosine, and S1P (A), different chain length species of ceramide (B), monohexosylceramide (C), and sphingomyelin (D) were determined by LC-ESI-MS/MS. Numbers indicate chain length, followed by the number of double bonds in the fatty acid. Data are averages of triplicate determinations and are expressed as picomoles of lipid per million cells. Similar results were found in two additional experiments.
Spns2−/− mice have decreased blood levels of S1P and dihydro-S1P and increased levels of very long chain ceramides
Recent studies suggest that Spns2 functions as a S1P transporter in vascular endothelial cells but not in erythrocytes or platelets and that the plasma S1P concentration in Spns2−/− mice was markedly reduced compared with that in wild type mice, suggesting that Spns2 is responsible, at least in part, for transporting S1P into the blood (12, 13). In contrast, others have reported that plasma S1P levels are not reduced in Spns2−/− mice (15). Therefore, next we used the same Spns2-targeted mouse line Spns2tm1a/tm1a, generated by the Wellcome Trust Sanger Institute, to examine effects on levels of S1P in blood. In these Spns2-knockout mice, not only S1P but also dihydo-S1P was reduced in plasma and whole blood by 25–30% compared with levels in wild-type littermates (Fig. 3A, B). Notably, although there were no changes in levels of their precursors sphingosine and dihydrosphingosine in blood, there were significant increases in very long chain ceramides Cer22:0, Cer24:1, and Cer24:0, whereas long-chain ceramide species were unaffected (Fig. 3C). However, levels of the complex sphingolipids monohexosylceramides and sphingomyelins were similar in both Spns2−/− and wild-type mice (data not shown).
Figure 3.
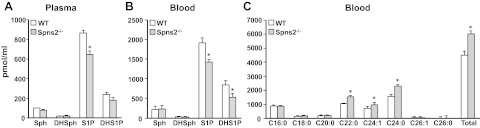
Blood levels of S1P and dihydro-S1P are reduced, whereas very long chain ceramides are increased in Spns2-knockout mice. Lipids were extracted from plasma (A) and whole blood (B, C) from wild-type (WT) and Spns2-knockout mice and dihydrosphingosine, dihydro-S1P, sphingosine, and S1P (A, B), and ceramide species (C) were determined by LC-ESI-MS/MS. Numbers indicate chain length followed by the number of double bonds in the fatty acid. *P < 0.05.
Spns2 deletion influences lymphocyte trafficking
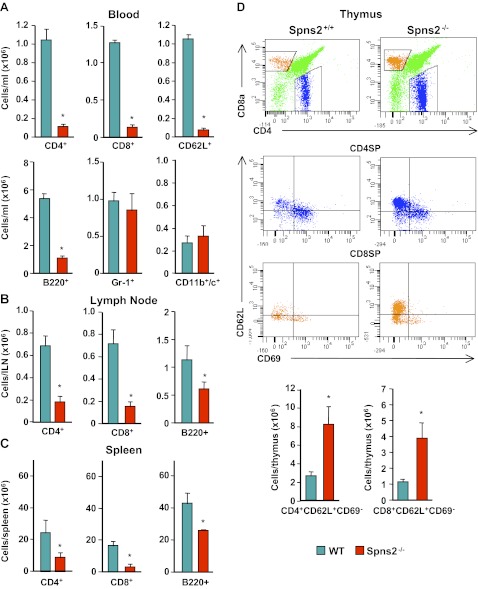
Lymphocyte egress from thymus and secondary lymphoid organs was markedly reduced in mice with conditional deletion of SphK1 and SphK2, resulting in undetectable levels of S1P in plasma and lymph (17). In agreement with others (12, 13, 15), we found that circulating lymphocytes in Spns2-knockout mice were significantly decreased, in particular CD4 and CD8 single-positive T cells, including those expressing the homing molecule L-selectin (CD62L), and B220-positive B cells (Fig. 4A). However, numbers of granulocytes (Gr-1+) and dendritic cells (CD11b+ and CD11c+) were not significantly altered (Fig. 4A). There was also a significant reduction in numbers of CD4 and CD8 T cells and B cells in the lymph nodes and spleen (Fig. 4B, C). In contrast, in the thymus, there was an increase in the proportion of mature CD4 and CD8 single-positive T cells expressing lower levels of the activation marker CD69 and higher levels of CD62L (Fig. 4D). These results substantiate the notion that Spns2 is involved in lymphocyte trafficking and egress of mature T cells from the thymus into the circulation.
Figure 4.
Aberrant lymphocyte trafficking in Spns2−/− mice. Lymphocytes of wild-type (WT) and Spns2−/− mice were analyzed by flow cytometry. A) Number of circulating CD3+ T cells that are CD4+, CD8+, or CD62L+; B220+ B cells; Gr-1+ granulocytes; and CD11b+/c+ dendritic cells. B, C) Total number of CD3+ T cells that are CD4+, CD8+, or CD62L+ and B220+ B cells in lymph nodes (B) and spleen (C). D) Flow cytometry analysis of wild-type and Spns2−/− thymocytes. Single-positive CD4+ and CD8+ T cells were gated for the maturation markers CD69 and CD62L. Representative flow cytometry plots (top panels) and total number of CD4+CD62L+CD69− and CD4+CD62L+CD69− T cells (bottom panels) are shown. *P < 0.01.
Deletion of Spns2 elevates lymph S1P
We were intrigued by the observation that SphK1-knockout mice, which have reductions in blood S1P similar to those of Spns2-knockout mice, do not have any defects in lymphocyte trafficking (25). Moreover, although it was initially assumed that the S1P gradient between the thymus and the blood is a primary determinant of egress of mature T cells, several studies have shown that plasma S1P is insufficient to promote thymic egress (17, 26, 27). Therefore, because separate sources provide S1P to plasma and lymph to help lymphocytes exit the low S1P environment of lymphoid organs (17), we next measured S1P levels in the lymph by LC-ESI-MS/MS. S1P levels are 150 ± 10 nM in lymph from wild-type mice, which is in agreement with previous reports (17, 28). Surprisingly, in contrast with blood, lymph S1P and dihydro-S1P levels in Spns2-knockout mice were elevated compared with those in wild-type littermate controls (Fig. 5A). Thus, although Spns2 is involved in transport of S1P from vascular endothelial cells into the blood (12, 13), it may not play a similar role in secretion of S1P from lymphatic endothelial cells into the lymphatic fluid.
Figure 5.
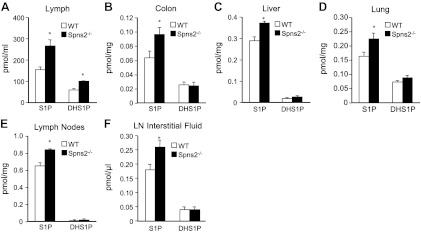
Increased levels of S1P in lymph and tissues of Spns2−/− mice. Lipids were extracted from lymph (A), colon (B), liver (C), lung (D), lymph nodes (E), and lymph node (LN) interstitial fluid (F) from wild-type (WT) and Spns2-knockout mice, and S1P and dihydro-S1P (DHS1P) were determined by LC-ESI-MS/MS. *P < 0.05.
Spns2 deficiency increases tissue S1P levels
Although tissue concentrations of S1P are high in adult mice, the importance of Spns2 in exporting S1P out of tissues has not been examined. Moreover, lymphocyte circulation is also inhibited when S1P is increased in tissues by inhibition or deletion of S1P lyase (16). Therefore, we next determined levels of S1P in tissues of Spns2−/− mice. Significant increases of S1P compared with those in littermate controls were detected in colon, liver, lung, and even lymph nodes (Fig. 5B–E) but not in several other tissues. The increases in S1P correlate with Spns2 transcript levels determined by quantitative PCR, for which the highest expression is in colon, liver, lung, and lymph nodes (ref. 15 and data not shown).
Tissue stromal cells are constantly bathed with transudate plasma, interstitial fluid, to bring nutrients and remove waste. Although never measured, S1P in the interstitial fluid of lymphoid organs has been assumed to be extremely low (in the nanomolar range), allowing lymphocytes to be drawn from regions of low S1P to the high levels of S1P in lymph (2). To quantitatively measure mass levels of S1P and dihydro-S1P, we isolated interstitial fluid from lymph nodes by a low-speed centrifugation method (21). Notably, S1P levels are higher than expected and also significantly increased in lymph node interstitial fluid from Spns2−/− mice (Fig. 5F). The levels of dihydro-S1P were similar in both wild-type and Spns2−/− mice, but because of the limited volume of interstitial fluid, both were close to the limit of detection.
Impaired lymphocyte egress from lymph nodes and aberrant lymphatic vessels in Spns2-deficient mice
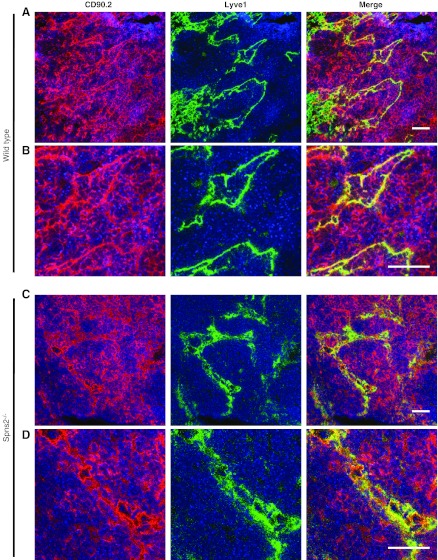
The S1P gradient, low in the lymph node parenchyma and high at the exit site, enables lymphocyte migration into the lymphatic vessel endothelial LYVE-1+ cortical sinuses of the lymph nodes via S1PR1 present on their cell surface (2). Consistent with the reduced lymphocyte numbers in the circulation detected by FACS analysis (Fig. 4) and the aberrant S1P gradient (Fig. 5), LYVE-1+ sinuses in lymph nodes of Spns2-deficient mice contained reduced numbers of lymphocytes and appeared to be collapsed (Fig. 6C, D). In contrast, LYVE-1+ sinuses in lymph nodes from wild-type littermates were replete with cells (Fig. 6A, B). Similarly, cortical sinuses were also empty in lymphatic SphK1/2-deficient mice (18). Taken together, these findings provide further evidence for the involvement of S1P and Spns2 in an early event of lymphocyte egress from lymph nodes.
Figure 6.
Impaired T-cell egress from lymph nodes in Spns2-deficient mice. Lymph nodes from wild-type (A, B) and Spns2−/− mice (C, D) were immunostained to visualize LECs with LYVE-1 (green), T cells with CD90.2 (red), and nuclei with Hoechst (blue). Confocal fluorescent images at ×40 (A, C) and ×95 (B, D). Scale bars = 100 μm.
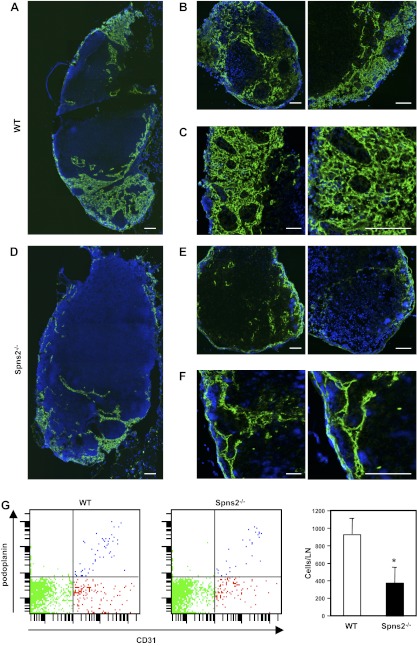
It has been suggested that S1P from LECs not only is responsible for lymphocyte egress but also is involved lymphatic vessel maturation (18). Therefore, we investigated the effect of Spns2 deficiency on lymphatic vessel structure, which should have intact S1P production. The tile scan technology that combines high magnification images to see macroscopic appearance with high resolution allowed visualization of the widespread lymphatic sinus-forming network in lymph nodes (Fig. 7A–C). Remarkably, we observed that LYVE-1+ lymphatic sinuses appeared collapsed with reduced lymphatic network formation in Spns2-deficient lymph nodes (Fig. 7D–F). This difference was even more striking at higher magnification at which the lymphatic vessel network appears completely collapsed in Spns2-deficient lymph nodes compared with that in lymph nodes of wild-type littermates (Fig. 7C, F).
Figure 7.
Lack of Spns2 disrupts the lymphatic vessel network in lymph nodes. A–F) Immunofluorescent analysis of lymph nodes from wild-type (WT) littermates and Spns2−/− mice stained for LYVE-1 (green) and Hoechst (blue). Confocal images of axillary lymph nodes from wild-type (A–C) and Spns−/− mice (D–F), showing lymphatic sinus stained with antibody against LYVE-1 with low magnification using tile scan technology (A, B, D, E) and with high magnification (C, F). Scale bars = 100 μm. G) Single-cell suspensions were prepared from axillary lymph nodes of wild-type and Spns2−/− mice by digestion with collagenase, and total numbers of LECs were quantified by FACS analysis. Data are means ± se of triplicate determinations.
We next quantified the number of LECs in these lymph nodes. Single-cell suspensions were analyzed by a FACS gating scheme that we previously established to separate LECs from BECs and from other cells (29). In brief, TER-119 and CD45 were used to gate out RBCs and leukocytes, respectively. LECs were quantified using gp38 (podoplanin), a specific marker for LECs, and CD31, a marker for both BECs and LECs. There were significantly fewer LECs (CD45−CD31+gp38+) in Spns2-null lymph nodes than in wild-type lymph nodes (Fig. 7G), consistent with their aberrant lymphatic network.
DISCUSSION
In this work, we have shown that Spns2 functions as a transporter for both S1P and dihydro-S1P from cells and tissues in which it is highly expressed. In agreement with others (12, 13), using mass spectrometry methods, we found that blood and plasma S1P levels were lower in Spns2-deficient mice. The levels of dihydro-S1P were also reduced, consistent with our results in cell culture that Spns2 also transports dihydro-S1P. In contrast, another group reported that these mice had no significant reduction in plasma S1P, determined by ELISA and the S1PR1 redistribution assay that measures internalization of this receptor (15). This result suggests that these semiquantitative assays are not sufficiently sensitive to accurately measure S1P levels.
Based on previous observations that endothelium-specific Spns2 deletion resulted in defective egress of lymphocytes similar to that in global knockout mice (13), it has been suggested that Spns2 functions mainly in BECs to secrete S1P into the blood and establish the S1P gradient required for T- and B-lymphocyte egress. However, our results suggest that reduction in blood levels of S1P is not the sole factor that influences this aberrant lymphocyte trafficking. This result is consistent with previous studies demonstrating that although SphK1-knockout mice have a similar 30–50% reduction in blood S1P levels, they do not have lymphopenia (25).
It is well established that mature single-positive T cells up-regulate S1PR1, allowing them to exit the thymus in response to a S1P gradient (2). This gradient is produced by neural crest-derived perivascular cells that surround the thymus (26), and, subsequently, the T cells encounter the endothelium and respond to the high levels of S1P in the blood maintained by RBCs (17) and endothelial cells (13, 30). In agreement with others (12, 13, 15), we have shown that Spns2 is important for egress of mature T lymphocytes from the thymus, most likely assisting in the maintenance of these gradients. Similarly, B-lymphocyte egress from secondary lymphoid organs requires S1P and S1PR1 (2). In the secondary lymphoid organs, such as peripheral lymph nodes and spleen, both T and B cells are decreased in Spns2−/− mice, but not as severely as in the blood. Intriguingly, the S1P levels in the peripheral lymph node are elevated, similar to those in mice treated with a S1P lyase inhibitor (16). The higher S1P levels in the lymph node would probably down-regulate S1PR1 expression on the lymphocytes required for their egress out of the lymph node. This might explain the higher ratio of lymphocytes in the secondary lymphoid organs vs. circulating in the blood in Spns2−/− mice.
Based on the extensive literature demonstrating a role for the S1P/S1PR1 axis in lymphocyte egress (2), we hypothesized that the S1P gradient was aberrant in Spns2−/− mice. We found that S1P is elevated in colon, liver, lung, and lymph nodes of these mice, all tissues with high Spns2 expression. One simple explanation for this result is that Spns2 transports S1P out of the cells, and thus S1P accumulates inside the cells of these tissues. We also surprisingly observed that S1P was higher in Spns2−/− lymphatic fluid than in that of wild-type mice. Thus, although it was shown that BECs release S1P into plasma in an Spns2-dependent manner (12, 13), the role of Spns2 in release of S1P from LECs into the lymph is different and probably more complex, because if it functions in a similar manner, lymph S1P levels would be expected to decrease when it is globally knocked out, rather than increased. Because it has been elegantly demonstrated that S1P produced by SphK1/2 in LECs is the major source of lymph S1P (18), it seems likely that either a different S1P transporter on LECs might be involved in its release into lymph or that in the absence of Spns2, there is a compensatory mechanism. What then accounts for the increased S1P levels in lymph from Spns2-deficient mice? We collected lymph from cisterna chyli, in which the major source of the fluid is from the intestine. It is well known that interstitial fluid in the intestine is drained into lymphatic vessels via button-like structures of the initial lymphatic vessels, and thus the compositions of interstitial fluid and the lymphatic fluid in the initial lymphatic vessels are considered to be equivalent (31). Taken together with our observation that S1P levels are higher in the colons of Spns2−/− mice as well as in other organs and in lymph node interstitial fluid, speculation that the interstitial fluid in these animals contributes S1P to the lymph is tempting. It is also possible that accumulation of S1P in BECs due to loss of Spns2 may contribute to increased levels of S1P in interstitial fluid and in lymphatic fluid. It has been assumed that levels of S1P in interstitial fluid are in the low nanomolar range to ensure expression of S1PR1 on lymphocytes and their exit from secondary lymphoid organs (2). Remarkably, however, we were able to detect significant levels of S1P in interstitial fluid (∼180 nM), which was 40% higher in the Spns2−/− mice. It seems unlikely that these high levels of S1P in lymph node interstitial fluid resulted from rupture of cells in the lymph nodes because there was no detectable amount of the intracellular protein actin.
Another important observation is that the lymph nodes of Spns2-deficient mice contain collapsed lymphatic vessels and emptied sinuses. The collapse of many cortical sinuses is strikingly similar to that seen in mice deficient in LEC-specific production of S1P and to the emptying of sinuses induced by treatment with the immunosuppressive drug FTY720, a S1PR1 modulator (18). Comparable cortical sinus disorganization and emptying of cortical sinuses were seen in lymph nodes responding to local inflammation induced by poly(I:C) that occurred as a result of CD69 induction in lymphocytes (32). Based on 3-dimensional reconstruction and intravital 2-photon microscopy in this model of local inflammation, it was suggested that the presence of lymphocytes within the sinuses is necessary to keep them open and that the fluid flow within the vessels is influenced by their cell content (32). However, the possibility that development of cortical sinus organization is also affected by Spns2 deficiency cannot be ruled out. In addition, we have previously observed that pharmacological inhibition of S1P production inhibited the tumor-induced increase of LECs in the lymph nodes (22). Intriguingly, LECs are also decreased in lymph nodes of Spns2-deficient mice. Taken together, these results suggest that either LECs are capable of transducing signals from extracellular S1P that mediate organization of lymphatic vessels or, alternatively, that optimum levels of S1P are required for the proper development of the lymphatic vasculature.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grants R37-GM043880 and U19-AI077435 (to S.S.) and K12-HD055881 and Susan G. Komen for the Cure Research Foundation Career Catalyst research grant KG090510 (to K.T.). E.Y.K. and J.C.A. were supported by NIH training grant T32HL094290. M.N. is a Japan Society for the Promotion of Science Postdoctoral Fellow. The Flow Cytometry and Confocal Microscopy Shared Resource Cores were supported in part by NIH grant P30CA16059 to the Massey Cancer Center and National Institute of Neurological Disorders and Stroke Center Core grant 5P30NS047463, respectively.
M.N. designed and performed the research and assisted in writing the manuscript; E.Y.K., A.Y., S.R., and N.C.H. performed the research and analyzed the data; J.C.A. measured sphingolipids; M.M. and S.M. analyzed the data and assisted in writing the manuscript; K.T. designed experiments, analyzed the data, and assisted in writing the manuscript; and S.S. designed experiments, analyzed data, and wrote the manuscript. The authors declare no conflicts of interest.
Footnotes
- ABC
- ATP-binding cassette
- BEC
- blood endothelial cell
- FACS
- fluorescence-activated cell sorter
- HEK
- human embryonic kidney
- LC-ESI-MS/MS
- liquid chromatography-electrospray ionization-tandem mass spectrometry
- LEC
- lymphatic endothelial cell
- LYVE-1
- lymphatic vessel endothelial hyaluronan receptor 1
- RBC
- red blood cell
- S1P
- sphingosine-1-phosphate
- siRNA
- small interfering RNA
- Spns2
- spinster 2
- SphK
- sphingosine kinase
- S1PR
- sphingosine-1-phosphate receptor
- TIME
- telomerase-immortalized human microvascular endothelial
REFERENCES
- 1. Spiegel S., Milstien S. (2011) The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cyster J. G., Schwab S. R. (2012) Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 [DOI] [PubMed] [Google Scholar]
- 3. Mitra P., Oskeritzian C. A., Payne S. G., Beaven M. A., Milstien S., Spiegel S. (2006) Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. U. S. A. 103, 16394–16399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato K., Malchinkhuu E., Horiuchi Y., Mogi C., Tomura H., Tosaka M., Yoshimoto Y., Kuwabara A., Okajima F. (2007) Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J. Neurochem. 103, 2610–2619 [DOI] [PubMed] [Google Scholar]
- 5. Takabe K., Kim R. H., Allegood J. C., Mitra P., Ramachandran S., Nagahashi M., Harikumar K. B., Hait N. C., Milstien S., Spiegel S. (2010) Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 285, 10477–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kobayashi N., Nishi T., Hirata T., Kihara A., Sano T., Igarashi Y., Yamaguchi A. (2006) Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J. Lipid Res. 47, 614–621 [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi N., Yamaguchi A., Nishi T. (2009) Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J. Biol. Chem. 284, 21192–21200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boujaoude L. C., Bradshaw-Wilder C., Mao C., Cohn J., Ogretmen B., Hannun Y. A., Obeid L. M. (2001) CFTR regulates uptake of sphingoid base phosphates and LPA. J. Biol. Chem. 276, 35258–35264 [DOI] [PubMed] [Google Scholar]
- 9. Meissner A., Yang J., Kroetsch J. T., Sauve M., Dax H., Momen A., Noyan-Ashraf M. H., Heximer S., Husain M., Lidington D., Bolz S. S. (2012) Tumor necrosis factor-α-mediated downregulation of the cystic fibrosis transmembrane conductance regulator drives pathological sphingosine-1-phosphate signaling in a mouse model of heart failure. Circulation 125, 2739–2750 [DOI] [PubMed] [Google Scholar]
- 10. Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A., Mochizuki N. (2009) The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323, 524–527 [DOI] [PubMed] [Google Scholar]
- 11. Hisano Y., Kobayashi N., Kawahara A., Yamaguchi A., Nishi T. (2011) The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J. Biol. Chem. 286, 1758–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hisano Y., Kobayashi N., Yamaguchi A., Nishi T. (2012) Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 7, e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukuhara S., Simmons S., Kawamura S., Inoue A., Orba Y., Tokudome T., Sunden Y., Arai Y., Moriwaki K., Ishida J., Uemura A., Kiyonari H., Abe T., Fukamizu A., Hirashima M., Sawa H., Aoki J., Ishii M., Mochizuki N. (2012) The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122, 1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osborne N., Brand-Arzamendi K., Ober E. A., Jin S. W., Verkade H., Holtzman N. G., Yelon D., Stainier D. Y. (2008) The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr. Biol. 18, 1882–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nijnik A., Clare S., Hale C., Chen J., Raisen C., Mottram L., Lucas M., Estabel J., Ryder E., Adissu H., Adams N. C., Ramirez-Solis R., White J. K., Steel K. P., Dougan G., Hancock R. E. (2012) The role of sphingosine-1-phosphate transporter spns2 in immune system function. J. Immunol. 189, 102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y., Cyster J. G. (2005) Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 [DOI] [PubMed] [Google Scholar]
- 17. Pappu R., Schwab S. R., Cornelissen I., Pereira J. P., Regard J. B., Xu Y., Camerer E., Zheng Y. W., Huang Y., Cyster J. G., Coughlin S. R. (2007) Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 [DOI] [PubMed] [Google Scholar]
- 18. Pham T. H., Baluk P., Xu Y., Grigorova I., Bankovich A. J., Pappu R., Coughlin S. R., McDonald D. M., Schwab S. R., Cyster J. G. (2010) Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207, 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwab S. R., Cyster J. G. (2007) Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8, 1295–1301 [DOI] [PubMed] [Google Scholar]
- 20. Matloubian M., Lo C. G., Cinamon G., Lesneski M. J., Xu Y., Brinkmann V., Allende M. L., Proia R. L., Cyster J. G. (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 [DOI] [PubMed] [Google Scholar]
- 21. Wiig H., Aukland K., Tenstad O. (2003) Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. Am. J. Physiol. Heart Circ. Physiol. 284, H416–H424 [DOI] [PubMed] [Google Scholar]
- 22. Nagahashi M., Ramachandran S., Kim E. Y., Allegood J. C., Rashid O. M., Milstien S., Spiegel S., Takabe K. (2012) Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by tumor-induced angiogenesis and lymphangiogenesis. Cancer Res. 72, 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., Spiegel S. (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brindley D. N., Pilquil C. (2009) Lipid phosphate phosphatases and signaling. J. Lipid Res. 50(Suppl), S225–S230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., Mandala S., Spiegel S., Proia R. L. (2004) Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 [DOI] [PubMed] [Google Scholar]
- 26. Zachariah M. A., Cyster J. G. (2010) Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science 328, 1129–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Breart B., Ramos-Perez W. D., Mendoza A., Salous A. K., Gobert M., Huang Y., Adams R. H., Lafaille J. J., Escalante-Alcalde D., Morris A. J., Schwab S. R. (2011) Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med. 208, 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sensken S. C., Bode C., Nagarajan M., Peest U., Pabst O., Graler M. H. (2010) Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J. Immunol. 184, 4133–4142 [DOI] [PubMed] [Google Scholar]
- 29. Nagahashi M., Ramachandran S., Rashid O. M., Takabe K. (2010) Lymphangiogenesis: a new player in cancer progression. World J. Gastroenterol. 16, 4003–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Venkataraman K., Lee Y. M., Michaud J., Thangada S., Ai Y., Bonkovsky H. L., Parikh N. S., Habrukowich C., Hla T. (2008) Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 102, 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baluk P., Fuxe J., Hashizume H., Romano T., Lashnits E., Butz S., Vestweber D., Corada M., Molendini C., Dejana E., McDonald D. M. (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grigorova I. L., Panteleev M., Cyster J. G. (2010) Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc. Natl. Acad. Sci. U. S. A. 107, 20447–20452 [DOI] [PMC free article] [PubMed] [Google Scholar]