Abstract
Lymphedema is a dreaded complication of cancer treatment. However, despite the fact that >5 million Americans are affected by this disorder, the development of effective treatments is limited by the fact that the pathology of lymphedema remains unknown. The purpose of these studies was to determine the role of inflammatory responses in lymphedema pathology. Using mouse models of lymphedema, as well as clinical lymphedema specimens, we show that lymphatic stasis results in a CD4+ T-cell inflammation and T-helper 2 (Th2) differentiation. Using mice deficient in T cells or CD4+ cells, we show that this inflammatory response is necessary for the pathological changes of lymphedema, including fibrosis, adipose deposition, and lymphatic dysfunction. Further, we show that inhibition of Th2 differentiation using interleukin-4 (IL-4) or IL-13 blockade prevents initiation and progression of lymphedema by decreasing tissue fibrosis and significantly improving lymphatic function, independent of lymphangiogenic growth factors. We show that CD4+ inflammation is a critical regulator of tissue fibrosis and lymphatic dysfunction in lymphedema and that inhibition of Th2 differentiation markedly improves lymphatic function independent of lymphangiogenic cytokine expression. Notably, preventing and/or reversing the development of pathological tissue changes that occur in lymphedema may be a viable treatment strategy for this disorder.—Avraham, T., Zampell, J. C., Yan, A., Elhadad, S., Weitman, E. S., Rockson, S. G., Bromberg, J., Mehrara, B. J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema.
Keywords: inflammation, lymphangiogenesis, lymphatic endothelial cell
Lymphedema is chronic tissue swelling that occurs when lymphatic transport capacity is exceeded. In the United States, lymphedema occurs most commonly as a complication of lymph node dissection for cancer treatment and affects 3–5 million patients, resulting in significant medical expenditures, decreased quality of life, and severe soft tissue infections (1, 2).
Although numerous studies have shown that lymphedema is characterized histologically by chronic inflammation and fibrosis (3, 4), the etiology of this disorder remains unknown. It is unclear, for instance, why an identical operation leads to lymphedema in some patients and not others. Perhaps the most perplexing aspect of lymphedema is the fact that progressive swelling, in most cases, occurs in a delayed fashion 1–5 yr after surgery (5). These clinical features suggest that injury and resultant lymphatic stasis are only the initiating events and that other pathological steps are required for lymphedema development.
On the basis of the clinical features of lymphedema, we hypothesized that lymphatic stasis initiates a cycle of inflammation → progressive tissue fibrosis → worsening lymphatic function (6). This cycle, over time, leads to end-organ failure of the lymphatic system and clinically apparent lymphedema. This hypothesis is supported by the fact that fibrosis is a common mode of organ failure, and, similar to lymphedema, fibrotic disorders are characterized by inflammation, replacement of parenchyma with scar, and progressive organ dysfunction (7). In addition, our hypothesis explains the delayed development of lymphedema after injury since the progressive fibrosis necessary to cause end-organ failure of the lymphatics takes time to develop. Fibrosis also provides a mechanism for the known risk factors of lymphedema, such as radiation, obesity, and infection, since these predisposing conditions contribute either directly or indirectly to fibrosis (8, 9).
The purpose of this study was to determine the role of inflammatory responses in the regulation of fibrosis and lymphatic dysfunction in lymphedema. We show that lymphatic stasis results in chronic T-cell inflammation and a mixed T-helper-1/T-helper-2 (Th1/Th2) response. In addition, we show that inhibition of T-cell inflammation or Th2 differentiation significantly attenuates the initiation and progression of fibrosis, improves lymphatic function, and markedly decreases pathological tissue changes, suggesting that this approach can serve as a novel treatment modality for lymphedema.
MATERIALS AND METHODS
Animal models
We used a well-described mouse-tail model (6, 10–12) in 10- to 14-wk-old female mice (C57BL/6J, Nude (B6.Cg-Foxn1nu), CD4-knockout [CD4KO; CBY.129S2(B6)-Cd4tm1mak/J]; Jackson Laboratories, Bar Harbor, ME, USA) to study the effects of lymphatic stasis on inflammation. Briefly, lymphatic stasis was induced by excising a 2-mm circumferential segment of skin and deep lymphatics 20 mm from the base of the tail (10). The wound was then covered with or without 0.1% rat tail collagen (Sigma-Aldrich, St. Louis, MO, USA) for 5 d. We, and others, have previously shown that covering the wound with collagen gel results in rapid wound repair and lymphatic regeneration across the wound within 3 wk of surgery. In contrast, leaving the wound uncovered results in delayed lymphatic regeneration with resultant sustained lymphatic stasis for at least 6 wk after surgery. Therefore, comparison of mouse tail wounds treated with or without collagen gel enabled us to compare the effects of temporary (collagen gel) and sustained lymphatic stasis (excision). Axillary lymph node dissection (ALND) and axillary incision without lymph node removal were performed in adult female C57BL/6J mice, as described previously (13).
Bleomycin-induced skin fibrosis was performed using a modification of a previously reported model (14). Briefly, 10 μl of bleomycin (0.5 U/ml; Sigma-Aldrich) was intradermally injected in the superior and inferior aspect of the tail twice weekly for 14 d; control animals were injected with phosphate-buffered saline (PBS). All animal studies were approved by the Resource Animal Research Center Institutional Animal Care and Use Committee at Memorial Sloan-Kettering Cancer Center.
Antibodies and inhibitors
We used well-described monoclonal antibodies against mouse interleukin-4 (IL-4; clone 11B11; 5 μg/g/dose; Bio-X-cell, West Lebanon, NH, USA) or rat antibody against mouse IL-13 (clone 38213; 5 μg/g; R&D Systems, Minneapolis, MN, USA) administered intraperitoneally (15, 16).Controls were treated with similar doses of isotype control antibodies (Bio-X-cell). To determine the role of IL-4 in the initiation of fibrosis, C57BL/6J mice were treated with IL-4 monoclonal antibody (mAb), IL-13mAb, or isotype control antibodies 24 h prior to, then weekly (IL-4mAb) or every 4 d (IL-13mAb) for 6 wk after surgery. To determine the role of IL-4 in progression of established lymphedema, wild-type mice underwent tail skin and lymphatic excision and were allowed to recover for 6 wk. Animals were then randomized to receive either IL-4mAb or control antibody weekly for 3 wk. A subset of this group was euthanized at this time point, while other animals were followed for an additional 3 wk after completion of treatment. A minimum of 6-8 animals was evaluated in each group.
Lymph node lymphangiogenesis was performed as described previously (17). Briefly, a mixture of 2% ovalbumin/complete Freund's adjuvant (OVA/CFA) was injected in the hind paw of adult female C57BL/6J mice. Animals were then immediately treated with IL-4mAb (5 μg/g weekly) or isotype antibody followed by weekly treatments for 2 wk, after which time popliteal lymph nodes were harvested and analyzed.
For blockade of Janus kinase 1/2 [JAK1/2; signal transducer and activator of transcription 3 (STAT3) inhibition] we used a well-described small molecule inhibitor of the JAK1/2 signaling (AZD1480, Astra-Zeneca Wilmington, DE, USA) administered at a dose of 60 mg/kg (in 0.5% HPMC/0.1% Tween 80) by daily oral gavage for 6 wk beginning immediately after surgery (18).
Evaluation of lymphatic function
Tail volumes were determined using the truncated cone formula, as previously reported (10). Subcutaneous tissue thickness was measured from the basal layer of epidermis to the underlying fascia of histological cross sections 10 mm distal to the surgical site by blinded reviewers at ×2 magnification in a minimum of 4 areas/animal (Mirax Imaging Software, Carl Zeiss, Munich, Germany).
Microlymphangiography was performed by injection of fluorescein isothiocyanate (FITC)-conjugated dextran (2,000 kDa, 10 mg/ml; Invitrogen, Carlsbad, CA, USA) using our previous methods (6). FITC-dextran was visualized 15 min following injection using the Lumar Stereoscope (Carl Zeiss, Peabody, MA, USA) and Metamorph imaging software (Molecular Devices, Sunnyvale, CA, USA), keeping exposure, gain, and magnification constant. Uptake of FITC-dextran in the proximal region of the tail was calculated and expressed as the ratio of average pixel intensity of regions proximal and distal to the surgical site.
Lymphoscintigraphy was performed by injecting 50 μl of technetium Tc 99m (99mTc) sulfur colloid in the distal tail (6). Peak lymph node uptake was calculated using X-SPECT camera (Gamma Medica, Northridge, CA, USA) and region-of-interest analysis performed to derive decay-adjusted activity using ASIPro software (CTI Molecular Imaging, Knoxville, TN, USA). Functional lymphatic vessel staining was performed by injecting tomato lectin (1 mg/ml; Sigma) 20 mm to the distal tip of the tail followed by euthanization 45 min later, as described previously (19).
Immunohistochemistry
Immunohistochemical staining was performed using our previous methods (6). Lymphatic vessels were identified using podoplanin or LYVE-1 antibodies (Abcam, Cambridge, MA, USA). Inflammatory cells were identified using antibodies against CD45 (R&D Systems, Minneapolis, MN, USA), CD4, CD8, IL-4, IL-13, interferon-γ (IFN-γ), and pSTAT3 (all from Abcam). Negative control sections were incubated with isotype control antibody or secondary antibody alone. Bright-field images were imaged with a Mirax slide scanner (Carl Zeiss, Jena, Germany).
Confocal microscopy for antigen colocalization [LYVE-1 and α-smooth muscle actin (SMA; Abcam), or CD4 and IFN-γ or IL-13] was performed as described previously and was visualized using the Nuance Multispectral Imaging System (LOT Oriel Group, Daimstadt, Germany; ref. 6). Specificity was confirmed using single-stained sections and negative controls. Images were captured using an Axioscope (Carl Zeiss; ref.6). Lymph node lymphatic vessel density was determined using Metamorph Offline software and expressed relative to node area (vessels/mm2), as described previously (20).
Fibrosis was assessed using our previously published methods to quantify Sirius Red staining and type I collagen immunohistochemistry (21). Sirius red scar index was calculated using polarized light microscopy to calculate the ratio of orange/red to yellow/green birefringence and analyzed using Metamorph Offline software. Type I collagen immunohistochemistry was performed using an antibody to mouse type I collagen (Abcam) and quantified as a ratio of the area of positively stained dermis and subcutaneous tissue within a fixed threshold to total tissue area/HPF.
Human lymphedema samples
Thirteen patients with postsurgical upper extremity lymphedema (grade I–III) were identified at the Stanford Center for Lymphatic and Venous Disorders. Full-thickness 5-mm skin punch biopsies were obtained from identical locations of the lymphedematous and contralateral normal limb and analyzed by immunohistochemistry for CD4, IL-4, and IL-13 expression. The number of positive cells was calculated in a minimum of 3 high-powered fields (HPF)/patient/limb by 2 reviewers using a Leica TCS microscope in a blinded procedure. The Institutional Review Boards of Stanford University and Memorial Sloan-Kettering Cancer Center approved all studies.
Flow cytometry
Skin and subcutaneous sections (1 cm) were digested with collagenase P (n=3–5 animals/group/time point). Cell suspensions were stained with fluorophore-conjugated antibodies to CD4 and TCRβ (Biolegend, San Diego, CA, USA) and analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and FlowJo software (Tree Star, Ashland, OR, USA).
Western blot analysis, ELISA
Protein was harvested from skin and subcutaneous tissues and quantified using the Bradford method (Bio-Rad, Hercules, CA, USA); Western blots were performed using our previous methods to quantify protein expression with all antibodies purchased from Abcam (13). Equal loading was confirmed with actin, and relative changes in band density was determined as a ratio of mean band density and was normalized to actin using ImageJ software (http://rsbweb.nih.gov/ij/). All experiments were performed in triplicate. For ELISA, serum was isolated from peripheral blood (n=3–5 animals/group) and analyzed according to the manufacturer's directions (eBioscience, San Diego, CA, USA).
Statistical analysis
The Student's t test was used to compare differences between 2 groups. Multigroup comparisons were performed using ANOVA with the Tukey-Kramer post hoc test. Analysis of clinical lymphedema samples was performed using the Wilcoxon matched-pair t test. Correlation between lymphedema grade and CD4+ infiltrate was evaluated by Spearman correlation. Data are presented as means ± sd unless otherwise noted, with P < 0.05 considered significant.
RESULTS
Sustained lymphatic stasis results in CD4+ cell inflammation
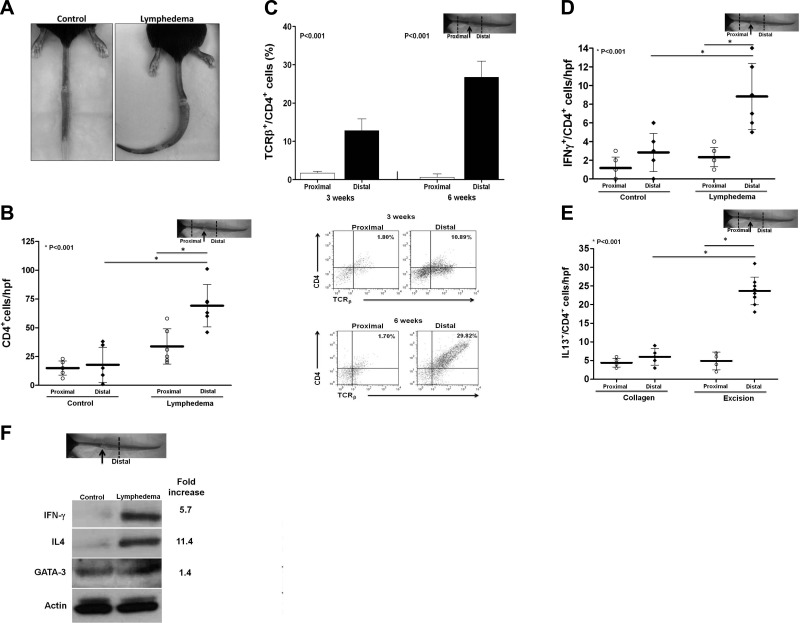
To determine how lymph stasis regulates inflammation, we used a mouse-tail model that enabled us to compare the effects of temporary (control) or sustained (lymphedema) lymphatic stasis (6, 10). As expected, at 6 wk after surgery, animals with lymphedema had significantly increased tail edema, decreased lymphatic function (10.3-fold decreased uptake of 99mTc), and increased CD45+ cell inflammation (4.3-fold) as compared with controls (Fig. 1A and Supplemental Fig. S1A, B). Gradients of lymphatic stasis comparing distal to proximal regions of the tail within the lymphedema group also demonstrated markedly increased infiltration by CD4+ cells (4-fold; Fig. 1B and Supplemental S1C, D). In fact, 70% of the CD45+ leukocytes were also CD4+ (Supplemental Fig. S1C). These findings were confirmed with flow cytometry, demonstrating a 7.5- and 38-fold increase, respectively, in percentage of mature T-helper cells 3 and 6 wk after surgery when comparing normal (proximal) and lymphedematous (distal) regions of the tail (Fig. 1C).
Figure 1.
Sustained lymphatic stasis results in CD4+ cell inflammation. A) Representative photograph demonstrating persistent tail lymphedema in an experimental mouse 6 wk postoperatively as compared with resolving edema in a control mouse. B) CD4+ cell counts proximal and distal to the wound in control and lymphedematous animals. C) Quantification (top panel) and representative flow cytometry analysis (bottom panel) demonstrating effects of gradients of lymph stasis on mature T-helper cell inflammation in mice with tail lymphedema 3 and 6 wk after surgery. Note accumulation of mature T-helper cells in the distal (lymphedematous) regions. D, E) Number of Th1 (D) and Th2 (E) cells in proximal and distal regions of control and lymphedematous mouse tails 6 wk after surgery. F) Representative Western blot and relative fold changes comparing protein expression in the distal regions of control and lymphedematous tails 6 wk after surgery.
Localization of Th1 (IFN-γ+/CD4+) and Th2 (IL-13+/CD4+, IL-4+, or GATA-3+) cells demonstrated a mixed Th1/Th2 inflammatory response to lymphedema (Fig. 1D, E and Supplemental Fig. S1E–H). These findings were supported by Western blot analysis, demonstrating increased Th1 and Th2 cytokine expression in lymphedematous tissues peaking 6 wk postoperatively (Fig. 1F and Supplemental Fig. S1H). Notably, although both groups demonstrated Th1 responses, Th2 inflammation and cytokine expression was primarily noted only in mice with lymphedema.
Lymph node dissection results in CD4+ cell inflammation
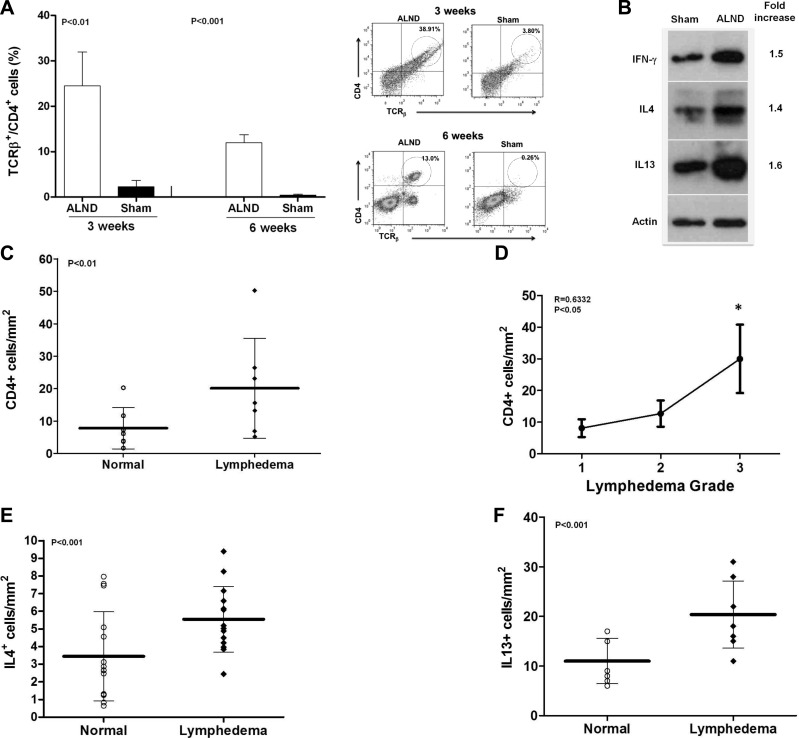
In support of our tail model findings, we found an 11- and 30-fold increase in mature CD4+ cells 3 and 6 wk postoperatively, respectively, when we compared mice that had undergone ALND with controls (Fig. 2A). Similarly, both Th1 and Th2 cytokines were up-regulated (1.4-1.6×) compared with controls (Fig. 2B).
Figure 2.
Lymph node dissection results in CD4+ cell inflammation. A) Quantification (left panel) and representative flow cytometry analysis (right panel) demonstrating accumulation of mature T-helper cells in upper extremity soft tissues after ALND 3 and 6 wk after surgery. B) Representative Western blot of protein harvested from the upper extremity 6 wk postoperatively with relative fold-increase comparing ALND to sham. C) Number of CD4+ cells in normal and lymphedematous upper extremities of lymphedema patients. D) Correlation of the number of CD4+ cells in the lymphedematous limb with severity of lymphedema. E, F) Number of IL-4+ cells/mm2 (E) and IL-13+ cells/mm2 (F) in normal and lymphedematous upper extremities of patients with lymphedema.
We confirmed these findings in patients with upper extremity lymphedema by comparing matched specimens taken from lymphedematous and normal arms. In this analysis, lymphedema was associated with a significant increase in the number of CD4+ cells (2.6-fold), the degree of CD4+ cell inflammation correlated with the severity of lymphedema (r=0.6332, P<0.05), and there were increased numbers of IL-4+ (1.5-fold) and IL-13+ cells (2.3-fold; Fig. 2C–F and Supplemental Fig. S1I).
CD4+ cells are necessary for fibrosis and lymphatic dysfunction
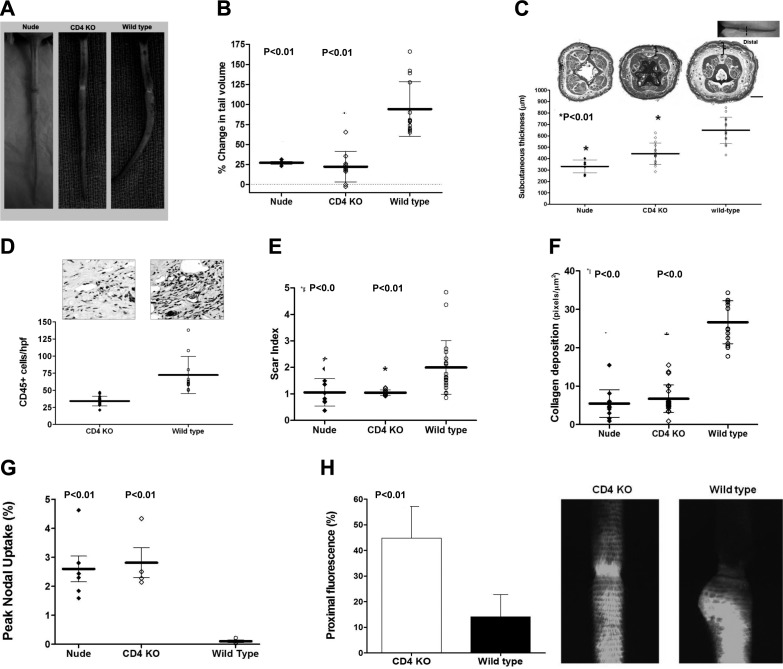
Using our tail model of lymphedema in nude, CD4KO, and wild-type mice, we found that loss of T-cell (nudes) or CD4+ cell inflammation significantly decreased tail lymphedema (3-to 4-fold), subcutaneous tissue thickness (1.9-fold nudes; 1.4-fold CD4KO), and CD45+ cell infiltration (2-fold; Fig. 3A–D). Nude and CD4KO mice also had significantly less tissue fibrosis (2-fold decreased scar index, 6-fold decreased type I collagen deposition) and markedly improved lymphatic function (3-fold increased 99mTc uptake; Fig. 3E--G). Using microlymphangiography, we detected lymphatic flow traversing the site of lymphatic incision resulting in a >4-fold increase in proximal tail fluorescence in CD4KO mice, as compared to controls (Fig. 3H).
Figure 3.
CD4+ cells are necessary for fibrosis and lymphatic dysfunction. A) Representative photographs of nude, CD4KO, and wild-type mouse-tails 6 wk after tail skin/lymphatic excision. Note fixed contracture (J shape) of wild-type mouse-tail. B) Tail volume changes (% change from preoperative) 6 wk after surgery. C) Subcutaneous tissue thickness and representative cross-sectional histology 6 wk after surgery. Gross photograph and site of tissue harvest (dashed line) are shown for orientation. Note markedly decreased subcutaneous adipose deposition (brackets) in nude and CD4KO mice. D) CD45+ cells/HPF in CD4KO and WT mouse tails 6 wk after tail skin and lymphatic excision. E, F) Scar index (E) and collagen deposition (F) of distal tail tissues 6 wk postoperatively. G) Peak nodal uptake of 99mTc injected in the distal tail 6 wk after surgery. H) Proximal tail fluorescence (left) and representative photographs (right) 6 wk after surgery, comparing CD4KO and wild-type mice.
Inhibition of Th2 differentiation decreases initiation and progression of fibrosis and improves lymphatic function
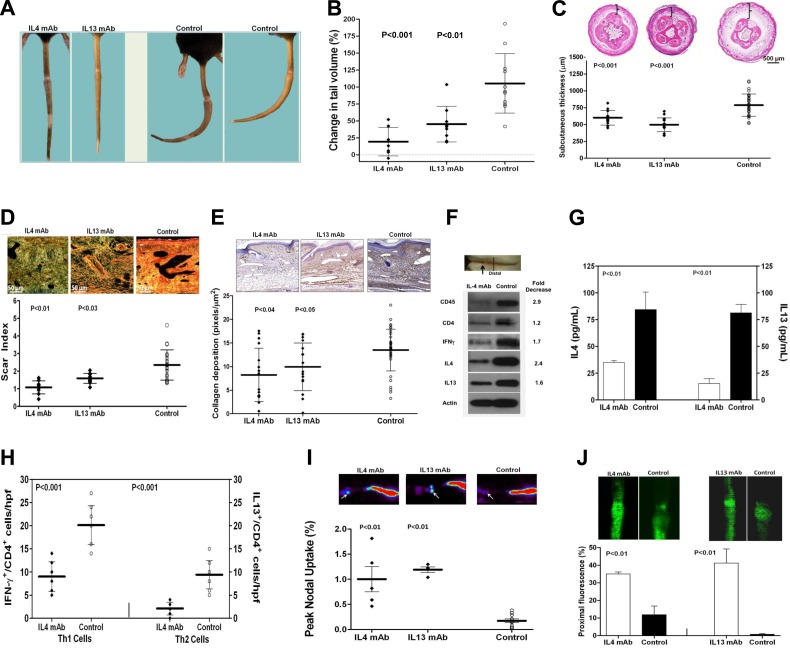
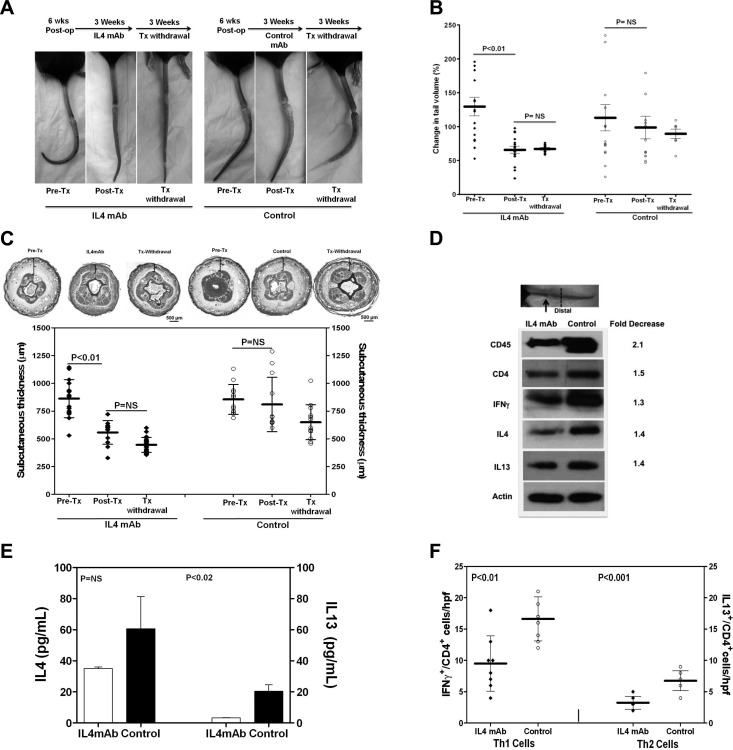
Because we found that the expression of Th2 cytokines, including IL-4 and IL-13, were significantly increased by lymphatic stasis, we next sought to determine whether Th2 differentiation regulates initiation of fibrosis in response to lymphatic stasis. To test this hypothesis, we treated wild-type mice with isotype control or monoclonal neutralizing antibodies against IL-4 or IL-13 (IL-4mAb or IL-13mAb) beginning immediately after tail skin and lymphatic excision. As expected, neutralization of IL-4 decreased IgE production, confirming that our treatment prevented Th2 differentiation since class switching to IgE is IL-4 dependent (P<0.02; not shown; ref. 22). Treatment with either IL-4mAb or IL-13mAb markedly decreased tail lymphedema as compared with controls (5- and 2.8-fold, respectively; Fig. 4A, B). Whereas control animals developed fixed tail contractures due to fibrosis (J configuration), the tails of IL-4mAb or IL-13mAb-treated animals remained pliable. Histologically, IL-4mAb or IL-13mAb-treated animals had significantly decreased subcutaneous edema and adipose deposition, scar index, and collagen deposition (Fig. 4C–E). IL-4 neutralization decreased tissue expression of CD45, CD4, IFN-γ, IL-4, and IL-13, as well as peripheral blood concentrations of IL-4 and IL-13 (Fig. 4F, G). These findings were corroborated by significantly decreased numbers of Th1 and Th2 cells in tail tissues from IL-4mAb-treated animals (2-fold; Fig. 4H). Decreased inflammation and fibrosis in IL-4mAb- or IL-13mAb-treated animals resulted in markedly improved lymphatic function (5–7-fold increased 99mTc uptake; 3.5–30-fold increase in relative proximal fluorescence; Fig. 4I, J).
Figure 4.
Inhibition of Th2 differentiation prevents initiation of fibrosis and improves lymphatic function. A) Representative photographs of tails from animals treated with IL-4mAb, IL-13mAb, or isotype control antibodies (control) for 6 wk beginning immediately after tail skin/lymphatic excision. Note lack of fibrotic contracture in the tails of IL-4/IL-13mAb-treated mice, as compared to fixed contracture of controls. B) Tail volume changes (% change from preoperative) 6 wk after surgery. C) Subcutaneous tissue thickness and representative cross-sectional histology 6 wk after surgery. Note marked decreased adipose deposition in IL-4mAb/IL-13mAb-treated mice (brackets). D) Scar index and representative histology (×40) of distal tail tissues 6 wk postoperatively. E) Collagen deposition and representative histology (×20) of distal tail tissues 6 wk postoperatively. F) Western blot analysis of distal tail tissues (arrow indicates wound; dotted line indicates harvest site) comparing IL-4mAb and control animals 6 wk after surgery. Fold decrease in expression in IL-4mAb animals relative to controls is shown. G) Serum IL-4 and IL-13 levels in animals treated with IL-4mAb or control antibody 6 wk after surgery. H) Number of Th1 (IFN-γ+/CD4+) and Th2 (IL-13+/CD4+) cells in distal tail tissues of IL-4mAb and control antibody-treated animals 6 wk postoperatively. I) Representative heat maps (top panels) and peak nodal uptake of 99mTc in sacral lymph nodes (white arrows) following distal tail injection 6 wk after surgery. J) Representative microlymphangiography and proximal tail fluorescence 6 wk after surgery.
To test the hypothesis that Th2 cytokines also regulate the progression of fibrosis and lymphatic dysfunction in established lymphedema, we performed tail skin and lymphatic excisions in wild-type mice and waited 6 wk for lymphedema to become established (Fig. 5A, B). At this point, animals were randomized into groups and treated either with IL-4mAb or isotype control antibodies for 3 wk, followed by a 3-wk period of treatment withdrawal.
Figure 5.
Inhibition of Th2 differentiation decreases established lymphedema. A–C) Representative photographs (A), tail volumes (B), and subcutaneous tissue thickness (C) of tails from animals 6 wk after skin excision and lymphatic disruption (pretx), following 3 wk treatment with IL-4/control antibodies (post-tx) beginning 6 wk after surgery, and following an additional 3-wk treatment withdrawal (post-tx withdrawal). D) Western blot of distal tail tissues from animals following 3-wk treatment with IL-4/control antibodies beginning 6 wk postoperatively (fold-decrease=normalized expression from IL-4mAb-treated animals relative to controls). E) Serum IL-4 and IL-13 levels in animals post-treatment with IL-4/control antibodies for 3 wk. F) Number of Th1/Th2 cells in distal tail tissues posttreatment with IL-4/control antibodies.
Measurement of tail volumes demonstrated significant improvements in lymphedema in animals treated with IL-4mAb (38% decrease), as compared with controls (Fig. 5A, B). Animals treated with IL-4mAb had almost complete resolution of tail lymphedema at the conclusion of the 3-wk treatment period with markedly decreased tissue fibrosis and tail contracture. In contrast, control animals had persistent tail lymphedema and tail fibrosis. These observations were confirmed by histological analysis demonstrating significantly decreased subcutaneous tissue thickness and adipose deposition in IL-4mAb-treated animals as compared with controls (40% reduction; Fig. 5C). Withdrawal of IL-4mAb did not result in recurrent fibrosis, tail swelling, or increased subcutaneous tissue thickness, although this analysis was somewhat weakened by the fact that control animals also had minor, though nonsignificant, spontaneous improvements in these measures by 12 wk postoperatively.
IL-4 neutralization markedly decreased tissue inflammation (1.4- to 2.1-fold decreased CD45, CD4, IFN-γ, IL-4, and IL-13 expression by Western blot) and serum levels of IL-4 (2-fold) and IL-13 (6-fold), although differences in free serum IL-4 concentrations as measured by ELISA did not quite reach statistical significance (Fig. 5D, E). It is possible that this lack of difference may reflect our inability to measure IL-4-neutralizing antibody complexes in the experimental animals. IL-4mAb treatment also significantly decreased the number of Th1 (1.7-fold) and Th2 (2.1-fold) cells in lymphedematous tissues (Fig. 5F).
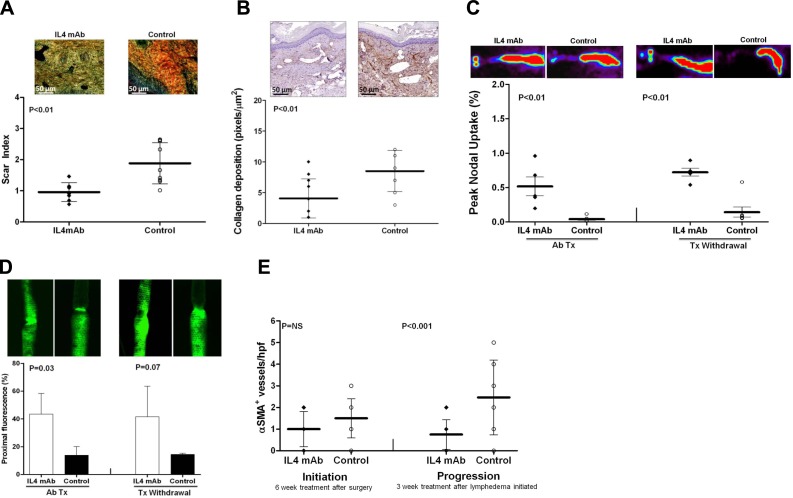
IL-4 blockade in animals with established lymphedema significantly decreased tissue fibrosis (2-fold decreased scar index and type I collagen deposition) and markedly improved lymphatic function (13-fold increased 99mTc uptake; 3.1-fold increased proximal lymphatic fluorescence; Fig. 6A–D). These improvements persisted, even after treatment withdrawal (5-fold increased 99mTc uptake; 2.9-fold increase proximal fluorescence).
Figure 6.
Inhibition of Th2 differentiation decreases established fibrosis and improves lymphatic function. Scar index (A; representative ×40 views) and collagen staining (B; representative ×20 views) of distal tail tissues posttreatment with IL-4/control antibodies. C) Representative heat maps (top panels) and peak nodal uptake of 99mTc in sacral lymph nodes following distal tail injection posttreatment (Ab Tx) and following treatment withdrawal (Tx withdrawal). D) Representative microlymphangiography and proximal tail fluorescence posttreatment (Ab Tx) and following treatment withdrawal (Tx withdrawal) with IL-4/control antibodies. E) Number of α-sma+ capillary lymphatic vessels/hpf.
We have previously observed that fibrosis in the mouse-tail either due to radiation or skin and lymphatic excision results in the expression of α-sma by capillary lymphatic vessels (an abnormal finding since capillary lymphatics lack pericyte coverage; ref. 10). Consistent with these observations, we found that IL-4 neutralization for 6 wk decreased the number of α-sma+ capillary lymphatics compared with controls; however, this difference did not reach statistical significance (Fig. 6E). In contrast, when we analyzed animals treated after lymphedema was established, we found significantly fewer α-sma+/LYVE-1+ capillary lymphatics (3.3-fold; Fig. 6E and Supplemental Fig. S2A). This finding correlated with our observation that IL-4mAb treatment resulted in decreased type I collagen deposition around capillary lymphatic vessels, suggesting that lymphatic and tissue fibrosis is decreased by IL-4mAb treatment (Supplemental Fig. S2B).
Inhibition of JAK1/2 does not prevent fibrosis or preserve lymphatic function
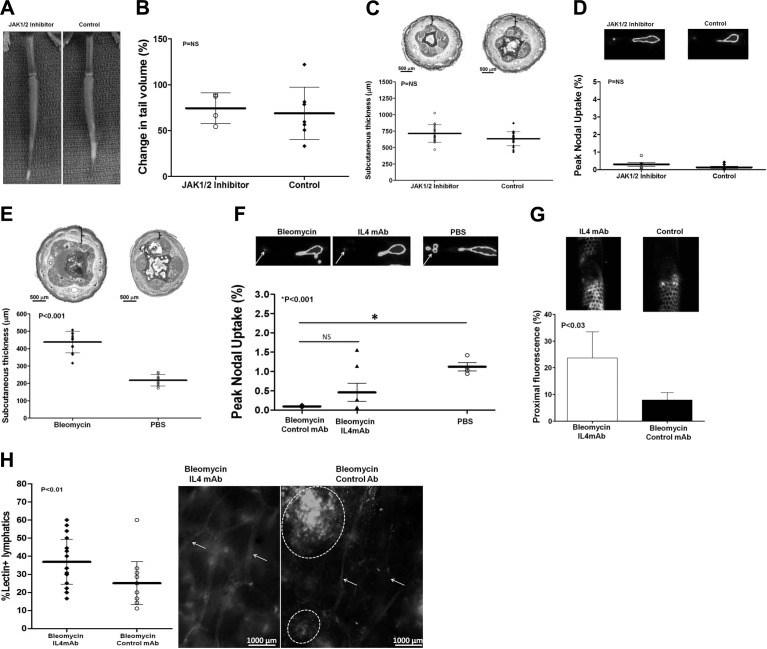
To test the hypothesis that inhibition of fibrosis is specific to Th2 differentiation rather than a generalized anti-inflammatory effect, we performed tail skin/lymphatic excision on wild-type mice followed by treatment with either an inhibitor of JAK1/2 (AZD1480) or vehicle control for 6 wk. We chose to block this pathway, since IL-4 and IL-13 signal through JAK3 and STAT6 rather than JAK1/2 and STAT3, and because JAK1/2 signaling by a variety of inflammatory cytokines, including IL-6, is an important regulator of acute and chronic inflammation (23). This is relevant to lymphedema, since we have noted that IL-6 expression is increased in our tail and ALND models (unpublished results), Karlsen et al. (24) have shown that IL-6 expression is significantly increased in lymphedematous tissues of mice with congenital lymphatic abnormalities, and Olszewski et al. (25) and Karlsen et al. (24) have shown that IL-6 is increased in lymphatic fluid of patients with lymphedema.
As expected, treatment with AZD1480 virtually completely blocked STAT3 phosphorylation (pSTAT3) by Western blot analysis and decreased the number of pSTAT3+ cells in lymphedematous tissues (Supplemental Fig. S3A, B). However, mice treated with AZD1480 had no gross or measureable decrease in tail swelling and no difference in subcutaneous tissue thickness and interstitial edema, as compared with controls (Fig. 7A–C). Notably, STAT3 inhibition did not decrease fibrosis or improve lymphatic function, suggesting that these outcomes are not related to generalized anti-inflammatory effects (Fig. 7D and Supplemental Fig. S3C, D). Taken together, these findings suggest that the pathological changes of lymphedema (i.e., fibrosis, adipose deposition, and lymphatic dysfunction) are not a consequence of STAT-3 activation. However, although we can hypothesize that STAT-6 activation by IL-4 or IL-13 is involved in this process, additional work is required to prove this hypothesis and is ongoing in our laboratory.
Figure 7.
Inhibition of JAK1/2 does not prevent fibrosis or preserve lymphatic function and fibrosis independently inhibits lymphatic function. A) Representative photographs of JAK1/2 inhibitor or control-treated mouse tails 6 wk postoperatively. B, C) Change in tail volume (B) and subcutaneous tissue thickness (C) in JAK1/2 inhibitor or control treated mice 6 wk postoperatively. D) Peak nodal uptake of 99mTc in JAK1/2 inhibitor or control-treated animals 6 wk postoperatively. E) Subcutaneous tissue thickness of animals treated with bleomycin or PBS. F, G) Peak nodal uptake of 99mTc by sacral lymph nodes (F) and microlymphangiography/proximal fluorescence (G) in animals treated with bleomycin, bleomycin and IL-4mAb, or PBS control. H) Quantification of lectin+ capillary lymphatics as a percentage of total lymphatics (left panel) and representative whole mount lectin stain (right panel) in bleomycin/IL-4mAb and bleomycin/control antibody-treated animals. White arrows indicate capillary lymphatics; dotted circles represent pooled areas of lectin in interstitial space.
Fibrosis independently inhibits lymphatic function
We have previously reported that inhibition of radiation-induced fibrosis significantly improves lymphatic transport, suggesting that fibrosis is an independent regulator of lymphatic function (26). To further test this hypothesis, we injected bleomycin intradermally into tails of wild-type mice to induce fibrosis without skin/lymphatic excision (27). Bleomycin injection resulted in severe tissue fibrosis, and this effect was associated with increased subcutaneous tissue thickness and severely impaired lymphatic function (12-fold decreased 99mTc; Fig. 7E, F). Inhibition of bleomycin-induced fibrosis with IL-4mAb improved lymphatic function (increased 99mTc uptake 5-fold, although, this difference did not quite reach statistical significance). However, we did note significantly improved lymphatic function using microlymphangiography and increased numbers of functional lymphatics as assessed by FITC-conjugated lectin uptake by lymphatic endothelial cells (1.5-fold increase; Fig. 7G, H). Using whole-mount staining, we found that IL-4mAb-treated animals had normal appearing, thin-walled lymphatics with interconnections (Fig. 7H). In contrast, control animals displayed distal pooling of injected lectin and markedly atrophic superficial lymphatic vessels lacking interconnections.
IL-4 blockade does not increase VEGF-A or VEGF-C expression
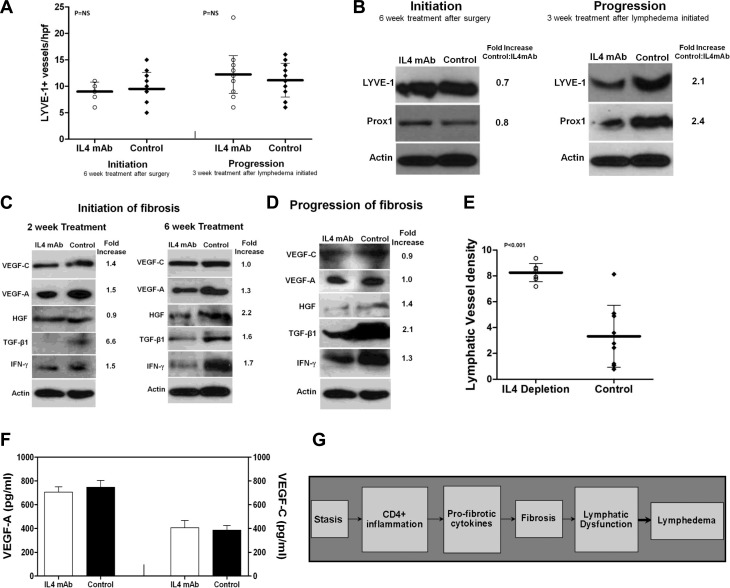
We have previously shown that lymphatic stasis regulates lymphatic function by regulating the ratio of prolymphangiogenic and antilymphangiogenic cytokines and that this effect is dependent on T-cell inflammation (17). Further, we and others have shown that inhibition of antilymphangiogenic cytokines, such as IFN-γ or transforming growth factor-β1 (TGF-β1), can increase lymphatic function independent of significant changes in lymphangiogenesis or VEGF-A/C expression (6, 10, 17, 28). Consistent with these observations, we found that treatment with IL-4mAb was not associated with significant changes in the number of LYVE-1+ or podoplanin+ lymphatic vessels or expression of lymphatic markers (LYVE-1 or PROX1) in the regions of the tail immediately distal to the wound (Fig. 8A, B). In fact, we noted a paradoxical decrease in LYVE-1 and PROX1 expression in IL-4mAb treated animals when they were treated after lymphedema had become established. However, consistent with our observations in microlymphangiography demonstrating improved flow of interstitial fluid across the wound, we found that IL-4mAb-treated animals more commonly exhibited lymphatic vessels that traversed the wound (Supplemental Fig. S4A).
Figure 8.
IL-4 blockade does not increase VEGF-A or VEGF-C expression. A) LYVE-1+ vessel counts in IL-4/control antibody-treated animals beginning immediately after surgery and continued for 6 wk (initiation) or beginning 6 wk after surgery and continued for 3 wk (progression). B) Representative Western blots (of triplicate experiments) for LYVE-1 and PROX1 expression in distal tail protein of animals treated with IL-4mAb/control antibody for 6 wk beginning immediately after surgery (left) or after treatment with IL-4mAb/control antibodies for 3 wk beginning 6 wk postoperatively (right). Normalized fold-increase in expression relative to controls is shown. C, D) Representative Western blots (of triplicate experiments) from distal tail protein isolated from animals treated with IL-4mAb/control antibody for 2 or 6 wk beginning immediately after surgery (C; initiation of fibrosis) or after treatment with IL-4/control antibodies for 3 wk beginning 6 wk after surgery (D; progression of fibrosis). Normalized fold-increase in expression relative to controls is shown. E) LYVE-1+ lymphatic vessel density of popliteal lymph nodes in animals treated with IL-4mAb/control antibodies 2 wk post-CFA/OVA injection. F) Expression of VEGF-A/C (by ELISA) in popliteal lymph nodes 2 wk post-CFA/OVA. G) Schema depicting proposed mechanism of lymphedema development.
Consistent with the lack of difference in lymphatic vessel counts, we found that protein expression of lymphangiogenic growth factors was little changed (VEGF-A and VEGF-C) or decreased (hepatocyte growth factor, HGF) by IL-4mAb treatment regardless of the time of treatment initiation (Fig. 8C, D). Notably, we did not note significant changes in the expression of VEGF-A, VEGF-C, or HGF when we analyzed tissue protein shortly after initiation of IL-4mAb treatment (2 wk) or even after prolonged treatment (6 wk). In contrast, the expression of antilymphangiogenic cytokines, such as IFN-γ or TGF-β1, was markedly decreased in IL-4mAb-treated animals at all time points examined. These findings together with our previous studies (17) suggest that inhibition of Th2 differentiation improves lymphatic function at least, in part, by inhibiting expression of antilymphangiogenic cytokines rather than directly increasing lymphangiogenesis by up-regulating expression of lymphangiogenic growth factors.
To determine how IL-4 regulates lymphangiogenesis in response to physiological inflammation, we analyzed the effects of IL-4 neutralization on popliteal lymph node lymphangiogenesis after CFA/OVA. Interestingly, in contrast to our tail model findings, we found that IL-4 neutralization significantly increased lymph node lymphatic vessel density 2 wk after CFA/OVA injection in the paw (Fig. 8E and Supplemental Fig. S4B). However, consistent with our tail model, IL-4mAb treatment did not increase VEGF-A or VEGF-C protein expression in the lymph node (Fig. 8F).
DISCUSSION
Our findings support the hypothesis that the pathophysiology of lymphedema is related to progressive lymphatic dysfunction occurring as a consequence of a CD4+ inflammation, Th2 differentiation, and fibrosis. The importance of T-cell inflammation in this sequence is highlighted by the observation that CD4+ inflammatory responses are a key difference between temporary and sustained lymphatic stasis, as well as the fact that loss of T cells or CD4+ cells markedly decreased inflammation, decreased fibrosis, and improved lymphatic function. The conclusion that lymphatic stasis initiates pathological changes by inducing inflammation is also consistent with previous studies demonstrating that lymphatic stasis potently induces the expression of endogenous danger signals (20) and up-regulates the expression of inflammation and fibrosis regulatory genes both clinically and in the mouse tail model (29); lymphedema is clinically associated with mononuclear cell tissue inflammation (3) and increased numbers of T lymphocytes in the lymphatic fluid (30); and lymphedema-associated inflammation is markedly decreased by decongestive therapy and resultant decreased limb swelling (31).
Our study also suggests that fibrosis is a critical step in the pathological sequence of lymphedema and that this response is dependent on Th2 differentiation. This hypothesis is supported by our observations that inhibition of T-cell inflammation or Th2 differentiation but not generalized inflammation (JAK1/2/STAT3 inhibitor) markedly decreases initiation and progression of fibrosis and improves lymphatic function; bleomycin-induced fibrosis independently impairs lymphatic function, and this effect is partially mitigated by IL-4mAb; and sustained lymphatic stasis is associated with fibrosis of lymphatic capillaries. The fibrosis hypothesis is also supported by our previous studies demonstrating that inhibition of fibrosis resulting from lymphatic vessel ligation or radiation using therapies aimed at the profibrotic growth factor TGF-β1 markedly improves lymphatic function, decreases T-cell inflammation, and markedly down-regulates IL-4 and IL-13 expression, suggesting that TGF-β1 and Th2 responses have independent and interactive roles in this process (6, 21, 26). Our results are also consistent with the fact that bleomycin-induced lung fibrosis is attenuated in nude mice (32) or mice depleted of CD4+ T cells (33). The finding that the composition of the extracellular matrix (ECM) can directly regulate interstitial fluid flow by altering the hydraulic conductivity of the skin (34), possibly as a consequence of changes in the compliance of lymphatic vessels (35) and their connection to the ECM by anchoring filaments (36), also supports the concept that fibrosis or mechanical changes in the ECM can regulate lymphatic function. This hypothesis is further directly supported by clinical anatomic studies demonstrating encasement of lymphatics in fibrotic shells in patients with chronic lymphedema (3, 4). On the basis of these findings, we hypothesize that lymphedema develops in a subset of patients as a consequence of cumulative loss of lymphatic function, resulting from lymphatic injury in combination with progressive fibrosis-induced lymphatic dysfunction (Fig. 8G). In other patients, lymphedema does not develop because of spontaneous resolution of lymphatic stasis (i.e., regeneration or lymphatic bypass channels; ref. 7) or inhibition of fibrosis resulting from other variables.
The finding that targeted blockade of the Th2 response can prevent initiation/progression of fibrosis and improve lymphatic function is important and clinically relevant since it suggests for the first time that inhibiting tissue changes that occur in response to stagnating lymphatic fluid can be a means of preventing or treating lymphedema. These findings are consistent with other fibrotic disorders involving the lung, liver, skin, and radiation-induced fibrosis demonstrating that inhibition of Th2 cytokines can decrease ECM deposition and preserve functional tissue parenchyma (37). The fact that targeting Th2 inflammation can markedly improve lymphatic function is important since broad-spectrum anti-inflammatory treatments (e.g., corticosteroids) are not clinically applicable, as these interventions have significant deleterious effects on wound healing and other biological processes. In fact, Rockson et al. (38) have shown that targeted inhibition of TNF-α, a cytokine with critical roles in acute inflammatory reactions, paradoxically results in increased inflammation and worsening lymphedema in the mouse-tail, suggesting that some inflammation is necessary for wound healing and lymphangiogenesis to occur.
Although it is clear that VEGF-C is necessary for lymphatic regeneration in most settings, it is also clear that this stimulus is not sufficient to restore lymphatic function in the context of lymphatic stasis, suggesting that other stimuli actively or passively inhibit this process (39, 40). These findings are consistent with previous reports from our laboratory and others demonstrating that inhibition of antilymphangiogenic cytokines can augment lymphatic function even in the context of normal or decreased VEGF-C expression (6, 10, 17, 28, 41). Similarly, in the current study, we noted that IL-4 neutralization improved lymphatic function in the mouse-tail and increased lymph node lymphangiogenesis without significantly changing the expression of lymphangiogenic cytokines. This is critical since previous experimental strategies for treating lymphedema have focused on repairing surgically damaged lymphatics using lymphangiogenic cytokines (e.g., VEGF-C; ref. 19); however, these approaches are contraindicated in most cancer patients since these molecules also potently promote tumor growth/metastasis (42). Therefore, the use of targeted antifibrotic strategies may enable treatment of cancer survivors without increasing oncologic risks.
Supplementary Material
Acknowledgments
The authors thank Ronald DeMatteo (Memorial Sloan-Kettering Cancer Center) for his helpful advice regarding planning and execution of the experiments.
This work was funded, in part, by the Plastic Surgery Educational Foundation and The Society of Memorial Sloan-Kettering Cancer Center grants to B.J.M. T.A. was funded in part by a fellowship grant from the Plastic Surgery Educational Foundation. T.A., J.Z., and E.W. were also supported by T32 Surgical Oncology Training Grant NIH T32 CA 009501. J.Z. was also partially supported from a grant (BC103691) from the U.S. Department of Defense. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the U.S. National Institutes of Health (NIH) under award R01HL111130.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported, in part, by NIH Small-Animal Imaging Research Program (SAIRP) grant R24 CA83084 and NIH Center grant P30 CA08748, are gratefully acknowledged.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ALND
- axillary lymph node dissection
- CD4KO
- CD4 knockout
- FITC
- fluorescein isothiocyanate
- IFN-γ
- interferon-γ
- IL-4
- interleukin-4
- IL-13
- interleukin-13
- JAK
- Janus kinase
- mAb
- monoclonal antibody
- OVA/CFA
- ovalbumin/complete Freund's adjuvant
- PBS
- phosphate-buffered saline
- STAT
- signal transducer and activator of transcription
- TGF-B1
- transforming growth factor-β1
- Th1
- T-helper 1
- Th2
- T-helper 2
REFERENCES
- 1. Pyszel A., Malyszczak K., Pyszel K., Andrzejak R., Szuba A. (2006) Disability, psychological distress and quality of life in breast cancer survivors with arm lymphedema. Lymphology 39, 185–192 [PubMed] [Google Scholar]
- 2. Rockson S. G., Rivera K. K. (2008) Estimating the population burden of lymphedema. Ann. N. Y. Acad. Sci. 1131, 147–154 [DOI] [PubMed] [Google Scholar]
- 3. Olszewski W. L., Jamal S., Manokaran G., Lukomska B., Kubicka U. (1993) Skin changes in filarial and non-filarial lymphoedema of the lower extremities. Trop. Med. Parasitol. 44, 40–44 [PubMed] [Google Scholar]
- 4. Suami H., Pan W. R., Taylor G. I. (2007) Changes in the lymph structure of the upper limb after axillary dissection: Radiographic and anatomical study in a human cadaver. Plast. Reconstr. Surg. 120, 982–991 [DOI] [PubMed] [Google Scholar]
- 5. Petrek J. A., Heelan M. C. (1998) Incidence of breast carcinoma-related lymphedema. Cancer 83, 2776–2781 [DOI] [PubMed] [Google Scholar]
- 6. Avraham T., Daluvoy S., Zampell J., Yan A., Haviv Y. S., Rockson S. G., Mehrara B. J. (2010) Blockade of transforming growth factor-β1 accelerates lymphatic regeneration during wound repair. Am. J. Pathol. 177, 3202–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wynn T. A. (2008) Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hinrichs C. S., Watroba N. L., Rezaishiraz H., Giese W., Hurd T., Fassl K. A., Edge S. B. (2004) Lymphedema secondary to postmastectomy radiation: Incidence and risk factors. Ann. Surg. Oncol. 11, 573–580 [DOI] [PubMed] [Google Scholar]
- 9. Beesley V., Janda M., Eakin E., Obermair A., Battistutta D. (2007) Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer 109, 2607–2614 [DOI] [PubMed] [Google Scholar]
- 10. Clavin N. W., Avraham T., Fernandez J., Daluvoy S. V., Soares M. A., Chaudhry A., Mehrara B. J. (2008) Tgf-β1 is a negative regulator of lymphatic regeneration during wound repair. Am. J. Physiol. Heart Circ. Physiol. 295, H2113–H2127 [DOI] [PubMed] [Google Scholar]
- 11. Zampell J., Elhadad S., Avraham T., Weitman E., Aschen S., Yan A., Mehrara B. J. (2012) Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am. J. Physiol. Cell Physiol. 302, C709–C719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldman J., Le T. X., Skobe M., Swartz M. A. (2005) Overexpression of vegf-c causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ. Res. 96, 1193–1199 [DOI] [PubMed] [Google Scholar]
- 13. Zampell J. C., Yan A., Avraham T., Daluvoy S., Weitman E. S., Mehrara B. J. (2012) Hif-1α coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J. 26, 1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castelino F. V., Seiders J., Bain G., Brooks S. F., King C. D., Swaney J. S., Lorrain D. S., Chun J., Luster A. D., Tager A. M. (2011) Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 63, 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romani L., Mencacci A., Grohmann U., Mocci S., Mosci P., Puccetti P., Bistoni F. (1992) Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J. Exp. Med. 176, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang G., Volk A., Petley T., Emmell E., Giles-Komar J., Shang X., Li J., Das A. M., Shealy D., Griswold D. E., Li L. (2004) Anti-il-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine 28, 224–232 [DOI] [PubMed] [Google Scholar]
- 17. Zampell J. C., Avraham T., Yoder N., Fort N., Yan A., Weitman E. S., Mehrara B. J. (2012) Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti-lymphangiogenic cytokines. Am. J. Physiol. Cell Physiol. 302, C392–C404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mankan A. K., Greten F. R. (2011) Inhibiting signal transducer and activator of transcription 3. Rationality and rationale design of inhibitors. Expert Opin. Investig. Drugs 20, 1263–1275 [DOI] [PubMed] [Google Scholar]
- 19. Tammela T., Saaristo A., Holopainen T., Lyytikka J., Kotronen A., Pitkonen M., Abo-Ramadan U., Yla-Herttuala S., Petrova T. V., Alitalo K. (2007) Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat. Med. 13, 1458–1466 [DOI] [PubMed] [Google Scholar]
- 20. Zampell J. C., Yan A., Avraham T., Andrade V., Malliaris S., Aschen S., Rockson S. G., Mehrara B. J. (2011) Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am. J. Physiol. Cell Physiol. 300, C1107–C1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Avraham T., Clavin N. W., Daluvoy S. V., Fernandez J., Soares M. A., Cordeiro A. P., Mehrara B. J. (2009) Fibrosis is a key inhibitor of lymphatic regeneration. Plast. Reconstr. Surg. 124, 438–450 [DOI] [PubMed] [Google Scholar]
- 22. Poulsen L. K., Hummelshoj L. (2007) Triggers of ige class switching and allergy development. Ann. Med. 39, 440–456 [DOI] [PubMed] [Google Scholar]
- 23. Neurath M. F., Finotto S. (2011) Il-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 22, 83–89 [DOI] [PubMed] [Google Scholar]
- 24. Karlsen T. V., Karkkainen M. J., Alitalo K., Wiig H. (2006) Transcapillary fluid balance consequences of missing initial lymphatics studied in a mouse model of primary lymphoedema. J. Physiol. 574, 583–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olszewski W. L., Jamal S., Lukomska B., Manokaran G., Grzelak I. (1992) Immune proteins in peripheral tissue fluid-lymph in patients with filarial lymphedema of the lower limbs. Lymphology 25, 166–171 [PubMed] [Google Scholar]
- 26. Avraham T., Yan A., Zampell J. C., Daluvoy S. V., Haimovitz-Friedman A., Cordeiro A. P., Mehrara B. J. (2010) Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-β1-mediated tissue fibrosis. Am. J. Physiol. Cell Physiol. 299, C589–C605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshizaki A., Iwata Y., Komura K., Ogawa F., Hara T., Muroi E., Takenaka M., Shimizu K., Hasegawa M., Fujimoto M., Tedder T. F., Sato S. (2008) Cd19 regulates skin and lung fibrosis via toll-like receptor signaling in a model of bleomycin-induced scleroderma. Am. J. Pathol. 172, 1650–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kataru R. P., Kim H., Jang C., Choi D. K., Koh B. I., Kim M., Gollamudi S., Kim Y. K., Lee S. H., Koh G. Y. (2011) T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34, 96–107 [DOI] [PubMed] [Google Scholar]
- 29. Tabibiazar R., Cheung L., Han J., Swanson J., Beilhack A., An A., Dadras S. S., Rockson N., Joshi S., Wagner R., Rockson S. G. (2006) Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 3, e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galkowska H., Olszewski W. L. (1986) Cellular composition of lymph in experimental lymphedema. Lymphology 19, 139–145 [PubMed] [Google Scholar]
- 31. Foldi E., Sauerwald A., Hennig B. (2000) Effect of complex decongestive physiotherapy on gene expression for the inflammatory response in peripheral lymphedema. Lymphology 33, 19–23 [PubMed] [Google Scholar]
- 32. Schrier D. J., Phan S. H., McGarry B. M. (1983) The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. A biochemical evaluation. Am. Rev. Respir. Dis. 127, 614–617 [DOI] [PubMed] [Google Scholar]
- 33. Xu J., Mora A. L., LaVoy J., Brigham K. L., Rojas M. (2006) Increased bleomycin-induced lung injury in mice deficient in the transcription factor t-bet. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L658–L667 [DOI] [PubMed] [Google Scholar]
- 34. Bennuru S., Maldarelli G., Kumaraswami V., Klion A. D., Nutman T. B. (2010) Elevated levels of plasma angiogenic factors are associated with human lymphatic filarial infections. Am. J. Trop. Med. Hyg. 83, 884–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rutkowski J. M., Markhus C. E., Gyenge C. C., Alitalo K., Wiig H., Swartz M. A. (2010) Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am. J. Pathol. 176, 1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rossi A., Weber E., Sacchi G., Maestrini D., Di Cintio F., Gerli R. (2007) Mechanotransduction in lymphatic endothelial cells. Lymphology 40, 102–113 [PubMed] [Google Scholar]
- 37. Aboul-Enein A, Eshmawy I, Arafa SA A. (1984) The role of lymphovenous communication in the development of post mastectomy lymphedema. Surgery 95, 562. [PubMed] [Google Scholar]
- 38. Nakamura K., Radhakrishnan K., Wong Y. M., Rockson S. G. (2009) Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS One 4, e8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldman j, Rutkowski J., Shields J., Pasquier M., Cui Y., Schmökel H., Willey S., Hicklin D., Pytowski B., Swartz M. (2007) Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 21, 1003–1012 [DOI] [PubMed] [Google Scholar]
- 40. Rutkowski J. M., Moya M., Johannes J., Goldman J., Swartz M. A. (2006) Secondary lymphedema in the mouse-tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc. Res. 72, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oka M., Iwata C., Suzuki H. I., Kiyono K., Morishita Y., Watabe T., Komuro A., Kano M. R., Miyazono K. (2008) Inhibition of endogenous TGF-β signaling enhances lymphangiogenesis. Blood 111, 4571–4579 [DOI] [PubMed] [Google Scholar]
- 42. Gu Y., Qi X., Guo S. (2008) Lymphangiogenesis induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome in breast carcinoma: A retrospective study of 61 cases. Clin. Exp. Metastasis 25, 717–725 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.