Abstract
Genetic variants in the fatty acid (FA) translocase FAT/CD36 associate with abnormal postprandial lipids and influence risk for the metabolic syndrome. CD36 is abundant on apical enterocyte membranes in the proximal small intestine, where it facilitates FA uptake and FA-initiated signaling. We explored whether CD36 signaling influences FA-mediated secretion of cholecystokinin (CCK) and secretin, peptides released by enteroendocrine cells (EECs) in the duodenum/jejunum, which regulate events important for fat digestion and homeostasis. CD36 was immunodetected on apical membranes of secretin- and CCK-positive EECs and colocalized with cytosolic granules. Intragastric lipid administration to CD36−/− mice released less secretin (−60%) and CCK (−50%) compared with wild-type mice. Likewise, diminished secretin and CCK responses to FA were observed with CD36−/− intestinal segments in vitro, arguing against influence of alterations in fat absorption. Signaling mechanisms underlying peptide release were examined in STC-1 cells stably expressing human CD36 or a signaling-impaired mutant (CD36K/A). FA stimulation of cells expressing CD36 (vs. vector or CD36K/A) released more secretin (3.5- to 4-fold) and CCK (2- to 3-fold), generated more cAMP (2- to 2.5-fold), and enhanced protein kinase A activation. Protein kinase A inhibition (H-89) blunted secretin (80%) but not CCK release, which was reduced (50%) by blocking of calmodulin kinase II (KN-62). Coculture of STC-1 cells with Caco-2 cells stably expressing CD36 did not alter secretin or CCK release, consistent with a minimal effect of adjacent enterocytes. In summary, CD36 is a major mediator of FA-induced release of CCK and secretin. These peptides contribute to the role of CD36 in fat absorption and to its pleiotropic metabolic effects.—Sundaresan, S., Shahid, R., Riehl, T. E., Chandra, R., Nassir, F., Stenson, W. F., Liddle, R. A., Abumrad, N. A. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin.
Keywords: CCK, cAMP, calcium
Dietary fatty acids (FAs) trigger release of a number of peptides, including cholecystokinin (CCK) and secretin, by enteroendocrine cells (EECs) (1, 2). CCK is secreted by I cells (3, 4) and secretin by S cells (5–7), localized primarily to the duodenum and jejunum. Both peptides influence fat absorption and participate in nutrient sensing and regulation of energy balance (1, 8, 9). CCK regulates gallbladder contraction, secretions by the stomach and acinar pancreas, and colonic motility (10). Secretin plays an important role in buffering the acidic chyme in the intestinal lumen by stimulating water and bicarbonate secretion and enhances CCK effects on the acinar pancreas and gallbladder (11). In addition, both CCK and secretin have prosatiety effects (12, 13).
A number of cellular surface receptors for FA, including the family of G-protein-coupled receptors (GPRs) and the scavenger receptor CD36, have been identified. Long-chain FAs are recognized by GPR120, GPR40, and CD36 (14–16). CD36 facilitates cellular FA uptake (14) and, like the GPRs (17, 18), mediates FA-induced signal transduction, influencing cellular calcium for release of neurotransmitters (19) and arachidonic acid and prostaglandins (20). In taste cells, FA sensing by CD36 mediates fat perception and preference (21, 22) and induces the cephalic phase of digestion (22). In humans, oral FA detection thresholds are higher in subjects carrying single-nucleotide polymorphisms (SNPs) that reduce CD36 levels (23).
CD36 and the GPRs have distinct distribution patterns in the intestine. CD36 is abundant in the proximal small intestine (24, 25) on villi enterocytes, where it contributes to FA and cholesterol uptake (25) and to signaling events important for initiating chylomicron formation (26–28). The GPRs (GPR120 and GPR40) are more abundant in distal segments, localize primarily to EECs, and were shown to mediate FA-induced peptide release (17, 29). The signaling pathways involved in peptide release involve cAMP and protein kinase A (PKA) as well as PKA-independent mechanisms (30–32). Based on its ability for FA sensing and signal transduction, CD36 could contribute to the regulation of EEC peptide release. However, this possibility had been unexplored and was examined in the present work. Using CD36−/− and wild-type (WT) mice, we documented in vivo and in vitro, using intestinal segments, robust effects of CD36 on FA-induced release of secretin and CCK. In studies using STC-1 cells, we examined the mechanisms underlying CD36-mediated effects and demonstrated involvement of cAMP and calcium.
MATERIALS AND METHODS
Materials
Antibodies used are listed in Table 1. FA-free bovine serum albumin (BSA) fraction IV, PKA inhibitor H-89 dihydrochloride (N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide), cAMP inhibitor MDL-12,330A hydrochloride [cis-N-(2-phenylcyclopentyl)-azacyclotridec-1-en-2-amine], and calmodulin kinase inhibitor II (CaM-KII) KN-62 were from Sigma-Aldrich (St. Louis, MO, USA).
Table 1.
Antibodies
| Antibodies | Host | Source | Dilution |
|---|---|---|---|
| CD36 | Goat | R&D Systems (Minneapolis, MN, USA) | 1:100 |
| β-Actin | Mouse | Santa Cruz Biotechnology (Santa Cruz, CA, USA) | 1:10,000 |
| Chromogranin A | Rabbit | Abcam (Cambridge, MA, USA | 1:200 |
| GFP | Chicken | Abcam | 1:1000 |
| Phospho-PKA substrates (RRXS*/T*) | Rabbit | Cell Signaling Technology (Danvers, MA, USA) | 1:1000 |
| PKA C-α | Rabbit | Cell Signaling Technology | 1:1000 |
| Ran | Goat | Santa Cruz Biotechnology | 1:10,000 |
| Secretin | Rabbit | Phoenix Pharmaceuticals (Belmont, CA, USA) | 1:1000 |
Animals
CD36-null (CD36−/−) and WT mice on the C57BL/6 background were housed in a facility with a 12-h light-dark cycle and fed chow ad libitum (Purina, St. Louis, MO, USA). Female mice, 3–4 mo old, were denied access to food for 16 h before euthanasia. Mouse care and use followed guidelines of the animal ethics committee of Washington University School of Medicine (St. Louis, MO, USA). Transgenic CCK-green fluorescent protein (GFP) mice, used for CCK immunohistochemistry, were developed by the Mutant Mouse Regional Resource Center (University of Missouri, St. Louis, MO, USA). In these mice, GFP expression is driven by the CCK promoter, allowing detection of CCK-positive cells by immunofluorescence (33). Animals were denied access to food for 16 h with ad libitum access to water before the small intestine was harvested.
Evaluation of gastric emptying
Gastric emptying was measured as described previously (34). In brief, 1 ml of phenol red (100 μg/ml) was administered orally to mice after overnight food withdrawal, and stomachs were collected 15 min later. The residual phenol (A) was recovered by rinsing with 10 ml of Na2HPO4 solution (0.1 M), and aliquots were diluted 1.5-fold (S1) and 5-fold (S2) before measurement of absorbance at 570 nm (BioTek Instruments, Winooski, VT, USA). The concentration was obtained from a calibration curve. Dye recovered from stomachs of mice sacrificed immediately after dye administration served as controls (B). Percentage of gastric emptying was calculated as 100 − (A/B) × 100.
Immunohistochemistry
Mice were administered a mixture of 5-bromo-2′-deoxyuridine (120 mg/kg) and 5-fluoro-2′-deoxyuridine (12 mg/kg) intraperitoneally 90 min before sacrifice to label S-phase cells. Proximal and distal small intestines were opened longitudinally, fixed in 10% formalin, and paraffin embedded. Cut sections (5 μm) were deparaffinized, followed by antigen retrieval (99°C, 18 min) in a pressurized chamber (Biocare Medical, Concord, CA, USA). Sections were incubated (1 h) in donkey serum (2%) and BSA (3%) to block nonspecific binding and then were incubated overnight (4°C) with primary antibodies, followed by fluorescently labeled (Alexa Fluor) secondary antibodies (1 h, 1:200). Images were taken using a Zeiss inverted fluorescent microscope (Carl Zeiss Inc., Thornwood, NY, USA). For colocalization, doubly positive cells at ×400 were counted for 5 mice/antigen. For CCK, immunohistochemical analysis was performed as described previously (33).
Secretin and CCK release in vivo
After overnight food withdrawal, mice were administered an intragastric load of olive oil or saline (16 μl/g body weight), and peptide release into the blood was measured 30 min later. CCK and secretin were extracted on C18 columns (Waters, Milford, MA, USA) and quantified. Plasma CCK was measured by bioassay using amylase secretion from isolated rat pancreatic acini (35). Plasma from 3 mice was pooled to obtain adequate sensitivity (36). Secretin was measured by enzyme-linked immunosorbent assay (ELISA; Phoenix Pharmaceuticals, Belmont, CA, USA).
Secretion by proximal intestinal loops
The proximal small intestine isolated from WT and CD36−/− mice after 16 h of food withdrawal was cut into two 10-cm segments, starting at 2 cm after the pylorus. The pieces were filled with Hanks' balanced saline solution (HBSS) with or without 100 or 300 μM linoleic acid (LA; plus protease and phosphatase inhibitors), tied at both ends, and incubated for 1 h at 37°C. The media were collected, lyophilized, rehydrated with 500 μl, and assayed. Secretin was measured by ELISA, and CCK was quantified by radioimmunoassay (RIA) using 125I-labeled CCK-8 and antisera 92128, which recognize biologically active CCK (37).
STC-1 and Caco-2 cells
The mouse EEC line (STC-1) was cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) with 20% fetal bovine serum (FBS). STC-1 cells stably expressing human WT or mutated CD36 (CD36K/A) with C-terminal lysines 469 and 472 substituted by alanine (38) were generated by electroporation (Nucleofector Kit V; Lonza, Cologne, Germany) followed by selection in gentamicin (400 μg/ml). Caco-2 cells expressing WT CD36 or the mutated form CD36K/A were generated as described for STC-1 cells and maintained in DMEM with 20% FBS.
Cocultures of enterocytes (Caco-2 cells) with EECs (STC-1 cells) were established by serial seeding. First, 3.0 × 106 differentiated Caco-2 cells expressing CD36 or empty vector were seeded in DMEM containing 10% FBS. Twelve hours later, 1 ml of culture medium containing 0.3 × 106 STC-1 cells, with or without CD36 expression, was added to the dish. A 1:10 ratio of STC-1 cells to Caco-2 cells was chosen to simulate the relative physiological distribution. One day later, the mixed culture was washed, serum starved for 8 h, and incubated (60 min, 37°C) in HBSS (with Ca2+ and Mg2+) with 50 and 200 μM LA (plus 5 μM BSA) or with BSA alone (controls). Secretin and CCK release was determined by ELISA and RIA (37), respectively.
Peptide release by STC-1 cells
Cells (1×106) in 6-well plates were serum starved in DMEM and then were stimulated (1 h) by addition of 50–200 μM FA; LA, docosahexaenoic acid (DHA), oleic acid (OA), and palmitic acid (PA) complexed to 5 μM BSA were added in HBSS (no glucose) containing aprotinin (200 kallikrein-inhibiting units/ml). Media were collected for secretin analyses by ELISA. Cells were lysed for protein assays in buffer [20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM Na2EDTA; 1 mM EGTA; 1% Triton X-100; 2.5 mM sodium pyrophosphate; 1 mM glycerol 2-phosphate; 1 mM Na3VO4; 1 μg/ml leupeptin; and 1 mM phenylmethanesulfonyl fluoride (PMSF)] containing a protease inhibitor mix (Roche Diagnostics, Inc., Indianapolis, IN, USA). CCK release was measured by RIA (37).
Measurement of cAMP, PKA activation, and intracellular Ca2+
Intracellular cAMP was measured using the DetectX Direct High Sensitivity Cyclic AMP Chemiluminescent Immunoassay Kit (Arbor Assays, Ann Arbor, MI, USA). In brief, serum-starved STC-1 cells (5×105/well) were stimulated with FA (100 μM) complexed with BSA (5 μM) or BSA alone (30 min, 37°C) in HBSS containing 1 mM 3-isobutyl-1-methylxanthine (IBMX). Cellular protein was determined in lysates from duplicate wells (DC Protein Assay; Bio-Rad Laboratories, Hercules, CA, USA). PKA activation was determined from phosphorylation of PKA substrates (with the RRXS/T motif) and nuclear content of the catalytic PKA subunit PKA-Cα. Intracellular calcium was measured as described previously (20), using Fura-2/AM dye (2.5 μM).
Western blot analyses
Protein signals were detected using the Odyssey Infrared System (Li-Cor Biosciences, Lincoln, NE, USA) as described previously (20). Cell proteins separated and transferred to polyvinylidene fluoride membranes were blocked and incubated overnight with primary antibodies (4°C) and then for 1 h with infrared dye-labeled secondary antibodies (room temperature).
Statistical analyses
Statistical analyses were performed with GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA, USA). Differences obtained by 1-way ANOVA were considered significant at values of P ≤ 0.05. The Bonferroni test was performed to identify groups that were different.
RESULTS
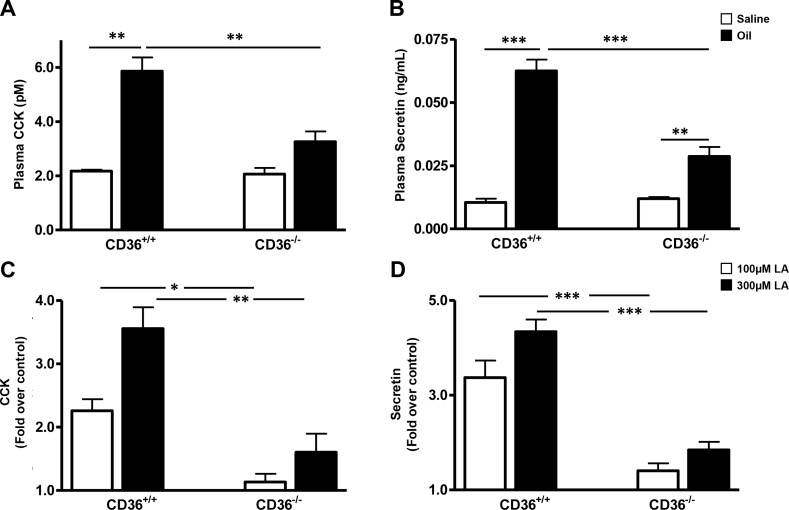
CD36−/− mice show reduced levels of intestinal peptides in response to a lipid load: CD36 is abundant in the proximal intestine, where it has been shown to facilitate uptake of FA and cholesterol (25) and to promote chylomicron formation (26). We examined the influence of CD36 deletion on fat-induced secretion of CCK and secretin, peptides with important roles in fat absorption that are released by EECs localized primarily in proximal segments (3–7). Plasma CCK and secretin levels were measured in WT and CD36−/− mice 30 min after an intragastric load of olive oil. CD36−/− mice had 50% lower CCK (Fig. 1A) and 60% lower secretin levels (Fig. 1B) than WT mice. Peptide levels were similar in saline-administered WT and CD36−/− mice.
Figure 1.
CCK and secretin release in vivo or in vitro by incubated intestinal segments from WT and CD36−/− mice. A, B) In vivo. After overnight food withdrawal, mice were administered an intragastric load of olive oil or saline (16 μl/g body weight), and peptide release into the blood was measured 30 min later. CCK (A) and secretin (B) were extracted on C18 columns and quantified by bioassay or ELISA, respectively. All assays were performed on plasma pooled from 3 mice per genotype per treatment. Data are means ± se [n of pooled (3/pool) samples=3]. C, D) Intestinal segments in vitro. Proximal intestinal segments (10-cm length, beginning at 2 cm after the pylorus) isolated from mice after 16 h of food withdrawal, were filled with HBSS buffer with or without 100 or 300 μM LA, tied at both ends, and incubated at 37°C for 1 h. CCK (C) and secretin (D) release was determined by RIA or ELISA, respectively. Data are means ± se (n=3 separate mice per genotype per treatment). Release data are expressed as fold increase above buffer controls without LA. *P < 0.05; **P < 0.01; ***P < 0.001.
CD36 deficiency alters gut fat absorption by impairing chylomicron formation and shifting more luminal fat to distal parts of the small intestine (26, 28). These changes might contribute to the reduction of fat-induced CCK and secretin release in CD36−/− mice by altering fat exposure of EECs in the proximal part of the intestine. We first compared gastric emptying rates in WT and CD36−/− mice and found no significant differences between the two groups (68.45±4.34 vs. 71.32±6.39, n=4). To directly examine the role of CD36 in FA-induced release of secretin and CCK independent of fat absorption, intestinal segments were isolated from WT and CD36−/− mice and tested for FA-induced peptide release. Secretin and CCK release were similar in WT and CD36−/− segments under basal conditions (data not shown). Addition of 100 and 300 μM LA enhanced CCK release from WT segments by 2.3- and 3.6-fold above basal. No enhancement was observed in CD36−/− segments with 100 μM LA, and a 1.6-fold enhancement occurred at 300 μM. Secretin release from WT segments increased 3.3- and 4.3-fold above basal in response to 100 and 300 μM LA. In contrast, the same LA concentrations increased release only 1.4- and 1.8-fold in CD36−/− segments. These data suggested a major role of CD36 in fat-induced EEC secretion of CCK and secretin that was independent of the effects of CD36 on luminal fat absorption.
CD36 expression in the small intestine and colocalization with chromogranin A
CD36 is expressed apically on enterocytes of the small intestine (39) after a proximal to distal decreasing gradient (24, 25), and its levels are down-regulated by small amounts of dietary fat (40). Information about CD36 expression on EECs and whether it is regulated by dietary fat is unavailable so this was examined. Consistent with previous observations, CD36 was abundant on epithelial cells of the duodenum and jejunum and less abundant in the ileum (Supplemental Fig. S1A−C). No CD36 signal was detected in intestines from CD36−/− mice (Supplemental Fig. S1D). Epithelial CD36 expression was down-regulated in intestines from fed (Supplemental Fig. S2C, D) compared with unfed mice (Supplemental Fig. S2A, B). CD36 was expressed on nonepithelial cells throughout the small intestine, and nonepithelial expression also declined with feeding (Supplemental Fig. S2).
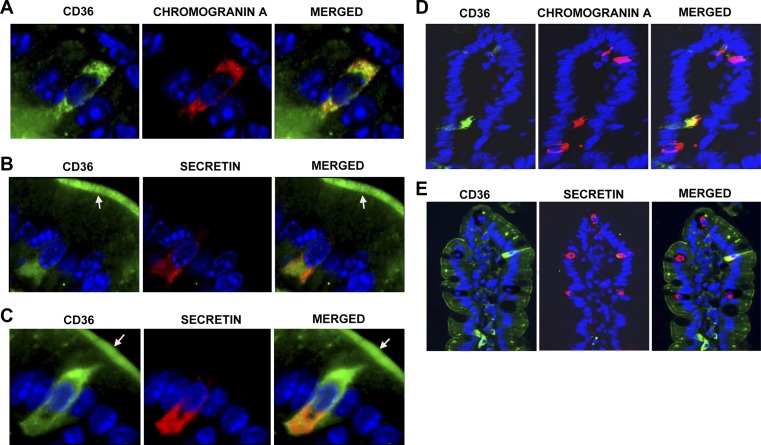
We next examined expression of CD36 on EECs. Chromogranin A is an essential component of secretory vesicles typical of EECs and a marker for most cell types of the enteroendocrine lineage (41). We costained for chromogranin A and CD36 in intestinal sections and found that a subset of chromogranin A-positive cells express CD36 in the proximal (Fig. 2A) and distal small intestine (data not shown), as determined by merge of fluorescent signals. To quantify the distribution of CD36 across chromogranin A-positive cells, doubly positive cells (for chromogranin A and CD36) were counted and expressed as a percentage of cells positive for chromogranin A only. Sections from 5 mice were used for quantification. This process demonstrated that ∼35 and 16% of chromogranin A-positive EECs in the proximal and distal small intestine, respectively, stained for both CD36 and chromogranin A (Table 2). Images at ×200 showed that CD36 is expressed on one-third of chromogranin A-positive cells (Fig. 2D) in the proximal small intestine.
Figure 2.
CD36 expression on intestinal EECs. A) CD36 (green) on chromogranin A (red)-positive EECs. B, C) Colocalization of CD36 with secretin (red)-positive cells. CD36 staining may be present on apical membranes (arrows) of secretin-positive cells. Nuclei are blue (DAPI). Under the same conditions, no CD36 signal is detected in CD36−/− intestines (Supplemental Fig. S1D). Immunoreactivity was visualized using Alexa Fluor 488 and 594 conjugates and a Zeiss fluorescence microscope (×400). D, E) CD36 is expressed on one-third of chromogranin A-positive EECs (D) and on one-fifth of secretin-positive (E) EECs (×200). Formalin-fixed, paraffin-embedded sections were deparaffinized and incubated sequentially with relevant antibodies (Supplemental Table S1). Five unfed mice (overnight food withdrawal) were tested per peptide (n=5). Similar results were obtained with fed mice (data not shown).
Table 2.
Distribution of CD36 across EECs positive for chromogranin A, secretin, and CCK along the duodenal-ileum axis in SI
| Location | Doubly positive cells |
||
|---|---|---|---|
| Chromogranin A and CD36 | Secretin and CD36 | CCK and CD36 | |
| Proximal SI | 28/80 (35.0%) | 8/39 (20.5%) | 5/100 (5%) |
| Distal SI | 4/25 (16.0%) | 1/11 (9.1%) | |
Sections from 5 individual mice were used for determination of colocalization of CD36 with chromogranin A and secretin; sections from 3 mice were used for CCK. Colocalization, determined by doubly positive cells, is expressed as a percentage (n=5). SI, small intestine.
CD36 is expressed on subpopulations of EECs
Our in vivo data showed that CD36 deletion reduced release of secretin and CCK. We examined whether CD36 expression can be detected on the subpopulations of EECs involved in release of these peptides. The CD36 signal was detected in the cytoplasm of a subset of secretin-producing cells in the proximal (Fig. 2B, C) and distal (data not shown) small intestines. Approximately 21 and 9% of secretin-positive cells in the proximal and distal small intestine, respectively, expressed CD36 (Table 2 and Fig. 2E). Secretin-positive cells in these sections were also positive for chromogranin A (data not shown). EECs project their apical membrane into the intestinal lumen, and all secretin-positive EECs may express CD36 on their apical membrane (Fig. 2B, C; arrows).
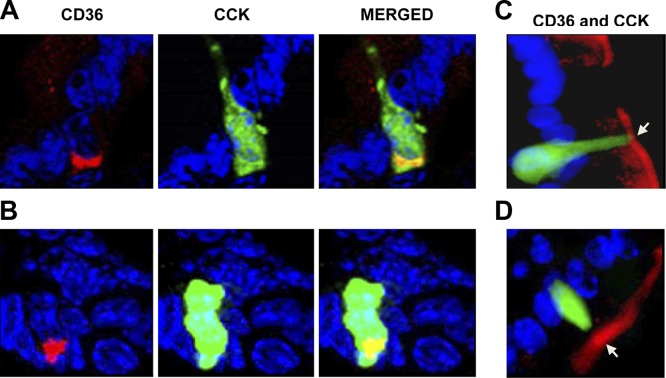
Colocalization of CD36 and CCK was studied using transgenic mice expressing the GFP driven by the CCK promoter. A small subset of CCK-secreting cells (∼5%) exhibited intracellular costaining for CD36 (Fig. 3A, B and Table 2). Based on immunofluorescence staining, CCK-positive cells may have apical CD36 expression (Fig. 3C, D; arrows).
Figure 3.
CD36 expression on CCK-positive cells in proximal intestines of CCK-GFP transgenic mice. A, B) Subset of CCK-positive cells (green) express cytoplasmic CD36 (red). C, D) CD36 may also be present on apical membranes (arrows) of CCK-positive cells. Frozen OCT-embedded sections were fixed in 10% formalin, blocked, and incubated with CD36 and GFP antibodies. Immunoreactivity was visualized as in the legend to Fig. 2 (×400). Three mice [fed (A, B) or unfed overnight (C, D)] were tested for CCK (n=3).
CD36 enhances secretin and CCK release by STC-1 cells
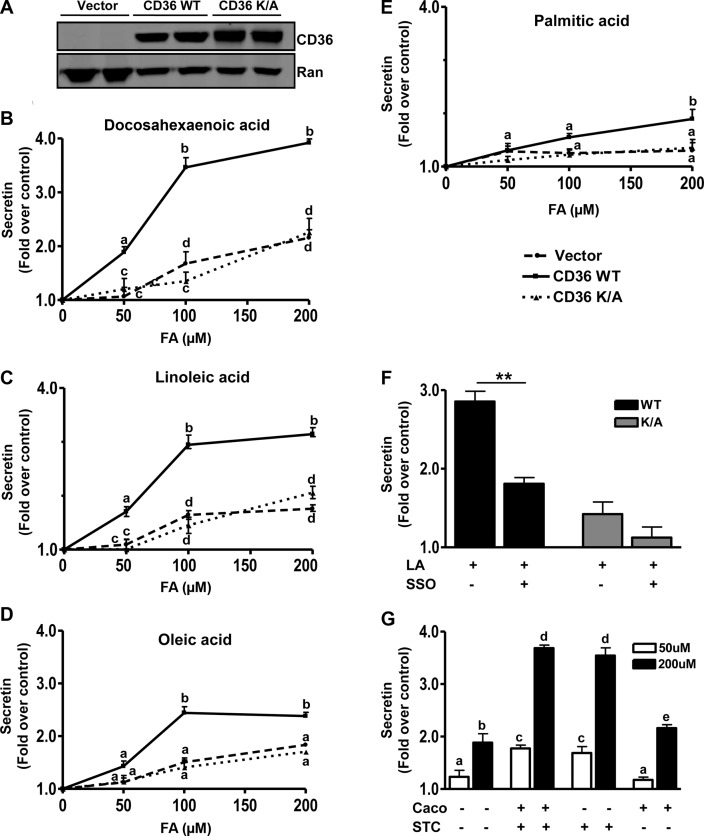
To demonstrate direct CD36 involvement in FA-induced peptide secretion and to better understand the signaling mechanisms involved, studies were conducted in the EEC line STC-1 derived from an endocrine tumor of the mouse small intestine. The STC-1 cell line is a mix of EEC subpopulations (42) that secrete a myriad of gut hormones including secretin and CCK (43) and has been used extensively to investigate the mechanisms underlying peptide release. No expression of CD36 in STC-1 cells was detected, and cells stably expressing WT human CD36 and a control line expressing empty vector were generated (Fig. 4A). A cell line stably expressing a mutated form of CD36 (CD36K/A) that lacks CD36-mediated signaling to intracellular calcium was also used. The C terminus of CD36 is important for signal transduction (44, 45). In the CD36K/A mutant lysines K469 and K472 in the C terminus were substituted with alanine. As we reported previously, the CD36K/A mutant has lost the ability to regulate calcium influx, calcium-induced phospholipase activation, and translocation to membranes (20), but it has normal FA uptake activity (38). We hypothesized that this mutant might have impaired regulation of granule exocytosis as a result of its defect in calcium signaling. All cells were treated with 50, 100, or 200 μM FA (bound to 5 μM BSA), and the effect on peptide secretion was monitored.
Figure 4.
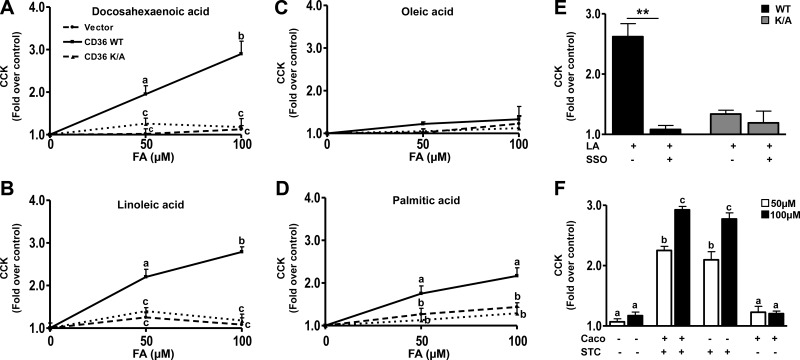
CD36 enhances FA-induced secretin release from STC-1 cells. A) Western blot showing CD36 expression in STC-1 cells stably expressing empty vector, human CD36 WT, or the CD36K/A mutant. Ran is the loading control. B−E) Secretin release. STC-1 cells were incubated with 50, 100, and 200 μM FA (with 5 μM BSA) or BSA alone (control) in HBSS (no glucose) for 60 min at 37°C, and secretin release was measured after DHA (B), LA (C), OA (D), and PA (E). Release is expressed as fold increase over BSA controls after normalization to cell protein. Data are means ± se of triplicates from 4 experiments (n=12). F) LA-induced secretin release in the presence of the CD36 inhibitor, SSO (20 μM, 15 min). Cells were preincubated with 20 μM SSO for 15 min before stimulation with 100 μM LA. G) Coculture of Caco-2 and STC-1 cells, with or without stable expression of CD36. STC-1 cells expressing CD36 (STC +) or empty vector (STC −) were seeded with Caco-2 cells with (Caco +) or without (Caco −) CD36 expression. Cocultures were serum starved overnight and then were incubated in HBSS with 50 or 200 μM LA (plus 5 μM BSA) or BSA alone (controls) for 60 min at 37°C. Secretin release was determined and is expressed as fold increase over BSA alone after normalization to cell protein. Data are means ± se of triplicates from 3 experiments (n=8–12). Points with different letter symbols are significantly different (P<0.05). **P < 0.01.
Secretin release was induced 2-fold above basal by 50 μM DHA, in CD36-expressing STC-1 cells, and no enhancement was observed in cells expressing either CD36K/A or the empty vector (Fig. 4B). Similar data were observed with LA (Fig. 4C) and linolenic acid (data not shown). OA and PA were ineffective (Fig. 4D, E). As the FA concentration was increased to 100 μM, secretin release increased to 3.5 times basal with DHA (Fig. 4B) and to 3 and 2 times basal with LA (Fig. 4C) and OA (Fig. 4D), respectively. However, modest enhancement of release (1.5–1.8 times basal) could now be observed in control (vector and CD36K/A) cells. At 200 μM FA, the stimulatory effects of DHA, LA, and OA on secretin release were slightly more than those at 100 μM, and PA had a small effect. To further validate the CD36 specificity of observed effects, the irreversible CD36 inhibitor sulfo-N-succinimidyl oleate (SSO; ref. 46) was used. In cells preincubated with SSO (20 μM, 15 min) and then treated with 100 μM FA, secretin release was blunted (Fig. 4F), whereas no significant effect was observed on CD36-independent release (vector and CD36K/A-expressing cells).
CCK
DHA at 50 μM stimulated CCK release by 2-fold from cells expressing CD36 with no effect on empty vector or CD36K/A-expressing cells (Fig. 5A). Similar effects were observed with LA (Fig. 5B). PA induced a modest 30% increase (Fig. 5D), whereas OA was ineffective (Fig. 5C). At higher concentrations of FA (200 μM), DHA (Fig. 5A) and LA (Fig. 5B) induced almost 3-fold enhancement of CCK release, whereas PA (Fig. 5D) increased it by approximately 2-fold. OA was still ineffective. No enhancement was observed in cells expressing vector or CD36K/A. The specificity of CD36 involvement was validated further by SSO producing almost complete inhibition of CCK release (Fig. 5E).
Figure 5.
CD36 mediates FA-induced CCK release. A−D) CCK release from STC-1 cells expressing empty vector, CD36 WT, or the mutant CD36K/A in response to DHA (A), LA (B), OA (C), and PA (D). E) LA-induced CCK release after treatment with SSO (20 μM, 15 min). Cells were preincubated with 20 μM SSO for 15 min before stimulation with 100 μM LA. STC-1 cells were FA treated (as in the legend to Fig. 4), and CCK release was determined by RIA. Data are means ± se of triplicates from 3 experiments (n=9). Data points with different letter symbols are significantly different (P<0.05). *P < 0.05; **P < 0.01. F) Coculture of Caco-2 and STC-1 cells, with (+) or without (−) stable expression of CD36. Cocultures were serum starved for 8 h and then were tested for CCK release as in the legend to Fig. 4. CCK was determined by RIA and is expressed as fold over cells with BSA alone after normalization to cell protein. Data are means ± se of triplicates from 2 experiments (n=4 for HBSS controls; n=6 for treatments). Points with different letter symbols are significantly different (P<0.05). **P < 0.01.
Enterocyte coculture on secretin and CCK release by STC-1 cells
Our experiments using intestinal segments in vitro suggested that CD36 regulation of EEC release of secretin and CCK was independent of changes in fat absorption (Fig. 1). To examine this result further we tested whether CD36 expression on enterocytes influences peptide secretion by neighboring EECs. Studies were conducted using coculture of STC-1 cells with Caco-2 cells, a well-studied model of enterocytes (47–49). Differentiated Caco-2 cells with stable expression of CD36, which are responsive to FA signaling (data not shown), were generated and used for these experiments. Cocultures of Caco-2 and STC-1 cells, with (+) or without (−) stable expression of CD36 were tested for LA-induced release of secretin and CCK. At 50 μM, LA enhanced secretin release in STC-1 cells stably expressing CD36 (+), compared with that in empty vector controls (−) by∼2-fold above basal whether the coculture contained Caco-2 cells expressing CD36 (+) or empty vector (−) (Fig. 4G). A similar trend was observed with 200 μM LA. Secretin release increased similarly and ∼4-fold over basal in cocultures containing STC-1 (+) cells whether the cocultured Caco-2 cells did or did not express CD36. In contrast with 50 μM LA, 200 μM LA enhanced secretion ∼2-fold in STC-1 cells not expressing CD36 (Fig. 4G). For CCK release, the trends were similar to those observed with secretin (Fig. 5F). LA at 50 and 200 μM stimulated CCK release by 2- to 2.2- and 2.7- to 2.9-fold, respectively, in STC-1 cells stably expressing CD36 (+) compared with that in vector controls (−), independent of CD36 expression in Caco-2 cells. These data suggested limited contribution of CD36 expression on adjacent enterocytes to FA-stimulated peptide release.
cAMP production is involved in CD36-dependent peptide release
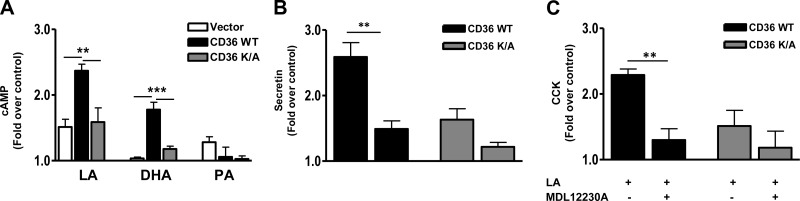
cAMP regulates a variety of secretory events including EEC release of secretin and CCK (30, 50). Its role in CD36-mediated effects was examined. LA treatment increased intracellular cAMP (∼50%) compared with that in BSA controls, and this effect was strongly amplified in CD36-expressing cells in which cAMP increased by 240% (Fig. 6A). DHA increased cAMP (∼88%) only in these cells, whereas PA had no effect (Fig. 6A). Further, the cAMP inhibitor MDL12230A (10 μM, 30 min) reduced CD36-dependent release of both secretin (Fig. 6B) and CCK (Fig. 6C). MDL12230A had no significant effect on release of these peptides in the CD36K/A mutant. These data implicated cAMP generation in CD36-mediated FA-induced release of secretin and CCK.
Figure 6.
CD36-mediated enhancement of peptide release involves cAMP generation. A) Intracellular cAMP levels in response to LA, DHA, and PA in STC-1 cells with vector, CD36 WT, and CD36K/A. STC-1 cells were FA treated at the indicated concentration for 30 min (in the presence of the phosphodiesterase inhibitor IBMX, 0.5 mM), and cAMP was measured in cell lysates. B, C) Secretin (B) and CCK (C) release after treatment with the cAMP inhibitor MDL12230A (10 μM, 30 min) in the presence of 100 μM LA. Data are means ± se of triplicates from 3 experiments (n=9). **P < 0.01; ***P < 0.001.
CD36-mediated secretin release is dependent on PKA activation
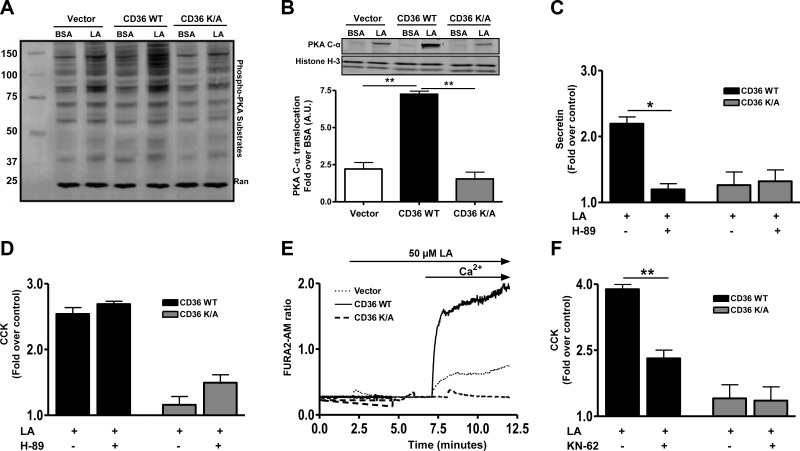
A major effector of cAMP is PKA, which, when activated, phosphorylates an array of protein substrates (51). We determined the effect of FA on PKA activity by measuring phosphorylation of its substrates (RRXS*/T* motifs). Addition of LA (Fig. 7A) or DHA (data not shown) in the presence of 0.5 mM IBMX (to block cAMP degradation) strongly enhanced phosphorylation of PKA substrates in STC-1 cells expressing WT CD36 but not in cells expressing the signaling impaired mutant CD36K/A (Fig. 7A). Under the same conditions, PA treatment was ineffective (Supplemental Fig. S3). Activation of the PKA tetramer associates with translocation of the catalytic subunit to the nucleus (51). LA treatment induced nuclear translocation of catalytic PKA C-α in control cells, and this effect was amplified 3-fold in cells expressing CD36 WT (Fig. 7B). LA increased nuclear protein kinase C content by ∼2.4-fold in vector cells, by 7.4-fold in CD36WT cells, and by 1.7-fold in CD36K/A cells. The responses of vector and CD36K/A-expressing cells were similar and significantly less than that of CD36WT cells (P<0.01). Pretreatment with the PKA inhibitor H-89 (10 μM, 30 min) reduced secretin release only in CD36 WT-expressing cells (Fig. 7C), suggesting that release involved PKA activation. In contrast, CD36-mediated, FA-induced CCK release was not affected by H-89 (Fig. 7D), implicating PKA-independent mechanisms downstream of cAMP.
Figure 7.
CD36-mediated effects on peptide release involve activation of PKA and CaM-KII. A, B) Phospho-PKA substrates (with RRXS*/T* motif) in cell lysates (A) and PKA C-α in nuclear fractions (B) of STC-1 cells expressing vector, CD36 WT, or CD36K/A after LA stimulation (in the presence of 0.5 mM IBMX). Histone H-3 was used as a loading control for nuclear lysates (n=4). C, D) LA-induced secretin (C) and CCK release (D) after treatment with the PKA inhibitor H-89. E) Representative intracellular calcium transients in STC-1 cells stably expressing vector, CD36 WT, or CD36K/A. F) LA (100 μM) induced CCK release in CD36-expressing STC-1 cells with or without treatment with the CaM-KII inhibitor KN-62 (2.5 μM, 15 min). Data are means ± se of triplicates from 3 experiments (n=9). *P < 0.05; **P < 0.01.
CD36-mediated CCK release involves CaM-KII
An increase in intracellular calcium after cAMP generation has been implicated in release of CCK (50, 52). CD36 was recently reported to influence calcium flux in Chinese hamster ovary, macrophages, and taste bud cells (20, 22). We investigated effect of CD36 expression on calcium transients in response to LA in the absence or presence of extracellular calcium. A rapid increase in intracellular calcium was observed in LA-treated STC-1 cells expressing CD36 WT on addition of medium calcium, whereas no effect was observed in cells expressing the vector or CD36K/A mutant (Fig. 7E). Calcium influx activates CaM-KII, which is involved in release of CCK (32). Indeed, treatment with the CaM-KII inhibitor KN-62 (2.5 μM, 15 min) blunted CCK release in CD36-expressing cells (Fig. 7F), supporting involvement of CaM-KII in CD36-mediated CCK release.
DISCUSSION
CD36 contributes to different steps of the fat absorption process (53); it mediates fat perception in taste bud cells and the initiation of the cephalic phase of digestion (22). It also influences intestinal fat processing and chylomicron formation (25, 26, 28). This study documented a novel and potentially important metabolic function of CD36: its mediation of gut peptide release in response to dietary FA. We showed that CD36 is expressed on EECs positive for secretin and CCK and the release of these peptides in response to intragastric fat was halved in CD36−/− mice. This reduction was most likely independent of changes in fat absorption because it was reproduced in isolated intestinal segments. Using the EEC line STC-1 with stable expression of CD36 or a signaling-impaired CD36 mutant, specific CD36 mediation of FA-induced peptide release was demonstrated to involve increases in cellular cAMP and calcium.
CD36 regulates peptide release
CD36 deletion was associated with 50–60% lower plasma levels of secretin and CCK at 30 min after an intragastric triglyceride load. In vitro, the effect of FA to induce release of these peptides was specific to cells expressing functional WT CD36. The calcium signaling-impaired CD36K/A mutant was ineffective despite the fact that it expresses normally, localizes to the plasma membrane, and functions in FA uptake (20, 38). Our findings using WT and CD36−/− mice, given a high oil load, support the existence of both CD36-dependent and CD36-independent peptide release because secretin and CCK release was suppressed by 60 and 50%, respectively. The work in vitro with proximal intestinal segments and with STC-1 cells showed that the CD36-independent component becomes more apparent as the FA concentration is increased and the high-affinity CD36-mediated pathway is saturated. In vivo, this could imply that one function of CD36 is to enable secretin and CCK release at relatively low FA concentrations possibly early during the digestion process, thus optimizing subsequent absorption. Other FA receptors such as GPR120 (17) and GPR40 (2), which are localized more distally in the intestine, would contribute to FA-induced FA release at a later stage in the absorptive process. GPR120 has been mainly implicated in mediation of FA-induced incretin release (17, 18). Its role in CCK release has been briefly documented in STC-1 cells, in which small interfering RNA directed against GPR120 but not GPR40 inhibited a 2-fold effect of linolenic acid to induce CCK secretion by 30–40% (54). More convincing evidence documented involvement of GPR40 in long-chain FA-induced release of CCK (2) in vivo using GPR40−/− mice. A load of intragastric olive oil comparable to that used in our study resulted at 30 min in a 4.2-fold increase of plasma CCK vs. a 2.8-fold increase in GPR40−/− mice, meaning a reduction of 33%. These findings together with our observation of a 50% reduction of plasma CCK, 30 min after oil gavage in CD36−/− mice (Fig. 1) support the interpretation that CD36 and GPR40 might account for most FA-induced CCK release in vivo. In the case of secretin, our study provides, to our knowledge, the first information related to the FA receptor involved in secretin release by documenting a major role of CD36 based on the 60% reduction of plasma secretin release in CD36−/− mice, given a high intragastric oil load (Fig. 1).
CD36-mediated FA signaling enhances cAMP generation and calcium transients
Endocrine cells release granules in response to factors that increase intracellular Ca2+ or cAMP levels. The cAMP pathway modulates granule exocytosis in a variety of cells (51) and has been implicated in release of gut peptides (30, 50). We showed that CD36 signaling regulates cAMP levels and that this was involved in its enhancement of FA-induced release of secretin and CCK. FA activation of PKA, the major cAMP effector, and enhanced nuclear PKA content were documented in CD36-expressing cells. However, only release of secretin was markedly attenuated by the PKA inhibitor H-89, whereas that of CCK was not, suggesting PKA-independent mechanisms. CCK release is triggered by an increase in intracellular Ca2+ that is coupled to cAMP generation (50) and CaM-KII activation (32). Consistent with CD36 regulation of this pathway, FA induced an increase in intracellular Ca2+ specifically in STC-1 cells expressing CD36, and the CCK response was suppressed by the CaM-KII inhibitor KN-62. The differential effects of signaling inhibitors, implying involvement of distinct pathways downstream of cAMP in the regulation of secretin vs. CCK release, probably reflect the contribution of different cell subpopulations because the STC-1 cell line derived from an endocrine tumor of the intestine is heterogeneous (42, 43). Our data with STC-1 cells are consistent with previous work supporting involvement of Ca2+ and cAMP in release of secretory granules in most cell types and with selectivity of granule exocytosis, reflecting variable sensitivity to Ca2+ and selective downstream phosphorylation pathways (55). Some EECs in the mouse intestine synthesize and secrete more than one peptide, including CCK and secretin (56). There is also evidence for different composition of granules in CCK- or secretin-releasing EECs (57), consistent with differential regulation of granule release. The possibility that some secretion of CCK and secretin may occur from the same STC-1 subpopulation cannot be ruled out.
In summary, our data document CD36 expression on secretin- and CCK-releasing EECs and its role in FA-induced release of these peptides in vivo. The more abundant localization of CD36 in the proximal than in the distal intestine and the relatively higher FA affinity of CD36-mediated vs. CD36-independent peptide release suggest that CD36 would play a critical role in the early phase of fat absorption. Other FA receptors such as GPR40, which is more abundant in the distal intestine, would contribute at later phases of absorption. Our data, together with previous findings, suggest that CD36 and GPR40 account for most FA-induced CCK release and that CD36 is the predominant regulator of FA-induced secretin release. CCK and secretin play important regulatory roles in fat absorption because they influence gastric emptying and acid secretion and pancreatic and intestinal bicarbonate secretion in addition to gallbladder contractility and intestinal motility (3, 58). Both peptides influence satiety and energy homeostasis (12, 13). Regulation of FA-induced CCK and secretin release contributes to the role of CD36 in intestinal handling of dietary fat. In humans, common SNPs in the CD36 gene influence plasma lipids (59), and CD36 deficiency is characterized by altered chylomicron production (60). In addition, SNPs that reduce the CD36 level associate with diminished oral FA sensitivity (23). Whether intestinal FA sensing is also altered in these subjects remains to be determined. FA sensing in the small intestine with consequent effects on gut peptide release and on satiety were reported to be attenuated in subjects with reduced oral FA sensitivity or after high-fat feeding (8).
Supplementary Material
Acknowledgments
The authors acknowledge valuable assistance by Terri Pietka (Adipocyte Biology and Molecular Nutrition Core, Nutrition and Obesity Research Center, Washington University School of Medicine; P30-DK056341) and are grateful to Dr. Ondrej Kuda for help with the calcium flux studies and to Dr. Doug Hanahan for the permission to obtain STC-1 cells from American Type Culture Collection (Manassas, VA, USA).
This work was supported by funding from U.S. National Institutes of Health grants DK033301, DK60022 (to N.A.A.), DK33165, DK55753 (to W.F.S.), and DK091946 (to R.A.L.).
R.S., T.E.R., R.C., and F.N. assisted in data acquisition and reviewed the manuscript; W.F.S. and R.A.L. critically reviewed the manuscript; S.S. obtained and analyzed data; and S.S. and N.A.A. were involved in development of the study concept and design, data analysis and interpretation, and manuscript preparation.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BSA
- bovine serum albumin
- CaM-KII
- calmodulin kinase II
- CCK
- cholecystokinin
- DHA
- docosahexaenoic acid
- DMEM
- Dulbecco's modified Eagle's medium
- EEC
- enteroendocrine cell
- ELISA
- enzyme-linked immunosorbent assay
- FA
- fatty acid
- FBS
- fetal bovine serum
- GFP
- green fluorescent protein
- GPR
- G-protein-coupled receptor
- HBSS
- Hanks' balanced saline solution
- IBMX
- 3-isobutyl-1-methylxanthine
- LA
- linoleic acid
- OA
- oleic acid
- PA
- palmitic acid
- PKA
- protein kinase A
- PMSF
- phenylmethanesulfonyl fluoride
- RIA
- radioimmunoassay
- SNP
- single-nucleotide polymorphism
- SSO
- sulfo-N-succinimidyl oleate
- WT
- wild type
REFERENCES
- 1. Beglinger C., Degen L. (2004) Fat in the intestine as a regulator of appetite—role of CCK. Physiol. Behav. 83, 617–621 [DOI] [PubMed] [Google Scholar]
- 2. Liou A. P., Lu X., Sei Y., Zhao X., Pechhold S., Carrero R. J., Raybould H. E., Wank S. (2011) The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 140, 903–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liddle R. A. (2000) Regulation of cholecystokinin secretion in humans. J. Gastroenterol. 35, 181–187 [DOI] [PubMed] [Google Scholar]
- 4. Buchan A. M., Polak J. M., Solcia E., Capella C., Hudson D., Pearse A. G. (1978) Electron immunohistochemical evidence for the human intestinal I cell as the source of CCK. Gut 19, 403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lam I. P., Lee L. T., Choi H. S., Chow B. K. (2006) Localization of small heterodimer partner (SHP) and secretin in mouse duodenal cells. Ann. N. Y. Acad. Sci. 1070, 371–375 [DOI] [PubMed] [Google Scholar]
- 6. Polak J. M., Bloom S., Coulling I., Pearse A. G. (1971) Immunofluorescent localization of secretin in the canine duodenum. Gut 12, 605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polak J. M., Coulling I., Bloom S., Pearse A. G. (1971) Immunofluorescent localization of secretin and enteroglucagon in human intestinal mucosa. Scand. J. Gastroenterol. 6, 739–744 [DOI] [PubMed] [Google Scholar]
- 8. Little T. J., Feinle-Bisset C. (2011) Effects of dietary fat on appetite and energy intake in health and obesity—oral and gastrointestinal sensory contributions. Physiol. Behav. 104, 613–620 [DOI] [PubMed] [Google Scholar]
- 9. Sclafani A., Ackroff K. (2012) Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R1119–R1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berna M. J., Jensen R. T. (2007) Role of CCK/gastrin receptors in gastrointestinal/metabolic diseases and results of human studies using gastrin/CCK receptor agonists/antagonists in these diseases. Curr. Top. Med. Chem. 7, 1211–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chey W. Y., Chang T. M. (2003) Secretin, 100 years later. J. Gastroenterol. 38, 1025–1035 [DOI] [PubMed] [Google Scholar]
- 12. Cheng C. Y., Chu J. Y., Chow B. K. (2011) Central and peripheral administration of secretin inhibits food intake in mice through the activation of the melanocortin system. Neuropsychopharmacology 36, 459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dockray G. J. (2012) Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 19, 8–12 [DOI] [PubMed] [Google Scholar]
- 14. Hajri T., Abumrad N. A. (2002) Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu. Rev. Nutr. 22, 383–415 [DOI] [PubMed] [Google Scholar]
- 15. Oh D. Y., Lagakos W. S. (2011) The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care 14, 322–327 [DOI] [PubMed] [Google Scholar]
- 16. Vinolo M. A., Hirabara S. M., Curi R. (2012) G-protein-coupled receptors as fat sensors. Curr. Opin. Clin. Nutr. Metab. Care 15, 112–116 [DOI] [PubMed] [Google Scholar]
- 17. Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11, 90–94 [DOI] [PubMed] [Google Scholar]
- 18. Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., Olefsky J. M. (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El-Yassimi A., Hichami A., Besnard P., Khan N. A. (2008) Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 283, 12949–12959 [DOI] [PubMed] [Google Scholar]
- 20. Kuda O., Jenkins C. M., Skinner J. R., Moon S. H., Su X., Gross R. W., Abumrad N. A. (2011) CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J. Biol. Chem. 286, 17785–17795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan N. A., Besnard P. (2009) Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim. Biophys. Acta 1791, 149–155 [DOI] [PubMed] [Google Scholar]
- 22. Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., Besnard P. (2005) CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115, 3177–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pepino M. Y., Love-Gregory L., Klein S., Abumrad N. A. (2012) The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 53, 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lobo M. V., Huerta L., Ruiz-Velasco N., Teixeiro E., de la Cueva P., Celdran A., Martin-Hidalgo A., Vega M. A., Bragado R. (2001) Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J. Histochem. Cytochem. 49, 1253–1260 [DOI] [PubMed] [Google Scholar]
- 25. Nassir F., Wilson B., Han X., Gross R. W., Abumrad N. A. (2007) CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 282, 19493–19501 [DOI] [PubMed] [Google Scholar]
- 26. Drover V. A., Ajmal M., Nassir F., Davidson N. O., Nauli A. M., Sahoo D., Tso P., Abumrad N. A. (2005) CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Invest. 115, 1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsieh J., Longuet C., Maida A., Bahrami J., Xu E., Baker C. L., Brubaker P. L., Drucker D. J., Adeli K. (2009) Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology 137, 997–1005, 1005.e1–1005.e4 [DOI] [PubMed] [Google Scholar]
- 28. Nauli A. M., Nassir F., Zheng S., Yang Q., Lo C. M., Vonlehmden S. B., Lee D., Jandacek R. J., Abumrad N. A., Tso P. (2006) CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 131, 1197–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edfalk S., Steneberg P., Edlund H. (2008) Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57, 2280–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang C. H., Chey W. Y., Erway B., Coy D. H., Chang T. M. (1998) Modulation of secretin release by neuropeptides in secretin-producing cells. Am. J. Physiol. 275, G192–G202 [DOI] [PubMed] [Google Scholar]
- 31. Parker H. E., Reimann F., Gribble F. M. (2010) Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev. Mol. Med. 12, e1. [DOI] [PubMed] [Google Scholar]
- 32. Prpic V., Basavappa S., Liddle R. A., Mangel A. W. (1994) Regulation of cholecystokinin secretion by calcium-dependent calmodulin kinase II: differential effects of phenylalanine and cAMP. Biochem. Biophys. Res. Commun. 201, 1483–1489 [DOI] [PubMed] [Google Scholar]
- 33. Chandra R., Samsa L. A., Vigna S. R., Liddle R. A. (2010) Pseudopod-like basal cell processes in intestinal cholecystokinin cells. Cell Tissue Res. 341, 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki S., Suzuki H., Horiguchi K., Tsugawa H., Matsuzaki J., Takagi T., Shimojima N., Hibi T. (2010) Delayed gastric emptying and disruption of the interstitial cells of Cajal network after gastric ischaemia and reperfusion. Neurogastroenterol. Motil. 22, 585–593, e126 [DOI] [PubMed] [Google Scholar]
- 35. Liddle R. A., Goldfine I. D., Williams J. A. (1984) Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology 87, 542–549 [PubMed] [Google Scholar]
- 36. Tashiro M., Samuelson L. C., Liddle R. A., Williams J. A. (2004) Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G784–G790 [DOI] [PubMed] [Google Scholar]
- 37. Rehfeld J. F. (1998) Accurate measurement of cholecystokinin in plasma. Clin. Chem. 44, 991–1001 [PubMed] [Google Scholar]
- 38. Smith J., Su X., El-Maghrabi R., Stahl P. D., Abumrad N. A. (2008) Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J. Biol. Chem. 283, 13578–13585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poirier H., Degrace P., Niot I., Bernard A., Besnard P. (1996) Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP). Eur. J. Biochem. 238, 368–373 [DOI] [PubMed] [Google Scholar]
- 40. Tran T. T., Poirier H., Clement L., Nassir F., Pelsers M. M., Petit V., Degrace P., Monnot M. C., Glatz J. F., Abumrad N. A., Besnard P., Niot I. (2011) Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J. Biol. Chem. 286, 25201–25210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Varndell I. M., Lloyd R. V., Wilson B. S., Polak J. M. (1985) Ultrastructural localization of chromogranin: a potential marker for the electron microscopical recognition of endocrine cell secretory granules. Histochem. J. 17, 981–992 [DOI] [PubMed] [Google Scholar]
- 42. Cheung A. T., Dayanandan B., Lewis J. T., Korbutt G. S., Rajotte R. V., Bryer-Ash M., Boylan M. O., Wolfe M. M., Kieffer T. J. (2000) Glucose-dependent insulin release from genetically engineered K cells. Science 290, 1959–1962 [DOI] [PubMed] [Google Scholar]
- 43. Rindi G., Grant S. G., Yiangou Y., Ghatei M. A., Bloom S. R., Bautch V. L., Solcia E., Polak J. M. (1990) Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice. Heterogeneity of hormone expression. Am. J. Pathol. 136, 1349–1363 [PMC free article] [PubMed] [Google Scholar]
- 44. Lipsky R. H., Eckert D. M., Tang Y., Ockenhouse C. F. (1997) The carboxyl-terminal cytoplasmic domain of CD36 is required for oxidized low-density lipoprotein modulation of NF-κB activity by tumor necrosis factor-α. Recept. Signal Transduct. 7, 1–11 [PubMed] [Google Scholar]
- 45. Silverstein R. L. (2009) Type 2 scavenger receptor CD36 in platelet activation: the role of hyperlipemia and oxidative stress. Clin. Lipidol. 4, 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harmon C. M., Luce P., Beth A. H., Abumrad N. A. (1991) Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J. Membr. Biol. 121, 261–268 [DOI] [PubMed] [Google Scholar]
- 47. Hussain M. M. (2000) A proposed model for the assembly of chylomicrons. Atherosclerosis 148, 1–15 [DOI] [PubMed] [Google Scholar]
- 48. Levy E., Bendayan M. (2000) Use of immunoelectron microscopy and intestinal models to explore the elaboration of apolipoproteins required for intraenterocyte lipid transport. Microsc. Res. Tech. 49, 374–382 [DOI] [PubMed] [Google Scholar]
- 49. Mathias A., Duc M., Favre L., Benyacoub J., Blum S., Corthesy B. (2010) Potentiation of polarized intestinal Caco-2 cell responsiveness to probiotics complexed with secretory IgA. J. Biol. Chem. 285, 33906–33913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scott L., Prpic V., Capel W. D., Basavappa S., Mangel A. W., Gettys T. W., Liddle R. A. (1996) β-Adrenergic regulation of cholecystokinin secretion in STC-1 cells. Am. J. Physiol. 270, G291–G297 [DOI] [PubMed] [Google Scholar]
- 51. Seino S., Shibasaki T. (2005) PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 85, 1303–1342 [DOI] [PubMed] [Google Scholar]
- 52. Sidhu S. S., Thompson D. G., Warhurst G., Case R. M., Benson R. S. (2000) Fatty acid-induced cholecystokinin secretion and changes in intracellular Ca2+ in two enteroendocrine cell lines, STC-1 and GLUTag. J. Physiol. 528 (Pt. 1), 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abumrad N. A., Davidson N. O. (2012) Role of the gut in lipid homeostasis. Physiol. Rev. 92, 1061–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanaka T., Katsuma S., Adachi T., Koshimizu T. A., Hirasawa A., Tsujimoto G. (2008) Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn-Schmiedebergs Arch. Pharmacol. 377, 523–527 [DOI] [PubMed] [Google Scholar]
- 55. Burgoyne R. D., Morgan A. (2003) Secretory granule exocytosis. Physiol. Rev. 83, 581–632 [DOI] [PubMed] [Google Scholar]
- 56. Habib A. M., Richards P., Cairns L. S., Rogers G. J., Bannon C. A., Parker H. E., Morley T. C., Yeo G. S., Reimann F., Gribble F. M. (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153, 3054–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Portela-Gomes G. M., Stridsberg M., Johansson H., Grimelius L. (1997) Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J. Histochem. Cytochem. 45, 815–822 [DOI] [PubMed] [Google Scholar]
- 58. Lam I. P., Siu F. K., Chu J. Y., Chow B. K. (2008) Multiple actions of secretin in the human body. Int. Rev. Cytol. 265, 159–190 [DOI] [PubMed] [Google Scholar]
- 59. Love-Gregory L., Sherva R., Schappe T., Qi J. S., McCrea J., Klein S., Connelly M. A., Abumrad N. A. (2011) Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 20, 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Masuda D., Hirano K., Oku H., Sandoval J. C., Kawase R., Yuasa-Kawase M., Yamashita Y., Takada M., Tsubakio-Yamamoto K., Tochino Y., Koseki M., Matsuura F., Nishida M., Kawamoto T., Ishigami M., Hori M., Shimomura I., Yamashita S. (2009) Chylomicron remnants are increased in the postprandial state in CD36 deficiency. J. Lipid Res. 50, 999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.