Abstract
Neutrophil apoptosis and subsequent nonphlogistic clearance by surrounding phagocytes are key to the successful resolution of neutrophilic inflammation, with dysregulated apoptosis reported in multiple human inflammatory diseases. Enhancing neutrophil apoptosis has proresolution and anti-inflammatory effects in preclinical models of inflammation. Here we investigate the ability of the flavones apigenin, luteolin, and wogonin to induce neutrophil apoptosis in vitro and resolve neutrophilic inflammation in vivo. Human neutrophil apoptosis was assessed morphologically and by flow cytometry following incubation with apigenin, luteolin, and wogonin. All three flavones induced time- and concentration-dependent neutrophil apoptosis (apigenin, EC50=12.2 μM; luteolin, EC50=14.6 μM; and wogonin, EC50=28.9 μM). Induction of apoptosis was caspase dependent, as it was blocked by the broad-spectrum caspase inhibitor Q-VD-OPh and was associated with both caspase-3 and caspase-9 activation. Flavone-induced apoptosis was preceded by down-regulation of the prosurvival protein Mcl-1, with proteasomal inhibition preventing flavone-induced Mcl-1 down-regulation and apoptosis. The flavones abrogated the survival effects of mediators that prolong neutrophil life span, including lipoteichoic acid, peptidoglycan, dexamethasone, and granulocyte-macrophage colony stimulating factor, by driving apoptosis. Furthermore, wogonin enhanced resolution of established neutrophilic inflammation in a zebrafish model of sterile tissue injury. Wogonin-induced resolution was dependent on apoptosis in vivo as it was blocked by caspase inhibition. Our data show that the flavones induce neutrophil apoptosis and have potential as neutrophil apoptosis-inducing anti-inflammatory, proresolution agents.—Lucas, C. D., Allen, K. C., Dorward, D. A., Hoodless, L. J., Melrose, L. A., Marwick, J. A., Tucker, C. S., Haslett, C., Duffin, R., Rossi, A. G. Flavones induce neutrophil apoptosis by down-regulation of Mcl-1 via a proteasomal-dependent pathway.
Keywords: inflammation, resolution, polyphenols
Neutrophils are key cells of the innate immune system that are rapidly recruited to sites of infection or tissue injury where they act to ensure host protection against invading pathogens (1). This is achieved by processes including neutrophil phagocytosis, degranulation, and production of reactive oxygen species (ROS) or by extruding strands of DNA coated with antimicrobial peptides into the surrounding environment (NETosis). However, as these neutrophil functions can lack specificity and can be injurious to host tissues, it is important that neutrophil activity is tightly regulated to prevent perpetuation of inflammation (2). Despite having a life span of only hours under physiological situations, neutrophil longevity is extended in inflammatory environments by delaying apoptosis, a form of programmed cell death. Nevertheless, once an episode of acute neutrophilic inflammation is complete, it is essential that neutrophil recruitment is halted and that recruited neutrophils undergo apoptosis, before disposal of the apoptotic cells by surrounding phagocytes such as macrophages, to ensure efficient resolution of inflammation (3). These processes allow fine control of neutrophil function, prevent neutrophil death by necrosis with consequent inflammation, and modulate macrophage phenotype toward anti-inflammatory cytokine production (4, 5).
The natural resolution process of neutrophil apoptosis followed by phagocytosis is impaired in numerous human inflammatory disease states, with delayed apoptosis seen in conditions such as rheumatoid arthritis (6) and acute respiratory distress syndrome (7) while macrophage phagocytosis is defective in diseases such as systemic lupus erythematosus (8) and asthma (9). It is becoming increasingly clear that strategies that manipulate this physiological resolution process have both anti-inflammatory and proresolution potential, with agents that either enhance macrophage phagocytosis or drive neutrophil apoptosis demonstrating utility in preclinical models of both acute and chronic inflammation. In particular, the induction of neutrophil apoptosis by strategies such as cyclin-dependent kinase inhibition (10–12), ligation of TNF-related apoptosis inducing ligand (TRAIL; refs. 13, 14), lipid mediators (15–17), antimicrobial agents (18), or modulators of intracellular survival pathways (19) have all shown benefit in preclinical models of neutrophilic inflammation.
Flavones are phenolic compounds from the class of flavonoids present in a wide variety of plant material and form a core component of many herbal medicines (20). The health benefits of a diet high in plant material have long been known, with flavone-rich foods suggested to be protective against the development of atherosclerosis and dementia, although the exact mechanism of action remains unknown (21, 22). One possibility relates to flavones acting as potential anti-inflammatory compounds secondary to their antioxidant properties (23) as well as inhibiting several proinflammatory signaling cascades and transcription factors (24–27). Furthermore, anti-inflammatory and antitumor effects of flavones have been demonstrated in vivo due to their ability to influence cellular apoptosis. However, the ability of flavones to influence proresolution pathways such as neutrophil apoptosis has not previously been studied.
Here we demonstrate that the flavones apigenin, luteolin, and wogonin drive primary human neutrophil apoptosis in a caspase-dependent fashion even in the presence of powerful neutrophil prosurvival factors. In addition, we show that the flavones induce neutrophil apoptosis by degradation of the key endogenous neutrophil survival protein myeloid cell leukemia-1 (Mcl-1) in a proteasomal-dependent fashion. Furthermore, we investigate the ability of wogonin to enhance resolution of established neutrophilic inflammation in a zebrafish model of sterile tissue injury.
MATERIALS AND METHODS
Neutrophil isolation and culture
Granulocytes were isolated from the peripheral blood of healthy donors as described previously (11, 28, 29); ethics approval was obtained from the Lothian Research Ethics Committee (approval no. 08/S1103/38). Neutrophil isolates were >96% pure (with 1-3% contaminating eosinophils) as confirmed by morphological appearance using light microscopy. Cells were resuspended at 5 × 106/ml in Iscove's modified Dulbecco's medium (PAA, Pasching, Austria) plus 5% autologous serum and cultured at 37°C with 5% CO2 with apigenin, luteolin, wogonin, lipoteichoic acid (from Staphylococcus aureus), and peptidoglycan (from Staphylococcus aureus) (all from Sigma, Dorset, UK); and Q-VD-OPh, dexamethasone, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (all from R&D, Abingdon, UK), alone or in combination.
Assessment of viability and apoptosis
Viability, apoptosis, and necrosis were assessed by flow cytometry using annexin-V-conjugated fluorescein isothiocynate (FITC; Roche, Welwyn Garden City, UK) and propidium iodide (PI; Sigma) as described previously (10, 28). Briefly, annexin-V was diluted 1:500 in binding buffer (HBSS with 2.5 mM Ca2+) and 280 μl was added to 20 μl of cells. After 10 min of incubation at 4°C, 1 μl of PI (1 mg/ml) was added to each sample before immediate analysis on a FACScan flow cytometer (BD, Oxford, UK). Neutrophil apoptosis was confirmed by cyto-centrifugation of 80 μl of sample at 300 rpm for 3 min. Samples were then fixed with methanol and stained with Diff-Quick (Gamidor, Didcot, UK) before morphological analysis (30).
Assessment of neutrophil activation
Shape change, CD11b up-regulation, and CD62L shedding were assessed as described previously (28). Neutrophils were resuspended in HBSS with cations and incubated with or without the flavones (all at 50 μM) on a shaking heat block for 30 min before addition of N-formyl-Met-Leu-Phe (fMLP; 10 nM; Sigma) or control medium for a further 15 min. Neutrophils were then incubated with appropriate antibodies (CD11b, Biolegend, Cambridge, UK; CD62L, BD Pharmingen) and analyzed by flow cytometry.
ROS generation was assessed by dihydrorhodamine (DHR) fluorescence as described previously (28). Neutrophils resuspended in HBSS with cations were loaded with DHR (2 μM; Invitrogen, Carlsbad, CA, USA) for 10 min and then incubated with or without the flavones (all at 50 μM) or the protein kinase C inhibitor Ro 31-8220 (1 μM; Calibochem, San Diego, CA, USA) on a shaking heat block for 30 min before stimulation with phorbol 12-myristate 13-acetate (PMA; 300 nM; Sigma) or control for a further 15 min, and DHR fluorescence was analyzed by flow cytometry (FL-1).
Intracellular calcium was analyzed by spectrofluorimetry of fura-2 (2 μM; Sigma) labeled neutrophils as described previously (31) in response to addition of the flavones (all at 50 μM) or the formyl peptide receptor 1 antagonist cyclosporine H (Enzo Lifesciences, Exeter, UK) and subsequently by fMLP (10 nM) and platelet-activating factor (PAF; 10 nM; Sigma).
Western blotting
Following incubation with compounds as per figure legends, neutrophils (5×106/ml) were lysed for 10 min with 0.1% Nonidet P40 in Tris-buffered saline (TBS) containing a protease inhibitor cocktail as described previously (32). Following BCA protein assay (Thermo Scientific, Cramlington, UK) to ensure equal protein loading, lysates were run on a 12% SDS gel (Thermo Fisher Scientific) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% dried milk (Marvel, Knighton, UK) in TBS/0.1% Tween-20 before incubation with antibodies against Mcl-1 (1:500; Santa-Cruz, Santa Cruz, Biotechnology, CA, USA), GAPDH (1:10,000; Sigma), cleaved caspase-3 (1:1000, Cell Signaling, Danvers, MA, USA), and cleaved caspase-9 (1:1000; Cell Signaling). After 3 washes with TBS/0.1% Tween-20, membranes were incubated with the appropriate horseradish-peroxidase-conjugated secondary antibodies (1:2500; Dako Cambridgeshire, UK). Finally, blots were incubated with ECL prime (GE Healthcare, Little Chalfont, UK), exposed to light-sensitive film (Kodak, Rochester, NY, USA) and processed through an X-ray developer (Xograph Imaging Systems, Tetbury, UK).
In vivo sterile tissue injury
Transgenic zebrafish [Tg(mpx:eGFPi114); ref. 33; a kind gift from Dr. Steve Renshaw, University of Sheffield, Sheffield, UK], which have fluorescently labeled neutrophils, were maintained according to standard protocols. Three day postfertilization larvae were anesthetized in tricaine and the tail fin transected by a sterile scalpel (34). Individual fish were then imaged using a fluorescent microscope (×8 view), and neutrophil numbers at the site of tissue injury were counted at 0, 4, and 24 h post-tail median fin transection. Zebrafish were exposed to flavones or DMSO controls as per figure legends from 4 h post-tail fin transection onward with stock solutions diluted in embryo medium (35) such that the final concentration of DMSO never exceeded 0.2%. For the experiments with the caspase inhibitor zVAD-FMK (zVAD; Tocris Bioscience, Bristol, UK), 2 nl of 100 mM zVAD (or DMSO control) was microinjected into the yolk sac 3.5 h after tail fin transaction, and zebrafish were exposed to wogonin from 4 h onward.
Data handling and statistical analysis
All flow cytometry data were analyzed using FlowJo software (Treestar, Ashland, OR, USA). Analysis of zebrafish images was carried out using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). All neutrophil experiments were conducted in duplicate using cells isolated from ≥3 different donors per experiment, and results are expressed as means ± se. Data were analyzed by 1-way ANOVA with a Newman-Keuls multiple comparison post hoc test, or by a 2-tailed unpaired t test for zebrafish experiments (Prism 5; GraphPad, San Diego, CA, USA), with significance accepted at values of P < 0.05.
RESULTS
Flavones apigenin, luteolin, and wogonin drive primary human neutrophil apoptosis in a time- and concentration-dependent manner
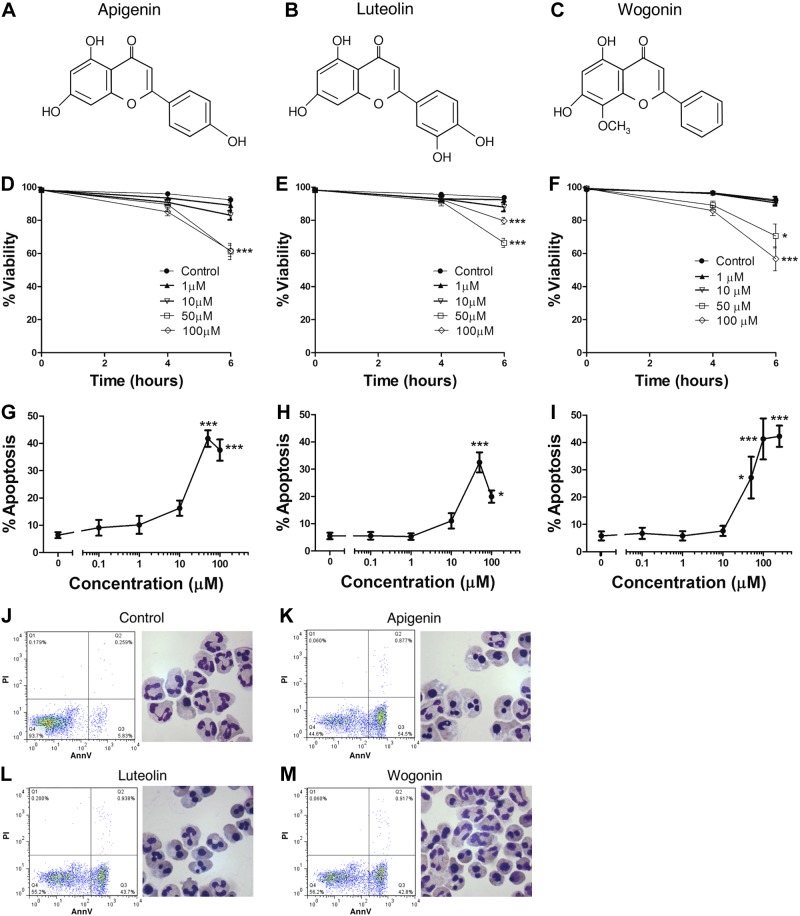
To investigate the ability of the flavones to induce apoptosis, primary human neutrophils were incubated with increasing concentrations of apigenin, luteolin, and wogonin (chemical structures given in Fig. 1A–C) for 4–6 h before viability and apoptosis assessment by flow cytometry and morphological examination. All three flavones resulted in a time- and concentration-dependent decrease in neutrophil viability (Fig. 1D–F) secondary to the induction of neutrophil apoptosis (Fig. 1G–I). After 6 h of flavone treatment at concentrations ≥50 μM, there was a marked increase in annexin V positive cells (Fig. 1J–M). Similarly, cellular morphology revealed the appearance of cells with characteristic changes of apoptosis (such as nuclear condensation and cellular shrinkage) in flavone-treated groups. EC50 calculation revealed values of 12.2 μM for apigenin, 14.6 μM for luteolin, and 28.9 μM for wogonin at 6 h.
Figure 1.
Flavones apigenin, luteolin, and wogonin induce human neutrophil apoptosis in a time- and concentration-dependent manner. A–C) Chemical structures of the flavones apigenin (A), luteolin (B), and wogonin (C). Neutrophils were cultured for 4 or 6 h in increasing concentrations of the flavones apigenin, luteolin, and wogonin before neutrophil viability, apoptosis and necrosis determination by flow cytometric analysis of annexin-V (AnnV)/PI binding. D–F) A time- and concentration-dependent reduction in neutrophil viability is seen with apigenin (D), luteolin (E), and wogonin (F); n ≥ 4. G–I) Reduction in neutrophil viability is secondary to induction of neutrophil apoptosis with concentration response curves at 6 h of culture shown for apigenin (G), luteolin (H), and wogonin (I); n ≥ 4. J–M) Representative flow cytometry plots of annexin-V (AnnV; x axis)/PI (y axis) binding and representative cyto-centrifuge preparations after staining with Diff-Quick are shown at 6 h for control (J), 50 μM apigenin (K), 50 μM luteolin (L), and 50 μM wogonin (M). Cyto-centrifuge preparations (×1000 view) demonstrate increased numbers of neutrophils with apoptotic morphology in the flavone-treated group. Data are expressed as means ± se, analyzed by ANOVA with a Newman-Keuls multiple comparison test. *P < 0.05, ***P < 0.001 vs. control.
To investigate the effect of flavones on other neutrophil functions, we measured shape change, CD11b up-regulation, CD62L shedding, ROS generation, and intracellular calcium flux both in response to treatment with the flavones as well as with subsequent stimulation with neutrophil agonists (Supplemental Fig. S1). The flavones did not induce shape change or inhibit fMLP-induced shape change (Supplemental Fig. S1A–C). Although the flavones did not themselves induce changes in CD11b, the subsequent response to fMLP-induced CD11b was unchanged with apigenin (Supplemental Fig. S1D), reduced with luteolin (Supplemental Fig. S1E), and increased with wogonin (Supplemental Fig. S1F). Luteolin and wogonin both caused partial down-regulation of CD62L; however, fMLP-induced CD62L shedding was unchanged by pretreatment with the flavones (Supplemental Fig. S1G–I). PMA-induced ROS production was attenuated by all three flavones, consistent with their known role as antioxidants; however, the flavones by themselves did not induce ROS generation (Supplemental Fig. S1J–K). The protein kinase C inhibitor Ro 31-8220 (Ro) was included as a positive control, as ROS generation is known to be regulated by protein kinase C. Transient increases in intracellular calcium are essential for multiple neutrophil functions. The flavones did not cause a rise in intracellular calcium, with flavone-treated neutrophils still able to increase intracellular calcium in response to fMLP and PAF (Supplemental Fig. S1L). In contrast, the formyl peptide receptor 1 antagonist cyclosporine H markedly inhibited the intracellular calcium rise in response to fMLP.
Flavones induce caspase-dependent apoptosis in primary human neutrophils
Caspase enzymes play a critical role in the process of apoptosis. To investigate whether the caspase pathway was involved in flavone-induced neutrophil apoptosis, a highly effective pharmacological pan caspase inhibitor (QVD-OPh) was used. Flavone-induced apoptosis was dependent on caspase enzyme activation, as coincubation of neutrophils with Q-VD-OPh abolished the ability of the flavones to induce apoptosis at both 6 and 20 h of incubation (Fig. 2). Coincubation of flavones and Q-VD-OPh also prevented progression of neutrophils into secondary necrosis which was evident at the 20h time point (Fig. 2B). The temporal distribution of the necrosis, with apoptosis occurring before the onset of necrosis, as well as the observation that the necrosis can be abrogated by inhibition of apoptosis, confirms this as secondary necrosis. Furthermore, Q-VD-OPh also prevented constitutive neutrophil apoptosis, consistent with published literature (Fig. 2B and ref. 36).
Figure 2.
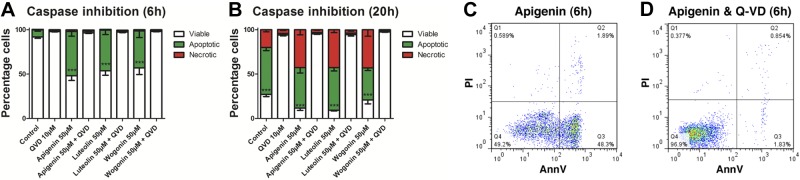
Flavones induce caspase-dependent apoptosis in primary human neutrophils. A, B) Neutrophils were cultured with the flavones apigenin, luteolin, and wogonin (all at 50 μM) with and without the broad spectrum caspase inhibitor Q-VD-OPh (Q-VD; 10 μM) before neutrophil viability, apoptosis, and necrosis assessment by flow cytometric analysis of annexin-V/PI binding at both 6 h (A) and 20 h (B); n ≥ 3. The presence of Q-VD-OPh dramatically inhibited the ability of flavones to induce apoptosis. C, D) Representative flow cytometry plots of annexin-V (x axis)/PI (y axis) binding are shown at 6 h for 50 μM apigenin (C) and 50 μM apigenin plus 10 μM Q-VD-OPh (D). Data are expressed as means ± se, analyzed by ANOVA with a Newman-Keuls multiple comparison test. ***P < 0.001 vs. Q-VD-OPh-treated sample.
Flavones induce apoptosis of neutrophils by down-regulation of Mcl-1 via a proteasomal-dependent pathway
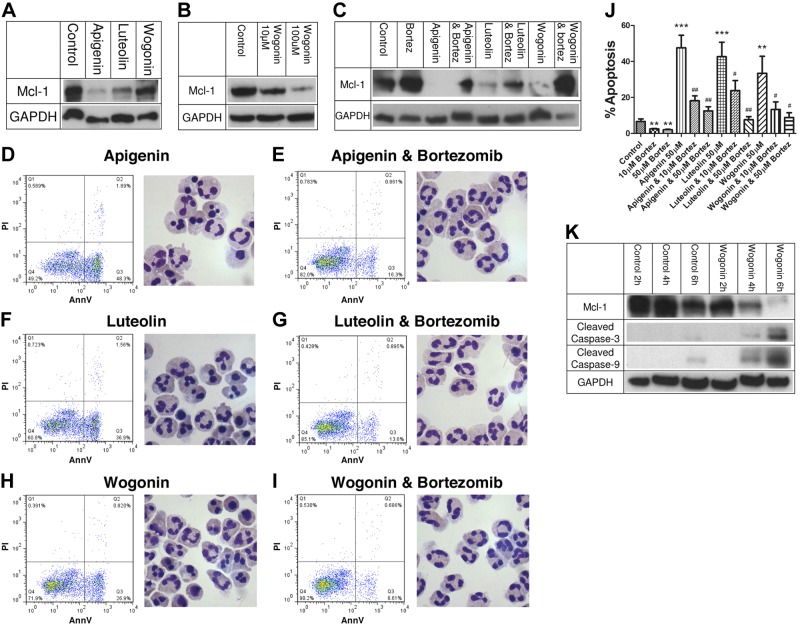
To further investigate the molecular mechanisms behind the flavones' ability to induce neutrophil apoptosis, Western blotting was carried out to determine levels of the key neutrophil intracellular survival protein Mcl-1 after 4 h of culture. The flavones apigenin and luteolin (both at 50 μM) caused significant down-regulation of Mcl-1 at the protein level (Fig. 3A). Although wogonin appeared to have only a small effect on Mcl-1 levels at 50 μM at this time point, a higher concentration of wogonin (100 μM) caused marked Mcl-1 down-regulation at 4 h (Fig. 3B). This is consistent with the relatively reduced potency of wogonin compared with the other two flavones. To investigate the mechanism behind the flavone-induced down-regulation of Mcl-1, neutrophils were cultured with the flavones apigenin, luteolin, and wogonin (all at 50 μM) with and without the proteasomal inhibitor bortezomib (at 10 μM) (37) for 4 h before Western blotting for Mcl-1. Proteasomal inhibition reduced the loss of Mcl-1 in flavone-treated neutrophils as well as reduced Mcl-1 loss in neutrophils undergoing constitutive apoptosis (control vs. bortezomib-treated neutrophils; Fig. 3C). Proteasomal inhibition, by reducing flavone-induced Mcl-1 down-regulation, also prevented flavone-induced neutrophil apoptosis as determined by flow cytometry and neutrophil morphology (Fig. 3D–I). The effects of proteasomal inhibition by bortezomib were concentration dependent, with a higher concentration of bortezomib (50 μM) reducing flavone-induced apoptosis back toward control levels (Fig. 3J). To further investigate the temporal association between flavone-induced Mcl-1 down-regulation and caspase activation, Western blotting was carried out at 2, 4, and 6 h in both control and wogonin-treated (50 μM) neutrophils (Fig. 3K). Mcl-1 down-regulation was evident by 4 h of culture, with accelerated caspase-3 cleavage in wogonin-treated cells from this time onward. In addition, wogonin caused early and accelerated caspase-9 cleavage, consistent with apoptosis induction via the intrinsic pathway of apoptosis (Fig. 3K). Furthermore, all 3 flavones enhanced loss of mitochondrial outer membrane potential in neutrophils (data not shown), a key event in the intrinsic pathway of apoptosis.
Figure 3.
Flavones induce apoptosis of neutrophils by down-regulation of Mcl-1 via a proteasomal dependent pathway. A) Neutrophils were cultured for 4 h in either control medium or 50 μM of the flavones apigenin, luteolin, and wogonin before Western blotting for Mcl-1 (40 kDa) and GAPDH (37 kDa). B) Neutrophils were cultured for 4 h in either control medium, 10 or 100 μM of the flavone wogonin before Western blotting for Mcl-1 and GAPDH. C) To investigate whether Mcl-1 down-regulation was dependent on proteasomal degradation of Mcl-1, neutrophils were cultured with the flavones apigenin, luteolin, and wogonin (all at 50 μM) with and without the proteasomal inhibitor bortezomib (Bortez; 10 μM) for 4 h before Western blotting for Mcl-1 and GAPDH. D–I) Proteasomal inhibition attenuated down-regulation of Mcl-1 as well as attenuated the ability of the flavones to induce apoptosis, with representative flow cytometry plots of annexin-V (x axis)/PI (y axis) binding and representative cyto-centrifuge preparations after staining with Diff-Quick shown for apigenin (50 μM) alone (D), apigenin plus bortezomib (Bortez; 10 μM; E), luteolin (50 μM) alone (F), luteolin plus bortezomib (G), wogonin (50 μM) alone (H), and wogonin plus bortezomib (I), all at 6 h. J) Cumulative apoptosis data for n = 4 experiments. K) To further investigate flavone-induced down-regulation of Mcl-1 and activation of caspases, neutrophils were cultured for 2, 4, or 6 h in either control medium or wogonin (50 μM) before lysis and Western blotting for Mcl-1, cleaved caspase-3 (17/19 kDa), cleaved caspase-9 (35/37 kDa), and GAPDH. All Western blots representative of n ≥ 3 separate experiments. Data are expressed as means ± se, analyzed by ANOVA with a Newman-Keuls multiple comparison test. **P < 0.01, ***P < 0.001 vs. control; #P < 0.05, ##P < 0.01 vs. flavone-treated sample.
Prosurvival effects of multiple inflammatory mediators on neutrophil life span can be overridden by flavones
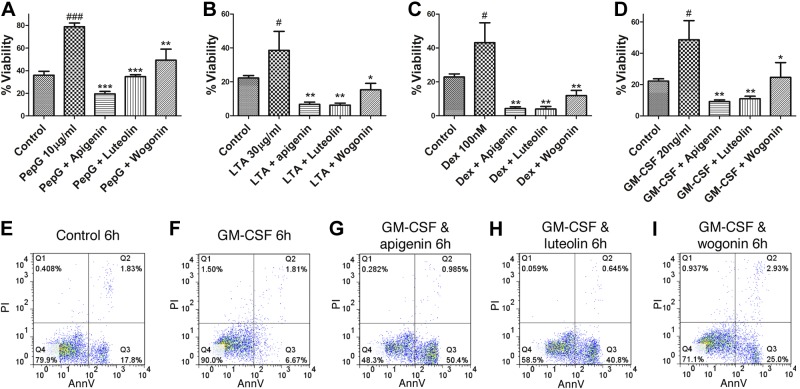
Neutrophils are exposed to inflammatory mediators at sites of inflammation that extend their life span by delaying apoptosis. As the flavones were able to induce apoptosis in unstimulated human neutrophils, we investigated the ability of flavones to counteract the prosurvival effects of multiple inflammatory mediators. The bacterial cell wall components peptidoglycan and lipoteichoic acid, the synthetic glucocorticoid dexamethasone, and GM-CSF all extended neutrophil life span at 20 h of culture (Fig. 4). The flavones apigenin, luteolin, and wogonin were able to override the antiapoptotic, prosurvival effect of all four mediators studied by inducing neutrophil apoptosis (Fig. 4).
Figure 4.
Prosurvival effects of multiple inflammatory mediators on neutrophil life span can be overridden by flavones. Neutrophils were cultured in the presence of the neutrophil prosurvival factors peptidoglycan (PepG; 10 μg/ml), LTA (30 μg/ml), dexamethasone (Dex; 100 nM), or granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng/ml) with and without the flavones apigenin, luteolin and wogonin (all at 100μM) before flow cytometric analysis of annexin-V/PI binding. A–D) Neutrophil viability after 20 h for peptidoglycan (A), LTA (B), dexamethasone (C), and GM-CSF (D); n = 3–4. D–I) Representative flow cytometry plots of annexin-V (x axis)/PI (y axis) binding for control (E), GM-CSF alone (20 ng/ml; F), GM-CSF and apigenin (G), GM-CSF and luteolin (H), and GM-CSF and wogonin (I) at 6 h of culture. Data are expressed as means ± se, analyzed by ANOVA with a Newman-Keuls multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001 vs. prosurvival factor alone; #P < 0.05, ###P < 0.001 vs. control.
Wogonin enhances resolution of sterile neutrophilic inflammation
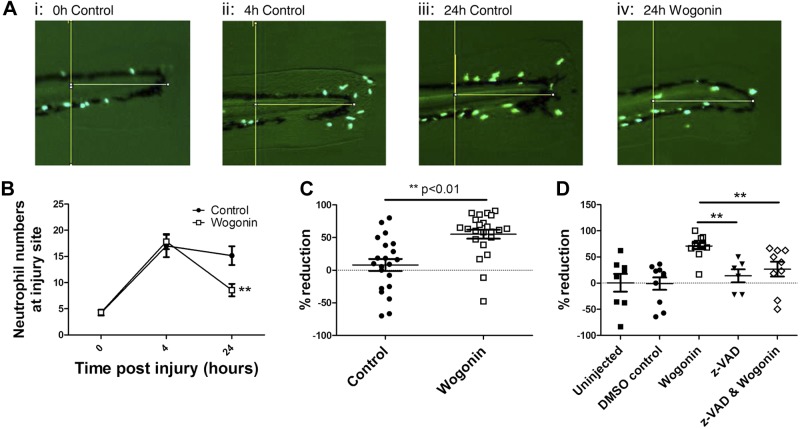
Having demonstrated that the flavones were able to induce neutrophil apoptosis in vitro, we examined the ability of flavones to enhance resolution in established neutrophilic inflammation using a zebrafish model of sterile tissue injury. Initial experiments suggested that wogonin had the most potent effect in our in vivo zebrafish model (data not shown), and this was therefore used as the lead compound for all in vivo experiments. By use of transgenic zebrafish [Tg(mpx:eGFP)], which have fluorescently labeled neutrophils, neutrophil presence at the site of injury can be serially imaged in a single organism. Following sterile transection of the tail fin, neutrophils are observed at the site of injury within 1 h (data not shown) with peak neutrophil numbers observed at around 4 h postinjury (Fig. 5B). By 24 h postinjury, spontaneous resolution of the neutrophilic inflammation begins with reductions in neutrophil numbers observed (Fig. 5A, B and ref. 34). Addition of wogonin (50 μM) at the peak of inflammation (4 h post-tail fin transection) was able to enhance resolution of established neutrophilic inflammation as assessed by cellular infiltration to the site of injury (Fig. 5). The percentage reduction in neutrophils from peak of inflammation to the 24 h time point in each individual fish is also shown (Fig. 5C). This gave a resolution interval (Ri; the time taken for neutrophil numbers at the site of inflammation to decline to half maximal (38, 39) for wogonin of ∼23 h, whereas the Ri for control zebrafish was >48 h (data not shown). To assess whether wogonin was causing enhanced resolution of neutrophilic inflammation by induction of apoptosis in vivo, separate zebrafish were microinjected with the caspase inhibitor zVAD to inhibit apoptosis. Treatment with zVAD attenuated the ability of wogonin to reduce the neutrophilic inflammation, confirming wogonin-induced caspase-dependent apoptosis was occurring in vivo (Fig. 5D).
Figure 5.
The flavone wogonin enhances resolution of sterile neutrophilic inflammation. At 3 d postfertilization, transgenic zebrafish [Tg(mpx:eGFPi114)], which have fluorescently labeled (eGFP) neutrophils, underwent tailfin transection to induce sterile tissue injury. Individual fish were then serially imaged at 0, 4, and 24 h postinjury with quantification of neutrophil numbers at the site of injury with wogonin (50 μM) or vehicle control added at 4 h postinjury. A) Representative images (× 8 view) taken by fluorescent microscope with a GFP filter at 0 h (i), 4 h (ii), 24 h control (iii), and 24 h wogonin-treated (iv). B) Cumulative data (n≥23, 5 separate experiments). C) Percentage reduction in neutrophil numbers between 4 and 24 h for each fish. D) To assess the role of apoptosis in the wogonin-treated fish, zebrafish were microinjected with the caspase inhibitor zVAD at 3.5 h postinjury, with addition of wogonin to the medium at 4 h postinjury. Neutrophil numbers were imaged at 4 and 24 h; percentage reduction in neutrophil numbers between these time points is shown (n≥6, 2 separate experiments). Data are expressed as means ± se, analyzed by a 2-tailed unpaired t test (B, C) or by ANOVA with a Newman-Keuls multiple comparison test (D). **P < 0.01 vs. control (B, C) or wogonin (D).
DISCUSSION
Neutrophils are central to host immunity, with a key role in the defense against invading bacteria, fungi, and viruses. Their normally short life span in the circulation can be manipulated and extended at sites of inflammation to ensure an appropriate host response is mounted in response to infection or tissue injury. However, uncontrolled neutrophil recruitment or inappropriate neutrophil longevity is pathophysiologically involved in inflammatory diseases with unresolved neutrophilic inflammation and low levels of neutrophil apoptosis seen in multiple human inflammatory disease states. Pharmacological manipulation of such defective resolution pathways is therefore an attractive avenue for the development of novel proresolution anti-inflammatory treatments (40). Modulation of granulocyte apoptosis either by inhibition of kinases, by ligation of death receptors, or by use of lipid mediators have demonstrated efficacy in multiple preclinical models of inflammatory disease (10, 11, 13, 15). Furthermore, the efficacy of glucocorticosteroids in the treatment of inflammatory diseases such as asthma is thought to be linked to their ability to enhance eosinophil apoptosis, with increased eosinophil apoptosis seen in asthma following commencement of steroid treatment (41). This is in contrast to use of glucocorticosteroids in neutrophilic inflammation where their relative lack of efficacy may be linked to their ability to inhibit neutrophil apoptosis (42).
Here we have shown that polyphenolic plant-derived compounds, the flavones, induce primary neutrophil apoptosis in vitro. Apigenin (derived from parsley), luteolin (present in a wide variety of leaves as well as celery and peppers), and wogonin (derived from Scutellaria and an active ingredient in the Japanese herbal supplement sho-saiko-to) were all able to drive neutrophil apoptosis in a time- and concentration-dependent fashion. Furthermore, the flavones were able to override multiple powerful prosurvival mediators, including bacterial constituents [e.g., lipoteichoic acid (LTA) and peptidoglycan] and host-derived factors (e.g., GM-CSF), frequently found at increased concentration at sites of inflammation where they act to delay neutrophil apoptosis. This ability of flavones to override survival factors and drive apoptosis is important to ensure that such neutrophil apoptosis-inducing effects are not lost at sites of inflammation in vivo. An apoptosis-inducing effect of flavones on neutrophils is in contrast to their studied effects on cardiomyocytes, neurones, and lymphocytes, where inhibition of apoptosis is observed (43–45). It is not entirely clear why the effect of flavones on apoptosis varies between cell types; however, recent evidence has suggested that flavones prevent cardiomyocyte apoptosis by inhibiting glycogen synthase kinase-3β (GSK-3β) induced p53 translocation in this cell type (46). By comparison, polyphenolic compounds, including flavones, have been demonstrated to exert a proapoptotic effect on malignant cells both in vitro and in vivo (47–49). However, it is clear that pharmacological induction of apoptosis is a highly cell type specific event with dexamethasone delaying neutrophil apoptosis but inducing rapid apoptosis in the closely related eosinophil.
Apoptosis can proceed via either an extrinsic pathway on ligation of a death receptor (including TNF-α, Fas, and TRAIL) or an intrinsic pathway in response to cellular stress (withdrawal of survival factors, ischemia, etc.), chemotherapeutic agents, or ultraviolet damage. The intrinsic pathway is controlled by the balance of pro- vs. antiapoptotic proteins from the B-cell lymphoma 2 (Bcl-2) family, which together regulate mitochondrial outer membrane permeabilization and release of proapoptotic substances (such as cytochrome c) from mitochondria (40). Both pathways eventually act through activation of enzymes called caspases (cysteine aspartic specific proteases), which are responsible for the numerous morphological and biochemical changes of apoptosis. Flavone-induced neutrophil apoptosis was critically dependent on activity of caspase enzymes, as apoptosis was completely blocked in vitro by use of the broad-spectrum caspase inhibitor Q-VD-OPh. Consistent with this dependence on activated caspases for the action of flavones, we observed that wogonin caused accelerated activation of caspase-3 which was evident by 4 h, before the induction of apoptosis, which was observable by 6 h. Furthermore, the proresolution effect of wogonin in vivo was dependent on activation of caspases as it was inhibited by the broad-spectrum caspase inhibitor zVAD (Fig. 5D).
Both extrinsic and intrinsic pathways of apoptosis can affect neutrophil life span, with TRAIL and Fas ligation causing neutrophil apoptosis while TNF-α causes early apoptosis but late neutrophil survival dependent on NF-κB activation (32, 50). Cytokine-mediated neutrophil survival occurs secondary to Bcl-2 member alterations such as Bax down-regulation (a proapoptotic member; refs. 51, 52) while pharmacological induction of neutrophil apoptosis by cyclin-dependent kinase inhibitors or sodium salicylayte can do so by down-regulating prosurvival Bcl-2 members (28, 53). Neutrophils lack the main Bcl-2 antiapoptotic homologue Bcl-2 itself and instead have increased dependence on the antiapoptotic Mcl-1. Mcl-1 is an unusual Bcl-2 member, as it has a very short half-life of only a few hours, secondary to PEST domains [proline (P), glutamic acid (E), serine (S), threonine (T)] and constitutive ubiquitination, which target the protein for proteasomal degredation. The importance of Mcl-1 in regulating neutrophil survival is revealed by myeloid specific deletion of Mcl-1 in mice, which leads to neutropaenia without any observable defects in macrophage number or function (54). Furthermore, Mcl-1 levels can be influenced by neutrophil prosurvival factors, with GM-CSF, hypoxia, and cAMP cyclic adenosine monophosphate (cAMP) all promoting neutrophil longevity with enhanced Mcl-1 levels (55–58). Flavone-induced neutrophil apoptosis occurs following down-regulation of Mcl-1, with levels of Mcl-1 falling before induction of apoptosis suggesting that the flavone-induced decrease in Mcl-1 is a key and early event in the induction of neutrophil apoptosis. Flavone-induced down-regulation was dependent on activity of the proteasome as use of the proteasomal inhibitor bortezomib (37) increased Mcl-1 levels in flavone-treated neutrophils. Furthermore, enhanced levels of Mcl-1 secondary to proteasome inhibition prevented both constitutive and flavone-induced neutrophil apoptosis, confirming the key role of Mcl-1 levels in governing the onset of neutrophil apoptosis. Whether flavones enhance Mcl-1 targeting for proteasomal degredation or increase the general activity of the proteasome remains to be investigated. Such alterations in Mcl-1 levels following flavone treatment, accelerated appearance of cleaved caspase-9, and enhanced loss of neutrophil mitochondrial outer membrane potential (data not shown) together confirm that flavones act via the intrinsic pathway of apoptosis in neutrophils.
Anti-inflammatory properties of flavones have been suggested for some time, with a high dietary intake in humans associated with a reduced incidence of inflammatory diseases, such as atherosclerosis (21). The proposed mechanism behind such anti-inflammatory properties of flavones in humans centers around their ability to act as antioxidant agents (Supplemental Fig. S1 and ref. 23). While their antioxidant abilities may play a role in the protection against atheroma, such antioxidant activity is unlikely to be the mechanism behind flavone-induced neutrophil apoptosis, especially considering that the flavones by themselves did not induce changes in neutrophil ROS generation. Furthermore, antioxidant molecules traditionally cause enhanced neutrophil life span by delaying neutrophil apoptosis (59, 60), while production of neutrophil ROS is associated with the induction of neutrophil apoptosis in a variety of experimental situations (61).
Preclinical investigation of flavones have also revealed anti-inflammatory properties with luteolin attenuating endotoxin-mediated lung injury and bleomycin-induced lung fibrosis in mice (62–64), concomitant baicalin (a flavone) and antibiotics showing beneficial effects in experimental meningitis (65), and baicalin alone improving survival in a mouse model of polymicrobial sepsis secondary to caecal ligation and puncture (45). In these studies, the suggested mechanism behind the anti-inflammatory effects of flavones have varied from inhibition of intracellular signaling cascades [such as mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase (PI3K)], to reduced cytokine production (such as TNF-α and IL-1), to enhanced clearance of bacteria, and to inhibition of lymphocyte apoptosis. In several of these studies, reduced levels of neutrophils in inflammatory exudates have been observed (45, 63, 64, 66), but to our knowledge our study is the first to characterize the direct effects of flavones on the regulation of neutrophil life span. Furthermore, we have demonstrated the utility of such neutrophil-apoptosis inducing effects in vivo, by using wogonin to enhance the resolution of established neutrophilic inflammation following sterile tissue injury. Our initial in vivo experiments in the zebrafish suggested wogonin had the most potent proresolution effects (data not shown), despite having the highest EC50 of all three compounds tested. This may in part relate to the poor water solubility of the flavone compounds, a factor of vital importance in the aqueous environment of the zebrafish. This issue of drug solubility and poor absorption in the zebrafish has been reported by others (67) and was also demonstrated with use of the broad spectrum caspase inhibitor zVAD, which we found was only effective following microinjection (Fig. 5 and data not shown). Our studies have clearly shown a role for apoptosis in the wogonin-induced resolution of zebrafish inflammation and like the mammalian system it has recently become evident that proresolution processes in the zebrafish also involve switching from proinflammatory lipid synthesis to the active generation of anti-inflammatory and proresolution lipids (68). Thus, we believe that careful future lipidomic analysis of proinflammatory, anti-inflammatory, and proresolution lipid profiles in the zebrafish in response to flavone treatment is warranted to better understand inflammation resolution in our model.
Although apigenin has previously been reported to be potentially proinflammatory in a rat model of intrapleural carrageenan-induced inflammation due to an increase in total number of inflammatory cells (19), differential inflammatory cell counts were not given and total exudate volume was unchanged with apigenin administration. In this experimental setup, it would be key to know whether apigenin administration had effects on the relative numbers of neutrophils in the exudate fluid, especially as others have reported anti-inflammatory effects of apigenin in rodents in other experimental models (69). Alternatively, it may well be possible that apigenin may exert differential effects when administered at different phases of the inflammatory response or that effects may be inflammatory stimulus specific.
Although any potential neutrophil apoptosis-inducing proresolution therapy needs to be balanced with the absolute requirement of functioning neutrophils for protection against invading pathogens, preclincal experiments have not revealed any impaired ability to control nonsterile inflammation. For example, induction of neutrophil apoptosis along with antimicrobial therapy in a model of bacterial meningitis leads to both reduced inflammation and enhanced recovery from infection (12), while proresolving lipid mediators such as resolvin E1 reduce neutrophil number while also enhancing bacterial clearance in a rodent model of pneumonia (17). Conversly, delaying neutrophil apoptosis by deletion of the proapoptotic Bcl-2 family member p53 upregulated modulator of apoptosis (PUMA) leads to enhanced inflammation and an inability to control bacterial infection (70). These findings may in part be explained by stimulated, inflammatory neutrophils having altered sensitivity toward apoptosis-inducing stimuli. For example, TRAIL-deficient mice show normal circulating neutrophil numbers and normal constitutive neutrophil apoptosis but demonstrate impaired apoptosis when exposed to an inflammatory environment (13), while the cyclin-dependent kinase inhibitor induced down-regulation of Mcl-1 is accelerated in the presence of GM-CSF (10). Such findings suggest that neutrophil apoptosis-inducing strategies such as flavones may allow ongoing antimicrobial clearance while targeting excessive inflammation even in nonsterile human inflammatory diseases.
In summary, we have demonstrated that flavones drive neutrophil apoptosis in a caspase-dependent fashion to override survival factor-induced delay of apoptosis by down-regulating the key neutrophil survival protein Mcl-1 in a proteasomal-dependent fashion. Enhanced resolution of inflammation is seen following addition of wogonin to already established neutrophilic inflammation in vivo. As such, flavones may be a novel class of neutrophil apoptosis-inducing anti-inflammatory compounds for the treatment of human inflammatory disease.
Supplementary Material
Acknowledgments
The authors thank Dr. Steve Renshaw (University of Sheffield, Sheffield, UK) for use of the transgenic zebrafish used in our in vivo experiments and Fiona Rossi and Shonna Johnston for assistance with flow cytometry.
This work was funded by the Wellcome Trust (WT094415) and the UK Medical Research Council (G0601481).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Bcl-2
- B-cell lymphoma 2
- cAMP
- cyclic adenosine monophosphate
- fMLP
- N-formyl-Met-Leu-Phe
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- GSK-3β
- glycogen synthase kinase-3β
- LTA
- lipoteichoic acid
- Mcl-1
- myeloid cell leukemia-1
- PAF
- platelet-activating factor
- PI
- propidium iodide
- PMA
- phorbol 12-myristate 13-acetate
- PUMA
- p53 upregulated modulator of apoptosis
- ROS
- reactive oxygen species
- TRAIL
- TNF-related apoptosis inducing ligand
REFERENCES
- 1. Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 [DOI] [PubMed] [Google Scholar]
- 2. Duffin R., Leitch A. E., Fox S., Haslett C., Rossi A. G. (2010) Targeting granulocyte apoptosis: mechanisms, models, and therapies. Immunol. Rev. 236, 28–40 [DOI] [PubMed] [Google Scholar]
- 3. Serhan C. N., Brain S. D., Buckley C. D., Gilroy D. W., Haslett C., O'Neill L. A., Perretti M., Rossi A. G., Wallace J. L. (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fox S., Leitch A. E., Duffin R., Haslett C., Rossi A. G. (2010) Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J. Innate Immun. 2, 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michlewska S., Dransfield I., Megson I. L., Rossi A. G. (2009) Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 23, 844–854 [DOI] [PubMed] [Google Scholar]
- 6. Raza K., Scheel-Toellner D., Lee C. Y., Pilling D., Curnow S. J., Falciani F., Trevino V., Kumar K., Assi L. K., Lord J. M., Gordon C., Buckley C. D., Salmon M. (2006) Synovial fluid leukocyte apoptosis is inhibited in patients with very early rheumatoid arthritis. Arthritis Res. Ther. 8, R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fialkow L., Fochesatto Filho L., Bozzetti M. C., Milani A. R., Rodrigues Filho E. M., Ladniuk R. M., Pierozan P., de Moura R. M., Prolla J. C., Vachon E., Downey G. P. (2006) Neutrophil apoptosis: a marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit. Care 10, R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munoz L. E., Lauber K., Schiller M., Manfredi A. A., Herrmann M. (2010) The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 6, 280–289 [DOI] [PubMed] [Google Scholar]
- 9. Fitzpatrick A. M., Holguin F., Teague W. G., Brown L. A. (2008) Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J. Allergy Clin. Immunol. 121, 1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rossi A. G., Sawatzky D. A., Walker A., Ward C., Sheldrake T. A., Riley N. A., Caldicott A., Martinez-Losa M., Walker T. R., Duffin R., Gray M., Crescenzi E., Martin M. C., Brady H. J., Savill J. S., Dransfield I., Haslett C. (2006) Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12, 1056–1064 [DOI] [PubMed] [Google Scholar]
- 11. Leitch A. E., Lucas C. D., Marwick J. A., Duffin R., Haslett C., Rossi A. G. (2012) Cyclin-dependent kinases 7 and 9 specifically regulate neutrophil transcription and their inhibition drives apoptosis to promote resolution of inflammation. Cell Death Differ. 29, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koedel U., Frankenberg T., Kirschnek S., Obermaier B., Hacker H., Paul R., Hacker G. (2009) Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 5, e1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGrath E. E., Marriott H. M., Lawrie A., Francis S. E., Sabroe I., Renshaw S. A., Dockrell D. H., Whyte M. K. (2011) TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J. Leukoc. Biol. 2011, 855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leitch A. E., Lucas C. D., Rossi A. G. (2011) Neutrophil apoptosis: hot on the TRAIL of inflammatory resolution. J. Leukoc. Biol. 90, 841–843 [DOI] [PubMed] [Google Scholar]
- 15. El Kebir D., Jozsef L., Pan W., Wang L., Petasis N. A., Serhan C. N., Filep J. G. (2009) 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am. J. Respir. Crit. Care Med. 180, 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiang N., Fredman G., Backhed F., Oh S. F., Vickery T., Schmidt B. A., Serhan C. N. (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seki H., Fukunaga K., Arita M., Arai H., Nakanishi H., Taguchi R., Miyasho T., Takamiya R., Asano K., Ishizaka A., Takeda J., Levy B. D. (2010) The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 184, 836–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fischer C. D., Beatty J. K., Zvaigzne C. G., Morck D. W., Lucas M. J., Buret A. G. (2011) Anti-Inflammatory benefits of antibiotic-induced neutrophil apoptosis: tulathromycin induces caspase-3-dependent neutrophil programmed cell death and inhibits NF-kappaB signaling and CXCL8 transcription. Antimicrob. Agents Chemother. 55, 338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sawatzky D. A., Willoughby D. A., Colville-Nash P. R., Rossi A. G. (2006) The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute inflammation in vivo. Am. J. Pathol. 168, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havsteen B. H. (2002) The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 96, 67–202 [DOI] [PubMed] [Google Scholar]
- 21. McCullough M. L., Peterson J. J., Patel R., Jacques P. F., Shah R., Dwyer J. T. (2012) Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 95, 454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rossi L., Mazzitelli S., Arciello M., Capo C. R., Rotilio G. (2008) Benefits from dietary polyphenols for brain aging and Alzheimer's disease. Neurochem. Res. 33, 2390–2400 [DOI] [PubMed] [Google Scholar]
- 23. Serafini M., Peluso I., Raguzzini A. (2010) Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 69, 273–278 [DOI] [PubMed] [Google Scholar]
- 24. Huang C. H., Jan R. L., Kuo C. H., Chu Y. T., Wang W. L., Lee M. S., Chen H. N., Hung C. H. (2010) Natural flavone kaempferol suppresses chemokines expression in human monocyte THP-1 cells through MAPK pathways. J. Food Sci. 75, H254–259 [DOI] [PubMed] [Google Scholar]
- 25. Yi Lau G. T., Leung L. K. (2010) The dietary flavonoid apigenin blocks phorbol 12-myristate 13-acetate-induced COX-2 transcriptional activity in breast cell lines. Food Chem. Toxicol. 48, 3022–3027 [DOI] [PubMed] [Google Scholar]
- 26. Chen S. R., Xu X. Z., Wang Y. H., Chen J. W., Xu S. W., Gu L. Q., Liu P. Q. (2010) Icariin derivative inhibits inflammation through suppression of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways. Biol. Pharm. Bull. 33, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 27. Mann G. E., Rowlands D. J., Li F. Y., de Winter P., Siow R. C. (2007) Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc. Res. 75, 261–274 [DOI] [PubMed] [Google Scholar]
- 28. Leitch A. E., Riley N. A., Sheldrake T. A., Festa M., Fox S., Duffin R., Haslett C., Rossi A. G. (2010) The cyclin-dependent kinase inhibitor R-roscovitine down-regulates Mcl-1 to override pro-inflammatory signalling and drive neutrophil apoptosis. Eur. J. Immunol. 40, 1127–1138 [DOI] [PubMed] [Google Scholar]
- 29. Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr., Henson P. M. (1985) Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 119, 101–110 [PMC free article] [PubMed] [Google Scholar]
- 30. Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. (1989) Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83, 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Flaherty J. T., Rossi A. G. (1993) 5-hydroxyicosatetraenoate stimulates neutrophils by a stereospecific, G protein-linked mechanism. J. Biol. Chem. 268, 14708–14714 [PubMed] [Google Scholar]
- 32. Ward C., Chilvers E. R., Lawson M. F., Pryde J. G., Fujihara S., Farrow S. N., Haslett C., Rossi A. G. (1999) NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 274, 4309–4318 [DOI] [PubMed] [Google Scholar]
- 33. Renshaw S. A., Loynes C. A., Trushell D. M., Elworthy S., Ingham P. W., Whyte M. K. (2006) A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976–3978 [DOI] [PubMed] [Google Scholar]
- 34. Loynes C. A., Martin J. S., Robertson A., Trushell D. M., Ingham P. W., Whyte M. K., Renshaw S. A. (2010) Pivotal Advance: Pharmacological manipulation of inflammation resolution during spontaneously resolving tissue neutrophilia in the zebrafish. J. Leukoc. Biol. 87, 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Westerfield M. (2007) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), University of Oregon Press, Eugene, OR, USA [Google Scholar]
- 36. Wardle D. J., Burgon J., Sabroe I., Bingle C. D., Whyte M. K., Renshaw S. A. (2011) Effective caspase inhibition blocks neutrophil apoptosis and reveals Mcl-1 as both a regulator and a target of neutrophil caspase activation PLoS One 6, e15768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hideshima T., Richardson P., Chauhan D., Palombella V. J., Elliott P. J., Adams J., Anderson K. C. (2001) The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 61, 3071–3076 [PubMed] [Google Scholar]
- 38. Bannenberg G. L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K. H., Hong S., Serhan C. N. (2005) Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 [DOI] [PubMed] [Google Scholar]
- 39. Schwab J. M., Chiang N., Arita M., Serhan C. N. (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dorward D. A., Lucas C. D., Rossi A. G., Haslett C., Dhaliwal K. (2012) Imaging inflammation: molecular strategies to visualize key components of the inflammatory cascade, from initiation to resolution. Pharmacol. Ther. 135, 182–199 [DOI] [PubMed] [Google Scholar]
- 41. Woolley K. L., Gibson P. G., Carty K., Wilson A. J., Twaddell S. H., Woolley M. J. (1996) Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am. J. Respir. Crit. Care Med. 154, 237–243 [DOI] [PubMed] [Google Scholar]
- 42. Meagher L. C., Cousin J. M., Seckl J. R., Haslett C. (1996) Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J. Immunol. 156, 4422–4428 [PubMed] [Google Scholar]
- 43. Qi L., Pan H., Li D., Fang F., Chen D., Sun H. (2011) Luteolin improves contractile function and attenuates apoptosis following ischemia-reperfusion in adult rat cardiomyocytes. Eur. J. Pharmacol. 668, 201–207 [DOI] [PubMed] [Google Scholar]
- 44. Yamamoto Y., Shioda N., Han F., Moriguchi S., Nakajima A., Yokosuka A., Mimaki Y., Sashida Y., Yamakuni T., Ohizumi Y., Fukunaga K. (2009) Nobiletin improves brain ischemia-induced learning and memory deficits through stimulation of CaMKII and CREB phosphorylation. Brain Res. 1295, 218–229 [DOI] [PubMed] [Google Scholar]
- 45. Zhu J., Wang J., Sheng Y., Zou Y., Bo L., Wang F., Lou J., Fan X., Bao R., Wu Y., Chen F., Deng X., Li J. (2012) Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One 7, e35523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin C. L., Tseng H. C., Chen R. F., Chen W. P., Su M. J., Fang K. M., Wu M. L. (2011) Intracellular zinc release-activated ERK-dependent GSK-3beta-p53 and Noxa-Mcl-1 signaling are both involved in cardiac ischemic-reperfusion injury. Cell Death Differ. 18, 1651–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jacquemin G., Shirley S., Micheau O. (2010) Combining naturally occurring polyphenols with TNF-related apoptosis-inducing ligand: a promising approach to kill resistant cancer cells? Cell. Mol. Life Sci. 67, 3115–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brizuela L., Dayon A., Doumerc N., Ader I., Golzio M., Izard J. C., Hara Y., Malavaud B., Cuvillier O. (2010) The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB J. 24, 3882–3894 [DOI] [PubMed] [Google Scholar]
- 49. Walter A., Etienne-Selloum N., Brasse D., Khallouf H., Bronner C., Rio M. C., Beretz A., Schini-Kerth V. B. (2010) Intake of grape-derived polyphenols reduces C26 tumor growth by inhibiting angiogenesis and inducing apoptosis. FASEB J. 24, 3360–3369 [DOI] [PubMed] [Google Scholar]
- 50. Akgul C., Edwards S. W. (2003) Regulation of neutrophil apoptosis via death receptors. Cell. Mol. Life Sci. 60, 2402–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dibbert B., Weber M., Nikolaizik W. H., Vogt P., Schoni M. H., Blaser K., Simon H. U. (1999) Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc. Natl. Acad. Sci. U. S. A. 96, 13330–13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moulding D. A., Akgul C., Derouet M., White M. R., Edwards S. W. (2001) BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis. J. Leukoc. Biol. 70, 783–792 [PubMed] [Google Scholar]
- 53. Derouet M., Thomas L., Moulding D. A., Akgul C., Cross A., Moots R. J., Edwards S. W. (2006) Sodium salicylate promotes neutrophil apoptosis by stimulating caspase-dependent turnover of Mcl-1. J. Immunol. 176, 957–965 [DOI] [PubMed] [Google Scholar]
- 54. Dzhagalov I., St John A., He Y. W. (2007) The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 109, 1620–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Derouet M., Thomas L., Cross A., Moots R. J., Edwards S. W. (2004) Granulocyte macrophage colony-stimulating factor signaling and proteasome inhibition delay neutrophil apoptosis by increasing the stability of Mcl-1. J. Biol. Chem. 279, 26915–26921 [DOI] [PubMed] [Google Scholar]
- 56. Leuenroth S. J., Grutkoski P. S., Ayala A., Simms H. H. (2000) Suppression of PMN apoptosis by hypoxia is dependent on Mcl-1 and MAPK activity. Surgery 128, 171–177 [DOI] [PubMed] [Google Scholar]
- 57. Kato T., Kutsuna H., Oshitani N., Kitagawa S. (2006) Cyclic AMP delays neutrophil apoptosis via stabilization of Mcl-1. FEBS Lett. 580, 4582–4586 [DOI] [PubMed] [Google Scholar]
- 58. Akgul C., Turner P. C., White M. R., Edwards S. W. (2000) Functional analysis of the human MCL-1 gene. Cell. Mol. Life. Sci. 57, 684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Oishi K., Machida K. (1997) Inhibition of neutrophil apoptosis by antioxidants in culture medium. Scand. J. Immunol. 45, 21–27 [DOI] [PubMed] [Google Scholar]
- 60. Watson R. W., Redmond H. P., Wang J. H., Condron C., Bouchier-Hayes D. (1996) Neutrophils undergo apoptosis following ingestion of Escherichia coli. J. Immunol. 156, 3986–3992 [PubMed] [Google Scholar]
- 61. Simon H. U., Haj-Yehia A., Levi-Schaffer F. (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5, 415–418 [DOI] [PubMed] [Google Scholar]
- 62. Lee J. P., Li Y. C., Chen H. Y., Lin R. H., Huang S. S., Chen H. L., Kuan P. C., Liao M. F., Chen C. J., Kuan Y. H. (2010) Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol. Sin. 31, 831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kuo M. Y., Liao M. F., Chen F. L., Li Y. C., Yang M. L., Lin R. H., Kuan Y. H. (2011) Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NFkappaB pathways in mice with endotoxin-induced acute lung injury. Food Chem. Toxicol. 49, 2660–2666 [DOI] [PubMed] [Google Scholar]
- 64. Chen C. Y., Peng W. H., Wu L. C., Wu C. C., Hsu S. L. (2010) Luteolin ameliorates experimental lung fibrosis both in vivo and in vitro: implications for therapy of lung fibrosis. J. Agric. Food Chem. 58, 11653–11661 [DOI] [PubMed] [Google Scholar]
- 65. Tang Y. J., Zhou F. W., Luo Z. Q., Li X. Z., Yan H. M., Wang M. J., Huang F. R., Yue S. J. (2010) Multiple therapeutic effects of adjunctive baicalin therapy in experimental bacterial meningitis. Inflammation 33, 180–188 [DOI] [PubMed] [Google Scholar]
- 66. Birrell M. A., McCluskie K., Wong S., Donnelly L. E., Barnes P. J., Belvisi M. G. (2005) Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J. 19, 840–841 [DOI] [PubMed] [Google Scholar]
- 67. Milan D. J., Peterson T. A., Ruskin J. N., Peterson R. T., MacRae C. A. (2003) Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 107, 1355–1358 [DOI] [PubMed] [Google Scholar]
- 68. Tobin D. M., Roca F. J., Oh S. F., McFarland R., Vickery T. W., Ray J. P., Ko D. C., Zou Y., Bang N. D., Chau T. T., Vary J. C., Hawn T. R., Dunstan S. J., Farrar J. J., Thwaites G. E., King M. C., Serhan C. N., Ramakrishnan L. (2012) Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148, 434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rithidech K. N., Tungjai M., Reungpatthanaphong P., Honikel L., Simon S. R. (2012) Attenuation of oxidative damage and inflammatory responses by apigenin given to mice after irradiation. Mutat. Res. 15, 00268–00269 [DOI] [PubMed] [Google Scholar]
- 70. Garrison S. P., Thornton J. A., Hacker H., Webby R., Rehg J. E., Parganas E., Zambetti G. P., Tuomanen E. I. (2010) The p53-target gene puma drives neutrophil-mediated protection against lethal bacterial sepsis. PLoS Pathog. 6, e1001240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.