Abstract
Xenopus laevis adults mount effective immune responses to ranavirus Frog Virus 3 (FV3) infections and clear the pathogen within 2–3 weeks. In contrast, most tadpoles cannot clear FV3 and succumb to infections within a month. While larval susceptibility has been attributed to ineffective adaptive immunity, the contribution of innate immune components has not been addressed. Accordingly, we performed a comprehensive gene expression analysis on FV3-infected tadpoles and adults. In comparison to adults, leukocytes and tissues of infected tadpoles exhibited modest (10–100 time lower than adult) and delayed (3 day later than adult) increase in expression of inflammation-associated (TNF-α, IL-1β and IFN-γ) and antiviral (Mx1) genes. In contrast, these genes were readily and robustly upregulated in tadpoles upon bacterial stimulation. Furthermore, greater proportions of larval than adult PLs were infected by FV3. Our study suggests that tadpole susceptibility to FV3 infection is partially due to poor virus-elicited innate immune responses.
Keywords: Anti-viral immunity, Viral infection, Amphibian, Innate immunity
Introduction
The tadpole and adult forms of the amphibian Xenopus laevis each display distinct immune systems. This peculiarity affords a unique opportunity to compare and contrast immune responses in the same organism. Although both tadpoles and adults are immunocompetent, both B and T cell responses are weaker in larvae (Reviewed in (Du Pasquier et al., 1989; Robert and Ohta, 2009). In particular, there is no consistent expression of MHC class I protein until metamorphosis, although thymic derived CD8 T cells are present (Flajnik and Du Pasquier, 1988; Flajnik et al., 1986). Further weakness of larval adaptive immunity includes a poor switch from IgM to IgY, an affinity of antibody lower than adult, and incomplete skin graft rejection capacity (Chardonnens and Du Pasquier, 1973; DiMarzo and Cohen, 1982b; Hsu and Du Pasquier, 1984). Besides the observed absence of NK cells until metamorphosis (Horton et al., 2003), little is known about tadpole innate immune responses.
We have developed X. laevis as a reliable model system to explore the evolution of viral immunity as well as to better evaluate host factors involved in susceptibility to emerging infectious diseases caused by ranavirus (RV) pathogens (Chinchar et al., 2009). RVs have become a major concern for captive and wild amphibians, fish, and other ectothermic species worldwide. In fact, ranavirus infections were the leading causes of amphibian mortality in the US between 1996–2001 (Green et al., 2002; Schloegel et al., 2010). We have focused our study on Frog Virus 3 (FV3), which is the main member and the type species of the RV genus. FV3 is a large (200 nm) poxvirus-like, double stranded DNA virus that is infectious in both enveloped and non-enveloped form (reviewed in Chinchar et al. (2009)). FV3 or FV3-like viruses are now found worldwide, infecting many different amphibian species, making it a serious global threat (Duffus et al., 2008; Gray et al., 2007; Mazzoni et al., 2009; Pearman et al., 2004).
The Xenopus adaptive immune response elicited during FV3 infection has been well characterized (Gantress et al., 2003; Robert et al., 2005). Adult frogs develop an effective CD8 T cell responses and clear FV3 within 2–3 weeks (Morales and Robert, 2007). Potent specific antibodies are also generated against FV3 in adults (Maniero et al., 2006). Recently, we began to characterize innate immune responses at an early stage of FV3 infection in adults that includes a rapid up-regulation of genes encoding the pro-inflammatory cytokines TNF-α and IL-1β (Morales et al., 2010). In contrast to adults, most Xenopus tadpoles (~90%) are unable to clear the virus and die within a few weeks after infection (Gantress et al., 2003). The high susceptibility of larval stages to FV3 infection is also documented for other anuran species in natural (Gray et al., 2009; Gray et al., 2007) and captive population (Mazzoni et al., 2009). The weaker or immature adaptive immune effector functions in tadpoles may explain this higher susceptibility. Indeed, our attempts to generate protective immunity by immunization and to detect an anti-FV3 antibody response have so far been unsuccessful. However, the variability of survival times observed among individuals suggests that the tadpole immune system is not completely inactive or ignorant of FV3 infection. Therefore, we postulate that in Xenopus tadpoles, some innate immune responses are elicited upon FV3 infection.
To assess this possibility and begin to characterize innate immunity in tadpoles, we determined the expression profiles of several relevant inflammation-associated genes (TNF-α, IL-1β, IFN-γ) and the type I IFN-inducible Myxovirus-resistance 1 (Mx1) gene during the early phase of FV3 infection. Surprisingly, the expression changes of these genes upon FV3 infections is delayed and of lower in magnitude in tadpoles compared to adults, which may be one of the reasons for the high susceptibility of tadpoles to FV3.
Results
Changes in inflammation-associated gene expression during FV3 infection
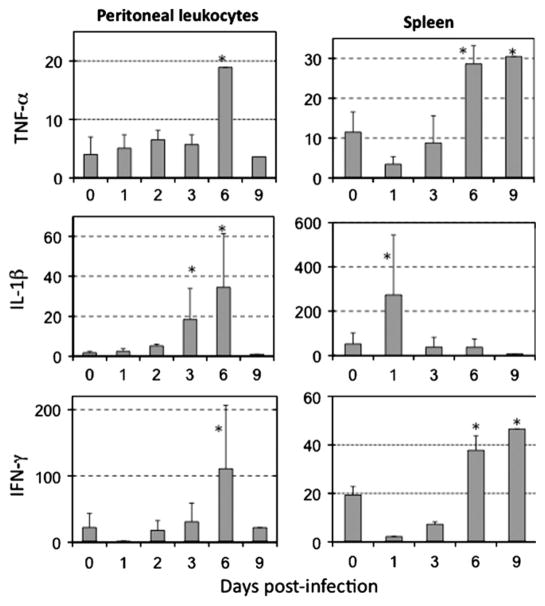
In adult X. laevis, increased expression of the pro-inflammatory genes IL-1β and TNF-α by peritoneal leukocyte (PL) can be detected as early as 1 day post-infection (dpi; (Morales et al., 2010). We investigated whether a similar gene expression kinetics are elicited in tadpoles upon FV3 infections. For this purpose, we used outbred pre-metamorphic tadpoles at developmental stage 56 (3–4 week post-fertilization; Supplementary Fig. 1), when the spleen is well developed and immune responses can be detected (review in Robert and Ohta (2009)). Tadpoles were infected for 1 to 9 days by a single i.p. injection of 1 × 104 PFU of FV3, and PLs and tissues were collected from 3 individuals at each time point for qPCR analysis. To obtain sufficient amounts of RNA from PLs and spleens, we pooled respective samples from three tadpoles. In three independent experiments using this approach (Fig. 1), we detected a consistently delayed (6 dpi) increases of TNF-α expression in PLs. Similarly, IL-1β gene expression by PLs exhibited delayed increases (6 dpi), albeit with greater variation in the magnitude of response (Fig. 1). For the spleen, which represents both a primary and the only secondary lymphoid organ in Xenopus, similar delayed increases of TNF-α gene expression were observed, whereas the mRNA levels of IL-1β were elevated by 1 dpi and subsided subsequently (Fig. 1).
Fig. 1.
Low and delayed increases of inflammation-associated gene expression by tadpole peritoneal and splenic leukocytes during FV3 infection. Quantitative gene expression analysis (qPCR assay) was performed on PLs and spleen harvested from the same pre-metamorphic tadpoles (st 56) infected by i.p. injection of FV3 (1 × 104 PFU) for 1, 2, 3, 6, and 9 day. The primers used were specific for Xenopus TNF-α, IL-1β, and IFN-γ. Control cells for this experiment were the peritoneal and splenic leukocytes removed on the day of infection (day 0). Each data point represents 3 different experiments where cells from 3–5 tadpoles were pooled. The expression level was determined using the delta delta CT method and the results are expressed as the means ± SD of relative quantification, with GAPDH used as endogenous control. *Significantly greater expression of cytokines in cells of infected tadpoles in comparison with uninfected controls by Student’s t-test, p ≤ 0.005.
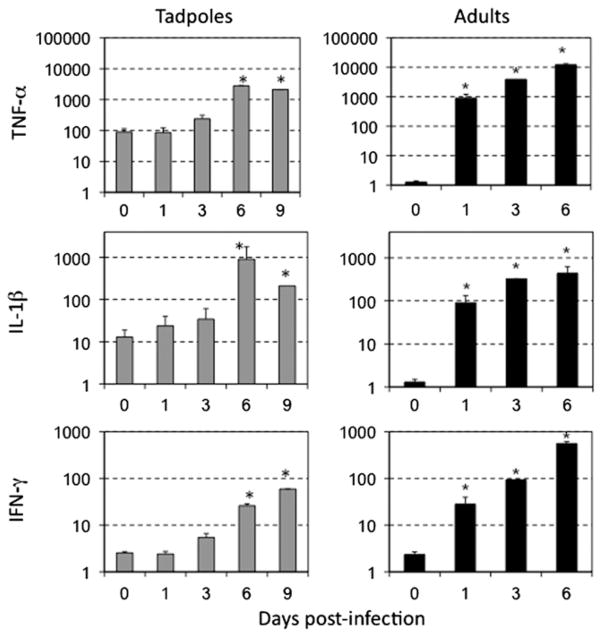
Since activated leukocytes expressing pro-inflammatory genes may have accumulated at the sites of infection, we examined the immune gene expression profiles in several tadpole organs. Given that the adult kidney is the main target of FV3 infection (Robert et al., 2005), we focused on this tissue to compare gene expression between adults and tadpoles during FV3 infections. As expected, adults exhibited rapid and marked increases of TNF-α (1000 × on average) and IL-1β (100 × on average) mRNA levels as early as 1 dpi, with further increases at 3 and 6 dpi (Fig. 2). Interestingly, the basal mRNA levels of TNF-α and IL-1β in uninfected tadpole kidneys were significantly higher than those seen in uninfected adult kidneys (100 × and 10 ×, respectively; Fig. 2). In addition, the expression levels of these two genes remained at basal at 1 and 3 dpi, and only modestly increased at 6 dpi (10 ×; Fig. 2). Similarly, relatively delayed and modest increases of TNF-α, IL-1β and IFN-γ expression were found in tadpole liver tissues (Supplementary Fig. 2).
Fig. 2.
Low and delayed increases of inflammation-associated gene expression in FV3 infected kidney of tadpoles compared to adults. A qPCR assay was performed on kidneys from ip-infected pre-metamorphic tadpoles (1 × 104 PFU FV3) at 1, 2, 3, 6 and 9 dpi, and adults (1 × 106 PFU FV3) at 1, 3 and 6 dpi using primers specific for Xenopus TNF-α, IL-1β, and IFN-γ. Control cells for this experiment were kidneys obtained from tadpoles on the day of infection (day 0). Each data point represents 3 different experiments where kidneys of 3 individuals were processed separately. The data determined by the delta delta CT method are expressed as the means fold change in expression ± SD against GAPDH endogenous control. Statistical significance between control and infected animals: *p < 0.005.
In mammals, IFN-γ is a critical effector cytokine initially produced by activated NK cells during innate immune response, and later on during adaptive immune responses by CD8 T and CD4 T helper 1 (Th1) cells (Schoenborn and Wilson, 2007). An IFN-γ homologue has been identified and partially characterized in Xenopus tropicalis, using its fully sequenced genome (Qi and Nie, 2008). Using this sequence we cloned, sequenced and characterized by phylogenetic analysis the X. laevis IFN-γ homologue (data not shown, GenBank accession number: JN634068). We designed and validated primers specific for this gene and here report the first expression analysis of the X. laevis IFN-γ in FV3-infected adults (Fig. 2). Significant increases of IFN-γ gene expression were already detectable at 1 dpi (20 × on average above non-infected controls), whereas greater increases (>1000×) occurred at the peak of the response, 6 dpi.
We then examined the IFN-γ gene expression in various tissues of FV3-infected pre-metamorphic tadpoles. In several experiments, we detected no significant increases in IFN-γ mRNA levels above uninfected controls at 1 and 3 dpi in kidneys, whereas at 6 and 9 dpi this cytokine was consistently increased 30–40 fold over respective controls (Fig. 2). Significant increases of IFN-γ expression were also observed in larval PLs and spleen (Fig. 1, bottom panel), and to a lesser degree in liver tissues (Supplementary Fig. 2).
Kinetics of virus load in tadpoles
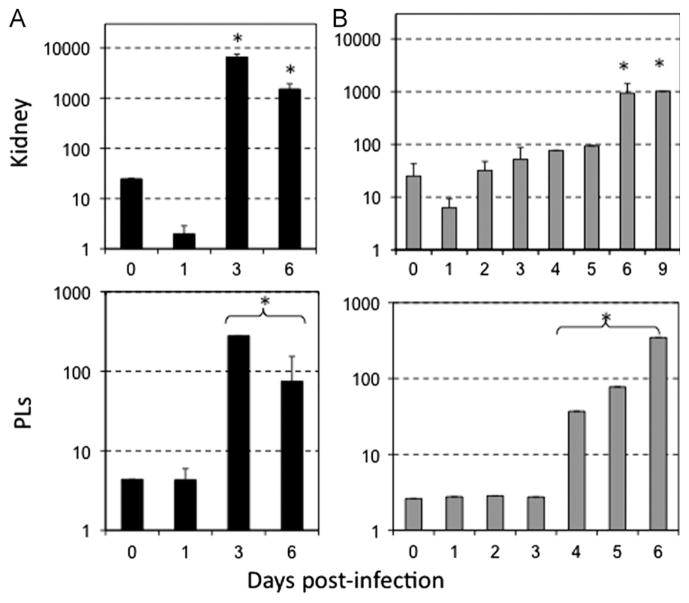
Since the less robust and more delayed inflammation-associated gene expression changes observed in infected tadpoles could be attributed to slower infection kinetics and/or lower virus loads, it was important to evaluate the degree of FV3 infections in tadpoles. For this purpose, we monitored the virus load over time for different tadpole tissues by qPCR using primers specific for the FV3 DNA polymerase II (νPol, 60R). Significant amplification of vPol as compared to time 0 (tissues taken just after infection) was already observed at 1 dpi in intestine, liver and kidney tissues, which indicate that viral growth and productive infection in tadpoles are initiated as early as it is in adults (Fig. 3). Furthermore, the increase in vPol DNA over 2 to 3 log peaked at 6 dpi, consistent with what has been observed in adults (Chen et al., 2011). Interestingly, the increase of virus load was substantially higher (100 ×) in the tadpole kidney than the liver and the intestine (Fig. 3). This indicates that as in adult Xenopus, the kidney is the main target of FV3 in tadpoles (Table 1).
Fig. 3.
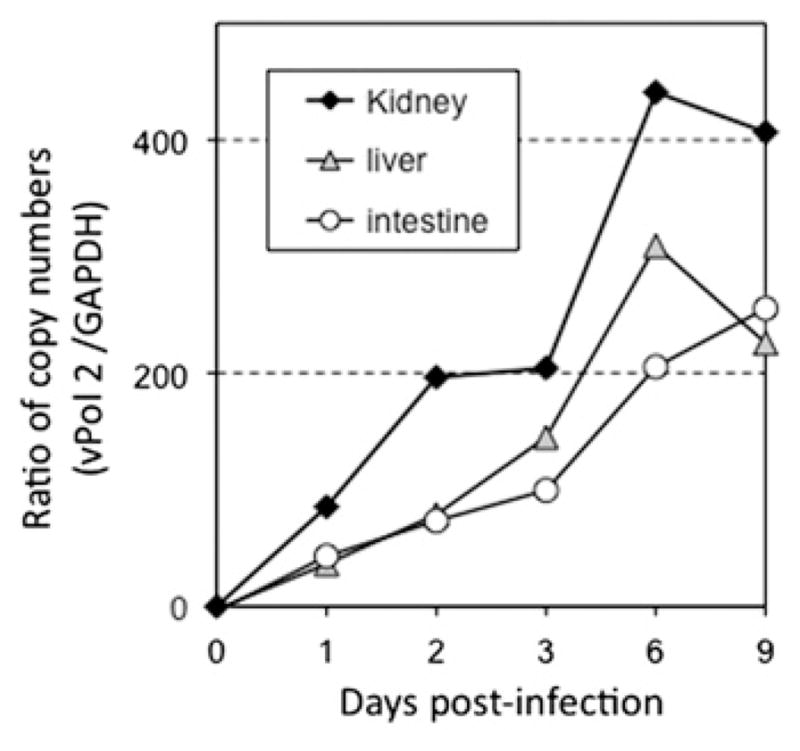
Detection of viral replication in vivo by qPCR. Pre-metamorphic (st 56) X. laevis tadpoles were injected i.p. with 1 × 105 PFU of FV3. DNA was extracted from kidney, liver and intestine of three pooled tadpoles at 1, 2, 3, 6, and 9 dpi or from uninfected tadpoles. Viral replication was determined by qPCR with primers specific for FV3 DNA polymerase (ORF 60R). The replication of DNA polymerase was standardized to GAPDH. Data are expressed as means fold change in expression ± SD against GAPDH endogenous control (note that SD is minimal and fall within the size of the symbols).
Table 1.
Comparison of FV3 infectivity between tadpole and adult stages of X. laevis.
ND: Not determined due to little or no cytopathicity at 1:50 or less dilution.
Pre-metamorphic tadpoles (st 56, 3 week-old) were infected by i.p. injection with 1 × 104 PFU of FV3.
Two-year old, three-inch outbred adults were infected by i.p. injection with 1 × 106 PFU of FV3.
Value expressed as PFU × 104/μg of total protein of kidney lysate (see the Materials and methods section).
As an additional parameter to compare the virus loads in kidney tissues of adults and tadpoles during the early stage of FV3 infection, we determined the numbers of infectious particles retrieved from lysates of kidney tissues from tadpoles and adults at different times after infection using a 50% endpoint dilution method (Reed and Muench, 1983). When the values obtained were normalized with the amount of total protein of each lysate, the virus loads at 6 dpi (peak of infection) were similar for tadpoles and adults (Table 2).
Table 2.
Primers sequences and uses.
| Primers name | Sequences |
|---|---|
| FV3 Polymerase 2 F | ACGAGCCCGACGAAGACTACATAG |
| R | TGGTGGTCCTCAGCATCCTTTG |
| Interleukin-1β F | AAC AGAAGATGG CCAAGACTC |
| R | ATG CAA CCG ATT CAA AGC |
| TNF-α F | GCTCAAGGATAACTCCATCG |
| R | AACCAAGTGGCACCTGAATG |
| IFN-γ F | CTGAGGAAATACTTTAACTCCATTGACC |
| R | TTGTAACATCTCCCACCTGTATTGTC |
| GAPDH F | ACCCCTTCATCGACTTGGAC |
| R | GGAGCCAGACAGTTTGTAGTG |
| β2-m Consensus-F1 | TGACGGTGAATCCTGGAGAC |
| β2-m Consensus-58R | CGATAGCCGTGACAATGAGC |
| β-actin-ex2-F1 | CCGGTGGTCAAGGTTTACACTG |
| β-actin-ex2-R1 | TAGAGATCAGTGATTGGATGA |
F, forward.
R, reverse.
β2-m, β2-microglobulin.
We conclude from these data, that the infection kinetics and infectivity of FV3 at early stage of infection is comparable between tadpole and adult. In addition, in both Xenopus tadpoles and adults the kidney is the main target of FV3.
Changes of inflammation-associated gene expression in tadpoles following bacterial stimulation
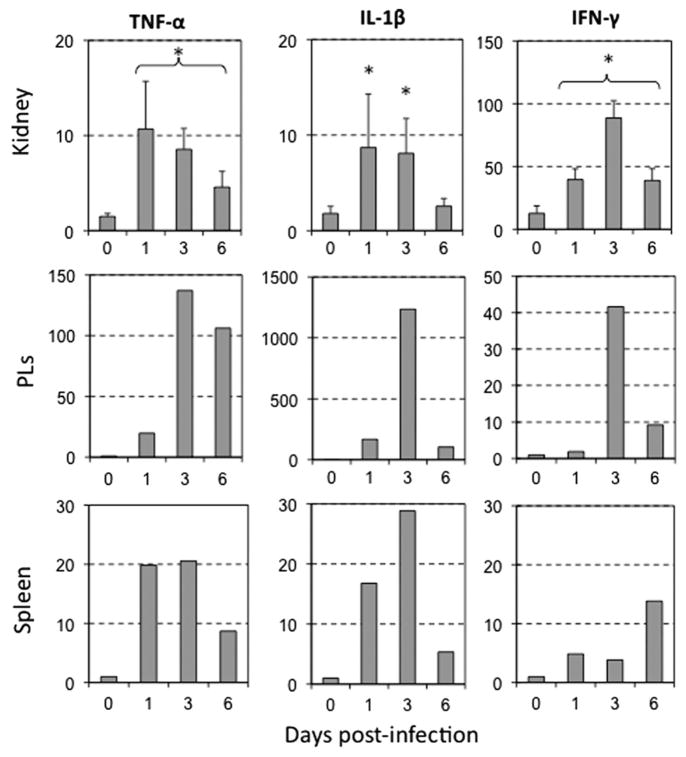
To determine if the poor and delayed anti-FV3 pro-inflammatory responses in tadpoles could be due to a general weakness of its immune system, we used a previously established bacterial stimulation protocol (Marr et al., 2007). Kidneys were collected from 3 separate tadpoles at 1, 3 and 6 days following intraperitoneal challenge with heat-killed E. coli. Unlike FV3 infections, significant increases in TNF-α, IL-1β and IFN-γ gene expression were already detected at 1 and 3 day post-stimulation (Fig. 4). Pooled PLs and splenocytes also revealed rapid and robust increases in expression of TNF-α and IL-1β genes, albeit with some delay in IFN-γ levels. Nevertheless, based on the more rapid enhanced expression of these three genes, we conclude that tadpoles are capable of acute inflammatory responses, which suggests that the low and delayed responses upon FV3 infection are virus-related.
Fig. 4.
Increase of inflammation-associated gene expression in kidneys, peritoneal and splenic leukocytes of tadpoles challenged with heat-killed bacteria. A qPCR assay was performed on kidneys, PLs and spleen from untreated and immunized pre-metamorphic tadpoles (st 56) at 1, 3 and 6 day following i.p. injection of 5 μl of heat-killed bacteria using primers specific for Xenopus TNF-α, IL-1β, and IFN-γ. Data from the kidneys are from three individuals processed separately, whereas PLs and spleens were pooled. The data determined by the delta delta CT method are expressed as the means fold change in expression ± SD against GAPDH endogenous control. Statistical significance between control and infected animals: *p < 0.005.
Change in expression of type I IFN-inducible gene in tadpoles during FV3 infection
At the present time type I interferons are not characterized in X. laevis, and multiple IFN-α/β gene model candidates are present in the sequenced genome of X. tropicalis. Importantly, in mammals (Cilloniz et al.; Yu et al., 2010) and in fish (Campbell et al., 2011; Jorgensen et al., 2007), the expression of the type I IFN-inducible Myxovirus-resistance 1 (Mx1) gene is induced by type I interferons and/or by viral infections. Thus Mx1 serves as a reliable marker of the IFN response. Accordingly, we identified putative X. tropicalis and X. laevis homologs of the Mx1 gene by bioinformatics approaches. Subsequently, we identified several cDNA clones in the X. laevis EST database (Unigene Xl.56887) that we obtained and fully sequenced. The X. laevis (Xl)Mx1 gene encodes a putative molecule of 624 amino acids with overall conserved domain architecture, especially at the N-terminus that contains a GTP-binding domain and a dynamin family signature. The identity of this putative Mx1 gene (gene bank accession number: JN634067) was further evaluated by a phylogenetic analysis (Supplementary Fig. 3). Both X. tropicalis and X. laevis branch together with a high bootstrap value and form a cluster independent of mammalian, avian and teleost Mx1 genes. We designed specific primers and determined XlMx1 expression profile during FV3 infection. The XlMx1 gene expression in adults was markedly induced in kidneys and PLs at 3 and 6 dpi (Fig. 5). The XlMx1 expression was also significantly increased in spleens at 3 dpi (data not shown). Compared to adults, the increased XlMx1 mRNA levels in tadpole kidneys were modest (10 ×) and delayed with a significant difference only at 6 dpi onward. However, it is noteworthy that unlike cytokine genes, the increase of XlMx1 expression in tadpole PLs was greater and less delayed (Fig. 5).
Fig. 5.
Delayed induced expression of type I interferon-inducible Mx gene in kidneys and PLs of tadpoles infected by FV3 compared to adults. qPCR assay performed on kidneys and PLs of uninfected and ip-infected (1 × 106 PFU FV3) adults at 1, 3 and 6 dpi, and pre-metamorphic tadpoles (1 × 104 PFU FV3) at 1, 2, 3, 6 and 9 dpi, using primers specific for XlMx1. Data from adult kidneys, larval kidneys and adult PLs are from 3 experiments using for each three separate individuals, whereas larval PLs were pools of 3 individuals. The data determined by the delta delta CT method are expressed as the means fold change in expression ± SD against GAPDH endogenous control. Statistical significance between control and infected animals: *p < 0.005. (A) Adults, (B) Tadpoles.
Effects of FV3 infections on tadpole PLs
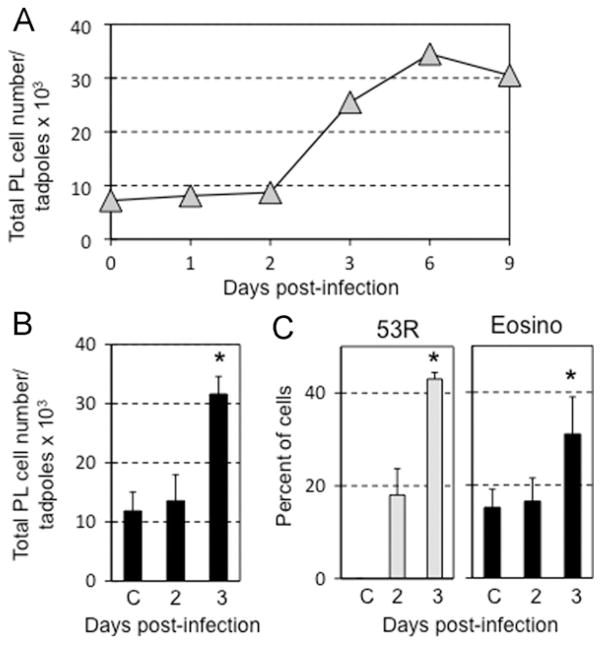
Our previous work revealed that during early stages of FV3 infection of Xenopus adults, the total numbers of PLs increased (Morales et al., 2010). In addition, a minor fraction of these PLs (less than 20%), consisting mostly of macrophages, became infected by FV3 as determined by fluorescence microscopy using a rabbit polyclonal antibody (Ab) specific for 53R, a putative 54.7-kDa myristoylated viral protein that is critical for FV3 replication (Robert et al., 2011). This Ab is FV3-specific and does not stain uninfected Xenopus PLs.
We were interested in determining if tadpole PLs were also infected by FV3. For this purpose, PLs were collected from pools of 5 to 10 pre-metamorphic tadpoles at different times after FV3 infection, counted and stained for immunofluorescence microscopy. To minimize potential side effects of ip injections on PLs, we infected animals by oral ingestion. Similar to adults, the total numbers of PLs increased significantly from 3 dpi and peaked at 6 dpi (Fig. 6A and B). We also noted an increase in the relative fractions of mononuclear eosinophilic cells at 3 dpi (Fig. 6C). Notably, the fraction of PLs infected by FV3 and positively stained by the anti-53R Ab also markedly increased. At 3 dpi more than 40% of tadpole PLs were infected, which is more than twice the number of infected adult PLs observed on average (Fig. 6C). Further microscopic observations by phase contrast and fluorescence microscopy analysis indicated that most infected PLs exhibited mononuclear phagocyte morphology (i.e., one well-defined nucleus, no granulations; Fig. 7). To further determine if these infected monocytic PLs were macrophages, we used a mouse monoclonal Ab (anti-HAM56) specific for macrophage antigen (HAM56), and reported to specifically cross-reacts with Xenopus macrophages (Nishikawa et al., 1998). This mAb allowed us to visualize infected peritoneal macrophages in Xenopus adults (Robert et al., 2011). Surprisingly, we did not observe any specific staining of tadpole PLs. Given the other reported differences between tadpole and adult peritoneal macrophages including morphological differences (Hsu, pers. comm.), MHC class I expression and response to bacterial stimulation (Marr et al., 2005), it is possible that they also differ in the expression of the HAM antigen.
Fig. 6.
Increase of infected and total numbers of tadpole PLs during FV3 infection. (A) One experiment where PLs were collected from 10 pre-metamorphic tadpoles uninfected, or at 1 up to 9 day following FV3 infection by oral ingestion (105 PFU in a volume of 5 μl) and counted on a hemacytometer. Data are expressed as average total PL cell number per tadpole × 103. (B) Average total number PLs from three different experiments of five pooled pre-metamorphic tadpoles uninfected or FV3 infected by oral ingestion for 2 and 3 day. (0) Day 0 control cells obtained on the day of infection. Data are expressed as total PL cell number per tadpole × 103 ± standard deviation. *p < 0.05 by ANOVA between uninfected and 3 dpi, or between 2 and 3 dpi. (C) PLs from B were cytocentrifuged on microscope slides, fixed with formaldehyde, permeabilized with ethanol, then stained with a rabbit anti-53R and FITC-conjugated donkey anti-rabbit Abs. Cells were then stained with the DNA dye Hoechst-33258 (Blue) mounted in anti-fade medium and visualized with a Leica DMIRB inverted fluorescence microscope. (0) Day 0 control cells obtained on the day of infection. Data are presented as average percent of infected 53R+ cells determined in 10 randomly chosen fields from two different experiments. *p < 0.05 by Student t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
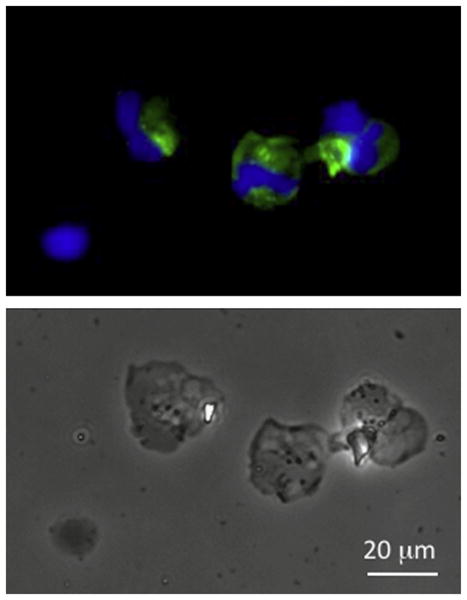
Infected tadpole peritoneal monocytic leukocytes visualized by immunofluorescence microscopy. PLs were harvested from 10 pooled pre-metamorphic tadpoles 3 day after FV3 infection by oral ingestion. Cells were cytocentrifuged on microscope slides, fixed with formaldehyde, permeabilized with ethanol, then stained with a rabbit anti-53R and FITC-conjugated donkey anti-rabbit Abs. Cells were then stained with the DNA dye Hoechst-33258 (Blue) mounted in anti-fade medium and visualized with a Leica DMIRB inverted fluorescence microscope. The upper panel shows the immunofluorescence image of the same cells shown in bright field in the lower panel. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We conclude that tadpole non-granulocytic leukocytes of the peritoneal cavity are less resistant to FV3 infections than those of adults.
Discussion
The immaturity of the Xenopus tadpole immune system has been so far mainly documented for its adaptive arm (Du Pasquier, Schwager, and Flajnik, 1989; Robert and Ohta, 2009). Accordingly, it is generally assumed that tadpoles rely on efficient innate immune defenses. This study provides evidence that this is perhaps not always the case, and that the immaturity or weakness of the tadpole immune system also includes some innate immune components. Indeed, our data indicate that the response of several genes critical for early host anti-viral immune defenses is slower and is of lower magnitude in Xenopus tadpoles than in adults. Aside from fundamental interests, our results are also relevant in the context of conservation biology, since RVs such as FV3 are causing emerging infectious diseases in many different ectothermic vertebrate species worldwide including anuran amphibians. Notably, RVs are particularly lethal for pre-metamorphic stages (Chinchar et al., 2009; Keesing et al., 2010).
Although tadpoles are immunocompetent, multiple indications of immaturity of their immune system have been reported. These include, lower antibody affinity to model antigens such as DNP-KLH (Hsu, 1998; Hsu and Du Pasquier, 1984), and poorer isotype switch from IgM to IgY (the IgG functional equivalent isotype) as compared to adult frogs (Du Pasquier et al., 2000). Since in mammals as well as in Xenopus, isotype switching is dependent on T cell help (Du Pasquier et al., 2000), this implies an overall less effective T cell response in tadpoles. This is supported by the ability of improving the tadpole class switch by the adoptive transfer of syngeneic adult splenocytes (Hsu and Du Pasquier, 1984). Additional evidence of an immature tadpole T cell effector functions include poor anti-tumor responses (Robert, Guiet, and Du Pasquier, 1995) and slower skin graft rejection (DiMarzo and Cohen, 1982a). Of particular relevance to viral immunity is the fact that although tadpoles do have CD8 T cells, there is no consistent MHC class I protein expression in the thymus until metamorphosis, and only limited class I surface expression on splenocytes during late pre-metamorphic stages (Flajnik and Du Pasquier, 1988; Flajnik et al., 1986; Rollins-Smith et al., 1997). We have clearly demonstrated the critical involvement of CD8 T cells in resistance to FV3 by Xenopus adults that are MHC class I competent (Morales and Robert, 2007). As such, we have postulated that the higher susceptibility of tadpoles to FV3 may be in part due to an inefficient CD8 T cell responses (Gantress et al., 2003).
While all these studies collectively support that tadpoles have a poor adaptive immune system, the efficacy and/or maturity of the tadpole innate immune system is still poorly characterized. It is known that tadpole macrophages are morphologically different from adult macrophages, and are MHC class I negative but class II+ (Hsu, pers. comm). No NK cell activity or NK cells (characterized by expression of the 1F8 marker) have been found in tadpoles until metamorphosis (Horton et al., 2003). However, it is difficult to draw conclusions towards the capacity of innate effector functions based on such observations. Accordingly, using a natural pathogen such as FV3 is more informative. The present investigation clearly show that the responses of three important inflammation-associated genes TNF-α, IL-1β and IFN-γ in susceptible tadpoles are less robust and delayed during early stage of FV3 infection, both by immune cells and in infected tissues, as compared to resistant adults.
The role of TNF-α in immune responses against viral infection is well documented in mammals (reviewed in Bartee et al. (2008)). This cytokine is among the first to be produced in response to viral infection and in turn triggers multiple antiviral mechanisms as well as pro-inflammatory cascades including IL-1β (review in Bartee et al. (2008)). TNF-α is critically involved in immune responses against a plethora of viruses including poxvirus (Diel et al., 2011), its gene expression is rapidly increased by macrophages upon viral infection, and can inhibit viral replication (Bartee et al., 2008). Aside from mammals, robust (i.e., in the range of 100 fold increase) up-regulation of TNF-α gene expression upon acute viral infection has been reported in fish (Huang et al., 2011; Ordas et al., 2007; Purcell et al., 2004; Purcell et al., 2006; Xiao et al., 2007), and in Xenopus adults (Morales et al., 2010). Some of the biological activities of this mediator have also been obtained with recombinant TNF-α proteins of bony fish species including apoptotic activity in the mandarin fish (Xiao et al., 2007) as well as nitric oxide production and recruitment of inflammatory cells in turbot, Scophthalmus maximus (Ordas et al., 2007). In Xenopus, TNF-α activates NF-κB, which is consistent with its involvement in antiviral immune responses (Mawaribuchi et al., 2008). Therefore, the low and delayed increases in TNF-α gene expression in tissues, splenocytes and PLs of infected tadpoles suggest that overall the early responses to FV3 infection are poor.
The delayed and low levels of IL-1β induction during FV3 infection, especially at the main site of infection in kidneys, further substantiate the idea that the innate immune response developed against FV3 by Xenopus tadpoles are relatively meager and delayed. IL-1β is another important cytokine largely responsible for the acute phase response and acute protein synthesis. Rapid and robust increases of IL-1β gene expression have been observed for various viral infections in rainbow trout, Oncorhynchus mykiss (Cuesta and Tafalla, 2009; Purcell et al., 2004; Purcell et al., 2006). In Xenopus adults it is strongly up-regulated 24 h following LPS treatment or FV3 infections (Morales et al., 2010; Zou et al., 2000). Therefore, the low IL-1β increase only 3 day post-infection in tadpoles further argues that a suboptimal innate immune response is elicited at early stages of FV3 infection.
Although IFN-γ is typically produced in large amounts by CD8 and Th1 CD4 T cell effectors during the adaptive phase of immune responses, it is also produced earlier by innate cell effectors such as NK and NKT cells (Schoenborn and Wilson, 2007). The low levels of IFN-γ expression in most tissues including leukocytes of uninfected and early stage infected (1–3 dpi) tadpoles could be explained by the absence of NK cells until metamorphosis (Horton et al., 2003). While increase of IFN-γ expression at 6 dpi would be consistent with the development of an adaptive response and infiltration of activated T cells in infected tissues as it the case in adults (Morales and Robert, 2007). However, the magnitude of the IFN-γ response is low compared to adults, which is in agreement with previous studies suggesting a poor T cell responses against FV3 in tadpoles (Gantress et al., 2003).
Collectively, as already underlined, our observations suggest that the larval susceptibility to FV3 is at least in part a consequence of poor innate immune responses. However, it is worth noting that in comparison to adult frogs, the X. laevis tadpoles possessed significantly greater baseline mRNA levels of the TNF-α and IL-1β (but not IFN-γ or Mx1) transcripts. This is reminiscent of what has been reported for bony fish, where TNF-α and IL-1β (Bird et al., 2002; Grayfer et al., 2008; Hirono et al., 2000) exhibit constitutive gene expression, whereas IFN-γ mRNA levels are more tightly transcriptionally regulated and require stimulus for induction (Grayfer and Belosevic, 2009; Grayfer et al., 2010; Igawa et al., 2006). Perhaps during FV3 infections, the tadpoles’ strategy is to avoid exacerbating the already high inflammatory cytokine levels. Possibly, the tissue damage resulting from later stages of tadpole-FV3 infections may be due to these further increases in inflammation-associated gene expression, accounting for the tadpole mortalities. In spite of this, it is equally (if not more) likely that adult frogs possess more efficient transcriptional regulation of inflammatory cytokines than do tadpoles.
Mx1 proteins are dynamin-like GTPases that mediate antiviral defenses through as of yet poorly understood mechanisms (Sadler and Williams, 2008). Mx1 gene homologs have been identified and characterized in several fish species (Robertsen, 2006). As in mammals, fish Mx1 gene expression is induced by type I interferons and following viral infections (Purcell et al., 2004; Purcell et al., 2006). In Xenopus, XlMx1 has an open reading frame coding for 624 amino acids that displays the characteristic features of Mx1 proteins including: a highly conserved tripartite GTP-binding domain (GXXXXGKS, DXXG, and T/NKXD) and a dynamin family signature at the N-terminus, a “central interactive domain” in the middle, and a GTPase effector domain with leucine zipper motifs, which are essential for Mx1 oligomerization, at the C-terminus end (Haller et al., 2007). Further evidence of the potential role of XlMx1 in Xenopus antiviral immune response is its tight regulation of expression upon FV3 infection. In adults, the XlMx1 gene is expressed at very low levels in non-infected animals, but is rapidly induced upon FV3 infection, both in infected kidney and in leukocytes. This implies that the type I IFN response is intact and readily induced upon FV3 infection in adult Xenopus. Although originally identified as a host factor against RNA viruses, DNA viruses are also susceptible to the activities of Mx1 protein (Netherton et al., 2009). Therefore, the involvement of Mx1 molecules in response to RV, also a DNA virus, is likely. Interestingly, although XlMx1 expression in tadpoles was also increased in kidney, it was induced with a delay of 3 day and with a more modest increase in infected kidneys compared to adults. This suggests of a delay in type I IFN responses in tadpoles, although this still needs to be confirmed by a more direct monitoring of type I IFN gene products. Alternatively, the delay in XlMx1 induction may result from a less efficient or incomplete signaling cascade involving pattern-recognition receptors and other sensors of viral products (Sadler and Williams, 2008). The induction of XlMx1 response occurred earlier and was stronger in PLs compared to kidneys of infected tadpoles. This may suggest a previously unsuspected infection these cells, which remains to be determined.
Taken together, the less robust and delayed increases in gene expression of several key inflammation-associated cytokines combined with delays in induction of a critical type I-inducible gene, provide convergent evidence that elements of the tadpole innate immune system are not fully efficient toward responding to FV3 infections. Perhaps this is in part due to the fact that, as reported here, innate immune cells of monocytic origin are targeted by FV3 infections. The current study confirms that tadpole macrophages have a different morphology compared to adult macrophages. It will be interesting to learn whether the infection of these Xenopus tadpole immune cells is a contributing factor to the less efficient tadpole anti-viral immunity and the enhanced susceptibility of this developmental stage to ranavirus infection.
Materials and methods
Animals
Outbred young adults and pre-metamorphic (stage 56–58) tadpoles were obtained from our Xenopus laevis Research Resource for Immunology at the University of Rochester (http://www.urmc.rochester.edu/smd/mbi/xenopus/index.htm). All animals were handled under strict laboratory and UCAR regulations (Approval number 100577/2003-151), minimizing discomfort at all times. Tadpoles were infected either by (1) intraperitoneal (i.p.) injection with 1 × 104 PFU in 10 μl volume) of FV3 using a glass Pasteur pipette whose small end had been pulled in a flame; (2) oral ingestion of 10 μl (1 × 105 PFU) delivered in the pharynx with a pipetman; or water bath exposure (1 h in 2 ml of water containing 5 × 106 PFU). Controls were sham-infected with the same amount of APBS. At different time points post-infection (Day 0, 1, 3, 6 and 9) tadpoles were euthanized by immersion in 1% tricaine methane sulfonate (MS-222) buffered with bicarbonate and peritoneal leukocytes were first collected by i.p. puncture with Pasteur pipettes. Tissues including spleen, kidney, liver, and intestine were dissected and directly homogenized on ice. Adults (2 year-old) were injected i.p. with 1 × 106 PFU in 0.1 ml volume.
FV3 stock and infection
Fathead minnow cells (FHM; American Type Culture Collection, ATCC No.CCL-42) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Invitrogen), penicillin (100 U/ml) and streptomycin (100 μg/ml) with 5% CO2 at 37 °C. FV3 was grown by a single passage on FMH cells, purified by ultracentrifugation on a 30% sucrose cushion and quantified by plaque assay on FMH monolayer under an overlay of 1% methylcellulose (Morales et al., 2010).
To determine the number of infectious particles in the kidney of infected tadpoles and adults, the whole kidney for one individual at 2, 6 and 12 dpi was lysed in hypotonic 30 mM NaHCO3 buffer by 3 freeze-thaw cycles. The total amount of proteins for each lysate was determined by the Bradford assay and the virus titer by the 50% endpoint dilution method (Reed and Muench, 1983).
Bacterial stimulation
E. coli (XL1-blue, Strategene, La Jolla, Ca.) cultured overnight at 37 °C, were boiled for 1 h, centrifuged and resuspended in 0.1 volume (approximately 108 bacteria/ml) of Xenopus cell culture medium (Marr et al., 2007). Tadpoles were injected i.p. with 5 μl of heat-killed bacteria mixture.
PCR, RT-PCR and quantitative real-time PCR (qPCR)
RNA and DNA were extracted from cells and tissues using Trizol reagent following the manufacturer’s protocol (Invitrogen). 0.5 to 1.0 μg of total RNA in 20 μl was used to synthesize cDNA with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). 1 μl of cDNA template was used in all RT-PCRs and 50 ng DNA for PCR. Minus RT controls were included for every reaction, and all primers spanned at least one intron (Table 2). A water-only control was included in each reaction. PCR products were separated on 1.5% agarose gels and stained with ethidium bromide. Sizes of the products were determined using standardized markers of 1 kb plus from Invitrogen (Carlsbad, CA).
SYBR green-based real-time PCR (qPCR) was performed using an ABI 7300 real-time PCR system and PerfeCTa® SYBR Green FastMix, ROX (Quanta) using the delta delta CT method. Briefly, 3 μl of diluted cDNA or genomic DNA (120 ng) was amplified in a mixture of 50 μl containing 200 nM of each primer and 1 × SYBR green FastMix containing 1 × ROX passive reference dye. Gene copy numbers were calculated using ABI sequence detection system software (SDS). Each sample was run in three replicates. Melting curve analysis was carried out after each PCR run to ensure the specificity of the reaction.
Cytospin and staining
200,000 cells (200 μl volume) were cytocentrifuged using a Shandon Southern cytospin centrifuge (600 rpm, 5 min.), fixed with 3.7% formalin for 1 min., permeabilized with 100% cold methanol (−20 °C) and briefly washed with APBS. After blocking with 1% BSA in APBS for 1 h, the cells were incubated overnight with rabbit anti-FV3 53R serum, or normal rabbit serum as negative control. After washing, cells were incubated with DyLight 488-conjugated F(ab′)2 donkey anti-rabbit IgG (H+L) (Jackson ImmunoReaserch, PA) and a fluorescent DNA intercalator (Hoechst-33258). Preparations were mounted in anti-fade medium (Molecular Probes, Oregon) and visualized with a Leica DMIRB inverted fluorescence microscope with a cooled charge-couple device (Cooke) controlled by Image-Pro software (Media Cybernetics).
Phylogenetic analysis
Available deduced amino acid sequences of Mx1 homologs were retrieved from GenBank using ENTREZ at the NCBI. Multiple nucleotide and amino acid sequence alignments of Mx1 genes were generated using Clustal X. Phylogenetic analysis was performed using molecular evolutionary genetics analysis (MEGA, version 4.1). Phylogenetic analysis was performed using molecular evolutionary genetics analysis (MEGA, version 4.1). The neighbor joining method with pairwise deletion of gaps and p-distances (proportion of differences) was used to generate the tree. Numbers on nodes represent percentages of 1000 bootstrap replicates supporting each partition.
Statistics
A one-way analysis of variance (ANOVA) for Independent or correlated samples was performed using an online database available through Vassar Stat a website for statistical computation (http://faculty.vassar.edu/lowry//anova1u.html). A standard weighted-means analysis was done on independent samples k = 5 for all samples with n > 5.
Supplementary Material
Acknowledgments
The expert animal husbandry provided by Tina Martin and David Albright is gratefully appreciated. We would like to thank Jennifer Sabogal, Tanya Cruz Luna and Megan Green for their significant technical contribution to this work. We also thank Dr. Nicholas Cohen, for his critical reviews of the manuscript. This research was supported by grants R24-AI-059830 from the NIH and IOB-0923772 from NSF.
Abbreviations
- ANOVA
one-way analysis of variance
- IE
immediate-early
- FV3
Frog Virus 3
- MOI
multiplicity of infection
- PFU
plaque forming units
- i.p
intraperitoneal injection
- Qpcr
quantitative real-time PCR
- p.i
post-infection
- dpi
days post-infection
- RV
Ranavirus
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.virol.2012.07.001.
References
- Bartee E, Mohamed MR, McFadden G. Tumor necrosis factor and interferon: cytokines in harmony. Curr Opin Microbiol. 2008;11 (4):378–383. doi: 10.1016/j.mib.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird S, Wang T, Zou J, Cunningham C, Secombes CJ. The first cytokine sequence within cartilaginous fish: IL-1 beta in the small spotted catshark (Scyliorhinus canicula) J Immunol. 2002;168 (7):3329–3340. doi: 10.4049/jimmunol.168.7.3329. [DOI] [PubMed] [Google Scholar]
- Campbell S, McBeath A, Secombes C, Snow M, Collet B. Interferon response following infection with genetically similar isolates of viral haemorrhagic septicaemia virus (VHSV) exhibiting contrasting virulence in rainbow trout. Fish Shellfish Immunol. 2011;30 (1):287–294. doi: 10.1016/j.fsi.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Chardonnens X, Du Pasquier L. Induction of skin allograft tolerance during metamorphosis of the toad Xenopus laevis: a possible model for studying generation of self tolerance to histocompatibility antigens. Eur J Immunol. 1973;3 (9):569–573. doi: 10.1002/eji.1830030909. [DOI] [PubMed] [Google Scholar]
- Chen G, Ward BM, Yu EK, Chinchar VG, Robert J. Improved knockout methodology reveals that Frog Virus 3 mutants lacking either the 18K immediate-early gene or the truncated vIF2-{alpha} gene are defective for replication and growth in vivo. J Virol. 2011 doi: 10.1128/JVI.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchar VG, Hyatt A, Miyazaki T, Williams T. Family Iridoviridae: poor viral relations no longer. Curr Top Microbiol Immunol. 2009;328:123–170. doi: 10.1007/978-3-540-68618-7_4. [DOI] [PubMed] [Google Scholar]
- Cilloniz C, Pantin-Jackwood MJ, Ni C, Carter VS, Korth MJ, Swayne DE, Tumpey TM, Katze MG. Molecular signatures associated with Mx1-mediated resistance to highly pathogenic influenza virus infection: mechanisms of survival. J Virol. 86(5):2437–2446. doi: 10.1128/JVI.06156-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta A, Tafalla C. Transcription of immune genes upon challenge with viral hemorrhagic septicemia virus (VHSV) in DNA vaccinated rainbow trout (Oncorhynchus mykiss) Vaccine. 2009;27 (2):280–289. doi: 10.1016/j.vaccine.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Diel DG, Luo S, Delhon G, Peng Y, Flores EF, Rock DL. A nuclear inhibitor of NF-kappaB encoded by a poxvirus. J Virol. 2011;85 (1):264–275. doi: 10.1128/JVI.01149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarzo SJ, Cohen N. An in vivo study of the ontogeny of alloreactivity in the frog, Xenopus laevis. Immunology. 1982a;45 (1):39–48. [PMC free article] [PubMed] [Google Scholar]
- DiMarzo SJ, Cohen N. Immunogenetic aspects of in vivo allotolerance induction during the ontogeny of Xenopus laevis. Immunogenetics. 1982b;16 (2):103–116. doi: 10.1007/BF00364398. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Robert J, Courtet M, Mussmann R. B-cell development in the amphibian Xenopus. Immunol Rev. 2000;175:201–213. doi: 10.1111/j.1600-065x.2000.imr017501.x. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Schwager J, Flajnik MF. The immune system of Xenopus. Annu Rev Immunol. 1989;7:251–275. doi: 10.1146/annurev.iy.07.040189.001343. [DOI] [PubMed] [Google Scholar]
- Duffus AL, Pauli BD, Wozney K, Brunetti CR, Berrill M. Frog Virus 3-like infections in aquatic amphibian communities. J Wildl Dis. 2008;44 (1):109–120. doi: 10.7589/0090-3558-44.1.109. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Du Pasquier L. MHC class I antigens as surface markers of adult erythrocytes during the metamorphosis of Xenopus. Dev Biol. 1988;128 (1):198–206. doi: 10.1016/0012-1606(88)90282-5. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Kaufman JF, Hsu E, Manes M, Parisot R, Du Pasquier L. Major histocompatibility complex-encoded class I molecules are absent in immunologically competent Xenopus before metamorphosis. J Immunol. 1986;137 (12):3891–3899. [PubMed] [Google Scholar]
- Gantress J, Maniero GD, Cohen N, Robert J. Development and characterization of a model system to study amphibian immune responses to iridoviruses. Virology. 2003;311 (2):254–262. doi: 10.1016/s0042-6822(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Miller DL, Hoverman JT. Ecology and pathology of amphibian ranaviruses. Dis Aquat Organ. 2009;87 (3):243–266. doi: 10.3354/dao02138. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Miller DL, Schmutzer AC, Baldwin CA. Frog Virus 3 prevalence in tadpole populations inhabiting cattle-access and non-access wetlands in Tennessee, USA. Dis Aquat Organ. 2007;77 (2):97–103. doi: 10.3354/dao01837. [DOI] [PubMed] [Google Scholar]
- Grayfer L, Belosevic M. Molecular characterization, expression and functional analysis of goldfish (Carassius aurutus L.) interferon gamma. Dev Comp Immunol. 2009;33 (2):235–246. doi: 10.1016/j.dci.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Grayfer L, Garcia EG, Belosevic M. Comparison of macrophage antimicrobial responses induced by type II interferons of the goldfish (Carassius auratus L.) J Biol Chem. 2010;285 (31):23537–23547. doi: 10.1074/jbc.M109.096925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayfer L, Walsh JG, Belosevic M. Characterization and functional analysis of goldfish (Carassius auratus L.) tumor necrosis factor-alpha. Dev Comp Immunol. 2008;32 (5):532–543. doi: 10.1016/j.dci.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Green DE, Converse KA, Schrader AK. Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996–2001. Ann NY Acad Sci. 2002;969:323–339. doi: 10.1111/j.1749-6632.2002.tb04400.x. [DOI] [PubMed] [Google Scholar]
- Haller O, Staeheli P, Kochs G. Interferon-induced Mx proteins in antiviral host defense. Biochimie. 2007;89 (6–7):812–818. doi: 10.1016/j.biochi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hirono I, Nam BH, Kurobe T, Aoki T. Molecular cloning, characterization, and expression of TNF cDNA and gene from Japanese flounder Paralychthys olivaceus. J Immunol. 2000;165 (8):4423–4427. doi: 10.4049/jimmunol.165.8.4423. [DOI] [PubMed] [Google Scholar]
- Horton TL, Stewart R, Cohen N, Rau L, Ritchie P, Watson MD, Robert J, Horton JD. Ontogeny of Xenopus NK cells in the absence of MHC class I antigens. Dev Comp Immunol. 2003;27 (8):715–726. doi: 10.1016/s0145-305x(03)00040-5. [DOI] [PubMed] [Google Scholar]
- Hsu E. Mutation, selection, and memory in B lymphocytes of exothermic vertebrates. Immunol Rev. 1998;162:25–36. doi: 10.1111/j.1600-065x.1998.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Hsu E, Du Pasquier L. Ontogeny of the immune system in Xenopus. I Larval immune response. Differentiation. 1984;28:109–115. [Google Scholar]
- Huang X, Huang Y, Ouyang Z, Cai J, Yan Y, Qin Q. Roles of stress-activated protein kinases in the replication of Singapore grouper iridovirus and regulation of the inflammatory responses in grouper cells. J Gen Virol. 2011 doi: 10.1099/vir.0.029173-0. [DOI] [PubMed] [Google Scholar]
- Igawa D, Sakai M, Savan R. An unexpected discovery of two interferon gamma-like genes along with interleukin (IL)-22 and -26 from teleost: IL-22 and -26 genes have been described for the first time outside mammals. Mol Immunol. 2006;43 (7):999–1009. doi: 10.1016/j.molimm.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Jorgensen JB, Johansen A, Hegseth MN, Zou J, Robertsen B, Collet B, Secombes CJ. A recombinant CHSE-214 cell line expressing an Mx1 promoter-reporter system responds to both interferon type I and type II from salmonids and represents a versatile tool to study the IFN-system in teleost fish. Fish Shellfish Immunol. 2007;23 (6):1294–1303. doi: 10.1016/j.fsi.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogich T, Ostfeld RS. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468 (7324):647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniero GD, Morales H, Gantress J, Robert J. Generation of a long-lasting, protective, and neutralizing antibody response to the ranavirus FV3 by the frog Xenopus. Dev Comp Immunol. 2006;30 (7):649–657. doi: 10.1016/j.dci.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Marr S, Goyos A, Gantress J, Maniero GD, Robert J. CD91 up-regulates upon immune stimulation in Xenopus adult but not larval peritoneal leukocytes. Immunogenetics. 2005;56 (10):735–742. doi: 10.1007/s00251-004-0736-4. [DOI] [PubMed] [Google Scholar]
- Marr S, Morales H, Bottaro A, Cooper M, Flajnik M, Robert J. Localization and differential expression of activation-induced cytidine deaminase in the amphibian Xenopus upon antigen stimulation and during early development. J Immunol. 2007;179 (10):6783–6789. doi: 10.4049/jimmunol.179.10.6783. [DOI] [PubMed] [Google Scholar]
- Mawaribuchi S, Tamura K, Okano S, Takayama S, Yaoita Y, Shiba T, Takamatsu N, Ito M. Tumor necrosis factor-alpha attenuates thyroid hormone-induced apoptosis in vascular endothelial cell line XLgoo established from Xenopus tadpole tails. Endocrinology. 2008;149 (7):3379–3389. doi: 10.1210/en.2007-1591. [DOI] [PubMed] [Google Scholar]
- Mazzoni R, de Mesquita AJ, Fleury LF, de Brito WM, Nunes IA, Robert J, Morales H, Coelho AS, Barthasson DL, Galli L, Catroxo MH. Mass mortality associated with a Frog Virus 3-like ranavirus infection in farmed tadpoles Rana catesbeiana from Brazil. Dis Aquat Organ. 2009;86 (3):181–191. doi: 10.3354/dao02096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales HD, Abramowitz L, Gertz J, Sowa J, Vogel A, Robert J. Innate immune responses and permissiveness to ranavirus infection of peritoneal leukocytes in the frog Xenopus laevis. J Virol. 2010;84 (10):4912–4922. doi: 10.1128/JVI.02486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales HD, Robert J. Characterization of primary and memory CD8 T-cell responses against ranavirus (FV3) in Xenopus laevis. J Virol. 2007;81 (5):2240–2248. doi: 10.1128/JVI.01104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherton CL, Simpson J, Haller O, Wileman TE, Takamatsu HH, Monaghan P, Taylor G. Inhibition of a large double-stranded DNA virus by MxA protein. J Virol. 2009;83 (5):2310–2320. doi: 10.1128/JVI.00781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa A, Murata E, Akita M, Kaneko K, Moriya O, Tomita M, Hayashi H. Roles of macrophages in programmed cell death and remodeling of tail and body muscle of Xenopus laevis during metamorphosis. Histochem Cell Biol. 1998;109 (1):11–17. doi: 10.1007/s004180050197. [DOI] [PubMed] [Google Scholar]
- Ordas MC, Costa MM, Roca FJ, Lopez-Castejon G, Mulero V, Meseguer J, Figueras A, Novoa B. Turbot TNFalpha gene: molecular characterization and biological activity of the recombinant protein. Mol Immunol. 2007;44 (4):389–400. doi: 10.1016/j.molimm.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Pearman PB, Garner TW, Straub M, Greber UF. Response of the Italian agile frog (Rana latastei) to a ranavirus, Frog Virus 3: a model for viral emergence in naive populations. J Wildl Dis. 2004;40 (4):660–669. doi: 10.7589/0090-3558-40.4.660. [DOI] [PubMed] [Google Scholar]
- Purcell MK, Kurath G, Garver KA, Herwig RP, Winton JR. Quantitative expression profiling of immune response genes in rainbow trout following infectious haematopoietic necrosis virus (IHNV) infection or DNA vaccination. Fish Shellfish Immunol. 2004;17 (5):447–462. doi: 10.1016/j.fsi.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Purcell MK, Nichols KM, Winton JR, Kurath G, Thorgaard GH, Wheeler P, Hansen JD, Herwig RP, Park LK. Comprehensive gene expression profiling following DNA vaccination of rainbow trout against infectious hematopoietic necrosis virus. Mol Immunol. 2006;43 (13):2089–2106. doi: 10.1016/j.molimm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Qi ZT, Nie P. Comparative study and expression analysis of the interferon gamma gene locus cytokines in Xenopus tropicalis. Immunogenetics. 2008;60 (11):699–710. doi: 10.1007/s00251-008-0326-y. [DOI] [PubMed] [Google Scholar]
- Reed L, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1983;27:493–497. [Google Scholar]
- Robert J, George E, De Jesus Andino F, Chen G. Waterborne infectivity of the ranavirus Frog Virus 3 in Xenopus laevis. Virology. 2011 doi: 10.1016/j.virol.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Guiet C, Du Pasquier L. Ontogeny of the alloimmune response against a transplanted tumor in Xenopus laevis. Differentiation. 1995;59 (3):135–144. doi: 10.1046/j.1432-0436.1995.5930135.x. [DOI] [PubMed] [Google Scholar]
- Robert J, Morales H, Buck W, Cohen N, Marr S, Gantress J. Adaptive immunity and histopathology in Frog Virus 3-infected Xenopus. Virology. 2005;332 (2):667–675. doi: 10.1016/j.virol.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Robert J, Ohta Y. Comparative and developmental study of the immune system in Xenopus. Dev Dyn. 2009;238 (6):1249–1270. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsen B. The interferon system of teleost fish. Fish Shellfish Immunol. 2006;20 (2):172–191. doi: 10.1016/j.fsi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Flajnik MF, Blair PJ, Davis AT, Green WF. Involvement of thyroid hormones in the expression of MHC class I antigens during ontogeny in Xenopus. Dev Immunol. 1997;5 (2):133–144. doi: 10.1155/1997/38464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8 (7):559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloegel LM, Daszak P, Cunningham AA, Speare R, Hill B. Two amphibian diseases, chytridiomycosis and ranaviral disease, are now globally notifiable to the World Organization for Animal Health (OIE): an assessment. Dis Aquat Organ. 2010;92 (2–3):101–108. doi: 10.3354/dao02140. [DOI] [PubMed] [Google Scholar]
- Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- Xiao J, Zhou ZC, Chen C, Huo WL, Yin ZX, Weng SP, Chan SM, Yu XQ, He JG. Tumor necrosis factor-alpha gene from mandarin fish, Siniperca chuatsi: molecular cloning, cytotoxicity analysis and expression profile. Mol Immunol. 2007;44 (14):3615–3622. doi: 10.1016/j.molimm.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zeng Z, Huang Z, Lian J, Yang J, Deng Q, Zeng W. Increased mRNA expression of interferon-induced Mx1 and immunomodulation following oral administration of IFN-alpha2b-transformed B. longum to mice. Arch Microbiol. 2010;192 (8):633–638. doi: 10.1007/s00203-010-0589-1. [DOI] [PubMed] [Google Scholar]
- Zou J, Bird S, Minter R, Horton J, Cunningham C, Secombes CJ. Molecular cloning of the gene for interleukin-1beta from Xenopus laevis and analysis of expression in vivo and in vitro. Immunogenetics. 2000;51 (4–5):332–338. doi: 10.1007/s002510050627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.