Abstract
Recent technological advances have led to an improved understanding of central serous chorioretinopathy (CSC): new pathophysiological insights, new imaging techniques for diagnosis and management, and new treatments. The primary role of the choroid has become more widely accepted with widespread use of indocyanine green angiography. Optical coherence tomography (OCT), and particularly enhanced depth imaging OCT, demonstrate a thickened and engorged choroid. Adaptive optics, fundus autofluorescence, multifocal electroretinography, microperimetry, and contrast sensitivity testing reveal that patients with even a mild course suffer previously undetected anatomic and functional loss. While focal laser and photodynamic therapy are the current standard of care for persistent subretinal fluid in CSC, they are not appropriate in all cases, and the optimal timing of intervention remains unclear.
Keywords: central serous chorioretinopathy, diffuse retinal pigment epitheliopathy, photodynamic therapy, corticosteroids, indocyanine green angiography, fundus autofluorescence, optical coherence tomography
I. INTRODUCTION
Central serous chorioretinopathy (CSC) is a disorder characterized by serous retinal detachment and/or retinal pigment epithelial (RPE) detachment, changes most often confined to the macula, and associated with leakage of fluid through the RPE into the subretinal space. CSC is seen frequently in most retina practices, classically in young male patients with no associated systemic conditions. CSC has been known by many names since the original description by von Graefe in 1866,221 and these names reflect the course of progress in our understanding of the pathogenesis of the disease. von Graefe described the disease as a recurrent central retinitis, whereas Horniker in 1922 called it capillarospastic central retinitis to reflect his belief that vasospasm was the mechanism.77 Other names of the 20th century include central angiospastic retinopathy and central serous retinopathy.138 Maumenee described fluorescein angiographic (FA) characteristics; fluorescein leakage at the level of the RPE revealed that the choroid and RPE were the primary tissues involved.136 Gass further characterized the angiographic findings and coined the term central serous chorioretinopathy.58 Since we now understand that hyperpermeability of the choroid causes leakage through the RPE, resulting in a neurosensory retinal detachment, CSC is the preferred term.138
A. Epidemiology
CSC has historically been viewed as primarily affecting men in their third and fourth decades, but the average age reported in recent large studies has ranged from 4573 to 51 years.200 Spaide and colleagues reported a mean of 51 years, but they observed that older patients were more likely to present with diffuse RPE loss and secondary choroidal neovascularization (CNV),200 suggesting that the onset of disease occurred years before presentation. Some patients are initially asymptomatic and may not be diagnosed until they develop more advanced disease. Likewise, the large retrospective case control series published by Haimovici and colleagues in 2004 included patients with evidence of chronic disease.73 They reported a mean age of 45 years. Kitzmann and colleagues (2008) assessed for age of onset and reported a mean of 41 years.97 Age-related macular degeneration (AMD) with choroidal neovascularization (CNV) may resemble CSC, and this must be ruled out in patients over 50.
The lone population-based study of CSC reported an annual incidence of 9.9 cases per 100,000 men and 1.7 per 100,000 women in Olmstead County, Minnesota.97 Reported male to female ratios range from 2.7:1216 to 7:1.206 CSC is thought to be less common in African Americans than in Caucasians, Hispanics, and Asians,235 although this has been disputed.44
Numerous risk factors for CSC have been reported, but the most consistent is the use of glucocorticoids. This association was suspected as early as 1966 by Jain and Singh,86 but was first reported in detail in 1985 and 1985 by Wakakura and Ishikawa and Harada and Harada.20,74,223 Haimovici et al reported an odds ratio of 10.3 (95% CI 4.0–26.4) for corticosteroid use in their case control study of 312 patients with CSC.73 Tittl and colleagues reported an odds ratio of 3.17 (95% CI 1.3–7.7) in their 230 patients.216 A smaller prospective study (38 cases) also supports the association.88 CSC appears to be associated with elevated levels of endogenous corticosteroids, as in patients with Cushing’s syndrome.19 Glucocorticosteroids have at times been used to treat CSC,91,120,162 but given the strong association between CSC and steroid use, they should be avoided whenever possible. Furthermore, patients with CSC should be questioned exhaustively about all forms of steroid use to eliminate the possibility that a skin cream, joint injection, nasal spray, inhalant, or other commonly overlooked form of glucocorticoid could be a contributing factor.
Pregnancy is another recognized risk factor for CSC. Plasma cortisol levels are elevated during pregnancy, particularly during the third trimester.42 Haimovici and colleagues had 18 pregnant patients among their 312 cases (versus just 2/312 age and gender-matched controls, P<0.0001) and calculated an odds ratio of 9.3 (95% CI 2.1–40.6).73 Chumbley and Frank describe an otherwise healthy young woman who developed active CSC during each of four successive pregnancies, with spontaneous resolution after giving birth.40 Pregnancy-associated CSC occurs most often in the third trimester, tends to present with distinctive white subretinal exudation, and usually resolves spontaneously after delivery.20
The historic association between CSC and type A personality is controversial. While this is a challenging relationship to study, Yannuzzi’s study of CSC and personality types supports the association.235 His study relied on a comparison of personality surveys of CSC patients with matched controls that predominantly had diabetic retinopathy, rhegmatogenous retinal detachment, and refractive error. Others have found use of psychotropic medication to be a risk factor, which may suggest that psychological stresses are associated with CSC.216 Additional associations including systemic hypertension,73,216 gastroesophageal reflux disease (GERD),124 and use of alcohol73 or sympathomimetic agents139,209,242 require further confirmation. Kitzmann and colleagues small (74 eyes) case control series failed to confirm any of the above associations with CSC, including corticosteroid use.97
B. Clinical Findings
CSC can occur in an acute or chronic form. The designation of chronicity in CSC is somewhat arbitrary. Some authors have defined chronicity as persistent fluid for at least six months,239 whereas recent clinical trials have used three months with persistent fluid.36,172,191 While the acute form can sometimes be recurrent, it generally resolves spontaneously with minimal sequelae. Chronic CSC, however, can result in widespread RPE damage, sometimes referred to as diffuse retinal pigment epitheliopathy (DRPE). These patients have longstanding subretinal fluid that cannot be reabsorbed efficiently because of choroidal disease and extensive dysfunction and loss of RPE. The presence of chronic fluid leads to photoreceptor death and may result in permanent visual loss. Furthermore, chronic CSC is more likely to be complicated by CNV that can cause severe visual loss. Not all patients with acute CSC go on to develop chronic disease, and the reasons for the varied courses are not well understood. There may be additional unknown differences in the pathophysiology of acute and chronic CSC.
A newer classification scheme based on the status of the retinal pigment epithelium employs the terms classic CSC and DRPE. Classic cases have minimal focal RPE damage and discrete leaks at the level of the RPE, and DRPE cases have extensive RPE damage and diffuse leakage.200 Additionally, a minority of patients develop the bullous variant which occurs when inferiorly gravitating fluid results in a bullous serous retinal detachment.59
The hallmark of CSC is the presence of a serous detachment of the neurosensory retina in the posterior pole, sometimes associated with a serous RPE detachment. These findings are usually apparent on slit-lamp biomicroscopy, and they are easily detected and quantified with optical coherence tomography (OCT). In long-standing CSC, some eyes will develop intraretinal fluid and cystoid edema (Figure 1). The most common fluorescein angiographic pattern in CSC is a single pinpoint leak at the level of the RPE (Figure 2). Some patients will present with multiple pinpoint leaks or a smokestack fluorescein pattern (Figure 3). Fluorescein angiography may show evidence of CSC episodes limited to the extramacular area (Figure 4) that typically go undetected as they are asymptomatic. Those presenting with DRPE have extensive RPE disease and diffuse fluorescein leakage. In long-standing CSC, findings may include RPE atrophy that exposes underlying choroidal vascular patterns, areas of RPE pigment clumping, and even bone spicules. Other important imaging findings include evidence of gravity-driven descending tracts of subretinal fluid on fluorescein angiography or fundus autofluorescence (FAF) (Figure 5). These tracts are typically hyperautofluorescent when the fluid first occurs, but then become increasingly hypoautofluorescent as RPE cells are damaged in the pathway of the fluid. On mid-phase indocyanine green (ICG) angiography, there are often plaques of hyperfluorescent inner choroidal staining both in the posterior pole and in the periphery. As shown in Figure 6, CSC often extends beyond the macula.201 Hence, CSC can be regarded as a disorder of the posterior pole rather than purely a maculopathy. Enhanced depth imaging (EDI) reveals a thickened choroid.80 These imaging findings are discussed further below.
Figure 1.

Cystoid macular edema in end-stage CSC. (A) Time-domain OCT of an eye with CSC and subretinal fluid (between arrows). Best-corrected visual acuity is 20/40. (B) SD-OCT of the same eye four years later, now with cystoid macular edema (arrows), subretinal fluid, and best-corrected visual acuity of 20/400. The eye had not responded to two PDT treatments, multiple anti-VEGF injections, and a trial of oral acetazolamide. CNV had been ruled out with fluorescein angiography and indocyanine green angiography. (C) SD-OCT of the same eye one month later. Macular atrophy is apparent after spontaneous resolution of cystoid edema. Best-corrected visual acuity is 20/500.
Figure 2.

Fluorescein angiogram demonstrating a single pinpoint leak at twenty seconds (A) and ten minutes (B).
Figure 3.

Fluorescein angiogram showing a smokestack leakage pattern at thirty seconds, two minutes, and five minutes.
Figure 4.

Fluorescein angiogram of an asymptomatic eye with extramacular evidence of CSC.
Figure 5.
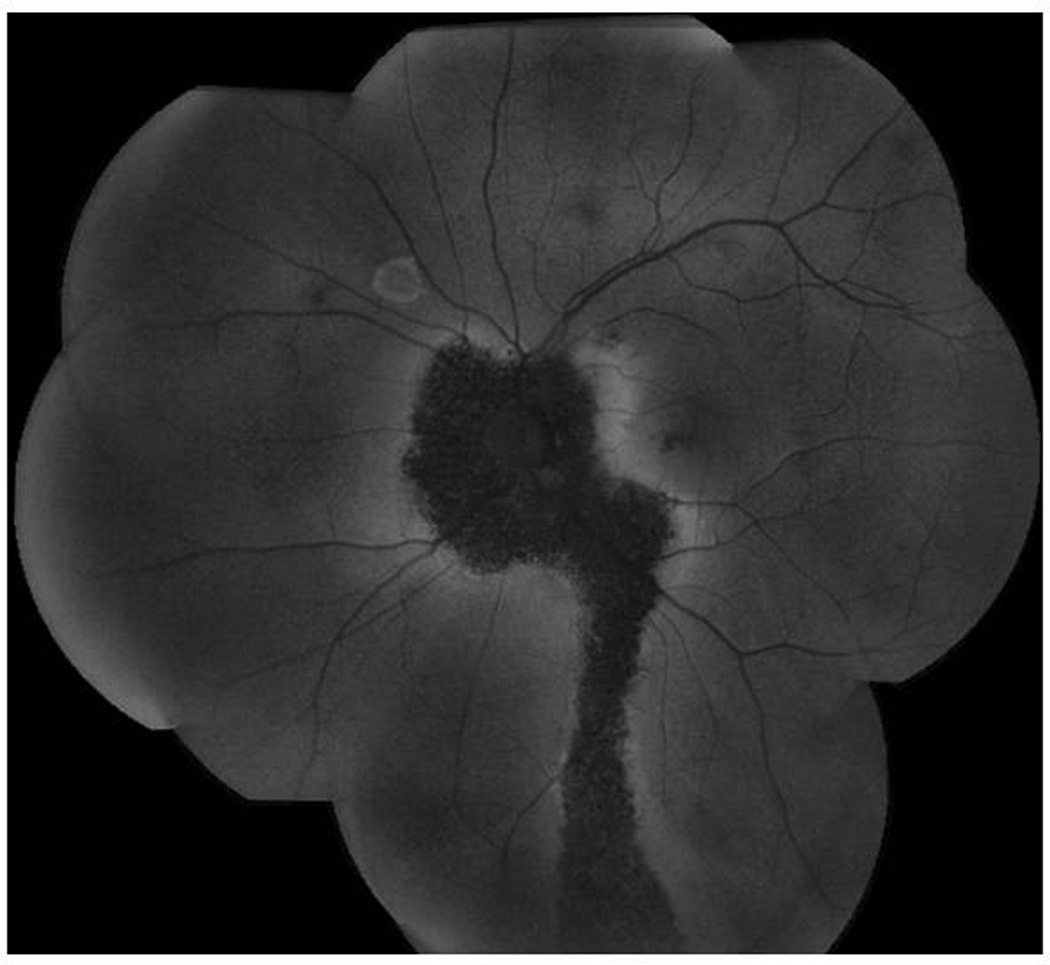
Fundus autofluorescence (FAF) of a descending tract in CSC. The marked hypoautofluorescence suggests chronic disease and irreversible RPE damage. There is also a hyperfluorescent ring superiorly due to a retinal pigment epithelial detachment.
Figure 6.

Numerous hyperfluorescent plaques on mid-phase indocyanine green (ICG) angiography demonstrating inner choroidal staining.
II. PATHOPHYSIOLOGY
The pathophysiology of CSC remains poorly understood despite advances in imaging techniques and numerous studies of the disease. Clinical findings in CSC have given rise to several theories of pathogenesis, each of which may in part explain the disease process. The major theories of the pathophysiology of CSC, including etiologies originating in the choroid, RPE, and hormonal milieu, are presented here in Part A. In Part B, there is a discussion of pathophysiological insights that have been gained through new examination and imaging technologies.
A. Theories of Pathogenesis
1. Role of the Choroid
The current understanding of the pathogenesis of CSC emphasizes the role of the choroid. The choroid is thought to be hyperpermeable in CSC, possibly as a result of stasis, ischemia, or inflammation.239 The staining of the inner choroid seen on mid-phase ICG angiography is the primary evidence of choroidal hyperpermeability.156,167,201 The primary role of the choroid is further supported by the EDI OCT finding of a thickened choroid in both eyes of patients with CSC.80 Interestingly, the areas of staining on ICG do not correspond with thickening on EDI OCT, and their significance remains to be fully explained.204
Hyperpermeable choroidal vessels are thought to produce increased tissue hydrostatic pressure, which promotes the formation of retinal pigment epithelial detachments (PEDs), overwhelms the barrier function of the RPE, and leads to areas of fluid accumulation between the retina and the RPE. Since areas of choroidal staining are usually contiguous with foci of RPE leakage on FA,156,218,238 the hypothesis of a mechanistic relationship between the two findings is reasonable. Not all areas of choroidal staining are associated with RPE leaks, suggesting that in some instances the RPE may be able to withstand the stresses posed by choroidal disease.
The underlying cause of choroidal thickening on OCT and staining on ICG is not known. Theories that integrate these findings with corticosteroid use and sympathomimetic agents have been advocated. Although the connection between sympathomimetic agents and CSC is less established, their potential for effects on choroidal vessels and blood flow is clear. Corticosteroids influence the transcription of myriad genes, including those for some adrenergic receptors.15,181 Furthermore, steroids are known to potentiate vascular reactivity through a variety of pathways.234 CSC may result from impaired choroidal vascular autoregulation induced by steroids, catecholamines, or sympathomimetic agents. A monkey model of CSC produced by repeated intravenous administration of epinephrine (epinephrine only in one monkey, epinephrine plus steroid in a second animal) showed damage to choriocapillaris endothelium and overlying RPE.241 Furthermore, there was extensive fibrin leakage into Bruch membrane and fibrin clots in the choriocapillaris at the sites of endothelial cell loss. Although this model has been criticized as inducing manifestations of hypertensive disease rather than true CSC, it did produce a strikingly similar constellation of findings including serous retinal detachment and characteristic changes in fluorescein angiography.242
Further evidence for choroidal vasculopathy in CSC comes from studies that indicate that areas with mid-phase inner choroidal staining also have delayed choroidal filling, suggesting choroidal lobular ischemia156,167,201 with associated areas of venous dilatation. Elevated serum levels of plasminogen activator inhibitor-1, an inhibitor of physiologic fibrinolysis, in CSC have led to suggestions of a thrombotic mechanism for these vascular changes.26,28,79,233
Tittl and colleagues measured fundus pulsation amplitudes in CSC eyes and control eyes with a laser interferometer and demonstrated greater amplitudes in CSC eyes.214 Affected eyes were also significantly different from unaffected eyes in the same patients, while systemic parameters were similar between study subjects and controls, suggesting that the pulsatile component of choroidal blood flow is altered locally in the affected eye in CSC. In a separate study, this group demonstrated that subfoveal choroidal blood flow is significantly greater in inactive chronic CSC patients than in controls during exercise.215 The evidence for a choroidal vasculopathy in CSC is strong, but the underlying mechanism of choroidal disease remains to be determined.
2. Role of the Retinal Pigment Epithelium
RPE dysfunction plays a significant role in the pathogenesis of CSC. This is perhaps most easily appreciated in cases of DRPE, in which widespread loss of RPE cells is apparent on clinical exam and FAF, and where the loss of RPE barrier and pumping functions in the setting of an engorged choroid results in chronic subretinal fluid. Before widespread acceptance of an underlying choroidal pathology, RPE theories of pathogenesis predominated. The focal areas of leakage through the RPE that are characteristic of classic CSC were one of the first clues to the pathogenesis of the disease. These pinpoint leaks were seen as focal defects in the RPE that were thought to be primarily responsible for the accumulation of subretinal fluid. However, Negi and Marmor showed that focal RPE defects promoted flow of fluid out of the subretinal space toward the choroid rather than vice versa.149 An alternate theory is that focal loss of polarity of RPE cells leads to active fluid pumping into the subretinal space.205
The role of the RPE in CSC pathogenesis remains poorly understood. Perhaps the most complete theory states that increased tissue hydrostatic pressure in the choroid overwhelms the barrier function of the RPE and leads to areas of fluid accumulation between the retina and the RPE. Some refer to the pinpoint areas of leakage seen in acute CSC as micro-rips or blowouts. PEDs are common in CSC and could also represent a form of RPE decompensation in response to high choroidal hydrostatic pressure. The CSC monkey model,241 described above, and ICG findings of perfusion delays in areas of leakage indicate that local choroidal ischemia could contribute to RPE damage. Epinephrine has been associated with RPE apoptosis in vitro,195 so a direct effect unrelated to perfusion could also contribute to pathogenesis. Inflammatory and hormonal causes for focal RPE incompetence have also been suggested.62,178,239
Spectral domain OCT studies of the RPE in CSC have shown RPE defects in the location of a fluorescein leakage in some patients.54 These studies mirror the small RPE defects found histopathologically in the monkey model.241 Many RPE defects are too small for reliable detection on OCT. Another OCT-based study of the RPE showed that RPE abnormalities are present in nearly all asymptomatic, fluid-free contralateral eyes of CSC patients.68 This suggests that RPE damage is not necessarily a late sequela of CSC, but occurs even in formse frustes.
The success of focal photocoagulation of isolated leaks suggests that treatment at the level of the RPE may be effective in some cases.60 Pharmacologic interventions targeting the RPE (discussed further below), however, have not yet yielded a treatment breakthrough.51,164
3. Hormonal Factors
The strong association between CSC and glucocorticosteroid use suggests a role in pathogenesis. Both serum glucocorticoid57 and serum catecholamine levels72,209 are elevated in active CSC. The complexity of corticosteroid pharmacology and the interconnected nature of the hypothalamic-pituitary-adrenal axis and the autonomic nervous system suggest a number of possible explanations for these associations.
Glucocorticoids could potentially impact the course of the disease by affecting the choroid, Bruch’s membrane, or the RPE.20 Proposed mechanisms in the choroid include effects on vascular autoregulation via increased transcription of adrenergic receptors15,181or potentiation of vascular reactivity,234 effects from steroid-induced systemic hypertension,216 or a prothrombotic effect.20 Corticosteroids could affect Bruch membrane via their known inhibition of collagen synthesis.152 Finally, epithelial water and ion transport are altered by corticosteroids,16,72,182,197 and this might impair the barrier function of the RPE. Arndt and colleagues studied RPE tissue in vitro and showed that administration of hydrocortisone decreased the transepithelial membrane potential and resistence.8 In vivo, IV prednisolone reduced the rise in standing potential induced by glucose infusion.
Interestingly, local ocular glucocorticoid use is usually not associated with CSC.18,82,100 Intravitreal and periocular steroid treatments are common, yet reports of CSC after local ocular steroid injections are rare; however, almost every form of exogenous glucocorticoid has been associated with exacerbation, and ocular and systemic glucocorticoids should be avoided in CSC.
CSC has not been described after treatment with sympathomimetic agents alone, so it may be that the association with elevated catecholamine levels is related to simultaneously increased levels of corticosteroids in a physiologic stress response. Furthermore, beta-blocking agents have not been effective in treating or preventing CSC.22,166
4. H. pylori
Several recent papers have reported an association between H. pylori infection and CSC,3,10,41,64,135,140 and some have noted a beneficial effect in CSC in patients treated for H. pylori.64,168 H. pylori is a Gram-negative bacterium that causes gastritis and has also been associated with a variety of extragastric conditions, including thrombotic disease.25 Thrombotic disease is a pathway through which infection could cause CSC. Immune-mediated damage to choroidal endothelial cells resulting from molecular mimicry is one proposed mechanism.65 The largest series to date found H. pylori infection in 31/78 (40%) French CSC patients versus a 25% infection rate in the general population (p=0.0036).3 Kitzmann and colleagues, however, found no patients with a known history of H. pylori infection in their 74 cases.97
A randomized, controlled trial comparing triple therapy H. pylori treatment with observation in H. pylori-infected acute CSC patients found that the time to fluid resorption was significantly reduced in the treatment group (9.3 vs 11.6 weeks, p=0.015).168 There was no visual acuity benefit. Follow up visits were at weeks 2, 4, 6, 8, 12 and 16. The four week interval between study visits after week 8 limits the precision of the time to resorption outcome measurements in this study. Larger studies are warranted to confirm the association between H. pylori and CSC.
5. Genetics
A review of the CSC literature reveals numerous reports of familial CSC.5,70,119,157,161,222,224,230 Perhaps the most compelling evidence for a genetic contribution to pathogenesis comes from Weenink and colleagues224 who found CSC-like pathology in 14/27 (52%) families of chronic CSC patients. Only a small percentage of affected relatives reported symptoms..
Just one population-based prevalence study has been conducted to date, and this was in a predominantly white, American population. Nonetheless, CSC is thought to have a higher prevalence in whites, Hispanics, and Asians than in African Americans.44,138,236 Further studies of the genetics of CSC are warranted, including studies of single nucleotide polymorphisms (SNPs) that may help identify individuals at risk to allow for appropriate counseling and closer monitoring. Studies of SNPs could help identify those at greatest risk of developing CSC and may be helpful in predicting those who are more likely to progress to chronic CSC or DRPE.
6. Cytokine Analyses
Aqueous samples from CSC eyes have been analyzed for various growth factors and cytokines.114,192 Aqueous vascular endothelial growth factor (VEGF) levels are not elevated in CSC. Levels of IL-6, IL-8, and monocyte chemoattractant protein-1 do not differ from controls, while interferon gamma and TNF-α have been undetectable in these eyes. This provides further evidence against an inflammatory etiology. Platelet-derived growth factor (PDGF) levels appear to be lower than in controls. PDGF is an RPE mitogen, and it is secreted by endothelial cells to recruit blood vessel mural cells.30 PDGF-related RPE dysfunction or vascular incompetence could contribute to the pathogenesis of CSC.
B. Pathophysiological Insights From New Imaging and Examination Technologies
1. Spectral Domain Optical Coherence Tomography
The first spectral domain OCT device (SD-OCT) was approved by the FDA in 2006. Since then, SD-OCT has become the standard for OCT imaging worldwide given its ability to acquire high definition images of ocular structures rapidly. While OCT allows for ready detection of known manifestations of CSC, including serous retinal detachment (Figure 7) and serous PED,144 the high resolution images have allowed for detailed study of subtle findings in CSC and have enhanced our understanding of the disease.
Figure 7.

(A) Enhanced depth imaging (EDI) OCT of an area with CSC-related subretinal fluid. The choroid is abnormally thick (502 microns). (B) Corresponding FAF in which a line shows the location of the EDI OCT. Note central hypoautofluorescence and surrounding hyperautofluorescence suggestive of RPE damage.
Perhaps the most important and clinically useful application of SD-OCT in CSC has been the ability to image the choroid with EDI OCT (Figure 7). EDI OCT can be performed with commercially available SD-OCT units. The choroid has been shown to be abnormally thick in CSC in both the affected and the fellow eye.80,130 In one study the mean age-adjusted choroidal thickness was 368 microns in CSC patients and 242 microns in controls.95 This thickening is thought to be related to choroidal vascular disease and the apparent choroidal hyperpermeability seen on ICG. Treatment response to PDT can be in part evaluated with EDI OCT, which typically shows about a 20% reduction in subfoveal choroidal thickness one year post-half fluence treatment.127,129
The thickness of the outer nuclear layer, as measured with SD-OCT, appears to correlate with acuity in CSC.133 In one study, the mean thickness was 74.6 µm in patients with resolved CSC and acuity worse than 20/20. It was 103 µm in CSC patients who saw 20/20 or better and 125 µm in normal age-matched controls. The distance from the internal limiting membrane to the external limiting membrane appears to be decreased in CSC, and this finding corresponds with outer nuclear layer thinning, possibly from photoreceptor apoptosis.132
SD-OCT has proven useful for the study of punctate precipitates and white material seen in as many as 65% of CSC eyes in the area of serous retinal detachment.101,128 These precipitates are hyperreflective on OCT and can be found both within the retina and in the subretinal space (Figure 8).134,203 The punctate precipitates and white material often occur together, suggesting that they are related substances. Numerous hypotheses about these substances are proposed: they may be accumulations of shed photoreceptor outer segments in the subretinal space, accumulations of fibrin or lipid, or macrophages clearing the subretinal space of such debris (see fundus autofluorescence section below).
Figure 8.

Punctate hyperautofluorescence resulting in a granular appearance on FAF (A, arrow) often corresponds to hyperreflective lesions in the outer retina (B, arrow) and subretinal space on OCT. They have been hypothesized to be macrophages engorged with phagocytosed outer segments.
OCT suggests that photoreceptor outer segments elongate in eyes with active serous detachment.132 Photoreceptor length is thought to correspond to the distance from the inner segment/outer segment (IS/OS) junction to the outermost retinal structure on SDOCT. Outer segments may elongate on the outer surface of the detached retina when not apposed with the RPE and eventually shed into the subretinal space (Figures 9 and 10). This OCT finding is frequently present when punctate precipitates or white deposits are seen clinically.134
Figure 9.

SD-OCT of a fovea-involving a serous retinal detachment in a patient with acute CSC. Note the lack of significant reflective debris on the outer retina or in the subretinal space.
Figure 10.

(A) SD-OCT from the initial visit of a patient who had symptoms of CSC for ten months. Note the accumulation of material on the outer retina (arrow). This accumulation is thought to represent shed photoreceptor outer segments, engorged macrophages, and other inflammatory debris such as fibrin. (B) SD-OCT of the same eye two months later showing that the accumulation of this material has increased (arrow), perhaps due to elongation of outer segments.
RPE abnormalities can also be readily imaged with SD-OCT. The area around a leak on FA frequently has a PED,54,141 sometimes with a detectable breach in the RPE layer that is believed to be the site of fluorescein transmission from the choroid. Asymptomatic contralateral eyes, despite ostensibly normal macular raster scans, have been shown to have notable RPE abnormalities on en face RPE maps or c-scans.68 These RPE changes consist of multiple bumps, and they may represent early RPE decompensation.
OCT findings, particularly disruption of the IS/OS junction, correlate with acuity outcomes.96,133,154,163 Integrity of the IS/OS junction has been correlated with visual acuity in many macular diseases.151,153 Unsurprisingly, foveal thickness is less in affected eyes after resolution of fluid in chronic CSC than in fellow eyes, reflecting the risks of long term atrophy in CSC.45 Foveal thickness at presentation and after resolution appear to correlate with acuity outcomes.56 Those with more foveal thinning and atrophy have worse vision.
2. Fundus Autofluorescence
Fundus autofluorescence (FAF) is a novel imaging technique that relies on the autofluorescent properties of the breakdown products of cellular components, especially photoreceptor outer segments. While confocal systems such as the Heidelberg Spectralis® (Heidelberg Engineering, Heidelberg, Germany) provide high resolution images by focusing on the RPE alone, non-confocal systems such as the Topcon retinal camera (Topcon Medical Systems, Oakland, New Jersey, USA) have the advantage of simultanaeous imaging of the RPE and outer retina. The primary cellular breakdown product, lipofuscin, contains various fluorophores (such as the pyridinium bisretinoid A2E) that can release light when stimulated by light of specific wavelengths. Lipofuscin accumulates in RPE cells due to incomplete lysosomal degradation of photoreceptor outer segment disks. In the setting of age-related macular degeneration (AMD), hyperautofluorescence is often due to excessive accumulation of lipofuscin within the RPE, while hypoautofluorescence is frequently a result of RPE atrophy;185,202 however, the mechanisms of FAF change deserve separate consideration in the setting of CSC.
Autofluorescence often differs in acute versus chronic CSC. In acute CSC, there may be no FAF findings initially.128 Hyperautofluorescence characteristically develops within the area of subretinal fluid over months, although macular pigment may obscure FAF changes.203 The hyperautofluorescence tends to accumulate along the borders of the detachment, especially inferiorly134,203 and usually disappears with resolution of subretinal fluid in acute CSC. There also may be areas of hyperautofluorescence that correspond to punctate precipitates and white material seen on clinical exam128,134 (see SD-OCT section above). In chronic CSC, autofluorescence abnormalities reflect areas of chronic RPE damage or areas of active serous detachment. There are sometimes large areas of FAF changes or descending tracts consistent with past gravity-related guttering of subretinal fluid (Figure 5). FAF changes in chronic CSC have been characterized as granular or confluent hypoautofluorescence and hyperautofluorescence.81 These patterns often coexist with areas with different degrees of RPE damage.
Spaide has postulated that areas of hyperautofluorescence seen in acute CSC are indicative of lipofuscin components within accumulating photoreceptor outer segments on the outer surface of the detached retina.199,203 The inability of the separated RPE to process old outer segments is thought to lead to increasing hyperautofluorescence with increased duration of serous retinal detachment. Spaide found that areas of hyperautofluorescence in CSC corresponded to areas of accumulation of material on the outer surface of the retina, as visualized with time-domain OCT.203 The thickness of this material was proportional to the amount of hyperautofluorescence.203 A more recent study using SD-OCT has proposed that these areas of thickened outer retina represent elongated photoreceptor outer segments.134 Accumulation of shed outer segments in subretinal fluid may explain the relative hyperautofluorescence seen at the inferior margin of serous retinal detachments.
The small granular/punctate areas of hyperautofluorescence seen in CSC correspond to the punctate subretinal white dots/precipitates seen clinically, and these may represent macrophages engorged with phagocytosed outer segments.134,203 While lipid and fibrin are proposed components of these white deposits, they do not autofluoresce and therefore do not explain the autofluorescent deposits. The punctate FAF findings correspond to hyperreflective lesions in the outer retina and subretinal space on OCT (Figure 8). Not all punctate precipitates are hyperautofluorescent, and those that do not autofluoresce may be composed of lipid or fibrin.
Accumulation of autofluorescent material in the subretinal space may contribute to RPE damage in CSC.128 A2-PE and A2E, autofluorescent byproducts of visual cycle proteins, have been shown to promote production of reactive oxygen species that would impact nearby RPE cells and photoreceptors.92
In DRPE, advanced and irreversible FAF changes predominate. FAF changes highlight areas of RPE damage. Imamura and colleagues grouped significant hypoautofluorescence findings into three categories: granular hypoautofluorescence, confluent hypoautofluoescence, and descending tracts.81 In their large cohort, they found equal incidences of descending tracts from the macula and from the optic nerve head (9.1% for each). OCT studies have shown that areas of hypoautofluorescence correlate with RPE atrophy and associated atrophy of the outer retina.203 The granular pattern of hypoautofluorescence appears to signify incomplete loss of RPE tissue.
While FAF imaging has shed light on the pathophysiology of CSC, this imaging modality has functional utility as well. Spaide and colleagues reported that normalized central macular FAF predicts visual acuity.203 Decreased central macular autofluorescence was associated with decreased visual acuity. In the largest retrospective cohort examining the correlation between FAF and visual acuity, both granular and confluent patterns of hypoautofluorescence were found to be strongly correlated with visual function.81 In that study, hyperautofluorescence did not reach a statistically significant correlation with acuity in multivariate prediction analyses.
Infrared186 (IR) and near infrared autofluorescence has been investigated in CSC.177 Sekiryu and colleagues also looked at the short-wave autofluorescent properties in CSC eyes. Areas of simultaneous punctate hyper-IR autofluorescence and hyper-short wave autofluorescence in CSC eyes are believed to signify RPE hyperplastic clumps, since hyper-IR autofluorescence may come from melanin while hyper-short wave autofluorescence appears to be associated with lipofuscin fluorophores. RPE clumping in these areas is consistent with OCT findings. Study of these modalities is in an early stage, and the significance of the findings at new wavelengths has yet to be fully elucidated.
3. Multifocal Electroretinography (mfERG)
Studies using mfERG indicate that there is more widespread retinal dysfunction in CSC than is appreciated on clinical exam. Marmor and Tan were among the first to investigate mfERG in CSC, and they argued that mfERG depression may extend beyond the border of observed serous detachment and could also be found in fellow eyes.126,147 Vajaranant and colleagues, however, found that mfERG changes predominantly corresponded with areas of clinically apparent disease.219 mfERG amplitudes improve markedly after resolution of subretinal fluid, but do not return to normal levels.38,210,211
Multifocal ERG findings tend to correlate with function. Correlation between acuity, first order amplitudes, and implicit times has been documented.107,240 Implicit times in the paracentral rings correlate with OCT findings as well, including macular volume, area of subretinal fluid, and thickness of subretinal fluid. Given that mfERG correlates with activity and function in CSC, mfERG is a reasonable modality for documenting disease course and has been used as a primary outcome measure in a study of half dose verteporfin PDT for acute CSC. Significant improvements in OCT findings and vision at twelve months correlated with mfERG amplitude ratios.229
4. Microperimetry
Microperimetry allows for functional testing of different points in the macula, thereby providing more information about the health of the macula as a whole than visual acuity alone. Microperimetric data in CSC appears to correlate well with anatomic findings (Figure 11).
Figure 11.

(A) Fundus autofluorescence (FAF) showing several paramacular areas of autofluorescence abnormalities. The hypoautofluorescent descending tract inferotemporal to the macula represents the area of greatest RPE damage. (B) A microperimetry pattern used to study the inferior macula and mid-periphery shows decreased sensitivity in the areas of autofluorescence abnormality, with a particularly dense scotoma in the area of the descending tract seen in (A). Units are decibels.
Ojima and colleagues compared microperimetry with SD-OCT and found that retinal sensitivity in areas of RPE irregularity or IS/OS disruption is significantly decreased.155 Microperimetry findings have also been successfully correlated with mfERG implicit times and visual acuity.94
Retinal sensitivity as measured with a microperimeter improves with treatment and with resolution of subretinal fluid.55,173,187 Sensitivity generally does not return to normal levels after resolution of subretinal fluid, even in patients with 20/20 visual acuity.159 This highlights the need to balance the risks and benefits of early treatment in eyes with persistent subretinal fluid.
5. Adaptive Optics
Adaptive optics (AO) is a technology that corrects for the optical aberrations of an eye and thereby allows for imaging of individual photoreceptors. A wavefront sensor detects higher order aberrations in the optical system, and a deformable mirror or another form of phase modulator corrects the aberrations.158 Ooto and colleagues have investigated CSC eyes with an AO scanning laser ophthalmoscope (SLO) device..158 They examined eyes with resolved subretinal fluid; most of their patients had acute CSC that resolved spontaneously. Eyes with resolved CSC had fewer cones per square millimeter than controls (31,290 +/− 14,300 at 0.2 mm from the fovea versus 67,900 +/− 9120 in controls).
They documented this dramatic loss of photoreceptors in a cohort where most patients (29/45) had 20/20 or better visual acuity. In fact, the cone density in CSC eyes with preserved IS/OS junctions was significantly less than controls (42,380 +/− 6390 versus 67,900 +/− 9120 in controls). Cone density at 0.2 mm from the fovea correlated with visual acuity and also with OCT findings. CSC eyes with intact IS/OS junctions had greater average cone densities than CSC eyes with IS/OS junction gaps on OCT. There was no difference in cone density between eyes treated with PDT or focal laser and untreated eyes. The loss of photoreceptors, even in eyes with relatively good acuity, underscores the frequently subclinical functional loss that patients can experience after even a single episode of CSC.
III. TREATMENT
Acute CSC is typically a self-limited process.98 Recovery of visual acuity typically occurs within one to four months, coinciding with reattachment of the neurosensory retina, with few recognized visual sequelae.98,148,239 Recurrences are common, however, occurring in approximately 30–50% of patients by one year.121 Patients with frequent recurrences or chronic neurosensory retinal detachment may develop RPE atrophy and neurosensory retinal changes that results in permanent loss of visual function, including visual acuity, color vision, and contrast sensitivity.14,23,50,103,122 Although the terms classic CSC and DRPE seem to be more helpful in determining treatment approaches, the terms acute and chronic (fluid persisting >3 months) will be used in this discussion.
Observation is the standard initial management in most cases of acute or classic CSC, but there are instances when treatment may be desirable. These include cases of CSC with persistent macular subretinal fluid or reduced visual acuity, cases in which rapid recovery of vision is required for vocational or other reasons, and also where untreated CSC has previously resulted in a poor visual outcome in the fellow eye. Treatment should also be considered in patients who experience multiple recurrences.
The aims of treatment of CSC are to induce reattachment of the neurosensory retina, improve or preserve visual acuity, and prevent recurrences. It is important to consider the temporal course of the fluid and the RPE status when choosing among different treatment options. Treatment of DRPE cases may not offer significant visual benefit if RPE atrophy and photoreceptor damage is extensive, but may prevent further toxicity to the photoreceptors in the setting of chronic fluid. CNV can arise in the setting of CSC and requires rapid detection and treatment. Retinal hemorrhage and lipid in conjunction with subretinal fluid suggest CNV may be present. Diagnostic fluorescein or indocyanine green angiography can confirm the presence of CNV.
Only limited randomized clinical trials evaluating CSC treatment modalities have been performed, but they are critical for guiding evidence-based treatment. Non-randomized trials are less helpful for providing definitive data regarding efficacy given the waxing and waning course of CSC. Several pilot studies provide insight about the feasibility of larger trials. In the following discussion the quality of the evidence for a particular intervention is rated as good, fair, or poor based on the U.S. Preventive Task Force definitions (Table 1).1
Table 1.
Quality of Evidence, U.S. Preventive Services Task Force Definitions1
| Good | Evidence includes consistent results from well-designed, well-conducted studies in representative populations that directly assess effects on health outcomes |
| Fair | Evidence is sufficient to determine effects on health outcomes, but the strength of the evidence is limited by the number, quality, or consistency of the individual studies, generalizability to routine practice, or indirect nature of the evidence on health outcomes |
| Poor | Evidence is insufficient to assess the effects on health outcomes because of limited number or power of studies, important flaws in their design or conduct, gaps in the chain of evidence, or lack of information on important health outcomes. |
A. Observation and Risk Factor modification (Quality of Evidence – Fair)
Management of classic CSC usually involves careful observation with risk factor modification. In most cases, a three month period is acceptable to allow subretinal fluid to resolve spontaneously.98,236 Visual acuity characteristically returns to normal after a first episode.
Risk factor modification most frequently consists of discontinuing corticosteroid use (see section below). Other risk factor modifications have been proposed. As CSC has been associated with high baseline cortisol levels and Type A personalities, it has been suggested that activities to reduce overall stress levels may be of benefit; however, their utility has not been established.61,75,139,198,213,216,231
Obstructive sleep apnea has been associated with CSC, possibly because some patients with sleep apnea have higher basal levels of endogenous catecholamines.99,111 Interestingly, one report demonstrated a rapid (one week) resolution in a patient with bilateral CSC after starting treatment with a continuous positive airway pressure machine.85
Use of 5-phosphodiesterase inhibitors (eg., sildenafil and tadalafil) has been associated with CSC.4,52 Although some improved after stopping such medications, the only large study of this association, a case control study with 577 cases, failed to confirm an association.53
B. Discontinuation of Steroids (Quality of Evidence – Good)
Given the association between steroid use and CSC, discontinuation of such treatment when practical is warranted to aid resolution of CSC and prevent complications of chronic disease.32,73 In 1966 Jain and Singh described a case of CSC that resolved after stopping oral steroids prescribed to treat Reiter syndrome.86 Several subsequent case reports demonstrated a link between reduction or discontinuation of systemic corticosteroids and resolution of CSC.2,112,166,223,227 The largest series to date was a noncomparative case series of 24 eyes with CSC, most of which were initially misdiagnosed and therefore inappropriately on systemic steroids. Nearly 90% had resolution of subretinal fluid and RPE leaks after discontinuation of steroids, with approximately 14% of eyes requiring adjunctive laser photocoagulation.189
Although there are no randomized trials of steroid discontinuation for CSC, a consensus has developed about the role of corticosteroids in CSC based on epidemiologic data (discussed previously), case series, and challenge/re-challenge reports. Further study in the form of large clinical trials are unlikely.
For patients using glucocorticosteroids, consultation with the prescribing physician is necessary to manage discontinuation or taper of these medications.87,112 Steroid-sparing agents may be of utility in patients requiring long-term immunomodulation. Nasal sprays,71 joint injections,6,12,90,143 and steroid skin creams47,48,89 have been associated with disease, and we routinely counsel patients to discontinue such medications if medically possible.
C. Helicobacter Pylori Treatment (Quality of Evidence – Poor)
As discussed above, Helicobacter pylori (H. pylori) infection has recently been implicated in the pathophysiology of CSC3,64,135 Some, therefore, have recommended testing and treatment of concomitant H. pylori infection in patients with CSC.65 The first report suggesting a relationship between H. pylori infection and CSC was a case report that described a patient with chronic recurrent CSC that seemed to wax and wane with infection and re-treatment.64 The one, randomized, unmasked trial of H. pylori treatment is discussed above.168.
D. Anti-glucocorticosteroids (Quality of Evidence – Poor)
Patients with CSC commonly have elevated levels of serum cortisol.57,72,244 resulting in trials of medications targeting cortisol pathways.87 This includes ketoconazole, mifepristone (RU486), finasteride, rifampin, and anti-adrenergics. There have been no randomized, controlled trials of these medications for CSC.
Ketoconazole, an anti-fungal agent, has been investigated by two groups as a treatment for CSC.66,137 Ketoconazole interferes with endogenous glucocorticoid production in part by inhibiting the conversion of 11 β-deoxycortisol to cortisol. Golshahi and colleagues retrospectively investigated ketoconazole (200 mg daily) in patients with new onset subretinal fluid, either as a first episode or a recurrence. They compared 15 study patients to 15 historical controls and found no difference between the two groups in terms of visual acuity or OCT parameters. Meyerle and colleagues prospectively studied five patients with chronic CSC in an uncontrolled pilot study. They used a higher dose (600 mg daily) and showed reduced serum cortisol levels, stable acuity, and anatomic improvement at 8 weeks.
Nielsen and colleagues investigate oral mifepristone, an abortefacient with glucocorticoid receptor antagonist properties in 16 chronic CSC subjects.150 Some patients responded well to the treatment, but the overall results were mixed. Seven subjects (44%) gained 5 or more letters after 12 weeks of treatment, and 7 subjects had improved OCT findings.
Finasteride, which is FDA-approved for benign prostatic hypertrophy (BPH) and male-pattern hair loss, is a 5-alpha-reductase inhibitor. 5-alpha-reductase converts testosterone to the potent androgen dihydrotestosterone (DHT). Given the overwhelmingly male demographics of CSC and its relationship to steroid hormones, this oral anti-androgen treatment has been investigated in CSC. In a pilot study of 5 patients with chronic CSC, the central subfield macular thickness, subretinal fluid volume, and serum DHT declined.51 The changes in center-subfield macular thickness and subretinal fluid volume paralleled serum DHT concentrations. Interestingly, subretinal fluid volume worsened in four out of five participants following discontinuation of finasteride. Three out of these four participants qualified for repeat finasteride treatment according to the investigational protocol, and when finasteride was re-instituted all of these participants experienced a reduction in subretinal fluid volume. A phase II, randomized trial sponsored by the National Eye Institute will provide more definitive data. Rifampin, another oral agent that proposed as a treatment of CSC, is thought to suppress endogenous glucocorticoid production, but has only been investigated in a small series.169,207
The rationale for adrenergic blockade to treat CSC is that CSC patients seem to have high serum catecholamine levels. Glucocorticosteroid use may affect adrenergic pathways by increasing expression of adrenergic receptors.69,181 Furthermore, Yoshioka’s monkey model suggest that inhibition of adrenergic receptors, particularly alpha receptors, prevents development of experimental CSC.243 Beta-blocking agents have therefore been investigated for CSC, but have not proven effective.22,166 There is one early report of prompt resolution of chronic CSC with an alpha blocking agent, but this has not been confirmed.76
E. Carbonic Anhydrase Inhibitors (Quality of Evidence – Poor)
Oral acetazolamide has been investigated as a treatment for CSC on the basis that inhibition of carbonic anhydrase IV in the RPE seems to promote resorption of subretinal fluid and retinal adhesion.43,208,228 The only clinical study to date investigating acetazolamide is a comparative, non-randomized, cohort study of 15 patients with acute CSC treated with acetazolamide compared to seven controls.164 Patients treated with acetazolamide had faster subjective improvements and resorption of subretinal fluid, with a mean time to resolution of 3.3 +/− 1.1 weeks in the treatment group and 7.7 +/− 1.5 weeks in the control group (P < 0.0001). There was no detectable difference in final visual acuity or recurrence rates between the two groups.
F. Anti-VEGF Agents (Quality of Evidence – Poor)
Anti-VEGF agents are not considered first-line treatments for either acute or chronic CSC, but several small trials have yielded suggestive results. Although VEGF levels are not elevated in aqueous samples from CSC eyes,114,192 some have hypothesized that hypoxic conditions in the choroid or RPE could lead to compartmentalized VEGF expression not detected in aqueous samples. Given this hypothesis and the remarkable success of these drugs in other disorders, numerous small trials have been undertaken.
1. Randomized, Controlled Trials
There are two small, randomized trials of anti-VEGF agents for CSC. The first was a trial of a single 1.25 mg bevacizumab injection in eyes with subretinal fluid for less than three months, most of which were experiencing recurrences. There was no difference in the clinical course of the 12 study eyes and the 12 controls.115 They did not report time to resolution of subretinal fluid, and the single injection design may be a limitation.
Bae and colleagues compared half-fluence PDT to intravitreal ranibizumab (0.5 mg) in a randomized trial of 16 eyes with chronic CSC (defined as recurrent CSC or persistent fluid for greater than six months).11 Visual acuity improved in both groups, but only two of 8 eyes in the ranibizumab group became fluid free after three months of treatment versus 6 of 8 PDT-treated eyes. These findings in this small study suggest that half-fluence PDT may be superior to intravitreal ranibizumab as a treatment for chronic CSC and requires further investigation..
2. Other Studies
Many uncontrolled studies report favorable results for intravitreal bevacizumab in CSC,7,83,110,113,117,118,183,188,217 and some comparative studies suggest a need for further study.9,109,115 Artunay and colleagues conducted a prospective, controlled, open-label, nonrandomized, trial of a single 2.5 mg intravitreal bevacizumab injection in thirty previously untreated eyes with chronic CSC.9 Treated eyes had a significantly better rate of stability or improvement in acuity (15/15 vs. 10/15 at 6 months) and a significantly higher rate of fluid resolution. A retrospective study comparing as needed bevacizumab injections to PDT for chronic CSC revealed similar improvements in acuity, greater foveal thinning in PDT eyes, and more fluid recurrence in injected eyes.109 The largest series published to date of intravitreal bevacizumab treatment for CSC included mostly patients having a first episode of CSC with a mean duration of about four months.117 Most patients (82.5%) had resolution of subretinal fluid with one or two injections. Classic CSC has a largely favorable natural history, so the lack of controls in this study is significant. An uncontrolled series of ten acute CSC eyes showed favorable anatomical and visual results.188
Anti-VEGF agents have a clearer role in eyes with CSC-related CNV. Intravitreal bevacizumab has been used successfully for CNV of diverse etiologies,67 and several reports confirm its utility for CSC-related CNV.34,123,145,165 Similarly, ranibizumab appears to be effective in the treatment of CSC-related CNV.31,102
G. Aspirin (Quality of Evidence – Poor)
Previous work has shown that patients with CSC demonstrate increased levels of plasminogen activator inhibitor compared to controls.79,232 This finding has led to a hypothesis that hypercoagulability plays a role in CSC pathogenesis. A study by Caccavale and colleagues investigated an aspirin regimen (100mg daily for 1 month followed by 100mg on alternating days for 5 months) in 109 patients.27 In this non-randomized, open label case series, aspirin appeared to hasten recovery of acuity, reduce the rate of recurrence, and result in a slightly better visual outcome (logMAR +0.07 +/− 0.13 or Snellen 20/23 versus +0.17 +/− 0.13 or 20/30, P<0.0001 at 2 years) in a comparison with a historical control group.
H. Laser Photocoagulation (Quality of Evidence – Good)
Focal laser photocoagulation is commonly used to expedite the absorption of subretinal fluid in acute and chronic CSC. Some reports indicate that laser treatment is associated with a decreased rate of recurrences, but this remains controversial (see below). Typically, laser burns are applied to areas of focal leakage that have been identified on FA as the principal sources of subretinal fluid. The mechanism of subretinal fluid resolution after laser photocoagulation treatment is not known; photocoagulation may seal focal defects in the RPE monolayer, promote a healing response and recruitment of healthy RPE cells, or directly stimulate pumping function of RPE cells near the leak. Although the first reports of thermal laser for acute CSC described the use of the xenon laser, the argon laser is used more commonly.142
1. Randomized, Controlled Trials
Leaver and Williams in 1979 reported faster resolution of subretinal fluid (6 vs. 16 weeks, P<0.001) in eyes treated with focal argon laser photocoagulation to the leakage site.108 This randomized trial included 70 eyes with subfoveal fluid and acuity of 6/12 or better. No duration of disease was stipulated, although minimal apparent RPE disease was required. They did not find a visual acuity benefit in the treatment group at six months.
Robertson and Ilstrup studied 42 eyes with “recent onset” CSC in a randomized, controlled trial comparing argon laser photocoagulation of the leakage site (“direct”) to sham treatment or treatment away from the leakage site (“indirect”).176 Just seven eyes underwent direct treatment, but they had a shorter duration of neurosensory retinal detachment (6 +/− 2.2 weeks) compared to the control groups (13.2 +/− 6.1 weeks for indirect and 16.3 weeks +/− 6.3 weeks for sham). Direct laser was not, however, directly compared with sham as direct laser was only given to eyes with extramacular leakage sites. No recurrences occurred over the 18 month follow-up period in the direct treatment eyes.
2. Other Studies
Burumcek and colleagues conducted a non-randomized study of laser versus observation in 45 eyes. They found a shorter time to fluid resolution and fewer recurrences in the laser group than in an untreated comparison group.24 They also reported a better final visual acuity in the treated group over almost 5 years of follow-up.
While the time to fluid absorption is consistently less in laser trials, reported recurrence rates after focal laser have varied. Ficker and colleagues reported the long-term follow up of the Leaver and Williams study.49 While just 44 eyes were available for long-term analysis, follow-up ranged from 6.4 years to 12.1 years. Recurrence rates were similar between the two groups (53% of controls and 44% of treated eyes, P=0.79). Final acuity and color discrimination were also similar. Two treated eyes developed extrafoveal CNV at the treatment site, but both eyes maintained excellent vision without further treatment for CNV. Drawbacks of this study include the number of patients lost to follow-up and limited power.
Brancato and colleagues reported a comparative series of 87 eyes with a mean of about eight years follow-up.21 The recurrence rate in the treated group was 40.5%, and in control eyes it was 42%. A retrospective study of 157 cases by Gilbert and colleagues also found no difference in long-term outcomes with laser.63
For chronic CSC, laser treatment to areas of leakage may decrease the amount of subretinal fluid. Yannuzzi and colleagues reported an uncontrolled series of 18 DRPE eyes treated with grid laser in areas of leakage, finding high rates of resolution of macular detachment and vision stability.237 These findings have not been confirmed in controlled studies, and there is some concern that grid laser to areas of damaged RPE may further impair RPE function. Focal laser does not appear to have a role in CSC with bullous serous detachment.17
Some general guidelines should be followed when planning laser treatment for CSC. Leakage sites should be greater than 375 µm from the fovea, and treatment should be performed with reference to a current FA. Other considerations include the duration of the attack (as fluid often resolves spontaneously), status of the fellow eye, and the patient’s life circumstances and desire for treatment. Treatment sites need to be monitored long-term for CNV. Leakage not amenable to laser, including subfoveal leaks and diffuse leakage, may be amenable to other treatment modalities (see below).
I. Photodynamic Therapy (Quality of Evidence – Good)
Verteporfin photodynamic therapy (PDT) has shown promise for not only promoting resolution of acute CSC but also preventing recurrences. Studies in chronic disease have yielded favorable results as well. The ICG finding of mid-phase hyperfluorescent choroidal plaques consistent with choroidal hyperpermeability suggests that treatment targeted at the choroidal vasculature might address the root cause of the disease. Treatment successes in early reports in 200329,33,212,238 using standard, Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) protocol PDT led to larger studies of standard PDT and eventually to numerous studies of reduced fluence PDT for CSC.
1. Randomized, Controlled Trial
Chan and colleagues performed a randomized, double-masked, controlled trial of half dose PDT versus placebo in 63 eyes with acute CSC.35 Participants had a baseline acuity of 20/200 or better and a duration of subretinal fluid of less than three months. All participants were experiencing either a first or second episode, and pregnant patients and those with exogenous corticosteroid exposure were excluded. In this report, 37 of 39 (94.9%) patients in the PDT group did not have subretinal fluid on OCT at one year compared to only 11 of 21 (57.9%) in the placebo group (P=0.001), suggesting that half dose PDT may be preferable to observation even in acute cases. Visual acuity was stable or improved in all patients in the PDT group compared to 78.9% of those in the placebo group. Although this is not a sufficient sample size to establish safety in this patient population, no adverse events occurred. Wu and colleagues reported mfERG results from a subset of the same study.229 Amongst 34 eligible eyes in the substudy, the P1 amplitudes in rings one and two were significantly greater in treated eyes (P = 0.030 and P = 0.018). N1 amplitudes were not significantly different.
2. Other Studies
The largest series of standard fluence PDT to date suggest a beneficial treatment effect. Yannuzzi and colleagues used ICG-guided PDT in 20 eyes with chronic CSC, and treatment led to complete resolution of macular detachment in 60% of eyes, with stable or improved vision in all eyes over 6.8 months follow-up.238 Ruiz-Moreno and colleagues have reported a series of 82 eyes with chronic CSC treated with standard PDT.180 All 82 eyes had complete resolution of subretinal fluid. Thirteen were treated more than once. There was a statistically significant improvement in acuity for the cohort (mean gain of 1.9 +/− 2.4 Snellen lines). Two patients developed CNV in the follow-up period, and nine eyes developed RPE hyperplasia at the treatment site. Moon and colleagues treated 41 eyes, mostly with persistent first episodes of CSC, with standard PDT and found 88% fluid resolution at 4–6 weeks.146 They compared PDT-treated eyes with a retrospective control group of eyes treated with focal laser for extrafoveal leaks. There was a significantly greater rate of foveal RPE atrophy in the PDT group. In some eyes, foveal RPE atrophy was progressive after PDT and associated with vision loss. Ozmert and colleagues demonstrated that fundus autofluorescence intensity increased following standard PDT and returned to baseline when subretinal fluid resolved.160
Risks of PDT include choroidal ischemia, RPE atrophy, and iatrogenic CNV.104,106,170,171,226 RPE rip has also been reported after PDT for CSC.93 The risks of PDT are thought to be decreased when reduced fluence PDT is used. Reduced fluence treatment involves decreasing the laser treatment time, lowering power to reduce energy output, or altering the interval between infusion and laser treatment. Reduced dose PDT, as in the randomized trial above, is also used. Several groups have evaluated the utility of reduced fluence PDT, and two have compared full fluence to half fluence. Reibaldi and colleagues treated 19 eyes with chronic CSC with standard fluence PDT and 23 with half fluence.172 They found no difference in the rate of vision improvement or fluid resolution. One eye in the standard group developed CNV in the follow-up period, and eight had severe choriocapillaris non-perfusion in the treated area. These complications did not occur in the low fluence group. Shin and colleagues retrospectively reported similar results in 67 eyes, and the half fluence group avoided the choriocapillaris hypoperfusion and retinal atrophy seen in the standard treatment group.191 Numerous other small trials of reduced dose or reduced fluence PDT have found favorable results.7,116,179,193
PDT has been shown to induce choroidal vascular remodeling and thinning of treated choroid.131,184 In CSC, even half-fluence PDT has been shown to reduce choroidal vascular congestion and decrease subfoveal choroidal thickness.127,129 Mid-phase ICG plaques, suggestive of choroidal hyperpermeability, disappear after treatment with PDT but not after successful treatment with focal laser. Microperimetry studies indicate functional benefit in CSC eyes treated with half-fluence PDT, even when no visual acuity improvement has occurred.46,55,187
Maruko and colleagues report a case of excessive iatrogenic choroidal thinning after half fluence PDT in an elderly patient who went from 321 µm pre-treatment to 64 µm at twelve months.129 Visual acuity nonetheless improved over the same period. Elderly patients may be at risk for severe choroidal thinning with PDT.125
Zhao and colleagues tested various verteporfin doses in the treatment of acute CSC and concluded that a 30% dose may be optimal.245 Patients treated with 30–70% doses (5 patients) had complete resolution of fluid, while 10% and 20% doses (3 patients) required re-treatment. An additional seven patients had successful anatomic resolution with 30% dosing. These findings require confirmation in larger trials.
Although PDT has mainly been used to treat acute or chronic subretinal fluid from CSC, PDT has also been described in the treatment of PED. To date there are two case reports describing PDT for chronic PED.37 Chang and colleagues describe a case of a chronic PED without concomitant subretinal fluid in a patient with 20/50 vision.37 After full fluence PDT the PED resolved with recovery of vision to 20/20 at 14 weeks post-PDT. However, caution must be exercised in the setting of large PEDs as there is a risk of RPE rip.
Performing PDT in CSC eyes often involves ICG angiogram guidance. Areas of presumed choroidal hyperpermeability (hyperfluorescent plaques on mid-phase ICG) are identified and treated with a spot size large enough to cover the plaque when practical. If there is diffuse involvement of the posterior pole, the spot is moved during the treatment to cover more territory. Areas of severe RPE loss should be avoided given past reports of RPE atrophy with PDT treatment. The absence of characteristic plaques on ICG may predict a poor response to PDT.84 Use of FA guidance rather than ICG guidance for PDT has been reported in a small series with favorable results.105
J. Diode Micropulse Laser (Quality of Evidence – Poor)
Diode micropulse laser treatment targets a series of ultrashort 810 nm laser pulses at the tissue of interest.196 These repetitive bursts allow for lower total energy use, and they help minimize damage to surrounding tissues from harmful thermal effects. In CSC the laser targets focal leaks at the level of the RPE. Retina-sparing photocoagulation is of particular interest in CSC given the frequent proximity of leaks to the fovea. One of the challenges of diode micropulse laser treatment is that no visible burn is created, and the surgeon is not able to visually confirm laser uptake. Ricci and colleagues have attempted to overcome this drawback with ICG-guided diode laser treatment.174,175
1. Randomized, Controlled Trial
Verma and colleagues randomized acute CSC patients with a single focal leak to diode micropulse laser or standard argon laser photocoagulation.220 All 30 eyes had resolution of fluid and similar final visual acuities at twelve weeks, but the diode group had significantly better final contrast sensitivity. Patients reported no persistent scotomata (versus 3/15 with a persistent scotoma in the argon group). The primary outcome measure for this study is not specified.
2. Other Studies
Diode laser for CSC was first described by Bandello and colleagues in 2003, who treated five patients who had complete fluid resolution within one month.13 Subsequently, Chen and colleagues reported a series of 26 eyes in which 14 of 15 eyes with focal leaks had fluid resolution, and just 5 of 11 eyes with diffuse leakage cleared all fluid.39
The ICG-assisted technique of Ricci and colleagues deserves separate mention because it is thought to benefit from RPE uptake of the ICG molecule, which has an absorption peak at 805–810 nm.174,175 The 810 nm diode laser energy would therefore be absorbed by ICG molecules, allowing for a more targeted treatment. Furthermore, post-operative imaging with an ICG filter allows for confirmation that the laser spots have treated the desired area. Ricci and colleagues have reported a series of seven eyes with persistent subretinal fluid, all of which were fluid free at one year post-treatment.
K. Transpupillary Thermotherapy (TTT) (Quality of Evidence – Poor)
TTT is a long-pulse, low-energy, 810 nm near-infrared laser used in the treatment of choroidal tumours. TTT is purported to induce choroidal vascular thrombosis by raising the temperature of the choroid. Some authors argue that TTT offers an option for treatment in cases where the site of focal leakage is juxtafoveal and therefore unsafe to treat with thermal laser.190 The utility of TTT in treating chronic CSC has been investigated in a handful of small studies.
Wei and colleagues were the first to report the use of TTT for CSC, describing the complete resolution of subretinal fluid four weeks after TTT in a case of chronic CSC with no observed visual improvement.225 In a case series of 14 patients with chronic, macula-involving CSC, Hussain and colleagues reported resolution of subretinal fluid on OCT in 64% of eyes at one month and 79% at two and three months, with approximately 50% demonstrating three lines of visual gain and 86% achieving 20/40 vision or better.78
In the largest study to date, Shukla and colleagues investigated TTT in chronic CSC. In this unmasked, nonrandomized, prospective cohort study, 25 eyes were treated with TTT (with a spot diameter of 0.5mm for 60 seconds) and were compared to an unmatched control group of 15 eyes which were observed.194 In the group treated with TTT, 84% experienced complete resolution of neurosensory retinal detachment and focal leakage on FA at one month, rising to 96% at three months. One patient developed CNV. Visual acuity improved by one line or more in 92% of cases compared to 33% of the control group, with 88% of TTT treated eyes attaining 20/30 vision or better. At baseline, controls had a longer duration of disease, and the outcomes of controls were likely negatively impacted by this difference. Given the lack of well-controlled, long-term studies demonstrating safety and efficacy of TTT, the precise role for TTT in the management of CSC remains to be defined.
IV. CONCLUSION
Recent technological advances have fostered collaborative efforts leading to a greater understanding of the pathogenesis of CSC. Advances in imaging techniques have not only resulted in more accurate phenotyping essential for diagnosis and management, but have also provided the foundation for the development of new treatments. Gass was ahead of his time when he emphasized the primary role of the choroid, as opposed to the retina, in CSC and coined the term central serous chorioretinopathy. Today, choroidal disease in CSC is widely accepted with widespread use of ICG angiography. OCT, and particularly EDI OCT, has confirmed his hypothesis by implicating a thickened and engorged choroid. Fundus autofluorescence (FAF) has also established a critical role for diagnosis and monitoring, and provided pathophysiological insights.
The application of adaptive optics, mfERG, microperimetry, and contrast sensitivity testing demonstrates that patients with even a mild course suffer significant anatomic and functional loss that was previously undetected. While focal laser and PDT are the current standard of care for persistent subretinal fluid in CSC, these treatments are not appropriate in all cases and the optimal timing of intervention with these modalities remains unclear. Recent imaging findings illustrating subclinical damage put a new onus on the ophthalmic community to develop treatments that are effective at the earliest sign of disease to prevent photoreceptor damage.
V. METHOD OF LITERATURE SEARCH
In preparing this review, we conducted a systematic review of the literature using PubMed® databases with the following search terms: “central serous retinopathy,” “central serous chorioretinopathy,” and “central serous.” We included case reports only if they contributed new information about characteristics, diagnosis, or treatment of the disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
REFERENCES
- 1.U.S. Preventive Services Task Force Ratings: Grade Definitions. Guide to Clinical Preventive Services, Third Edition: Periodic Updates. 2000–2003. [Accessed 10/10/2011]. at http://www.uspreventiveservicestaskforce.org/3rduspstf/ratings.htm. [Google Scholar]
- 2.Abu el-Asrar AM. Central serous chorioretinopathy complicating systemic corticosteroid therapy. Eur J Ophthalmol. 1997;7:297–300. doi: 10.1177/112067219700700317. [DOI] [PubMed] [Google Scholar]
- 3.Ahnoux-Zabsonre A, Quaranta M, Mauget-Faysse M. Prevalence of Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy: a complementary study. J Fr Ophtalmol. 2004;27:1129–1133. doi: 10.1016/s0181-5512(04)96281-x. [DOI] [PubMed] [Google Scholar]
- 4.Aliferis K, Petropoulos IK, Farpour B, Matter MA, Safran AB. Should central serous chorioretinopathy be added to the list of ocular side effects of phosphodiesterase 5 inhibitors? Ophthalmologica. 2012;227:85–89. doi: 10.1159/000333824. [DOI] [PubMed] [Google Scholar]
- 5.Amalric P, Gourinat P, Rebiere P. Is central serous choroiditis sometimes hereditary? Bull Soc Ophtalmol Fr. 1971;71:163–168. [PubMed] [Google Scholar]
- 6.Anupama B, Puthran N, Hegde V, Andrews S. Plantar fasciitis and impaired vision: A case report. Foot (Edinb) 2010;20:151–153. doi: 10.1016/j.foot.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Arevalo JF, Espinoza JV. Single-session combined photodynamic therapy with verteporfin and intravitreal anti-vascular endothelial growth factor therapy for chronic central serous chorioretinopathy: a pilot study at 12-month follow-up. Graefes Arch Clin Exp Ophthalmol. 2011;249:1159–1166. doi: 10.1007/s00417-011-1651-7. [DOI] [PubMed] [Google Scholar]
- 8.Arndt C, Sari A, Ferre M, et al. Electrophysiological effects of corticosteroids on the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:472–475. [PubMed] [Google Scholar]
- 9.Artunay O, Yuzbasioglu E, Rasier R, Sengul A, Bahcecioglu H. Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res. 2010;35:91–98. doi: 10.3109/02713680903428306. [DOI] [PubMed] [Google Scholar]
- 10.Asensio-Sanchez VM, Rodriguez-Delgado B, Garcia-Herrero E, Cabo-Vaquera V, Garcia-Loygorri C. Central serous chorioretinopathy as an extradigestive manifestation of Helicobacter pylori gastric infection. Arch Soc Esp Oftalmol. 2008;83:177–182. [PubMed] [Google Scholar]
- 11.Bae SH, Heo JW, Kim C, et al. A Randomized Pilot Study of Low-Fluence Photodynamic Therapy Versus Intravitreal Ranibizumab for Chronic Central Serous Chorioretinopathy. Am J Ophthalmol. 2011 doi: 10.1016/j.ajo.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Balakrishnan S, Apsingi S, Manjure SB. Sudden loss of visual acuity following intra-articular steroid injection in to the knee joint: a case report. Cases J. 2008;1:428. doi: 10.1186/1757-1626-1-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandello F, Lanzeto P, et al. FFe. Non-visible sub-threshold micropulse diode laser treatment of idiopathic central serous chorioretinopathy. A pilot study. Invest Ophthalmol Vis Sci. 2003;44:4858. [Google Scholar]
- 14.Baran NV, Gurlu VP, Esgin H. Long-term macular function in eyes with central serous chorioretinopathy. Clin Experiment Ophthalmol. 2005;33:369–372. doi: 10.1111/j.1442-9071.2005.01027.x. [DOI] [PubMed] [Google Scholar]
- 15.Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006;27:413–426. doi: 10.1183/09031936.06.00125404. [DOI] [PubMed] [Google Scholar]
- 16.Bastl CP. Regulation of cation transport by low doses of glucocorticoids in in vivo adrenalectomized rat colon. J Clin Invest. 1987;80:348–356. doi: 10.1172/JCI113079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battaglia Parodi M, Da Pozzo S, Ravalico G. Photodynamic therapy in chronic central serous chorioretinopathy. Retina. 2003;23:235–237. doi: 10.1097/00006982-200304000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Baumal CR, Martidis A, Truong SN. Central serous chorioretinopathy associated with periocular corticosteroid injection treatment for HLA-B27-associated iritis. Arch Ophthalmol. 2004;122:926–928. doi: 10.1001/archopht.122.6.926. [DOI] [PubMed] [Google Scholar]
- 19.Bouzas EA, Scott MH, Mastorakos G, Chrousos GP, Kaiser-Kupfer MI. Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol. 1993;111:1229–1233. doi: 10.1001/archopht.1993.01090090081024. [DOI] [PubMed] [Google Scholar]
- 20.Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47:431–448. doi: 10.1016/s0039-6257(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 21.Brancato R, Scialdone A, Pece A, Coscas G, Binaghi M. Eight-year follow-up of central serous chorioretinopathy with and without laser treatment. Graefes Arch Clin Exp Ophthalmol. 1987;225:166–168. doi: 10.1007/BF02175443. [DOI] [PubMed] [Google Scholar]
- 22.Browning DJ. Nadolol in the treatment of central serous retinopathy. Am J Ophthalmol. 1993;116:770–771. doi: 10.1016/s0002-9394(14)73483-x. [DOI] [PubMed] [Google Scholar]
- 23.Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand. 2001;79:417–421. doi: 10.1034/j.1600-0420.2001.079004417.x. [DOI] [PubMed] [Google Scholar]
- 24.Burumcek E, Mudun A, Karacorlu S, Arslan MO. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology. 1997;104:616–622. doi: 10.1016/s0161-6420(97)30262-0. [DOI] [PubMed] [Google Scholar]
- 25.Byrne MF, Kerrigan SW, Corcoran PA, et al. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology. 2003;124:1846–1854. doi: 10.1016/s0016-5085(03)00397-4. [DOI] [PubMed] [Google Scholar]
- 26.Caccavale A, Imparato M, Romanazzi F, Negri A, Porta A, Ferentini F. A new strategy of treatment with low-dosage acetyl salicylic acid in patients affected by central serous chorioretinopathy. Med Hypotheses. 2009;73:435–437. doi: 10.1016/j.mehy.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Low-dose aspirin as treatment for central serous chorioretinopathy. Clin Ophthalmol. 2010;4:899–903. doi: 10.2147/opth.s12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Central serous chorioretinopathy: a pathogenetic model. Clin Ophthalmol. 2011;5:239–243. doi: 10.2147/OPTH.S17182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003;23:752–763. doi: 10.1097/00006982-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carneiro AM, Silva RM, Veludo MJ, et al. Ranibizumab treatment for choroidal neovascularization from causes other than age-related macular degeneration and pathological myopia. Ophthalmologica. 2011;225:81–88. doi: 10.1159/000317908. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho-Recchia CA, Yannuzzi LA, Negrao S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109:1834–1837. doi: 10.1016/s0161-6420(02)01117-x. [DOI] [PubMed] [Google Scholar]
- 33.Chan WM, Lam DS, Lai TY, Tam BS, Liu DT, Chan CK. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87:1453–1458. doi: 10.1136/bjo.87.12.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (avastin) for choroidal neovascularization secondary to central serous chorioretinopathy, secondary to punctate inner choroidopathy, or of idiopathic origin. Am J Ophthalmol. 2007;143:977–983. doi: 10.1016/j.ajo.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008;115:1756–1765. doi: 10.1016/j.ophtha.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Chan WM, Lai TY, Lai RY, Tang EW, Liu DT, Lam DS. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008;28:85–93. doi: 10.1097/IAE.0b013e318156777f. [DOI] [PubMed] [Google Scholar]
- 37.Chang MA, Bressler SB. Photodynamic therapy for chronic pigment epithelial detachment in central serous chorioretinopathy. Can J Ophthalmol. 2009;44:221–222. doi: 10.3129/i09-043. [DOI] [PubMed] [Google Scholar]
- 38.Chappelow AV, Marmor MF. Multifocal electroretinogram abnormalities persist following resolution of central serous chorioretinopathy. Arch Ophthalmol. 2000;118:1211–1215. doi: 10.1001/archopht.118.9.1211. [DOI] [PubMed] [Google Scholar]
- 39.Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology. 2008;115:2229–2234. doi: 10.1016/j.ophtha.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Chumbley LC, Frank RN. Central serous retinopathy and pregnancy. Am J Ophthalmol. 1974;77:158–160. doi: 10.1016/0002-9394(74)90667-9. [DOI] [PubMed] [Google Scholar]
- 41.Cotticelli L, Borrelli M, D'Alessio AC, et al. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16:274–278. doi: 10.1177/112067210601600213. [DOI] [PubMed] [Google Scholar]
- 42.Cousins L, Rigg L, Hollingsworth D, et al. Qualitative and quantitative assessment of the circadian rhythm of cortisol in pregnancy. Am J Obstet Gynecol. 1983;145:411–416. doi: 10.1016/0002-9378(83)90309-5. [DOI] [PubMed] [Google Scholar]
- 43.Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988;106:1190–1195. doi: 10.1001/archopht.1988.01060140350030. [DOI] [PubMed] [Google Scholar]
- 44.Desai UR, Alhalel AA, Campen TJ, Schiffman RM, Edwards PA, Jacobsen GR. Central serous chorioretinopathy in African Americans. J Natl Med Assoc. 2003;95:553–559. [PMC free article] [PubMed] [Google Scholar]
- 45.Eandi CM, Chung JE, Cardillo-Piccolino F, Spaide RF. Optical coherence tomography in unilateral resolved central serous chorioretinopathy. Retina. 2005;25:417–421. doi: 10.1097/00006982-200506000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Ehrlich R, Mawer NP, Mody CH, Brand CS, Squirrell D. Visual function following photodynamic therapy for central serous retinopathy: a comparison of automated macular microperimery versus best corrected visual acuity. Clin Experiment Ophthalmol. 2011 doi: 10.1111/j.1442-9071.2011.02654.x. [DOI] [PubMed] [Google Scholar]
- 47.Ezra N, Taban M, Behroozan D. Central serous chorioretinopathy associated with topical corticosteroids in a patient with psoriasis. J Drugs Dermatol. 2011;10:918–921. [PubMed] [Google Scholar]
- 48.Fernandez CF, Mendoza AJ, Arevalo JF. Central serous chorioretinopathy associated with topical dermal corticosteroids. Retina. 2004;24:471–474. doi: 10.1097/00006982-200406000-00027. [DOI] [PubMed] [Google Scholar]
- 49.Ficker L, Vafidis G, While A, Leaver P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988;72:829–834. doi: 10.1136/bjo.72.11.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Folk JC, Thompson HS, Han DP, Brown CK. Visual function abnormalities in central serous retinopathy. Arch Ophthalmol. 1984;102:1299–1302. doi: 10.1001/archopht.1984.01040031049021. [DOI] [PubMed] [Google Scholar]
- 51.Forooghian F, Meleth AD, Cukras C, Chew EY, Wong WT, Meyerle CB. Finasteride for chronic central serous chorioretinopathy. Retina. 2011;31:766–771. doi: 10.1097/IAE.0b013e3181f04a35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraunfelder FW, Fraunfelder FT. Central serous chorioretinopathy associated with sildenafil. Retina. 2008;28:606–609. doi: 10.1097/IAE.0b013e31815ec2c8. [DOI] [PubMed] [Google Scholar]
- 53.French DD, Margo CE. Central serous chorioretinopathy and phosphodiesterase-5 inhibitors: a case-control postmarketing surveillance study. Retina. 2010;30:271–274. doi: 10.1097/IAE.0b013e3181b7740f. [DOI] [PubMed] [Google Scholar]
- 54.Fujimoto H, Gomi F, Wakabayashi T, Sawa M, Tsujikawa M, Tano Y. Morphologic changes in acute central serous chorioretinopathy evaluated by fourier-domain optical coherence tomography. Ophthalmology. 2008;115:1494–1500. 500 e1–500 e2. doi: 10.1016/j.ophtha.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Fujita K, Yuzawa M, Mori R. Retinal sensitivity after photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy: short-term results. Retina. 2011;31:772–778. doi: 10.1097/IAE.0b013e3181f049d3. [DOI] [PubMed] [Google Scholar]
- 56.Furuta M, Iida T, Kishi S. Foveal thickness can predict visual outcome in patients with persistent central serous chorioretinopathy. Ophthalmologica. 2009;223:28–31. doi: 10.1159/000161880. [DOI] [PubMed] [Google Scholar]
- 57.Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81:962–964. doi: 10.1136/bjo.81.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(Suppl):1–139. [PubMed] [Google Scholar]
- 59.Gass JD. Bullous retinal detachment. An unusual manifestation of idiopathic central serous choroidopathy. Am J Ophthalmol. 1973;75:810–821. doi: 10.1016/0002-9394(73)90887-8. [DOI] [PubMed] [Google Scholar]
- 60.Gass JD. Photocoagulation treatment of idiopathic central serous choroidopathy. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:456–467. [PubMed] [Google Scholar]
- 61.Gelber GS, Schatz H. Loss of vision due to central serous chorioretinopathy following psychological stress. Am J Psychiatry. 1987;144:46–50. doi: 10.1176/ajp.144.1.46. [DOI] [PubMed] [Google Scholar]
- 62.Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond) 2010;24:1743–1756. doi: 10.1038/eye.2010.130. [DOI] [PubMed] [Google Scholar]
- 63.Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68:815–820. doi: 10.1136/bjo.68.11.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giusti C. Central serous chorioretinopathy: a new extragastric manifestation of Helicobacter pylori?: Analysis of a clinical case. Clin Ter. 2001;152:393–397. [PubMed] [Google Scholar]
- 65.Giusti C. Association of Helicobacter pylori with central serous chorioretinopathy: hypotheses regarding pathogenesis. Med Hypotheses. 2004;63:524–527. doi: 10.1016/j.mehy.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 66.Golshahi A, Klingmuller D, Holz FG, Eter N. Ketoconazole in the treatment of central serous chorioretinopathy: a pilot study. Acta Ophthalmol. 2009 doi: 10.1111/j.1755-3768.2008.01467.x. [DOI] [PubMed] [Google Scholar]
- 67.Gunther JB, Altaweel MM. Bevacizumab (Avastin) for the treatment of ocular disease. Surv Ophthalmol. 2009;54:372–400. doi: 10.1016/j.survophthal.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Gupta P, Gupta V, Dogra MR, Singh R, Gupta A. Morphological changes in the retinal pigment epithelium on spectral-domain OCT in the unaffected eyes with idiopathic central serous chorioretinopathy. Int Ophthalmol. 2010;30:175–181. doi: 10.1007/s10792-009-9302-2. [DOI] [PubMed] [Google Scholar]
- 69.Hadcock JR, Malbon CC. Regulation of beta-adrenergic receptors by "permissive" hormones: glucocorticoids increase steady-state levels of receptor mRNA. Proc Natl Acad Sci U S A. 1988;85:8415–8419. doi: 10.1073/pnas.85.22.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haik GM, Perez LF, Murtagh JJ. Central serous retinopathy. Consecutive development in daughter and mother. Am J Ophthalmol. 1968;65:612–615. [PubMed] [Google Scholar]
- 71.Haimovici R, Gragoudas ES, Duker JS, Sjaarda RN, Eliott D. Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology. 1997;104:1653–1660. doi: 10.1016/s0161-6420(97)30082-7. [DOI] [PubMed] [Google Scholar]
- 72.Haimovici R, Rumelt S, Melby J. Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology. 2003;110:698–703. doi: 10.1016/S0161-6420(02)01975-9. [DOI] [PubMed] [Google Scholar]
- 73.Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111:244–249. doi: 10.1016/j.ophtha.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 74.Harada T, Harada K. Six cases of central serous choroidopathy induced by systemic corticosteroid therapy. Doc Ophthalmol. 1985;60:37–44. doi: 10.1007/BF00164568. [DOI] [PubMed] [Google Scholar]
- 75.Harrington DO. Psychosomatic interrelationships in ophthalmology. Am J Ophthalmol. 1948;31:1241–1251. doi: 10.1016/0002-9394(48)91014-9. [DOI] [PubMed] [Google Scholar]
- 76.Heinrich MR. Central serous retinopathy and alpha-blockaders. Bull Soc Ophtalmol Fr. 1974;74:681–683. [PubMed] [Google Scholar]
- 77.Horniker E. Su di una forma retinite centrale di origine vasoneurotica (retinite central capillaro spastica) Ann Otal. 1927;55:578–600. [Google Scholar]
- 78.Hussain N, Khanna R, Hussain A, Das T. Transpupillary thermotherapy for chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2006;244:1045–1051. doi: 10.1007/s00417-005-0175-4. [DOI] [PubMed] [Google Scholar]
- 79.Iijima H, Iida T, Murayama K, Imai M, Gohdo T. Plasminogen activator inhibitor 1 in central serous chorioretinopathy. Am J Ophthalmol. 1999;127:477–478. doi: 10.1016/s0002-9394(98)00378-x. [DOI] [PubMed] [Google Scholar]
- 80.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 81.Imamura Y, Fujiwara T, Spaide RF. Fundus autofluorescence and visual acuity in central serous chorioretinopathy. Ophthalmology. 2011;118:700–705. doi: 10.1016/j.ophtha.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 82.Imasawa M, Ohshiro T, Gotoh T, Imai M, Iijima H. Central serous chorioretinopathy following vitrectomy with intravitreal triamcinolone acetonide for diabetic macular oedema. Acta Ophthalmol Scand. 2005;83:132–133. doi: 10.1111/j.1600-0420.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 83.Inoue M, Kadonosono K, Watanabe Y, Kobayashi S, Yamane S, Arakawa A. Results of one-year follow-up examinations after intravitreal bevacizumab administration for chronic central serous chorioretinopathy. Ophthalmologica. 2011;225:37–40. doi: 10.1159/000314709. [DOI] [PubMed] [Google Scholar]
- 84.Inoue R, Sawa M, Tsujikawa M, Gomi F. Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol. 2010;149:441–446. e1–e2. doi: 10.1016/j.ajo.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 85.Jain AK, Kaines A, Schwartz S. Bilateral central serous chorioretinopathy resolving rapidly with treatment for obstructive sleep apnea. Graefes Arch Clin Exp Ophthalmol. 2010;248:1037–1039. doi: 10.1007/s00417-009-1257-5. [DOI] [PubMed] [Google Scholar]
- 86.Jain IS, Singh K. Maculopathy a corticosteroid side-effect. J All India Ophthalmol Soc. 1966;14:250–252. [PubMed] [Google Scholar]
- 87.Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology. 2002;109:1765–1766. doi: 10.1016/s0161-6420(02)01303-9. [DOI] [PubMed] [Google Scholar]
- 88.Karadimas P, Bouzas EA. Glucocorticoid use represents a risk factor for central serous chorioretinopathy: a prospective, case-control study. Graefes Arch Clin Exp Ophthalmol. 2004;242:800–802. doi: 10.1007/s00417-004-0885-z. [DOI] [PubMed] [Google Scholar]
- 89.Karadimas P, Kapetanios A, Bouzas EA. Central serous chorioretinopathy after local application of glucocorticoids for skin disorders. Arch Ophthalmol. 2004;122:784–786. doi: 10.1001/archopht.122.5.784. [DOI] [PubMed] [Google Scholar]
- 90.Kassam AA, White W, Ling RH, Kitson JB. Loss of visual acuity due to central serous retinopathy after steroid injection into the shoulder bursa. J Shoulder Elbow Surg. 2011;20:e5–e6. doi: 10.1016/j.jse.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 91.Kayazawa F. Central serous choroidopathy with exudative retinal detachment. Ann Ophthalmol. 1982;14:1035–1042. [PubMed] [Google Scholar]
- 92.Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res. 2006;82:828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Kim SW, Oh J, Oh IK, Huh K. Retinal pigment epithelial tear after half fluence PDT for serous pigment epithelial detachment in central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging. 2009;40:300–303. doi: 10.3928/15428877-20090430-14. [DOI] [PubMed] [Google Scholar]
- 94.Kim SW, Oh J, Huh K. Correlations among various functional and morphological tests in resolved central serous chorioretinopathy. Br J Ophthalmol. 2011 doi: 10.1136/bjo.2011.204503. [DOI] [PubMed] [Google Scholar]
- 95.Kim SW, Oh J, Kwon SS, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31:1904–1911. doi: 10.1097/IAE.0b013e31821801c5. [DOI] [PubMed] [Google Scholar]
- 96.Kim SW, Oh J, Huh K. Correlations among various functional and morphological tests in resolved central serous chorioretinopathy. Br J Ophthalmol. 2012;96:350–355. doi: 10.1136/bjo.2011.204503. [DOI] [PubMed] [Google Scholar]
- 97.Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology. 2008;115:169–173. doi: 10.1016/j.ophtha.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 98.Klein ML, Van Buskirk EM, Friedman E, Gragoudas E, Chandra S. Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol. 1974;91:247–250. doi: 10.1001/archopht.1974.03900060257001. [DOI] [PubMed] [Google Scholar]
- 99.Kloos P, Laube I, Thoelen A. Obstructive sleep apnea in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1225–1228. doi: 10.1007/s00417-008-0837-0. [DOI] [PubMed] [Google Scholar]
- 100.Kocabora MS, Durmaz S, Kandemir N. Exacerbation of central serous chorioretinopathy following intravitreal triamcinolone injection. Graefes Arch Clin Exp Ophthalmol. 2008;246:1783–1786. doi: 10.1007/s00417-008-0932-2. [DOI] [PubMed] [Google Scholar]
- 101.Kon Y, Iida T, Maruko I, Saito M. The optical coherence tomography-ophthalmoscope for examination of central serous chorioretinopathy with precipitates. Retina. 2008;28:864–869. doi: 10.1097/IAE.0b013e3181669795. [DOI] [PubMed] [Google Scholar]
- 102.Konstantinidis L, Mantel I, Zografos L, Ambresin A. Intravitreal ranibizumab in the treatment of choroidal neovascularization associated with idiopathic central serous chorioretinopathy. Eur J Ophthalmol. 2010;20:955–958. doi: 10.1177/112067211002000524. [DOI] [PubMed] [Google Scholar]
- 103.Koskela P, Laatikainen L, von Dickhoff K. Contrast sensitivity after resolution of central serous retinopathy. Graefes Arch Clin Exp Ophthalmol. 1994;232:473–476. doi: 10.1007/BF00195356. [DOI] [PubMed] [Google Scholar]
- 104.Koytak A, Erol K, Coskun E, Asik N, Ozturk H, Ozerturk Y. Fluorescein angiography-guided photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy. Retina. 30:1698–1703. doi: 10.1097/IAE.0b013e3181da4354. [DOI] [PubMed] [Google Scholar]
- 105.Koytak A, Erol K, Coskun E, Asik N, Ozturk H, Ozerturk Y. Fluorescein angiography-guided photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy. Retina. 2010;30:1698–1703. doi: 10.1097/IAE.0b013e3181da4354. [DOI] [PubMed] [Google Scholar]
- 106.Lai TY, Chan WM, Li H, Lai RY, Liu DT, Lam DS. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006;90:869–874. doi: 10.1136/bjo.2006.090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lai TY, Lai RY, Ngai JW, Chan WM, Li H, Lam DS. First and second-order kernel multifocal electroretinography abnormalities in acute central serous chorioretinopathy. Doc Ophthalmol. 2008;116:29–40. doi: 10.1007/s10633-007-9075-8. [DOI] [PubMed] [Google Scholar]
- 108.Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1979;63:674–677. doi: 10.1136/bjo.63.10.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee JY, Chae JB, Yang SJ, Kim JG, Yoon YH. Intravitreal bevacizumab versus the conventional protocol of photodynamic therapy for treatment of chronic central serous chorioretinopathy. Acta Ophthalmol. 2011;89:e293–e294. doi: 10.1111/j.1755-3768.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- 110.Lee ST, Adelman RA. The Treatment of Recurrent Central Serous Chorioretinopathy with Intravitreal Bevacizumab. J Ocul Pharmacol Ther. 2011 doi: 10.1089/jop.2011.0045. [DOI] [PubMed] [Google Scholar]
- 111.Leveque TK, Yu L, Musch DC, Chervin RD, Zacks DN. Central serous chorioretinopathy and risk for obstructive sleep apnea. Sleep Breath. 2007;11:253–257. doi: 10.1007/s11325-007-0112-3. [DOI] [PubMed] [Google Scholar]
- 112.Levy J, Marcus M, Belfair N, Klemperer I, Lifshitz T. Central serous chorioretinopathy in patients receiving systemic corticosteroid therapy. Can J Ophthalmol. 2005;40:217–221. doi: 10.1016/S0008-4182(05)80040-7. [DOI] [PubMed] [Google Scholar]
- 113.Li XJ, Zhang JS. Intravitreal bevacizumab injection for chronic central serous chorioretinopathy. Chin Med J (Engl) 2010;123:2145–2147. [PubMed] [Google Scholar]
- 114.Lim JW, Kim MU, Shin MC. Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina. 2010;30:1465–1471. doi: 10.1097/IAE.0b013e3181d8e7fe. [DOI] [PubMed] [Google Scholar]
- 115.Lim JW, Ryu SJ, Shin MC. The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy. Korean J Ophthalmol. 2010;24:155–158. doi: 10.3341/kjo.2010.24.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lim JW, Kang SW, Kim YT, Chung SE, Lee SW. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. Br J Ophthalmol. 2011;95:514–517. doi: 10.1136/bjo.2010.182121. [DOI] [PubMed] [Google Scholar]
- 117.Lim JW, Kim MU. The efficacy of intravitreal bevacizumab for idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249:969–974. doi: 10.1007/s00417-010-1581-9. [DOI] [PubMed] [Google Scholar]
- 118.Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010;30:100–106. doi: 10.1097/IAE.0b013e3181bcf0b4. [DOI] [PubMed] [Google Scholar]
- 119.Lin E, Arrigg PG, Kim RY. Familial central serous choroidopathy. Graefes Arch Clin Exp Ophthalmol. 2000;238:930–931. doi: 10.1007/s004179900110. [DOI] [PubMed] [Google Scholar]
- 120.Lin JM, Tsai YY. Retinal pigment epithelial detachment in the fellow eye of a patient with unilateral central serous chorioretinopathy treated with steroid. Retina. 2001;21:377–379. doi: 10.1097/00006982-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 121.Loo RH, Scott IU, Flynn HW, Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22:19–24. doi: 10.1097/00006982-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 122.Maaranen TH, Tuppurainen KT, Mantyjarvi MI. Color vision defects after central serous chorioretinopathy. Retina. 2000;20:633–637. doi: 10.1097/00006982-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 123.Mandal S, Sinha S, Abbas Z, Venkatesh P. Intravitreal bevacizumab for subfoveal choroidal neovascularization complicating active central serous chorioretinopathy. Indian J Ophthalmol. 2011;59:338–339. doi: 10.4103/0301-4738.82021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mansuetta CC, Mason JO, 3rd, Swanner J, et al. An association between central serous chorioretinopathy and gastroesophageal reflux disease. Am J Ophthalmol. 2004;137:1096–1100. doi: 10.1016/j.ajo.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 125.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 126.Marmor MF, Tan F. Central serous chorioretinopathy: bilateral multifocal electroretinographic abnormalities. Arch Ophthalmol. 1999;117:184–188. doi: 10.1001/archopht.117.2.184. [DOI] [PubMed] [Google Scholar]
- 127.Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117:1792–1799. doi: 10.1016/j.ophtha.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 128.Maruko I, Iida T, Ojima A, Sekiryu T. Subretinal dot-like precipitates and yellow material in central serous chorioretinopathy. Retina. 2011;31:759–765. doi: 10.1097/IAE.0b013e3181fbce8e. [DOI] [PubMed] [Google Scholar]
- 129.Maruko I, Iida T, Sugano Y, Furuta M, Sekiryu T. One-Year Choroidal Thickness Results after Photodynamic Therapy for Central Serous Chorioretinopathy. Retina. 2011 doi: 10.1097/IAE.0b013e31822bf6b1. [DOI] [PubMed] [Google Scholar]
- 130.Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011;31:1603–1608. doi: 10.1097/IAE.0b013e31820f4b39. [DOI] [PubMed] [Google Scholar]
- 131.Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T. Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2011;151:594 e1–603 e1. doi: 10.1016/j.ajo.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 132.Matsumoto H, Kishi S, Otani T, Sato T. Elongation of photoreceptor outer segment in central serous chorioretinopathy. Am J Ophthalmol. 2008;145:162–168. doi: 10.1016/j.ajo.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 133.Matsumoto H, Sato T, Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol. 2009;148:105 e1–110 e1. doi: 10.1016/j.ajo.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 134.Matsumoto H, Kishi S, Sato T, Mukai R. Fundus autofluorescence of elongated photoreceptor outer segments in central serous chorioretinopathy. Am J Ophthalmol. 2011;151:617 e1–623 e1. doi: 10.1016/j.ajo.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 135.Mauget-Faysse M, Kodjikian L, Quaranta M, et al. Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy. Results of the first prospective pilot study. J Fr Ophtalmol. 2002;25:1021–1025. [PubMed] [Google Scholar]
- 136.Maumenee AE. Symposium: macular diseases, clinical manifestations. Trans Am Acad Ophthalmol Otolaryngol. 1965;69:605–613. [PubMed] [Google Scholar]
- 137.Meyerle CB, Freund KB, Bhatnagar P, Shah V, Yannuzzi LA. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina. 2007;27:943–946. doi: 10.1097/IAE.0b013e318050ca69. [DOI] [PubMed] [Google Scholar]
- 138.Meyerle CBSRF. Central serous chorioretinopathy. In: Albert DMJ, Azar D, Blodi B, editors. Albert & Jakobiec's Principles & Practice of Ophthalmology. 3rd ed. Philadelphia: Saunders; 2008. [Google Scholar]
- 139.Michael JC, Pak J, Pulido J, de Venecia G. Central serous chorioretinopathy associated with administration of sympathomimetic agents. Am J Ophthalmol. 2003;136:182–185. doi: 10.1016/s0002-9394(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 140.Misiuk-Hojlo M, Michalowska M, Turno-Krecicka A. Helicobacter pylori--a risk factor for the developement of the central serous chorioretinopathy. Klin Oczna. 2009;111:30–32. [PubMed] [Google Scholar]
- 141.Mitarai K, Gomi F, Tano Y. Three-dimensional optical coherence tomographic findings in central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2006;244:1415–1420. doi: 10.1007/s00417-006-0277-7. [DOI] [PubMed] [Google Scholar]
- 142.Mitsui Y, Matsubara M, Kanagawa M. Xenon light-exposure as a treatment of central serous retinopathy (a preliminary report) Nippon Ganka Kiyo. 1969;20:291–294. [PubMed] [Google Scholar]
- 143.Mondal LK, Sarkar K, Datta H, Chatterjee PR. Acute bilateral central serous chorioretinopathy following intra-articular injection of corticosteroid. Indian J Ophthalmol. 2005;53:132–134. doi: 10.4103/0301-4738.16181. [DOI] [PubMed] [Google Scholar]
- 144.Montero JA, Ruiz-Moreno JM. Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J Ophthalmol. 2005;89:562–564. doi: 10.1136/bjo.2004.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Montero JA, Ruiz-Moreno JM, Fernandez-Munoz M. Intravitreal bevacizumab to treat choroidal neovascularization following photodynamic therapy in central serous choroidopathy. Eur J Ophthalmol. 2011 doi: 10.5301/EJO.2011.6290. [DOI] [PubMed] [Google Scholar]
- 146.Moon JW, Yu HG, Kim TW, Kim HC, Chung H. Prognostic factors related to photodynamic therapy for central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:1315–1323. doi: 10.1007/s00417-009-1104-8. [DOI] [PubMed] [Google Scholar]
- 147.Moschos M, Brouzas D, Koutsandrea C, Stefanos B, Loukianou H, Papantonis F. Assessment of central serous chorioretinopathy by optical coherence tomography and multifocal electroretinography. Ophthalmologica. 2007;221:292–298. doi: 10.1159/000104758. [DOI] [PubMed] [Google Scholar]
- 148.Mudvari SS, Goff MJ, Fu AD, et al. The natural history of pigment epithelial detachment associated with central serous chorioretinopathy. Retina. 2007;27:1168–1173. doi: 10.1097/IAE.0b013e318156db8a. [DOI] [PubMed] [Google Scholar]
- 149.Negi A, Marmor MF. Experimental serous retinal detachment and focal pigment epithelial damage. Arch Ophthalmol. 1984;102:445–449. doi: 10.1001/archopht.1984.01040030359038. [DOI] [PubMed] [Google Scholar]
- 150.Nielsen JS, Jampol LM. Oral mifepristone for chronic central serous chorioretinopathy. Retina. 2011;31:1928–1936. doi: 10.1097/IAE.0b013e31821c3ef6. [DOI] [PubMed] [Google Scholar]
- 151.Oh J, Smiddy WE, Flynn HW, Jr, Gregori G, Lujan B. Photoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgery. Invest Ophthalmol Vis Sci. 2010;51:1651–1658. doi: 10.1167/iovs.09-4420. [DOI] [PubMed] [Google Scholar]
- 152.Oikarinen AI, Uitto J, Oikarinen J. Glucocorticoid action on connective tissue: from molecular mechanisms to clinical practice. Med Biol. 1986;64:221–230. [PubMed] [Google Scholar]
- 153.Oishi A, Otani A, Sasahara M, et al. Photoreceptor integrity and visual acuity in cystoid macular oedema associated with retinitis pigmentosa. Eye. 2008 doi: 10.1038/eye.2008.266. [DOI] [PubMed] [Google Scholar]
- 154.Ojima Y, Hangai M, Sasahara M, et al. Three-dimensional Imaging of the Foveal Photoreceptor Layer in Central Serous Chorioretinopathy Using High-speed Optical Coherence Tomography. Ophthalmology. 2007 doi: 10.1016/j.ophtha.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 155.Ojima Y, Tsujikawa A, Hangai M, et al. Retinal sensitivity measured with the micro perimeter 1 after resolution of central serous chorioretinopathy. Am J Ophthalmol. 2008;146:77–84. doi: 10.1016/j.ajo.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 156.Okushiba U, Takeda M. Study of choroidal vascular lesions in central serous chorioretinopathy using indocyanine green angiography. Nihon Ganka Gakkai Zasshi. 1997;101:74–82. [PubMed] [Google Scholar]
- 157.Oosterhuis JA. Familial central serous retinopathy. Graefes Arch Clin Exp Ophthalmol. 1996;234:337–341. doi: 10.1007/BF00220710. [DOI] [PubMed] [Google Scholar]
- 158.Ooto S, Hangai M, Sakamoto A, et al. High-resolution imaging of resolved central serous chorioretinopathy using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 117:1800–1809. 9 e1–9 e2. doi: 10.1016/j.ophtha.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 159.Ozdemir H, Karacorlu SA, Senturk F, Karacorlu M, Uysal O. Assessment of macular function by microperimetry in unilateral resolved central serous chorioretinopathy. Eye (Lond) 2008;22:204–208. doi: 10.1038/sj.eye.6702563. [DOI] [PubMed] [Google Scholar]
- 160.Ozmert E, Batioglu F. Fundus autofluorescence before and after photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmologica. 2009;223:263–268. doi: 10.1159/000210386. [DOI] [PubMed] [Google Scholar]
- 161.Park DW, Schatz H, Gaffney MM, McDonald HR, Johnson RN, Schaeffer D. Central serous chorioretinopathy in two families. Eur J Ophthalmol. 1998;8:42–47. doi: 10.1177/112067219800800110. [DOI] [PubMed] [Google Scholar]
- 162.Peabody RR, Zweng HC, Little HL. Treatment of persistent central serous retinopathy. Arch Ophthalmol. 1968;79:166–169. doi: 10.1001/archopht.1968.03850040168011. [DOI] [PubMed] [Google Scholar]
- 163.Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139:87–99. doi: 10.1016/j.ajo.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 164.Pikkel J, Beiran I, Ophir A, Miller B. Acetazolamide for central serous retinopathy. Ophthalmology. 2002;109:1723–1725. doi: 10.1016/s0161-6420(02)01157-0. [DOI] [PubMed] [Google Scholar]
- 165.Pikkel J, Rumelt S. Intravitreal bevacizumab for choroidal neovascularization secondary to laser photocoagulation for central serous chorioretinopathy. Eur J Ophthalmol. 2011 doi: 10.5301/ejo.5000036. 0. [DOI] [PubMed] [Google Scholar]
- 166.Polak BC, Baarsma GS, Snyers B. Diffuse retinal pigment epitheliopathy complicating systemic corticosteroid treatment. Br J Ophthalmol. 1995;79:922–925. doi: 10.1136/bjo.79.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 168.Rahbani-Nobar MB, Javadzadeh A, Ghojazadeh L, Rafeey M, Ghorbanihaghjo A. The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy. Mol Vis. 2011;17:99–103. [PMC free article] [PubMed] [Google Scholar]
- 169.Ravage Z, Packo K. ARVO 2011. Ft. Lauderdale, FL: 2011. Rifampin for treatment of central serous chorioretinopathy. [Google Scholar]
- 170.Reibaldi M, Cardascia N, Longo A, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 149:307 e2–315 e2. doi: 10.1016/j.ajo.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 171.Reibaldi M, Boscia F, Avitabile T, et al. Low-fluence photodynamic therapy in longstanding chronic central serous chorioretinopathy with foveal and gravitational atrophy. Eur J Ophthalmol. 2009;19:154–158. doi: 10.1177/112067210901900126. [DOI] [PubMed] [Google Scholar]
- 172.Reibaldi M, Cardascia N, Longo A, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 2010;149:307 e2–315 e2. doi: 10.1016/j.ajo.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 173.Reibaldi M, Boscia F, Avitabile T, et al. Functional retinal changes measured by microperimetry in standard-fluence vs low-fluence photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2011;151:953 e2–960 e2. doi: 10.1016/j.ajo.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 174.Ricci F, Missiroli F, Cerulli L. Indocyanine green dye-enhanced micropulsed diode laser: a novel approach to subthreshold RPE treatment in a case of central serous chorioretinopathy. Eur J Ophthalmol. 2004;14:74–82. doi: 10.1177/112067210401400115. [DOI] [PubMed] [Google Scholar]
- 175.Ricci F, Missiroli F, Regine F, Grossi M, Dorin G. Indocyanine green enhanced subthreshold diode-laser micropulse photocoagulation treatment of chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:597–607. doi: 10.1007/s00417-008-1014-1. [DOI] [PubMed] [Google Scholar]
- 176.Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol. 1983;95:457–466. doi: 10.1016/0002-9394(83)90265-9. [DOI] [PubMed] [Google Scholar]
- 177.Roisman L, Lavinsky D, Magalhaes F, et al. Fundus Autofluorescence and Spectral Domain OCT in Central Serous Chorioretinopathy. J Ophthalmol. 2011;2011:706–849. doi: 10.1155/2011/706849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011;22:166–173. doi: 10.1097/ICU.0b013e3283459826. [DOI] [PubMed] [Google Scholar]
- 179.Rouvas A, Stavrakas P, Theodossiadis PG, et al. Long-term results of half-fluence photodynamic therapy for chronic central serous chorioretinopathy. Eur J Ophthalmol. 2011 doi: 10.5301/ejo.5000051. 0. [DOI] [PubMed] [Google Scholar]
- 180.Ruiz-Moreno JM, Lugo FL, Armada F, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Acta Ophthalmol. 2010;88:371–376. doi: 10.1111/j.1755-3768.2008.01408.x. [DOI] [PubMed] [Google Scholar]
- 181.Sakaue M, Hoffman BB. Glucocorticoids induce transcription and expression of the alpha 1B adrenergic receptor gene in DTT1 MF-2 smooth muscle cells. J Clin Invest. 1991;88:385–389. doi: 10.1172/JCI115315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sandle GI, McGlone F. Acute effects of dexamethasone on cation transport in colonic epithelium. Gut. 1987;28:701–706. doi: 10.1136/gut.28.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schaal KB, Hoeh AE, Scheuerle A, Schuett F, Dithmar S. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009;19:613–617. doi: 10.1177/112067210901900415. [DOI] [PubMed] [Google Scholar]
- 184.Schmidt-Erfurth U, Michels S, Barbazetto I, Laqua H. Photodynamic effects on choroidal neovascularization and physiological choroid. Invest Ophthalmol Vis Sci. 2002;43:830–841. [PubMed] [Google Scholar]
- 185.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 186.Sekiryu T, Iida T, Maruko I, Saito K, Kondo T. Infrared fundus autofluorescence and central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2010;51:4956–4962. doi: 10.1167/iovs.09-5009. [DOI] [PubMed] [Google Scholar]
- 187.Senturk F, Karacorlu M, Ozdemir H, Karacorlu SA, Uysal O. Microperimetric changes after photodynamic therapy for central serous chorioretinopathy. Am J Ophthalmol. 2011;151:303 e1–309 e1. doi: 10.1016/j.ajo.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 188.Seong HK, Bae JH, Kim ES, Han JR, Nam WH, Kim HK. Intravitreal bevacizumab to treat acute central serous chorioretinopathy: short-term effect. Ophthalmologica. 2009;223:343–347. doi: 10.1159/000224782. [DOI] [PubMed] [Google Scholar]
- 189.Sharma T, Shah N, Rao M, et al. Visual outcome after discontinuation of corticosteroids in atypical severe central serous chorioretinopathy. Ophthalmology. 2004;111:1708–1714. doi: 10.1016/j.ophtha.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 190.Sharma T, Parikh SD. Transpupillary Thermotherapy for Juxtafoveal Leak in Central Serous Chorioretinopathy. Ophthalmic Surg Lasers Imaging. 2010:1–3. doi: 10.3928/15428877-20100215-23. [DOI] [PubMed] [Google Scholar]
- 191.Shin JY, Woo SJ, Yu HG, Park KH. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2011;31:119–126. doi: 10.1097/IAE.0b013e3181e378f2. [DOI] [PubMed] [Google Scholar]
- 192.Shin MC, Lim JW. Concentration of cytokines in the aqueous humor of patients with central serous chorioretinopathy. Retina. 2011;31:1937–1943. doi: 10.1097/IAE.0b013e31820a6a17. [DOI] [PubMed] [Google Scholar]
- 193.Shinojima A, Kawamura A, Mori R, Fujita K, Yuzawa M. Detection of morphologic alterations by spectral-domain optical coherence tomography before and after half-dose verteporfin photodynamic therapy in chronic central serous chorioretinopathy. Retina. 2011;31:1912–1920. doi: 10.1097/IAE.0b013e3182252aa8. [DOI] [PubMed] [Google Scholar]
- 194.Shukla D, Kolluru C, Vignesh TP, Karthikprakash S, Kim R. Transpupillary thermotherapy for subfoveal leaks in central serous chorioretinopathy. Eye (Lond) 2008;22:100–106. doi: 10.1038/sj.eye.6702449. [DOI] [PubMed] [Google Scholar]
- 195.Sibayan SA, Kobuch K, Spiegel D, et al. Epinephrine, but not dexamethasone, induces apoptosis in retinal pigment epithelium cells in vitro: possible implications on the pathogenesis of central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2000;238:515–519. doi: 10.1007/pl00007893. [DOI] [PubMed] [Google Scholar]
- 196.Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: evolution and clinical applications. Surv Ophthalmol. 2010;55:516–530. doi: 10.1016/j.survophthal.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 197.Smith PR, Benos DJ. Epithelial Na+ channels. Annu Rev Physiol. 1991;53:509–530. doi: 10.1146/annurev.ph.53.030191.002453. [DOI] [PubMed] [Google Scholar]
- 198.Spahn C, Wiek J, Burger T, Hansen L. Psychosomatic aspects in patients with central serous chorioretinopathy. Br J Ophthalmol. 2003;87:704–708. doi: 10.1136/bjo.87.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Spaide R. Autofluorescence from the outer retina and subretinal space: hypothesis and review. Retina. 2008;28:5–35. doi: 10.1097/IAE.0b013e318158eca4. [DOI] [PubMed] [Google Scholar]
- 200.Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070–2079. doi: 10.1016/s0161-6420(96)30386-2. discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 201.Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16:203–213. doi: 10.1097/00006982-199616030-00004. [DOI] [PubMed] [Google Scholar]
- 202.Spaide RF. Fundus autofluorescence and age-related macular degeneration. Ophthalmology. 2003;110:392–399. doi: 10.1016/S0161-6420(02)01756-6. [DOI] [PubMed] [Google Scholar]
- 203.Spaide RF, Klancnik JM., Jr Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology. 2005;112:825–833. doi: 10.1016/j.ophtha.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 204.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 205.Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol. 1986;224:321–324. doi: 10.1007/BF02150023. [DOI] [PubMed] [Google Scholar]
- 206.Spitznas M, Huke J. Number, shape, and topography of leakage points in acute type I central serous retinopathy. Graefes Arch Clin Exp Ophthalmol. 1987;225:437–440. doi: 10.1007/BF02334172. [DOI] [PubMed] [Google Scholar]
- 207.Steinle NC, Gupta N, Yuan A, Singh RP. Oral rifampin utilisation for the treatment of chronic multifocal central serous retinopathy. Br J Ophthalmol. 2011 doi: 10.1136/bjophthalmol-2011-300183. [DOI] [PubMed] [Google Scholar]
- 208.Steinsapir KD, Tripathi RC, Tripathi BJ, Ernest JT. Inhibition of ocular gamma glutamyl transpeptidase by acetazolamide. Exp Eye Res. 1992;55:179–181. doi: 10.1016/0014-4835(92)90106-3. [DOI] [PubMed] [Google Scholar]
- 209.Sun J, Tan J, Wang Z, Yang H, Zhu X, Li L. Effect of catecholamine on central serous chorioretinopathy. J Huazhong Univ Sci Technolog Med Sci. 2003;23:313–316. doi: 10.1007/BF02829525. [DOI] [PubMed] [Google Scholar]
- 210.Suzuki K, Hasegawa S, Usui T, et al. Multifocal Electroretinogram in Central Serous Chorioretinopathy. Jpn J Ophthalmol. 2000;44:571. doi: 10.1016/s0021-5155(00)00243-4. [DOI] [PubMed] [Google Scholar]
- 211.Suzuki K, Hasegawa S, Usui T, et al. Multifocal electroretinogram in patients with central serous chorioretinopathy. Jpn J Ophthalmol. 2002;46:308–314. doi: 10.1016/s0021-5155(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 212.Taban M, Boyer DS, Thomas EL. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol. 2004;137:1073–1080. doi: 10.1016/j.ajo.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 213.Tewari HK, Gadia R, Kumar D, Venkatesh P, Garg SP. Sympathetic-parasympathetic activity and reactivity in central serous chorioretinopathy: a case-control study. Invest Ophthalmol Vis Sci. 2006;47:3474–3478. doi: 10.1167/iovs.05-1246. [DOI] [PubMed] [Google Scholar]
- 214.Tittl M, Polska E, Kircher K, et al. Topical fundus pulsation measurement in patients with active central serous chorioretinopathy. Arch Ophthalmol. 2003;121:975–978. doi: 10.1001/archopht.121.7.975. [DOI] [PubMed] [Google Scholar]
- 215.Tittl M, Maar N, Polska E, Weigert G, Stur M, Schmetterer L. Choroidal hemodynamic changes during isometric exercise in patients with inactive central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2005;46:4717–4721. doi: 10.1167/iovs.05-0268. [DOI] [PubMed] [Google Scholar]
- 216.Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128:63–68. doi: 10.1016/s0002-9394(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 217.Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports) Graefes Arch Clin Exp Ophthalmol. 2008;246:1235–1239. doi: 10.1007/s00417-008-0856-x. [DOI] [PubMed] [Google Scholar]
- 218.Uyama M, Matsunaga H, Matsubara T, Fukushima I, Takahashi K, Nishimura T. Indocyanine green angiography and pathophysiology of multifocal posterior pigment epitheliopathy. Retina. 1999;19:12–21. doi: 10.1097/00006982-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 219.Vajaranant TS, Szlyk JP, Fishman GA, Gieser JP, Seiple W. Localized retinal dysfunction in central serous chorioretinopathy as measured using the multifocal electroretinogram. Ophthalmology. 2002;109:1243–1250. doi: 10.1016/s0161-6420(02)01065-5. [DOI] [PubMed] [Google Scholar]
- 220.Verma L, Sinha R, Venkatesh P, Tewari HK. Comparative evaluation of diode laser versus argon laser photocoagulation in patients with central serous retinopathy: a pilot, randomized controlled trial [ISRCTN84128484] BMC Ophthalmol. 2004;4:15. doi: 10.1186/1471-2415-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.von Graefe A. Ueber centrale recidivierende retinitis. Graefes Arch Clin Exp Ophthalmol. 1866;12:211–215. [Google Scholar]
- 222.von Winning CH, Oosterhuis JA, Renger-van Dijk AH, Hornstra-Limburg H, Polak BC. Diffuse retinal pigment epitheliopathy. Ophthalmologica. 1982;185:7–14. doi: 10.1159/000309216. [DOI] [PubMed] [Google Scholar]
- 223.Wakakura M, Ishikawa S. Central serous chorioretinopathy complicating systemic corticosteroid treatment. Br J Ophthalmol. 1984;68:329–331. doi: 10.1136/bjo.68.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Weenink AC, Borsje RA, Oosterhuis JA. Familial chronic central serous chorioretinopathy. Ophthalmologica. 2001;215:183–187. doi: 10.1159/000050855. [DOI] [PubMed] [Google Scholar]
- 225.Wei SY, Yang CM. Transpupillary thermotherapy in the treatment of central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging. 2005;36:412–415. [PubMed] [Google Scholar]
- 226.Williams MA, Mulholland C, Silvestri G. Photodynamic therapy for central serous chorioretinopathy using a reduced dose of verteporfin. Can J Ophthalmol. 2008;43:123. doi: 10.3129/i07-205. [DOI] [PubMed] [Google Scholar]
- 227.Williamson J, Nuki G. Macular lesions during systemic therapy with depot tetracosactrin. Br J Ophthalmol. 1970;54:405–409. doi: 10.1136/bjo.54.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wolfensberger TJ, Chiang RK, Takeuchi A, Marmor MF. Inhibition of membrane-bound carbonic anhydrase enhances subretinal fluid absorption and retinal adhesiveness. Graefes Arch Clin Exp Ophthalmol. 2000;238:76–80. doi: 10.1007/s004170050013. [DOI] [PubMed] [Google Scholar]
- 229.Wu ZH, Lai RY, Yip YW, Chan WM, Lam DS, Lai TY. Improvement in multifocal electroretinography after half-dose verteporfin photodynamic therapy for central serous chorioretinopathy: a randomized placebo-controlled trial. Retina. 2011;31:1378–1386. doi: 10.1097/FTD.0b013e31820beb02. [DOI] [PubMed] [Google Scholar]
- 230.Wyman GJ. Central serous retinopathy in twins. Am J Ophthalmol. 1963;55:1265. doi: 10.1016/0002-9394(63)90204-6. [DOI] [PubMed] [Google Scholar]
- 231.Wynn PA. Idiopathic central serous chorioretinopathy--a physical complication of stress? Occup Med (Lond) 2001;51:139–140. doi: 10.1093/occmed/51.2.139. [DOI] [PubMed] [Google Scholar]
- 232.Yamada R, Yamada S, Ishii A, Tane S. Evaluation of tissue plasminogen activator and plasminogen activator inhibitor-1 in blood obtained from patients of idiopathic central serous chorioretinopathy. Nippon Ganka Gakkai Zasshi. 1993;97:955–960. [PubMed] [Google Scholar]
- 233.Yamada R, Yamada S, Ishii A, Tane S. Evaluation of tissue plasminogen activator and plasminogen activator inhibitor-1 in blood obtained from patients of idiopathic central serous chorioretinopathy. Nihon Ganka Gakkai Zasshi. 1993;97:955–960. [PubMed] [Google Scholar]
- 234.Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol. 2004;2:1–12. doi: 10.2174/1570161043476483. [DOI] [PubMed] [Google Scholar]
- 235.Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc. 1986;84:799–845. [PMC free article] [PubMed] [Google Scholar]
- 236.Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina. 1987;7:111–131. doi: 10.1097/00006982-198700720-00009. [DOI] [PubMed] [Google Scholar]
- 237.Yannuzzi LA, Slakter JS, Kaufman SR, Gupta K. Laser treatment of diffuse retinal pigment epitheliopathy. Eur J Ophthalmol. 1992;2:103–114. doi: 10.1177/112067219200200301. [DOI] [PubMed] [Google Scholar]
- 238.Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003;23:288–298. doi: 10.1097/00006982-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 239.Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010;149:361–363. doi: 10.1016/j.ajo.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 240.Yip YW, Ngai JW, Fok AC, et al. Correlation between functional and anatomical assessments by multifocal electroretinography and optical coherence tomography in central serous chorioretinopathy. Doc Ophthalmol. 2010;120:193–200. doi: 10.1007/s10633-010-9213-6. [DOI] [PubMed] [Google Scholar]
- 241.Yoshioka H, Katsume Y. Studies on experimental central serous chorioretinopathy. A light and electron microscopy. Nihon Ganka Gakkai Zasshi. 1982;86:738–749. [PubMed] [Google Scholar]
- 242.Yoshioka H, Katsume Y, Akune H. Experimental central serous chorioretinopathy in monkey eyes: fluorescein angiographic findings. Ophthalmologica. 1982;185:168–178. doi: 10.1159/000309239. [DOI] [PubMed] [Google Scholar]
- 243.Yoshioka H. The etiology of central serous chorioretinopathy. Nihon Ganka Gakkai Zasshi. 1991;95:1181–1195. [PubMed] [Google Scholar]
- 244.Zakir SM, Shukla M, Simi ZU, Ahmad J, Sajid M. Serum cortisol and testosterone levels in idiopathic central serous chorioretinopathy. Indian J Ophthalmol. 2009;57:419–422. doi: 10.4103/0301-4738.57143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhao MW, Zhou P, Xiao HX, et al. Photodynamic therapy for acute central serous chorioretinopathy: the safe effective lowest dose of verteporfin. Retina. 2009;29:1155–1161. doi: 10.1097/IAE.0b013e3181a6c028. [DOI] [PubMed] [Google Scholar]


