Abstract
Personality plays an important role in determining human health and risk of earlier death. However, the mechanisms underlying those associations remain unknown. We moved away from testing hypotheses rooted in the activities of modern humans, by testing whether these associations are ancestral and one side of a trade-off between fitness costs and benefits. We examined personality predictors of survival in 283 captive western lowland gorillas (Gorilla gorilla gorilla) followed for 18 years. We found that of four gorilla personality dimensions—dominance, extraversion, neuroticism and agreeableness—extraversion was associated with longer survival. This effect could not be explained by demographic information or husbandry practices. These findings suggest that understanding how extraversion and other personality domains influence longevity requires investigating the evolutionary bases of this association in nonhuman primates and other species.
Keywords: animal, behaviour, temperament, mortality, sociability, zoo
1. Introduction
A large body of literature indicates that who we are or our ‘character’ has major consequences related to our health [1]. Most strikingly, studies indicate that lower levels of neuroticism and higher levels of conscientiousness, agreeableness, openness to experience and aspects of extraversion linked to positive affect, and activity are related to reduced risk of all-cause mortality [1,2].
Humans are not the only primate species for which personality is a determinant of health. For example, studies of rhesus macaques found that ‘nervous temperament’ was associated with more neutrophils, lymphocytes and both CD4+ and CD8+ T cells [3], and that sociability was associated with better immune response directly or by moderating the effects of stressful situations [4]. These and similar studies suggest that insights into personality evolution can be gained from studying personality and health outcomes in closely related species [5]. To these ends, we examined personality and longevity in western lowland gorillas (Gorilla gorilla gorilla).
Western lowland gorillas, henceforth gorillas, and humans shared a common ancestor approximately 10 Ma. Sequencing demonstrated that for approximately 30 per cent of the genome, gorillas are closer to humans or chimpanzees than the latter two species are to each other [6]. This phylogenetic proximity is reflected in gorilla personalities, which resemble those of their hominid cousins. Gorilla personality includes reliable, validated dimensions labelled dominant, extroverted, fearful, and understanding [7,8]. The first is not a measure of rank, but resembles dimensions associated with competitive prowess and labelled dominance or confidence in other primates [9]. The latter three resemble dimensions labelled extraversion, neuroticism, and agreeableness, respectively, in humans, chimpanzees and orangutans [10–12].1
We predicted that gorillas lower in neuroticism and higher in extraversion and agreeableness would live longer. Should this be the case, the most parsimonious explanation would be that associations between these personality dimensions and mortality in humans were present in the common ancestor of gorillas and humans. Moreover, based on a review of primate social hierarchies and health, we predicted that gorillas lower in dominance will experience more stress and, consequently, have poorer health [13]. In addition, we tested for interactions of personality and other potential predictors of mortality. For example, given the influence of social instability on rhesus personality and immune functioning [3,4], we tested whether there was an interaction between personality and the number of transfers between facilities an individual experienced.
2. Material and methods
(a). Subjects
We derived our sample from 298 gorillas whose personalities were rated in 1993 [7]. These gorillas represented over 98 per cent of gorillas in the North American Gorilla Species Survival Plan (SSP) over 1 year in age and lived in 43 North American institutions accredited by the Association of Zoos and Aquariums. For our study, we excluded 15 of these gorillas: eight had unknown rearing histories, one had missing personality data, five died from fire exposure, and one died from gas exposure.
At the time their personalities were rated, these gorillas (mean age = 16.5 years ± 10.8 s.d.) included 130 males (mean age = 14.4 years ± 10.1 s.d.) and 153 females (mean age = 18.4 years ± 11.1 s.d.) living in 42 institutions. Ninety-one subjects were wild-born; 82 were captive-born and parent-raised; and 110 were captive-born and hand-raised.
(b). Mortality surveillance
We used the Gorilla SSP studbook to gather data on survival time from 1 March 1993 through to 15 August 2011. If a gorilla died during this period, we coded their mortality status as 1 and defined survival as the number of days between 1 March 1993 and date of death. If a gorilla was still alive we coded their mortality status as 0 and survival time was defined as 6741, the number of days between 1 March 1993 and 15 August 2011 (the censoring date).
(c). Personality
Gorilla personality was assessed using the Gorilla Behavior Index (GBI; appendix B in [7]). The GBI includes 25 behavioural adjectives paired with brief descriptors, e.g. ‘Active: moves about a lot.’ Ratings were made on a 1 (‘the item is weakly represented’) to 5 (‘the item is very strong and conspicuous, approaching the extreme’) scale. We computed z-scores for the personality dimensions based on factor definitions from the previous study ([7]; table 1). For a more detailed description of the rating procedure, see the electronic supplementary material.
Table 1.
Nested comparisons of accelerated failure time models to test for interaction effects. (n = 283. −2LL, −2 log likelihood of model; χ2, model chi-square; d.f., model degrees of freedom; Δχ2 and Δd.f., chi-square and degrees of freedom difference between the baseline and comparison models; p-value, significance of Δχ2 with Δd.f; AIC, Akaike's information criterion.)
| model | −2LL | χ2 | d.f. | Δχ2 | Δd.f. | p-value | AIC |
|---|---|---|---|---|---|---|---|
| baseline | 2418.2 | 65.78 | 10 | 2438.2 | |||
| add sex × personality | 2413.8 | 70.22 | 14 | 4.44 | 4 | 0.350 | 2441.8 |
| add age × personality | 2415.6 | 68.36 | 14 | 2.58 | 4 | 0.630 | 2443.6 |
| add background × personality | 2413.6 | 70.38 | 18 | 4.60 | 8 | 0.799 | 2449.6 |
| add transfers × personality | 2415.0 | 69.05 | 18 | 3.27 | 8 | 0.916 | 2451.0 |
(d). Covariates
Because sex and age effects on personality have been found in chimpanzees [14] and gorillas [8], respectively, we included these variables in our models. This ensured that any significant effects of personality could not be explained by their association with sex or age. To rule out confounds related to rearing, we included two sets of coded variables derived from information in the studbook. The first set included two dummy coded variables. One captive-born, mother-reared gorillas to wild-born gorillas. The other compared captive-born, hand-reared gorillas to wild-born gorillas. The second set included two dummy-coded variables indicating number of transfers (no transfers, 1 transfer, 2+ transfers) to new facilities before the personality ratings.
(e). Data analysis
We fitted six survival models using accelerated failure time analysis [15]. Based on preliminary analyses, we specified a Weibull distribution for survival time. Analyses were conducted using the survreg function in R [16]. In each model, predictors were entered simultaneously and thus were net of all other predictors. For ease of interpretation, associations between survival time and the predictors were expressed via the deceleration estimate (ĉ), which indicates the percentage difference in lifespan associated with a 1 unit change in the predictor. This estimate is computed by determining the antilog of the predictors' effects, i.e. raising the base of the natural log (e) to the power of a predictor's parameter estimates (b), and multiplying the value by 100.
The baseline model included sex, age in years at the time of the personality assessment, rearing type, birth type, number of transfers and the personality dimensions. This model was then compared with four models, each of which included four terms representing the interaction between one covariate and each of the four personality variables. The first tested for sex × personality interactions. The second tested for age × personality interactions. The third tested for rearing × personality interactions. The fourth tested for transfers × personality interactions. We compared models using difference χ2 tests and Akaike's information criteria (AIC; [17]).
3. Results
Over the follow-up period, 119 subjects died. Days to death ranged from 93 to 6741 (median = 3923, mean = 3614.2 ± 1942.9 s.d.). Age at death ranged from 2.4 to 55.7 years (mean = 31.7 ± 11.7 s.d.) and was normally distributed with half of the deaths occurring at 34.1 years or younger.
The baseline accelerated failure time model in which survival time was predicted by sex, age, background, number of transfers, and the four personality dimensions had the lowest AIC; none of the models that added interaction terms significantly improved model fit (table 1). The baseline model (table 2) indicated that females lived longer than males and that each year in age was associated with reduced survival time, though neither effect was significant. In this same model, being captive-born and mother-reared versus being wild-born was not related to survival time. There was also no significant effect of background; captive-born gorillas, whether mother- or hand-raised, did not differ in length of life from their wild-born counterparts. Compared with subjects that were not transferred, there was no significant effect of being transferred one time or being transferred two or more times. In terms of personality, only the effects of extraversion were significant, with each standard deviation being associated with just over a 30 per cent increase in lifespan (see figure 1).
Table 2.
Parameter estimates from the baseline model. (n = 283. ĉ, deceleration estimate; 95% CI, 95% confidence interval.)
| parameter | estimates |
|||
|---|---|---|---|---|
| b | s.e. | p-value | ĉ (95% CI) | |
| intercept | 9.202 | 0.422 | <0.001 | |
| female sex | 0.187 | 0.150 | 0.212 | 1.205 (0.899, 1.617) |
| age at personality rating | −0.024 | 0.015 | 0.103 | 0.976 (0.949, 1.005) |
| captive-born, mother-reareda | 0.402 | 0.297 | 0.177 | 1.494 (0.834, 2.676) |
| captive-born, hand-reareda | 0.412 | 0.246 | 0.094 | 1.510 (0.932, 2.447) |
| 1 transferb | 0.154 | 0.221 | 0.486 | 1.166 (0.757, 1.797) |
| 2+ transfersb | 0.331 | 0.241 | 0.170 | 1.392 (0.867, 2.235) |
| extraversion | 0.272 | 0.120 | 0.023 | 1.312 (1.038, 1.658) |
| dominance | −0.051 | 0.072 | 0.479 | 0.950 (0.826, 1.094) |
| neuroticism | 0.108 | 0.072 | 0.134 | 1.114 (0.967, 1.283) |
| agreeableness | 0.004 | 0.077 | 0.958 | 1.004 (0.864, 1.167) |
| log(scale) | −0.317 | 0.084 | <0.001 | |
aEffect compared with being wild-born.
bEffect compared with never being transferred.
Figure 1.
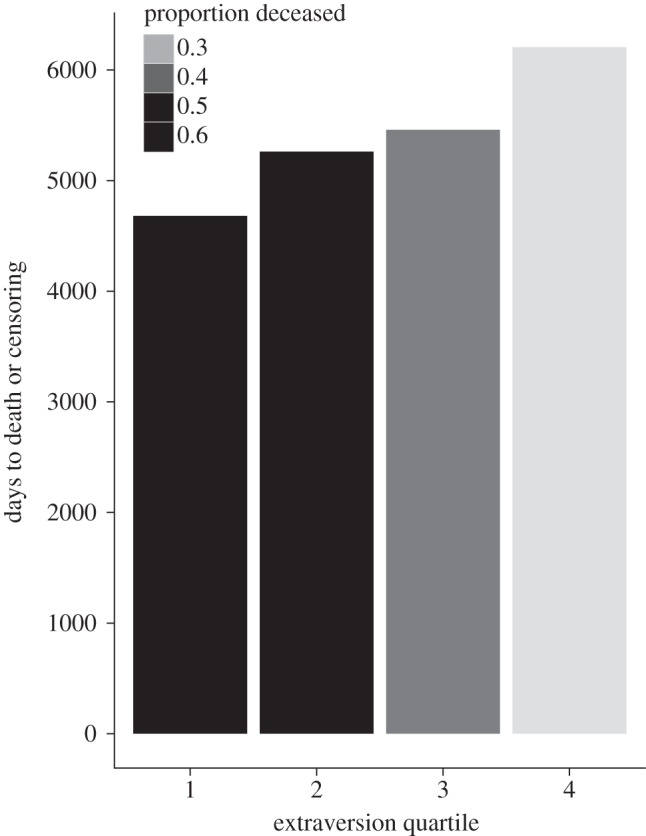
Unadjusted days to death or censoring for quartiles of extraversion (n = 283). Figure by the authors, licensed under a Creative Commons Attribution 3.0 Unported Licence and published under the terms of this licence. For more details see http://creativecommons.org/licenses/by/3.0/.
We conducted two additional analyses. The first sought to determine whether the extraversion effects were influenced by a higher mortality rate in infancy and was based on 179 subjects that were at least 10 years old at the time of the initial assessment. The effect of extraversion in this subsample was significant (ĉ = 1.354, 95% CI = 1.046, 1.754, p = 0.022). The second was conducted to determine whether the non-significant effects of age and number of transfers were attributable to the confounding of age and number of transfers, i.e. older animals would have been transferred more throughout their lives than younger animals. To do so, we fitted three additional models. The first only included sex and age as predictors. The second only included sex and number of transfers. The third only included sex, age, and number of transfers. The first model revealed that older animals had shorter survival times (ĉ = 0.951, 95% CI = 0.937, 0.965, p < 0.001). The second model revealed that, although subjects transferred one time did not have significantly different survival times than subjects who were never transferred (ĉ1 versus 0 = 0.699, 95% CI = 0.468, 1.042, p = 0.079), subjects transferred two or more times lived just under half as long as those who were never transferred (ĉ2+ versus 0 = 0.511, 95% CI = 0.352, 0.743, p < 0.001). The third model revealed that, after adjusting for age, the difference in survival time between individuals transferred once and those not transferred was not significant (ĉ1 versus 0 = 1.090, 95% CI = 0.727, 1.632, p = 0.677). The same was true for the difference between subjects that were transferred two or more times and those who were not transferred (ĉ2+8 versus 0 = 1.167, 95% CI = 0.770, 1.769, p < 0.466). Thus, the absence of significant age and transfer effects in our model are probably explained by these effects being confounded by other predictors, including personality.
4. Discussion
More extraverted gorillas lived longer than their more introverted peers; this association was not confounded by age or sex, rearing condition, or how many times the gorilla was transferred. This finding also did not reflect infant mortality or the deaths of very young gorillas. This finding is consistent with human studies [1,2] and suggests that the association between extraversion and longevity may have been present in the common ancestor shared by humans and gorillas. We would thus expect to find similar associations between extraversion and longevity in chimpanzees and bonobos who share this common ancestor [6].
These results suggest several causal mechanisms. First, like rhesus macaque sociability [4], gorilla extraversion could be a biomarker for differences in the functioning of the immune system. Second, gorilla extraversion could be related to stronger social ties and support that, as in humans, buffer individuals from the effects of environmental stressors [18]. Evidence consistent with this includes a study that showed an association between extraversion and higher rates of affiliation in a subsample of these gorillas [8]. Another possibility is that low extraversion could be linked to cardiovascular disease, which is the primary cause of mortality in captive gorillas [19].
Our other predictions were not supported. Neuroticism and agreeableness were not associated with survival. One possible explanation is that the association between these two personality dimensions and mortality emerged before the homo–pan split, approximately 2–4 Myr later [6,20]. If so, we would expect that neuroticism and agreeableness would be associated with chimpanzee and bonobo longevity. Alternatively, these null results may be an artefact of captivity as regular veterinary care, adequate nutrition, and lack of predation may buffer against untoward effects of higher neuroticism and lower agreeableness. Finally, these non-significant results may be attributable to gorilla social structure. Adult lowland gorillas typically live in cohesive single-male groups [21]. On the other hand, chimpanzees, bonobos and humans live in large multi-male–multi-female groups characterized by fission–fusion dynamics [22]. In the latter type of social groups, increased aggression associated with lower agreeableness [23] may lead to more frequent conflict with other group members and, hence, higher stress levels and hypothalamic-pituitary-adrenal axis activation. Similarly, living in large, complex and ever changing groups may lead to mortality differentials between individuals who differ in their susceptibility to stress. If differences in social structure were responsible, we would expect to find associations between these personality dimensions and longevity in chimpanzees, who live in large complex groups ([24]; but see [21]), but not in orangutans, a semisolitary species [25].
Contrary to our prediction, dominance was not associated with longevity. One possible explanation is that the zoo environment mitigated the effects of low dominance. For example, among wild gorillas, male and female dominance are related to competing over mates and food, respectively. As both are probably reduced or eliminated in captive environments, the consequences of related behaviours or physiological responses may be reduced. If so, we would expect to find a positive association between dominance and survival time among wild gorillas.
One limitation of the study was that data on cause of death, health outcomes and blood chemistry were unavailable. We were thus limited in our ability to understand the route by which extraversion led to longer life. Future researchers should attempt to replicate these findings and, together with zoological parks, collect these data for new studies on personality and health in gorillas and the other great apes.
Another limitation is that we cannot conclusively rule out the possibility that the association between extraversion and longevity may be confounded by characteristics of the gorillas' enclosures or social groups. For example, it may be that gorillas who were housed in small social groups appeared to be lower in extraversion and that these small social groups led to poorer health. To examine the possibility of confounding by zoo characteristics, we conducted two supplementary analyses. First, we tested whether social group size was a potential confound. This involved fitting a model identical to the baseline model, but including the number of subjects with personality data in each zoo as a proxy for social group size. The effects of extraversion held (ĉ = 1.318, 95% CI = 1.042, 1.667, p = 0.021). Second, we tested for the possibility of any other potential confounds related to the zoo environment or animal husbandry. Like the previous supplementary analysis, this involved fitting a model identical to the baseline model, but including the zoological park identity as a categorical variable. In short, we statistically adjusted for any differences across zoological parks in the housing and husbandry of the gorillas. The effects of extraversion in this model also held, and were somewhat stronger (ĉ = 1.558, 95% CI = 1.188, 2.043, p = 0.001). Thus, it is unlikely that the effects of extraversion were confounded by zoo level differences in housing and husbandry. In fact, differences among zoological parks seem to have ‘masked’ the effects of personality. Still, future researchers could learn much about this association by examining the degree to which these this association can be explained by specific differences in husbandry procedures, social group composition, physical environments, and enrichment.
This study revealed that the association between dispositions related to sociability, activity, and positive affect with longevity may have evolved at least 10 Ma. In doing so, it highlights ancestral fitness benefits of personality traits that might explain what kind of selection pressures maintain personality variability in humans [26] and our gorilla cousins. These findings also highlight how understanding the natural history of personality is vital to insuring the continued health and well-being of gorillas and other great apes, including ourselves.
Acknowledgements
Personality data collection was funded by Zoo Atlanta, the Georgia Institute of Technology, and a Lincoln Park Zoological Society's Dr Scholl's Graduate Research Fellowship to K.C.G. We thank the curators and keepers who completed the ratings.
Endnote
For consistency, we adopted labels used in previous studies.
References
- 1.Deary IJ, Weiss A, Batty GD. 2010. Intelligence and personality as predictors of illness and death: how researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychol. Sci. Pub. Interest 11, 53–79 10.1177/1529100610387081 (doi:10.1177/1529100610387081) [DOI] [PubMed] [Google Scholar]
- 2.Chapman BP, Roberts B, Duberstein P. 2011. Personality and longevity: knowns, unknowns, and implications for public health and personalized medicine. J. Aging Res. 2011 759170. 10.4061/2011/759170 (doi:10.4061/2011/759170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capitanio JP, Mendoza SP, Cole SW. 2011. Nervous temperament in infant monkeys is associated with reduced sensitivity of leukocytes to cortisol's influence on trafficking. Brain Behav. Immun. 25, 151–159 10.1016/j.bbi.2010.09.008 (doi:10.1016/j.bbi.2010.09.008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capitanio JP. 2011. Individual differences in emotionality: social temperament and health. Am. J. Primatol. 73, 507–515 10.1002/ajp.20870 (doi:10.1002/ajp.20870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosling SD, Graybeal A. 2007. Tree thinking: a new paradigm for integrating comparative data in psychology. J Gen. Psychol. 134, 259–277 10.3200/GENP.134.2.259-278 (doi:10.3200/GENP.134.2.259-278) [DOI] [PubMed] [Google Scholar]
- 6.Scally A, et al. 2012. Insights into hominid evolution from the gorilla genome sequence. Nature 483, 169–175 10.1038/nature10842 (doi:10.1038/nature10842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold KC, Maple TL. 1994. Personality assessment in the gorilla and its utility as a management tool. Zoo Biol. 13, 509–522 10.1002/zoo.1430130513 (doi:10.1002/zoo.1430130513) [DOI] [Google Scholar]
- 8.Kuhar CW, Stoinski TS, Lukas KE, Maple TL. 2006. Gorilla behavior index revisited: age, housing and behavior. Appl. Anim. Behav. Sci. 96, 315–326 10.1016/j.applanim.2005.06.004 (doi:10.1016/j.applanim.2005.06.004) [DOI] [Google Scholar]
- 9.Freeman HD, Gosling SD. 2010. Personality in nonhuman primates: a review and evaluation of past research. Am J. Primatol. 72, 653–671 10.1002/ajp.20833 (doi:10.1002/ajp.20833) [DOI] [PubMed] [Google Scholar]
- 10.Digman JM. 1990. Personality structure: emergence of the five-factor model. Annu. Rev. Psychol. 41, 417–440 10.1146/annurev.ps.41.020190.002221 (doi:10.1146/annurev.ps.41.020190.002221) [DOI] [Google Scholar]
- 11.King JE, Figueredo AJ. 1997. The five-factor model plus dominance in chimpanzee personality. J. Res. Pers. 31, 257–271 10.1006/jrpe.1997.2179 (doi:10.1006/jrpe.1997.2179) [DOI] [Google Scholar]
- 12.Weiss A, King JE, Perkins L. 2006. Personality and subjective well-being in orangutans (Pongo pygmaeus and Pongo abelii). J. Pers. Soc. Psychol. 90, 501–511 10.1037/0022-3514.90.3.501 (doi:10.1037/0022-3514.90.3.501) [DOI] [PubMed] [Google Scholar]
- 13.Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308, 648–652 10.1126/science.1106477 (doi:10.1126/science.1106477) [DOI] [PubMed] [Google Scholar]
- 14.King JE, Weiss A, Sisco MS. 2008. Aping humans: age and sex effects in chimpanzee (Pan troglodytes) and human (Homo sapiens) personality. J. Comp. Psychol. 122, 418–427 10.1037/a0013125 (doi:10.1037/a0013125) [DOI] [PubMed] [Google Scholar]
- 15.Swindell WR. 2008. Accelerated failure time models provide a useful statistical framework for aging research. Exp. Gerontol. 44, 190–200 10.1016/j.exger.2008.10.005 (doi:10.1016/j.exger.2008.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therneau T. 2012. A package for survival analysis in S. R package v. 2.36-12. See http://cran.r.project.org/web/packages/survival/index.html.
- 17.Akaike H. 1987. Factor analysis and AIC. Psychometrika 52, 317–332 10.1007/BF02294359 (doi:10.1007/BF02294359) [DOI] [Google Scholar]
- 18.Cohen S, Wills TA. 1985. Stress, social support, and the buffering hypothesis. Psychol. Bull. 98, 310–357 10.1037/0033-2909.98.2.310 (doi:10.1037/0033-2909.98.2.310) [DOI] [PubMed] [Google Scholar]
- 19.Murphy HW, Dennis P, Devlin W, Meehan T, Kutinski I. 2011. Echocardiographic parameters of captive western lowland gorillas (Gorilla gorilla gorilla). J. Zoo Wildl. Med. 42, 572–579 10.1638/2010-0139.1 (doi:10.1638/2010-0139.1) [DOI] [PubMed] [Google Scholar]
- 20.Purvis A. 1995. A composite estimate of primate phylogeny. Phil. Trans. R. Soc. Lond. B 348, 405–421 10.1098/rstb.1995.0078 (doi:10.1098/rstb.1995.0078) [DOI] [PubMed] [Google Scholar]
- 21.Parnell RJ. 2002. Group size and structure in western lowland gorillas (Gorilla gorilla gorilla) at Mbeli Bai, Republic of Congo. Am. J. Primatol. 56, 193–206 10.1002/ajp.1074 (doi:10.1002/ajp.1074) [DOI] [PubMed] [Google Scholar]
- 22.Aureli F, et al. 2008. Fission–fusion dynamics: new research frameworks. Cur. Anthropol. 49, 627–654 10.1086/586708 (doi:10.1086/586708) [DOI] [Google Scholar]
- 23.Bettencourt B, Talley A, Benjamin AJ, Valentine J. 2006. Personality and aggressive behavior under provoking and neutral conditions: a meta-analytic review. Psychol. Bull. 132, 751–777 10.1037/0033-2909.132.5.751 (doi:10.1037/0033-2909.132.5.751) [DOI] [PubMed] [Google Scholar]
- 24.Kalpers J, Williamson EA, Robbins MM, McNeilage A, Nzamurambaho A, Lola N, Mugiri G. 2003. Gorillas in the crossfire: population dynamics of the Virunga mountain gorillas over the past three decades. Oryx 37, 326–337 10.1017/S0030605303000589 (doi:10.1017/S0030605303000589) [DOI] [Google Scholar]
- 25.Galdikas BMF. 1985. Orangutan sociality at Tanjung-Puting. Am. J. Primatol. 9, 101–119 10.1002/ajp.1350090204 (doi:10.1002/ajp.1350090204) [DOI] [PubMed] [Google Scholar]
- 26.Penke L, Denissen JJA, Miller GF. 2007. The evolutionary genetics of personality. Eur. J. Pers. 21, 549–587 10.1002/per.629 (doi:10.1002/per.629) [DOI] [Google Scholar]


