Abstract
Vertebrates have achieved great evolutionary success due in large part to the anatomical diversification of their jaw complex, which allows them to inhabit almost every ecological niche. While many studies have focused on mechanisms that pattern the jaw skeleton, much remains to be understood about the origins of novelty and diversity in the closely associated musculature. To address this issue, we focused on parrots, which have acquired two anatomically unique jaw muscles: the ethmomandibular and the pseudomasseter. In parrot embryos, we observe distinct and highly derived expression patterns for Scx, Bmp4, Tgfβ2 and Six2 in neural crest-derived mesenchyme destined to form jaw muscle connective tissues. Furthermore, immunohistochemical analysis reveals that cell proliferation is more active in the cells within the jaw muscle than in surrounding connective tissue cells. This biased and differentially regulated mode of cell proliferation in cranial musculoskeletal tissues may allow these unusual jaw muscles to extend towards their new attachment sites. We conclude that the alteration of neural crest-derived connective tissue distribution during development may underlie the spatial changes in jaw musculoskeletal architecture found only in parrots. Thus, parrots provide valuable insights into molecular and cellular mechanisms that may generate evolutionary novelties with functionally adaptive significance.
Keywords: adaptive evolution, evolutionary novelty, jaw muscles, neural crest mesenchyme, parrots
1. Introduction
Within vertebrates, birds (class Aves) are the second largest group, consisting of over 10 000 species. Although birds display wide variations in body size, coloration and habitat, their body plan is highly uniform among almost all lineages, possibly owing to the biomechanical constraints of flight. Even the morphology of the jaw complex, which is highly variable in other groups of vertebrates, is less diverse in birds, being basically composed of toothless upper and lower beaks and a consistent number of jaw muscles (figure 1a, left). An exception to this is parrots (order Psittaciformes), which have acquired two novel jaw muscles that are anatomically peculiar and adaptive for feeding on hard-shelled nuts and seeds via strong adduction of the mandible [1–4]. The ethmomandibular (Musculus ethmomandibularis) originates high on the rostral part of the interorbital septum and inserts into the dorsomedial aspect of the mandible, passing the lateral surface of the palatine bone (figure 1a, right). The pseudomasseter (Musculus pseudomasseter) covers the jugal bone laterally, bearing a superficial resemblance to the mammalian masseter muscle. In no other avian taxon is the jugal bone covered with jaw muscle fibres. Therefore, parrots can serve as a useful model for understanding developmental mechanisms that establish evolutionary novelties not only in birds but in vertebrates in general.
Figure 1.
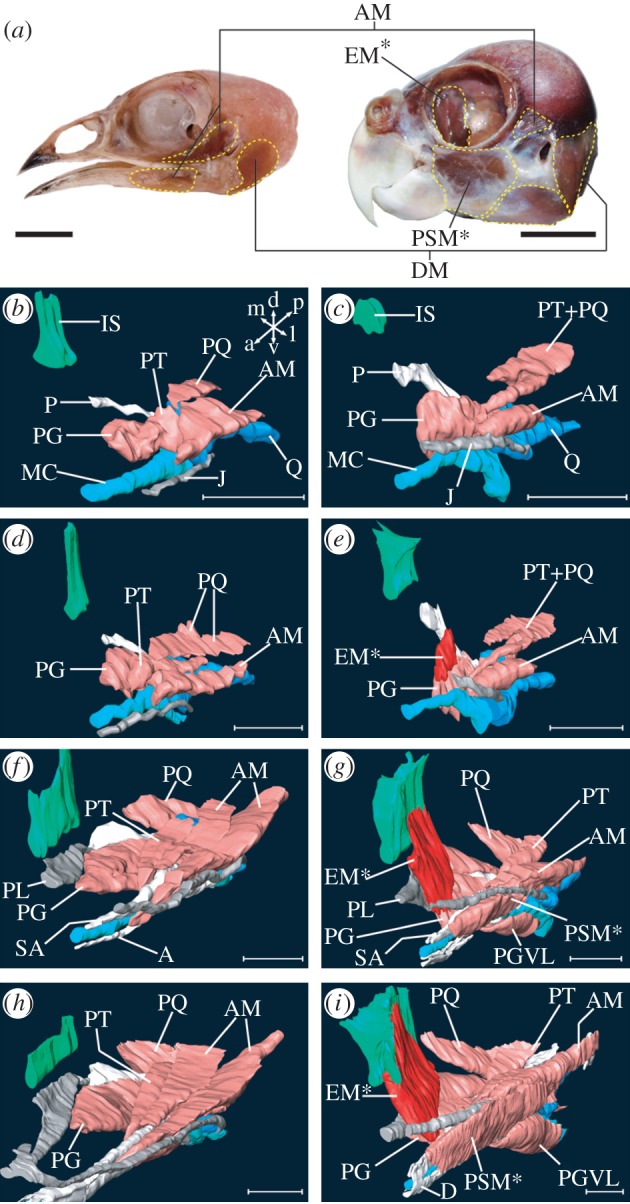
Morphogenesis of novel jaw muscles in parrots. (a) Quail (left) and parrot (right) heads with jaw muscles (yellow dashed lines). (b) Three-dimensional model of the jaw complex that is primarily composed of muscular, cartilaginous and intramembranous bone tissues, of quail at stage 30 reconstructed form a series of histological sections. Orientation of the model is indicated at right-upper corner: a, anterior; d, dorsal; l, lateral; m, medial; p, posterior; v, ventral. This orientation is applicable for other three-dimensional models. (c) Parrot at stage 30. (d) Quail at stage 32. (e) Parrot at stage 32. (f) Quail at stage 36. (g) Parrot at stage 36. (h) Quail at stage 38. (i) Parrot at stage 38. A, angular bone; AM, mandibular adductor muscle; D, dentary bone; DM, mandibular depressor muscle; EM, ethmomandibular muscle; IS, interorbital septum; J, jugal bone; MC, Meckel's cartilage; P, pterygoid bone; PG, pterygoideus muscle; PGVL, ventrolateral part of pterygoideus muscle; PL, palatine bone; PQ, quadrate protractor muscle; PSM, pseudomasseter muscle; PT, pseudotemporal muscle; Q, quadrate cartilage; SA, surangular bone. Asterisked muscles are specific to parrots. Scale bars, (a) 10 mm, (b–i) 1 mm.
Developmentally, jaw muscle fibres are derived from cranial paraxial mesoderm (reviewed by Noden & Francis-West [5]). By contrast, both the jaw skeleton and associated connective tissues, including ligaments, tendons and endomysia distributed in individual muscles, form from cranial neural crest mesenchyme (reviewed by Noden & Francis-West [5]). A series of studies, including mutant screens [6], tissue ablation [7] and hetero-specific transplantation [8,9], have revealed that the cranial neural crest has a crucial role in the differentiation and shaping of mesoderm-derived cranial muscles. Based on these results, we hypothesized that the neural crest is elemental not only in maintaining the highly conserved patterns for individual jaw muscles but also in the evolution of novel jaw muscles.
To test this hypothesis, we have taken a comparative approach in this study. First, we described the pattern of jaw morphogenesis in both parrot and non-parrot birds, using computer-aided reconstructions of serial sections. Second, we analysed gene expression patterns in the cranial musculoskeletal tissues for two avian lineages. In this analysis, differentiated muscle fibres were specifically stained by immunohistochemistry with an anti-skeletal muscle myosin antibody. To label cranial mesoderm-derived muscle precursors as well as differentiated muscles, we performed in situ hybridization of MyoD [10]. We used Runx2 as a marker for early osteoblasts [11], and Scx, Six2 and Tenascin-C as markers for connective tissues [12–14]. We also examined expression pattern of genes involved in signalling pathways known to regulate connective tissue development in vertebrates: BMP, FGF and TGF-β [15–23]. In this study, we focused on Bmp4, Fgf8 and Tgfβ2, which are expressed in tendinous connective tissues and affect tendon development. Third, we assayed for differences in the proliferation of cells distributed in a differentiated jaw muscle, and cells in surrounding non-muscle connective tissues of parrot and non-parrot bird embryos. This approach enabled us to assess the potential role of cell proliferation in the patterning of novel jaw muscles. Our analyses on the molecular and cellular levels reveal that parrot embryos exhibit an alteration of jaw connective tissue distribution. This spatial alteration may be linked to neural crest development, and ultimately may allow parrots to acquire novel jaw muscles that have been a key innovation for their highly successful granivorous lifestyle.
2. Results
(a). Morphogenesis of novel jaw muscles in parrots
Understanding how and when the novel jaw muscles of parrots develop is prerequisite information for identifying potential molecular and cellular mechanisms. Thus, we first described morphogenesis of differentiated jaw muscles and associated skeletons of parrot embryos, using three-dimensional reconstructions of serial sections. We compared parrot jaw muscle morphology with that of stage-matched quail (order Galliformes), which was used as a generalized and more basal representative of birds. At stage 30, the spatial pattern of jaw muscle precursors is quite similar in the two species (see figure 1b,c and electronic supplementary material, movie files). However, we observed a unique dorsal projection of the anterior part of the pterygoideus muscle precursor in parrot embryos. This corresponds to initiation of the ethmomandibular development. Subsequently (stage 32–34), this muscle projection further grew in a dorsal direction in parrots (figure 1e). Corresponding muscle projection was never seen in stage-matched quail (figure 1d). At stage 36, the ethmomandibular of parrots reached the cartilage of the interorbital septum, passing the lateral side of the palatine bone (figure 1g). In stage-matched quail embryos, the pterygoideus muscle attached to the ventrolateral edge of the palatine bone and no muscle covered the lateral surface of the bone (figure 1f). Initiation of development of the pseudomasseter, another novel jaw muscle of parrots, was observed in embryos at stage 36. This muscle branched off from the parent muscle (i.e. the mandibular adductor), and grew in a dorsolateral direction, almost covering the lateral surface of the jugal bone (figure 1g). The architectural patterning of the jaw muscles was almost completed in both parrot and quail by stage 38 (figure 1h,i). In parrot embryos, the dorsal attachment site of the ethmomandibular further broadened over the interorbital septum (figure 1i). Although the pseudomasseter was not separated completely from the medially located mandibular adductor, the lateral surface of the jugal bone was completely covered by the muscle in the middle part of the head (figure 1i).
(b). Unique expression of Scx, Bmp4 and Tgfβ2 in connective tissues associated with the ethmomandibular muscle
In parrot embryogenesis, the ethmomandibular develops earlier than the pseudomasseter. Spatially, the ethmomandibular develops in a more medial location close to the palate, compared with the pseudomasseter, which forms at a more lateral region of the head (figure 1). In stage 32 parrot embryos, MyoD-positive ethmomandibular muscle precursor overlay medially located and Runx2-positive palatine bone anlagen from a lateral direction (figure 2b,e). Between the muscle and the bone, an Scx-positive elongated tendinous layer was detected (figure 2f, arrowheads). In stage-matched quail embryos, the pterygoideus muscle, a parent muscle of the ethmomandibular acquired in parrots, approached the palatine bone via an underdeveloped tendinous tissue (figure 2a,c,d). The lateral surface of their palatine bone was never overlaid by any muscular or tendinous tissues (figure 2a,c,d).
Figure 2.
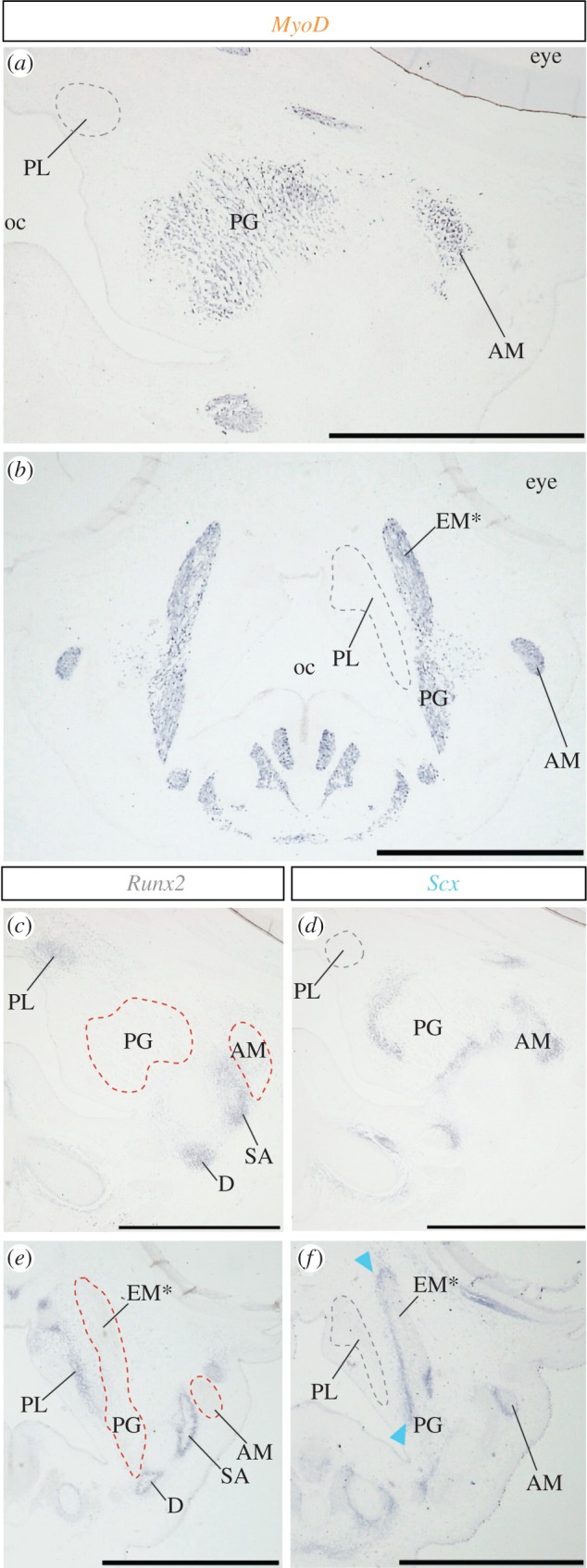
Substantial alteration of cranial musculoskeletal tissue arrangement in parrot embryos revealed by gene expression analysis. (a,b) MyoD expression in jaw muscles of quail and parrot embryos at stage 32, respectively. (c) Runx2 and (d) Scx expression in adjacent sections of (a). (e) Runx2 and (f) Scx expression in adjacent sections of (b). Note Scx expression in tendinous tissue that covers the palatine bone laterally in parrot embryo (arrowheads). oc, oral cavity. Other abbreviations as in figure 1. Scale bars, 1 mm.
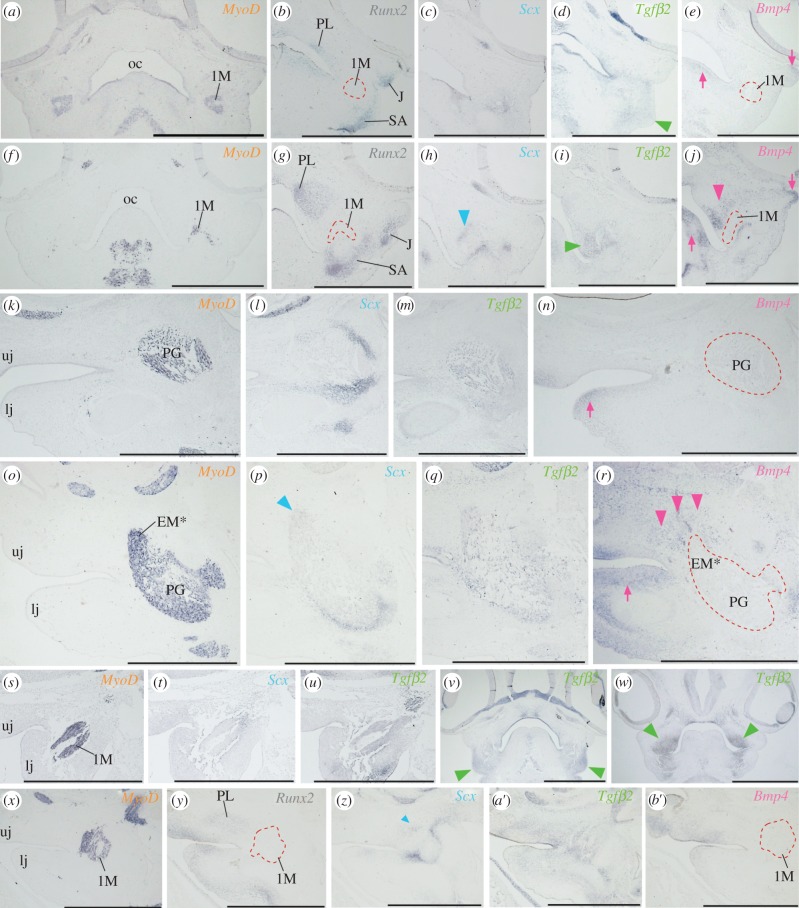
In younger parrot embryos (at stages 28–29), an Scx-positive tendon primordial layer uniquely formed between the dorsomedial edge of MyoD-positive jaw muscle and Runx2-positive palatine bone precursors (figure 3f–h,o,p). Because we detected this Scx-positive tendinous layer in most anterior portion of the jaw muscle precursor, it probably corresponds to future dorsomedial tendon of the ethmomandibular. In stage-matched galliform embryos, an Scx-positive tendinous layer was not detected between MyoD-positive jaw muscle and Runx2-positive palatine bone precursors (figure 3a–c,k,l). Instead, Scx-positive mesenchyme was diffusively distributed around the jaw muscle precursors. To screen candidate signalling pathways that govern the development of such spatially unique tendon in parrots, we examined expression patterns of Bmp4, Fgf8 and Tgfβ2, which are reportedly expressed in tendinous tissues, and compared them in embryos of two avian lineages.
Figure 3.
Unique expression of Bmp4 and Tgfβ2 in connective tissues associated with the ethmomandibular muscle. (a) MyoD expression in jaw muscle precursors (1M) of quail embryo at stage 28. (b) Runx2, (c) Scx, (d) Tgfβ2 and (e) Bmp4 expression in adjacent sections. (f) MyoD expression in jaw muscles of parrot embryo at stage 28. (g) Runx2, (h) Scx, (i) Tgfβ2 and (j) Bmp4 expression in adjacent sections. (k) Parasagittal section of chicken embryo at stage 29, where cranial muscles were labelled with MyoD probe. (l) Scx, (m) Tgfβ2 and (n) Bmp4 expression in adjacent sections. (o) Parasagittal section of parrot embryo at stage 29, where cranial muscular tissue was labelled with MyoD probe. (p) Scx, (q) Tgfβ2 and (r) Bmp4 expression in adjacent sections. (s) Parasagittal section of chicken embryo at stage 25, where cranial muscle precursors were labelled with MyoD probe. (t) Scx and (u) Tgfβ2 expression in adjacent sections. (v) Frontal section of quail embryo and (w) of parrot embryo at stage 26, where expression domain of Tgfβ2 was shown. (x) Parasagittal section of parrot embryo at stage 25, where cranial muscle precursors were labelled with MyoD probe. (y) Runx2, (z) Scx, (a′) Tgfβ2 and (b′) Bmp4 expression in adjacent sections. Arrowheads in (d), (h), (i), (j), (p), (r) (v), (w) and (z) indicate gene expression in neural crest-derived connective tissues associated with jaw muscles. Arrows in (e), (j), (n) and (r) indicate gene expression in neural crest-derived mesenchyme that is distant from jaw muscle tissues. lj, lower jaw; uj, upper jaw. Other abbreviations as in figure 1. Scale bars, 1 mm.
Although Fgf8 was expressed only in the oral epithelium in early stage (stages 24–28) embryos of both parrot and galliform birds (see the electronic supplementary material, figure S1), Bmp4 and Tgfβ2 were expressed in neural crest-derived connective tissues associated with the jaw muscles. In galliform embryos at stages 28–29, expression of Bmp4 was detected at the distal mesenchyme of the jaws and the mesenchyme within the eyelid primordium (figure 3e,n, arrows). By contrast, in stage-matched parrot embryos, unique expression of Bmp4 was detected in the mesenchyme associated with the dorsal part of the ethmomandibular (figure 3j,r, arrowheads), as well as in the distal mesenchyme of the lower jaw and the mesenchyme within the eyelid primordium (figure 3j,r, arrows). In both parrot and galliform embryos at stages 28–29, expression of Tgfβ2 was detected in the mesenchyme associated with the jaw muscle precursors (figure 3d,i,m,q). In galliform embryos at stages 24–26, Tgfβ2 was detected in the mesenchyme associated with jaw muscle precursors formed in the lower jaw primordium (figures 3s,u and 4v, arrowheads; electronic supplementary material, figure S2). In. (doi:10.1007/s00442-004-1809-7) these embryos, an Scx-positive tendon precursor was diffusively distributed around the jaw muscle precursor (figure 3t). In stage-matched parrot embryos, Tgfβ2 was expressed in neural crest-derived mesenchyme distributed in both upper and lower jaw primordia (figure 3w, arrowheads; figure 3a′; electronic supplementary material, figure S2). In the upper jaw primordium, Tgfβ2 was expressed in the mesenchyme distributed in proximity to both Runx2-positive palatine bone precursor and MyoD-positive jaw muscle precursor (figure 3w,a′). In these embryos, unique expression of Scx was just initiated in a population of mesenchyme located between jaw muscle and palatine bone precursors (figure 3y,z, arrowhead). We could not observe expression of Bmp4 in the mesenchyme adjacent to the palatine bone and jaw muscle precursors of these embryos (figure 3b′). The results on expression domains for each gene analysed in parrot and galliform bird embryos are summarized in the electronic supplementary material, table S1.
Figure 4.
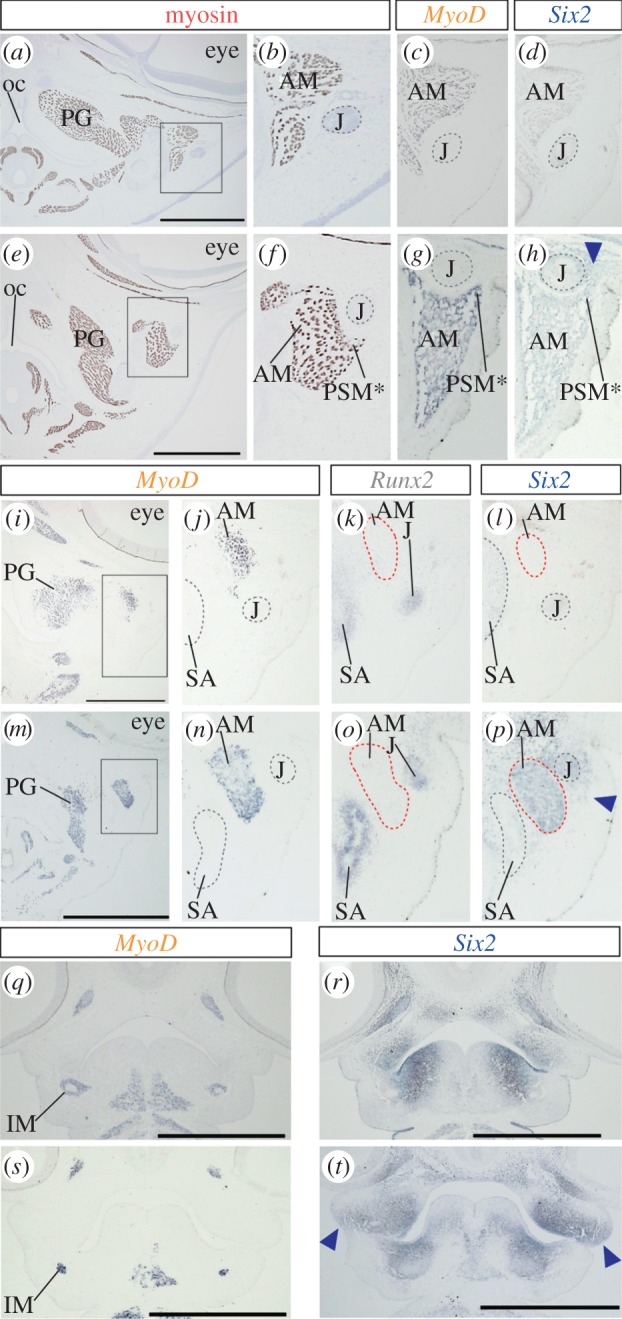
Unique expression of Six2 in tendon of the pseudomasseter muscle. (a) Frontal section of a quail embryo at stage 36, where skeletal muscles were stained with myosin antibody. (b) Higher magnification of the rectangle in (a). (c) Frontal section of chicken embryo at stage 36, where MyoD expression in jaw muscle tissue was shown. (d) Expression of Six2 in section adjacent to (c). (e) Frontal section of a parrot embryo at stage 36, where skeletal muscles were stained with myosin antibody. (f) Higher magnification of the rectangle in (e). (g) MyoD expression in the mandibular adductor muscle in parrot embryo at equivalent stage. (h) Six2 expression in the tendon of the pseudomasseter muscle. (i) MyoD expression in cranial muscles of chicken embryo at stage 32. (j) Higher magnification of the rectangle in (i). (k) Runx2 and (l) Six2 expression in adjacent sections. (m) MyoD expression in cranial muscles of parrot embryo at stage 32. (n) Higher magnification of the rectangle (m). (o) Runx2 and (p) Six2 expression in adjacent sections. (q) MyoD and (r) Six2 expression in frontal section of quail at stage 26. (s) MyoD and (t) Six2 expression in parrot embryo at stage 26. Arrowheads in (h), (p) and (t) indicate unique expression of Six2 in neural crest-derived connective tissue or mesenchyme in parrot embryos. Abbreviations as in figure 1. Scale bars, 1 mm.
(c). Unique expression of Six2 in tendon of the pseudomasseter muscle
Of two novel jaw muscles of parrots, the pseudomasseter begins to develop at a relatively late embryonic stage (figure 1). The pseudomasseter was first recognized in parrot embryos at stage 36, as a small dorsolateral projection of mandibular adductor muscle (figure 4a–c,e–g). As described in a previous study [2], we found the condensation of connective tissue at the dorsal tip of the muscle (figure 4h, arrowhead). This connective tissue condensation occupied the domain lateral to the jugal bone and expressed connective tissue-marker genes: Scx, Six2 and Tenascin-C (see figure 4h and electronic supplementary material, figure S3). This type of connective tissue condensation was absent in the corresponding domain of galliform quail and chicken embryos (figure 4d). In parrot embryos at stage 32, where the pseudomasseter has not yet formed (figure 4i–p), only Six2 was expressed in the connective tissue cells distributed at the domain lateral to Runx2-positive jugal bone and MyoD-positive mandibular adductor muscle (figure 4p, arrowhead). In adjacent sections, Scx and Tenascin-C were not expressed in corresponding connective tissue cells (see the electronic supplementary material, figure S3). Expression of Six2 was never detected at the corresponding domain in stage-matched galliform embryos (figure 4l). In younger embryos (at stages 26 and 24), no interspecific difference was observed in the spatial pattern of skeletal muscle precursors that express MyoD (figure 4q,s). By contrast, substantial differences were observed in the domain of Six2 expression between parrot and galliform birds (figure 4r,t). The expression domain of Six2 in neural crest-derived mesenchyme broadened in a lateral direction in the upper jaw primordium of parrot embryos (figure 4t, arrowheads; electronic supplementary material, figure S2).
(d). Cell proliferation activity in jaw muscle and surrounding connective tissue
Through the earlier-mentioned analyses, we found that both the ethmomandibular and pseudomasseter muscles of parrots acquired new tendons during embryogenesis. The formation of these muscles was initially achieved by expansion of the muscle into the new attachment sites where the new tendons were located. Based on those observations, we hypothesized that active cell proliferation in neural crest-derived endomysial connective tissues, which are distributed in the jaw muscle mass, drives this expansion. To test this hypothesis, we examined the level of cell proliferation activity in the cells composing a differentiated jaw muscle and the cells within surrounding connective tissue. We used an antibody specific for the mitotic marker phospho-histone H3 (PHH3; see electronic supplementary material, figure S4). For this experiment, we chose parrot embryos at stage 32 (n = 3) where a distinct growth of the ethmomandibular in an anterodorsal direction was observed (figure 1e). Then, we compared the numerical data on cell proliferation with that obtained from stage-matched quail embryos (n = 2) to find interspecific differences (table 1). As a result, although we did not see any remarkable interspecific differences in the mode of cell proliferation, cell proliferation was always more active (at least 50% more active on average) within jaw muscle compared with surrounding connective tissue in both species. Within jaw muscle, a majority of the cells undergoing proliferation (PHH3-positive) were not myosin-positive differentiated myocytes but myosin-negative cells (at least over 90% of the total number of cells counted).
Table 1.
Comparison of the ratio of cell proliferation in jaw muscle and non-muscular tissues surrounding muscle.
| species | specimen | tissue | total no. cells (=A) | no. PHH3-positive cells (=B) | B/A (%) |
|---|---|---|---|---|---|
| quail | 1 | muscle | 1228 | 30 (myosin+ : myosin− = 2:28) | 2.44 |
| non-muscle | 913 | 15 | 1.64 | ||
| 2 | muscle | 987 | 27 (0 : 27) | 2.74 | |
| non-muscle | 797 | 14 | 1.76 | ||
| parrot | 1 | muscle | 3892 | 52 (1 : 51) | 1.34 |
| non-muscle | 1003 | 8 | 0.80 | ||
| 2 | muscle | 5974 | 166 (1 : 165) | 2.78 | |
| non-muscle | 817 | 17 | 2.08 | ||
| 3 | muscle | 4601 | 90 (3 : 87) | 1.96 | |
| non-muscle | 1017 | 12 | 1.18 |
3. Discussion
To date, much attention has been paid to the anatomical diversification of the bones and cartilages of the jaws [24–26], and especially the fundamental role of the cranial neural crest mesenchyme in this process (reviewed in recent studies [27–29]). By contrast, few studies have identified developmental mechanisms that account for the diversity of jaw muscle morphology. Previously, we conducted hetero-specific neural crest transplantations between quail and duck, to test whether quail neural crest-derived connective tissues could confer species-specific identity to duck mesoderm-derived jaw muscles [9]. In these chimaeras, duck host mesoderm-derived jaw muscles acquired quail-like shape and attachment sites owing to the presence of quail donor neural crest-derived connective tissues. Based on these results, we concluded that neural crest mesenchyme regulates the species-specific jaw muscle morphology [9].
Interestingly, the number and organization of jaw muscles have been extremely modified in a few vertebrate taxa. For example, pufferfish (order Tetraodontiformes) show substantial alteration of jaw muscle morphology, principally caused by duplication of mandibular adductor muscles [30]. Similarly, parrots have acquired two novel jaw muscles that are anatomically peculiar and not possessed by any other avian lineages [1,2,4]. However, molecular and cellular mechanisms underlying the acquisition of such novel jaw muscles have not been investigated in these non-model vertebrates. Migration of neural crest mesenchyme from the neural tube into the first pharyngeal arch is relatively more advanced in parrots than in galliforms [31]. Such temporal shifts in neural crest cell migration may influence the organization of the jaw muscles [31]. To test our hypothesis that neural crest mesenchyme plays a crucial role not only in determining shape and attachment sites of individual jaw muscles but also in the evolution of architecturally unique new jaw muscles, we performed a series of comparative analyses using parrot embryos.
In this study, we observed unique expression patterns for several genes that are expressed in the neural crest-derived connective tissues of parrots. Regarding the ethmomandibular, we speculate that spatially unique expression of Tgfβ2, Scx and Bmp4 in neural crest-derived connective tissue may be important in its development. TGF-β signalling is crucial for the development of cranial neural crest-derived tissues [25,32,33]. In this study, we focused on Tgfβ2, which is expressed in cranial neural crest-derived mesenchyme [15,34,35]. In stage 24 parrot embryos, we observed Tgfβ2 expression in the neural crest-derived mesenchyme distributed in both the upper and lower jaw primordia. In subsequent stages (stages 25–26), the Tgfβ2 expression domain was expanded into the mesenchyme that ultimately differentiates into the palatine bone and the mesenchyme associated with the jaw muscle precursors. At the periphery of the Tgfβ2-positive connective tissue, Scx-positive tendon precursor appeared. Corresponding tendon primordia did not form in the head of galliform embryos. This situation mimics the observation that TGF-β2-soaked beads transplanted into cranial or appendicular tissues can upregulate Scx expression [15,20,21,22]. Thus, we speculate that the spatial alteration of Tgfβ2 expression in neural crest-derived mesenchyme may be crucial in enabling the formation of the new tendon necessary to attach the ethmomandibular in parrots.
BMP signalling also plays essential roles in the development of craniofacial tissues. In this study, we focused on Bmp4, which is expressed in neural crest-derived tissues, and regulates the development and morphology of the neural crest-derived skeleton [24,36–38]. In stage 28 parrot embryos, we observed unique expression of Bmp4 in the neural crest-derived mesenchyme distributed between the palatine bone and the ethmomandibular muscle precursors. In these embryos, Scx-positive tendon precursors have already formed at the dorsal tip of the ethmomandibular precursor. Bmp4 is expressed in tendon, and regulates proliferation and differentiation of muscles through activation of BMP signalling [23]. Also, Bmp4 expression in tendons is regulated by Scx [16], which is a downstream target of TGF-β signalling. Currently, much remains to be understood about how TGF-β signalling may affect the unique expression pattern of Bmp4 in the neural crest-derived mesenchyme of parrot embryos. We speculate that spatial alteration of Bmp4 expression in neural crest-derived mesenchyme may affect proliferation and differentiation of the ethmomandibular, and thus allow this muscle to expand into a new attachment site in the head of parrots.
FGF signalling is known to function in tendon development [19]. In this study, we focused on Fgf8, which upregulates Scx expression in trunk tendons of vertebrate embryos [17,18]. Although we detected Fgf8 expression in tendinous and fascial tissues associated with jaw muscles in late-stage parrot embryos (at stage 36 or older), its expression was not observed in the neural crest-derived mesenchyme associated with the jaw muscle precursors in early-stage parrot embryos (younger than stage 36). These results may relate to a lessor contribution of FGF signalling in the evolution of the novel jaw muscles in birds.
Through comparative analysis of cell proliferation among tissues, we found that the cells within the jaw muscle proliferate more actively than the surrounding connective tissue cells in avian embryos. Of these actively proliferating cells within the jaw muscle, a majority were myosin-negative cells. Unfortunately, our method is unable to distinguish mesoderm-derived and undifferentiated muscle progenitor cells from neural crest-derived mesenchyme. However, we speculate that a majority of the cells inside the jaw muscle could be the latter because there is an increase in the number of neural crest-derived mesenchymal cells inside muscles after stage 27 [5,39], and muscle progenitor cells tend to be located at the periphery of the muscle [23]. Synthesizing the results above, we propose a working hypothesis about the developmental mechanism underlying the evolution of the ethmomandibular. This hypothesis will be tested in future studies (see the electronic supplementary material, figure S5).
Another novel jaw muscle of parrots, the pseudomasseter, develops at a relatively late embryonic stage (after stage 36) and at the lateral part of the head. We speculate that spatially unique expression of Six2 in neural crest-derived mesenchyme may be crucial in pseudomasseter development. Six2 is a transcription factor that regulates development of urogenital and digestive organs, and its transcripts are also expressed in connective tissues such as tendons and ligaments [12,13]. In the head, Six2 is expressed in neural crest-derived mesenchyme [40] and regulates skull development [41,42]. In stage 32 parrot embryos where the pseudomasseter has not formed, Six2-positive neural crest-derived connective tissue was observed in the domain lateral to both the jugal bone and the mandibular adductor muscle precursors. In younger parrot embryos (stage 24–26), the Six2 expression domain already broadened laterally in the upper jaw primordium, where future jugal bone develops. By contrast, expression of Six2 was more biased into the medial portion of the upper jaw primordium in galliform embryos. Expression of Six2 in neural crest-derived mesenchyme distributed in pharyngeal arches is regulated by Hoxa2 [43,44]. Alteration in Hox gene regulation may account for spatially unique expression of Six2 that eventually forms the tendon of the pseudomasseter in parrots.
In conclusion, although the two novel jaw muscles of parrots form at different times and at different locations, they both appear to share a common developmental process, which is that their differentiation is preceded (and possibly even presaged) by the spatially unique populations of closely associated neural crest-derived connective tissues. We predict that this unique population of connective tissues can account for the evolution of novel jaw muscles (figure 5). As a next step, the nature of the interaction among genes that are uniquely expressed in neural crest-derived mesenchyme of parrots needs to be better defined, and the precise function of each of these genes should be tested in the context of novel jaw muscle development. Furthermore, understanding the interactions of adjacent cranial tissues (such as cranial nerves and blood vessels) with the jaw muscles will also be important for explaining how parrots and other vertebrate taxa can evolve novel features in their musculoskeletal systems.
Figure 5.
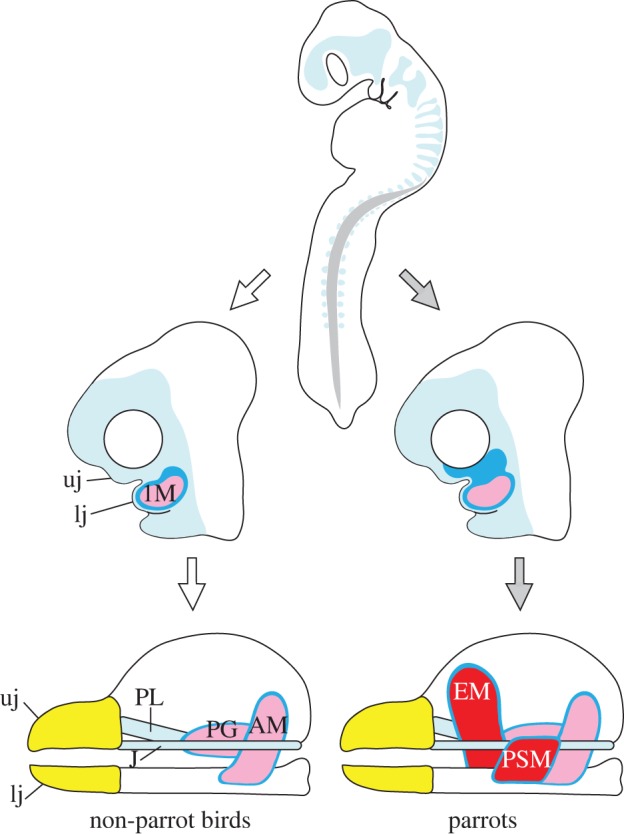
Schematic diagram depicting possible association of neural crest mesenchyme in the evolution of novel jaw muscles. Neural crest mesenchyme (pale blue) delaminates from the neural tube and migrates in a ventral direction in the head and trunk of early-stage embryos. In parrot embryos, development of populations of neural crest mesenchyme that later differentiates into jaw connective tissues (dark blue) is uniquely regulated, and this eventually leads to unique distribution of the mesenchyme (top black arrow), compared with the situation in non-parrot bird embryos (top white arrow). In parrots, spatially unique jaw muscle connective tissues that are derived from neural crest mesenchyme affect the organization of mesoderm-derived jaw muscles, and this eventually leads to the formation of novel jaw muscles with adaptive significance (bottom black arrow) that are absent in non-parrot birds (bottom white arrow). Abbreviations as in figure 1.
4. Methods
A full description of methods, including sample collection, staging of embryos, cloning of genes, analysis of gene expression and tissue-marker protein localization, and comparative analysis of cell proliferation, can be found in the electronic supplementary material. All animal experiments were approved by the University of Tsukuba Committee for Animal Care.
Acknowledgements
We thank Kiyoshi Matsukawa, who provided us with fertilized eggs of cockatiel, and Hiroshi Wada, who allowed the use of facilities for experiments and analyses. The A4.1025 antibody was obtained from DSHB, maintained by University of Iowa under the auspices of the NICHD. This study was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan to M.T. (22770077); Narishige Zoological Science Award to M.T.; and NIDCR R01 DE016402 to R.A.S.
References
- 1.Homberger DG. 2003. The comparative biomechanics of a prey–predator relationship: the adaptive morphologies of the feeding apparatus of Australian Black-Cockatoos and their foods as a basis for the reconstruction of the evolutionary history of the Psittaciformes. In Vertebrate biomechanics and evolution (eds Bels VL, Gasc J-P, Casinos A.), pp. 203–228 Oxford, UK: BIOS Scientific Publisher [Google Scholar]
- 2.Tokita M. 2004. Morphogenesis of parrot jaw muscles: understanding the development of an evolutionary novelty. J. Morphol. 259, 69–81 10.1002/jmor.10172 (doi:10.1002/jmor.10172) [DOI] [PubMed] [Google Scholar]
- 3.Tokita M, Kiyoshi T, Armstrong KN. 2007. Evolution of craniofacial novelty in parrots through developmental modularity and heterochrony. Evol. Dev. 9, 590–601 10.1111/j.1525-142X.2007.00199.x (doi:10.1111/j.1525-142X.2007.00199.x) [DOI] [PubMed] [Google Scholar]
- 4.Zusi RL. 1993. Patterns of diversity in the avian skull. In Skull, vol. 2 (eds Hanken J, Hall BK.), pp. 391–437 Chicago, IL: University of Chicago Press [Google Scholar]
- 5.Noden DM, Francis-West P. 2006. The differentiation and morphogenesis of craniofacial muscles. Dev. Dyn. 235, 1194–1218 10.1002/dvdy.20697 (doi:10.1002/dvdy.20697) [DOI] [PubMed] [Google Scholar]
- 6.Schilling TF, Walker C, Kimmel CB. 1996. The chinless mutation and neural crest cell interactions in zebrafish jaw development. Development 122, 1417–1426 [DOI] [PubMed] [Google Scholar]
- 7.Rinon A, et al. 2007. Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development 134, 3065–3075 10.1242/dev.002501 (doi:10.1242/dev.002501) [DOI] [PubMed] [Google Scholar]
- 8.Noden DM. 1983. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev. Biol. 96, 144–165 10.1016/0012-1606(83)90318-4 (doi:10.1016/0012-1606(83)90318-4) [DOI] [PubMed] [Google Scholar]
- 9.Tokita M, Schneider RA. 2009. Developmental origins of species-specific muscle pattern. Dev. Biol. 331, 311–325 10.1016/j.ydbio.2009.05.548 (doi:10.1016/j.ydbio.2009.05.548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noden DM, Marcucio R, Borycki AG, Emerson CP., Jr 1999. Differentiation of avian craniofacial muscles. I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Dev. Dyn. 216, 96–112 (doi:10.1002/(SICI)1097-0177(199910)216:2<96::AID-DVDY2>3.0.CO;2-6) [DOI] [PubMed] [Google Scholar]
- 11.Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. 2007. Regulation of skeletogenic differentiation in cranial dermal bone. Development 134, 3133–3144 10.1242/dev.002709 (doi:10.1242/dev.002709) [DOI] [PubMed] [Google Scholar]
- 12.Dreyer SD, Naruse T, Morello R, Zabel B, Winterpacht A, Johnson RL, Lee B, Oberg KC. 2004. Lmx1b expression during joint and tendon formation: localization and evaluation of potential downstream targets. Gene Expr. Patterns 4, 397–405 10.1016/j.modgep.2004.01.006 (doi:10.1016/j.modgep.2004.01.006) [DOI] [PubMed] [Google Scholar]
- 13.Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BNR, Hartenstein V, Zipursky SL, Gruss P. 1995. Homeobox genes and connective tissue patterning. Development 121, 693–705 [DOI] [PubMed] [Google Scholar]
- 14.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. 2001. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 [DOI] [PubMed] [Google Scholar]
- 15.Anthwal N, Chai Y, Tucker AS. 2008. The role of transforming growth factor-beta signalling in the patterning of the proximal processes of the murine dentary. Dev. Dyn. 237, 1604–1613 10.1002/dvdy.21567 (doi:10.1002/dvdy.21567) [DOI] [PubMed] [Google Scholar]
- 16.Blitz E, et al. 2009. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon–skeleton junction. Dev. Cell. 17, 861–873 10.1016/j.devcel.2009.10.010 (doi:10.1016/j.devcel.2009.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brent AE, Schweitzer R, Tabin CJ. 2003. A somitic compartment of tendon progenitors. Cell 113, 235–248 10.1016/S0092-8674(03)00268-X (doi:10.1016/S0092-8674(03)00268-X) [DOI] [PubMed] [Google Scholar]
- 18.Brent AE, Tabin CJ. 2004. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131, 3885–3896 10.1242/dev.01275 (doi:10.1242/dev.01275) [DOI] [PubMed] [Google Scholar]
- 19.Edom-Vovard F, Schuler B, Bonnin M-A, Teiller M-A, Duprez D. 2002. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 247, 351–366 10.1006/dbio.2002.0707 (doi:10.1006/dbio.2002.0707) [DOI] [PubMed] [Google Scholar]
- 20.Hosokawa R, Oka K, Yamaza T, Iwata J, Urata M, Xu X, Bringas P, Nonaka K, Chai Y. 2010. TGF-β mediated FGF10 signaling in cranial neural crest cells controls development of myogenic progenitor cells through tissue-tissue interactions during tongue morphogenesis. Dev. Biol. 341, 186–195 10.1016/j.ydbio.2010.02.030 (doi:10.1016/j.ydbio.2010.02.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oka K, Oka S, Hosokawa R, Bringas P, Brockhoff HC, Nonaka K, Chai Y. 2008. TGF-β mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev. Biol. 321, 303–309 10.1016/j.ydbio.2008.03.046 (doi:10.1016/j.ydbio.2008.03.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. 2009. Recruitment and maintenance of tendon progenitors by TGFβ signaling are essential for tendon formation. Development 136, 1351–1361 10.1242/dev.027342 (doi:10.1242/dev.027342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Noulet F, Edom-Vovard F, Le Grand F, Duprez D. 2010. Bmp signaling at the tips of skeletal muscles regulates the number of fetal muscle progenitors and satellite cells during development. Dev. Cell. 18, 643–654 10.1016/j.devcel.2010.02.008 (doi:10.1016/j.devcel.2010.02.008) [DOI] [PubMed] [Google Scholar]
- 24.Albertson RC, Streelman JT, Kocher TD, Yelick PC. 2005. Integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies. Proc. Natl Acad Sci USA 102, 16 287–16 292 10.1073/pnas.0506649102 (doi:10.1073/pnas.0506649102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallarino R, Grant PR, Grant BR, Herrel A, Kuo WP, Abzhanov A. 2011. Two developmental modules establish 3D beak-shape variation in Darwin's finches. Proc. Natl Acad. Sci. USA 108, 4057–4062 10.1073/pnas.1011480108 (doi:10.1073/pnas.1011480108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider RA, Helms JA. 2003. The cellular and molecular origins of beak morphology. Science 299, 565–568 10.1126/science.1077827 (doi:10.1126/science.1077827) [DOI] [PubMed] [Google Scholar]
- 27.Le Douarin NM, Creuzet S, Couly G, Dupin E. 2004. Neural crest cell plasticity and its limits. Development 131, 4637–4650 10.1242/dev.01350 (doi:10.1242/dev.01350) [DOI] [PubMed] [Google Scholar]
- 28.Minoux M, Rijli FM. 2010. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137, 2605–2621 10.1242/dev.040048 (doi:10.1242/dev.040048) [DOI] [PubMed] [Google Scholar]
- 29.Trainor PA, Melton KR, Manzanares M. 2003. Origins and plasticity of neural crest cells and their roles in jaw and craniofacial evolution. Int. J. Dev. Biol. 47, 541–553 [PubMed] [Google Scholar]
- 30.Friel JP, Wainwright PC. 1997. A model system of structural duplication: homologies of adductor mandibulae muscles in tetraodontiform fishes. Syst. Biol. 46, 441–463 10.1093/sysbio/46.3.441 (doi:10.1093/sysbio/46.3.441) [DOI] [Google Scholar]
- 31.Tokita M. 2006. Cranial neural crest cell migration in cockatiel Nymphicus hollandicus (Aves: Psittaciformes). J. Morphol. 340, 333–340 10.1002/jmor.10408 (doi:10.1002/jmor.10408) [DOI] [PubMed] [Google Scholar]
- 32.Iwata J-I, Hosokawa R, Sanchez-Lara PA, Urata M, Slavkin H, Chai Y. 2010. Transforming growth factor-β regulates basal transcriptional regulatory machinery to control cell proliferation and differentiation in cranial neural crest-derived osteoprogenitor cells. J. Biol. Chem. 285, 4975–4982 10.1074/jbc.M109.035105 (doi:10.1074/jbc.M109.035105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. 1997. Tgfβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development 124, 2659–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzpatrick DR, Denhez F, Kondaiah P, Akhurst RJ. 1990. Differential expression of TGF beta isoforms in murine palatogenesis. Development 109, 585–595 [DOI] [PubMed] [Google Scholar]
- 35.Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Nakajima A, Shuler CF, Moses HL, Chai Y. 2003. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 130, 5269–5280 10.1242/dev.00708 (doi:10.1242/dev.00708) [DOI] [PubMed] [Google Scholar]
- 36.Bonilla-Claudio M, Wang J, Bai Y, Klysik E, Selever J, Martin JF. 2012. Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development 139, 709–719 10.1242/dev.073197 (doi:10.1242/dev.073197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merrill AE, Eames BF, Weston SJ, Heath T, Schneider RA. 2008. Mesenchyme-dependent BMP signaling directs the timing of mandibular osteogenesis. Development 135, 1223–1234 10.1242/dev.015933 (doi:10.1242/dev.015933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu P, Jiang T-X, Suksaweang S, Widelitz R, Chuong C-M. 2004. Molecular shaping of the beak. Science 305, 1465–1466 10.1126/science.1098109 (doi:10.1126/science.1098109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grenier J, Teillet M-A, Grifone R, Kelly RG, Duprez D. 2009. Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE 4, e4381. 10.1371/journal.pone.0004381 (doi:10.1371/journal.pone.0004381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nonomura K, Takahashi M, Wakamatsu Y, Takano-Yamamoto T, Osumi N. 2010. Dynamic expression of Six family genes in the dental mesenchyme and the epithelial ameloblast stem/progenitor cells during murine tooth development. J. Anat. 216, 80–91 10.1111/j.1469-7580.2009.01167.x (doi:10.1111/j.1469-7580.2009.01167.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fogelgren B, et al. 2008. Misexpression of Six2 is associated with heritable frontonasal dysplasia and renal hypoplasia in 3H1 Br mice. Dev. Dyn. 237, 1767–1779 10.1002/dvdy.21587 (doi:10.1002/dvdy.21587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He G, Tavella S, Hanley KP, Self M, Oliver G, Grifone R, Hanley N, Ward C, Bobola N. 2010. Inactivation of Six2 in mouse identifies a novel genetic mechanism controlling development and growth of the cranial base. Dev. Biol. 344, 720–730 10.1016/j.ydbio.2010.05.509 (doi:10.1016/j.ydbio.2010.05.509) [DOI] [PubMed] [Google Scholar]
- 43.Kutejova E, Engist B, Mallo M, Kanzler B, Bobola N. 2005. Hoxa2 downregulates Six2 in the neural crest-derived mesenchyme. Development 132, 469–478 10.1242/dev.01536 (doi:10.1242/dev.01536) [DOI] [PubMed] [Google Scholar]
- 44.Kutejova E, Engist B, Self M, Oliver G, Kirilenko P, Bobola N. 2008. Six2 functions redundantly immediately downstream of Hoxa2. Development 135, 1463–1470 10.1242/dev.017624 (doi:10.1242/dev.017624) [DOI] [PubMed] [Google Scholar]