Abstract
Ostracod crustaceans are the most abundant fossil arthropods. The Silurian Pauline avibella gen. et sp. nov., from the Herefordshire Lagerstätte, UK, is an extremely rare Palaeozoic example with soft-part preservation. Based on its soft-part morphology, especially the exceptionally preserved limbs and presence of lateral eyes, it is assigned to the myodocopid myodocopes. The ostracod is very large, with an epipod on the fifth limb pair, as well as gills implying the presence of a heart and an integrated respiratory–circulatory system as in living cylindroleberidid myodocopids. Features of its shell morphology, however, recall halocyprid myodocopes and palaeocopes, encouraging caution in classifying ostracods based on the carapace alone and querying the interpretation of their shell-based fossil record, especially for the Palaeozoic, where some 500 genera are presently assigned to the Palaeocopida.
Keywords: exceptional preservation, Herefordshire Lagerstätte, Myodocopida, Myodocopa, Ostracoda, Silurian
1. Introduction
The shells of ostracods occur profusely throughout the stratigraphic record from the Early Ordovician onwards [1–3] in a wide range of aqueous environments [4]. The overwhelming majority of the estimated 33 000 living and fossil species [5] are benthic/nektobenthic. Pelagic forms, exclusively members of the Myodocopa, one of the two major living ostracod groups, arose via an ecological shift in the Silurian [6,7]. Myodocopes typically have weakly calcified valves and, consequently, a scant fossil record [8–11], which begins in the Late Ordovician [12]. The soft-parts of ostracods are rarely fossilized [13,14]. From the Palaeozoic a single ostracod-type limb is known from the Late Cambrian [15]; a podocopid specimen with some soft-parts [16] and an undescribed, partially open carapace with poorly preserved limbs [17] from the Devonian; and three myodocope species, each based on an essentially completely preserved specimen, from the Herefordshire (Silurian) Konservat-Lagerstätte, UK [18–20]. Here, we describe a new ostracod with soft-parts from the Herefordshire deposit, Pauline avibella gen. et sp. nov. (Myodocopa), based on two specimens.
The mid-Silurian (approx. 425 Myr BP) Herefordshire biota [21] has yielded unrivalled anatomical data from a diversity of invertebrate species that range up to several centimetres in size: brachiopods, a polychaete worm, a gastropod and aplacophorans, a pycnogonid, chelicerates, a marrellomorph, a possible stem lineage mandibulate, a barnacle, a phyllocarid, ostracods and a stem-group asteroid [20,22–25]. There are also many forms awaiting investigation, including sponges, brachiopods, molluscs, arthropods and echinoderms.
2. Material and methods
The Herefordshire fossils occur as three-dimensional calcitic in-fills within calcareous nodules hosted in a volcaniclastic layer [26]. The ostracods are reconstructed as ‘virtual fossils’ using the custom SPIERS software suite [27,28]. The original datasets resulting from serial grinding are housed at the University Museum of Natural History, Oxford (OUMNH). The fossils were ground and photographed at 20 µm intervals; extraneous material was removed digitally; fossil-matrix ambiguities were resolved prior to generating a colour-coded reconstruction using ray tracing.
3. Systematic palaeontology
Phylum Arthropoda, Subphylum Crustacea, Class Ostracoda.
Subclass: Myodocopa Sars [29].
Order: Myodocopida Sars [29].
Family: Cylindroleberididae Müller [30].
Genus: Pauline gen. nov.
Derivation of name: for Pauline Siveter, in memoriam. Gender: feminine.
Diagnosis: large cylindroleberidid. Carapace elongate with an adductorial sulcus, an anterior lobal complex, a prominent wing-like posterolateral lobal structure and a simple anterior gape. Exopod of second antenna has about 20 long setae distally.
Type species: Pauline avibella sp. nov.
Derivation of name: Latin avis, bird+bella, beautiful; fancied resemblance of the posterodorsal lobal structure to a bird's wing.
Diagnosis: as for the genus (monotypic).
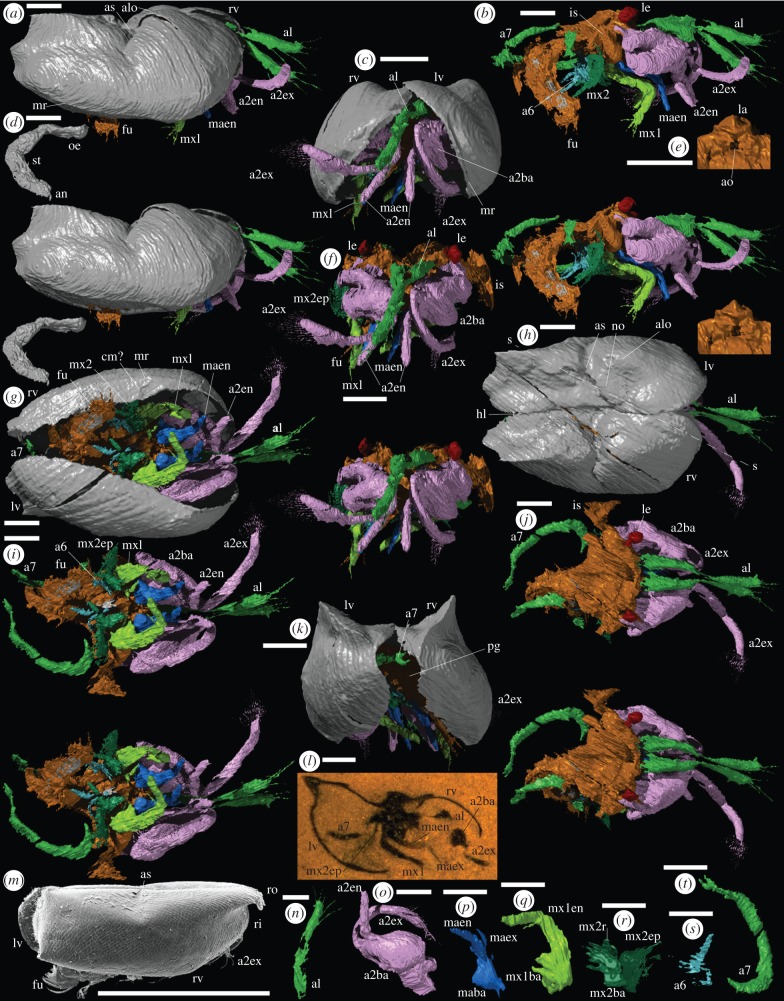
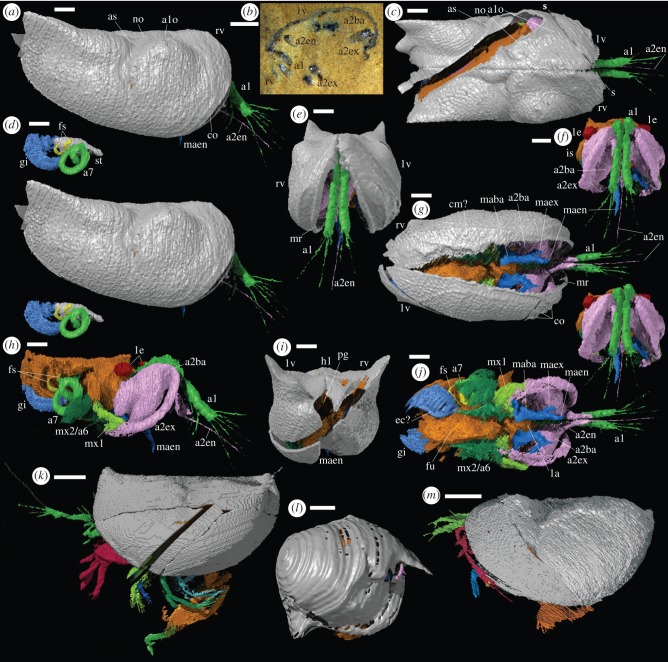
Material: two carapaces with soft-parts. The holotype, OUMNH C.29614 (figure 1l), reconstructed in three-dimensions (figure 1a–k,n–t); and the slightly less completely preserved OUMNH C.29613 (figure 2b), reconstructed in three-dimensions with some loss along the plane of cut (figure 2a,c–j).
Figure 1.
(a–l,n–t) Holotype of Pauline avibella, carapace with soft-parts (OUMNH C.29614): (a–k, n–t) ‘virtual’ reconstructions; (l) specimen in rock. The exact boundary between structures such as body and limbs, as indicated by colour changes, is somewhat arbitrary. (a) External right lateral view (stereo-pair). (b) Right lateral view (stereo-pair), valves omitted. (c) Anterior view. (d) Right lateral view (stereo-pair) of gut system. (e) Oblique ventral view of labrum and atrium oris. (f) Anterior view (stereo-pair), valves omitted. (g) Ventral view. (h) Dorsal view. (i) Ventral view (stereo-pair), valves omitted. (j) Dorsal view (stereo-pair), valves omitted. (k) Posterior view. (l) Lateral oblique section. (n–t) Oblique posterior approximately medial view of left limbs: (n) first antenna, (o) second antenna, (p) mandible, (q) first maxilla. (r) second maxilla. (s) sixth limb. (t) seventh limb. (m) Halocyprid myodocope Discoconchoecia pseudodiscophora (Rudjakov [31]), carapace, right lateral view (OUMNH RY.37); Recent, Sea of Japan, depth 320 m. All scale bars 1 mm. a1, first antenna; a2ba, a2en, a2ex, basipod, endopod and exopod of second antenna; a6, sixth limb; a7, seventh limb; alo, anterior lobe; an, anus; ao, atrium oris; as, adductorial sulcus; co, costa(e); cm?, contact margin structure?; ec?, epibranchial canal?; fs, finger-like structure; fu, furca; gi, gills; hl, hinge line; is, isthmus; la, labrum; le, lateral eye; lv, left valve; maba, maen, maex, basipod, endopod and exopod of mandible; mr, marginal ridge; mx1, first maxilla; mx1ba, mx1en, basipod and endopod of first maxilla; mx2, second maxilla; mx2ba, mx2ep, mx2r, basipod, epipod and ramus of second maxilla; no, node; oe, oesophagus; pg, posterior gape; ri, rostral incisure; ro, rostrum; rv, right valve; s-s, line of section through specimen (h,l); st, stomach.
Figure 2.
Silurian Myodocopida. (a–j) Pauline avibella, carapace with soft-parts (OUMNH C.29613): (a, c–j) ‘virtual’ reconstructions; (b) specimen in rock. The exact boundary between structures such as body and limbs, as indicated by colour changes, is somewhat arbitrary; the gap in data marks the line of split of the specimen in the nodule. (a) External right lateral view (stereo-pair). (b) Oblique section. (c) Dorsal view. (d) Lateral view (stereo-pair) of stomach, gills, seventh limb and finger-like projections (other soft-parts and valves omitted). (e) Anterior view. (f) Anterior view (stereo-pair), valves omitted. (g) Ventral view. (h) Right lateral view, valves omitted. (i) Posterior view. (j) Ventral view, valves omitted. (k–m) Herefordshire Lagerstätte myodocopids; ‘virtual’ reconstructions of holotype carapaces with soft-parts: (k) Nymphatelina gravida (OUMNH C.29600), left lateral view; (l) Nasunaris inflata (OUMNH C.29612), right lateral view; (m) Colymbosathon ecplecticos (OUMNH C.29570), left lateral view. All scale bars 1 mm. Abbreviations: as for figure 1; s-s, line of section through specimen (b,c).
Locality and stratigraphy: Herefordshire, UK; Wenlock Series, Silurian (further locality information by application to OUMNH).
Description: maximum length, height and width (in dorsal view) of the carapace are 6400 µm, 3000 µm and 4500 µm (holotype), and 10 200 µm, 5300 µm and 5500 µm (OUMNH C.29613). Valves gape at about 70° (holotype) and 25° (OUMNH C.29613) (figures 1c and 2e), and outline an ovoid posterior gape (figures 1k and 2i). The valve is bordered by a narrow marginal ridge that is less prominent posteriorly (figures 1a,c,g and 2e,g). A wide, thin, incomplete lamella-like feature of uncertain interpretation (contact margin structure?) extends adaxially from the ventral to posterior regions of the marginal ridge of the right valve, and indicates some measure of left over right valve overlap (figures 1g and 2g). A large, globose anterior lobe is gently rounded dorsally; its crest reaches just above the hinge line in the holotype, but is more subdued in OUMNH C.29613 (figures 1a and 2a). A smaller, more weakly developed node occurs between the anterior lobe and the hinge line (figures 1h and 2c). An adductorial sulcus occurs at mid-length; it is weakly Z-shaped, widest dorsally, and extends to just below valve mid-height (figures 1a,h and 2a,c). The ventral two-thirds of the valve behind the adductorial sulcus are gently inflated; above this a prominent wing-like lobal structure curves gently above the hinge line with posterior projections at mid-height and dorsally (figures 1a,h,k and 2a,c,i). The external surface of the valve of the holotype is smooth; in OUMNH C.29613, it is finely reticulate with a few weakly developed narrow costae close and parallel to the anterior margin of the valve (figures 1a and 2a,e,g).
The first antenna (i.e. antennula; figures 1a–c,f–j,n and 2a,c,e–h,j) originates close to the sagittal plane. It consists of two parts of similar length, separated by a geniculation (probable articulation) that is strongly compressed transversely. The distal part of the limb projects beyond the carapace and bears two long setae ventrally, which may correspond to separate podomeres; it terminates in three or four long setae. Exsagittal to the first antenna a pedunculate, ovoid, presumed compound lateral eye (ommatidia not discernable) lies in the anterior lobe of the valve (figures 1b,f,j and 2f,h). A medial eye is not evident. The basipod of the second antenna (i.e. antenna; figures 1a–c,f–j,o and 2f–h,j) is large, globose and almond-shaped with a marked lateral axial depression. The exopod is long, stout, ovoid in cross section, curved and slightly wider in the distal one-quarter, which bears about 20 long, fine, closely set and parallel setae; podomeres are not discernable (as in Recent cylindroleberidids, the distribution of setae may reflect individual podomeres, including multiple setae on the terminal podomere). The right exopod of the holotype projects beyond the carapace, whereas the left is preserved curving ventrally and posteriorly inside the domicilium (figure 1c,g). The endopod arises just below the exopod. It is stout, about half the length of the exopod, and consists of a proximal part that is geniculate with a longer distal part bearing a very long terminal seta that is preserved only on one limb in OUMNH C.29613 and preserved as a small stub in the holotype (figures 1b,g,i and 2a,c,f–h,j).
The mandible (figures 1b,g,i,p and 2f–h,j) has a broadly triangular-shaped limb base (presumed basipod and coxa) sited just over the atrium oris and adjacent to the conical-shaped labrum (figure 1e). The inner edge of the limb base bears a few weakly developed, presumed enditic processes. The endopod is stout, gently tapered and consists of a proximal part separated from a distal part of similar length by a geniculum. The exopod (evident in the holotype only in the left limb) arises adjacent to the endopod and is of similar length to the proximal part of the endopod but much narrower. The limb base (presumed basipod and proximal endite) of the first maxilla (i.e. maxillula) is large, broad, with 2–3 long, well-developed enditic processes along its inner edge adjacent to the atrium oris (figure 1b,g,i,q). The ramus (presumed endopod) is large, blade-like and comprises subequal proximal and distal parts (podomeres?) separated by a marked geniculation (figures 1b,i and 2h,j). As seen in the holotype, the ramus has at least three fine setae distally (best evident in the right limb; figure 1b) and a long fine seta that originates ventrally near the geniculum.
The limb base of the second maxilla (i.e. fifth limb) bears at least five setiferous enditic processes arranged in one or (as evident on the left limb of the holotype) two rows that lie just outside, and project towards, the atrium oris (figure 1b,g,i,r). A large, posteriorly concave, lamellar epipod projects laterally from the limb base (figure 1r), which also bears a single short tapered ramus (conventionally the exopod in myodocopids, but see Boxshall [32]; figure 1i,r). The second maxilla and sixth limb in OUMNH C.29613 cannot be fully resolved due to preservational factors. The sixth limb in the holotype is also poorly preserved; it is small, with a stout, tapered ramus projecting posteroventrally from a lamellar limb base that bears five long, medial enditic processes, evident on the best preserved (right) limb, the more distal of which project less anteromedially and more ventrally (figure 1b,g,i,s). The body isthmus (best preserved on the left side in the holotype) coincides with the adductorial sulcus (figure 1a,b,f,i,j). The seventh limb is vermiform and over 3000 µm long; it arises from the body just behind the isthmus and is similar in width throughout with a spanner-shaped termination (figures 1b,i–k,t and 2d,h,j).
Flanking the hind body region in OUMNH C.29613 (figure 2d,h,j) there are at least four paired sets of thin overlapping lamellae interpreted as gills. The outer edge of the outer right lamella is thickened, possibly representing the site of an (efferent) epibranchial canal. Two small, short, curved finger-like projections of unknown function originate from the body area between the seventh limb and the gills (evident on the right side in OUMNH C.29613; figure 2d,h,j). The posterior part of the body is poorly preserved in the holotype. The furca protrudes from the carapace (figure 1a,b,g,i). Each furcal lamella preserves a row of at least three to four long curved claws, though the large size of the furca suggests that more were probably present. The preserved gut includes oesophagus and stomach (figures 1d and 2d). The gender of the specimens cannot be determined.
4. Discussion
Lateral eyes and a vermiform seventh limb occur only in myodocopid ostracodes, and gills are known only in the Cylindroleberididae, to which P. avibella is assigned. The morphology of most of the other limbs, including the presence of an epipod only on the second maxilla, is also compatible with that assignment, as is the large size. Furthermore, the seventh limb of P. avibella has an indented distal termination as that in Recent cylindroleberidids [33, fig .15], but the associated comb of fine bristles found in living representatives is not evident, there is no setate comb on the second maxilla, and the (poorly resolved) sixth limb is not flap-like. Pauline avibella differs from almost all cylindroleberidids in lacking a rostrum and rostral incisure and in having a long, well-defined adductorial sulcus. Specimen OUMNH C.29613 differs from the holotype in being larger and preserving gills; other, minor differences in lobation (e.g. more acute posterior wing-like structures) and valve ornament may represent ontogenetic variation. At around 1 cm long, the larger specimen is comparable in size to adults of many Recent and fossil myodocopid species, which range up to about 30 mm in length [5,9,34].
Supposed myodocopids recorded from the fossil record number many tens of species, of which some 20 have been assigned to the Cylindroleberididae; however, only six, including five cylindroleberidids, are confirmed as myodocopids based on preserved soft-parts ([9,10] and references herein). Of the six species, the carapace morphology of the two post-Palaeozoic forms, the cylindroleberidids Triadocypris spitzbergensis Weitschat ([35] Triassic) and Juraleberis jubata Vannier & Siveter ([36] Jurassic), accords with that of Recent myodocopids. The overall carapace morphology of P. avibella, with its sub-oblong shape, long straight dorsal margin, well-developed lobation and long, prominent adductorial sulcus is unlike that of other extant or supposed fossil myodocopids. It may represent the shell form of the cylindroleberidid stem, in which a rostrum and rostral incisure had yet to develop, or perhaps belong more basally and indicate that the cylindroleberidid soft-anatomy is relatively primitive. The carapace morphology of P. avibella in many respects recalls that of certain halocypridid Halocyprida myodocopes, such as Discoconchoecia (figure 1m) [37,38], a group unknown from the fossil record. However, the limb morphology and lateral eyes of P. avibella clearly place it within the Myodocopida and not the Halocyprida. A marked adductorial sulcus and prominent lobation are features typical of Palaeocopida [39], the diverse and abundant Palaeozoic ostracod group that is known from carapaces alone and comprises some 500 genera (D. J. Siveter 2008, unpublished data), although the majority of palaeocopes characteristically have an adventral structure(s), which is lacking in P. avibella. P. avibella and the three other known Palaeozoic myodocope species with soft-parts, Nymphatelina gravida, Nasunaris inflata and Colymbosathon ecplecticos (figure 2a, k–m; [18–20]), show a diversity of carapace morphologies even though their soft-parts indicate that all four are myodocopids and all but Nymphatelina are cylindroleberidids (these assignments receive independent support from molecular/morphological analysis of fossil and Recent ostracods [40]). This confirms that carapace morphology alone is an inadequate basis for suprageneric assignment of (Recent) myodocopes [41]. The discovery of P. avibella with a soft-part morphology that is at odds with the appearance of the carapace encourages caution in interpreting the affinities of Palaeozoic ostracods based merely on shell morphology [1,19,20]. The current taxonomic assignment of many fossil ostracods, especially Palaeozoic forms, may be flawed.
The depositional setting of the Herefordshire Lagerstätte within the Welsh Basin included water depths of 150–200 m [21], a niche favoured by Silurian myodocopids [7,34]. The two powerful anterior limbs and especially the large basipod of the second antenna suggest that P. avibella was a swimmer. Its limited known distribution and its morphology suggest a nektobenthic lifestyle, similar to most Recent myodocopids [42]. In lacking a setate comb on the second maxilla, P. avibella apparently differed from living cylindroleberidids in not being a filter (comb)-feeder. With mandibles and first maxillae bearing endites and a furca with well-developed claws, P. avibella would have been capable of a feeding strategy similar to other Recent myodocopids, which scavenge, prey or are detritivores on or near the substrate [42]. P. avibella, a very large ostracod with gills and epipods, likely had a respiratory–circulatory system with a heart similar to that of living cylindroleberidids [43,44].
Acknowledgements
We thank the Natural Environment Research Council (grant no. NE/F017227/1) and English Nature for support; Carolyn Lewis for technical work; Robin Smith, John Whittaker and Hotaruika Museum, Toyama Prefecture, Japan, for Recent material; Mark Williams, David Horne and an anonymous reviewer for comments on the manuscript; T. H. Oakley and co-workers for a preprint of their manuscript; David Edwards and the late Roy Fenn for general assistance.
References
- 1.Siveter DJ. 2008. Ostracods in the Palaeozoic? Senckenbergiana Lethaea 88, 1–9 10.1007/BF03043973 (doi:10.1007/BF03043973) [DOI] [Google Scholar]
- 2.Williams M, Siveter DJ, Salas MJ, Vannier J, Popov LE, Ghobadi Pour M. 2008. The earliest ostracods: the geological evidence. Senckenbergiana Lethaea 88, 11–21 10.1007/BF03043974 (doi:10.1007/BF03043974) [DOI] [Google Scholar]
- 3.Hou X-G, Williams M, Siveter DJ, Siveter DJ, Aldridge RJ. 2010. Soft-part anatomy of the Early Cambrian bivalved arthropods Kunyangella and Kunmingella: significance for the phylogenetic relationships of Bradoriida. Proc. R. Soc. B 277, 1835–1841 10.1098/rspb.2009.2194 (doi:10.1098/rspb.2009.2194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horne DJ. 2003. Key events in the ecological radiation of the Ostracoda. In Bridging the gap: trends in the ostracode biological and geological sciences, vol. 9 (eds Park LE, Smith AJ.), pp. 181–201 (Paleontol. Soc. Pap) New Haven, CT: The Paleontological Society. [Google Scholar]
- 5.Horne DJ, Cohen A, Martens K. 2002. Taxonomy, morphology and biology of Quaternary and living Ostracoda. In The Ostracoda: applications in Quaternary Research, vol. 131 (eds Holmes JA, Chivas A.), pp. 5–36 (Geophys. Monogr) Washington, DC: American Geophysical Union. [Google Scholar]
- 6.Siveter DJ. 1984. Habitats and modes of life of Silurian ostracodes. In The autecology of Silurian organisms, vol. 32 (eds Bassett MG, Lawson JD.), pp. 71–85 (Spec. Pap. Palaeont) London, UK: The Palaeontological Association. [Google Scholar]
- 7.Siveter DJ, Vannier JMC, Palmer D. 1991. Silurian myodocopes: pioneer pelagic ostracodes and the chronology of an ecological shift. J. Micropalaeontol. 10, 151–173 10.1144/jm.10.2.151 (doi:10.1144/jm.10.2.151) [DOI] [Google Scholar]
- 8.Siveter DJ, Vannier JMC. 1990. The Silurian myodocope ostracode Entomozoe from the Pentland Hills, Scotland; its taxonomic, ecological and phylogenetic significance and the affinity of the bolbozoid myodocopes. Trans. Roy. Soc. Edinb. Earth Sci. 81, 45–67 10.1017/S0263593300005125 (doi:10.1017/S0263593300005125) [DOI] [Google Scholar]
- 9.Kornicker LS, Sohn IG. 2000. Myodocopid Ostracoda from the Late Permian of Greece and a basic classification for Paleozoic and Mesozoic Myodocopida. Smithson Contrib. Paleobiol. 91, 1–33 [Google Scholar]
- 10.Kornicker LS, Van Bakel BWM, Fraaiji RHB, Jagt JWM. 2006. Revision of Mesozoic Myodocopina (Ostracoda) and a new genus and species, Mesoleberis hollandica, from the Upper Cretaceous of Belgium and The Netherlands. Zootaxa 1246, 15–54 [Google Scholar]
- 11.Olempska E, Belka Z. 2010. Hydrothermal vent myodocopid ostracods from the Eifelian (Middle Devonian) of southern Morocco. Geobios 43, 519–529 10.1016/j.geobios.2010.06.001 (doi:10.1016/j.geobios.2010.06.001) [DOI] [Google Scholar]
- 12.Gabbott SE, Siveter DJ, Aldridge RJ, Theron JN. 2003. The earliest myodocopes: ostracodes from the late Ordovician Soom Shale Lagerstätte of South Africa. Lethaia 36, 151–160 10.1080/00241160310004620 (doi:10.1080/00241160310004620) [DOI] [Google Scholar]
- 13.Smith RJ. 2000. Morphology and ontogeny of Cretaceous ostracods with preserved appendages from Brazil. Palaeontology 43, 63–98 10.1111/1475-4983.00119 (doi:10.1111/1475-4983.00119) [DOI] [Google Scholar]
- 14.Wilkinson IP, Wilby PR, Williams M, Siveter DJ, Page AA, Leggitt L, Riley DA. 2010. Exceptionally preserved ostracods from a Middle Miocene palaeolake, California, USA. J. Geol. Soc. Lond. 167, 817–825 10.1144/0016-76492009-178 (doi:10.1144/0016-76492009-178) [DOI] [Google Scholar]
- 15.Harvey THP, Velez MI, Butterfield NJ. 2012. Exceptionally preserved crustaceans from western Canada reveal a cryptic Cambrian radiation. Proc. Natl Acad. Sci. USA 109, 1589–1594 10.1073/pnas.1115244109 (doi:10.1073/pnas.1115244109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olempska E, Horne DJ, Szaniawski H. 2012. First record of preserved soft parts in a Palaeozoic podocopid (Metacopina) ostracod, Cytherellina submagna: phylogenetic implications. Proc. R. Soc. B 279, 564–570 10.1098/rspb.2011.0943 (doi:10.1098/rspb.2011.0943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller AKJ. 1979. Body appendages of Paleozoic ostracodes. In Proc. 7th Int. Symp. on Ostracodes, pp. 5–9 Belgrade, Yugoslavia: Serbian Geological Society [Google Scholar]
- 18.Siveter DJ, Sutton MD, Briggs DEG, Siveter DJ. 2003. An ostracode crustacean with soft parts from the Lower Silurian. Science 302, 1749–1751 10.1126/science.1091376 (doi:10.1126/science.1091376) [DOI] [PubMed] [Google Scholar]
- 19.Siveter DJ, Siveter DJ, Sutton MD, Briggs DEG. 2007. Brood care in a Silurian ostracod. Proc. R. Soc. B 274, 465–469 10.1098/rspb.2006.3756 (doi:10.1098/rspb.2006.3756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siveter DJ, Briggs DEG, Siveter DJ, Sutton MD. 2010. An exceptionally preserved myodocopid ostracod from the Silurian of Herefordshire, UK. Proc. R. Soc. B 277, 1539–1544 10.1098/rspb.2009.2122 (doi:10.1098/rspb.2009.2122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briggs DEG, Siveter DJ, Siveter DJ. 1996. Soft-bodied fossils from a Silurian volcaniclastic deposit. Nature 382, 248–250 10.1038/382248a0 (doi:10.1038/382248a0) [DOI] [Google Scholar]
- 22.Briggs DEG, Siveter DJ, Siveter DJ, Sutton MD. 2008. Virtual fossils from 425 million-year-old volcanic ash. Am. Sci. 96, 474–481 10.1511/2008.75.474 (doi:10.1511/2008.75.474) [DOI] [Google Scholar]
- 23.Sutton MD, Briggs DEG, Siveter DJ, Siveter DJ. 2011. A soft-bodied lophophorate from the Silurian of England. Biol. Lett. 7, 146–149 10.1098/rsbl.2010.0540 (doi:10.1098/rsbl.2010.0540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briggs DEG, Siveter DJ, Siveter DJ, Sutton MD, Garwood RJ, Legg D. 2012. A Silurian horseshoe crab illuminates the evolution of chelicerate limbs. Proc. Natl Acad. Sci. USA 109, 15 702–15 705 10.1073/pnas.1205875109 (doi:10.1073/pnas.1205875109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton MD, Briggs DEG, Siveter DJ, Siveter DJ, Sigwart JD. 2012. A Silurian armoured aplacophoran: implications for molluscan phylogeny. Nature 490, 94–97 10.1038/nature11328 (doi:10.1038/nature11328) [DOI] [PubMed] [Google Scholar]
- 26.Orr PJ, Briggs DEG, Siveter DJ, Siveter DJ. 2000. Three-dimensional preservation of a non-biomineralized arthropod in concretions in Silurian volcaniclastic rocks from Herefordshire, England. J. Geol. Soc. Lond. 157, 173–186 10.1144/jgs.157.1.173 (doi:10.1144/jgs.157.1.173) [DOI] [Google Scholar]
- 27.Sutton MD, Briggs DEG, Siveter DJ, Siveter DJ. 2001. Methodologies for the visualization and reconstruction of three-dimensional fossils from the Silurian Herefordshire Lagerstätte. Palaeontol. Electron. 4, art. 2 (http://palaeo-electronica.org/2001_1/s2/issue1_01.htm). [Google Scholar]
- 28.Sutton MD, Garwood RJ, Siveter DJ, Siveter DJ. 2012. SPIERS and VAXML: a software toolkit for tomographic visualisation, and a format for virtual specimen exchange. Palaeontol. Electron. 15, 14 [Google Scholar]
- 29.Sars GO. 1866. Oversigt af Norges marine ostracoder. Norske Vidensk Akad. Forhandl 1865, 1–130 [Google Scholar]
- 30.Müller GW. 1906. Ostracoda. Die Ostracoden der Siboga-Expedition. In Siboga-Expeditie, Uitkomsten op Zoologisch, Botanisch, Oceanographisch en H. M. Siboga, Monographien, vol. 30, pp. 40 Leiden, The Netherlands: E. J. Brill [Google Scholar]
- 31.Rudjakov Yu. A. 1962. Ostracoda Myodocopa of the family Halocypridae from the north-west Pacific. Trudy Inst. Okeanol. Akad. Nauk SSSR 58, 172–201 [Google Scholar]
- 32.Boxshall GA. 1998. Comparative limb morphology in major crustacean groups: the coxa-basis joint in postmandibular limbs. In Arthropod Phylogeny (eds Fortey RA, Thomas RT.), pp. 155–167 London, UK: Chapman & Hall [Google Scholar]
- 33.Kornicker LS. 1981. Revision, distribution, ecology, and ontogeny of the ostracode Subfamily Cyclasteropinae (Myodocopina: Cylindroleberididae). Smithson Contrib. Zool. 319, 1–548 [Google Scholar]
- 34.Perrier V, Vannier J, Siveter DJ. 2011. Silurian bolbozoids and cypridinids (Myodocopa) from Europe: pioneer pelagic ostracods. Palaeontology 54, 1361–1391 10.1111/j.1475-4983.2011.01096.x (doi:10.1111/j.1475-4983.2011.01096.x) [DOI] [Google Scholar]
- 35.Weitschat W. 1983. Myodocopid ostracodes with preserved appendages from the Lower Triassic of Spitzbergen. Paläontologische Zeitschrift 57, 309–323 [Google Scholar]
- 36.Vannier J, Siveter DJ. 1996. On Juraleberis jubata gen. et sp. nov. Stereo Atlas Ostracod Shells, 22, 86–95 (for 1995) [Google Scholar]
- 37.Angel M. 1993. Pelagic Marine Ostracoda In Synopses of the British Fauna (New Series), vol. 48 (eds Kermack DM, Barnes RSK, Crothers JH.), pp. 239 London, UK: Linnean Society of London and The Estuarine and Coastal Sciences Association. Field Studies Council [Google Scholar]
- 38.Blachowiak-Samolyk K, Angel M. 2011. An Atlas of Southern Ocean Ostracoda. Southampton, UK: Southampton Oceanography centre; See http://deep.iopan.gda.pl/ostracoda. [Google Scholar]
- 39.Vannier JMC, Siveter DJ, Schallreuter REL. 1989. The composition and palaeogeographical significance of the Ordovician ostracode faunas of Southern Britain, Baltoscandia and Ibero-Amorica. Palaeontology 32, 163–222 [Google Scholar]
- 40.Oakley TH, Wolfe JM, Lindgren AR, Zaharoff AK. 2012. Phylotranscriptomics to bring the understudied into the fold; monophyletic Ostracoda, fossil placement and pancrustacean phylogeny. Mol. Biol. Evol. (doi:10.1093/molbev/mss216). [DOI] [PubMed] [Google Scholar]
- 41.Cohen AC, Morin JG. 2003. Sexual morphology, reproduction and the evolution of bioluminescence in Ostracoda. In Bridging the gap: trends in the ostracode biological and geological sciences, vol. 9 (eds Park LE, Smith AJ.), pp. 37–70 Paleontol. Soc. Pap New Haven, CT: The Paleontological Society. [Google Scholar]
- 42.Vannier J, Abe K, Ikuta K. 1998. Feeding in myodocopid ostracods: functional morphology and laboratory observations from videos. Mar. Biol. 132, 391–408 10.1007/s002270050406 (doi:10.1007/s002270050406) [DOI] [Google Scholar]
- 43.Vannier J, Abe K, Ikuta K. 1996. Gills of cylindroleberid ostracodes exemplified by Leuroleberis surugaensis from Japan. J. Crust. Biol. 16, 453–468 10.2307/1548735 (doi:10.2307/1548735) [DOI] [Google Scholar]
- 44.Vannier J, Abe K. 1998. Size, body plan and respiration in the Ostracoda. Palaeontology 38, 843–873 [Google Scholar]