Abstract
Vertebrates that eavesdrop on heterospecific alarm calls must distinguish alarms from sounds that can safely be ignored, but the mechanisms for identifying heterospecific alarm calls are poorly understood. While vertebrates learn to identify heterospecific alarms through experience, some can also respond to unfamiliar alarm calls that are acoustically similar to conspecific alarm calls. We used synthetic calls to test the role of specific acoustic properties in alarm call identification by superb fairy-wrens, Malurus cyaneus. Individuals fled more often in response to synthetic calls with peak frequencies closer to those of conspecific calls, even if other acoustic features were dissimilar to that of fairy-wren calls. Further, they then spent more time in cover following calls that had both peak frequencies and frequency modulation rates closer to natural fairy-wren means. Thus, fairy-wrens use similarity in specific acoustic properties to identify alarms and adjust a two-stage antipredator response. Our study reveals how birds respond to heterospecific alarm calls without experience, and, together with previous work using playback of natural calls, shows that both acoustic similarity and learning are important for interspecific eavesdropping. More generally, this study reconciles contrasting views on the importance of alarm signal structure and learning in recognition of heterospecific alarms.
Keywords: alarm call, acoustic properties, eavesdropping, Malurus cyaneus, risk assessment, superb fairy-wren
1. Introduction
Alarm calling is a fast and effective means of signalling danger, and some animals not only respond to conspecific alarm calls but can also identify the alarm calls of other species. Interspecific eavesdropping on alarm calls occurs in most vertebrate taxa, including reptiles [1,2], birds [3–7] and mammals [8–10]. As well as increasing the likelihood that predators are detected, heterospecific alarm calls can provide early warnings of danger [3,11,12] and information complementing that conveyed by conspecific calls [12,13]. The ability to recognize heterospecific alarm calls can also allow eavesdropping species to reduce vigilance in the presence of heterospecifics, and consequently increase foraging efficiency [14] or the amount of time spent foraging [15,16].
Although the benefits of responding to heterospecific alarm calls are clear, the means by which vertebrates distinguish alarm calls from the many benign calls in their environment is poorly understood. Although responses to conspecific alarm calls often seem to be innate [17,18], both learning and acoustic similarity appear to be important for identifying heterospecific alarm calls [19,20]. Most studies suggest that responses to heterospecific alarm calls are acquired through experience and learning [21–23]. One study showed that golden-mantled squirrels, Spermophilus lateralis, acquired responses to a novel sound after being trained to associate the sound with the appearance of a model predator, demonstrating how antipredator responses could develop [24]. Thus, learning which heterospecific calls signal danger appears to be widespread. However, a few playback experiments show that birds respond to heterospecific alarm calls that they have had no opportunity to learn [19,25]. These responses to unfamiliar alarm calls might have been due to the heterospecific calls sharing acoustic properties with the conspecific calls of the species tested, consistent with the view that alarm calls share acoustic structures that prompt unlearned responses [26].
In addition to simply identifying heterospecific alarm calls, some eavesdropping species minimize the cost of reacting to alarms by tailoring responses according to the risk of immediate threat. The degree of risk represented by a heterospecific alarm call depends on: (i) the likelihood that the call indicates danger; (ii) whether the danger is relevant to the eavesdropping species; and (iii) the perceived urgency of the call. This risk can depend on both the calling species and type of alarm call. For example, New Holland honeyeaters, Phylidonyris novaehollandiae, respond to the alarm calls of one heterospecific that produces reliable information but usually ignore alarm calls of another species whose alarms are less reliably associated with danger to honeyeaters [27]. More subtly, red-breasted nuthatches, Sitta canadensis, mob more vigorously in response to mobbing alarm call variants of black-capped chickadees, Poecille atricapillus, that indicate more threatening predators than calls indicating less threatening predators [28]. In both examples, eavesdropping species respond more strongly to heterospecific calls indicating greater risk, a strategy that could allow them to maintain safety while minimizing unnecessary energy expenditure [28].
Superb fairy-wrens, Malurus cyaneus, provide a good model for examining the mechanism of heterospecific alarm call recognition because they appear to rely on both learning and acoustic structure. Fairy-wrens respond to playback of the aerial alarm calls of white-browed scrubwrens, Sericornis frontalis, and noisy miners, Manorina melanocephala, only in locations where these species are locally common, thus suggesting that they must learn to recognize these calls [20,22]. By contrast, fairy-wrens respond to unfamiliar heterospecific alarm calls that are acoustically similar to conspecific calls [19], showing that learning is not always necessary. The responses to unfamiliar calls appeared to be affected by similarity in specific acoustic properties—peak frequency and frequency modulation rate—rather than similarity in general acoustic structure. However, the use of a small set of natural calls with diverse characteristics made it difficult to conclude whether these specific acoustic properties were key features in call identification. Furthermore, the correlations between behavioural responses and acoustic features might depend on the sample of calls used, and could have been confounded by unmeasured differences, including those associated with phylogenetic relatedness [19].
We used calls synthesized on computer to test the role of specific acoustic properties in the identification of aerial alarm calls by superb fairy-wrens. Aerial alarm calls are a type of ‘flee call’ that signal the presence of fast-moving threats, and thus the need to respond rapidly by increasing vigilance or fleeing to cover to avoid danger [29]. We presented fairy-wrens with synthetic calls that varied systematically in peak frequency and frequency modulation rate, the acoustic properties that appear to affect fairy-wren responses to unfamiliar heterospecific alarm calls [19]. The synthetic calls encapsulated variation in these acoustic features found in natural heterospecific calls within three taxonomic families of Australian birds. By using synthetic calls rather than using natural calls, we were able to modify peak frequency and frequency modulation rate of calls while keeping other acoustic features constant, allowing us to investigate their particular effects on antipredator responses. A system for instantly identifying signals of imminent danger is crucial for maximizing an individual's chances of survival, and so it is important to discover the acoustic features that prompt responses to alarm calls. Such features could be important for learning responses to heterospecific calls as well as responding to unfamiliar calls that resemble familiar alarm calls.
2. Methods
(a). Study site and species
We studied superb fairy-wrens around Lake Burley Griffin and in the Australian National Botanic Gardens (35°16′ S, 149°6′ E) in Canberra, Australia. The superb fairy-wren is a small (9–12 g) passerine that breeds cooperatively and forages largely on the ground [30]. Groups defend their territories until the end of the breeding season, after which they join other groups to form flocks that often include other bird species [31].
Fairy-wrens signal the presence of airborne threats such as predatory birds by producing aerial alarm calls. These calls contain one or more elements, each consisting of a single band that is rapidly frequency-modulated about a constant carrier frequency (mean peak frequency: 9.1 kHz; frequency range: 8–11 kHz; figure 1 [4]). Fairy-wrens produce calls containing more elements when threats are closer, prompting stronger responses by conspecific and heterospecific listeners [33]. Conspecifics respond to multi-element calls by (i) immediately fleeing to cover, and (ii) then waiting in cover before re-emerging, with the amount of time spent in cover increasing with an increasing number of elements in the call [33].
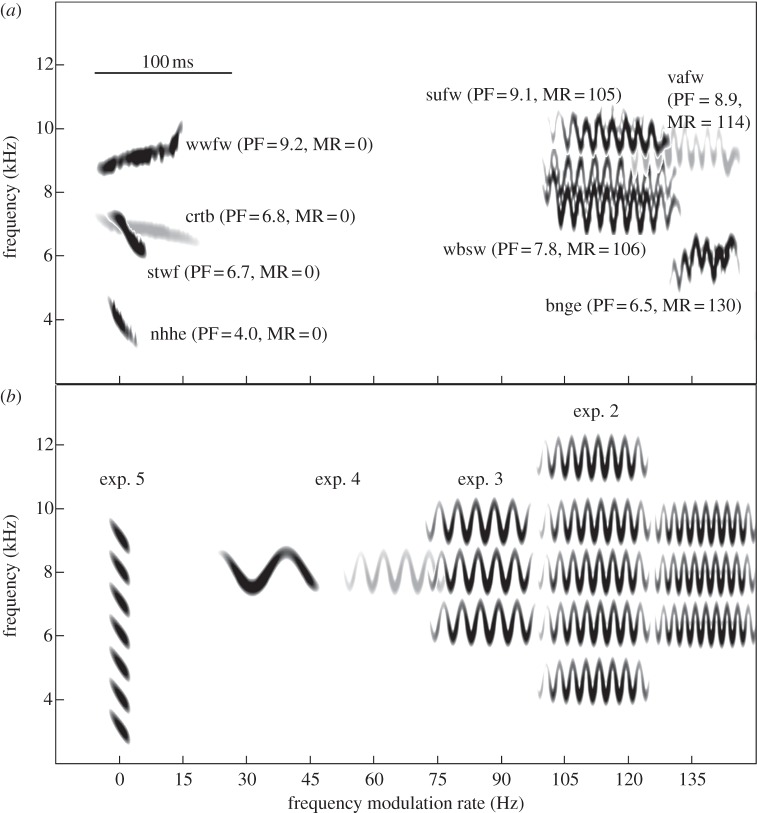
Figure 1.
Spectrograms of call elements. (a) Natural aerial alarm calls from species in three passerine families, including the Maluridae: superb fairy-wren (sufw), white-winged fairy-wren (wwfw), variegated fairy-wren (vafw); Acanthizidae: southern whiteface (stwf), chestnut-rumped thornbill (crtb), brown gerygone (bnge), white-browed scrubwren (wbsw); and Meliphagidae: New Holland honeyeater (nhhe). (b) Synthetic calls used in experiments 2 (4.1 and 11.1 kHz only), 3, 4 and 5. The mean peak frequency (PF, kHz) and mean frequency modulation rate (MR, Hz) is shown next to the natural call of each species [19]. The beginning of each element is aligned with the frequency modulation rate. Some elements are lighter in shade to distinguish calls that overlap. Spectrograms were produced in Raven Pro 1.3 [43] and set to Blackman window function, a temporal grid resolution of 0.295 ms with 94.9% overlap and a frequency grid resolution of 86.1 Hz.
(b). Playback experiments
We conducted five playback experiments to test how fairy-wrens respond to synthetic alarm calls differing systematically in their acoustic properties. Playbacks of natural calls suggest that the similarity of peak frequency and frequency modulation rate to conspecific calls are the key acoustic features that affect response (above). Therefore, in our first four experiments, we manipulated peak frequency, frequency modulation rate or both, creating synthetic calls that ranged from highly similar (mimicking superb fairy-wren calls) to highly dissimilar (comparable with an acoustically distinct heterospecific call; figure 1). To achieve this range of similarity, we used the ‘base properties’—element duration, amplitude envelope and constancy of carrier frequency—of superb fairy-wren alarm calls, and then synthesized calls with different peak frequencies and frequency modulation rates while holding base properties constant. In the fifth experiment, we tested whether peak frequency had the predicted effect on response when using synthetic calls with quite different base properties. These base properties were those of New Holland honeyeater aerial alarm calls, which have short elements that decline rapidly in frequency and lack frequency modulation. Together, the synthesized calls in these five experiments covered an ‘acoustic landscape’ [34] of aerial alarm calls occupied by species in the families Maluridae (fairy-wrens), Acanthizidae (scrubwrens, thornbills and allies) and Meliphagidae (honeyeaters), and therefore assess responses to heterospecific variation (figure 1).
Experiment 1 tested whether synthetic calls were suitable for investigating superb fairy-wren responses to aerial alarm calls. For synthetic calls to be suitable, a version mimicking natural fairy-wren calls should prompt the same response as natural calls themselves. We therefore presented fairy-wrens with a natural fairy-wren alarm call, a synthetic call mimicking fairy-wren alarm calls, and a synthetic call mimicking the piping or ‘bell’ contact call of crimson rosellas, Platycercus elegans [4,35]. Rosellas are harmless parrots common to the study site, and fairy-wrens do not respond to playback of natural rosella piping calls [19,33]. We used synthetic contact calls to control for any alarming properties of synthetic calls and to ensure that birds were not simply responding to any sound of similar amplitude. Each synthetic fairy-wren and rosella call was made unique by generating calls at slightly different peak frequencies within 1 s.d. of the mean for natural calls to ensure that responses were to this type of call and not to a single exemplar. We presented each set of three calls to 15 fairy-wren groups in June 2006.
Experiment 2 used synthetic calls to test the effect of varying peak frequency on fairy-wren responses, and thus whether peak frequency is important for alarm call identification. The mean peak frequency of fairy-wren aerial alarm calls is 9.1 kHz [4]. We presented fairy-wrens with synthetic calls with the base properties of superb fairy-wren alarms but generated at eight different peak frequencies: 4.1, 5.1, 6.1, 7.1, 8.1, 9.1, 10.1 and 11.1 kHz. Keeping base properties constant isolated the effect of peak frequency, while using fairy-wren base properties meant that our playbacks were ‘calibrated’ against a version of high similarity, mimicking superb fairy-wren calls. Natural calls composed from fairy-wren and white-browed scrubwren aerial alarm calls served as positive controls, and synthetic rosella contact calls once again served as a neutral control. We presented each of the eight synthetic calls 10 times and 10 unique replicates of each control call to 110 different fairy-wren groups in July and August 2006.
Experiment 3 investigated the combined effects of peak frequency and frequency modulation rate on antipredator behaviour [19]. While fairy-wren aerial alarm calls have high peak frequencies and frequency modulation rates, heterospecific alarm calls often have lower frequencies and vary widely in modulation rate, or are not modulated [19,27]. We presented fairy-wrens with nine synthetic calls generated with three measures for peak frequency (kHz): 9.1 (fairy-wren natural mean), 7.6 and 6.1; and three measures for frequency modulation rate (number of frequency cycles per second, Hz): 130, 105 (fairy-wren natural mean: 105.2 ± 8.0 s.d. [19]) and 75. Playbacks had base properties of fairy-wren calls, and natural fairy-wren alarm calls were used as a positive control. We presented each of the nine synthetic calls 10 times, and 10 unique natural fairy-wren alarm calls once to 100 different fairy-wren groups in October and November 2009.
Experiment 4 tested the response of fairy-wrens to synthetic calls with very low frequency modulation rates. As all synthetic calls with peak frequencies similar to conspecific calls prompted fairy-wrens to flee regardless of modulation rate (see §3), the modulation rates tested in experiment 3 might not have been low enough to reveal the effects of modulation rate, or to represent heterospecific alarm calls with low or no frequency modulation. Therefore, we conducted an additional experiment, this time using synthetic calls with very low modulation rates. Synthetic calls had a peak frequency of 9.1 kHz, modulation rates of 105, 60 and 20 Hz, and base properties of superb fairy-wren calls. We presented each synthetic call 10 times to 30 different fairy-wren groups in November and December 2009.
Experiment 5 tested the effect of peak frequency on fairy-wren responses to synthetic calls with base properties of New Holland honeyeater aerial alarm calls, which are completely different to those of superb fairy-wrens. Honeyeater alarm call elements have a low peak frequency (mean ± s.d. = 4.0 ± 0.23 kHz), much shorter duration (47.9 ± 6.2 ms) and a declining tone with no frequency modulation (5.5–3.1 kHz) [20]. Honeyeaters are common at the study site, where fairy-wrens respond strongly to honeyeater calls [20,27], almost certainly as a result of learning to recognize that they signal danger [20,22]. We generated synthetic calls with the base properties of honeyeater calls and peak frequencies of 3, 4, 5, 6, 7, 8 and 9 kHz (figure 1), with the 4 kHz version mimicking natural honeyeater calls. Calls made from natural honeyeater calls served as a positive control, and natural rosella calls served as a neutral control. We presented 108 calls to 12 fairy-wren groups in Canberra over six weeks in January and February 2011. We made a counterintuitive and therefore strong prediction based on the previous experiments using synthetic calls and our work on learned recognition in fairy-wrens [20,22]. We predicted that as peak frequency increased from a natural honeyeater 4 kHz, fairy-wren responses would first decline but then increase as peak frequencies approached the natural fairy-wren 9 kHz. Responses would first decline if peak frequency was used to recognize honeyeater calls, but then increase if peak frequency was used to classify unfamiliar calls as potential alarm calls. We therefore predicted a concave curvilinear relationship between peak frequency and response. We included 3 kHz calls to test whether responses would also decline below the natural 4 kHz.
(c). Generating playback calls
We composed multi-element natural alarm calls for playback using high-quality elements selected from recordings of fairy-wren, scrubwren and honeyeater aerial alarm calls (methods following Magrath et al. [4]; figure 1). Four-element fairy-wren and scrubwren calls were constructed by repeating a single element at an interval (time from the end of one element to the start of the next element) of 45 ms, and eight-element honeyeater calls by repeating an element at 60 ms intervals, a natural timing for these species. Each natural alarm call was composed using a unique element to include natural variation and increase external validity. We filtered out all sound below 4 kHz in fairy-wren and scrubwren calls, and all sound below 2 kHz in honeyeater calls. Multi-element aerial alarm calls represent urgent danger, and playback of multi-element conspecific alarm calls almost always provokes fairy-wrens to immediately flee (98 of 99 trials; data from [4,19,20,27,33]). Using multi-element alarm calls therefore results in unambiguous responses.
We generated synthetic calls using Audition v. 3.0 (Adobe Systems Inc.), with base properties taken from mean values of fairy-wren (experiments 1–4) or honeyeater (experiment 5) aerial alarm calls. Synthetic calls with the base properties of fairy-wren calls were produced by generating a pure tone with a specified frequency and frequency modulation rate, which were then subjected to a fast Fourier transformation filter to mimic the frequency spectrum of natural calls. Next, an amplitude envelope was applied to the tone to create a pattern of increasing and then decreasing amplitude. In the light of additional data on mean natural properties, synthetic calls in experiments 3 and 4 had a slightly shorter duration than those in experiments 1 and 2 (90 versus 104 ms). Synthetic calls based on honeyeater calls were produced by generating a 49 ms pure tone with a descending frequency, applying an amplitude envelope of increasing then decreasing amplitude. Like the natural alarm calls, multi-element synthetic calls were constructed by repeating the same element.
(d). Broadcasting playback calls
Playback sounds were uncompressed wave files played from a Sony CD Walkman D-EJ751 in experiments 1 and 2, and from a Roland Edirol R-09 HR solid-state playback and recording device (20–40 000 Hz) in experiments 3–5. Calls were broadcast via a Kemo Electronics integrated amplifier (20–25 000 Hz) and a Response Dome Tweeter speaker (1500–20 000 Hz), which were mounted around the observer's waist. Playback call amplitude was measured in Raven Pro v. 1.3 and calibrated against a tone that had its amplitude determined using a sound level meter. To ensure that calls were audible, we presented calls at an element amplitude of 57 dB at 8 m, which is around 1 s.d. above the mean amplitude for superb fairy-wren aerial alarm calls [4], and a level at which they always respond to conspecific alarm calls.
Calls within a set of treatments were presented in a predetermined random order, and each set completed before the next set was started. Experiments 2–4 followed an independent design in which each treatment was presented to a different group and focal bird. In experiments 1 and 5, each fairy-wren group was presented with a complete set of calls, and in experiment 5, playbacks were carried out only on territories where honeyeaters had recently been observed, to ensure fairy-wren familiarity with their calls. We used the same playback procedure as a study that tested fairy-wren responses to playback of unfamiliar heterospecific alarm calls [19]. We presented calls from a distance of around 8 m from the focal bird and recorded whether it immediately scanned for danger or fled to cover. If it fled to cover, the duration of time spent in cover (s) was recorded, if possible, but capped at 60 s (experiments 3–5). Fairy-wrens in the botanic gardens were identified by coloured leg bands, and groups living around the lake were distinguished by location.
(e). Statistical analyses
Experiment 1 was based on a simple matched design, so we used a Cochran's Q test to assess whether immediate response (stay or flee) depended on playback type. In experiments 2–4, we used generalized linear models (GLMs) with binomial error to analyse immediate responses, and used GLMs with a normal distribution to analyse the time spent in cover for those birds that fled to cover. The acoustic properties modified in each experiment were fitted as explanatory variables and tested using the Wald statistic. We then removed non-significant terms one by one until only significant effects remained. Fairy-wren responses to synthetic calls in experiment 5 were analysed using generalized linear mixed models (GLMMs; family binomial with logit link) with group identity as the random term, and binomial response as the dependent variable. We tested our specific prediction that response would decline and then increase from 4 to 9 kHz by using peak frequency as an ordered factor, and testing for linear, quadratic and cubic effects. Analyses were complicated by the fact that responses were uniform (all or none responded) in some categories, leading to incorrect estimates and standard errors [36,37]. To overcome this problem, we created a single random exception in each category where responses were uniform; for example, we changed a single response to ‘stay’ in categories in which all fled, and a single response to ‘flee’ in categories in which all stayed. This approach led to a conservative analysis, where it was harder to detect significant patterns because we decreased variation among treatments. Analyses of experiments 1–4 were carried out in Genstat v. 13 (VSN International), and the analysis of experiment 5 was carried out in R v. 2.15.1 using the lmer function in package lme4 [38,39]. Significance levels were set at p < 0.05. Data are available at the Dryad repository: doi:10.5061/dryad.vp5b7.
3. Results
(a). Experiment 1: response to natural versus synthetic calls
Fairy-wrens responded identically to natural alarm calls and synthetic calls designed to mimic them, showing that the use of synthetic calls was appropriate for subsequent experiments. Birds immediately fled to all natural and synthetic alarm calls, but did not show any response to the synthetic rosella calls (n = 15 experiments; Cochran's Q = 20.0, d.f. = 2, p < 0.001).
(b). Experiment 2: response to variation in peak frequency
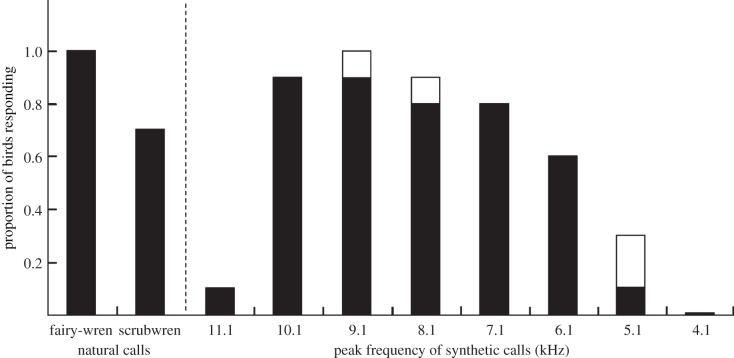
Fairy-wrens varied their responses to novel synthetic calls of different peak frequencies, their probability of fleeing increasing as the frequency of synthetic calls approached that of conspecific alarm calls (GLM: 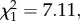 p = 0.008; figure 2). Flee rate gradually increased with increasing peak frequency up to 9 kHz and then rapidly declined at 11 kHz.
p = 0.008; figure 2). Flee rate gradually increased with increasing peak frequency up to 9 kHz and then rapidly declined at 11 kHz.
Figure 2.
The results of experiment 2, showing the proportion of fairy-wrens immediately responding to synthetic calls varying in peak frequency (raw data; n = 10 for each column). Natural alarm calls of fairy-wrens (mean peak frequency: 9.1 kHz) and white-browed scrubwrens (mean peak frequency: 7.1 kHz) served as positive controls. Synthetic calls had fairy-wren base properties (see text). Synthetic crimson rosella contact calls, the neutral control, did not elicit any response and are not represented here. Black bars, fleeing; white bars, scanning.
(c). Experiment 3: response to variation in both peak frequency and frequency modulation rate
Fairy-wrens responded more strongly to novel synthetic calls with peak frequencies and modulation rates that were more similar to conspecific calls. As in experiment 2, fairy-wrens had a greater probability of fleeing to cover after synthetic calls with peak frequencies of 9.1 kHz rather than lower frequencies (GLM: 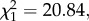 p < 0.001; figure 3a). The amount of time birds spent in cover after fleeing also increased as peak frequency approached the natural mean (GLM:
p < 0.001; figure 3a). The amount of time birds spent in cover after fleeing also increased as peak frequency approached the natural mean (GLM: 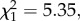 p = 0.011; figure 3b). A comparison of responses with similar versus different modulation rates showed that birds had a higher probability of fleeing to synthetic calls with similar modulation rates (105 versus 75 and 130 Hz; two-sample binomial test: p < 0.001). There was also a trend for birds to spend more time in cover in response to synthetic calls with frequency modulation rates similar to conspecific calls (0.15 ± 0.08 s.e.; GLM prior to dropping modulation rate:
p = 0.011; figure 3b). A comparison of responses with similar versus different modulation rates showed that birds had a higher probability of fleeing to synthetic calls with similar modulation rates (105 versus 75 and 130 Hz; two-sample binomial test: p < 0.001). There was also a trend for birds to spend more time in cover in response to synthetic calls with frequency modulation rates similar to conspecific calls (0.15 ± 0.08 s.e.; GLM prior to dropping modulation rate: 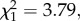 p = 0.057). We did not detect interactions between peak frequency and modulation rate in their effects on the proportion of birds fleeing (GLM:
p = 0.057). We did not detect interactions between peak frequency and modulation rate in their effects on the proportion of birds fleeing (GLM: 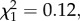 p = 0.726) or on time in cover (GLM:
p = 0.726) or on time in cover (GLM: 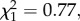 p = 0.385).
p = 0.385).
Figure 3.
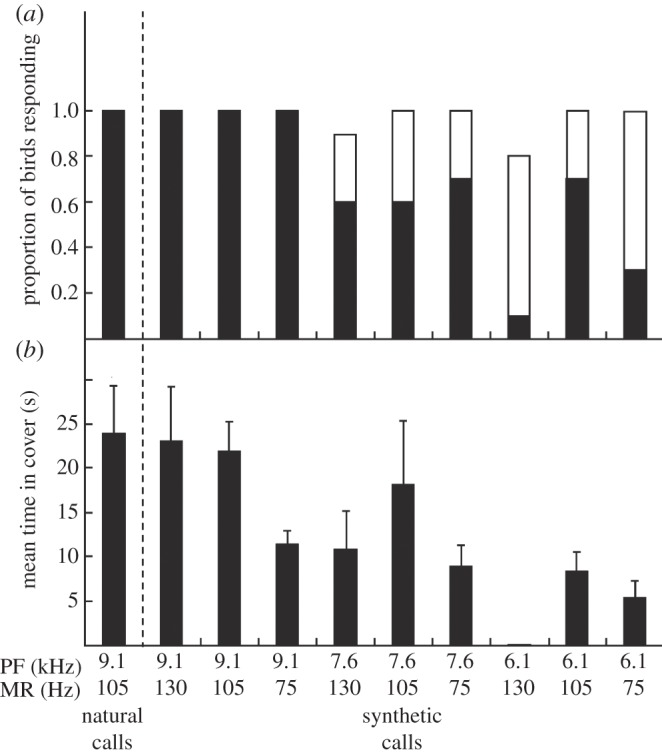
The results of experiment 3, showing the immediate response of fairy-wrens to synthetic aerial alarm calls varying in both peak frequency (PF) and frequency modulation rate (MR; n = 10 playbacks in each column; raw data shown): (a) the proportion of focal birds fleeing to cover or scanning; and (b) the observed mean (±s.e.) duration (s) the focal bird spent in cover before re-emerging. Natural fairy-wren alarm calls served as a positive control; synthetic calls had fairy-wren base properties (see text). Black bars, fleeing; white bars, scanning.
(d). Experiment 4: response to very low frequency modulation rates
The response of fairy-wrens to synthetic calls with the peak frequency of fairy-wren calls but very low modulation rates showed that modulation rate primarily affected time in cover rather than the decision to flee. Birds spent more time in cover after fleeing synthetic calls with higher modulation rates (GLM: 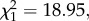 p = 0.001), but modulation rate did not affect the probability that birds fled to cover (GLM:
p = 0.001), but modulation rate did not affect the probability that birds fled to cover (GLM: 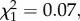 p = 0.787; figure 4). However, there was a trend for fairy-wrens to flee less often in response to calls with low modulation rates (20 and 60 Hz) than to calls with the modulation rate of conspecific calls (105 Hz; two-sample binomial test: p = 0.053).
p = 0.787; figure 4). However, there was a trend for fairy-wrens to flee less often in response to calls with low modulation rates (20 and 60 Hz) than to calls with the modulation rate of conspecific calls (105 Hz; two-sample binomial test: p = 0.053).
Figure 4.
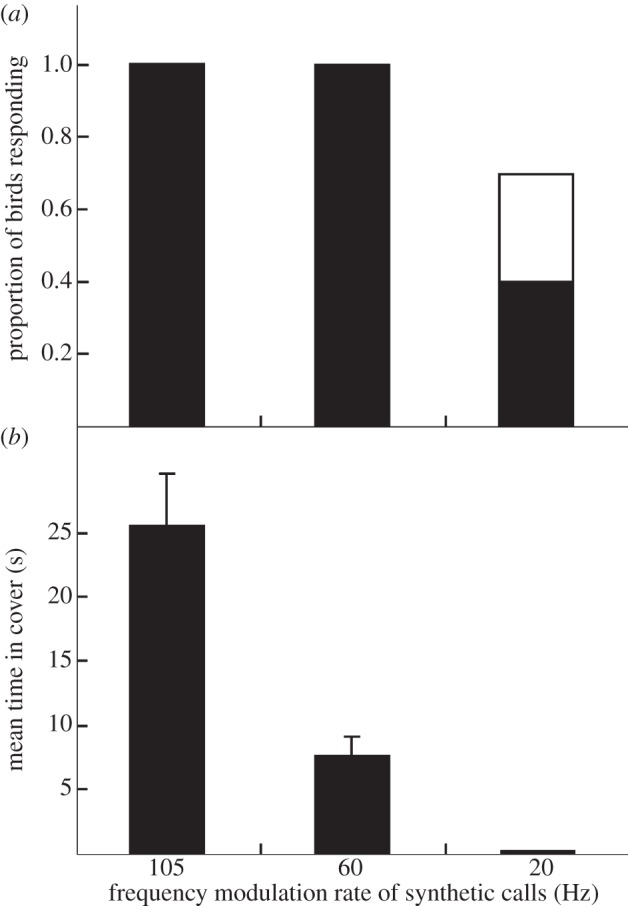
The results of experiment 4, showing the immediate response of fairy-wrens to synthetic aerial alarm calls with very low frequency modulation rates (n = 10 for each column; raw data shown): (a) the proportion of focal birds fleeing to cover or scanning; and (b) the mean (±s.e.) duration (s) the focal bird spent in cover before re-emerging. Synthetic calls had fairy-wren base properties (see text). Black bars, fleeing; white bars, scanning.
(e). Experiment 5: response to variation in peak frequency using honeyeater base characteristics
Peak frequency determined fairy-wren responses to synthetic calls in the nonlinear manner predicted. Fairy-wrens responded to synthetic calls with peak frequencies similar either to honeyeater calls (4 kHz) or fairy-wren calls (9 kHz), but showed little response to calls with peak frequencies in between, producing the predicted concave nonlinear pattern of responses (figure 5; GLMM: flee or stay: linear term, z = 1.83, p = 0.07; quadratic term, z = 4.05, p = 0.0005; higher-order terms, p > 0.2; no response versus any response: linear term, z = 1.02, p = 0.3; quadratic term, z = 3.68, p = 0.0002; cubic term, z = 3.56, p = 0.0004; higher-order terms, p > 0.9). The significant cubic term results from a plateauing of any response above 8 kHz (figure 5). Synthetic calls with the peak frequency of honeyeater calls (4 kHz) prompted immediate flight by all birds, identical to the response to natural honeyeater calls. They also spent similar time in cover (t-test: t = −0.069, d.f. = 22, p = 0.50). Synthetic calls with the peak frequency of fairy-wren calls (9 kHz) provoked responses by 9/12 birds, including 5/12 that fled to cover. Synthetic calls with peak frequencies deviating only 1 kHz from the mean of honeyeater and fairy-wren calls caused a dramatic decline in response: only 3/12 fled to 3 kHz calls and 0/12 fled to 5 kHz calls, while only 1/12 fled to 8 kHz, although 10/12 of these calls provoked scanning.
Figure 5.
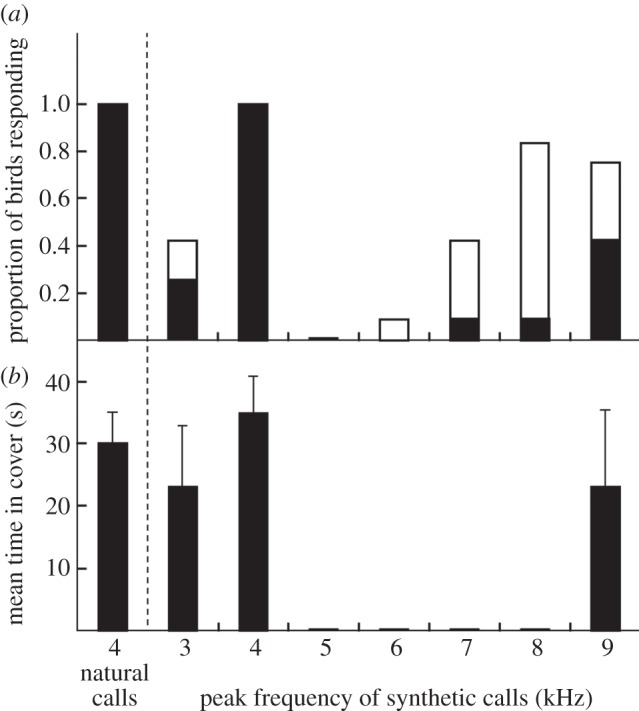
The results of experiment 5, showing the immediate response of superb fairy-wrens to natural and synthetic calls with the base properties of New Holland honeyeater alarm calls and varying in peak frequency (n = 12 for each column; raw data shown): (a) the proportion of focal birds fleeing to cover or scanning; and (b) the mean (±s.e.) duration (s) the focal bird spent in cover before re-emerging. Natural crimson rosella contact calls, the neutral control, did not elicit any response and are not shown here. Black bars, fleeing; white bars, scanning.
4. Discussion
Our study shows that superb fairy-wrens identify alarm calls and adjust antipredator responses by comparing the specific acoustic properties of sounds with conspecific alarm calls and familiar heterospecific alarm calls. Fairy-wrens had a higher probability of fleeing in response to synthetic calls with peak frequencies more similar to conspecific calls, regardless of basic acoustic structure, and then spent more time in cover following calls with both peak frequencies and modulation rates closer to that of conspecific calls. Similarity in peak frequency was also critical in recognition of the alarm calls of a familiar heterospecific. Thus, peak frequency is likely to play a key role in the identification of both unfamiliar and familiar alarm calls. Our results complement studies suggesting that fairy-wrens need to learn to recognize acoustically different heterospecific calls [20,22], by supporting the long-standing proposal that acoustic similarity can allow birds to eavesdrop on the alarm calls of other species without requiring learning [40].
Peak frequency appears to be a key acoustic feature used for initial alarm call response. Synthetic calls with peak frequencies closer to fairy-wren calls increased the likelihood that birds immediately fled to cover, regardless of their basic acoustic structure (experiments 2–5), and suggests that individuals generalize their response from conspecific alarm calls [41]. These results are consistent with a playback experiment that tested the response of fairy-wrens to the unfamiliar alarm calls of eight heterospecific passerines [19]. Birds usually fled only to playback of heterospecific alarm calls with peak frequencies greater than 8 kHz, and therefore similar to conspecific alarms. Relative to most bird species studied, the aerial alarm calls of fairy-wrens have a very high peak frequency, and the highest among the calls collected from birds in our study site. The lack of alarm calls reaching frequencies higher than those of fairy-wren calls might explain why no fairy-wrens responded even by scanning to presentation of the 11 kHz synthetic calls. Alternatively, sound at this frequency might exceed the limit of sensitive fairy-wren hearing. We suggest that peak frequency may be used for initial call classification because it is more resistant to environmental degradation than fine acoustic structure, and because birds are in general highly sensitive to variation in frequency [42]. Generalizing from conspecific calls to unfamiliar calls with similar peak frequencies is likely to be advantageous in areas where fairy-wren species live sympatrically with congeners and other species that have similar high-frequency alarm calls.
The response of fairy-wrens to synthetic calls with the base properties of honeyeater calls demonstrates that similarity to conspecific peak frequency is important even for novel alarm calls that are otherwise very different from fairy-wren calls. Fairy-wrens responded more strongly as peak frequency rose from 5 to 9 kHz, the peak frequency of fairy-wren alarms, suggesting generalization from conspecific calls. Those that fled to 9 kHz synthetic calls also spent the same amount of time in cover as fairy-wrens that fled in response to conspecific calls in experiment 3 (t-test: t = 0.12, d.f. = 12, p = 0.91). However, not all birds fled to synthetic calls with the peak frequency of conspecific calls, which shows that other acoustic features, or a combination of features, also affected the decision to flee. The relatively strong response of fairy-wrens to synthetic calls with a peak frequency of 8 kHz might have been due to the base acoustic structure of honeyeater alarm call elements, which descends from 1 kHz above to 1 kHz below the peak frequency (figure 1). These elements therefore started at 9 kHz, the peak frequency of superb fairy-wren calls, which could explain almost universal vigilance, but only one bird fleeing.
In addition to responding more strongly to novel alarm calls with peak frequencies approaching their own, fairy-wrens also responded strongly to synthetic calls of similar frequency to honeyeater calls, thereby producing the predicted curvilinear relationship between peak frequency and response. We suggest the response to low-frequency alarm calls is a result of learning. Across three studies including the present study, fairy-wrens have fled to 100 per cent of natural honeyeater alarm call playbacks [20,27]. This is despite fairy-wrens typically not responding to unfamiliar heterospecific alarm calls with peak frequencies lower than 7 kHz [19], and never responding to 4 kHz synthetic calls with the base properties of superb fairy-wren alarms (experiment 2). Additional evidence for learned recognition by fairy-wrens includes responding to the aerial alarm calls of noisy miners, another honeyeater with low-frequency alarm calls that lack frequency modulation, only in areas where they live closely with noisy miners [22]. Furthermore, fairy-wrens usually scanned rather than fleeing in response to honeyeater calls played in reverse (ascending frequency rather than descending frequency), even though reversed calls are similar in most acoustic properties, including peak frequency [20]. This suggests that fairy-wrens learn to recognize the specific features of honeyeater alarm calls, rather than merely responding to some general acoustic feature of alarm calls. Having learnt the specific features of these heterospecific calls, they then generalize, but with weaker responses, to some calls of similar frequency but different structure (calls played in reverse [20]) and calls of different peak frequency but similar structure (3 kHz synthetic calls with base properties of honeyeaters; figure 5). Overall, fairy-wrens responded to novel alarm calls at least partly according to their similarity to conspecific or familiar heterospecific alarm calls, suggesting both innate and learned generalization.
The asymmetrical response of fairy-wrens to synthetic calls with peak frequencies above and below the mean peak frequency of fairy-wren calls might be a consequence of fairy-wrens learning to respond to heterospecific alarms that are similar to conspecific alarms but lower in frequency. Fairy-wrens usually fled in response to synthetic calls with a peak frequency of 6.1 and 7.1 kHz (figure 2), peak frequencies deviating more than 1 kHz below the mean of conspecific calls but within 1 kHz of the mean peak frequency of scrubwren calls (mean ± s.d. = 7.2 ± 0.39 [4]). In Canberra, where scrubwrens are common, fairy-wrens flee to playback of scrubwren aerial alarm calls [4,20,27], and respond appropriately to the risk-based information conveyed [33]. Yet despite scrubwren alarm calls sharing several acoustic features with fairy-wren calls, fairy-wrens nonetheless appear to require experience to recognize scrubwren alarm calls [20]. Fairy-wrens in our study site might therefore have developed a ‘learning-based bias’ as a result of generalizing responses from learnt stimuli to novel stimuli that are similar [43].
Fairy-wrens used peak frequency to rapidly assess whether to flee to cover and then modified time in cover based on peak frequency and modulation rate. Although all birds fled in response to synthetic calls with peak frequencies similar to conspecific calls and modulation rates of 60 Hz or greater (figures 3 and 4), birds responding to 60 Hz synthetic calls spent on average less than half as much time in cover as those responding to synthetic calls with the modulation rate of conspecific calls (105 Hz; figure 4). Conservative two-stage responses ensure that fairy-wrens flee to calls that are likely to indicate immediate danger, while minimizing the amount of energy and time that is wasted by fleeing or remaining in cover after low-risk alarms or non-alarm calls. The only synthetic calls with conspecific peak frequencies that failed to provoke fleeing in all cases were those modulated at 20 Hz, which prompted only 40 per cent of birds to flee, none of which remained in cover (figure 4). The limited response to synthetic calls modulated at 20 Hz contrasts with the response of fairy-wrens to the unfamiliar alarm calls of white-winged fairy-wrens, Malurus leucopterus (figure 1), which have a peak frequency of 9.2 kHz and are not frequency-modulated, and yet provoked 83 per cent of fairy-wrens to flee and spend on average 6.9 s in cover [19]. The different results using natural versus synthetic calls suggest undetected acoustic differences between the calls of these two fairy-wren species, highlighting the importance of using synthetic calls to control for acoustic variation.
In conclusion, synthetic calls served as a vital tool for investigating the effects of specific acoustic properties on alarm call identification and risk-based responses by fairy-wrens. The synthetic calls included acoustic variation spanning much of the natural variation in calls produced by species in the Maluridae and related families, and therefore explored heterospecific calls in the acoustic landscape. The results show that similarity in acoustic structure to either conspecific or familiar heterospecific alarm calls can prompt responses to novel calls, suggesting that both innate and learned responses can be generalized to unfamiliar alarm calls. These findings suggest how individuals will respond to unfamiliar heterospecific calls based on key acoustic properties, and imply that birds will learn not to respond to any non-alarm calls that are acoustically similar to conspecific or familiar heterospecific alarm calls. Such predictions are important in understanding the relative importance of shared ancestry, evolutionary convergence and learning in heterospecific eavesdropping. We suggest that the ability to learn responses to heterospecific alarm calls and generalize from learned responses reduces selection on alarm call convergence, and therefore helps to explain alarm call diversity.
Acknowledgements
We thank Janet Gardner for providing recordings and Hwan-Jin Yoon for statistical advice. We are grateful to Anastasia Dalziell, Tonya Haff, Branislav Igic, Naomi Langmore and two anonymous referees for comments on the manuscript. This study was carried out with approval from the Australian National University Ethics Committee and under permits from the Australian National Botanic Gardens, Environment ACT, and the Australian Bird and Bat Banding Scheme. Funding was provided by a grant to R.D.M. from the Australian Research Council.
References
- 1.Vitousek MN, Adelman JS, Gregory NC, St Clair JJH. 2007. Heterospecific alarm call recognition in a non-vocal reptile. Biol. Lett. 3, 632–634 10.1098/rsbl.2007.0443 (doi:10.1098/rsbl.2007.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito R, Mori A. 2010. Vigilance against predators induced by eavesdropping on heterospecific alarm calls in a non-vocal lizard Oplurus cuvieri cuvieri (Reptilia: Iguania). Proc. R. Soc. B 277, 1275–1280 10.1098/rspb.2009.2047 (doi:10.1098/rspb.2009.2047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nuechterlein GL. 1981. ‘Information parasitism’ in mixed colonies of western grebes and Forster's terns. Anim. Behav. 29, 985–989 10.1016/S0003-3472(81)80051-6 (doi:10.1016/S0003-3472(81)80051-6) [DOI] [Google Scholar]
- 4.Magrath RD, Pitcher BJ, Gardner JL. 2007. A mutual understanding? Interspecific responses by birds to each other's aerial alarm calls. Behav. Ecol. 18, 944–951 10.1093/beheco/arm063 (doi:10.1093/beheco/arm063) [DOI] [Google Scholar]
- 5.Forsman JT, Monkkonen M. 2001. Responses by breeding birds to heterospecific song and mobbing call playbacks under varying predation risk. Anim. Behav. 62, 1067–1073 10.1006/anbe.2001.1856 (doi:10.1006/anbe.2001.1856) [DOI] [Google Scholar]
- 6.Griffin AS, Savani RS, Hausmanis K, Lefebvre L. 2005. Mixed-species aggregations in birds: zenaida doves, Zenaida aurita, respond to the alarm calls of carib grackles, Quiscalus lugubris. Anim. Behav. 70, 507–515 10.1016/j.anbehav.2004.11.023 (doi:10.1016/j.anbehav.2004.11.023) [DOI] [Google Scholar]
- 7.Hurd CR. 1996. Interspecific attraction to the mobbing calls of black-capped chickadees (Parus atricapillus). Behav. Ecol. Sociobiol. 38, 287–292 10.1007/s002650050244 (doi:10.1007/s002650050244) [DOI] [Google Scholar]
- 8.Shriner WM. 1998. Yellow-bellied marmot and golden-mantled ground squirrel responses to heterospecific alarm calls. Anim. Behav. 55, 529–536 10.1006/anbe.1997.0623 (doi:10.1006/anbe.1997.0623) [DOI] [PubMed] [Google Scholar]
- 9.Fichtel C. 2004. Reciprocal recognition of sifaka (Propithecus verreauxi verreauxi) and redfronted lemur (Eulemur fulvus rufus) alarm calls. Anim. Cogn. 7, 45–52 10.1007/s10071-003-0180-0 (doi:10.1007/s10071-003-0180-0) [DOI] [PubMed] [Google Scholar]
- 10.Lea AJ, Barrera JP, Tom LM, Blumstein DT. 2008. Heterospecific eavesdropping in a nonsocial species. Behav. Ecol. 19, 1041–1046 10.1093/beheco/arn064 (doi:10.1093/beheco/arn064) [DOI] [Google Scholar]
- 11.Burger J. 1984. Grebes nesting in gull colonies: protective associations and early warning. Am. Nat. 123, 327–337 10.1086/284207 (doi:10.1086/284207) [DOI] [Google Scholar]
- 12.Goodale E, Kotagama SW. 2005. Alarm calling in Sri Lankan mixed-species bird flocks. Auk 122, 108–120 10.1642/0004-8038(2005)122[0108:ACISLM]2.0.CO;2 (doi:10.1642/0004-8038(2005)122[0108:ACISLM]2.0.CO;2) [DOI] [Google Scholar]
- 13.Bshary R, Noë R. 1997. Red colobus and Diana monkeys provide mutual protection against predators. Anim. Behav. 54, 1461–1474 10.1234/12345678 (doi:10.1234/12345678) [DOI] [PubMed] [Google Scholar]
- 14.Radford AN, Bell MBV, Hollen LI, Ridley AR. 2010. Singing for your supper: sentinel calling by kleptoparasites can mitigate the cost to victims. Evolution 65, 900–906 10.1111/j.1558-5646.2010.01180.x (doi:10.1111/j.1558-5646.2010.01180.x) [DOI] [PubMed] [Google Scholar]
- 15.Sullivan KA. 1984. Information exploitation by downy woodpeckers in mixed-species flocks. Behaviour 91, 294–311 10.1163/156853984X00128 (doi:10.1163/156853984X00128) [DOI] [Google Scholar]
- 16.Dolby AS, Grubb TCJ. 1998. Benefits to satellite members in mixed-species foraging groups: an experimental analysis. Anim. Behav. 56, 501–509 10.1006/anbe.1998.0808 (doi:10.1006/anbe.1998.0808) [DOI] [PubMed] [Google Scholar]
- 17.Herzog M, Hopf S. 1984. Behavioral responses to species-specific warning calls in infant squirrel monkeys reared in social isolation. Am. J. Primatol. 7, 99–106 10.1002/ajp.1350070204 (doi:10.1002/ajp.1350070204) [DOI] [PubMed] [Google Scholar]
- 18.Hollén LI, Radford AN. 2009. The development of alarm call behaviour in mammals and birds. Anim. Behav. 78, 791–800 10.1016/j.anbehav.2009.07.021 (doi:10.1016/j.anbehav.2009.07.021) [DOI] [Google Scholar]
- 19.Fallow PM, Gardner JL, Magrath RD. 2011. Sound familiar? Acoustic similarity provokes responses to unfamiliar heterospecific alarm calls. Behav. Ecol. 22, 401–410 10.1093/beheco/ARQ221 (doi:10.1093/beheco/ARQ221) [DOI] [Google Scholar]
- 20.Magrath RD, Pitcher BJ, Gardner JL. 2009. Recognition of other species’ aerial alarm calls: speaking the same language or learning another? Proc. R. Soc. B 276, 769–774 10.1098/rspb.2008.1368 (doi:10.1098/rspb.2008.1368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser MD. 1988. How infant vervet monkeys learn to recognize starling alarm calls: the role of experience. Behaviour 105, 187–201 10.1163/156853988X00016 (doi:10.1163/156853988X00016) [DOI] [Google Scholar]
- 22.Magrath RD, Bennett TH. 2012. A micro-geography of fear: learning to eavesdrop on alarm calls of neighbouring heterospecifics. Proc. R. Soc. B 279, 902–909 10.1098/rspb.2011.1362 (doi:10.1098/rspb.2011.1362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakrishnan U, Coss RG. 2000. Recognition of heterospecific alarm vocalizations by bonnet macaques (Macaca radiata). J. Comp. Psychol. 114, 3–12 10.1037//0735-7036.114.1.3 (doi:10.1037//0735-7036.114.1.3) [DOI] [PubMed] [Google Scholar]
- 24.Shriner WM. 1999. Antipredator responses to a previously neutral sound by free-living adult golden-mantled ground squirrels, Spermophilus lateralis (Sciuridae). Ethology 105, 747–757 10.1046/j.1439-0310.1999.00454.x (doi:10.1046/j.1439-0310.1999.00454.x) [DOI] [Google Scholar]
- 25.Johnson FR, McNaughton EJ, Shelley CD, Blumstein DT. 2003. Mechanisms of heterospecific recognition in avian mobbing calls. Aust. J. Zool. 51, 577–585 10.1071/ZO03031 (doi:10.1071/ZO03031) [DOI] [Google Scholar]
- 26.Rendall D, Owren MJ, Ryan MJ. 2009. What do animal signals mean? Anim. Behav. 78, 233–240 10.1016/j.anbehav.2009.06.007 (doi:10.1016/j.anbehav.2009.06.007) [DOI] [Google Scholar]
- 27.Magrath RD, Pitcher BJ, Gardner JL. 2009. An avian eavesdropping network: alarm signal reliability and heterospecific response. Behav. Ecol. 20, 745–752 10.1093/beheco/arp055 (doi:10.1093/beheco/arp055) [DOI] [Google Scholar]
- 28.Templeton CN, Greene E. 2007. Nuthatches eavesdrop on variations in heterospecific chickadee mobbing calls. Proc. Natl Acad. Sci. USA 104, 5479–5482 10.1073/pnas.0605183104 (doi:10.1073/pnas.0605183104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradbury JW, Vehrencamp SL. 1998. Principles of animal communication. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 30.Higgins PJ, Peter JM, Steele WK. 2001. Handbook of Australian, New Zealand and Antarctic birds. Vol. 5: tyrant-flycatchers to chats. Melbourne, Australia: Oxford University Press [Google Scholar]
- 31.Bell HL. 1980. Composition and seasonality of mixed-species feeding flocks of insectivorous birds in the Australian Capital Territory. Emu 80, 227–232 [Google Scholar]
- 32.Charif RA, Waack AM, Strickman LM. 2008. Raven Pro 1.3 user‘s manual. Ithaca, NY: Cornell Laboratory of Ornithology [Google Scholar]
- 33.Fallow PM, Magrath RD. 2010. Eavesdropping on other species: mutual interspecific understanding of urgency information in avian alarm calls. Anim. Behav. 79, 411–417 10.1016/j.anbehav.2009.11.018 (doi:10.1016/j.anbehav.2009.11.018) [DOI] [Google Scholar]
- 34.Ryan MJ, Rand AS. 2003. Sexual selection in female perceptual space: how female Túngara frogs perceive and respond to complex population variation in acoustic mating signals. Evolution 57, 2608–2618 10.1111/j.0014-3820.2003.tb01503.x (doi:10.1111/j.0014-3820.2003.tb01503.x) [DOI] [PubMed] [Google Scholar]
- 35.Higgins PJ. 1999. Handbook of Australian, New Zealand and Antarctic birds. Vol. 4: parrots to dollarbird. Melbourne, Australia: Oxford University Press [Google Scholar]
- 36.Hauck WW, Jr, Donner A. 1977. Wald's test as applied to hypotheses in logit analysis. J. Am. Stat. Assoc. 72, 851–953 10.1080/01621459.1977.10479969 (doi:10.1080/01621459.1977.10479969) [DOI] [Google Scholar]
- 37.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer [Google Scholar]
- 38.R Core Team 2012. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/ [Google Scholar]
- 39.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer [Google Scholar]
- 40.Marler P. 1957. Specific distinctiveness in the communication signals of birds. Behaviour 11, 13–38 10.1163/156853956X00066 (doi:10.1163/156853956X00066) [DOI] [Google Scholar]
- 41.Ghirlanda S, Enquist M. 2003. A century of generalization. Anim. Behav. 66, 15–36 10.1006/anbe.2003.2174 (doi:10.1006/anbe.2003.2174) [DOI] [Google Scholar]
- 42.Dooling R. 2004. Audition: can birds hear everything they sing? In Nature‘s music: the science of birdsong (eds Marler P, Slabbekoorn H.), pp. 206–225 New York, NY: Academic Press [Google Scholar]
- 43.ten Cate C, Rowe C. 2007. Biases in signal evolution: learning makes a difference. Trends Ecol. Evol. 22, 380–387 10.1016/j.tree.2007.03.006 (doi:10.1016/j.tree.2007.03.006) [DOI] [PubMed] [Google Scholar]