Abstract
In diverse pollinator communities, interspecific interactions may modify the behaviour and increase the pollination effectiveness of individual species. Because agricultural production reliant on pollination is growing, improving pollination effectiveness could increase crop yield without any increase in agricultural intensity or area. In California almond, a crop highly dependent on honey bee pollination, we explored the foraging behaviour and pollination effectiveness of honey bees in orchards with simple (honey bee only) and diverse (non-Apis bees present) bee communities. In orchards with non-Apis bees, the foraging behaviour of honey bees changed and the pollination effectiveness of a single honey bee visit was greater than in orchards where non-Apis bees were absent. This change translated to a greater proportion of fruit set in these orchards. Our field experiments show that increased pollinator diversity can synergistically increase pollination service, through species interactions that alter the behaviour and resulting functional quality of a dominant pollinator species. These results of functional synergy between species were supported by an additional controlled cage experiment with Osmia lignaria and Apis mellifera. Our findings highlight a largely unexplored facilitative component of the benefit of biodiversity to ecosystem services, and represent a way to improve pollinator-dependent crop yields in a sustainable manner.
Keywords: biodiversity, blue orchard bee, ecosystem service, interspecific interactions, Osmina lignaria, wild bees
1. Introduction
There are a growing number of examples of a positive relationship between diversity and ecosystem services [1–3]. As an ecosystem service, pollination can increase the fruit or seed quality or quantity of 39 of the world's 57 major crops [4], and a more diverse pollinator community has been found to improve pollination service [5–7]. For some crops, wild bees are more effective pollinators on a per visit basis than honey bees [8,9] and/or can functionally complement the dominant visitor [6,10–12]. A less explored reason is that in diverse communities, interspecific interactions potentially alter behaviour in ways that increase pollination effectiveness [13]. Little is known about how community composition affects pollinator behaviour and the role such species interactions play in determining diversity–ecosystem service relationships.
Interspecific interactions can result in non-additive impacts of diversity on ecosystem functions. Examples include the facilitation of resource capture in diverse groups of aquatic arthropods [14], and non-additive increases in pest suppression and alfalfa (Medicago sativa L.) production in enclosures with diverse natural enemy guilds [15]. In diverse communities, one mechanism by which species interactions may augment function is the potential to modify the behaviour and the resulting effectiveness of the ecosystem service providers. Interactions with non-Apis bees cause Apis mellifera L. to move more often between rows of sunflower (Helianthus annuus L., planted with alternate rows of male and female cultivars) [13,16], increasing their pollination efficiency (number of seeds produced per visit) [13]. Such changes in pollinator movement are particularly important in crop species with separate male and female flowers, and those with self-incompatibility (e.g. almond Prunus dulcis Mill.). As well as direct interaction and disturbance [13,16], avoidance of interspecific chemical cues [17,18] and resource competition [19] have the potential to alter pollinator foraging movements.
Global human population growth is putting greater pressure on agricultural production [20,21]. There is concern over how to meet the increasing demand for food, while at the same time safeguarding ecosystems and biodiversity [22–24]. In the future, land currently under agricultural production will have to be more intensively managed to increase yields and/or more land will have to be converted to agriculture [25–27]. Given the negative impact agriculture has already had on biodiversity [28–30], it is important that future steps to increase production be made environmentally sustainable [22].
In the last 50 years, the fraction of agricultural production requiring biotic pollination has more than tripled [31]. When compared with crops that are not pollinator-dependent, those that are moderately pollinator-dependent have shown slower growth in yield and faster expansion in area from 1961 to 2006 [32]. Almond is a mass-flowering, varietally self-incompatible crop species, highly dependent on biotic pollination [4]. Almond orchards are generally planted with alternating rows of two or more varieties. Planting a single variety per row facilitates harvest, but complicates pollination because pollen must be transferred between rows to achieve fruit set. To allow for management activities, trees between rows are further apart than those within the same row (local standard of 6.7 m between rows and 4.9 m within rows). Apis mellifera tend to forage within a tree and then move down the same row, probably because less effort is required to move to the next tree in the same row or because the rows act as visual markers that influence movement [33]. This foraging pattern means A. mellifera tend to move more incompatible pollen, limiting their pollination effectiveness.
In almond, we investigated whether the presence of non-Apis bees affected the behaviour and pollination service of the dominant pollinator species, A. mellifera. Often almond orchards are isolated from natural habitat and non-Apis bees can be completely absent [7]. Therefore, we were able to compare A. mellifera behaviour and pollination effectiveness in diverse bee communities (orchards with non-Apis bees) with orchards lacking non-Apis bees. Here, we refer to pollinator effectiveness as the probability an ovule is fertilized following a single visit [34]. We complemented our intensive field sampling with observations in a controlled cage environment, where A. mellifera were introduced along with the blue orchard bee Osmia lignaria Say [35]. We hypothesized that where non-Apis bees were present, such as in sunflower [13,16], interspecific interactions would cause A. mellifera to more frequently move between rows. We further hypothesized that an increase in between-row movements by A. mellifera would increase their pollination effectiveness and increase fruit set (figure 1).
Figure 1.
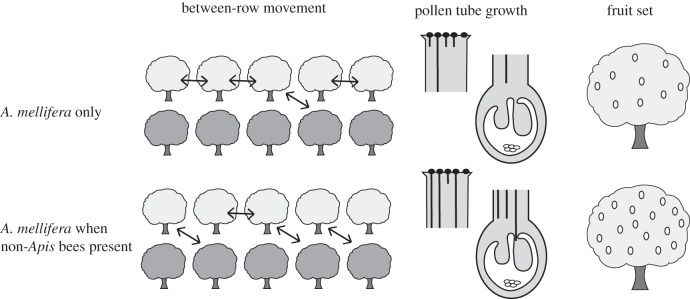
The impact of the presence of non-Apis bees on Apis mellifera movement, pollination effectiveness and fruit set in almond orchards. For pollen tube growth, the top half of the style depicts pollen deposition and the initiation of pollen tube growth. The second part depicts pollen tube growth to the base of the style and the potential consequences for fertilization.
2. Material and methods
Fieldwork took place from February to July 2008–2011 in 25 almond orchards. All orchards in the study were located in northern California (37°41′–38°57′ N and 120°43′–122°14′ W). The minimum distance between sites was 1 km (average 2.8 km), and the average tree height was 6.2 m in orchards with non-Apis bees and 6.1 m in orchards without.
(a). Open orchards: movement between trees
In 2011, A. mellifera movements were observed in five orchards isolated from natural habitat, where non-Apis bees were not present. The number of movements by A. mellifera was counted between two trees of different varieties across the orchard row for 1 min. This was repeated a minimum of four times, counting movements between the same two rows, between different adjacent trees. The number of movements by A. mellifera was also counted between two adjacent trees of the same variety within the same orchard row. These two counts were repeated a minimum of eight times down a row along adjacent trees (four times along one row and four times along the other). The same observations were made in five orchards where non-Apis bees were present [8]. Four of the orchards contained wild bees such as Bombus vosnesenskii Rad. and Bombus melanopygus Nyl., and the fifth contained the managed native blue orchard bee O. lignaria.
(b). Open orchards: single visit pollination effectiveness
In 2009, we categorized 14 almond orchards either as having non-Apis bees (n = 7) or lacking non-Apis bees (n = 7), based on standard observations of flower visitors (80 min per orchard). In each orchard, we covered a set of almond branches with mesh bags before flowering to exclude pollinator visits. Once an orchard was in bloom, the bags were removed, and the branches with the previously unvisited flowers were removed from the trees and immediately presented to foraging A. mellifera. After an A. mellifera had visited one of these flowers, the flower was removed from the branch, its petals and anthers were removed, and it was placed in a 1.5 ml microcentrifuge tube containing 0.5 ml of water, such that the stigma did not touch the tube's surface and the pedicle was in water. These flowers were left at room temperature, away from direct sunlight for 72 h to allow pollen tubes to grow. After 72 h, the pistils were fixed in FAA (10 : 7 : 2 : 1 ethanol 95%, H2O, formalin, acetic acid) [36] and stored at 4°C until further processing. From each of the 14 orchards, an average of 30 stigmas were processed after a single honey bee visit (min. 20, max. 42).
To examine pollen tube growth, pistils were removed from the FAA and the tissue softened by boiling in 5 per cent sodium sulphite (Na2SO3) for 30 min. They were then soaked in tap water for 20 min, and incubated for 24 h in a decolourized solution of 0.1 per cent aniline blue dye, dissolved in 0.1 N K3PO4 (potassium phosphate) [36]. The softened stained pistils were squashed onto a microscope slide to reveal pollen tubes. The slides were examined using a fluorescent microscope (Nikon Eclipse 80i with a CFL-FITC filter). For each slide, the numbers of pollen tubes initiating growth on the stigma and reaching the base of the style were scored. A flower was considered successfully pollinated if a pollen tube reached the base of the style.
(c). Open orchards: fruit set
In 2009, we measured fruit set in each of nine orchards with non-Apis bees and nine without non-Apis bees. We marked 1 m lengths of branches on five trees on the outer row of each orchard. To calculate fruit set, the number of flowers on each marked branch section was counted and in July the number of developing fruits on the same section of branch was recorded. In 2008, standardized observations of flower visitors were conducted in the same orchards [7]. In the nine orchards with non-Apis bees (those used for the pollination effectiveness measurements were a subset of these), overall 18 different non-Apis bee species/morphospecies were observed visiting almond flowers, with Andrena cerasifolii (Cockerell) being the most common non-Apis visitor (see the electronic supplementary material, table S1 for a species list of non-Apis bees, and electronic supplementary material, table S2 for the number of flower visits by A. mellifera and non-Apis bees).
(d). Cage experiment
In 2011, three large cages (20 × 13 × 3 m) were set up in one of the study orchards. Each cage contained two rows of four trees (see the electronic supplementary material, figure S1). One row was the Monterey variety and the other the Carmel variety. The cages were stocked with bees at the initiation of bloom (13 February). One cage received a four-frame nucleus A. mellifera colony with two entrances, set up so that one entrance opened into the cage and one entrance to the open orchard. One cage received 32 female and 32 male individuals of O. lignaria. Wooden nesting blocks, water and loose soil were provided for nesting. In the third cage both A. mellifera and O. lignaria were stocked at opposite ends of the cage as described previously. Observational scans were made of the frequency of flower visits in the cages to assess the number of foraging bees within each cage. In each scan, a group of flowers was observed for 20 s, and the number of flowers observed and the frequency of flower visits recorded. Scans were repeated in different sections of each tree on different days (a total of 43 min of observation in each cage).
Many of the same methods as detailed above for the open orchards were used in the cages, with the following differences. The movement between adjacent trees was recorded during 1 min observations. Three observations were made down one row, three down the other row and four between the rows. These observations were conducted on 5 days in the A. mellifera cage (50 min total observation) and 4 days in the mixed A. mellifera/O. lignaria cage (40 min). Single-visit pollination effectiveness was measured for A. mellifera in the A. mellifera cage (n = 28) and the mixed cage (n = 27). For each visit, the number of pollen grains on the stigma, the number of pollen tubes initiating growth and the number of pollen tubes reaching the base of the style were counted. The fruit set was estimated as above by marking two branches on each of the eight trees per cage. Per visit fruit set was estimated by dividing the fruit set by the average flower visitation rate in the cage.
(e). Statistical analysis
Data from the open orchards on A. mellifera movement (the proportion of movement across versus down rows), pollen tube growth and fruit set were analysed using generalized linear mixed models (table 1). All models were simplified by stepwise deletion. Analysis of variance was used to compare the loss of explanatory power from the removal of an explanatory variable, and if p ≥ 0.05 the variable was dropped [38]. A Mann–Whitney U-test was performed on the data from flower visitor observations in the orchards where fruit set was recorded. The visitation rate of A. mellifera at the orchard edge was compared between the orchards with non-Apis bees present and those without. For the cage data, means and standard errors were calculated for informal comparison between the cages, as replications at the cage level were not possible. All analyses were carried out in R v. 2.14.1 [39] (supporting data are provided in the electronic supplementary material, tables S3 and S4).
Table 1.
A summary of the design of the generalized linear models used to analyse the data in open orchards.
| response variable | explanatory variables | random variables | error structure |
|---|---|---|---|
| proportion flights across rows | non-Apis bees present | orchard/day | binomial |
| pollen tube at base of style? | non-Apis bees present | orchard | binomial |
| no. pollen tubes initiating growth | non-Apis bees present | orchard, subjecta | Poisson |
| no. pollen tubes at base of style | non-Apis bees present | orchard | Poisson |
| proportion fruit set | non-Apis bees present, rate of flower visitationb | orchard | binomial |
3. Results
(a). Open orchard experiments
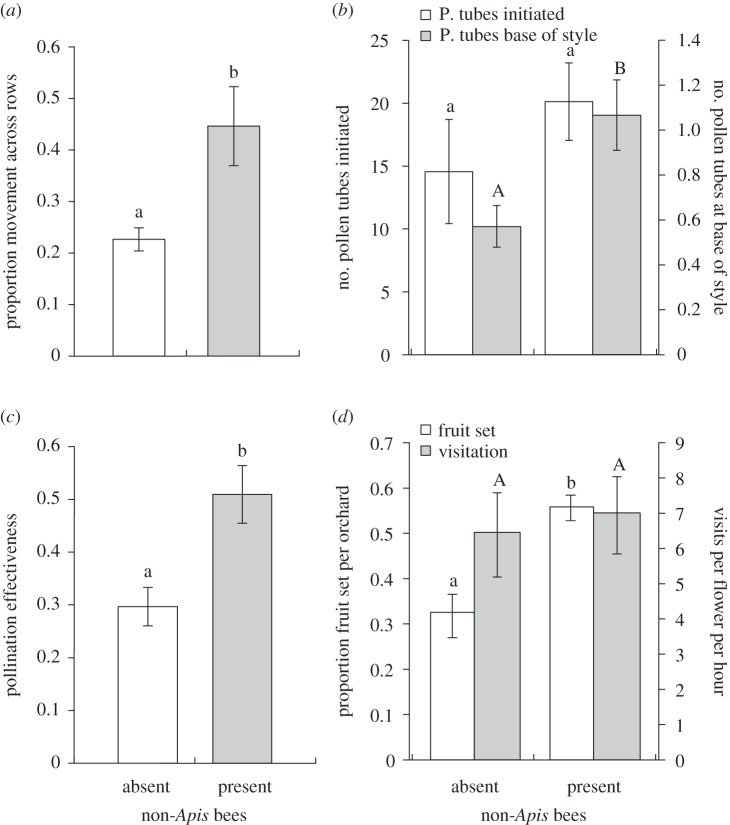
The average A. mellifera movement (at the orchard level±s.e.) was 1.4 ± 0.4 bees per minute across rows and 3.0±0.8 bees per minute down rows in orchards without non-Apis bees. In orchards with non-Apis bees present, the average A. mellifera movement was 1.2 ± 0.3 bees per minute across rows and 1.1 ± 0.4 bees per minute down rows. Apis mellifera made a greater proportion of flights across orchard rows (between varieties) in orchards with non-Apis bees present (figure 2a; χ2 = 5.56, d.f. = 1, p = 0.018). The number of pollen tubes that initiated growth following a single visit by an A. mellifera individual did not differ with the presence of non-Apis bees in the orchard (figure 2b; χ2 = 2.32, d.f. = 1, p = 0.128). However, in orchards with non-Apis bees present, single visits from A. mellifera were more likely to result in a pollen tube reaching the base of the flower's style, and thus the ovary (binomial analysis; figure 2c; χ2 = 8.57, d.f. = 1, p = 0.003). In addition, the number of pollen tubes per flower reaching the base of the style was greater in the orchards with non-Apis bees present (figure 2b; χ2 = 11.54, d.f. = 1, p < 0.001). The proportion fruit set was greater in orchards with non-Apis bees present (figure 2d; χ2 = 14.96, d.f. = 1, p < 0.001), irrespective of the visitation rate of all bees (χ2 = 0.30, d.f. = 1, p = 0.584). The visitation rate of A. mellifera was lower (W = 64, p = 0.040) in the orchards where non-Apis bees were present than where they were not (2.8 ± 0.6, 6.4 ± 1.5, mean visits per flower per hour ± s.e. respectively).
Figure 2.
(a) The proportion of Apis mellifera movements across orchard rows (of different tree varietie), rather than down orchard rows (of the same variety). Observations were carried out in orchards with non-Apis bees absent (n = 5) and present (n = 5). (b) The number of pollen tubes initiating growth (white bars) and reaching the base of the style (grey bars) following a single A. mellifera visit in orchards with non-Apis bees absent (n = 7) and present (n = 7). (c) The single-visit pollination effectiveness of A. mellifera, represented by the proportion of visits that resulted in a pollen tube reaching the base of the flower's style. (d) The average fruit set (white bars) per orchard and visitation rates (grey bars; all bees), where non-Apis bees were absent (n = 9) and present (n = 9). For each graph, error bars are the s.e. of the orchard mean and different letters above the bars indicate a significant difference (p < 0.05).
(b). Cage experiment
The proportion of A. mellifera flights between rows, single-visit pollen deposition and pollination effectiveness were similar between the cages with or without non-Apis bees (table 2). With an increasing number of pollen grains deposited, the number of pollen tubes initiating growth increased more sharply in the mixed cage than in the A. mellifera cage (see the electronic supplementary material, figure S2a). The number of pollen tubes reaching the base of the style showed a ‘humped’ relationship with the number of pollen grains deposited, such that at the highest level of deposition fewer tubes reached the ovary (see the electronic supplementary material, figure S2b). Fruit set was higher in the mixed cage than in the A. mellifera and O. lignaria cages (figure 3).
Table 2.
Data on Apis mellifera movement and pollination, and fruit set, in cages containing eight almond trees. One cage contained only A. mellifera (A. m), one contained a mixture of A. mellifera and Osmia lignaria (mixed), and one contained only O. lignaria (O. l). The figures for fruit set are the mean per tree per cage.
| cage (mean ± s.e.) |
||
|---|---|---|
| proportion flights between rows | A. m | 0.19 ± 0.04 |
| mixed | 0.25 ± 0.04 | |
| pollen deposition (single visit) | A. m | 59 ± 14 |
| mixed | 40 ± 8 | |
| pollen tubes initiated (single visit) | A. m | 12 ± 2 |
| mixed | 21 ± 4 | |
| pollen tubes base of style (single visit) | A. m | 0.5 ± 0.2 |
| mixed | 0.9 ± 0.3 | |
| pollination effectivenessa | A. m | 0.38 |
| mixed | 0.41 | |
| fruit set | A. m | 0.22 ± 0.03 |
| mixed | 0.30 ± 0.05 | |
| O. l | 0.15 ± 0.02 | |
aPollination effectiveness of A. mellifera, represented by the proportion of visits that resulted in a pollen tube reaching the base of the flower's style.
Figure 3.
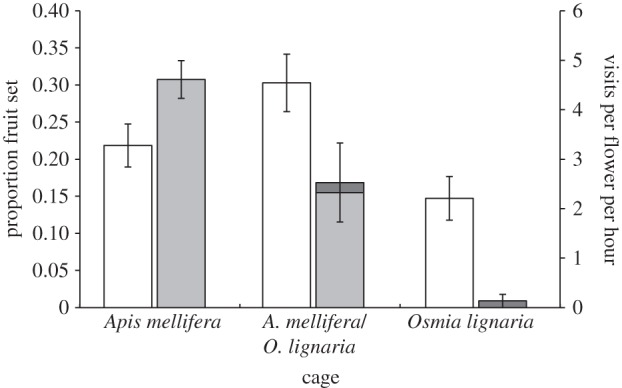
The average proportion fruit set per tree in three cages containing eight almond trees each. One cage contained only Apis mellifera, one contained only Osmia lignaria and one contained a mix of both (white bars represent fruit set and grey bars represent visitation). Owing to the different numbers of bees in the cages, the average flower visitation rates are also shown (A. mellifera in lighter grey, O. lignaria in darker grey). The error bars are the s.e. of the mean per tree.
We used the fruit set per A. mellifera visit, calculated from the A. mellifera cage and the fruit set per O. lignaria visit from the O. lignaria cage, to predict the fruit set in the mixed cage if there was no synergistic effect. Based on the visitation rates in the mixed cage and the fruit set per visit from the A. mellifera and O. lignaria cages, the mixed cage was predicted to have a proportion fruit set of 0.25. The mixed cage had a 5 per cent greater fruit set than predicted by its visitation rates.
4. Discussion
Our findings show that increased pollinator diversity can synergistically increase pollination service through species interactions that alter the behaviour and resulting functional quality of a dominant pollinator species. This highlights a largely unexplored facilitative component of the benefit of biodiversity to ecosystem services. Total bee visitation rates were similar between orchard types, and the visitation rate of A. mellifera was lower in orchards with non-Apis bees. However, A. mellifera pollination effectiveness was greater and fruit set was higher when non-Apis bees were present. Apis mellifera increased their proportion of movement between tree rows when non-Apis bees were present, thereby improving pollination effectiveness. More successful pollen tube growth translated into significantly higher fruit set in the orchards where non-Apis bees were present. The greater proportion of between row movements by A. mellifera individuals most probably resulted in the deposition of more compatible pollen, since pollen from the same variety generally does not set fruit (A. M. Klein & C. Kremen 2008, unpublished data). This synergistic effect of the presence of non-Apis bees suggests that maintaining biodiversity in agricultural ecosystems could provide unrecognized benefits, and it offers exciting opportunities for the integration of more diverse pollination systems to improve the longer-term sustainability of crop production for almond and similar crops.
Unlike in sunflower [13,16], very few direct interactions were observed between A. mellifera and non-Apis bees. One potential mechanism for the increased proportion of inter-row movement of A. mellifera in the presence of other non-Apis bees is linked to resource depletion. Because some non-Apis bees can fly at lower temperatures than A. mellifera [40,41], and therefore earlier in the day, it may be that if A. mellifera begin foraging and find flowers have already been depleted then they increase the distance of their foraging flights. Alternatively, it may be related to the scent marks left by non-Apis bees on the flowers [17]. Apis mellifera have been shown to avoid visiting flowers that have been marked by bumble bees [18], and it may be that as well as avoiding the flowers they also shift their foraging location. Given that A. mellifera tend to move down the same row, if a response to perceived resource competition is to shift foraging locations, this could be expected to involve movement across rows. However, at present the mechanism for the increased between-row movements when non-Apis bees are present is unknown.
In the controlled cage environment, the steeper increase in the number of pollen tubes initiated with increasing pollen deposition in the mixed cage and the higher fruit set supports the findings from the open orchards that more compatible pollen is being moved when pollinator communities are diverse. Because there was only one cage per treatment, the data from the cages are only descriptive, but they do support the findings from the open orchards. Future work should attempt to replicate similar treatments. The greater fruit set in the mixed cage when the visitation rate was lower than in the A. mellifera cage may be due to the slightly higher proportion of between-row movements in the mixed cage. Although the differences in movement were not great, the pollen tube data suggest that more pollen was moving between varieties in the mixed cage. The size of the cages limited the opportunity for between-tree flights. This and the relatively low visitation rate of O. lignaria in the cages compared with the open orchards (0.2 ± 0.1 versus 1.6 ± 0.5 flowers per hour, respectively, mean ± s.e.) may explain why the difference in pollen tube formation and fruit set between the cages was less than in the open orchards. The price of California almond in 2011 was approximately $1.79 per pound and production was estimated at 2670 lbs per acre (USDA National Agricultural Statistics). If a 5 per cent increase in fruit set as calculated from the controlled cage environment translated into an equivalent increase in production, farmers would make $239 more per acre (the average orchard size in our study was 56 acres). This estimate from cages may be a lower bound compared with open orchards.
Sampling effects and complementarity have been the primary explanations for a positive relationship between biodiversity and ecosystem function [42]. Here, we show a different mechanism, possibly due to interspecific competition, where community composition alters the behaviour of a service-providing organism with a positive knock-on effect for the ecosystem service. Our results show alterations in A. mellifera foraging behaviour when a diverse community of other bees are present, and suggest almond yield can be increased by encouraging wild bees in the orchards. As such, natural habitat near almond orchards should be conserved to protect wild bee communities [7]. The availability of A. mellifera is not predicted to increase at the same rate as demand for their services in agriculture [31]. Thus, increasing the pollination effectiveness of A. mellifera and conserving wild pollinator communities could help increase crop yields. The synergistic combination of A. mellifera and non-Apis bees represents a sustainable way to improve crop pollination services, but the generality of such effects still need to be tested across multiple crop systems.
Acknowledgements
We gratefully acknowledge the Alexander von Humbold Foundation, the Hellmann Foundation, the McDonnell 21st Century Foundation, the Chancellor's Partnership Fund of the University of California, Berkeley, the German Academic Exchange Programme for support of students and the German Science Foundation (KL 1849/4-1) for financial support. We thank several almond growers in Yolo, Colusa and Stanislaus Counties for their willingness to provide access to their orchards. We thank Stephen Peterson, AgPollen LLC for access to experimental orchards, and Drew Scofield for assistance with cage construction. We thank Sue Cobey (UC Davis) and the USDA ARS Pollinating Insect Research Unit with assistance from Glen Trostle (retired ARS) for providing bees and advice. We thank Lucas Garibaldi and Theresa Pitts-Singer for discussions, and Elisabeth Eilers, Christina Locke, Miriam Voss, Anika Hudewenz, Stephen Hendrix and Amber Sciligo for help in the field and laboratory. We thank Jeff Ollerton and two anonymous reviewers for their comments on the manuscript.
References
- 1.Benayas JMR, Newton AC, Diaz A, Bullock JM. 2009. Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science 325, 1121–1124 10.1126/science.1172460 (doi:10.1126/science.1172460) [DOI] [PubMed] [Google Scholar]
- 2.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67 10.1038/nature11148 (doi:10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 3.Hooper DU, et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108 10.1038/nature11118 (doi:10.1038/nature11118) [DOI] [PubMed] [Google Scholar]
- 4.Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313 10.1098/rspb.2006.3721 (doi:10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein AM, Steffan-Dewenter I, Tscharntke T. 2003. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. Lond. B 270, 955–961 10.1098/rspb.2002.2306 (doi:10.1098/rspb.2002.2306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoehn P, Tscharntke T, Tylianakis JM, Steffan-Dewenter I. 2008. Functional group diversity of bee pollinators increases crop yield. Proc. R. Soc. B 275, 2283–2291 10.1098/rspb.2008.0405 (doi:10.1098/rspb.2008.0405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein AM, Brittain C, Hendrix SD, Thorp R, Williams N, Kremen C. 2012. Wild pollination services to California almond rely on semi-natural habitat. J. Appl. Ecol. 49, 723–732 10.1111/j.1365-2664.2012.02144.x (doi:10.1111/j.1365-2664.2012.02144.x) [DOI] [Google Scholar]
- 8.Willmer PG, Bataw AAM, Hughes JP. 1994. The superiority of bumblebees to honeybees as pollinators: insect visits to raspberry flowers. Ecol. Entomol. 19, 271–284 10.1111/j.1365-2311.1994.tb00419.x (doi:10.1111/j.1365-2311.1994.tb00419.x) [DOI] [Google Scholar]
- 9.Javorek SK, Mackenzie KE, Vander Kloet SP. 2002. Comparative pollination effectiveness among bees (Hymenoptera: Apoidea) on lowbush blueberry (Ericaceae: Vaccinium angustifolium). Ann. Entomol. Soc. Am. 95, 345–351 10.1603/0013-8746(2002)095[0345:CPEABH]2.0.CO;2 (doi:10.1603/0013-8746(2002)095[0345:CPEABH]2.0.CO;2) [DOI] [Google Scholar]
- 10.Chagnon M, Ingras J, Oliveira DD. 1993. Complementary aspects of strawberry pollination by honey and indigenous bees (Hymenoptera). J. Econ. Entomol. 86, 416–420 [Google Scholar]
- 11.Brittain C, Kremen C, Klein AM. 2012. Biodiversity buffers pollination from changes in environmental conditions . Glob. Change Biol. 10.1111/gcb.12043 (doi:10.1111/gcb.12043) [DOI] [PubMed] [Google Scholar]
- 12.Albrecht M, Schmid B, Hautier Y, Müller CB. 2012. Diverse pollinator communities enhance plant reproductive success. Proc. R. Soc. B 279, 4845–4852 10.1098/rspb.2012.1621 (doi:10.1098/rspb.2012.1621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenleaf SS, Kremen C. 2006. Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc. Natl Acad. Sci. USA 103, 13 890–13 895 10.1073/pnas.0600929103 (doi:10.1073/pnas.0600929103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardinale BJ, Palmer MA, Collins SL. 2002. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 415, 426–429 10.1038/415426a (doi:10.1038/415426a) [DOI] [PubMed] [Google Scholar]
- 15.Cardinale BJ, Harvey CT, Gross K, Ives AR. 2003. Biodiversity and biocontrol: emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol. Lett. 6, 857–865 10.1046/j.1461-0248.2003.00508.x (doi:10.1046/j.1461-0248.2003.00508.x) [DOI] [Google Scholar]
- 16.Carvalheiro LG, Veldtman R, Shenkute AG, Tesfay GB, Pirk CWW, Donaldson JS, Nicolson SW. 2011. Natural and within-farmland biodiversity enhances crop productivity. Ecol. Lett. 14, 251–259 10.1111/j.1461-0248.2010.01579.x (doi:10.1111/j.1461-0248.2010.01579.x) [DOI] [PubMed] [Google Scholar]
- 17.Eltz T. 2006. Tracing pollinator footprints on natural flowers. J. Chem. Ecol. 32, 907–915 10.1007/s10886-006-9055-6 (doi:10.1007/s10886-006-9055-6) [DOI] [PubMed] [Google Scholar]
- 18.Stout JC, Goulson D. 2001. The use of conspecific and interspecific scent marks by foraging bumblebees and honeybees. Anim. Behav. 62, 183–189 10.1006/anbe.2001.1729 (doi:10.1006/anbe.2001.1729) [DOI] [Google Scholar]
- 19.Inouye DW. 1978. Resource partitioning in bumblebees: experimental studies of foraging behavior. Ecology 59, 672–678 10.2307/1938769 (doi:10.2307/1938769) [DOI] [Google Scholar]
- 20.Tilman D, et al. 2001. Forecasting agriculturally driven global environmental change. Science 292, 281–284 10.1126/science.1057544 (doi:10.1126/science.1057544) [DOI] [PubMed] [Google Scholar]
- 21.Foley JA, et al. 2011. Solutions for a cultivated planet. Nature 478, 337–342 10.1038/nature10452 (doi:10.1038/nature10452) [DOI] [PubMed] [Google Scholar]
- 22.Beddington J. 2010. Food security: contributions from science to a new and greener revolution. Philos. Trans. R. Soc. B 365, 61–71 10.1098/rstb.2009.0201 (doi:10.1098/rstb.2009.0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brussaard L, Caron P, Campbell B, Lipper L, Mainka S, Rabbinge R, Babin D, Pulleman M. 2010. Reconciling biodiversity conservation and food security: scientific challenges for a new agriculture. Curr. Opin. Environ. Sust. 2, 34–42 10.1016/j.cosust.2010.03.007 (doi:10.1016/j.cosust.2010.03.007) [DOI] [Google Scholar]
- 24.Perfecto I, Vandermeer J. 2010. The agroecological matrix as alternative to the land-sparing/agriculture intensification model. Proc. Natl Acad. Sci. USA 107, 5786–5791 10.1073/pnas.0905455107 (doi:10.1073/pnas.0905455107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilman D, Balzer C, Hill J, Befort BL. 2011. Global food demand and the sustainable intensification of agriculture. Proc. Natl Acad. Sci. USA 108, 20 260–20 264 10.1073/pnas.1116437108 (doi:10.1073/pnas.1116437108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phalan B, Onial M, Balmford A, Green RE. 2011. Reconciling food production and biodiversity conservation: land sharing and land sparing compared. Science 333, 1289–1291 10.1126/science.1208742 (doi:10.1126/science.1208742) [DOI] [PubMed] [Google Scholar]
- 27.Fischer J, et al. 2011. Conservation: limits of land sparing. Science 334, 593. 10.1126/science.334.6056.593-a (doi:10.1126/science.334.6056.593-a) [DOI] [PubMed] [Google Scholar]
- 28.Stoate C, Boatman ND, Borralho RJ, Carvalho CR, de Snoo GR, Eden P. 2001. Ecological impacts of arable intensification in Europe. J. Environ. Manag. 63, 337–365 10.1006/jema.2001.0473 (doi:10.1006/jema.2001.0473) [DOI] [PubMed] [Google Scholar]
- 29.Philpott SM, et al. 2008. Biodiversity loss in Latin American coffee landscapes: review of the evidence on ants, birds, and trees. Conserv. Biol. 22, 1093–1105 10.1111/j.1523-1739.2008.01029.x (doi:10.1111/j.1523-1739.2008.01029.x) [DOI] [PubMed] [Google Scholar]
- 30.Geiger F, et al. 2010. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 11, 97–105 10.1016/j.baae.2009.12.001 (doi:10.1016/j.baae.2009.12.001) [DOI] [Google Scholar]
- 31.Aizen MA, Harder LD. 2009. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 19, 915–918 10.1016/j.cub.2009.03.071 (doi:10.1016/j.cub.2009.03.071) [DOI] [PubMed] [Google Scholar]
- 32.Garibaldi LA, Aizen MA, Cunningham S, Klein AM. 2009. Pollinator shortage and global crop yield: looking at the whole spectrum of pollinator dependency. Commun. Integr. Biol. 2, 37–39 10.4161/cib.2.1.7425 (doi:10.4161/cib.2.1.7425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cranmer L, Mccollin D, Ollerton J. 2012. Landscape structure influences pollinator movements and directly affects plant reproductive success. Oikos 121, 562–568 10.1111/j.1600-0706.2011.19704.x (doi:10.1111/j.1600-0706.2011.19704.x) [DOI] [Google Scholar]
- 34.Ne'eman G, Jürgens A, Newstrom-Lloyd L, Potts SG, Dafni A. 2010. A framework for comparing pollinator performance: effectiveness and efficiency. Biol. Rev. Camb. Philos. Soc. 85, 435–451 10.1111/j.1469-185X.2009.00108.x (doi:10.1111/j.1469-185X.2009.00108.x) [DOI] [PubMed] [Google Scholar]
- 35.Bosch J, Kemp WP, Peterson SS. 2000. Management of Osmia lignaria (Hymenoptera: Megachilidae) populations for almond pollination: methods to advance bee emergence. Environ. Entomol. 29, 874–883 10.1603/0046-225x-29.5.874 (doi:10.1603/0046-225x-29.5.874). [DOI] [Google Scholar]
- 36.Currier HB. 1957. Callose substance in plant cells. Am. J. Bot. 44, 478–488 10.2307/2438916 (doi:10.2307/2438916) [DOI] [Google Scholar]
- 37.Maindonald J, Braun WJ. 2010. Data analysis and graphics using R: an example-based approach, 3rd edn Cambridge, UK: Cambridge University Press [Google Scholar]
- 38.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer [Google Scholar]
- 39.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 40.Tuell JK, Isaacs R. 2010. Weather during bloom affects pollination and yield of highbush blueberry. J. Econ. Entomol. 103, 557–562 10.1603/ec09387 (doi:10.1603/ec09387) [DOI] [PubMed] [Google Scholar]
- 41.Vicens N, Bosch J. 2000. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environ. Entomol. 29, 413–420 10.1603/0046-225x-29.3.413 (doi:10.1603/0046-225x-29.3.413) [DOI] [Google Scholar]
- 42.Reiss J, Bridle JR, Montoya JM, Woodward G. 2009. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 24, 505–514 10.1016/j.tree.2009.03.018 (doi:10.1016/j.tree.2009.03.018) [DOI] [PubMed] [Google Scholar]