Abstract
When animals live in cities, they have to adjust their behaviour and life histories to novel environments. Noise pollution puts a severe constraint on vocal communication by interfering with the detection of acoustic signals. Recent studies show that city birds sing higher-frequency songs than their conspecifics in non-urban habitats. This has been interpreted as an adaptation to counteract masking by traffic noise. However, this notion is debated, for the observed frequency shifts seem to be less efficient at mitigating noise than singing louder, and it has been suggested that city birds might use particularly high-frequency song elements because they can be produced at higher amplitudes. Here, we present the first phonetogram for a songbird, which shows that frequency and amplitude are strongly positively correlated in the common blackbird (Turdus merula), a successful urban colonizer. Moreover, city blackbirds preferentially sang higher-frequency elements that can be produced at higher intensities and, at the same time, happen to be less masked in low-frequency traffic noise.
Keywords: anthropogenic noise, bird song, phonetogram, urbanization, blackbird, acoustic communication
1. Introduction
Many animals rely on acoustic signals to find mating partners, deter rivals or avoid predators [1]. Especially, in long-range communication, environmental constraints can be severe, and it has often been shown that animals adjust their acoustic signals to the habitat acoustics to ensure effective signal transmission [2]. For example, dense vegetation scatters and absorbs high frequencies of vocalizations and reverberations either degrade [3,4] or reinforce [5,6] acoustic signals, depending on the signal structure. This constraint may account for the observation that forest birds often use more low frequency, tonal sounds with slow modulations in amplitude and frequency [7]. But even within a given habitat, the transmission properties of acoustic signals can change with the communication distance [8], the position of the signaller or the receiver [9,10], the season [11] or the time of day ([12,13], but see [14]). Diurnal fluctuation can also often be observed in the levels of ambient noise, which is another important constraint on acoustic communication [15]. If dominant noise frequencies overlap the signal spectrum, the resulting acoustic masking may disrupt information transfer between individuals and communication may break down. In the recent years, anthropogenic noise and its detrimental influence on acoustic communication received increasing interest, and several studies investigated whether and how animals can cope with this new man-made impact [15–17].
Birds use multiple tactics to mitigate acoustic masking by anthropogenic noise. For example, robins (Erithacus rubecula) in cities shift their singing activity more in the night to avoid noisy periods [18]. In addition to changes in singing activity, birds also reduce signal masking by changing the characteristics of their songs. Birds, as well as mammals, exhibit the Lombard effect, i.e. they increase the amplitude of their vocalizations in response to an increase in background noise (reviewed in [19]), which is used to communicate in the presence of anthropogenic noise [20]. It is also often observed that birds sing at higher frequencies at noisy locations [21–25], which is probably the result of vocal plasticity [26–28].
High songs are easier to detect in low-frequency noise [29,30], but the actual benefits in urban environments are a subject of debate [31]. A recent study demonstrated that the typical rises in frequency found in many city birds are too low to yield a considerable improvement in signal transmission [32]. By contrast, vocal amplitude adjustments are probably much more effective at maintaining the active space of the signal in noise. Therefore, Nemeth & Brumm [32] cautioned that the assumption that the observed small increases in frequency must be an adaptation to masking noise may be premature. Instead, they suggest that the increase in frequency might as well be the outcome of the increased amplitude of bird song in noise [33].
Such a concurrence of frequency and amplitude shifts could arise in two ways [33]. First, the increased song frequency could be an epiphenomenon of the Lombard effect [34]. In humans, such a passive increase of vocal pitch in Lombard speech has been clearly shown, but it is less well understood in songbirds (reviewed in [19]). There are only very few published data on the link between amplitude and frequency in bird vocalizations in response to increased levels of background noise. In laboratory experiments, budgerigars (Melopsittacus undulatus) [35] and elegant crested tinamous (Eudromia elegans) [36] increased both the amplitude and frequency of their calls when background noise levels increased, but whether this occurs in the field is unknown. By contrast, the begging calls of juvenile tree swallows (Tachycineta bicolour) were both louder and higher when experimentally exposed to noise in the field, but in the laboratory under similar conditions only call amplitude increased [37].
Second, there may be a general coupling between vocal amplitude and frequency related to biophysical properties of the sound source and vocal tract. Again, this relationship is better understood in humans than in birds. In absence of voluntary counter-adjustments, both the amplitude and frequency increase with increasing lung pressure [38]. In addition, the vocal tract acts as an impedance-matching filter that allows relatively greater power transfer from the source (the glottis in humans or the syrinx in birds) to the air at higher frequencies than at lower ones [38]. This means that, in humans, higher-frequency sounds tend to be louder than lower frequencies, and that it is relatively more efficient to further increase vocal amplitude at high frequencies than it is at low ones. This same relationship has been demonstrated in the ring dove (Streptopelia risoria), a non-passerine with a single vocal source (a tracheal syrinx) [39]. In songbirds, sound production is more complex, as they have two independently controlled sound sources (a tracheo-bronchial syrinx), the control of which often requires rapid bilateral coordination of central song control nuclei, respiratory and syringeal muscles, and the dynamic adjustment of the upper vocal tract to match the frequencies produced by either of the sides of the syrinx [40–43]. However, despite this more complex vocal production system, there is scattered evidence from at least four songbird species for a positive correlation between song frequency and amplitude [44–47]. Although in these studies, the question has not been investigated directly, they have potentially important implications for understanding changes in vocal behaviour that have been observed in urban species. If this relationship between amplitude and frequency exists in urban bird species, then switching to higher-frequency song elements would automatically lead to higher amplitude songs as well.
In this study, we investigated the relationships between song amplitude and frequency in a successful colonizer of urban areas, the common blackbird (Turdus merula). Two independent studies demonstrated that city blackbirds sing on average at higher frequencies than forest birds [22,48]. Here, we applied for the first time, to our knowledge, phonetic tools to address the behavioural ecology of animal communication. In particular, we measured the frequencies and amplitudes for each element in blackbird song motifs and created an average phonetogram for the species [49]. We then related this vocal profile to a detailed analysis of the song frequency use in city and forest males. This approach allowed new insights into questions such as why birds sing at higher frequencies in cities. Finally, we calculated the frequency-related amplitude differences of typical city and forest birds to test whether a switch from low- to high-frequency elements already leads to an effective increase in amplitude.
2. Material and methods
(a). Study birds
We analysed the songs of two sets of blackbirds: (i) a sample of hand-raised males recorded in sound-attenuating chambers to calculate a vocal range profile, and (ii) recordings from free-living city and forest blackbirds, which were used to investigate the different usage of songs from within their vocal frequency range. In the first set, we recorded 12 2-year old males, which were always kept in separate aviaries where they could not see but could hear each other. For the sound recordings, each bird was placed in a cage in a sound-attenuating chamber (105 × 57 cm and 70 cm high) for 2–3 days. Song activity was continuously recorded using the software Sound Analysis Pro [50]. Each cage had two perches at the same height close to the ground of the cage and cups with ad libitum water and food. Sound radiation patterns around singing birds show a clear frequency-dependent directionality [51–53]. Therefore, we placed the microphone (Behringer C2) vertically above the perches and midway between them, ca 22 cm from the bird's head. This set-up allowed us to minimize variation in song amplitude because of lateral head movements [54–56]. To measure the effect of variation in the birds’ position on the perch on the recorded amplitude and spectrum, we mounted the carcass of a male blackbird in a natural singing position on the perch while white noise and frequency-modulated sweeps (1–8 kHz, rise time 250 ms) were broadcast through the vocal tract of the carcass from a 1 cm diameter loudspeaker mounted in place of the syrinx [51]. The emitted sound was recorded with the same settings as the live birds, with the carcass positioned in the centre and at both ends of the perch (this variation in singing position was greater than the variation observed during the recording of the live birds, which usually sang from approximately the centre of the perch). The maximum difference in recorded sound pressure level was less than 2 dB in all cases (n = 20 repeated measurements at each of the three locations, standard deviations ranged between 0.08 and 0.19 dB) and the maximum difference in spectral energy distribution of the recorded white noise and of the sweeps was less than 1.6 dB per 1 Hz spectrum level unit within in the range of blackbird song motifs (1.2–3.5 kHz).
In the second dataset, we investigated habitat-related differences in song frequency usage from field recordings from 16 city and 17 forest birds described in [22]. In brief, territorial males were recorded in the inner city of Vienna and in the mature deciduous forest of the Vienna Woods. Owing to traffic noise, the mean background noise levels in the city territories was significantly higher than at the forest sites (LAeq: city: 54 dB; forest: 45; LLeq: city: 71 dB; forest: 60 dB). The noise spectra in the forest were dominated by the songs of other species. See Nemeth et al. [22] for further details, e.g. spectral noise profiles.
(b). Acoustic analyses
Common blackbirds have a discontinuous singing style, with strophes that can be divided into motif and twitter parts [57,58]. We restricted our analysis to the motif part of the song for two reasons. First, the motif portion of the song is higher in amplitude than the twitter elements and thus is crucial for long-range communication in this species [59]. Second, the frequency range of motif elements is narrower and frequencies are lower and thus this part of the song is more heavily affected by low-frequency anthropogenic noise [22,32]. For the analysis of the song recordings from our laboratory, we used an operational definition of motif elements that allowed an automatic sound processing. In particular, elements were classified as motif elements if they were tonal, had a peak frequency below 3.5 kHz and a bandwidth of less than 2.5 kHz [22,60]. The acoustic analyses were conducted using the automatic parameter measurement function of the software Avisoft SASLab Pro v. 5 (Raimund Specht, Berlin, Germany). Spectral parameters were measured with a frequency resolution of 22 Hz. Temporal parameters were measured separately with a resolution of 2.9 ms. As often occurs in field recordings from urban areas, the lower-pitched part of the songs were partly masked by low-frequency background noise, which impeded the reliable measurement of minimum frequencies [22,61,62]. Therefore, we restricted our comparison of field and laboratory recordings to peak frequencies, i.e. the frequency at the maximum amplitude in the spectrum. Sound pressure level was measured as root mean square values using a time window of 125 ms, which is equivalent to the ‘fast’ time-weighting setting of a sound pressure level meter with flat response curve. It has been shown in several species that mean song amplitude can differ considerably between individuals [55,56,63] and this is probably also true among blackbirds. However, as we were interested in within-bird differences in song amplitude rather than between-bird differences, we did not measure the calibrated sound pressure level of the birds. In order to investigate whether song amplitude within individuals varies in a consistent way with frequency, we normalized the amplitude values within each male by setting the maximum value of each bird to 0 dB with other amplitudes reported as negative dB relative to this reference. The captive birds commenced the day with soft vocalizations (cf. [64]) and then gradually increased their vocal amplitude, which may account for the comparably high variation in amplitude levels. We correlated the mean amplitude and frequency values of all investigated individuals based on the weighted amplitude averages measured in 100 Hz intervals (figure 1).
Figure 1.
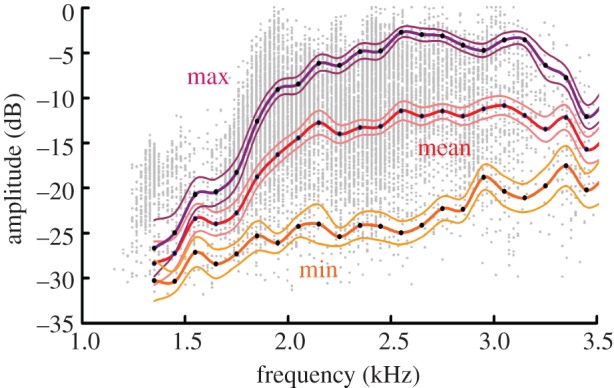
Relationship between peak song frequency and amplitude in blackbirds (n = 12 males recorded in sound-shielded chambers). Minimum peak frequency (min); maximum peak frequency (max) and mean peak frequency curves are based on the weighted amplitudes averages of all males, measured in 100 Hz intervals (black dots). Upper and lower lines denote standard errors above and below these averages. Individual peak frequencies of all measured motif elements of all males (i.e. 13 298 elements in 20 Hz intervals) are shown in grey.
In total, we analysed 13 298 motif elements from the birds in the sound-proof chambers (mean = 1108 elements per male, range = 176–2861 elements) and 4046 from the wild birds (1721 from city males (mean = 108, range = 31–287) and 2325 from forest males (mean = 137, range = 20–341)). See the electronic supplementary material, tables S1 and S2.
(c). Estimation of amplitude differences between city and forest blackbirds
In a next step, the vocal range of the captive birds and the distribution of peak frequencies in the free-living city and forest birds were used to estimate frequency-dependent amplitude differences between city and forest songs. Assuming that free-living blackbirds show similar frequency–amplitude relationships in their songs as our captive birds, we calculated the average amplitude values for each bird. This was done by multiplying the number of syllables per measured frequency interval with the average amplitude in this interval calculated from the averages of amplitudes in the laboratory. For these calculations, we used the average maximum amplitudes for each frequency, as we assume that in noise birds would sing closer to the upper limits of their vocal intensity [19]. The variation in the differences in amplitude between city and forest birds were calculated by taking the square root of the sum of the squared standard errors of the amplitude distribution in our samples of wild and captive birds. The calculated amplitudes of city and forest birds were compared with a two-sample t-test (two-tailed). The dataset was suitable for parametric testing, for the amplitude values in both groups did not deviate from normality (Kolmogorov Smirnov test: Ncity = 16, p = 0.892; Nforest = 17, p = 0.623) and the variances did not differ between the two groups (F-test: F = 0.664, Ncity = 17, Nforest = 16, p = 0.210).
We used a published model [32] to investigate the effects of amplitude and frequency changes on the communication distances in a noisy city environment (for details, see [32]). Again, we used the parameter variance measured in the captive and in the wild birds to get variance estimates for our predicted average communication distances in forest and city birds. The error propagation in our model was estimated by linear approximation.
3. Results
(a). Vocal range profile
We found a marked frequency-dependent variation in vocal amplitude in the analysed blackbirds, with a total amplitude range of the vocal profile of 26 dB (figure 1). In the lower-frequency range up to 2.2 kHz, which is the most relevant for potential masking by traffic noise, maximum, mean and also minimum amplitude values were strongly correlated with frequency (figure 1; Spearman rank correlation: n = 10 intervals, maximum amplitudes: R = 0.98, p < 0.001, mean amplitudes: R = 0.98, p < 0.001, minimum amplitudes: R = 0.93, p < 0.001).
(b). Frequency distribution of song elements in forest and city birds
The forest and city birds analysed showed a clear difference in their usage of different element frequencies (figure 2). The forest birds used the frequency band from 1.8 to 1.9 kHz most often (16% of all motif elements), whereas the city birds sang the highest number of elements in the range between 2.2 and 2.3 kHz. Forest males used frequencies below 2 kHz significantly more often than city birds did (Mann-Whitney U-test, Ncity = 16, Nforest = 17, Z = −4.467, p < 0.001).
Figure 2.

Distribution of motif elements in blackbird songs recorded in the city of Vienna and the Vienna Woods. The curves give average percentages of peak frequency values in 100 Hz intervals for 16 city (blue) and 17 forest birds (green), the error bars show standard errors of these values.
(c). Amplitude differences between forest and city birds
In the next step of our analysis, we used the maximum element amplitudes measured in the sound-shielded chambers to calculate potential amplitude differences between city and forest songs in the wild. We found that the use of high-frequency elements with higher amplitudes in city birds leads to an average increase in song amplitude of 2.5 ± 0.82 dB (mean ± s.e.) over the use of lower-frequency songs of forest birds (t-test:, Ncity = 16, Nforest = 17, t = 3.422, p = 0.002). The amplitude gain of city birds is even higher when only considering the elements that were used most frequently in the two populations: the city elements at the most commonly used frequency at 2.3 kHz had on average a 6 dB higher amplitude than elements at the frequency that were used most often by the forest birds at 1.8 kHz (figure 2).
4. Discussion
Our results show that higher-frequency song elements in blackbirds were also higher in amplitude, and that city birds preferentially sang higher-frequency (and thus higher amplitude) song elements than forest birds.
(a). Amplitude increases with frequency
Our phonetogram of blackbird song elements revealed a clear positive correlation of frequency and amplitude in the frequency range up to 2.2 kHz. The variation in amplitude with frequency was remarkably strong, for example, from 1.5 to 2.5 kHz the average maximum amplitude level increased by more than 15 dB. This finding corroborates earlier studies which also reported positive relationships between frequency and amplitude in other songbird species [44–47], suggesting that proximate mechanisms, such as physical impedances [65], biophysical limitations [41] or physiological constraints [66], may limit the production of loud vocalizations at the lower end of the frequency range.
(b). Different usage of song elements in city and forest birds
We found that city and forest blackbirds differed markedly in their usage of song elements based on element peak frequencies. Interestingly, both distributions were multimodal with maxima at roughly the same frequencies, except that city birds did not sing many elements below 2 kHz. In particular, city birds sang very few elements with peak frequencies around 1.8 kHz, which was a mode in forest birds. Instead, city males sang more elements at frequencies above 2.2 kHz. Biophysical constraints may shape vocal output, for example, if the two sides of the syrinx have different vocal ranges or resonant frequencies, although there are as yet few data on the relationship between amplitude and frequency control in the songbird syrinx [39,40,67].
Peak frequencies can be changed either by singing different element types or by shifting the spectral energy within the same syllable types, without changing the overall frequency contour of the element. To find out which is the case in blackbirds, one must do experiments comparing repertoire performance of individual males in noisy and quiet conditions. To this end, published methods for scoring blackbird song elements and repertoire size [57,68] can be used in combination with spectrographic analyses. The potential use of different song elements in blackbirds could be similar to the switching of song types in noise observed in great tits (Parus major) [69]. Our findings, however, offer an alternative explanation for such song type switching: by choosing higher-frequency elements, birds not only switch to elements that happen to be less heavily masked in low-frequency traffic noise, but may also exploit an indirect means to sing songs at higher amplitudes.
(c). Implications for communication in noise
Our results indicate that city blackbirds increase their vocal amplitude by singing at higher frequencies. To evaluate the quantitative importance of these increased amplitude values for mitigating acoustic masking by traffic noise, we used a published model for signal transmission of bird song that is based on song amplitude measurements in wild blackbirds and urban noise spectra experienced by birds in cities [32]. According to this model, the frequency shift observed in city blackbird song leads in average city noise to an increase in communication distance by 13 per cent when compared with lower-frequency forest songs. However, the average frequency-related amplitude increase of 2.5 dB found in this study will add an even greater gain, resulting in a total increase in communication distance of 43 per cent. While blackbirds can communicate with forest songs over a distance of up to 30 ± 2.1 m (mean ± s.e.) in city noise, city songs can be transmitted over 43 ± 2.8 m (mean ± s.e.). Thus, in traffic noise, the average transmission benefit of the frequency-dependent increase in song amplitude is much greater than the effect of the frequency shift itself.
The differences between low- and high-frequency vocalizations are even larger when considering selected motif elements rather than average values across entire songs.
The most common peak frequency in our forest sample was between 1.8 and 1.9 kHz, whereas the city birds produced most commonly song elements with a peak frequency between 2.3 and 2.4 kHz. By using these higher-pitched elements, which can be produced at higher amplitudes, instead of the lower-pitched elements between 1.8 and 1.9 kHz, which are commonly used in forests, blackbirds increase their communication distance from 19 ± 1.2 m to 60 ± 3.8 m (mean ± s.e.).
(d). What is the unit of analysis, songs or elements?
These considerations raise the question whether one should regard the averages of entire songs or certain elements as important for avian communication in noise. Some previous studies have related mean song frequency values to environmental noise levels [21,22,28,70,71]. Means are the expected values of a random variable from a uniform and ideally Gaussian distribution. In blackbirds, the distribution of song peak frequencies was multimodal, but certainly not normally distributed (figure 2). This may be attributed to the complex sound production mechanism in songbirds [40]. In cases in which bird songs consist of many different elements, the measurement of one mean frequency value of the entire song neglects this variation and may miss important biological variation within songs. Considering only the mean song frequencies in analyses of the vocal behaviour in urban and rural birds may therefore be misleading. In our model [32], we used published averages of song to calculate communication distances. As shown in the application of the model for the blackbird data reported in this study, a frequency shift for elements at the lower end of the vocal frequency range can have a strong effect on the active space of a song. The same applies to amplitude changes. Eventually, field studies that combine detailed measurements of frequency and amplitude shifts will help to gain a more accurate view of how songbirds adjust their vocal signals in urban noise.
(e). Explanations for higher-frequency city songs
Our study shows that the increased amplitude of higher-frequency blackbird song elements yields a greater release from masking than the frequency shifts alone. As shown in previous studies, birds exhibit the Lombard effect [19] and thus city blackbirds probably sing with a higher sound pressure level than forest birds across all frequencies. The resulting higher signal-to-noise ratio would add to the effect of the here described frequency–amplitude relationship. However, it is important to bear in mind that bird song is affected by many more selection pressures than just anthropogenic noise. Blackbird song, especially the motif elements, seems adapted to forest acoustics, where lower frequencies and more tonal elements transmit particularly well [3,59]. Thus, the greater numbers of low-frequency motif elements in forest birds can also be interpreted as an adaptation to closed habitats. Conversely, as shown for songs of great tits [31], there is also the possibility that city songs are adjusted to the structure of urban habitats. Furthermore, the song divergence between city and forest birds might also be explained by additional factors that are not related to signal transmission but to other aspects of urban ecology [22]. For instance, the breeding density of blackbirds is generally higher in urban habitats than in forests [72] and as a result, city birds may be engaging in more and more intense territorial interactions. Several studies have shown that a higher arousal is reflected in higher song frequencies in this species ([44,73,74], but see [75]). Supporting this view, a recent study on urban great tits [71] found that both noise level and breeding density predicted song frequency. Moreover, forest blackbirds have higher plasma testosterone levels than urban males [76], and a higher testosterone level may lead to lower song frequencies ([77], but see [78]), which could account for the lower song frequencies in forest blackbirds found in this and other studies. However, all these explanations are not mutually exclusive. The observed patterns of frequency–amplitude variation in this study hint at a physical coupling during sound production, which would operate independently of, and in addition to any changes owing to differences in arousal or testosterone level.
5. Conclusions
In summary, we show that peak frequency and amplitude were coupled in blackbird song, and that city birds preferentially sang higher-frequency elements that can be produced at higher sound intensities. Both the increased frequency and the related rise in amplitude reduce acoustic masking by low-frequency traffic noise but the frequency-dependent amplitude change has a greater effect. By choosing higher elements, city birds may further increase their capacity to sing at high amplitudes to mitigate acoustic masking by noise.
Acknowledgements
All work described has been carried out in accordance with ASAB/ABS's Guidelines for the treatment of Animals in Research.
We thank the animal caretakers and the technical staff of the Max Planck Institute for Ornithology for their support. Sophie Jaquier helped in the laboratory recordings and Lisa Trost gave logistic support. We also thank two anonymous reviewers for their comments. The recording of wild blackbirds was funded by the Hochschuljubiläumsfond der Stadt Wien (Nr H-2323/2007), all other data collection and analyses were supported by the Max Planck Society and the Deutsche Forschungsgemeinschaft (award BR 2309/6-1).
References
- 1.Bradbury JW, Vehrencamp SL. 2011. Principles of animal communication, 2nd edn Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- 2.Brumm H, Naguib M. 2009. Environmental acoustics and the evolution of bird song. Adv. Study Behav. 40, 1–33 10.1016/S0065-3454(09)40001-9 (doi:10.1016/S0065-3454(09)40001-9) [DOI] [Google Scholar]
- 3.Morton ES. 1975. Ecological sources of selection on avian sounds. Am. Nat. 109, 17–34 10.1086/282971 (doi:10.1086/282971) [DOI] [Google Scholar]
- 4.Wiley RH, Richards DG. 1978. Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav. Ecol. Sociobiol. 3, 69–94 10.1007/BF00300047 (doi:10.1007/BF00300047) [DOI] [Google Scholar]
- 5.Slabbekoorn H, Ellers J, Smith TB. 2002. Birdsong and sound transmission: the benefits of reverberations. Condor 104, 564–573 10.1650/0010-5422(2002)104[0564:BASTTB]2.0.CO;2 (doi:10.1650/0010-5422(2002)104[0564:BASTTB]2.0.CO;2) [DOI] [Google Scholar]
- 6.Nemeth E, Dabelsteen T, Pedersen SB, Winkler H. 2006. Rainforest as concert halls for birds: are reverberations improving sound transmission of long song elements? J. Acoust. Soc. Am. 119, 620–626 10.1121/1.2139072 (doi:10.1121/1.2139072) [DOI] [PubMed] [Google Scholar]
- 7.Wiley RH. 1991. Associations of song properties with habitats for territorial oscine birds of eastern North America. Am. Nat. 138, 973–993 10.1086/285263 (doi:10.1086/285263) [DOI] [Google Scholar]
- 8.Padgham M. 2004. Reverberation and frequency attenuation in forests: implications for acoustic communication in animals. J. Acoust. Soc. Am. 115, 402–410 10.1121/1.1629304 (doi:10.1121/1.1629304) [DOI] [PubMed] [Google Scholar]
- 9.Mathevon N, Aubin T, Dabelsteen T. 1996. Song degradation during propagation: importance of song post for the wren Troglodytes troglodytes. Ethology 102, 397–412 10.1111/j.1439-0310.1996.tb01135.x (doi:10.1111/j.1439-0310.1996.tb01135.x) [DOI] [Google Scholar]
- 10.Nemeth E, Winkler H, Dabelsteen T. 2001. Differential degradation of antbird songs in a Neotropical rainforest: adaptation to perch height? J. Acoust. Soc. Am. 110, 3263–3274 10.1121/1.1420385 (doi:10.1121/1.1420385) [DOI] [PubMed] [Google Scholar]
- 11.Blumenrath SH, Dabelsteen T. 2004. Degradation of great tit (Parus major) song before and after foliation: implications for vocal communication in a deciduous forest. Behaviour 141, 935–958 10.1163/1568539042360152 (doi:10.1163/1568539042360152) [DOI] [Google Scholar]
- 12.Brown TJ, Handford P. 2003. Why birds sing at dawn: the role of consistent sound transmission. Ibis 145, 120–129 10.1046/j.1474-919X.2003.00130.x (doi:10.1046/j.1474-919X.2003.00130.x) [DOI] [Google Scholar]
- 13.Henwood K, Fabrick A. 1979. A quantitative analysis of the dawn chorus: temporal selection for communicatory optimisation. Am. Nat. 114, 260–274 10.1086/283473 (doi:10.1086/283473) [DOI] [Google Scholar]
- 14.Dabelsteen T, Mathevon N. 2002. Why do songbirds sing intensively at dawn? A test of the acoustic transmission hypothesis. Acta Ethol. 4, 65–72 10.1007/s10211-001-0056-8 (doi:10.1007/s10211-001-0056-8) [DOI] [Google Scholar]
- 15.Brumm H, Slabbekoorn H. 2005. Acoustic communication in noise. Adv. Study Behav. 35, 151–209 10.1016/S0065-3454(05)35004-2 (doi:10.1016/S0065-3454(05)35004-2) [DOI] [Google Scholar]
- 16.Slabbekoorn H, Ripmeester EAP. 2008. Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83 10.1111/j.1365-294X.2007.03487.x (doi:10.1111/j.1365-294X.2007.03487.x) [DOI] [PubMed] [Google Scholar]
- 17.Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427 10.1016/j.tree.2010.04.005 (doi:10.1016/j.tree.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 18.Fuller RA, Warren PH, Gaston KJ. 2007. Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 3, 368–370 10.1098/rsbl.2007.0134 (doi:10.1098/rsbl.2007.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brumm H, Zollinger SA. 2011. The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour 148, 1173–1198 10.1163/000579511X605759 (doi:10.1163/000579511X605759) [DOI] [Google Scholar]
- 20.Brumm H. 2004. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 73, 434–440 10.1111/j.0021-8790.2004.00814.x (doi:10.1111/j.0021-8790.2004.00814.x) [DOI] [Google Scholar]
- 21.Slabbekoorn H, Peet M. 2003. Birds sing at a higher pitch in urban noise. Nature 424, 267. 10.1038/424267a (doi:10.1038/424267a) [DOI] [PubMed] [Google Scholar]
- 22.Nemeth E, Brumm H. 2009. Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Anim. Behav. 78, 637–641 10.1016/j.anbehav.2009.06.016 (doi:10.1016/j.anbehav.2009.06.016) [DOI] [Google Scholar]
- 23.Fernández-Juricic E, Poston R, Collibus KD, Morgan T, Bastain B, Martin C, Jones K, Treminio R. 2005. Microhabitat selection and singing behavior patterns of male house finches (Carpodacus mexicanus) in urban parks in a heavily urbanized landscape in the Western US. Urban Habitats 1, 49–69 [Google Scholar]
- 24.Mockford EJ, Marshall RC. 2009. Effects of urban noise on song and response behaviour in great tits. Proc. R. Soc. B 276, 2979–2985 10.1098/rspb.2009.0586 (doi:10.1098/rspb.2009.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potvin DA, Parris KM, Mulder RA. 2011. Geographically pervasive effects of urban noise on frequency and syllable rate of songs and calls in silvereyes (Zosterops lateralis). Proc. R. Soc. B 278, 2464–2469 10.1098/rspb.2010.2296 (doi:10.1098/rspb.2010.2296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verzijden MN, Ripmeester EAP, Ohms VR, Snelderwaard P, Slabbekoorn H. 2010. Immediate spectral flexibility in singing chiffchaffs during experimental exposure to highway noise. J. Exp. Biol. 213, 2575–2581 10.1242/jeb.038299 (doi:10.1242/jeb.038299) [DOI] [PubMed] [Google Scholar]
- 27.Bermúdez-Cuamatzin E, Ríos-Chelén AA, Gil D, Garcia CM. 2011. Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol. Lett. 7, 36–38 10.1098/rsbl.2010.0437 (doi:10.1098/rsbl.2010.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross K, Pasinelli G, Kunc HP. 2010. Behavioral plasticity allows short term adjustment to a novel environment. Am. Nat. 176, 456–464 10.1086/655428 (doi:10.1086/655428) [DOI] [PubMed] [Google Scholar]
- 29.Pohl NU, Slabbekoorn H, Klump GM, Langemann U. 2009. Effects of signal features and environmental noise on signal detection in the great tit, Parus major. Anim. Behav. 78, 1293–1300 10.1016/j.anbehav.2009.09.005 (doi:10.1016/j.anbehav.2009.09.005) [DOI] [Google Scholar]
- 30.Halfwerk W, Bot S, Buikx J, van der Velde M, Komdeur J, ten Cate C, Slabbekoorn H. 2011. Low-frequency songs lose their potency in noisy urban conditions. Proc. Natl Acad. Sci. USA 35, 14 549–14 554 10.1073/pnas.1109091108 (doi:10.1073/pnas.1109091108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mockford EJ, Marshall RC, Dabelsteen T. 2011. Degradation of rural and urban tit song: testing transmission efficiency. PLoS ONE 6, e28242. 10.1371/journal.pone.0028242 (doi:10.1371/journal.pone.0028242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemeth E, Brumm H. 2010. Birds and anthropogenic noise: are urban songs adaptive? Am. Nat. 176, 465–475 10.1086/656275 (doi:10.1086/656275) [DOI] [PubMed] [Google Scholar]
- 33.Nemeth E, Zollinger SA, Brumm H. 2012. Effect sizes and the integrative understanding of urban bird song. (A reply to Slabbekoorn et al.) Am. Nat. 180, 146–152 10.1086/665994 (doi:10.1086/665994) [DOI] [Google Scholar]
- 34.Zollinger SA, Brumm H. 2011. The Lombard effect. Curr. Biol. 21, R614–R615 10.1016/j.cub.2011.06.003 (doi:10.1016/j.cub.2011.06.003) [DOI] [PubMed] [Google Scholar]
- 35.Osmanski MS, Dooling RJ. 2009. The effect of altered auditory feedback on control of vocal production in budgerigars (Melopsittacus undulatus) . J. Acoust. Soc. Am. 126, 911–919 10.1121/1.3158928 (doi:10.1121/1.3158928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster S, Zollinger SA, Lesku JA, Brumm H. 2012 On the evolution of noise-dependent vocal plasticity in birds. Biol. Lett. 8, 913–916 10.1098/rsbl.2012.0676 (doi:10.1098/rsbl.2012.0676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard ML, Horn AG. 2005. Ambient noise and the design of begging signals. Proc. R. Soc. B 272, 651–656 10.1098/rspb.2004.3021 (doi:10.1098/rspb.2004.3021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Titze IR. 1994. Principles of voice production. Englewood Cliffs, NJ: Prentice Hall [Google Scholar]
- 39.Elemans CPH, Zaccarelli R, Herzel H. 2008. Biomechanics and control of vocalization in a non-songbird. J. R. Soc. Interface 5, 691–703 10.1098/rsif.2007.1237 (doi:10.1098/rsif.2007.1237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suthers RA, Zollinger SA. 2004. Producing song: the vocal apparatus. In Behavioral neurobiology of birdsong (eds Zeigler HP, Marler P.), pp. 109–129 New York, NY: New York Academy of Sciences; [DOI] [PubMed] [Google Scholar]
- 41.Riede T, Suthers RA, Fletcher NH, Blevins WE. 2006. Songbirds tune their vocal tract to the fundamental frequency of their song. Proc. Natl Acad. Sci. USA 103, 5543–5548 10.1073/pnas.0601262103 (doi:10.1073/pnas.0601262103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CZH, Herbst JA, Keller GB, Hahnloser RHR. 2008. Rapid interhemispheric switching during vocal production in a songbird. PLoS Biol. 6, 2154–2162 10.1371/journal.pbio.0060250 (doi:10.1371/journal.pbio.0060250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suthers RA, Zollinger SA. 2008. From brain to song: the vocal organ and vocal tract. In Neuroscience of birdsong (eds Zeigler HP, Marler P.), pp. 78–98 Cambridge, UK: Cambridge University Press [Google Scholar]
- 44.Dabelsteen T. 1984. An analysis of the full song of the blackbird Turdus merula with respect to message coding and adaptations for acoustic communication. Ornis Scand. 15, 227–239 10.2307/3675931 (doi:10.2307/3675931) [DOI] [Google Scholar]
- 45.Nelson BS. 2000. Avian dependence on sound pressure level as an auditory distance cue. Anim. Behav. 59, 57–67 10.1006/anbe.1999.1278 (doi:10.1006/anbe.1999.1278) [DOI] [PubMed] [Google Scholar]
- 46.Goller F, Cooper BG. 2008. Peripheral mechanisms of sensorimotor integration during singing. In Neuroscience of birdsong (eds Zeigler HP, Marler P.), pp. 99–114 Cambridge, UK: Cambridge University Press [Google Scholar]
- 47.Ritschard M, Brumm H. 2011. Effects of vocal learning, phonetics and inheritance on song amplitude in zebra finches. Anim. Behav. 82, 1415–1422 10.1016/j.anbehav.2011.09.026 (doi:10.1016/j.anbehav.2011.09.026) [DOI] [Google Scholar]
- 48.Ripmeester EAP, Kok JS, van Rijssel JC, Slabbekoorn H. 2010. Habitat-related birdsong divergence: a multi-level study on the influence of territory density and ambient noise in European blackbirds. Behav. Ecol. Sociobiol. 64, 409–418 10.1007/s00265-009-0857-8 (doi:10.1007/s00265-009-0857-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Titze IR. 1992. Acoustic interpretation of the voice range profile (phonetogram). J. Speech Hear. Res. 35, 21–34 [DOI] [PubMed] [Google Scholar]
- 50.Tchernichovski O, Lints TJ, Deregnaucourt S, Cimenser A, Mitra PP. 2004. Studying the song development process rationale and methods. In Behavioral neurobiology of birdsong (eds Zeigler HP, Marler P.), pp. 348–363 New York, NY: New York Academy of Sciences; [DOI] [PubMed] [Google Scholar]
- 51.Larsen ON, Dabelsteen T. 1990. Directionality of blackbird vocalization. Implications for vocal communication and its further study. Ornis Scand. 21, 37–45 10.2307/3676376 (doi:10.2307/3676376) [DOI] [Google Scholar]
- 52.Brumm H. 2002. Sound radiation patterns in nightingale (Luscinia megarhynchos) songs. J. Ornithol. 143, 468–471 [Google Scholar]
- 53.Patricelli GL, Dantzker MS, Bradbury JW. 2007. Differences in acoustic directionality among vocalizations of the male red-winged blackbird (Agelaius pheoniceus) are related to function in communication. Behav. Ecol. Sociobiol. 61, 1099–1110 10.1007/s00265-006-0343-5 (doi:10.1007/s00265-006-0343-5) [DOI] [Google Scholar]
- 54.Brumm H. 2009. Song amplitude and body size in birds. Behav. Ecol. Sociobiol. 63, 1157–1165 10.1007/s00265-009-0743-4 (doi:10.1007/s00265-009-0743-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brumm H, Zollinger SA, Slater PJB. 2009. Developmental stress affects song learning but not song complexity and vocal amplitude in zebra finches. Behav. Ecol. Sociobiol. 63, 1387–1395 10.1007/s00265-009-0749-y (doi:10.1007/s00265-009-0749-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nemeth E, Kempenaers B, Matessi G, Brumm H. 2012. Rock sparrow song reflects male age and reproductive success. PLoS ONE 7, e43259. 10.1371/journal.pone.0043259 (doi:10.1371/journal.pone.0043259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Todt D. 1970. Gesangliche Reaktionen der Amsel (Turdus merula L.) auf ihren experimentell reproduzierten Eigengesang. Z. vergl. Physiologie 66, 294–317 10.1007/BF00297831 (doi:10.1007/BF00297831) [DOI] [Google Scholar]
- 58.Dabelsteen T. 1981. The sound pressure level in the dawn song of the blackbird Turdus merula and a method for adjusting the level in experimental song to the level in natural song. Z. Tierpsychol. 56, 137–149 [Google Scholar]
- 59.Dabelsteen T, Larsen ON, Pedersen SB. 1993. Habitat-induced degradation of sound signals: quantifying the effects of communication sounds and bird location on blur ratio, excess attenuation, and signal-to-noise ratio in blackbird song. J. Acoust. Soc. Am. 93, 2206–2220 10.1121/1.406682 (doi:10.1121/1.406682) [DOI] [Google Scholar]
- 60.Dabelsteen T, Pedersen SB. 1990. Song and information about aggressive responses of blackbirds, Turdus merula: evidence from interactive playback experiments with territory owners. Anim. Behav. 40, 1158–1168 10.1016/S0003-3472(05)80182-4 (doi:10.1016/S0003-3472(05)80182-4) [DOI] [Google Scholar]
- 61.Beecher MD. 1988. Spectrographic analysis of animal vocalisations: implications of the uncertainty principle. Bioacoustics 1, 187–208 10.1080/09524622.1988.9753091 (doi:10.1080/09524622.1988.9753091) [DOI] [Google Scholar]
- 62.Zollinger SA, Podos J, Nemeth E, Goller F, Brumm H. 2012. On the relationship between, and measurement of, amplitude and frequency in bird song. Anim. Behav. 84, e1–e9 10.1016/j.anbehav.2012.04.026 (doi:10.1016/j.anbehav.2012.04.026) [DOI] [Google Scholar]
- 63.Brumm H, Ritschard M. 2011. Song amplitude affects territorial aggression of male receivers in chaffinches. Behav. Ecol. 22, 310–316 10.1093/beheco/arq205 (doi:10.1093/beheco/arq205) [DOI] [Google Scholar]
- 64.Brumm H, Hultsch H. 2001. Pattern amplitude is related to pattern imitation during the song development of nightingales. Anim. Behav 61, 747–754 10.1006/anbe.2000.1664 (doi:10.1006/anbe.2000.1664) [DOI] [Google Scholar]
- 65.Bradbury JW, Vehrencamp SL. 2011. Web Topic 2.9: radiation efficiency and sound radiator size. In Principles of animal communication, 2nd edn See http://sites.sinauer.com/animalcommunication2e (accessed 20 July 2012) [Google Scholar]
- 66.Wild JM, Goller F, Suthers RA. 1998. Inspiratory muscle activity during birdsong. J. Neurobiol 36, 441–453 (doi:10.1002/(SICI)1097-4695(19980905)36:3<441::AID-NEU11>3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- 67.Zollinger SA, Riede T, Suthers RA. 2008. Two-voice complexity from a single side of the syrinx in northern mockingbird Mimus polyglottos vocalizations. J. Exp. Biol. 211, 1978–1991 10.1242/jeb.014092 (doi:10.1242/jeb.014092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hesler N, Mundry R, Sacher T, Coppack T, Bairlein F, Dabelsteen T. 2012. Song repertoire size correlates with measures of body size in Eurasian blackbirds. Behaviour 149, 645–665 10.1163/156853912X649920 (doi:10.1163/156853912X649920) [DOI] [Google Scholar]
- 69.Halfwerk W, Slabbekoorn H. 2009. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim. Behav. 78, 1301–1307 10.1016/j.anbehav.2009.09.015 (doi:10.1016/j.anbehav.2009.09.015) [DOI] [Google Scholar]
- 70.Francis CD, Ortega CP, Cruz A. 2011. Different behavioural responses to anthropogenic noise by two closely related passerine birds. Biol. Lett. 7, 850–852 10.1098/rsbl.2011.0359 (doi:10.1098/rsbl.2011.0359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamao S, Watanabe M, Mori Y. 2011. Urban noise and male density affect songs in the great tit Parus major. Ethol. Ecol. Evol. 23, 111–119 10.1080/03949370.2011.554881 (doi:10.1080/03949370.2011.554881) [DOI] [Google Scholar]
- 72.Snow DW. 1958. A study in blackbirds. London, UK: George Allen Unwin Ltd [Google Scholar]
- 73.Dabelsteen T. 1985. Messages and meanings of bird song with special reference to the blackbird (Turdus merula) and some methodology problems. Biol. Skr. Dan. Vid. Sel. 25, 173–208 [Google Scholar]
- 74.Dabelsteen T, Pedersen SB. 1985. Correspondence between messages in the full song of the blackbird Turdus merula and meanings to territorial males, as inferred from responses to computerized modifications of natural song. Z. Tierpsychol. 69, 149–165 10.1111/j.1439-0310.1985.tb00142.x (doi:10.1111/j.1439-0310.1985.tb00142.x) [DOI] [Google Scholar]
- 75.Ripmeester EAP, De Vries AM, Slabbekoorn H. 2007. Do blackbirds signal motivation to fight with their song? Ethology 113, 1021–1028 10.1111/j.1439-0310.2007.01398.x (doi:10.1111/j.1439-0310.2007.01398.x) [DOI] [Google Scholar]
- 76.Partecke J, Van't Hof TJ, Gwinner E. 2005. Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J. Avian Biol. 36, 295–305 10.1111/j.0908-8857.2005.03344.x (doi:10.1111/j.0908-8857.2005.03344.x) [DOI] [Google Scholar]
- 77.Cynx J, Bean NJ, Rossman I. 2005. Testosterone implants alter the frequency range of zebra finch songs. Horm. Behav. 47, 446–451 10.1016/j.yhbeh.2004.11.018 (doi:10.1016/j.yhbeh.2004.11.018) [DOI] [PubMed] [Google Scholar]
- 78.Ritschard M, Laucht S, Dale J, Brumm H. 2011. Enhanced testosterone levels affect singing motivation but not song structure and amplitude in Bengalese finches. Physiol. Behav. 102, 30–35 10.1016/j.physbeh.2010.10.005 (doi:10.1016/j.physbeh.2010.10.005) [DOI] [PubMed] [Google Scholar]


