Abstract
Co-infection is ubiquitous in people in the developing world but little is known regarding the potential for one parasite to act as a risk factor for another. Using generalized linear mixed modelling approaches applied to data from school-aged children from Zanzibar, Tanzania, we determined the strength of association between four focal infections (i.e. Ascaris lumbricoides, Trichuris trichiura, hookworm and self-reported fever, the latter used as a proxy for viral, bacterial or protozoal infections) and the prevalence or intensity of each of the helminth infections. We compared these potential co-infections with additional risk factors, specifically, host sex and age, socioeconomic status and physical environment, and determined what the relative contribution of each risk factor was. We found that the risk of infection with all four focal infections was strongly associated with at least one other infection, and that this was frequently dependent on the intensity of that other infection. In comparison, no other incorporated risk factor was associated with all focal infections. Successful control of infectious diseases requires identification of infection risk factors. This study demonstrates that co-infection is likely to be one of these principal risk factors and should therefore be given greater consideration when designing disease-control strategies. Future work should also incorporate other potential risk factors, including host genetics which were not available in this study and, ideally, assess the risks via experimental manipulation.
Keywords: co-infection, infection risk, infection heterogeneity, childhood infection, soil-transmitted helminthiasis, fever
1. Introduction
Co-infection (where two or more virus, bacteria, protozoa or helminth species concomitantly infect an individual) is the norm in most natural systems, including people living in developing countries [1–5]. Infectious diseases are among the most important causes of childhood mortality and morbidity in the developing world [6–13]. In sub-Saharan Africa alone, approximately 1.5 million children die before their fifth birthday, owing to infectious diseases [14,15]. However, there is clearly heterogeneity between individuals, in the mix of infections an individual has and in the intensity of those infections [4,16,17]. Thus, some children have many infections and/or have high intensities of infection, whereas others have few infections and/or the intensity of those infections is low. Understanding what drives an individual's infections is essential if effective disease-control strategies are to be developed [18,19].
There are many possible risk factors associated with an individual's infections, including their physical environment [17,20–22], genetics [23,24], behaviour [25,26] and demographic factors [27,28]. A comparatively understudied risk factor for infection with one organism is co-infection with a second species. Any infection will change the within-host environment in some way, for example by inducing changes in host physiology (e.g. Leishmania spp. creating an ulcerous wound that bacterial opportunists can exploit [29]) and/or the host immune response [1,30,31] (e.g. hookworm-induced suppression of the pro-inflammatory cytokine IFN-γ causing increased parasitaemia with Plasmodium parasites [32]). Therefore, a second parasite species encountering an already infected host experiences a different environment than if that host was uninfected. Such changes to the host environment could potentially predispose, or protect, that host from a second infection, and/or modulate the intensity of that infection. There is increasing evidence from both laboratory investigations [33–35], and in particular from field studies of animals [36–38], that interspecific parasite interactions can be a powerful influence on parasite dynamics. However, in humans, co-infection is often considered as the co-incident result of other infection risk factors and it is rarely considered that one infection could alter the risk of infection by a second species.
There have been a few recent studies that consider the role of co-infection alongside other potential infection risk factors, focusing largely on helminth and Plasmodium infections [4,39–42]. However, these studies generally consider co-infection in terms of co-exposure, describing the geographical co-incident overlap of the parasite species’ infective stages, vectors or intermediate hosts, resulting from their shared requirements for particular climatic and geographical conditions. Despite this ‘co-incident overlap’ focus, these studies do provide evidence that associations between co-infecting species can remain, even after controlling for geographical and environmental attributes [39,40], suggesting that simple co-exposure is not a full explanation for observed parasite co-infections. Further, even where these studies incorporated behavioural and socioeconomic data, there was evidence that some positive associations between parasites persisted [39,40].
Traditional analytical approaches for detecting potential associations between infections (e.g. seeking simple correlations between infection intensities within hosts) do not allow for the effect of other potential infection risk factors to be considered [43,44]. In natural systems, many different factors can potentially act (either alone or together) to change a person's probability of becoming infected with a particular parasite. Therefore, in order to determine the relative importance of the different potential risk factors, advanced statistical methods, such as generalized linear mixed modelling (GLMM), must be used, which enable the simultaneous assessment of multiple factors and have a greater chance of detecting negative relationships between infections [43].
Here, we have used GLMMs applied to a dataset of infections with three common soil-transmitted helminths (STHs, i.e. Ascaris lumbricoides, Trichuris trichiura and hookworm) and self-reported fever in school-aged children from Zanzibar, Tanzania [20]; these data also included other potential infection risk factors (i.e. physical environment, demography, behaviour and socioeconomic status). GLMMs have previously been used to determine (or infer) the presence of both positive and negative interactions between parasites, in both simulated and wild animal parasite datasets [36,37,43]. We used this integrated approach to investigate the role of co-infection in infection risk after accounting for other infection risk factors.
2. Methods
(a). Participants and study design
The study design, description of the population studied, and field and laboratory procedures used have been reported elsewhere [20]. Briefly, in 2008, a community-based, cross-sectional epidemiological survey was carried out in two shehias (administrative areas) of Unguja, the main island of the Zanzibar archipelago. The Bandamaji shehia is located in the rural north of the island, whereas the peri-urban shehia Dole is located in the west of Zanzibar Town in the centre of Unguja. In this study, only infection data of school-aged children were examined. Helminth-control interventions (e.g. preventive chemotherapy) are primarily targeted at this age group [13], yet co-infection's influence on these children has been understudied. Approximately 330 individuals (adults and children) were enrolled in the main village of each shehia; 180 children, aged 5–16 years, provided complete information with 17 children providing one faecal sample, 51 providing two samples and the remaining 112 providing three samples, collected over a 3-day period.
Faecal egg counts (FECs) of A. lumbricoides, T. trichiura and hookworm were recorded for each child in the study; stool samples were examined using the Kato–Katz technique [45]. Strongyloides stercoralis and Schistosoma haematobium infections were also recorded using appropriate diagnostic techniques [20], but these infections were rare and therefore excluded from the present analysis. Malaria is close to elimination in Zanzibar [46–48], and hence was not assessed in this study.
For all children, a pre-tested, standardized questionnaire was used to record a wide range of commonly acknowledged infection risk factors, i.e. an individual's age, sex, soil-eating and shoe-wearing behaviour; their family's socioeconomic status and physical environment. In addition, self-reported status of (i) coughs, (ii) colds and (iii) fevers were recorded using a two-week recall period [20]. Fever was of non-specific aetiology but is a symptom usually associated with viral, bacterial or protozoal infection rather than helminth infection. Malaria is unlikely to make a notable contribution to these self-reports of fever, owing to its rarity in Zanzibar.
(b). Statistical analyses
We investigated the association of the potential infection risk factors, detailed earlier, together with co-infection, on the presence or absence of four focal infections (i.e. A. lumbricoides, T. trichiura, hookworm and self-reported fever). These focal infections were examined in four separate GLMMs using a binomial error structure and a logit link function in the statistical package ASReml v3 (VSN International Ltd., Hemel Hempstead, UK). In all models, repeated measures of FEC (i.e. from patients providing two or more faecal samples) were controlled for by incorporating each child's individual identification code in the random model. A cubic smoothing spline was fitted to age in all initial random models, which allowed potential nonlinear relationships between age and each of the four focal infections to be assessed. Fitting the spline controlled for any age-associated correlations in infection intensity, because apparent associations between infections can arise simply as a result of correlated host age-specific patterns of infection [43].
The initial detailed structure of each model is described in the electronic supplementary material, table S1, and the database used in the analyses is given in the comma separated value file ‘S2.csv’. In brief, all models contained the fixed effects of host age, sex, behaviour, physical environment and socioeconomic status; the infection data were in the form of ln(FEC+1) for A. lumbricoides and T. trichiura, and presence/absence for hookworm and self-reported fever. Presence/absence data rather than FEC were used for hookworm because of low prevalence (i.e. many uninfected individuals) which meant that normalization of these data was not possible by any attempted transformation. Where a STH was used as the dependent variable, presence/absence data on self-reported colds and coughs were also included as explanatory variables; where self-reported fever was the dependent variable, coughs and colds were excluded from the analysis as they are also symptoms of several of the causes of fever and, as such, would be confounding factors.
Likelihood ratio tests were used to compare the random model in each analysis. Following simplification of the random model, the fixed model was refined by stepwise deletion of insignificant terms (i.e. p > 0.05) using the Wald test and evaluation of the conditional F-statistics. Predictions from the final models are presented as prevalence of infection with a STH or self-reported fever (i.e. predictions range between 0 and 1), which can also be viewed as the risk of infection.
Effect sizes for each significant term in the minimal model of each focal infection were calculated as odds ratios (ORs). Where helminth FEC was a significant risk factor, ORs were calculated by comparing uninfected children (i.e. a FEC of zero) with children at the bootstrapped mean (zm) and bootstrapped maximum values (zx).
3. Results
Overall risk of infection (i.e. predicted prevalence) with T. trichiura was 0.40, A. lumbricoides 0.49, hookworm 0.26 and self-reported fever 0.43. Co-infection state was associated with a significant change in infection risk for all four focal infections. Conversely, none of the other factors (i.e. host age, sex, behaviour, socioeconomic status or physical environment) were significant risk factors for all infections, although at least one of these factors was significant in each of the four infection models.
(a). Focal infection with Trichuris trichiura
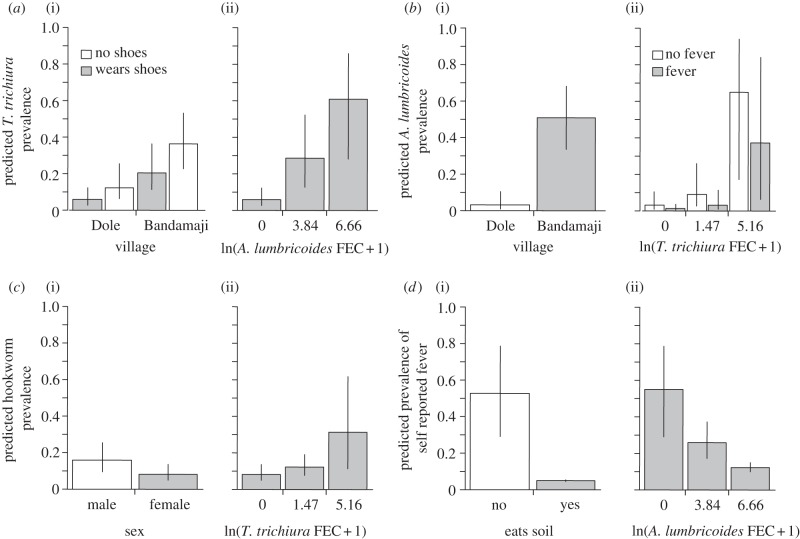
Both a child's village of residency (F1,451 = 10.6, p = 0.001) and shoe-wearing behaviour (F1,451 = 4.95, p = 0.028) were associated with a significant change in the risk of T. trichiura infection. Children resident in rural Bandamaji had substantially higher risk (OR = 4.06) of infection than those from the village in the peri-urban shehia of Dole (figure 1a(i)). Similarly, children who did not wear shoes had a higher risk of infection (OR = 2.25) than those who did (figure 1a(i)). By combining these two risks, the difference between a child living in Dole and wearing shoes compared with one living in Bandamaji and not wearing shoes gave an OR of 9.13.
Figure 1.
Risk factors associated with the four focal infections in school-aged children in Zanzibar. Generalized linear mixed modelling (GLMM) predicted prevalence (equating to risk of infection) of (a) Trichuris trichiura, (b) Ascaris lumbricoides, (c) hookworm and (d) self-reported fever, with the significant risk factors from each model. Section (i) of each graph presents the significant non-infection terms, with predictions made at a value of faecal egg counts (FECs) = 0 and no self-reported fever for model (a). Section (ii) of each graph presents the significant infection terms. Predictions for the infection terms were made with village set to Bandamaji for models (a) and (b), sex set to male for model (c) and no soil consumption for model (d). Error bars show the 95% confidence limits of the predictions.
Co-infection with A. lumbricoides was associated with a significantly higher risk of T. trichiura infection (F1,451 = 30.7, p < 0.001). Further, this risk was positively dependent on A. lumbricoides FEC (figure 1a(ii); comparing zero FEC with the mean FEC (zm) of A. lumbricoides, and OR = 6.22 comparing zero with maximum A. lumbricoides FEC (zx) OR = 24.57).
(b). Focal infection with Ascaris lumbricoides
A child's village of residency was also a significant risk factor for A. lumbricoides infection (F1,451 = 29.58, p < 0.001). Children of Bandamaji had a substantially higher (OR = 30.24) risk of A. lumbricoides infection than those living in Dole (figure 1b(i)).
Infection with T. trichiura (F1,451 = 14.94 p < 0.001) and self-reported fever (F1,451 = 5.31, p = 0.023) were associated with significant changes in the risk of A. lumbricoides infection. The T. trichiura FECs were positively associated with an increased risk of A. lumbricoides infection (figure 1b(ii); zm OR = 3.12 and zx OR = 54.07). Conversely, self-reported fever was associated with a lower risk of A. lumbricoides infection (figure 1b(ii), OR = 0.32). By combining these two risk factors, the difference between a child with fever and without T. trichiura infection and one without fever but with the mean for A. lumbricoides infection or maximum T. trichiura FEC gave ORs of 9.74 and 168.95, respectively.
(c). Focal infection with hookworm
A child's sex was a significant risk factor (F1,452 = 4.19, p = 0.044) for hookworm infection, with boys at higher risk (OR = 2.14) of hookworm infection compared with girls (figure 1c(i)). Trichuris trichiura infection was a significant risk factor for hookworm infection (F1,452 = 5.14, p = 0.025). Increasing T. trichiura FEC was positively associated with an increased risk of hookworm infection (figure 1c(ii) zm OR = 1.6, zx OR = 5.16).
(d). Focal infection of self-reported fever
A child's soil consumption behaviour was significantly associated with the risk of self-reported fever (F1,452 = 5.89, p = 0.016). Those who consumed soil had a substantially lower risk (OR = 0.04) of self-reported fever compared with those who did not consume soil (figure 1d(i)). Ascaris lumbricoides infection was significantly associated with self-reported fever (F1,452 = 8.89, p = 0.003) with increasing A. lumbricoides FEC associated with a decreasing risk of fever (figure 1d(ii) zm OR = 0.29, zx OR = 0.11).
4. Discussion
In recent years, studies in wild animals have shown the importance of co-infection in driving disease dynamics [36–38], highlighting the need for parasite-community-based approaches in the creation of parasite control strategies. However, this perspective is not apparent in the human disease literature, despite the growing understanding of the, often negative, consequences of co-infection for disease severity and treatment efficacy [49–52]. A limited number of recent human disease studies do consider co-infection [39–42] but the focus is clearly weighted towards explaining the causes of co-infection in terms of factors such as spatial overlap of infective stages, rather than considering the potential of infection with one species as a risk factor for other infections. We believe our study to be unique in assessing the potential importance of co-infection as an infection risk factor in humans while simultaneously looking at a wide range of other potential risk factors. It is also novel in attempting to answer this question by applying statistical techniques previously used to successfully answer similar questions in wild animal systems.
Here, we have considered how risk factors such as host age, sex, behaviour, socioeconomic status and physical environment, together with co-infection, act to govern the prevalence of focal infections in school-aged children. This integrated approach has enabled us to determine both the significance and effect size of these risk factors, thus determining their relative importance. These analyses have shown, for each of four focal infections, that co-infection remains a significant risk factor, even when other established risk factors are accounted for. Moreover, on average, co-infection was associated with a greater change in the risk of focal infection than the non-co-infection risk factors. For three of the focal infections (the exception being self-reported fever), ORs calculated by comparing zero FEC with the maximum (zx) of A. lumbricoides or T. trichiura FEC were higher than those of any non-co-infection risk factors. Further, even the mean A. lumbricoides FEC, is associated with a greater risk of T. trichiura infection than either the risk factors shehia- or shoe-wearing alone.
An important point to highlight is that the co-infection can also be associated with a decreased risk of infection, as seen in the relationship between fever and A. lumbricoides FEC. Although soil consumption is associated with the greatest change in risk for self-reported fever, the role of co-infection is still substantial, with a 55 per cent lower risk of self-reported fever associated with the mean value of A. lumbricoides FEC (zm) and an 80 per cent lower risk associated with maximum A. lumbricoides FEC (zx). The unknown aetiology of self-reported fever means that we must be particularly cautious in attempting to suggest a mechanism in this case. However, fever is often an immune-mediated consequence of viral or bacterial infections caused by T-helper 1 (Th1) type immune responses. Therefore, one possible explanation for the relationship between A. lumbricoides and fever could be antagonism between the Th1 and T-helper 2 (Th2) arms of the immune response [53]. The Th2 response acts against macroparasites, and the two branches of the immune response are mutually downregulatory. Notably, the role of co-infection is likely to be underestimated in our analysis, because our data consider only three STHs and self-reported fever, when in fact, many other infections are likely to occur in this study population. Overall, our results show that co-infection is a very important risk factor for childhood infection, particularly for STHs.
In comparison with the role of co-infection, host age, sex and behaviour were comparatively low risk factors for infection. However, soil-eating behaviour was associated with a substantially reduced infection risk for self-reported fever (figure 1d(ii)). This may at first seem counterintuitive because soil consumption is generally associated with ill-health [20,25,54–56] and geophagy behaviour will, presumably, expose individuals to a range of infections. However, clay-rich soils have also been associated with the binding of microbial toxins and with the colonization of normal gut flora [54], which may provide protection against some pathogenic infections [57].
Socioeconomic status and physical environment had no significant effect on either the prevalence of STHs or fever. Other studies did find such associations, for example, between housing type and either hookworm [58], or A. lumbricoides and T. trichiura [22] infections. However, unlike our work, the analyses in these studies did not account for such a wide range of potential infection risk factors, including co-infection, which might otherwise have altered the findings.
A child's home shehia was associated with a significant change in infection risk for both A. lumbricoides and T. trichiura. The two shehias differ in the mix of religions, professions, educational level and socioeconomic status of the inhabitants, as well as in their physical environment (Bandamaji being rural with much vegetation and many streams, Dole peri-urban with limited vegetation and few streams) [20]. Dole is a little larger (approx. 9 km2) than Bandamaji (approx. 7 km2), while the estimated population of Dole was 2968 and that of Bandamaji was 1124 at the time of the study in 2008. The significance of the risk and size of the ORs associated with shehia suggests that some environmental, behavioural, socioeconomic or other factor (or a combination of factors), which differ between these shehias, is still unaccounted for in our analyses. The availability and use of a more developed sanitary infrastructure in Dole may be a contributing factor, because this is likely to reduce environmental contamination with STH eggs and larvae in Dole [20,59]. However, because co-infection effects are equally evident in both shehias, despite their differences, it is unlikely that any within-shehia variation would explain the role of co-infection in infection risk.
While our study provides strong preliminary evidence that co-infection can significantly change the risk of infection, rather than being a simple correlate of other risk factors, it cannot prove causation, because the data are both cross-sectional and observational. Therefore, intervention studies, to test for causation, should be the next step. The range of infections we have explored is limited and focuses mainly on helminths. Importantly though, adding further species to the analysis is likely to extend rather than diminish the co-infection associations. Further, the use of self-reported fever expands the applicability of our findings, because fever is likely to be caused by viral, bacterial or protozoal infections rather than helminths.
Perhaps the greatest limitation of this study is that we lack information on host genetics, which can have substantial effects on infection status [23,24]. In future research, such genetic effects should be considered alongside co-infection and the other potential drivers of infection heterogeneity, as it is possible that susceptibility to one parasite species may result in susceptibility to other pathogenic organisms, or alternatively to increased resistance. Finally, it will be important to confirm these findings in other communities and to ascertain whether the findings are robust to different socioeconomic and environmental settings.
Similar to previous human disease studies [39,40], we have demonstrated the use of advanced statistical techniques, such as GLMM for the simultaneous assessment of a wide range of potential risk factors. However, the statistical approaches used in these studies and in our current work differ, which may account for the differences in perception regarding the importance of co-infection. An important step in this field of research will therefore be to determine the most appropriate methods to enable a clear determination of the relative importance of co-infection in driving disease dynamics. Further, these studies were conducted across very different environmental scales and their findings may suggest that, while co-infection may not be a major influence on risk of infection at very broad spatial scales, it could be an essential consideration at smaller spatial scales.
Ultimately, this study has shown that among school-aged children in Zanzibar, co-infection is likely to be a major risk factor for four focal infections. However, future studies should incorporate host genetics along with other risk factors, because of the potential for differential susceptibility to parasites, which could give rise to some of the co-infection risk patterns observed. Ideally, future work should test the importance of these risks by undertaking experimental manipulation, e.g. through chemotherapeutic interventions. Both model experimental systems, and human field study observations have demonstrated, for specific cases, that co-infection can alter disease outcome, parasite transmission potential and susceptibility of hosts to other infections [60,61]. Given this knowledge and the evidence from our study, associations between parasite species should not be dismissed as mere correlation without further investigation. Simple correlated exposure is not a sufficient explanation for the observed associations between infections in our study, as many other potential explanations for simple co-incident infection were accounted for. We suggest that, given the ubiquity of co-infection in human populations, the consequence of one infection for other infections in a host population is likely to be widespread. Theoretical studies have already demonstrated that interactions between co-infecting pathogens can have profound effects (both positive and negative) on pathogen control (e.g. leading to vaccine failure) [37]. Therefore, considering and accounting for co-infection must become a central tenet in studies of infection and disease. Ironically, it may be the multiplicity of infections that people face, which ultimately helps to improve public health.
Acknowledgements
The study was approved by the institutional research commission of the Swiss Tropical and Public Health Institute (Basel, Switzerland). Ethical clearance was obtained from the Ethics Committee of the Ministry of Health and Social Welfare, Zanzibar (reference no. 16) [20]. All participants in this study gave written informed consent, which was provided by the parent or guardian for the children involved. Additionally, children provided oral assent.
The original research that gave rise to the dataset used in this study was funded jointly by the Swiss National Science Foundation (project nos PPOOB-102883 and PPOOB-119129), European Union (FP6 STREP CONTRAST project, contract no. 032203), and the ‘Kommission für Reisestipendien’ of the Swiss Academy for Natural Sciences (SCNAT). A UK, Medical Research Council, Environmental and Social Ecology of Human Infectious Disease, Catalyst Grant awarded to M.E.V. and in which J.L. was also involved was the initial impetus for this work. Personal stipends from the ‘Emanuel Burckhardt Stiftung Basel’ and the ‘Forschungsfonds’ of the University of Basel allowed S.K. to be involved in the project.
References
- 1.Cox FEG. 2001. Concomitant infections, parasites and immune responses. Parasitology 122, S23–S38 10.1017/S003118200001698X (doi:10.1017/S003118200001698X) [DOI] [PubMed] [Google Scholar]
- 2.Raso G, et al. 2004. Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Côte d'Ivoire. Int. J. Epidemiol. 33, 1092–1102 10.1093/ije/dyh241 (doi:10.1093/ije/dyh241) [DOI] [PubMed] [Google Scholar]
- 3.Pullan R, Brooker S. 2008. The health impact of polyparasitism in humans: are we under-estimating the burden of parasitic diseases? Parasitology 135, 783–794 10.1017/S0031182008000346 (doi:10.1017/S0031182008000346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooker S, Clements ACA. 2009. Spatial heterogeneity of parasite co-infection: determinants and geostatistical prediction at regional scales. Int. J. Parasitol. 39, 591–597 10.1016/j.ijpara.2008.10.014 (doi:10.1016/j.ijpara.2008.10.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinmann P, Utzinger J, Du ZW, Zhou XN. 2010. Multiparasitism: a neglected reality on global, regional and local scale. Adv. Parasitol. 73, 21–50 10.1016/S0065-308X(10)73002-5 (doi:10.1016/S0065-308X(10)73002-5) [DOI] [PubMed] [Google Scholar]
- 6.Morris SK, Bassani DG, Awasthi S, Kumar R, Shet A, Suraweera W, Jha P. 2011. Diarrhea, pneumonia, and infectious disease mortality in children aged 5 to 14 years in India. PLoS ONE 6, e20119. 10.1371/journal.pone.0020119 (doi:10.1371/journal.pone.0020119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganatra HA, Zaidi AKM. 2010. Neonatal infections in the developing world. Semin. Perinatol. 34, 416–425 10.1053/j.semperi.2010.09.004 (doi:10.1053/j.semperi.2010.09.004) [DOI] [PubMed] [Google Scholar]
- 8.Thaver D, Zaidi AKM. 2009. Burden of neonatal infections in developing countries: a review of evidence from community-based studies. Pediatr. Infect. Dis. J. 28, S3–S9 10.1097/INF.0b013e3181958755 (doi:10.1097/INF.0b013e3181958755) [DOI] [PubMed] [Google Scholar]
- 9.Lawn JE, Wilczynska-Ketende K, Cousens SN. 2006. Estimating the causes of 4 million neonatal deaths in the year 2000. Int. J. Epidemiol. 35, 706–718 10.1093/ije/dy1043 (doi:10.1093/ije/dy1043) [DOI] [PubMed] [Google Scholar]
- 10.Guerrant RL, Blackwood BL. 1999. Threats to global health and survival: the growing crises of tropical infectious diseases: our ‘unfinished agenda’. Clin. Infect. Dis. 28, 966–986 10.1086/514765 (doi:10.1086/514765) [DOI] [PubMed] [Google Scholar]
- 11.Sacarlal J, et al. 2009. A 10 year study of the cause of death in children under 15 years in Manhica, Mozambique. BMC Public Health 9, 67. 10.1186/1471-2458-9-67 (doi:10.1186/1471-2458-9-67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slutsker L, Bloland P, Steketee RW, Wirima JJ, Heymann DL, Breman JG. 1996. Infant and second-year mortality in rural Malawi: causes and descriptive epidemiology. Am. J. Trop. Med. Hyg. 55, 77–81 [DOI] [PubMed] [Google Scholar]
- 13.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. 2008. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260 10.1016/S0140-6736(07)61690-0 (doi:10.1016/S0140-6736(07)61690-0) [DOI] [PubMed] [Google Scholar]
- 14.Kinney MV, Kerber KJ, Black RE, Cohen B, Nkrumah F, Coovadia H, Nampala PM, Lawn JE. 2010. Sub-Saharan Africa's mothers, newborns, and children: where and why do they die? PLoS Med. 7, e1000294. 10.1371/journal.pmed.1000294 (doi:10.1371/journal.pmed.1000294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friberg IK, et al. 2010. Sub-Saharan Africa's mothers, newborns, and children: how many lives could be saved with targeted health interventions? PLoS Med. 7, e1000295. 10.1371/journal.pmed.1000295 (doi:10.1371/journal.pmed.1000295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wanji S, Tendongfor N, Esum M, Atanga SN, Enyong P. 2003. Heterogeneity in the prevalence and intensity of loiasis in five contrasting bioecological zones in Cameroon. Trans. R. Soc. Trop. Med. Hyg. 97, 182–187 10.1016/S0035-9203(03)90114-3 (doi:10.1016/S0035-9203(03)90114-3) [DOI] [PubMed] [Google Scholar]
- 17.Brooker S, Alexander N, Geiger S, Moyeed RA, Stander J, Fleming F, Hotez PJ, Correa-Oliveira R, Bethony J. 2006. Contrasting patterns in the small-scale heterogeneity of human helminth infections in urban and rural environments in Brazil. Int. J. Parasitol. 36, 1143–1151 10.1016/j.ijpara.2006.05.009 (doi:10.1016/j.ijpara.2006.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolhouse MEJ, et al. 1997. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA 94, 338–342 10.1073/pnas.94.1.338 (doi:10.1073/pnas.94.1.338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolzoni L, Real L, De Giulio L. 2007. Transmission heterogeneity and control strategies for infectious disease emergence. PLoS ONE 2, e747. 10.1371/journal.pone.0000747 (doi:10.1371/journal.pone.0000747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopp S, Mohammed KA, Stothard JR, Khamis IS, Rollinson D, Marti H, Utzinger J. 2010. Patterns and risk factors of helminthiasis and anemia in a rural and a peri-urban community in Zanzibar, in the context of helminth control programs. PLoS Negl. Trop. Dis. 4, e681. 10.1371/journal.pntd.0000681 (doi:10.1371/journal.pntd.0000681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saathoff E, Olsen A, Kvalsvig JD, Appleton CC, Sharp B, Kleinschmidt I. 2005. Ecological covariates of Ascaris lumbricoides infection in schoolchildren from rural KwaZulu-Natal, South Africa. Trop. Med. Int. Health 10, 412–422 10.1111/j.1365-3156.2005.01406.x (doi:10.1111/j.1365-3156.2005.01406.x) [DOI] [PubMed] [Google Scholar]
- 22.Holland CV, Taren DL, Crompton DWT, Nesheim MC, Sanjur D, Barbeau I, Tucker K, Tiffany J, Rivera G. 1988. Intestinal helminthiases in relation to the socioeconomic environment of Panamanian children. Soc. Sci. Med. 26, 209–213 10.1016/0277-9536(88)90241-9 (doi:10.1016/0277-9536(88)90241-9) [DOI] [PubMed] [Google Scholar]
- 23.Abel L, Dessein AJ. 1997. The impact of host genetics on susceptibility to human infectious diseases. Curr. Opin. Immunol. 9, 509–516 10.1016/S0952-7915(97)80103-3 (doi:10.1016/S0952-7915(97)80103-3) [DOI] [PubMed] [Google Scholar]
- 24.Weatherall DJ. 1996. Host genetics and infectious disease. Parasitology 112, S23–S29 [PubMed] [Google Scholar]
- 25.Saathoff E, Olsen A, Kvalsvig JD, Geissler PW. 2002. Geophagy and its association with geohelminth infection in rural schoolchildren from northern KwaZulu-Natal, South Africa. Trans. R. Soc. Trop. Med. Hyg. 96, 485–490 10.1016/S0035-9203(02)90413-X (doi:10.1016/S0035-9203(02)90413-X) [DOI] [PubMed] [Google Scholar]
- 26.Pulford J, Hetzel MW, Bryant M, Siba PM, Mueller I. 2011. Reported reasons for not using a mosquito net when one is available: a review of the published literature. Malar. J. 10, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson RM, May RM. 1985. Helminth infections of humans: mathematical-models, population-dynamics and control. Adv. Parasitol. 24, 1–101 10.1016/S0065-308X(08)60561-8 (doi:10.1016/S0065-308X(08)60561-8) [DOI] [PubMed] [Google Scholar]
- 28.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367, 1521–1532 10.1016/S0140-6736(06)68653-4 (doi:10.1016/S0140-6736(06)68653-4) [DOI] [PubMed] [Google Scholar]
- 29.Ziaei H, Sadeghian G, Hejazi SH. 2008. Distribution frequency of pathogenic bacteria isolated from cutaneus leishmaniasis lesions. Korean J. Parasitol. 46, 191–193 10.3347/kjp.2008.46.3.191 (doi:10.3347/kjp.2008.46.3.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen JE, Maizels RM. 2011. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 11, 375–388 10.1038/nri2992 (doi:10.1038/nri2992) [DOI] [PubMed] [Google Scholar]
- 31.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. 2004. Helminth parasites: masters of regulation. Immunol. Rev. 201, 89–116 10.1111/j.0105-2896.2004.00191.x (doi:10.1111/j.0105-2896.2004.00191.x) [DOI] [PubMed] [Google Scholar]
- 32.Graham AL. 2008. Ecological rules governing helminth-microparasite coinfection. Proc. Natl Acad. Sci. USA 105, 566–570 10.1073/pnas.0707221105 (doi:10.1073/pnas.0707221105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page KR, Jedlicka AE, Fakheri B, Noland GS, Kesavan AK, Scott AL, Kumar N, Manabe YC. 2005. Mycobacterium-induced potentiation of type 1 immune responses and protection against malaria are host specific. Infect. Immun. 73, 8369–8380 10.1128/IAI.73.12.8369-8380.2005 (doi:10.1128/IAI.73.12.8369-8380.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lello J, Hussell T. 2008. Functional group/guild modelling of inter-specific pathogen interactions: a potential tool for predicting the consequences of co-infection. Parasitology 135, 825–839 10.1017/S0031182008000383 (doi:10.1017/S0031182008000383) [DOI] [PubMed] [Google Scholar]
- 35.Knowles SCL. 2011. The effect of helminth co-infection on malaria in mice: a meta-analysis. Int. J. Parasitol. 41, 1041–1051 10.1016/j.ijpara.2011.05.009 (doi:10.1016/j.ijpara.2011.05.009) [DOI] [PubMed] [Google Scholar]
- 36.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330, 243–246 10.1126/science.1190333 (doi:10.1126/science.1190333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lello J, Boag B, Fenton A, Stevenson IR, Hudson PJ. 2004. Competition and mutualism among the gut helminths of a mammalian host. Nature 428, 840–844 10.1038/nature02490 (doi:10.1038/nature02490) [DOI] [PubMed] [Google Scholar]
- 38.Ferrari N, Cattadori IM, Rizzoli A, Hudson PJ. 2009. Heligmosomoides polygyrus reduces infestation of Ixodes ricinus in free-living yellow-necked mice, Apodemus flavicollis. Parasitology 136, 305–316 10.1017/S0031182008005404 (doi:10.1017/S0031182008005404) [DOI] [PubMed] [Google Scholar]
- 39.Pullan RL, Kabatereine NB, Bukirwa H, Staedke SG, Brooker S. 2011. Heterogeneities and consequences of Plasmodium species and hookworm coinfection: a population based study in Uganda. J. Infect. Dis. 203, 406–417 10.1093/infdis/jiq063 (doi:10.1093/infdis/jiq063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooker SJ, Pullan RL, Gitonga CW, Ashton RA, Kolaczinski JH, Kabatereine N, Snow RW. 2012. Plasmodium–helminth coinfection and its sources of heterogeneity across East Africa. J. Infect. Dis. 205, 841–852 10.1093/infdis/jir844 (doi:10.1093/infdis/jir844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Righetti AA, Glinz D, Adiossan LG, Koua AY, Niamké S, Hurrell RF, Wegmüller R, N'goran EK, Utzinger J. 2012. Interactions and potential implications of Plasmodium falciparum-hookworm coinfection in different age groups in south-central Côte d'Ivoire. PLoS Negl. Trop. Dis. 6, e1889. 10.1371/journal.pntd.0001889 (doi:10.1371/journal.pntd.0001889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodburn PW, Muhangi L, Hillier S, Ndibazza J, Namujju PB, Kizza M, Ameke C, Omoding NE, Booth M, Elliott AM. 2009. Risk factors for helminth, malaria, and HIV infection in pregnancy in Entebbe, Uganda. PLoS Negl. Trop. Dis. 3, e473. 10.1371/journal.pntd.0000473 (doi:10.1371/journal.pntd.0000473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fenton A, Viney ME, Lello J. 2010. Detecting interspecific macroparasite interactions from ecological data: patterns and process. Ecol. Lett. 13, 606–615 10.1111/j.1461-0248.2010.01458.x (doi:10.1111/j.1461-0248.2010.01458.x) [DOI] [PubMed] [Google Scholar]
- 44.Paterson S, Lello J. 2003. Mixed models: getting the best use of parasitological data. Trends Parasitol. 19, 370–375 10.1016/S1471-4922(03)00149-1 (doi:10.1016/S1471-4922(03)00149-1) [DOI] [PubMed] [Google Scholar]
- 45.Katz N, Chaves A, Pellegrino J. 1972. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. São Paulo 14, 397–400 [PubMed] [Google Scholar]
- 46.Smith DL, Cohen JM, Moonen B, Tatem AJ, Sabot OJ, Ali A, Mugheiry SM. 2011. Solving the sisyphean problem of malaria in Zanzibar. Science 332, 1384–1385 10.1126/science.1201398 (doi:10.1126/science.1201398) [DOI] [PubMed] [Google Scholar]
- 47.Moonen B, Cohen JM, Tatem AJ, Cohen J, Hay SI, Sabot O, Smith DL. 2010. A framework for assessing the feasibility of malaria elimination. Malar. J. 9, 322. 10.1186/1475-2875-9-322 (doi:10.1186/1475-2875-9-322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanzibar Malaria Control Programme 2009. Malaria elimination on Zanzibar: a feasibility assessment. Zanzibar Malaria Control Programme, Ministry of Health and Social Welfare [Google Scholar]
- 49.Van Geertruyden JP, Menten J, Colebunders R, Korenromp E, D'Alessandro U. 2008. The impact of HIV-1 on the malaria parasite biomass in adults in sub-Saharan Africa contributes to the emergence of antimalarial drug resistance. Malar. J. 7, 134. 10.1186/1475-2875-7-134 (doi:10.1186/1475-2875-7-134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geertruyden J, Mwananyanda L, Chalwe V, Moerman F, Chilengi R, Kestens L, D'alessandro U. 2005. Higher risk of antimalarial treatment failure in HIV positive than in HIV negative individuals with clinical malaria [MIM-LM-234960]. Acta Trop. 95, S253 [Google Scholar]
- 51.Diallo TO, et al. 2010. Schistosomiasis coinfection in children influences acquired immune response against Plasmodium falciparum malaria antigens. PLoS ONE 5, e12764. 10.1371/journal.pone.0012764 (doi:10.1371/journal.pone.0012764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Remoue F, et al. 2003. Malaria co-infection in children influences antibody response to schistosome antigens and inflammatory markers associated with morbidity. Trans. R. Soc. Trop. Med. Hyg. 97, 361–364 10.1016/S0035-9203(03)90170-2 (doi:10.1016/S0035-9203(03)90170-2) [DOI] [PubMed] [Google Scholar]
- 53.Lello J. 2012. Co-infection: immunological considerations. In Immunity to parasitic infection (ed. Lamb TJ.), pp. 325–334 Chichester, UK: John Wiley and Sons Ltd [Google Scholar]
- 54.Bisi-Johnson MA, Obi CL, Ekosse GE. 2010. Microbiological and health related perspectives of geophagia: an overview. Afr. J. Biotechnol. 9, 5784–5791 [Google Scholar]
- 55.Geissler PW, Mwaniki DL, Thiong'o F, Michaelsen KF, Friis H. 1998. Geophagy, iron status and anaemia among primary school children in Western Kenya. Trop. Med. Int. Health 3, 529–534 10.1046/j.1365-3156.1998.00272.x (doi:10.1046/j.1365-3156.1998.00272.x) [DOI] [PubMed] [Google Scholar]
- 56.Kawai K, Saathoff E, Antelman G, Msamanga G, Fawzi WW. 2009. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am. J. Trop. Med. Hyg. 80, 36–43 [PMC free article] [PubMed] [Google Scholar]
- 57.Zimmermann MB, et al. 2010. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Côte d'Ivoire. Am. J. Clin. Nutr. 92, 1406–1415 10.3945/ajcn.110.004564 (doi:10.3945/ajcn.110.004564) [DOI] [PubMed] [Google Scholar]
- 58.Gomez J, Botto C, Zent S, Marin A, Sanchez J, Noguera C, Rangel T. 2004. Influence of housing type and settlement size of Piaroa Indian communities on the transmission of intestinal helminthiasis. Interciencia 29, 389–395 [Google Scholar]
- 59.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. 2012. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 9, e1001162. 10.1371/journal.pmed.1001162 (doi:10.1371/journal.pmed.1001162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu-Raddad LJ, Patnaik P, Kublin JG. 2006. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science 314, 1603–1606 10.1126/science.1132338 (doi:10.1126/science.1132338) [DOI] [PubMed] [Google Scholar]
- 61.Sokhna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, Diop M, Ly A, Druilhe P. 2004. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar. J. 3, 43. 10.1186/1475-2875-3-43 (doi:10.1186/1475-2875-3-43) [DOI] [PMC free article] [PubMed] [Google Scholar]