Abstract
Many malaria vector mosquitoes in Africa have an extreme preference for feeding on humans. This specialization allows them to sustain much higher levels of transmission than elsewhere, but there is little understanding of the evolutionary forces that drive this behaviour. In Tanzania, we used a semi-field system to test whether the well-documented preferences of the vectors, Anopheles arabiensis and Anopheles gambiae sensu stricto (s.s.) for cattle and humans, respectively, are predicted by the fitness they obtain from host-seeking on these species relative to other available hosts. Mosquito fitness was contrasted, when humans were fully exposed and when they were protected by a typical bednet. The fitness of both vectors varied between host species. The predicted relationship between host preference and fitness was confirmed in An. arabiensis, but not in An. gambiae s.s., whose fitness was similar on humans and other mammals. Use of typical, imperfect bednets generated only minor reductions in An. gambiae s.s. feeding success and fitness on humans, but was predicted to generate a significant reduction in the lifetime reproductive success of An. arabiensis on humans relative to cows. This supports the hypothesis that such human-protective measures could additionally benefit malaria control by increasing selection for zoophily in vectors.
Keywords: host specialization, selection, mosquito vectors, malaria, bednets
1. Introduction
Evolutionary change by pathogens and their invertebrate vectors is generally perceived as detrimental to disease control [1,2]. However, control measures could potentially generate selection upon disease vectors that provides them with a fitness reward for adopting phenotypes that reduce their transmission ability [3–5]. This approach could be amenable for vector-borne diseases where the host-specificity of parasites and their vectors differs. This mismatch presents an opportunity to reduce disease transmission by generating selection on vectors to shift their host use towards non-permissive species through ecological manipulation of the fitness benefits of host selection.
A potential candidate for such an approach is malaria, a disease caused by Plasmodium parasites transmitted by Anopheles mosquitoes. The host range of Anopheline species varies from avian and mammalian generalists, to those specific to one-host species [6]. By contrast, most human infectious Plasmodia can survive only in humans (Plasmodium knowlesi being an exception; [7]). Consequently, the degree to which Anopheles vectors specialize on humans (anthrophily) is a prime determinant of malaria transmission intensity [8], and any shift from anthrophily to feeding on other animals will reduce transmission (e.g. zooprophylaxis; [9]). Current malaria control strategies are based on reducing human exposure to mosquito bites and/or mosquito density [10]. Here, we investigate the potential for these approaches to generate additional benefits by creating an evolutionary incentive for mosquito vectors to switch their host species use from humans to other animals, that are commonly available in malaria endemic settings.
Prediction of the potential impact of control measures on the evolution of mosquito host range requires an understanding of the selective forces underpinning it. The host species range of haematophagous insects has undoubtedly been shaped by natural selection, but there has been relatively little empirical investigation of how host selection influences their fitness [6,11]. Theoretically, host specialization is predicted to arise due to a trade-off between the performance of foragers on different host types [12,13], with selection being generated for the development of preferences for those which provide the greatest fitness reward. By extension, environmental changes that diminish the fitness advantage associated with particular hosts could undermine selection for their continued preference. In the case of African malaria vectors, bednet usage is an example of an environmental change that could reduce fitness advantages associated with anthrophily. Should the expected fitness returns that mosquitoes obtain from attempting to feed on humans protected by bednets fall below those from foraging on other available animals, wide use of these interventions could generate selection on vectors to adopt more generalist feeding behaviours and/or switch their specialization to other host species. Both these phenomena could substantially reduce malaria transmission.
There has been speculation about the causes of anthrophily in African vectors (reviewed in [6]), but it remains unclear which factors are most responsible for driving it. Hypotheses include innate physiological or behavioural properties of humans that influence the fitness value of blood meals acquired from them, their high relative abundance and/or the environmental suitability of their habitats (houses) [6]. These can be grouped into two non-mutually exclusive routes through which selection for host specialization could arise: (i) on the basis of the relative abundance of hosts [12], and (ii) on the basis of the expected fitness obtained per host encounter. Bednet use does not directly influence human abundance, but will reduce the efficiency with which mosquitoes can extract blood on encounter. As vertebrate blood is vital for malaria vector reproduction and survival [6,14,15], interventions that interfere with the efficiency of blood extraction from a host could impair mosquito fitness and generate selection on host species use.
Although the coverage of insecticidal nets in Africa has increased dramatically over the past 10 years [16,17], untreated or poorly treated bednets remain the most common protective measure against mosquito biting in many locations [17]. We experimentally investigated how the fitness of the two most important African malaria vectors, Anopheles gambiae sensu stricto (s.s.) and Anopheles arabiensis, varied on encounter with different host species, and whether the use of such bednets reduced the relative fitness expected from foraging on humans relative to commonly available animal alternatives. We also tested whether the well-established preferences of these vectors towards specific host species are positively correlated with a fitness advantage from feeding upon them. These vectors are closely related and widely distributed throughout Africa [18], but vary in their host preference, with An. gambiae s.s. being almost exclusively anthrophilic [8] and An. arabiensis generally preferring cows over humans when both are available [19].
2. Material and methods
The study was conducted at the Ifakara Health Institute (IHI) in the Kilombero valley, Tanzania, where high levels of malaria transmission are sustained year-round by An. arabiensis, An. gambiae s.s. and Anopheles funestus. Experiments were conducted using An. arabiensis and An. gambiae s.s. from colonies at the IHI. The An. arabiensis colony was established a few months before the start of experiments with individuals from Sagamaganga village (approx. 15 km from IHI) and is maintained in a semi-field insectary [20]. The An. gambiae s.s. colony was established with individuals from Njage village in 1996 (approx. 70 km from IHI) and is maintained in an indoor insectary (26 ± 2.5°C, 80 ± 10% RH). Both colonies are maintained on human blood provided thrice weekly by arm feeding.
(a). Experimental set-up
An experimental hut (3.5 × 4 × 2.5 m) was built in a netting-enclosed chamber (9.1 × 9.6 × 3.7 m) of the IHI semi-field system (electronic supplementary material, S1) [20]. Mosquitoes could enter and exit the hut through its open eaves as they do in nature [21], or exit via the six windows. Mosquitoes leaving the hut were caught outside or in window exit traps. Mosquito feeding success and fitness were evaluated on humans and four other species commonly kept in or near houses in the Kilombero Valley: chickens, cattle, dogs and goats. Two sub-categories of cattle were tested: adult cows and calves. Within other host types, animals were approximately the same age and size. Humans were presented either exposed or sleeping under an untreated bednet. ‘Typical’ bednets were created following the World Health Organization's standard protocol for simulating the average condition of bednets in operational use by cutting six moderately sized holes into the sides (4 × 4 cm; [22]).
For each experiment, an individual from one of the seven host types was placed inside the hut at dusk. Human volunteers were provided with a bed and instructed to sleep and react to mosquito biting as normal (e.g. swatting as desired). Two hundred unfed An. arabiensis or An. gambiae s.s. females (4–6 days old) were then released into the chamber corners (maximum approx. 4.5 m from host). The next morning, the chamber and hut were intensively searched to recapture mosquitoes (by aspirator). Those recaptured were identified as being blood fed, unfed, live or dead. Six replicates (on different host individuals) were performed for each of the seven host types, for each mosquito species (84 trials in total). Experiments were run in one-week blocks within which seven nights of consecutive trials were performed. The order in which host species were used was randomly allocated over the week to minimize potential for carry-over effects.
(b). Fitness measurements
Mosquito feeding success was measured as: (i) the proportion of mosquitoes recaptured alive and blood fed, (ii) the proportion of mosquitoes dead at recapture, and (iii) blood meal size. For blood meal size measurement, mosquitoes visually identified as blood fed were moved into individual 30 ml tubes for 3 days (provided with 10% glucose solution) in the semi-field insectary. Mosquitoes were subsequently moved into individual paper cups lined with damp filter paper to stimulate oviposition, and the haematin content of excreta deposited in initial holding tubes was measured to provide an index of the mass of blood ingested [23]. Oviposition cups were inspected daily and the numbers of eggs laid within them were counted. Mosquitoes remained in holding cups and were monitored daily until death to estimate their host species-dependent survival.
(c). Statistical analyses
Variation in the probability of blood feeding, death on recapture and oviposition (all binomial), blood meal size and fecundity (continuous) were analysed using generalized linear mixed effect models (GLMM) with appropriate link functions in the R software package [24]. Here, ‘host species’ and ‘mosquito species’ were treated as fixed effects, and ‘host individual’ as a random effect. For each response variable, a maximal model was generated and the significance of fixed effects evaluated through stepwise deletion of terms using likelihood ratio tests (LRTs). For variables in which host species was identified as statistically significant, Dunnett's post hoc test (adjusting for multiple comparisons) was used to identify statistically significant two-way differences between the unprotected human reference group and other host types. The Cox proportional hazards model was used to test for differences in the post-feeding survival of mosquitoes due to host species. In these models, a frailty function [25] was used to incorporate the random effect of host individual, and host and mosquito species were fit as main effects [24]. Reported χ2 values refer to LRTs conducted on the output of GLMMs and z-values are for two-way comparison between a human reference group and other host species. ‘OR’ values are odds ratios from Cox proportional hazard models.
(d). Modelling the impact of host species on lifetime reproductive success
A mosquito life-history model was constructed assuming that to produce eggs, a female must acquire a blood meal during one night of seeking on the jth host type (with probability βj), survive the period between feeding and oviposition of dov days (with a daily survival probability sov,j) and oviposit (with probability γj) a total of ‘Fj’ eggs. We assumed that females who fed but did not obtain enough blood to trigger oviposition on one night (with probability = 1−βj*γj) can attempt to feed again on ‘k’ successive nights until they succeed or die. After oviposition, females can initiate another feeding cycle. Although the daily survival of unfed mosquitoes (sf) was assumed to be independent of host type, survival of mosquitoes between blood consumption and oviposition was assumed to be dependent on host type (sov,j). The expected number of eggs resulting from the first feeding cycle R(j), is thus:
and the lifetime reproductive success (LRS; estimated by R0j) expected from multiple feeding cycles i is given by 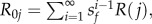 assuming age-independent survival.
assuming age-independent survival.
Most values for host-specific mosquito fitness traits were directly estimated from experiments described above, with the exception of survival between feeding and oviposition (sov,j). Rather than directly applying survival probabilities that were experimentally measured under semi-field conditions here (probably an overestimate of their value in nature), we estimated the odds of survival after feeding on different hosts relative to having fed on an unprotected human, and used this to adjust published values of the daily survival of human-fed mosquitoes in the field (see the electronic supplementary material, S3).
Confidence intervals around the predicted mean values of R0j were generated by conducting 10 000 simulations of the total LRS (R0) of an individual An. arabiensis or An. gambiae s.s., when feeding exclusively on each host type. Further simulations were conducted to assess the total LRS (R0) of An. arabiensis, when mixing its feeding between humans using a bednet and cows to varying degrees. Unlike An. gambiae s.s., which rarely feeds on anything other than humans in nature, An. arabiensis is known to be capable of feeding on humans and cattle to varying degrees depending on their local availability [6]. Uncertainty within each simulation was introduced by selecting the value of each host-specific parameter randomly from a Bernoulli (probability of feeding, surviving and oviposition) or normal distribution (number of eggs laid) with a mean and standard error from the appropriate statistical model. Observations over the first couple of trials of both vector species indicated that more than 90 per cent of fecund mosquitoes laid their eggs on the first day that an oviposition substrate was provided (4 days after feeding). It was thus assumed that the period between feeding and oviposition was independent of host species in this model.
To test for statistically significant differences in R0 between host types, bootstrapping analyses were performed on the 21 possible two-way host comparisons between the seven host types. Values of R0 for host types 1 and 2 were randomly drawn from their simulated distributions. The proportion of 10 000 such draws in which the R0 of one host type was greater than the other was used as an estimate of the probability that the LRS of mosquitoes on these host types was significantly different (if p < 0.05).
3. Results
The foraging success and subsequent fitness of 16 517 Anopheles vectors were tracked over 84 trials (see the electronic supplementary material, S4 and S5), and used to parameterize a life-history model for prediction of mosquito LRS on different host types. For all mosquito traits analysed, there was a statistically significant interaction between mosquito and host species (p < 0.001 except for fecundity where p = 0.03). Consequently, all subsequent statistical analyses were performed for each mosquito species separately. The random effect of ‘host individual’ was highly significant (p < 0.001) for all response variables examined, except for the proportion of An. arabiensis found dead at recapture (p = 0.02), and all results are from models including this random effect. Data and model results were used to address three questions.
(a). Does host species influence mosquito vector fitness?
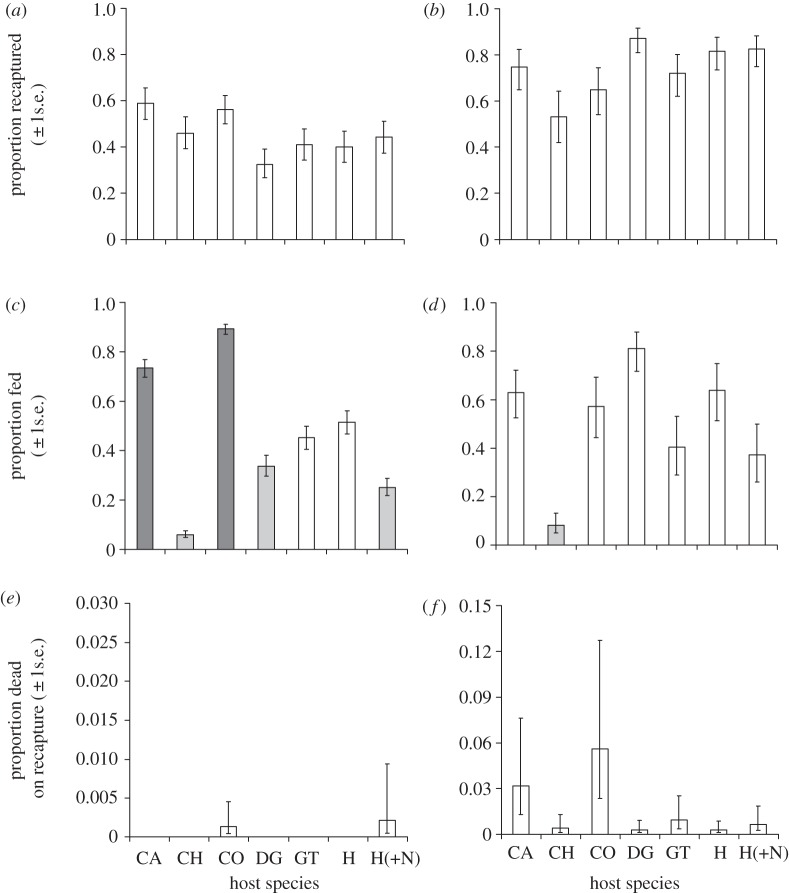
The proportion of mosquitoes recaptured did not vary between host species in An. arabiensis (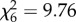 , p = 0.13; figure 1a) or An. gambiae s.s. (
, p = 0.13; figure 1a) or An. gambiae s.s. (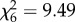 , p = 0.15; figure 1b). However, host species was a significant predictor of feeding probability in both An. arabiensis (
, p = 0.15; figure 1b). However, host species was a significant predictor of feeding probability in both An. arabiensis (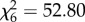 , p < 0.001; figure 1c) and An. gambiae s.s. (
, p < 0.001; figure 1c) and An. gambiae s.s. ( , p < 0.001; figure 1d). The proportion of mosquitoes dead on recapture was independent of host species (An. arabiensis:
, p < 0.001; figure 1d). The proportion of mosquitoes dead on recapture was independent of host species (An. arabiensis: 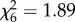 , p = 0.93, figure 1e; An. gambiae s.s.:
, p = 0.93, figure 1e; An. gambiae s.s.: 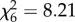 , p = 0.22; figure 1f).
, p = 0.22; figure 1f).
Figure 1.
Estimated proportions (±1 s.e.) of An. arabiensis (a,c,e) and An. gambiae s.s. (b,d,f) that were recaptured in trials with different host species (a,b), successfully obtained a blood meal (c,d) or died during host-seeking (e,f). Host types are: CH, chicken; CA, calf; CO, cow; DG, dog; GT, goat; H, unprotected human; H(+N), human sleeping under an untreated net. Colours indicate the nature of statistical differences between the ‘human without a net’ reference group and other host treatments (determined by Dunnett's post hoc test, adjusting for multiple comparisons). Dark grey indicates treatments that had a statistically higher value than the human reference group, light grey indicates treatments that had a statistically lower value than the reference group and white refers to treatments that were not significantly different from the reference group.
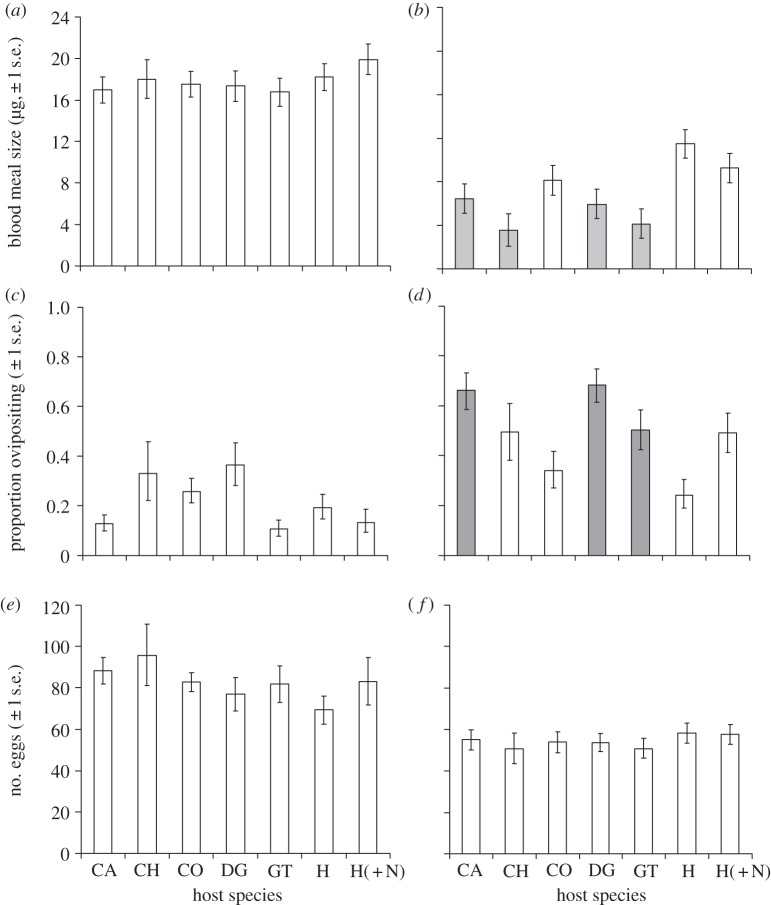
Whereas An. arabiensis obtained similarly sized blood meals from all hosts (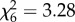 , p = 0.77; figure 2a), An. gambiae s.s. acquired larger meals from humans and cows than any other hosts (
, p = 0.77; figure 2a), An. gambiae s.s. acquired larger meals from humans and cows than any other hosts (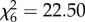 , p < 0.001; figure 2b). Host species influenced the probability of oviposition after blood feeding (An. arabiensis:
, p < 0.001; figure 2b). Host species influenced the probability of oviposition after blood feeding (An. arabiensis: 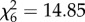 , p = 0.02, figure 2c; An. gambiae s.s.:
, p = 0.02, figure 2c; An. gambiae s.s.: 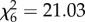 , p = 0.002, figure 2d), but not the number of eggs laid (An. arabiensis:
, p = 0.002, figure 2d), but not the number of eggs laid (An. arabiensis: 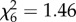 , p > 0.05; An. gambiae s.s.:
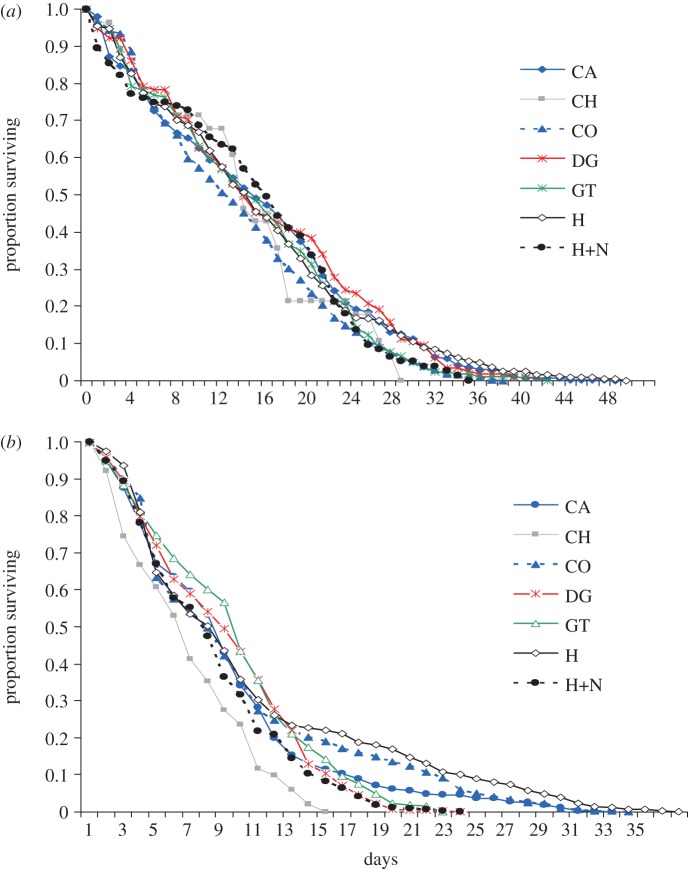
, p > 0.05; An. gambiae s.s.: 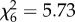 , p > 0.05; figure 2e,f). The impact of host species on mosquito survival also varied between mosquito species. Whereas An. arabiensis had similar survival on all host species (
, p > 0.05; figure 2e,f). The impact of host species on mosquito survival also varied between mosquito species. Whereas An. arabiensis had similar survival on all host species (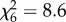 , p = 0.2; figure 3a and table 1), in An. gambiae s.s. the odds of mortality between the ‘best’ (humans and cows) and ‘worst’ host types (chickens) differed by 1.7-fold (
, p = 0.2; figure 3a and table 1), in An. gambiae s.s. the odds of mortality between the ‘best’ (humans and cows) and ‘worst’ host types (chickens) differed by 1.7-fold (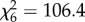 , p < 0.001; figure 3b and table 1). Combining these impacts of host species on mosquito fitness, the life-history model predicted the LRS of both An. arabiensis and An. gambiae s.s. to vary significantly between host species (see figure 4a,c and the electronic supplementary material, S6).
, p < 0.001; figure 3b and table 1). Combining these impacts of host species on mosquito fitness, the life-history model predicted the LRS of both An. arabiensis and An. gambiae s.s. to vary significantly between host species (see figure 4a,c and the electronic supplementary material, S6).
Figure 2.
Estimates (±1 s.e.) of the mean blood meal size (μg of hematin, a,b), oviposition rate (c,d) and number of eggs laid (e,f) by An. arabiensis (a,c,e) and An. gambiae s.s. (b,d,f) after feeding on different host types. Host type abbreviations are as specified in figure 1. Colours indicate the nature of statistical differences between the ‘human without a net’ reference group and all other host treatments, as detailed for figure 1.
Figure 3.
Survival of (a) An. arabiensis and (b) An. gambiae s.s. after taking a blood meal from different host species. Lines represent the survival function as estimated from fitting the Cox proportion hazard model. Host type abbreviations are as specified in Figure 1.
Table 1.
Relative odds of mortality in mosquito vectors after feeding on different host species. Numbers in brackets are 95% CIs.
| odds ratio (OR) of mortality |
||
|---|---|---|
| host species | An. arabiensis | An. gambiae s.s. |
| (a) relative to human without a net | ||
| goat | 1.25(1.02–1.54) | 1.44(1.23–1.69) |
| dog | 1.19(0.95–1.50) | 1.48(1.28–1.71) |
| chicken | 1.41(0.95–2.09) | 1.71(1.27–2.31) |
| calf | 1.08(0.91–1.29) | 1.48(1.26–1.72) |
| cow | 1.19(1.01–1.40) | 0.92(0.78–1.08) |
| human with untreated net | 1.08(0.85–1.38) | 1.83(1.56–2.14) |
| (b) relative to human with a net | ||
| goat | 1.16(0.89–1.50) | 0.79(0.68–0.92) |
| dog | 1.10(0.83–1.46) | 0.81(0.71–0.93) |
| chicken | 1.29(0.85–1.99) | 0.94(0.70–1.26) |
| calf | 0.99(0.79–1.27) | 0.81(0.69–0.94) |
| cow | 1.10(0.87–1.38) | 0.50(0.43–0.59) |
| human no net | 0.92(0.73–1.17) | 0.55(0.47–0.64) |
Figure 4.
Predicted distributions of the lifetime egg production of An. arabiensis: (a) feeding exclusively on hosts of different species and (b) taking a mixture of blood meals from cows and humans using bednets. The dotted black line represents An. arabiensis fitness under a ‘human-using-a-bednet’-only diet and the solid blue line a cow-only host diet. Dotted lines show expected distributions for variable proportions of cow-feeding (all other meals from humans using a bednet). Blue lines indicate host diets yielding a statistically significant advantage over an exclusive human-using-a-bednet diet (black dotted line). (c) shows the predicted distribution of An. gambiae s.s. lifetime egg production feeding exclusively on different host species. All distributions are based on 10 000 simulations, with host type abbreviations as specified in figure 1.
(b). Is mosquito fitness highest on naturally preferred host species?
In accordance with their natural feeding preference, An. arabiensis had greater feeding success on cows than any other host species (p < 0.001 in all cases; figure 1c): however An. arabiensis did not obtain larger blood meals (p > 0.05 in all pairwise comparisons; figure 2a), have higher oviposition probability (p > 0.05; figure 2d), egg production (p > 0.05 in all cases; figure 2e) or survival (p > 0.05; figure 3a) on cows than other host types. As a consequence of their higher feeding success, however, the LRS of An. arabiensis was predicted to be highest on cattle hosts (see figure 4a and the electronic supplementary material, S6).
The feeding probability of An. gambiae s.s. on their naturally preferred humans was no higher than on any other host type except chickens (figure 1d). Anopheles gambiae s.s. obtained significantly larger blood meals from exposed humans than from other host types except cows (z =−1.76, p = 0.31; figure 2b), but their oviposition probability and fecundity after feeding on humans were no higher than any other host species (figure 2d,f). The survival of An. gambiae s.s. was significantly higher after feeding on exposed humans than on other host type except cows (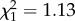 , p = 0.29; figure 3b and table 1). When all estimates of host-dependent fitness were combined to predict the LRS of An. gambiae s.s., there was no evidence of an advantage associated with human feeding (see figure 4c and the electronic supplementary material, S6).
, p = 0.29; figure 3b and table 1). When all estimates of host-dependent fitness were combined to predict the LRS of An. gambiae s.s., there was no evidence of an advantage associated with human feeding (see figure 4c and the electronic supplementary material, S6).
(c). Could the use of bednets alter the fitness value of humans relative to other host species?
Anopheles arabiensis was significantly more likely to feed on cows than on humans (z =−3.89, p = 0.002; figure 1c). This difference was even more pronounced when humans used bednets (z =−6.07, p < 0.001; figure 1c). The fecundity (figure 2c,e) and survival (figure 3a and table 1) of An. arabiensis that fed despite the presence of bednets were not significantly lower than on an unprotected human or other host species. Although the LRS of An. arabiensis was predicted to be highest on cows (figure 4a), the advantage of cattle over humans only achieved statistical significance when the latter was assumed to use bednets (see the electronic supplementary material, S6). Analysis of mixed human–cattle feeding strategies indicated that An. arabiensis that takes 60 per cent or greater of their blood meals from cows should have a significantly higher LRS than those who attempt to feed only on bednet-protected humans (see figure 4b and the electronic supplementary material, S6).
Use of bednets was associated with a moderate, but not statistically significant reduction in An. gambiae s.s. feeding success on humans (z = 1.49, p = 0.47; figure 1d). The oviposition and fecundity of An. gambiae s.s. that fed on people using bednets were no different from those who fed on unprotected people (figure 2d,f). However, the survival of An. gambiae s.s. that succeeded in feeding on humans using nets was significantly reduced relative to those who fed on fully exposed humans (table 1). The use of bednets was predicted to reduce the human-associated LRS of An. gambiae s.s. to below that predicted for several other host species (cattle, dogs and goats; figure 4c), however, these differences were not statistically significant after correcting for multiple comparisons (see the electronic supplementary material, S6).
4. Discussion
We show that the fitness which the malaria vectors An. arabiensis and An. gambiae s.s. derive from host encounter varies significantly between the host species most commonly available to them. However, evidence of positive correlations between the known natural host preferences of these vectors and their expected fitness from feeding on them was mixed. Whereas the LRS of An. arabiensis was predicted to be highest on its preferred cow hosts, that of An. gambiae s.s. was estimated to be relatively similar on their preferred humans and most other host species. This challenges the assumption that innate host-specific behavioural or physiological properties are responsible for the evolution of anthrophily in this important vector.
Evidence that untreated bednet use could reduce the relative rewards of anthrophily was also mixed. For An. arabiensis, a significant fitness advantage from foraging on cows instead of humans was only predicted if the latter use nets. Thus, in addition to the personal protection provided by such bednets [26], they may also be capable of imposing a cost on anthrophily that could exert selection for reduced human feeding in settings, where bednet coverage is high and where cattle are readily available. By contrast, protecting humans with ‘typical’, untreated bednets was predicted to have minimal impact on the fitness of An. gambiae s.s., and unlikely to reduce the fitness ranking of humans relative to other animal alternatives.
While not all of the mosquito fitness traits investigated here varied between host species, at least one did so for each vector. In An. arabiensis, host species primarily determined their probability of acquiring a blood meal, but not their post-feeding fitness. By contrast, under these experimental conditions An. gambiae s.s. had a similar feeding probability on all mammalian hosts, but variable reproductive success and survival afterwards. This suggests that there may be trade-offs in the value of host resources for different life-history processes. Although mosquito blood meal size and egg production have widely been correlated in previous work [27–29], the smaller blood meals associated with some host species here did not consistently translate into reduced egg production. Most previous studies have examined this relationship only within one host species, and it is possible that there are additional sources of haematological variation between host species that cause this relationship to breakdown, when comparing blood meals taken across them.
Evolutionary theory predicts that the fitness of specialists is highest when preferred resources are consumed [30]. Our life-history model predicted this to be true for An. arabiensis, whose LRS was estimated to be substantially higher on its naturally preferred cow hosts. However, although some An. gambiae s.s. fitness traits were highest on their preferred humans (blood meal size), there was no evidence of an overall advantage to their LRS associated with these hosts. Failure to detect correlations between host preference and performance have been documented in other insect systems [31], and attributed to ecological variation that modifies the quality of hosts in different environments. Similarly, our ability to detect host preference–performance relationships in An. gambiae s.s. may have been limited by experimental conditions. Here, we presented hosts to mosquitoes under a ‘no choice’ scenario in an indoor environment. This design was used to distinguish between fitness effects arising from innate biological properties of hosts (e.g. physiological and/or behavioural), from those arising indirectly due to variation in their use of habitats. While livestock is frequently kept inside buildings at night in our study area, in other settings livestock may be kept outside during vector activity periods. Anopheles gambiae s.s. has a strong preference for biting indoors [32,33], whereas, An. arabiensis bites hosts both indoors and outside. If An. gambiae's preference for feeding on humans is an indirect consequence of an advantage arising from indoor biting (irrespective of host species), the relative advantages of anthrophily in this vector species may be underestimated here relative to other environments, where animals are generally outside. Further investigation within this environmentally-realistic yet experimentally tractable system can help evaluate this hypothesis.
While this study demonstrates that untreated bednets have potential to diminish the relative fitness benefits of anthrophily in some malaria vector species, there has been relatively little evidence of such phenomena occurring in response to the use of this intervention in nature (reviewed in Lyimo and Ferguson [6]). A potential explanation is that our results indicate that the use of this intervention has relatively minor impacts on many mosquito fitness traits (e.g. in An. gambiae s.s.), and only led to statistically significant disadvantage of humans relative to animal hosts in a limited range of scenarios (An. arabiensis choosing between humans and cows). This reinforces the need to maintain good quality, intact and insecticidal-treated nets to reap the greatest epidemiological and evolutionary benefits for control.
Evaluation of the accuracy with which effects described here reflect the nature of selection acting on host species range in nature, will require further investigation of several areas that at present are intractable within the semi-field conditions used here. First, the host-specific feeding probabilities estimated here may be upwardly biased because they were measured under ‘no choice’ conditions (e.g. our observation that An. gambiae s.s. fed on all host species with similar probability contrasts with their known preference for humans in nature; [8]). Giving An. gambiae s.s. a choice between hosts may have significantly increased predicted feeding rates on humans at the expense of those estimated for other animals. However, this may not substantially alter our conclusions about the relative benefits of anthrophily, as An. gambiae s.s. were shown to be capable of feeding on other animals to the same degree as humans when no choice was available, with no consistent reduction in their fitness relative to those obtained from human blood meals. Ideally, this expectation could be confirmed by simultaneous measurement of mosquito host choice and subsequent fitness. Presently, this is not possible because the host choice of blood fed mosquitoes can only be confirmed by killing them to analyse their stomach contents, which prevents any further measurement of their fitness. Should non-invasive methods become available for blood meal identification, follow up investigation of mosquito fitness under choice scenarios should be pursued. Further investigation of other potential advantages of anthrophily beyond which they could be measured here, including habitat-dependent foraging success (higher inside houses) or benefits from host-seeking on aggregated populations, is encouraged. Finally, the requirement for large numbers of similarly aged, malaria-free mosquitoes required the use of insectary-reared mosquitoes in this study. Although both insectary colonies were initiated from mosquito populations in the local area and maintained on a natural blood source, the process of colonization can modify host discrimination behaviour [34]. Where possible, further study using F1 mosquitoes from wild populations is encouraged to identify potential biases arising from the use of colonized mosquitoes.
Our model predictions are based on several assumptions that also require validation for assessment of potential implications of these results to field. One is that the host-specific impacts on mosquito fitness measured are similar on all feeding cycles. Here, mosquito fitness was measured after one blood meal, whereas in nature, vectors feed every 2–4 days [35]. Repeated blood feeding could potentially cancel out or magnify the host-specific effects described here. A previous laboratory study showed that An. gambiae s.s. fed one blood meal using an artificial membrane feeder exhibited similar host-specific survival as documented here [15]. However, when mosquitoes were given two consecutive blood meals consisting of blood from humans followed by another animal, their longevity was similar [15]. This suggests that negative fitness effects arising from blood meals on poor quality hosts could be reduced by further meals from a ‘high quality’ hosts. Furthermore, mosquitoes may be able to increase their feeding frequency from what was assumed here to compensate for lower quality blood meals. Had mosquitoes been provided with an oviposition substrate earlier than the standard 4 day post-feeding period used here, it is possible that those fed on poorer quality host types could have brought forward their oviposition to increase future feeding opportunities. This phenomenon has not yet been documented in An. gambiae s.l., but is worthy of further investigation once reliable methods for individually marking and repeatedly sampling mosquitoes at different time points during their feeding cycle become available.
For most of the past 20 years, untreated bednets have been the primary vector control intervention in many malaria endemic regions including our study area. For example, recent estimates suggest that approximately 75–91% of households in the Kilombero Valley are covered by untreated nets [36]. However, in the past 5 years, these simple interventions are being rapidly replaced by the distribution of more effective insecticide-treated (ITN) and long-lasting insecticidal (LLINs) nets in many African countries. While increases in ITN and LLIN coverage over this period have been massive, the median proportion of households across sub-Saharan Africa reporting ownership of at least one ITN/LLIN is approximately 50 per cent [17]. Thus, there remains a significant proportion of households that do not have access to these more effective insecticidal interventions, and continue to rely on their untreated counterparts. Understanding the nature of selection that may have been generated by this widespread predecessor to ITN/LLINs can provide a useful framework for anticipating the future evolutionary changes these interventions may exert on mosquito behaviour. We hypothesize that the addition of insecticides to nets would substantially increase the fitness costs of anthrophily, and generate stronger selection for a shift away from human feeding; especially as results obtained here and our previous work [15] suggest that these vectors can reproduce and survive equally well on at least some of the commonly available alternative animal hosts.
At present, the genetic basis of host species preferences in malaria vectors is poorly understood, although early work [37] illustrated that An. gambiae can be selected for increased zoophily within a few generations (less than five). These experimental data combined with growing evidence from field settings that malaria vectors are modifying their feeding behaviour in response to insecticide-based interventions [38] suggest that their host preference is a phenotype that can evolve. Assuming such genetic variation exists, due caution would still be required before embarking on a strategy of using interventions to drive selection on mosquito host species choice. Specifically, it would need to be demonstrated that the epidemiological benefits of facilitating selection for zoophily would not be outweighed by the disadvantages of providing mosquitoes with alternative ‘refuge’ hosts that would allow their populations to be maintained, even when all humans are protected by LLINs [17,39]. However, these results highlight opportunities that interventions present for generating selection against mosquito behaviours that facilitate disease transmission. Opportunities to reduce human biting either through short-term diversion to non-permissive animal species (e.g. zooprophylaxis) or longer-term selection on anthrophily should be exploited as a means to reinforce control.
Acknowledgements
This study was approved by the Institutional Ethical Review Board (IRB) of the IHI (IHRDC/IRB/No.A015), the Medical Research Coordination Committee of the Tanzania National Institute for Medical Research (NIMR1HQ/R.8a/Vol.IX/708) and the University of Glasgow (for details see the electronic supplementary material, S2).
We thank IHI insectary technicians for rearing mosquitoes, Dr Sarah Moore for logistical support and Drs Gerry Killeen and Lisa Ranford-Cartwright for discussion. We also thank all human volunteers and livestock owners who participated in this research. This work was funded by the Faculty of Biomedical and Life Sciences Studentship to I.N.L., and a BBSRC David Phillips fellowship to H.M.F.
References
- 1.Koella JC, Penelope AL, Matthew BT, Andrew FR. 2009. Towards evolution-proof malaria control with insecticides. Evol. Appl. 9999, 1–12 10.1111/j.1752-4571.2009.00072.x (doi:10.1111/j.1752-4571.2009.00072.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gourley SA, Liu R, Wu J. 2011. Slowing the evolution of insecticide resistance in mosquitoes: a mathematical model. Proc. R. Soc. A 467, 2127–2148 10.1098/rspa.2010.0413 (doi:10.1098/rspa.2010.0413) [DOI] [Google Scholar]
- 3.Kurzban R, Egeth M. 2008. Applied Darwinian medicine: artificial selection for less-harmful parasites. Med. Hypotheses 71, 976–977 10.1016/j.mehy.2008.07.019 (doi:10.1016/j.mehy.2008.07.019) [DOI] [PubMed] [Google Scholar]
- 4.Ferguson H, Gandon S, McKinnon M, Read A. 2006. Malaria parasite virulence in mosquitoes and its implications for the introduction and efficacy of GMM malaria control programmes. In Genetically modified mosquitoes for malaria control (ed. Boete C.), pp. 103–116. Georgetown, TX: Landes Bioscience [Google Scholar]
- 5.Ferguson MH, Nicholas M, Takken W, Lyimo NI, Briët O, Lindsay WS, Smith AT. 2012. Selection of mosquito life-histories: a hidden weapon against malaria? Malar. J. 11, 1–9 10.1186/1475-2875-11-1 (doi:10.1186/1475-2875-11-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyimo IN, Ferguson HM. 2009. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 25, 189–196 10.1016/j.pt.2009.01.005 (doi:10.1016/j.pt.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Daneshvar C. 2010. Plasmodium knowlesi malaria in Malaysia. Med. J. Malaysia 65, 224–230 [PubMed] [Google Scholar]
- 8.Kiszewski A, Mellinger A, Spielman A, Malaney P, Sachs SE, Sachs J. 2004. A global index representing the stability of malaria transmission. Am. J. Trop. Med. Hyg. 70, 486–498 [PubMed] [Google Scholar]
- 9.Philip LB, Sungano M, Philip ET. 2009. Cattle, other domestic animal ownership, and distance between dwelling structures are associated with reduced risk of recurrent Plasmodium falciparum infection in southern Zambia. Trop. Med. Int. Health 14, 522–528 10.1111/j.1365-3156.2009.02270.x (doi:10.1111/j.1365-3156.2009.02270.x) [DOI] [PubMed] [Google Scholar]
- 10.Takken W, Bart GJK. 2009. Malaria vector control: current and future strategies. Trends Parasitol. 25, 101–104 10.1016/j.pt.2008.12.002 (doi:10.1016/j.pt.2008.12.002) [DOI] [PubMed] [Google Scholar]
- 11.Krasnov BR, Khokhlova IS, Burdelova NV, Mirzoyan NS, Degen AA. 2004. Fitness consequences of host selection in ectoparasites: testing reproductive patterns predicted by isodar theory in fleas parasitizing rodents. J. Anim. Ecol. 73, 815–820 10.1111/j.0021-8790.2004.00860.x (doi:10.1111/j.0021-8790.2004.00860.x) [DOI] [Google Scholar]
- 12.Futuyma DJ, Moreno G. 1988. The evolution of ecological speciation. Annu. Rev. Ecol. Syst. 19, 207–233 10.1146/annurev.ecolsys.19.1.207 (doi:10.1146/annurev.ecolsys.19.1.207) [DOI] [Google Scholar]
- 13.Agrawal AA. 2000. Host-range evolution: adaptation and trade-offs in fitness of mites on alternative hosts. Ecology 81, 500–508 10.1890/0012-9658(2000)081[0500:HREAAT]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[0500:HREAAT]2.0.CO;2) [DOI] [Google Scholar]
- 14.Harrington LC, Edman JD, Scott TW. 2001. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 38, 411–422 10.1603/0022-2585-38.3.411 (doi:10.1603/0022-2585-38.3.411) [DOI] [PubMed] [Google Scholar]
- 15.Lyimo IN, Keegan SP, Ranford-Cartwriwght LC, Ferguson HM. 2012. The impact of uniform and mixed species blood meals on the fitness of the mosquito vector Anopheles gambiae s.s.: does a specialist pay for diversifying its host species diet? J. Evol. Biol. 25, 452–460 10.1111/j.1420-9101.2011.02442.x (doi:10.1111/j.1420-9101.2011.02442.x) [DOI] [PubMed] [Google Scholar]
- 16.WHO 2009. World malaria report, pp. 3–8 Geneva, Switzerland: World Health Organization [Google Scholar]
- 17.WHO 2011. World malaria report, pp. 27–34 Geneva, Switzerland: World Health Organization [Google Scholar]
- 18.Coetzee M. 2004. Distribution of the African malaria vectors of the Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 70, 103–104 [PubMed] [Google Scholar]
- 19.Killeen GF, Seyoum A, Knols BGJ. 2004. Rationalizing historical success of malaria control in Africa in terms of mosquito resource availability management. Am. J. Trop. Med. Hyg. 71(Suppl. 2), 87–93 [PubMed] [Google Scholar]
- 20.Ferguson H, et al. 2008. Establishment of a large semi-field system for experimental study of African malaria vector ecology and control in Tanzania. Malar. J. 7, 158. 10.1186/1475-2875-7-158 (doi:10.1186/1475-2875-7-158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogoma SB, Lweitoijera DW, Ngonyani H, Furer B, Russell TL, Mukabana RW, Killeen GF, Moore SJ. 2010. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arborvirus and malaria vectors. PLoS Negl. Trop. Dis. 4, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO 2005. Guidelines for laboratory and field testing of long lasting insecticide mosquito nets, pp. 18–19 Geneva, Switzerland: World Health Organization [Google Scholar]
- 23.Briegel H. 1980. Determination of uric acid and hematin in a single sample of excreta from blood-fed insects. Experimentia 36, 1428. 10.1007/BF01960142 (doi:10.1007/BF01960142) [DOI] [Google Scholar]
- 24.Crawley MJ. 2007. The R book. Chichester, UK: Wiley [Google Scholar]
- 25.Hougaard P. 1995. Frailty models for survival data. Lifetime Data Anal. 1, 255–273 10.1007/BF00985760 (doi:10.1007/BF00985760) [DOI] [PubMed] [Google Scholar]
- 26.Clarke SE, Bogh C, Brown RC, Pinder M, Walraven GEL, Lindsay SW. 2001. Do untreated bednets protect against malaria?. Trans. R. Soc. Trop. Med. Hyg. 95, 457–462 10.1016/S0035-9203(01)90001-X (doi:10.1016/S0035-9203(01)90001-X) [DOI] [PubMed] [Google Scholar]
- 27.Ferguson HM, Rivero A, Read AF. 2003. The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology 127, 9–19 10.1017/S0031182003003287 (doi:10.1017/S0031182003003287) [DOI] [PubMed] [Google Scholar]
- 28.Roitberg BD, Gordon I. 2005. Does the Anopheles blood meal–fecundity curve, curve? J. Vector Ecol. 30, 83–86 [PubMed] [Google Scholar]
- 29.McCann S, Day JF, Allan S, Lord CC. 2009. Age modifies the effect of body size on fecundity in Culex quinquefasciatus Say (Diptera: Culicidae). J. Vector Ecol. 12, 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levins R. 1962. Theory of fitness in a heterogeneous environment. I. The fitness set and adaptive function. Am. Nat. 96, 361–373 10.1086/282245 (doi:10.1086/282245) [DOI] [Google Scholar]
- 31.Gripenberg S, Mayhew PJ, Parnell M, Roslin T. 2010. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 13, 383–393 10.1111/j.1461-0248.2009.01433.x (doi:10.1111/j.1461-0248.2009.01433.x) [DOI] [PubMed] [Google Scholar]
- 32.Gillies MT. 1954. Studies in house leaving and outside resting of Anopheles gambiae Giles and Anopheles funestus Giles in East Africa. II. The exodus from houses and the house resting population. Bull. Entomol. Res. 45, 375–387 10.1017/S000748530002719X (doi:10.1017/S000748530002719X) [DOI] [Google Scholar]
- 33.Gillies MT. 1954. Studies of house leaving and outside resting of Anopheles gambiae Giles and Anopheles funestus Giles in East Africa. I. The outside resting population. Bull. Entomol. Res. 45, 361–373 10.1017/S0007485300027188 (doi:10.1017/S0007485300027188) [DOI] [Google Scholar]
- 34.Lefèvre T, Gouagna L-C, Dabire KR, Elguero E, Fontenille D, Costantini C, Thomas F. 2009. Evolutionary lability of odour-mediated host preference by the malaria vector Anopheles gambiae. Trop. Med. Int. Health 14, 228–236 10.1111/j.1365-3156.2009.02206.x (doi:10.1111/j.1365-3156.2009.02206.x) [DOI] [PubMed] [Google Scholar]
- 35.Gillies MT. 1953. The duration of the gonotrophic cycle in Anopheles gambiae and Anopheles funestus, with a note on the efficiency of hand catching. East Afr. Med. J. 30, 129–135 [PubMed] [Google Scholar]
- 36.Russell T, et al. 2010. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar. J. 9, 187. 10.1186/1475-2875-9-187 (doi:10.1186/1475-2875-9-187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillies MT. 1964. Selection for host preference in Anopheles gambiae. Nature 203, 852–854 10.1038/203852a0 (doi:10.1038/203852a0) [DOI] [PubMed] [Google Scholar]
- 38.Lefevre T, Gouagna LC, Dabire KR, Elguero E, Fontenille D, Renaud F, Costantini C, Thomas F. 2009. Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible. Am. J. Trop. Med. Hyg. 81, 1023–1029 (doi:10.4269/ajtmh.2009.09–0124) [DOI] [PubMed] [Google Scholar]
- 39.Killeen GF, Smith TA. 2007. Exploring the contributions of bed nets, cattle, insecticides and excitorepellency malaria control: a deterministic model of mosquito host-seeking behaviour and mortality. Trans. R. Soc. Trop. Med. Hyg. 101, 867–880 10.1016/j.trstmh.2007.04.022 (doi:10.1016/j.trstmh.2007.04.022) [DOI] [PMC free article] [PubMed] [Google Scholar]