Abstract
Quantifying the fitness cost that parasites impose on wild hosts is a challenging task, because the epidemiological history of field-sampled hosts is often unknown. In this study, we used an internal marker of the parasite pressure on individual hosts to evaluate the costs of parasitism with respect to host body condition, size increase and reproductive potential of field-collected animals for which we also determined individual age. In our investigated system, the European eel Anguilla anguilla and the parasitic invader Anguillicoloides crassus, high virulence and severe impacts are expected because the host lacks an adaptive immune response. We demonstrated a nonlinear relationship between the severity of damage to the affected organ (i.e. the swimbladder, our internal marker) and parasite abundance and biomass, thus showing that the use of classical epidemiological parameters was not relevant here. Surprisingly, we found that the most severely affected eels (with damaged swimbladder) had greater body length and mass (+11% and +41%, respectively), than unaffected eels of same age. We discuss mechanisms that could explain this finding and other counterintuitive results in this host–parasite system, and highlight the likely importance of host panmixia in generating great inter-individual variability in growth potential and infection risk. Under that scenario, the most active foragers would not only have the greatest size increase, but also the highest probability of becoming repeatedly infected—via trophic parasite transmission—during their continental life.
Keywords: host–parasite interaction, pathogenicity, virulence, fitness cost, life-history traits, anguillicolosis
1. Introduction
Parasites, by their very nature, are expected to impose costs on their hosts, in diverting resources that could otherwise be used for host growth, maintenance and reproduction [1]. Assessing parasite cost on host fitness is a crucial but challenging task that can be rigorously quantified under controlled, experimental conditions [2,3]. However, when infected individuals are studied outside their ecological context, any observed reduction in their fitness might be a poor approximation of that incurred by the same hosts in natural systems. Indeed, hosts in their natural ecosystems are exposed to many other enemies and selective forces (e.g. competition, predation and sexual selection) and, under some circumstances, the presence of a specific parasite can even be beneficial to the host organism or populations [4]. However, field studies of parasite effects are problematic, because the epidemiological history of the host is rarely known. Here, for studying in natural conditions the impacts of a parasitic invader on its endangered eel host, we used a previously developed internal marker (an index measuring the cumulative damage to the infected organ) to evaluate the parasite pressure suffered by individual fish during their continental life.
The European eel Anguilla anguilla has a complex life cycle, with a growth phase occurring in continental waters and a panmictic reproduction occurring in the Sargasso Sea. Because of its snake-like appearance and abundance, this species was once considered to be ‘undesirable’ and locally ‘invasive’. However, things have dramatically changed. Since the 1970s, both scientific and commercial fishery data have recorded a continuous decline in stocks and recruitment [5,6]. In 2008, the European eel was classified as a ‘critically endangered’ species by the IUCN Red List [7], and is now considered to be ‘outside safe biological limits’ [8]. The causes of the decline have not yet been fully elucidated, but are almost certain to be multiple and synergistic [9]. Because of its long—catadromous—life cycle (living in fresh water but migrating to marine waters to breed), the European eel is exposed to many risk factors, including overfishing, pollution, habitat loss, migration barriers and oceanic changes [5,8,10].
Since the 1980s, the disease anguillicolosis caused by the invasive nematode worm Anguillicoloides crassus (formerly Anguillicola, see [11]) has emerged as a new threat to the already endangered eel. The parasite probably entered European waters via exotic eels imported from Taiwan for commercial culture [12]. Since the first record of its presence in Europe (in Germany, 1982), it has spread with remarkable speed, colonizing nearly all of the geographical range of its new host in just a few decades; it has now also been reported infecting the American eel (see [11,13] for reviews). In areas where the parasite has established it can rapidly reach prevalences of over 50 per cent among eels, in part because of its extensive use of intermediate and paratenic hosts (mostly crustaceans and small fishes; [11,13]). Larvae and adult worms, which invade the swimbladder wall and the swimbladder lumen, respectively, cause inflammatory reactions, haemorrhages and fibrosis, and this occasionally leads to the total collapse of the infected organ [14,15]. In addition, because of the bloodsucking activity of the adult parasites, infection affects the physiology and general metabolism of the eel [16–18]. The European eel lacks immune adaptation for resistance [19], and high parasite virulence and pathogenicity have been observed in the laboratory (see [13] for review). However, studies of life-history traits of infected eels under natural conditions have thus far produced ambiguous results. Because parameters such as body growth and condition are of primary interest to fishers, fish farmers and resource managers, the many reported studies have only further confounded the picture. While most works found no impact of infection, many others have reported either significant negative or positive effects on the host traits investigated (e.g. [20–22]; see also [13] for review).
However, a weakness of the methods applied in field studies has been the lack of age data for comparison of size differences and condition between infected and uninfected individuals. In most studies, the effect of A. crassus has been inferred from changes in host body mass as a function of body length, on the unverified assumption that an increase in length is not affected by infection. In addition, comparisons of eel body dimensions have generally been performed on the basis of the parasite load/biomass at the time of autopsy, with no consideration of the epidemiological history of the host. This is a critical missing parameter, given that eels probably experience multiple infection events between the stage at which they enter continental waters and the time that they initiate their reproductive seaward migration, some years later. As the life cycle of the nematode is short (a few months, [23]) relative to the continental phase of the eel (at least 3 years for the most rapidly maturing male eels, and up to 25 years for the largest female eels; [24]), the absence of parasites at autopsy does not preclude that an eel has been repeatedly infected and severely affected in the past.
Reliable and functional indices of swimbladder degeneration are now available to quantify the cumulative damage to the infected organ, and thereby evaluate the parasite pressure suffered by individual eels during their continental life phase (for review, see [13,25]). Consideration of the health status of the infected organ is particularly critical in this host–parasite system, for which infection state dependence has been demonstrated, whereby the tissue degradation caused by previous infections can limit and ultimately prevent the establishment of new parasite infections [26,27]. In the present study, we investigated the impact of past and current A. crassus infection(s) on body condition, size increase and silvering parameters of wild eels of known age, as determined by otolithometry.
2. Material and methods
(a). Data collection
The eels used in the study were obtained from a complex of brackish lagoons in the Rhône River delta, Camargue, southern France. Monthly sampling was conducted from March to December 2000 at two sites, Capelière (43°31′57″ N, 04°38′24″ E) and Malagroy (43°30′26″ N, 04°27′08″ E). At each site, two fyke nets (one with a funnel mesh size of 6 mm and a leading net length of 40 m, the other with a mesh size of 0.5 mm and a leading net length of 20 m) were used to catch all size classes of eels present in the lagoon system. The eels were collected every 24 h for four consecutive days per month and were immediately frozen until examined.
At each monthly sampling, 30 individuals spanning the range of eel lengths were selected. For each eel, the total body length (LT; from the most forward point of the head to the tip of the tail) was recorded to the nearest 1 mm, and the total body mass (MT) and somatic mass (MS) (i.e. eviscerated carcass mass) were recorded to the nearest 0.1 g. For eels more than or equal to 300 mm in length, the sex was determined by macroscopic observation of the gonads, using previously described criteria [28]. The gonads were then removed and weighed (MG) to the nearest 0.1 mg. Horizontal (Dh) and vertical (Dv) left eye diameters were measured to the nearest 0.1 mm.
The swimbladder of each fish was removed and examined macroscopically to determine its Swimbladder Degenerative Index score (SDI; [27]). The SDI was computed based on three criteria: (i) opacity of the swimbladder wall; (ii) the presence of pigmentation on the swimbladder wall and/or exudates in the swimbladder lumen; and (iii) the thickness of the swimbladder wall. For each criterion, the swimbladder was given a score of 0 (no degradation), 1 (moderate degradation) or 2 (severe degradation). Thus, the SDI ranges from 0 (no pathological signs observed) to 6 (extremely damaged). For each swimbladder, the number of A. crassus adults and pre-adults present in the lumen (excluding larvae within the swimbladder wall) and their combined weight (MA. crassus; to the nearest 0.1 mg) were determined.
The otoliths were removed from the head of each eel for age determination (otolithometry). Small otoliths were directly examined in 70 per cent ethanol, while larger ones were embedded in methacrylate resin, ground to the sagittal plane and stained with toluidine blue prior to microscopic examination [29]. The age of each individual was estimated by counting the number of yearly growth annuli, attributing an arbitrary ‘birth date’ of 1 April (the peak of eel recruitment occurring in late winter–early spring in the lagoons of Camargue; see [30]). Thus, based on the above procedure, eels that were caught during April and had no hyaline annulus were assigned to an age of one month, and so forth with monthly increments according to the sampling date and the number of yearly annuli [29].
(b). Estimates of eel body condition, size increase and silvering
Body condition was assessed using two estimators to ensure methodological reliability, as all available tools for studying body condition have limitations and relevance under particular circumstances [31,32]. Insofar as eels are characterized by allometric growth, we used Le Cren's relative condition factor (Krel), which compensates for changes in form or condition with increasing length [33]. The relative condition factor was calculated as: 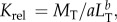 where MT is measured in grams and LT is measured in centimetres, and where a and b are, respectively, the allometric coefficient and the allometric exponent of the weight–length relationship of the European eel in Camargue lagoons. We used the a and b values calculated for sexually undifferentiated (n = 15 311), male (n = 1585) and female (n = 940) eels as part of long-term monitoring of the eel population at the sampling sites [34]. The second method used to assess the effect of A. crassus on host body condition involved analysis of covariance (ANCOVA); this enabled a direct estimate of the parasite effect on eel mass, while controlling for eel length [35].
where MT is measured in grams and LT is measured in centimetres, and where a and b are, respectively, the allometric coefficient and the allometric exponent of the weight–length relationship of the European eel in Camargue lagoons. We used the a and b values calculated for sexually undifferentiated (n = 15 311), male (n = 1585) and female (n = 940) eels as part of long-term monitoring of the eel population at the sampling sites [34]. The second method used to assess the effect of A. crassus on host body condition involved analysis of covariance (ANCOVA); this enabled a direct estimate of the parasite effect on eel mass, while controlling for eel length [35].
Quantification of the increase in eel size during the time spent in continental waters was used as a complementary approach. The two methods described above use the linear dimension of body size (i.e. length) as a scaling factor to derive the amount of extra body mass, which is an estimate of body condition. However, if the increase in length is influenced by infection, these estimators would be of no value for the purpose of the study. Hence, we also used an ANCOVA to investigate the effect of A. crassus on host body mass and body length, while controlling for the age of individual eels.
Eel silvering is characterized by multiple morphological and physiological changes that prepare the fish for their oceanic migration to the Sargasso Sea for reproduction. This involves an increase in eye diameter (for vision in the marine environment), and an increase in the gonad mass. The Ocular Index (IO; [36]) and the Gonadosomatic Index (IG; [37]) are standard and widely used estimators of these silvering-related changes. They were calculated as IO = [((Dh + Dv)/4)2 × π/LT] × 100 and IG = 100 × MG/MT. However, as these indices estimate changes in eye diameter and gonad mass as a function of eel length and mass, respectively, they may not be reliable if infection also affects host body dimensions. Therefore, in addition to the use of these indices in their original form (for comparison with previous studies on silvering), we also used an ANCOVA to investigate the effect of A. crassus infection on eye area (AE), calculated as AE = π × Dh × Dv/4, and on gonad mass (MG), while controlling for the age of individual eels.
(c). Factors describing past and current infections
The need to use an integrative measure of swimbladder health for evaluating the impact of infection has been increasingly recognized [38,39]. Moreover, we recently demonstrated a very strong association between the severity in the SDI scores and the functional loss in swimbladder gas volume, thereby definitively validating the biological significance of the index [25]. For statistical reasons (i.e. to ensure equal distribution of eel sample sizes, and homogeneity of variances among groups), in this study, the SDI was re-coded (SDI') using three classes. A value of 0 was assigned to eels having a healthy swimbladder (SDI values of 0 or 1), a value of 2 was assigned to eels having a severely damaged swimbladder (SDI values ≥ 4), and a value of 1 was assigned to eels having intermediate swimbladder damage (SDI values of 2 or 3).
The biomass of A. crassus (MA. crassus) was used to estimate the current parasite pressure affecting the host eels. As the nematode exhibits marked sexual dimorphism, with females being on average a factor of 10 heavier than males [11], it is likely that parasite biomass rather than parasite intensity (i.e. the number of parasite individuals per host) is a more relevant measure of parasite pressure, especially when considering host metabolism-related traits [40].
(d). Statistical analysis
General Linear Models were used with type III sums of squares (SS) to analyse the variations in eel body condition, size increase and silvering. In addition to host-related factors (i.e. individual age and sex) and parasite-related factors (i.e. SDI' and MA. crassus), to build models that are as robust as possible we also included the sampling site (Capelière and Malagroy) and the season during which the eels were caught as potential explanatory variables. Seasons were coded as ‘spring’ (for eels caught in March, April and May), ‘summer’ (for eels caught in June, July, August and September) and ‘autumn’ (for eels caught in October, November and December). The a priori full models are provided in the electronic supplementary material (see the electronic supplementary material, appendix S1). To determine the best subsets of explanatory variables, we calculated the Akaike's Information Criterion (AIC) to rank the reduced models. Only candidate models with an AIC value within 2 units of the best-fitting model (i.e. the candidate model with the lowest AIC) were considered to have substantial empirical support as a best-fitting model [41]. Model simplicity and factor significance (i.e. p < 0.05 for all explanatory variables of a given subset) were used as the ultimate criteria for selecting the best statistical model (see the electronic supplementary material). To test for model goodness of fit, residuals were plotted against fitted values to assess the generation of an approximately straight line (normal probability plots). Homogeneity of variances was tested using Levene's F-test. Transformations of raw variables were applied wherever necessary prior to analyses (see the electronic supplementary material, appendix S1). The importance of individual predictors was assessed using the squared semipartial correlation coefficient (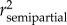 ), calculated as
), calculated as 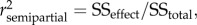 which represents the proportion of total variation accounted for by a factor over and above what is explained by other factors [42]. Fisher's least significant difference (Fisher's LSD) post-hoc test was performed to identify differences among groups (only parasite-related results are presented). Analyses were carried out using Statistica v. 6.0 software (StatSoft Inc.), and raw data were archived in the Dryad repository (doi:http://dx.doi.org/10.5061/dryad.34qf8).
which represents the proportion of total variation accounted for by a factor over and above what is explained by other factors [42]. Fisher's least significant difference (Fisher's LSD) post-hoc test was performed to identify differences among groups (only parasite-related results are presented). Analyses were carried out using Statistica v. 6.0 software (StatSoft Inc.), and raw data were archived in the Dryad repository (doi:http://dx.doi.org/10.5061/dryad.34qf8).
3. Results
Of the 277 eels examined in this study, 183 were at a sexually undifferentiated stage, 43 were males and 51 were females. The total body length (LT) ranged from 71 to 717 mm, the somatic mass (MS) ranged from 0.4 to 766.8 g (table 1) and the eel ages ranged from one to 90 months. The prevalence of A. crassus among all samples was 52.7 per cent, and the mean intensity was 4.1 ± 0.4 nematodes per infected eel, with a maximum of 37 (adults and pre-adults) recorded in a single eel. The full range of SDI scores (0–6) was recorded, from unaffected swimbladders to those that were totally degenerated with no internal lumen remaining (table 1). Figure 1 shows the curvilinear relationship between SDI scores and A. crassus abundance and biomass, and illustrates the negative effect of increased damage to the infected organ on the parasite's development (i.e. infection state dependence).
Table 1.
Morpho-anatomical characteristics, ages and A. crassus epidemiological parameters for the 277 sampled eels. s.d., standard deviation; 95% CI, 95% confidence interval; min.–max., minimum and maximum recorded values.
| eel data | mean ± s.d. | 95% CI | min.–max. |
|---|---|---|---|
| total body length (LT) (mm) | 266.7 ± 122.3 | 252.2 – 281.1 | 71 – 717 |
| somatic mass (MS) (gram) | 57.2 ± 101.1 | 45.2 – 69.1 | 0.4 – 766.8 |
| ocular index (IO) | |||
| males | 5.2 ± 2.4 | 4.5 – 5.9 | 2.3 – 11.0 |
| females | 4.2 ± 1.1 | 3.9 – 4.5 | 2.7 – 8.7 |
| gonadosomatic index (IG) | |||
| males | 0.38 ± 0.31 | 0.29 – 0.48 | 0.01 – 1.36 |
| females | 0.56 ± 0.44 | 0.44 – 0.68 | 0.11 – 1.88 |
| age (months) | 34.2 ± 15.1 | 32.4 – 36.0 | 1 – 90 |
| Anguillicoloides crassus | |||
| prevalence (%) | 52.7 ± 50.0 | 46.8 – 58.6 | n.a. |
| intensity (number of worms) | 4.1 ± 4.4 | 3.4 – 4.8 | 1 – 37 |
| SDI | 2.3 ± 1.5 | 2.2 – 2.5 | 0 – 6 |
Figure 1.
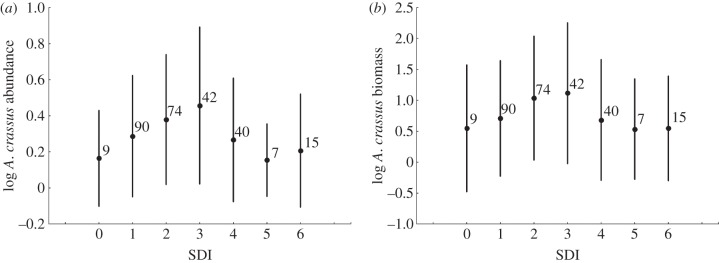
Correspondence between the values of the Swimbladder Degenerative Index (SDI) and current infection parameters in Anguillicoloides crassus. (a) Parasite abundance (mean ± s.d.; log10 + 1), (b) parasite biomass in milligrams (mean ± s.d.; log10 + 1). Numbers denote the sample size in the corresponding SDI groups.
(a). Body condition
None of the four candidate models of variation in relative condition factor (Krel) was significant (see table 2 and electronic supplementary material, S1). Variation in somatic mass (MS) was best explained by total body length (LT) ( , p < 0.0001), season (
, p < 0.0001), season (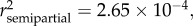 p = 0.0056), and sex (
p = 0.0056), and sex (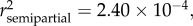 p = 0.0091) (see table 2 and electronic supplementary material S1).
p = 0.0091) (see table 2 and electronic supplementary material S1).
Table 2.
Best model variables in explaining the observed variation in three major life-history traits of the eel Anguilla anguilla during its continental phase in the study area (the Rhône River delta; n = 277). (For candidate models and model selection, see the electronic supplementary material, appendix S1. LT, total body length; SDI', re-coded Swimbladder Degenerative Index; site, sampling site.)
| eel traits dependent variables | explanatory variables | model significance |
|---|---|---|
| body condition | ||
| condition factor (Krel) | none | p > 0.05 |
| somatic mass (MS)a | LTa > season > sex | p < 0.0001, R2 = 0.99 (SS = 118.11, d.f. = 5, F = 7934.99) |
| size increase | ||
| total body length (LT)a | age > sex > season > SDI' > site | p < 0.0001, R2 = 0.83 (SS = 8.63, d.f. = 8, F = 162.24) |
| somatic mass (MS)a | age > sex > season > SDI' > site | p < 0.0001, R2 = 0.84 (SS = 99.27, d.f. = 8, F = 162.28) |
| silvering | ||
| ocular index (IO)b | season > sex | p < 0.0001, R2 = 0.42 (SS = 0.067, d.f. = 3, F = 21.88) |
| gonadosomatic index (IG)b | season > sex >site | p < 0.0001, R2 = 0.32 (SS = 0.22, d.f. = 4, F = 10.39) |
| eye area (AE)a | age > season | p < 0.0001, R2 = 0.54 (SS = 2.20, d.f. = 3, F = 34.79) |
| gonad mass (MG)a | age > sex > season | p < 0.0001, R2 = 0.49 (SS = 17.07, d.f. = 4, F = 21.74) |
alog10-transformed data.
barcsine-transformed data.
(b). Size increase
Variation in total body length (LT) was best explained by age ( p < 0.0001), sex (
p < 0.0001), sex (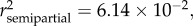 p < 0.0001), season (
p < 0.0001), season (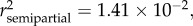 p < 0.0001), SDI' (
p < 0.0001), SDI' (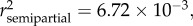 p = 0.0057) and sampling site (
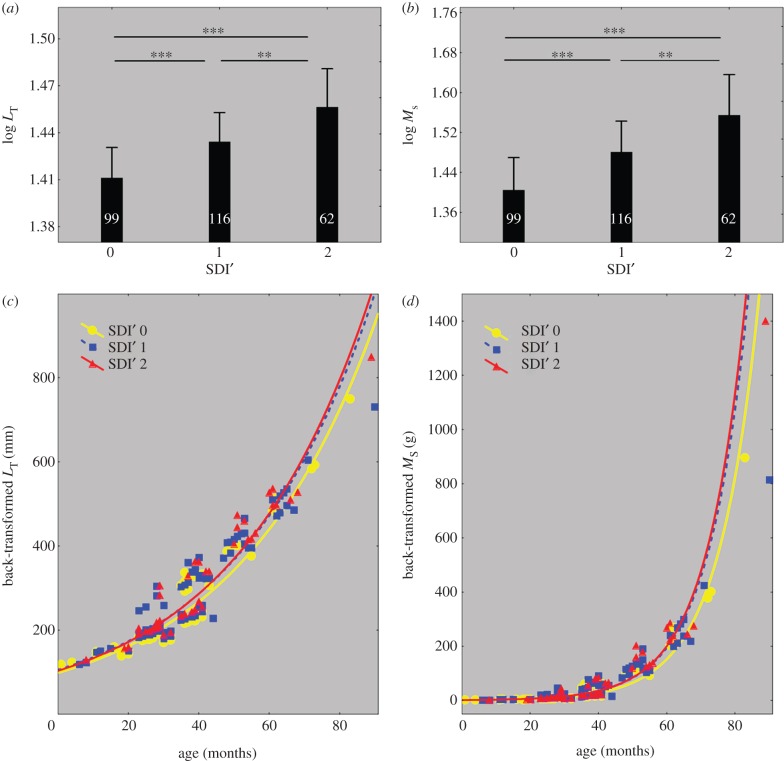
p = 0.0057) and sampling site (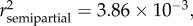 p = 0.0146) (see table 2 and electronic supplementary material S1). When controlled for age, eels with moderately (SDI' = 1) or severely (SDI' = 2) damaged swimbladders were significantly longer than eels with healthy swimbladders (SDI' = 0) (figure 2a,c). The corresponding size differences (calculated for a fixed age of 34 months following reciprocal transformation of the data) were +5.5 per cent and +11 per cent, respectively (for detailed calculations, see the electronic supplementary material, appendix S2). There was also a trend of a positive relationship between total body length (LT) and parasite biomass (MA. crassus) when the data were controlled for age (p = 0.08, in the first model; see electronic supplementary material).
p = 0.0146) (see table 2 and electronic supplementary material S1). When controlled for age, eels with moderately (SDI' = 1) or severely (SDI' = 2) damaged swimbladders were significantly longer than eels with healthy swimbladders (SDI' = 0) (figure 2a,c). The corresponding size differences (calculated for a fixed age of 34 months following reciprocal transformation of the data) were +5.5 per cent and +11 per cent, respectively (for detailed calculations, see the electronic supplementary material, appendix S2). There was also a trend of a positive relationship between total body length (LT) and parasite biomass (MA. crassus) when the data were controlled for age (p = 0.08, in the first model; see electronic supplementary material).
Figure 2.
Impact of A. crassus infection on eel size increase. In (a) and (b) adjusted means of body length and somatic mass (log10-transformed predicted values) for a given age of 34.19 months (covariate mean), according to the values of the SDI'. Vertical bars denote 95% confidence intervals. Significant difference between groups: ***p < 0.001, **p < 0.01. In (c) and (d) relationships between body length (back-transformed predicted values) and age, and somatic mass (back-transformed predicted values) and age, according to the values of the SDI'. Fitted lines using exponential function.
Variation in the somatic mass (MS) was best explained by age ( p < 0.0001), sex (
p < 0.0001), sex (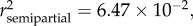 p < 0.0001), season (
p < 0.0001), season (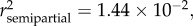 p < 0.0001), SDI' (
p < 0.0001), SDI' (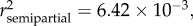 p = 0.0060) and sampling site (
p = 0.0060) and sampling site (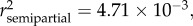 p = 0.0061) (see table 2 and electronic supplementary material, S1). When controlled for age, eels with moderately (SDI' = 1) or severely (SDI' = 2) damaged swimbladders were significantly heavier than eels with healthy swimbladders (SDI' = 0) (figure 2b,d). The corresponding size differences (calculated for a fixed age of 34 months following reciprocal transformation of the data) were +19.2 per cent and +41.2 per cent, respectively (for detailed calculations, see the electronic supplementary material, appendix S2). There was also a trend of a positive relationship between somatic mass (MS) and parasite biomass (MA. crassus), when the data were controlled for age (p = 0.06, in the first and second models; see electronic supplementary material).
p = 0.0061) (see table 2 and electronic supplementary material, S1). When controlled for age, eels with moderately (SDI' = 1) or severely (SDI' = 2) damaged swimbladders were significantly heavier than eels with healthy swimbladders (SDI' = 0) (figure 2b,d). The corresponding size differences (calculated for a fixed age of 34 months following reciprocal transformation of the data) were +19.2 per cent and +41.2 per cent, respectively (for detailed calculations, see the electronic supplementary material, appendix S2). There was also a trend of a positive relationship between somatic mass (MS) and parasite biomass (MA. crassus), when the data were controlled for age (p = 0.06, in the first and second models; see electronic supplementary material).
(c). Silvering parameters
Variation in the ocular index (IO) was best explained by season ( p < 0.0001) and sex (
p < 0.0001) and sex (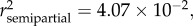 p = 0.0137) (see table 2 and electronic supplementary material, S1). Variation in the gonadosomatic index (IG) was best explained by season (
p = 0.0137) (see table 2 and electronic supplementary material, S1). Variation in the gonadosomatic index (IG) was best explained by season ( p = 0.0004), sex (
p = 0.0004), sex ( p = 0.0003) and sampling site (
p = 0.0003) and sampling site (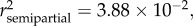 p = 0.0269) (see table 2 and electronic supplementary material, S1). Variation in the eye area (AE) was best explained by age (
p = 0.0269) (see table 2 and electronic supplementary material, S1). Variation in the eye area (AE) was best explained by age ( p < 0.0001) and season (
p < 0.0001) and season ( p < 0.0001) (see table 2 and electronic supplementary material, S1). Variation in the gonad mass (MG) was best explained by age (
p < 0.0001) (see table 2 and electronic supplementary material, S1). Variation in the gonad mass (MG) was best explained by age ( p < 0.0001), sex (
p < 0.0001), sex ( p < 0.0001) and season (
p < 0.0001) and season (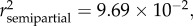 p = 0.0004) (see table 2 and electronic supplementary material, S1).
p = 0.0004) (see table 2 and electronic supplementary material, S1).
4. Discussion
Using swimbladder health as a measure of lifetime parasite pressure, we found that eels severely affected by A. crassus were significantly larger than unaffected eels (by 11% and 41% for body length and mass, respectively). This counterintuitive result echoes a number of previous field studies that also found positive or non-negative associations with infection (e.g. [20–22]; reviewed in [13]), and probably observed in other investigations but not reported accordingly (the ‘file drawer effect’, i.e. publication bias against non-significant or unexpected results; see [43]).
Positive effects on size during the time spent in continental waters were particularly unexpected in this host–parasite system, in view of the significant energetic drain that the nematode's bloodsucking diet is expected to impose on the host. In laboratory inoculation experiments [16–18], the data for most proxy parameters used (including haematocrit, glucose and cortisol) have suggested a physiological cost of infection. In a companion paper (using a subset of the same dataset), we revealed a significant enlargement of the spleen (up to a factor of two) in severely affected eels, which reflects either a defence or metabolic response to infection, or both [44]. The positive effect we revealed here is especially surprising, as we found no effect of infection on any of the silvering parameters investigated; in other words, the increased length and mass was not achieved at the expense of reproduction (contrary to the theory of resource allocation [45]).
Two main lines of argument can be advanced to explain this counterintuitive result: it either reflects a biological fact (i.e. infection does increase eel size) or a sampling artefact (i.e. a biased access to the true picture). Evidence of enhanced growth of infected hosts has been obtained for other fish–parasite systems. For instance, stickleback (Gasterosteus aculeatus) experimentally infected with the cestode Schistocephalus solidus show splenic enlargement and higher growth relative to uninfected fish [3]. The observed enhancement of growth was interpreted from multiple angles, including from the host and the parasite perspectives, and with respect to both proximal and evolutionary causes (for active parasite manipulation, see [46]; for strategic ‘adaptive’ host responses, see [47]). However, in our host–parasite system, long-term adaptive and strategic responses are unlikely to be involved because the interaction is quite recent on an evolutionary time scale (as evidenced by the lack of an efficient immune response in the host, see [19]). Rather, proximal and individual-related causes are more likely. Infected eels may attempt to compensate for the cost of infection by increased foraging activity (in support of this hypothesis, more food items have been recorded in the intestine of infected eels; see [48]). Using our dataset, we were able to show that the relative gut mass (estimated from the visceral mass divided by the total mass) was highly variable (from 2 to 30%) and positively correlated with parasite biomass (p < 0.05), suggesting that current infection and food intake are somehow linked in this host–parasite system. Such a ‘compensatory effect’ (hyperphagy) as an immediate response to infection (increased energy demand) has been observed in other helminth–fish systems [49,50]. Alternatively, it is possible that larger size results from a beneficial effect of the blood diet of adult worms, which may increase haematopoiesis and blood regeneration, in turn favouring metal elimination or food conversion [51,52].
The other line of explanation for the unexpected ‘positive’ parasite effects involves methodological artefacts and/or spurious interpretation of field data. Indeed, it may be difficult to differentiate the causes and consequences of infection [53]. For instance, in a cestode–smelt system, it was experimentally shown that infected hosts had a higher average rate of food ingestion and were larger in size prior to infection [54], which suggests that better foragers are more likely to get infected by trophically transmitted parasites. In support of this suggestion, we found among young eels a significant positive correlation between individual increase in length and parasite biomass (rs = +0.37, p = 0.001 for sexually undifferentiated eels having a healthy swimbladder, i.e. SDI < 2, n = 77). Following this argument, we propose here a general integrative scenario to account for the known A. crassus effects and the peculiarity of eel hosts. On the one hand, it has been shown that severely affected eels die first in a stressful environment [55,56], and are more likely to get caught [57,58]. On the other hand, the panmictic nature of eel reproduction [59] generates high inter-individual genetic variability, precludes local adaptation and favours phenotypic plasticity [60]. Eels are known to be extremely variable in terms of trophic activity and growth potential [61,62], to the extent that individual growth rates can influence the sexual orientation of gonads [24]. Using our dataset, we calculated that the increase in eel length during the time spent in continental waters was highly variable, ranging from 1.2 to 11.6 cm yr–1 for sexually undifferentiated eels (mean ± s.d. = 6.0 ± 1.8). We thus hypothesize that individuals undergoing a large increase in size are not only more likely to get infected (i.e. active eel foragers consume more intermediate/paratenic hosts; an idea first presented in [20]), but also more likely to get caught by fixed fishing gear, such as fyke nets. Conversely, infected eels initially in poor condition may be under-represented in field samples, either because they are less likely to be caught and/or to withstand infection.
An impaired functioning of the swimbladder is probably an insurmountable handicap for fish having to cross the Atlantic Ocean to reach their reproductive site in the Sargasso Sea (approx. 5000 km, with daily vertical migrations; see [63]). Referring to the most recent international collaborative effort on this subject ‘in case of heavy swimbladder infection and/or damage … [silver eels] … will never reach the spawning grounds and cannot contribute to recruitment’ [38, p. 233, 39]. If our assertions are correct, it means that anguillicolosis not only quantitatively removes a significant fraction of the effective reproductive pool, but also selectively affects the most active feeders and the highest quality individuals.
5. Conclusion
To our knowledge, this study is the first to evaluate the impact of an epizootic disease in nature using an internal marker of the host infection history. We showed that classical epidemiological parameters (i.e. parasite count or biomass) provide poor estimates of the true parasite pressure on individual hosts, as in this system, there is a mechanism of infection state dependence. Besides, this study also revealed the limitations of the descriptive correlational approach, and illustrated the difficulties in differentiating the causes and consequences of infection using cross-sectional samples. In the case of anguillicolosis, we suggest that the only realistic way to resolve the observed paradox (i.e. greater size/better condition among severely affected eels, observed in this and other studies) is to combine mark–recapture sampling with echo/radio imagery techniques to non-invasively assess the swimbladder condition among tagged eels (for reliability and applicability; [25,64]). Therefore, we call for scientists and eel managers to consider integrating an epidemiological dimension into their long-term monitoring programmes. Only then will it become possible to evaluate the true fitness cost (including mortality losses) imposed by this parasitic invader on its already endangered eel host.
Acknowledgements
The study conformed to the French legal requirements and was undertaken thanks to an annual permission of the Direction Départementale des Territoires et de la Mer des Bouches-du-Rhône, France according to the law R 436-9 of the Code of the Environment for the year 2000.
We thank Pascal Contournet, François Priour and Olivier Soulas for their help during sampling and dissections. We are grateful to Robert Poulin, Gilles Poizat and Anthony Acou for their comments on the manuscript. This study was supported by the Fondation MAVA, the NGO Migrateurs-Rhône-Méditerranée, the Agence de l'Eau, the Fédération Nationale de la Pêche en France, the PACA and LR régions, and the Conseil Général des Bouches-du-Rhône.
References
- 1.Begon M, Townsend C, Harper J. 2006. Ecology: from individuals to ecosystems, 4th edn. Malden, MA: Blackwell Publishing Ltd [Google Scholar]
- 2.Grenfell BT, Dobson AP. (eds) 1995. Ecology of infectious diseases in natural populations. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Arnott SA, Barber I, Huntingford FA. 2000. Parasite-associated growth enhancement in a fish-cestode system. Proc. R. Soc. Lond. B 267, 657–663 10.1098/rspb.2000.1052 (doi:10.1098/rspb.2000.1052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas F, Poulin R, Guégan J-F, Michalakis Y, Renaud F. 2000. Are there pros as well as cons to being parasitized? Parasitol. Today 16, 533–536 10.1016/S0169-4758(00)01790-7 (doi:10.1016/S0169-4758(00)01790-7) [DOI] [PubMed] [Google Scholar]
- 5.Dekker W. 2003. Worldwide decline of eel resources necessitates immediate action. Fisheries 28, 28–30 10.1577/1548-8446-28-12 (doi:10.1577/1548-8446-28-12) [DOI] [Google Scholar]
- 6.FAO 2011. Species Fact Sheets: Anguilla anguilla (Linnaeus, 1758). Rome: Food and Agriculture Organization of the United Nations; See http://www.fao.org/fishery/species/2203/en [Google Scholar]
- 7.IUCN 2011. Anguilla anguilla—International Union for Conservation of Nature and Natural Resources, Red List of Threatened Species, v. 2011.2. See http://www.iucnredlist.org [Google Scholar]
- 8.ICES 2011. Report of the 2011 session of the joint EIFAAC/ICES Working Group on eels. ICES CM 2011 / ACOM: 18, Lisbon, Portugal. See http://www.ices.dk/reports/ACOM/2011/WGEEL/wgeel_2011.pdf
- 9.Wirth T, Bernatchez L. 2003. Decline of North Atlantic eels: a fatal synergy? Proc. R. Soc. Lond. B 270, 681–688 10.1098/rspb.2002.2301 (doi:10.1098/rspb.2002.2301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durif CMF, Gjøsæter J, Vøllestad LA. 2011. Influence of oceanic factors on Anguilla anguilla (L.) over the twentieth century in coastal habitats of the Skagerrak, southern Norway. Proc. R. Soc. B 278, 464–473 10.1098/rspb.2010.1547 (doi:10.1098/rspb.2010.1547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moravec F. 2006. Dracunculoid and anguillicoloid nematodes parasitic in vertebrates. Prague, Czechoslovakia: Academia [Google Scholar]
- 12.Wielgoss S, Taraschewski H, Meyer A, Wirth T. 2008. Population structure of the parasitic nematode Anguillicola crassus, an invader of declining North Atlantic eel stocks. Mol. Ecol. 17, 3478–3495 10.1111/j.1365-294X.2008.03855.x (doi:10.1111/j.1365-294X.2008.03855.x) [DOI] [PubMed] [Google Scholar]
- 13.Lefebvre F, Fazio G, Crivelli AJ. 2012. Anguillicoloides crassus. In Fish parasites: pathobiology and protection (eds Woo PTK, Buchmann K.), pp. 320–336 Wallingford, CT: CABI [Google Scholar]
- 14.Molnár K, Baska F, Csaba G, Glávits R, Székely C. 1993. Pathological and histopathological studies of the swimbladder of eels Anguilla anguilla infected by Anguillicola crassus (Nematoda, Dracunculoidea). Dis. Aquat. Org. 15, 41–50 10.3354/dao015041 (doi:10.3354/dao015041) [DOI] [Google Scholar]
- 15.Würtz J, Taraschewski H, Pelster B. 1996. Changes in gas composition in the swimbladder of the European eel (Anguilla anguilla) infected with Anguillicola crassus (Nematoda). Parasitology 112, 233–238 10.1017/S003118200008481X (doi:10.1017/S003118200008481X) [DOI] [PubMed] [Google Scholar]
- 16.Boon JH, Cannaerts VMH, Augustijn H, Machiels MAM, De Charleroy D, Ollevier F. 1990. The effect of different infection levels with infective larvae of Anguillicola crassus on haematological parameters of European eel (Anguilla anguilla). Aquaculture 87, 243–253 10.1016/0044-8486(90)90062-R (doi:10.1016/0044-8486(90)90062-R) [DOI] [Google Scholar]
- 17.Haenen OLM, van Wijngaarden TAM, van der Heijden MHT, Höglund J, Cornelissen JBJW, van Leengoed LAMG, Borgsteede FHM, van Muiswinkel WB. 1996. Effects of experimental infections with different doses of Anguillicola crassus (Nematoda, Dracunculoidea) on European eel (Anguilla anguilla). Aquaculture 141, 41–57 10.1016/0044-8486(95)01213-3 (doi:10.1016/0044-8486(95)01213-3) [DOI] [Google Scholar]
- 18.Sures B, Knopf K, Kloas W. 2001. Induction of stress by the swimbladder nematode Anguillicola crassus in European eels, Anguilla anguilla, after repeated experimental infection. Parasitology 123, 179–184 10.1017/S003118200100823X (doi:10.1017/S003118200100823X) [DOI] [PubMed] [Google Scholar]
- 19.Knopf K. 2006. The swimbladder nematode Anguillicola crassus in the European eel Anguilla anguilla and the Japanese eel Anguilla japonica: differences in susceptibility and immunity between a recently colonized host and the original host. J. Helminthol. 80, 129–136 10.1079/JOH2006353 (doi:10.1079/JOH2006353) [DOI] [PubMed] [Google Scholar]
- 20.Koops H, Hartmann F. 1989. Anguillicola-infestations in Germany and in German eel imports. J. Appl. Ichthyol. 5, 41–45 10.1111/j.1439-0426.1989.tb00568.x (doi:10.1111/j.1439-0426.1989.tb00568.x) [DOI] [Google Scholar]
- 21.Kelly CE, Kennedy CR, Brown JA. 2000. Physiological status of wild European eels (Anguilla anguilla) infected with the parasitic nematode, Anguillicola crassus. Parasitology 120, 195–202 10.1017/S0031182099005314 (doi:10.1017/S0031182099005314) [DOI] [PubMed] [Google Scholar]
- 22.Costa-Dias S, Dias E, Lobón-Cerviá J, Antunes C, Coimbra J. 2010. Infection by Anguillicoloides crassus in a riverine stock of European eel, Anguilla anguilla. Fish. Manag. Ecol. 17, 485–492 10.1017/S0031182099005314 (doi:10.1017/S0031182099005314) [DOI] [Google Scholar]
- 23.De Charleroy D, Grisez L, Thomas K, Belpaire C, Ollevier F. 1990. The life cycle of Anguillicola crassus. Dis. Aquat. Org. 8, 77–84 10.3354/dao008077 (doi:10.3354/dao008077) [DOI] [Google Scholar]
- 24.Tesch F-W. 2003. The eel, 5th edn Oxford, UK: Blackwell Science [Google Scholar]
- 25.Lefebvre F, Fazio G, Palstra AP, Székely C, Crivelli AJ. 2011. An evaluation of indices of gross pathology associated with the nematode Anguillicoloides crassus in eels. J. Fish Dis. 34, 31–45 10.1111/j.1365-2761.2010.01207.x (doi:10.1111/j.1365-2761.2010.01207.x) [DOI] [PubMed] [Google Scholar]
- 26.Van Banning P, Haenen OLM. 1990. Effects of the swimbladder nematode Anguillicola crassus in wild and farmed eel, Anguilla anguilla. In Pathology in marine science (eds Perkins FO, Cheng TC.), pp. 317–330 New York, NY: Academic Press [Google Scholar]
- 27.Lefebvre F, Contournet P, Crivelli AJ. 2002. The health state of the eel swimbladder as a measure of parasite pressure by Anguillicola crassus. Parasitology 124, 457–463 10.1017/S0031182001001378 (doi:10.1017/S0031182001001378) [DOI] [PubMed] [Google Scholar]
- 28.Sinha VRP, Jones W. 1966. On the sex and distribution of the freshwater eel (Anguilla anguilla). J. Zool. 150, 371–385 10.1111/j.1469-7998.1966.tb03012.x (doi:10.1111/j.1469-7998.1966.tb03012.x) [DOI] [Google Scholar]
- 29.Mounaix B, Fontenelle G. 1994. Anguilles estuariennes et fluviales : apports de l'otolithométrie. Bull. Fr. Pêche Piscic. 335, 67–80 10.1051/kmae:1994005 (doi:10.1051/kmae:1994005) [DOI] [Google Scholar]
- 30.Lefebvre F, Sergent E, Acou A, Lecomte-Finiger R, Crivelli AJ. 2003. Recrutement des civelles (Anguilla anguilla) sur la côte méditerranéenne française : analyse comparée des caractéristiques biométriques et pigmentaires des saisons 1974-75 et 2000-01. Bull. Fr. Pêche Piscic. 368, 85–96 10.1051/kmae:2003038 (doi:10.1051/kmae:2003038) [DOI] [Google Scholar]
- 31.Bolger T, Connolly PL. 1989. The selection of suitable indices for the measurement and analysis of fish condition. J. Fish Biol. 34, 171–182 10.1111/j.1095-8649.1989.tb03300.x (doi:10.1111/j.1095-8649.1989.tb03300.x) [DOI] [Google Scholar]
- 32.Froese R. 2006. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J. Appl. Ichthyol. 22, 241–253 10.1111/j.1439-0426.2006.00805.x (doi:10.1111/j.1439-0426.2006.00805.x) [DOI] [Google Scholar]
- 33.Le Cren ED. 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 20, 201–219 10.2307/1540 (doi:10.2307/1540) [DOI] [Google Scholar]
- 34.Melià P, Bevacqua D, Crivelli AJ, De Leo GA, Panfili J, Gatto M. 2006. Age and growth of Anguilla anguilla in the Camargue lagoons. J. Fish Biol. 68, 876–890 10.1111/j.0022-1112.2006.00975.x (doi:10.1111/j.0022-1112.2006.00975.x) [DOI] [Google Scholar]
- 35.Garcìa-Berthou E. 2001. On the misuse of the residuals in ecology: testing regression residuals versus the analysis of covariance. J. Anim. Ecol. 70, 708–711 10.1046/j.1365-2656.2001.00524.x (doi:10.1046/j.1365-2656.2001.00524.x) [DOI] [Google Scholar]
- 36.Pankhurst NW. 1982. Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla (L.). J. Fish Biol. 21, 127–140 10.1111/j.1095-8649.1982.tb03994.x (doi:10.1111/j.1095-8649.1982.tb03994.x) [DOI] [Google Scholar]
- 37.Durif C, Dufour S, Elie P. 2005. The silvering process of Anguilla anguilla: a new classification from the yellow resident to the silver migrating stage. J. Fish Biol. 66, 1025–1043 10.1111/j.0022-1112.2005.00662.x (doi:10.1111/j.0022-1112.2005.00662.x) [DOI] [Google Scholar]
- 38.EELREP 2005. Estimation of the reproduction capacity of European eel. Final report of the EU project Q5RS-2001-01836. EU section: Quality of Life and Management of Living Resources
- 39.Palstra A, Heppener D, van Ginneken V, Székely C, van den Thillart G. 2007. Swimming performance of silver eels is severely impaired by the swim-bladder parasite Anguillicola crassus. J. Exp. Mar. Biol. Ecol. 352, 244–256 10.1016/j.jembe.2007.08.003 (doi:10.1016/j.jembe.2007.08.003) [DOI] [Google Scholar]
- 40.Poulin R. 2007. Are there general laws in parasite ecology? Parasitology 134, 763–776 10.1017/S0031182006002150 (doi:10.1017/S0031182006002150) [DOI] [PubMed] [Google Scholar]
- 41.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer [Google Scholar]
- 42.Cardinal RN, Aitken MRF. 2006. ANOVA for the behavioural sciences researcher. Mahwah, NJ: Lawrence Erlbaum Associates [Google Scholar]
- 43.Kotze DJ, Johnson CA, O'Hara RB, Vepsäläinen K, Fowler MS. 2004. Editorial: The Journal of Negative Results in Ecology and Evolutionary Biology. J. Negat. Results Ecol. Evol. Biol. 1, 1–5 See http://www.jnr-eeb.org/index.php/jnr/article/viewFile/8/6 [Google Scholar]
- 44.Lefebvre F, Mounaix B, Poizat G, Crivelli AJ. 2004. Impacts of the swimbladder nematode Anguillicola crassus on Anguilla anguilla: variations in liver and spleen masses. J. Fish Biol. 64, 435–447 10.1111/j.0022-1112.2004.00309.x (doi:10.1111/j.0022-1112.2004.00309.x) [DOI] [Google Scholar]
- 45.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 46.Phares K. 1996. An unusual host-parasite relationship: the growth hormone-like factor from plerocercoids of spirometrid tapeworms. Int. J. Parasitol. 26, 575–588 10.1016/0020-7519(96)00025-2 (doi:10.1016/0020-7519(96)00025-2) [DOI] [PubMed] [Google Scholar]
- 47.Ballabeni P. 1995. Parasite-induced gigantism in a snail: a host adaptation? Funct. Ecol. 9, 887–893 10.2307/2389987 (doi:10.2307/2389987) [DOI] [Google Scholar]
- 48.Moser ML, Patrick WS, Crutchfield JUJ. 2001. Infection of American eels, Anguilla rostrata, by an introduced nematode parasite, Anguillicola crassus, in North Carolina. Copeia 2001, 848–853 10.1643/0045-8511(2001)001[0848:IOAEAR]2.0.CO;2 (doi:10.1643/0045-8511(2001)001[0848:IOAEAR]2.0.CO;2) [DOI] [Google Scholar]
- 49.Loot G, Poulin R, Lek S, Guégan J-F. 2002. The differential effects of Ligula intestinalis (L.) plerocercoids on host growth in three natural population of roach, Rutilus rutilus (L.). Ecol. Freshw. Fish 11, 168–171 10.1034/j.1600-0633.2002.00006.x (doi:10.1034/j.1600-0633.2002.00006.x) [DOI] [Google Scholar]
- 50.Wright HA, Wootton RJ, Barber I. 2006. The effect of Schistocephalus solidus infection on meal size of three-spined stickleback. J. Fish Biol. 68, 801–809 10.1111/j.0022-1112.2006.00966.x (doi:10.1111/j.0022-1112.2006.00966.x) [DOI] [Google Scholar]
- 51.Boutilier RG, Dobson G, Hoeger U, Randall DJ. 1988. Acute exposure to graded levels of hypoxia in rainbow trout (Salmo gairdneri): metabolic and respiratory adaptations. Respir. Physiol. 71, 69–82 10.1016/0034-5687(88)90116-8 (doi:10.1016/0034-5687(88)90116-8) [DOI] [PubMed] [Google Scholar]
- 52.Lai JCC, Kakuta I, Mok HOL, Rummer JL, Randall D. 2006. Effects of moderate and substantial hypoxia on erythropoietin levels in rainbow trout kidney and spleen. J. Exp. Biol. 209, 2734–2738 10.1242/jeb.02279 (doi:10.1242/jeb.02279) [DOI] [PubMed] [Google Scholar]
- 53.Blanchet S, Thomas F, Loot G. 2009. Reciprocal effects between host phenotype and pathogens: new insights from an old problem. Trends Parasitol. 28, 364–369 10.1016/j.pt.2009.05.005 (doi:10.1016/j.pt.2009.05.005) [DOI] [PubMed] [Google Scholar]
- 54.Bourque J-F, Dodson JJ, Ryan DAJ, Marcogliese DJ. 2006. Cestode parasitism as a regulator of early life-history survival in an estuarine population of rainbow smelt Osmerus mordax. Mar. Ecol. Prog. Ser. 314, 295–307 10.3354/meps314295 (doi:10.3354/meps314295) [DOI] [Google Scholar]
- 55.Molnár K. 1993. Effect of decreased oxygen content on eels (Anguilla anguilla) infected by Anguillicola crassus (Nematoda: Dracunculoidea). Acta Vet. Hung. 41, 349–360 [PubMed] [Google Scholar]
- 56.Lefebvre F, Contournet P, Crivelli AJ. 2007. Interaction between the severity of the infection by the nematode Anguillicola crassus and the tolerance to hypoxia in the European eel Anguilla anguilla. Acta Parasitol. 52, 171–175 10.2478/s11686-007-0013-4 (doi:10.2478/s11686-007-0013-4) [DOI] [Google Scholar]
- 57.Sprengel G, Lüchtenberg H. 1991. Infection by endoparasites reduces maximum swimming speed of European smelt Osmerus eperlanus and European eel Anguilla anguilla. Dis. Aquat. Org. 11, 31–35 10.3354/dao011031 (doi:10.3354/dao011031) [DOI] [Google Scholar]
- 58.Sjöberg NB, Petersson E, Wickström H, Hansson S. 2009. Effects of the swimbladder parasite Anguillicola crassus on the migration of European silver eels Anguilla anguilla in the Baltic Sea. J. Fish Biol. 74, 2158–2170 10.1111/j.1095-8649.2009.02296.x (doi:10.1111/j.1095-8649.2009.02296.x) [DOI] [PubMed] [Google Scholar]
- 59.Als TD, et al. 2011. All roads lead to home: panmixia of European eel in the Sargasso Sea. Mol. Ecol. 20, 1333–1346 10.1111/j.1365-294X.2011.05011.x (doi:10.1111/j.1365-294X.2011.05011.x) [DOI] [PubMed] [Google Scholar]
- 60.Lenormand T. 2002. Gene flow and the limits to natural selection. Trends Ecol. Evol. 17, 183–189 10.1016/S0169-5347(02)02497-7 (doi:10.1016/S0169-5347(02)02497-7) [DOI] [Google Scholar]
- 61.Panfili J, Ximénès M-C, Crivelli AJ. 1994. Sources of variation in growth of the European eel (Anguilla anguilla) estimated from otoliths. Can. J. Fish. Aquat. Sci. 51, 506–515 10.1139/f94-053 (doi:10.1139/f94-053) [DOI] [Google Scholar]
- 62.Vøllestad LA. 1992. Geographic variation in age and length at metamorphosis of maturing European eel: environmental effects and phenotypic plasticity. J. Anim. Ecol. 61, 41–48 10.2307/5507 (doi:10.2307/5507) [DOI] [Google Scholar]
- 63.Aarestrup K, et al. 2009. Oceanic spawning migration of the European eel (Anguilla anguilla). Science 325, 1660 10.1126/science.1178120 (doi:10.1126/science.1178120) [DOI] [PubMed] [Google Scholar]
- 64.Székely C, Molnár K, Rácz OZ. 2005. Radiodiagnostic method for studying the dynamics of Anguillicola crassus (Nematoda: Dracunculoidea) infection and pathological status of the swimbladder in Lake Balaton eels. Dis. Aquat. Org. 64, 53–61 10.3354/dao064053 (doi:10.3354/dao064053) [DOI] [PubMed] [Google Scholar]