Abstract
Common vampire bats often regurgitate food to roost-mates that fail to feed. The original explanation for this costly helping behaviour invoked both direct and indirect fitness benefits. Several authors have since suggested that food sharing is maintained solely by indirect fitness because non-kin food sharing could have resulted from kin recognition errors, indiscriminate altruism within groups, or harassment. To test these alternatives, we examined predictors of food-sharing decisions under controlled conditions of mixed relatedness and equal familiarity. Over a 2 year period, we individually fasted 20 vampire bats (Desmodus rotundus) and induced food sharing on 48 days. Surprisingly, donors initiated food sharing more often than recipients, which is inconsistent with harassment. Food received was the best predictor of food given across dyads, and 8.5 times more important than relatedness. Sixty-four per cent of sharing dyads were unrelated, approaching the 67 per cent expected if nepotism was absent. Consistent with social bonding, the food-sharing network was consistent and correlated with mutual allogrooming. Together with past work, these findings support the hypothesis that food sharing in vampire bats provides mutual direct fitness benefits, and is not explained solely by kin selection or harassment.
Keywords: cooperation, Desmodus rotundus, helping, kin selection, reciprocity, sharing
1. Introduction
Cooperation poses an evolutionary puzzle whenever a donor pays a cost to help a recipient: what prevents individuals from receiving the reproductive benefits of donor cooperation without paying the costs? Several mechanisms can prevent such ‘cheating’ thereby ensuring that cooperative investments yield net inclusive fitness benefits [1]. The exploitation of altruism is often prevented through kin discrimination [2] or policing [3], whereas direct fitness cooperation can be enforced by behaviours that reward helpers, punish cheats or both [1,4–8]. To identify what mechanisms enforce or maintain cooperation, controlled experiments can directly test how individuals respond to cheating. The most successful of such experiments involve organisms that are easy to manipulate in the laboratory [6–13]. Studies using more cognitively complex organisms, such as non-human primates, are often limited to learned behaviours, such as pulling levers to deliver food to others [13–15], because inducing or manipulating natural helping acts that occur in the wild is difficult or impossible.
Common vampire bats (Desmodus rotundus) feed only on blood and die after 70 h of fasting [16], but unfed bats often receive food from roost-mates by regurgitation [17]. Vampire bat food sharing is potentially a powerful model for understanding the cognitive enforcement of cooperation, because this behaviour is completely natural, energetically costly, occurs between kin and non-kin and can be induced experimentally. Previous work found that free-ranging female vampire bats regurgitated blood mostly to their offspring (77 of 110 donations), but also fed adult females, preferentially close relatives and only frequent roost-mates (i.e. more than 60% co-roosting association [17]). Adult donations were predicted independently by relatedness and association [17]. A captive experiment that induced food sharing among unrelated bats found that bats returned food donations to their past donors on four of six possible occasions—more than expected by chance [17]. Although vampire bat food sharing has been a textbook example of reciprocity, this interpretation has been questioned owing to several alternative explanations [18–22].
Wilkinson originally suggested that food donating vampire bats obtain both direct and indirect fitness benefits [17], with direct benefits outweighing kin-selected benefits [23]. Under this scenario, cheating is prevented because bats donate preferentially to past donors and relatives. Hence, food-sharing decisions should integrate cues to kinship and future direct benefits (e.g. reciprocal donations or allogrooming [24]).
Others have suggested that non-kin food sharing might simply result from manipulation [20]. According to this ‘harassment hypothesis’, non-kin food-sharing benefits only recipients, not donors. Persistent begging by unfed bats might coerce conspecifics into food sharing. If so, donations should be solicited by recipients and directed primarily to dominant individuals.
Alternatively, donations to non-kin could simply be an incidental by-product of kin altruism. Hammerstein [20] suggested that olfactory kin recognition cues could have been miscalibrated by the lack of kin present in the captive experiment [19]. This ‘miscalibrated kin recognition hypothesis’ predicts that donors should donate almost exclusively to kin when in the more natural context of mixed relatedness.
Selection can favour indiscriminate altruism within social groups when the average within-group relatedness is high enough and the cost of helping is low enough. The ‘group-level altruism hypothesis’ predicts that donors indiscriminately help groupmates [22,25]. For example, Foster's [22] model of vampire bat food sharing ‘assumes that fed bats do not discriminate among unfed bats when giving blood’ presumably because the costs of discriminating kin are too high.
Several simulations have been developed to explain food sharing [22,23,25,26], yet no one has gathered additional empirical evidence regarding how vampire bats decide to share food (but see [27,28]). As a first step, we tested predictions of the above hypotheses by experimentally simulating unsuccessful foraging attempts in a captive colony of common vampire bats of mixed relatedness and equal familiarity. The original study [17] compared the explanatory roles of relatedness and association. Here, we directly compare relatedness and reciprocal help as predictors of food sharing, under conditions of equal association. We also evaluated alternative predictors of food sharing, including recipient age or size (as predicted by harassment) and food received from any groupmate (as predicted by generalized reciprocity [15,29]).
2. Methods
(a). Animals
All procedures were approved by the University of Maryland Institutional Animal Care and Use Committee (Protocol R-10-63). We did not test unhealthy bats, late pregnancy females, or mothers and their juveniles less than 4 months of age. We stopped testing males partway through the experiment since removing males coincided with increased aggression in the colony.
We fasted 11 males and nine females out of 25 common vampire bats, descended from multiple matrilines. Bats were housed at the Organization for Bat Conservation (Bloomfield Hills, MI, USA) in a flight cage large enough to allow them to freely associate during the study and for more than 2 years prior. All bats were uniquely marked with passive integrated transponder (PIT) tags and coloured bands, except for three juveniles (4–8 months) born during the study that were reliably identified by PIT tags and distinctive face and body marks.
(b). Fasting procedure
To induce food sharing, we removed and fasted a subject from the group for 24 h, then returned it to the cage with fed groupmates, and recorded subsequent social interactions for 2 h with a Sony Nightshot digital camcorder and infrared illumination. We measured the subject's mass immediately before reintroduction and after the 2 h observation period. We selected available bats randomly and without replacement to serve as subjects, and tested each subject 1–5 times. After the observation period, fasted bats were provided food.
(c). Behavioural data
We refer to subjects that received food as ‘recipients’ and partners that provided food as ‘donors’. To quantify food sharing, we measured mouth-licking bouts via frame-by-frame analysis in imovie 11. We defined mouth-licking bouts as periods where food could be passed that lasted at least 5 s and were separated by more than 5 s. We noted whether one bat clearly began licking a conspecific's mouth and classified bouts accordingly as initiated by the recipient, donor or ‘unknown’. We defined allogrooming as the licking of a conspecific at locations other than the mouth. To measure mean pairwise allogrooming rates, we randomly selected individuals for focal sample observations 1–4 times during non-trial days and counted the presence and direction of allogrooming with any conspecific every minute for 60 min.
We used mouth-licking time to estimate the amount of food sharing because it strongly correlated with mass gain during the 2 h trial (r = 0.90; 95% CI = 0.73–0.96). We pooled time spent donating food from multiple days to obtain a single measure of food sharing for each directional dyad that had an opportunity to share food in each direction (n = 312 dyads), except when we analysed sequences of sharing events (see the electronic supplementary material, S1).
(d). Pairwise relatedness
We extracted DNA from 2 to 3 mm biopsy punches using Qiagen DNeasy kits, then amplified and genotyped 13 microsatellite loci to estimate maximum-likelihood coefficients of relatedness (r) for each dyad using ML-Relate [30] (see the electronic supplementary material, S1). We jackknifed across loci to estimate standard errors (s.e.) for each r value (s.e. range = 0–0.035; s.e. mean = 0.005). Across all dyads, r = 0 for 59 per cent, r < 0.05 for 69 per cent and r > 0.25 for 20 per cent. Patterns of observed and expected heterozygosity indicated no history of inbreeding (see the electronic supplementary material, S1).
(e). Statistical analysis
The variance in mouth-licking times increased with the mean, so we log-transformed mean food-sharing times for each dyad (see the electronic supplementary material, figure S1). We therefore defined ‘food donated’ from bat A to B as ln ([total food shared A to B/chances for A to feed B]+1). We defined ‘food received’ similarly, except with the roles of A and B reversed. We z-transformed all variables to standardize scales.
To analyse dyadic data, we used a randomization approach to general linear models, where we permuted food donated to sets of predictor variables creating a null distribution of comparison F-values [31]. We first conducted univariate analyses to identify variables that predicted mean food donated across dyads and then performed a permuted multiple regression using the lmp function in the R package lmPerm. To choose the best model, we selected predictors and their interactions based on backward stepwise regression using Akaike and Bayesian information criteria in JMP v. 10. We interpreted interactions by examining correlations between two variables at several values of the other variables. To compare the relative importance of predictors, we averaged the sequential sum of squares over all orderings [32] for up to three predictors using the R package relaimpo [33]. We predicted amounts of food donated across directed dyads that could have shared food in both directions. We also predicted the presence or absence of food sharing across these dyads using logistic regression, and finally the amount of food donated only within dyads that did share food.
To determine whether individual food donations were exchanged in a reciprocal manner over time [34], we examined the sequence of sharing events across trials to test for correlations between food given and received within dyads (using both amounts and proportions, see the electronic supplementary material, S1). To test the effect of general help received, we compared the mean amount of food donated by a bat to all fasted partners before and after it was fed by others to determine whether it donated larger amounts after receiving food from others.
To test for symmetry and consistency of relationships, we used Mantel and randomization tests to compare network similarity for: (i) food sharing in subsequent fasting rounds, (ii) food sharing six months apart, (iii) allogrooming given and received, and (iv) food sharing given and received, using only bats that served as subjects and were available as donors in every round (see the electronic supplementary material, S1).
Finally, to assess the harassment hypothesis, we examined whether recipients or donors were more likely to initiate mouth-licking. We also tested two potential measures of coercion ability—recipient age and size (forearm length)—as potential predictors of food donated.
3. Results
(a). Pattern of food sharing
We induced food sharing on 48 out of 52 fasting trials over 780 days, and recorded 950 food-sharing bouts [35]. Food sharing occurred primarily between females and never between adult males (see the electronic supplementary material, figure S2). Sixty-three of the 98 dyads that shared food had relatedness estimates of less than 0.05. This percentage (64%) approaches that percentage expected (67%) if partners were chosen at random with respect to relatedness (i.e. 208 of 312 possible food-sharing dyads were related by less than 0.05).
In each trial, recipients were fed by an average of 3.9 donors (range = 1–7). Median donation time per dyad in a trial was 191 s (n = 204 donations, mean = 339 s, range = 5–3315 s). The total amount of food received from all donors during the 2 h period was typically about 5 per cent of an adult recipient's mass, which restored approximately 20 per cent of mass lost during 24 h of fasting (see the electronic supplementary material, S1).
(b). Predictors of food sharing across dyads
Univariate analyses showed that food donated was predicted by food received, allogrooming received, pairwise relatedness (figure 1) and donor sex (included as a binary variable, electronic supplementary material, figure S3). All correlations were also significant before log transformations (p < 0.0002 in all cases).
Figure 1.
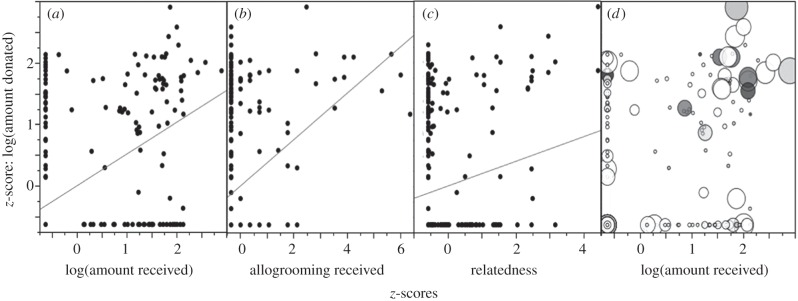
Relationships between food donated and predictor variables. z-score for log food donated was predicted by z-scores of (a) log food received (R2 = 0.27, p < 0.0002), (b) allogrooming received (R2 = 0.14, p < 0.0002) and (c) relatedness (R2 = 0.04, p < 0.0012). A bubble plot (d) shows multivariate relationships by scaling bubble size to relatedness and bubble darkness to allogrooming received.
The best multivariate model (adjusted R2 = 0.372, F5,306 = 37.8, p < 0.0002) included food received (β = 0.319, p < 0.0002), donor sex (β = 0.267, p < 0.0002), allogrooming received (β = 0.186, p < 0.0002) and the interaction between relatedness and food received (β = 0.069, p = 0.0276), but not relatedness (β = 0.052, p = 0.16). An interaction plot showed that the relationship between food donated and received increased in slope with higher relatedness. Food received was 8.5 times more important than relatedness for predicting food donated (figure 2).
Figure 2.
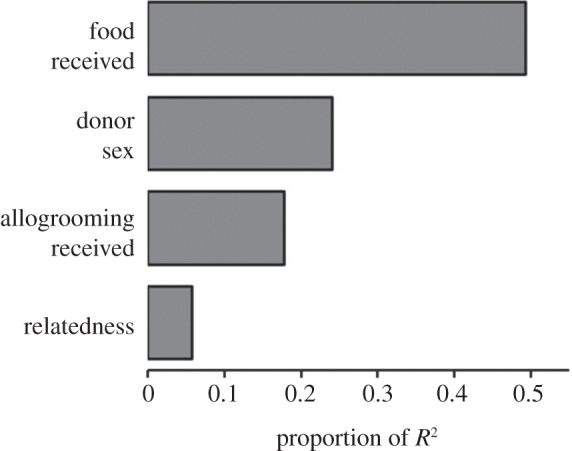
Relative importance on food donated of several predictors. Proportion of R2 is shown for four predictor variables. An interaction effect (see text) is not shown. The full model explained 38% of the variation in food donated.
Food received, donor sex and allogrooming received, but not relatedness, also predicted the presence of food sharing (see the electronic supplementary material, S1). Among the 98 food-sharing dyads, donation size was predicted independently by food received and relatedness, with the latter relationship driven by mother–offspring pairs (see the electronic supplementary material, S1).
(c). Predictors of food sharing across trials
Sequential analysis across trial days indicated that the amount of food donated and previously received were correlated when comparing the proportion of a donor's contribution to the total food received by a partner (R2 = 0.08, F1,160 = 13.9, p < 0.0002), but not when comparing the absolute amount of food given and received (R2 = 0.01, F1,160 = 2.4, p = 0.1).
We found no evidence that being fed in general increased subsequent food sharing, as expected by generalized reciprocity (see the electronic supplementary material, S1). Donation sizes could sometimes be compared both before and after the donor was fed within a round of trials. In these 28 cases, we failed to find a difference in presence of food sharing (paired t27 = 0.98, p = 0.34), total food donated (paired t27 =−1.3, p = 0.20) or food donated per recipient (paired t27 = 0.16, p = 0.87). When the donor was fed on the previous day, we found no difference between the amount donated on that day compared with the donor's average on other days (n = 9 donors and nine trials, paired t8 =−0.013, p = 0.99).
(d). Consistency of social relationships
Dyadic relationships were consistent and symmetrical over time. Contrary to random association, food-sharing networks were significantly similar when comparing patterns 8 days apart (15 bats, amount shared: p = 0.0298, presence of sharing: p = 0.0072) or six months apart (67 dyads, amount shared: p = 0.0238, presence of sharing: p < 0.0002). Amounts given and received were correlated for both the food-sharing (15 bats, amount shared: p = 0.0004) and allogrooming network (figure 3).
Figure 3.
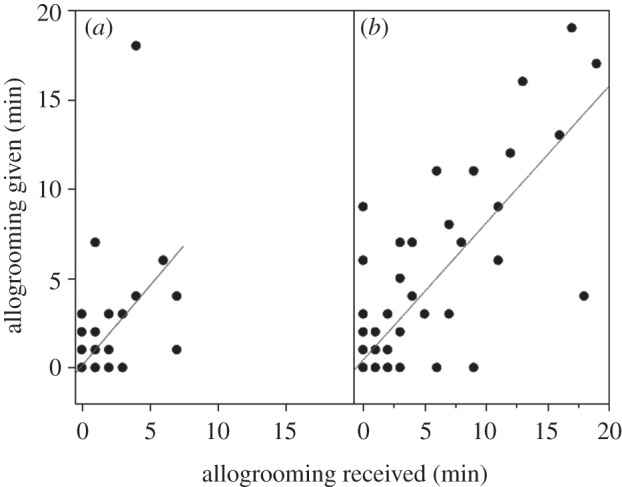
Allogrooming given correlates with allogrooming received. Allogrooming giving is plotted against allogrooming received for dyads that did not share food ((a) n = 214, r = 0.62, p < 0.0002) and dyads that did share food ((b) n = 98, r = 0.81, p < 0.0002). On non-trial days, dyads that shared food both gave and received more allogrooming than non-sharing dyads (F1,310 = 32.9 and 41.0, p < 0.0002 for both).
(e). Predictors of harassment
Donors initiated food sharing in 62 per cent of non-maternal food-sharing bouts. Mean duration did not differ between food-sharing bouts initiated by donors versus recipients (t = 1.4, n = 235, p = 0.16). We found no effect of recipient age (R2 = 0.006, p = 0.2) or forearm length (R2 = 0.004, p = 0.5) on the amount of food donated.
4. Discussion
(a). Predictors of food sharing
The relative importance of relatedness and reciprocal sharing in determining the food-sharing decisions of adult vampire bats was not directly comparable in previous work [17]. Here, we found that, among captive vampire bats where co-roosting association is held constant, the predictive role of reciprocal help greatly exceeds that of relatedness. Food received from a partner was the strongest and most robust predictor of both the presence and amount of food donated to that partner. The donor's sex, amount of allogrooming received and a positive interaction between food received and relatedness predicted food donated to a lesser extent.
Distinguishing the roles of direct and indirect fitness can be difficult because social behaviours, such as punishment or reciprocal help, can change the cost–benefit ratio in Hamilton's rule (r > c/b), leading to complex interactions between direct and indirect fitness benefits [1–3,23]. For example, the predictive roles of reciprocal help and relatedness in food sharing could interact positively or negatively. Since vampire bats under the age of 2 years fail to feed on 30 per cent of nights [17], the benefits of receiving food are probably age-dependent. We might therefore expect older bats to feed related young but not vice versa, causing a negative relationship between the predictors, reciprocal help and relatedness. Instead, we found a positive interaction: highly related pairs engaged in reciprocal sharing that was more symmetrical than unrelated pairs. For example, the largest donations were four females feeding their juvenile (4 and 8 months) or subadult male offspring (19 and 31 months); in all cases, the offspring reciprocated with large donations to the unfed mothers.
(b). Evidence for social bonds
Much emerging evidence links social bonds with direct fitness benefits in social mammals (see [36] and references therein). Wild female vampire bats have been observed still roosting together after 12 years [37], and several lines of evidence suggest that such long-term social relationships play a role in stabilizing food sharing. First, allogrooming appears to serve a social bonding function because it is uncorrelated with ectoparasite levels in the wild [24], and occurred commonly and symmetrically in the absence of visible ectoparasites (figure 3). Second, dyads that share food performed more allogrooming on non-test days than non-sharing dyads (figure 3). Third, food sharing and allogrooming were correlated across dyads (figure 1), and food-sharing patterns were significantly consistent over days and months. Finally, male vampire bats rarely share food in the wild where their social relationships are transient [37], but will share food in captivity [27] where male associations are more stable. Taken together with the relatively weak correlation between the exact amounts of within-dyad food donated and received between trials, these findings are consistent with long-term social bonds involving mutual exchange of both food and grooming over long periods, rather than short-term turn-taking or calculated reciprocity [14].
(c). Alternative explanations for non-kin food sharing
Contrary to predictions of the harassment hypothesis [20], donors were more likely than recipients to approach and initiate mouth-licking (see the electronic supplementary material, S2), even when excluding mother–offspring donations. We also found no relationship between food sharing and recipient age or forearm size, both potential correlates of harassment ability. The harassment hypothesis therefore seems untenable as the sole explanation for non-kin food sharing.
Can vampire bat food sharing be explained by indirect fitness alone? Contrary to predictions of the miscalibrated kin recognition hypothesis [18], our results show that non-kin food sharing prevailed in a colony of mixed relatedness and equal familiarity. In this study, relatedness did not predict the presence or amount of food sharing across dyads that could have shared food. Although relatedness predicted donation size for food-sharing dyads, the effect was largely driven by extended maternal care (see the electronic supplementary material, S1). One interpretation of these negative results is that kin discrimination is possible, but the indirect benefits of nepotism were overshadowed by the direct benefits of reciprocal food sharing. Alternatively, a group-level altruism hypothesis might predict that ‘kin discrimination’ is based on familiarity rather than phenotypic matching, leading to indiscriminate altruism within groups [22].
The fission–fusion social dynamics of wild vampire bats lead to unstable roosting group membership, and male dispersal and occasional recruitment of unrelated females lead to low average relatedness in groups (r = 0.02–0.11 based on genetic and pedigree analyses) [37]. Under such conditions, selection is not expected to favour kin recognition mechanisms based on familiarity alone. The multi-level selection model by Foster [22] suggests that indiscriminate altruism within groups can be favoured at mean group relatedness levels as low as 0.05, but this model assumes that bats are neither reciprocating nor nepotistic, as either strategy would make a system of indiscriminate altruism unstable. By contrast, we found that the network of food donations within the captive group was less random, more reciprocal and more consistent over time, than expected by chance.
Free-ranging common vampire bats preferentially feed relatives within roosts despite frequent roost-switching and co-roosting with non-kin [17,37], indicating that vampire bats are capable of kin discrimination. While the mechanisms for kin or individual discrimination are still unclear, auditory and olfactory cues are probable. Female bats of all species recognize juveniles through isolation calls, and adult common vampire bats often produced similar individual-specific contact calls when isolated [38]. Playback studies have demonstrated that such calls allow individual discrimination in the closest extant vampire bat species, Diaemus youngi [39]. Food-sharing bouts were preceded by allogrooming and sniffing (see the electronic supplementary material, S2), which suggest a role for odour. Additional studies are needed to test recognition mechanisms in this species.
(d). Evidence for reciprocity
The correlation we observed between food donated and received does not demonstrate that receiving food determines subsequent food donated within a dyad. For this reason, we avoided the term ‘reciprocity’ to prevent confusion because the term has broad, narrow, and sometimes contrasting, definitions in the literature [1,13–15,17–19]. Reciprocity could involve partner control through direct reward or punishment within dyads, or partner choice and switching based on the perceived relative value of different partners as cooperators [4–8,10–15]. Experiments are needed to test if and how donors respond to cheating.
We found that on average fasted bats were fed by three donors, so the costs of food sharing were often divided among partners. As expected, potential donors sometimes rejected begging recipients, but unexpectedly, some fasted subjects also appeared to reject food offers from some potential donors. This surprising observation may indicate that bats favour some food-sharing partners over others, with implications for modelling vampire bat cooperation as a biological market [4,40] rather than as an iterated dyadic interaction.
Acknowledgements
All procedures were approved by the University of Maryland Institutional Animal Care and Use Committee (protocol R-10-63).
We thank the Organization for Bat Conservation, Rob Mies, Jesse Fabian, Mary Smith and Matthew Mulkeen for their generous support. This work was funded by grants-in-aid of research from the Cosmos Club Foundation, American Society of Mammalogists, Explorer's Club Washington Group, and Sigma Xi. G.G.C. is supported by a Ford Foundation Predoctoral Fellowship, administered by the National Academy of Sciences.
References
- 1.West S, Griffin A, Gardner A. 2007. Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672 10.1016/j.cub.2007.06.004 (doi:10.1016/j.cub.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 2.Griffin A, West S. 2003. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634. 10.1126/science.1089402 (doi:10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- 3.Ratnieks F, Wenseleers T. 2008. Altruism in insect societies and beyond: voluntary or enforced? Trends Ecol. Evol. 23, 45–52 10.1016/j.tree.2007.09.013 (doi:10.1016/j.tree.2007.09.013) [DOI] [PubMed] [Google Scholar]
- 4.Noë R, Hammerstein P. 1994. Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11 10.1007/BF00167053 (doi:10.1007/BF00167053) [DOI] [Google Scholar]
- 5.Fruteau C, Lemoine S, Hellard E, van Damme E, Noë R. 2011. When females trade allogrooming for allogrooming: testing partner control and partner choice models of cooperation in two primate species. Anim. Behav. 81, 1223–1230 10.1016/j.anbehav.2011.03.008 (doi:10.1016/j.anbehav.2011.03.008) [DOI] [Google Scholar]
- 6.Kiers ET, Rousseau RA, West SA, Denison RF. 2003. Host sanctions and the legume–rhizobium mutualism. Nature 425, 78–81 10.1038/nature01931 (doi:10.1038/nature01931) [DOI] [PubMed] [Google Scholar]
- 7.Jandér KC, Herre EA. 2010. Host sanctions and pollinator cheating in the fig tree-fig wasp mutualism. Proc. R. Soc. B 277, 1481–1488 10.1098/rspb.2009.2157 (doi:10.1098/rspb.2009.2157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiers ET, et al. 2011. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333, 880–882 10.1126/science.1208473 (doi:10.1126/science.1208473) [DOI] [PubMed] [Google Scholar]
- 9.Diggle SP, Griffin AS, Campbell GS, West SA. 2007. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450, 411–414 10.1038/nature06279 (doi:10.1038/nature06279) [DOI] [PubMed] [Google Scholar]
- 10.Grutter AS, Bshary R. 2003. Cleaner wrasse prefer client mucus: support for partner control mechanisms in cleaning interactions. Proc. R. Soc. B 270, S242–S244 10.1098/rsbl.2003.0077 (doi:10.1098/rsbl.2003.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bshary R, Grutter AS. 2005. Punishment and partner switching cause cooperative behaviour in a cleaning mutualism. Biol. Lett. 1, 396–399 10.1098/rsbl.2005.0344 (doi:10.1098/rsbl.2005.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bshary R, Grutter AS. 2006. Image scoring and cooperation in a cleaner fish mutualism. Nature 441, 975–978 10.1038/nature04755 (doi:10.1038/nature04755) [DOI] [PubMed] [Google Scholar]
- 13.Noë R. 2006. Cooperation experiments: coordination through communication versus acting apart together. Anim. Behav. 71, 1–18 10.1016/j.anbehav.2005.03.037 (doi:10.1016/j.anbehav.2005.03.037) [DOI] [Google Scholar]
- 14.de Waal F, Brosnan S. 2006. Simple and complex reciprocity in primates. In Cooperation in primates and humans: mechanisms and evolution (eds Kappeler PM, van Schaik CP.), pp. 85–105 New York, NY: Springer [Google Scholar]
- 15.Rutte C, Taborsky M. 2008. The influence of social experience on cooperative behaviour of rats (Rattus norvegicus): direct versus generalised reciprocity. Behav. Ecol. Sociobiol. 62, 499–505 10.1007/s00265-007-0474-3 (doi:10.1007/s00265-007-0474-3) [DOI] [Google Scholar]
- 16.McNab BK. 1973. Energetics and distribution of vampire bats. J. Mammal 54, 131–144 10.2307/1378876 (doi:10.2307/1378876) [DOI] [Google Scholar]
- 17.Wilkinson GS. 1984. Reciprocal food sharing in the vampire bat. Nature 308, 181–184 10.1038/308181a0 (doi:10.1038/308181a0) [DOI] [Google Scholar]
- 18.Hammerstein P. 2003. Why is reciprocity so rare in social animals? A protestant appeal. In Genetic and cultural evolution of cooperation (ed. Hammerstein P.), pp. 83–93 Cambridge, MA: MIT Press [Google Scholar]
- 19.Stevens JR, Cushman FA, Hauser MD. 2005. Evolving the psychological mechanisms for cooperation. Annu. Rev. Ecol. Evol. Syst. 36, 499–518 10.1146/annurev.ecolsys.36.113004.083814 (doi:10.1146/annurev.ecolsys.36.113004.083814) [DOI] [Google Scholar]
- 20.Clutton-Brock T. 2009. Cooperation between non-kin in animal societies. Nature 461, 51–57 10.1038/nature08366 (doi:10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 21.Davies NB, Krebs JR, West SA. 2012. An introduction to behavioural ecology, 4th edn Oxford, UK: Wiley-Blackwell [Google Scholar]
- 22.Foster KR. 2004. Diminishing returns in social evolution: the not-so-tragic commons. J. Evol. Biol. 17, 1058–1072 10.1111/j.1420-9101.2004.00747.x (doi:10.1111/j.1420-9101.2004.00747.x) [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson G. 1988. Reciprocal altruism in bats and other mammals. Ethol. Sociobiol. 9, 85–100 10.1016/0162-3095(88)90015-5 (doi:10.1016/0162-3095(88)90015-5) [DOI] [Google Scholar]
- 24.Wilkinson G. 1986. Social allogrooming in the common vampire bat, Desmodus rotundus. Anim. Behav. 34, 1880–1889 10.1016/S0003-3472(86)80274-3 (doi:10.1016/S0003-3472(86)80274-3) [DOI] [Google Scholar]
- 25.Paolucci M, Conte R, Tosto GD. 2006. A model of social organization and the evolution of food sharing in vampire bats. Adaptive Behav. 14, 223–238 10.1177/105971230601400305 (doi:10.1177/105971230601400305) [DOI] [Google Scholar]
- 26.Witkowski M. 2007. Energy sharing for swarms modeled on the common vampire bat. Adaptive Behav. 15, 307–328 10.1177/1059712307082092 (doi:10.1177/1059712307082092) [DOI] [Google Scholar]
- 27.DeNault L, McFarlane D. 1995. Reciprocal altruism between male vampire bats, Desmodus rotundus. Anim. Behav. 49, 855–856 [Google Scholar]
- 28.Voigt CC, Voigt-Heucke SL, Schneeberger K. 2012. Isotopic data do not support food sharing within large networks of female vampire bats (Desmodus rotundus). Ethology 118, 260–268 10.1111/j.1439-0310.2011.02004.x (doi:10.1111/j.1439-0310.2011.02004.x) [DOI] [Google Scholar]
- 29.Pfeiffer T, Rutte C, Killingback T, Taborsky M, Bonhoeffer S. 2005. Evolution of cooperation through generalized reciprocity. Proc. R. Soc. B 272, 1115–1120 10.1098/rspb.2004.2988 (doi:10.1098/rspb.2004.2988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalinowski ST, Wagner AP, Taper ML. 2006. ML-Relate: a computer program for maximum likelihood estimation of relatedness and relationship. Mol. Ecol. Notes 6, 576–579 10.1111/j.1471-8286.2006.01256.x (doi:10.1111/j.1471-8286.2006.01256.x) [DOI] [Google Scholar]
- 31.Manly BFJ. 2007. Randomization, bootstrap and Monte Carlo methods in biology. Boca Raton, FL: CRC Press [Google Scholar]
- 32.Kruskal W. 1987. Relative importance by averaging over orderings. Am. Statist. 41, 6–10 10.1080/00031305.1987.10475432 (doi:10.1080/00031305.1987.10475432) [DOI] [Google Scholar]
- 33.Grömping U. 2006. Relative importance for linear regression in R: the package relaimpo. J. Stat. Softw. 17, 1–27 [Google Scholar]
- 34.de Waal F. 1997. The chimpanzee's service economy: food for grooming. Evol. Hum. Behav. 18, 375–386 10.1016/S1090-5138(97)00085-8 (doi:10.1016/S1090-5138(97)00085-8) [DOI] [Google Scholar]
- 35.Carter GG, Wilkinson GS. 2012. Data from: food sharing in vampire bats: reciprocal help predicts donations more than relatedness or harassment. Dryad Digital Repository. See http://dx.doi.org/10.5061/dryad.tg7b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210 10.1016/j.cub.2010.10.058 (doi:10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson G. 1985. The social organization of the common vampire bat I and II. Behav. Ecol. Sociobiol. 17, 111–134 [Google Scholar]
- 38.Carter GG, Logsdon R, Arnold BD, Menchaca A, Medellin RA. 2012. Adult vampire bats produce contact calls when isolated: acoustic variation by species, population, colony, and individual. PLoS ONE 7, e38791. 10.1371/journal.pone.0038791.t006 (doi:10.1371/journal.pone.0038791.t006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter GG, Skowronski M, Faure P, Fenton MB. 2008. Antiphonal calling allows individual discrimination in white-winged vampire bats. Anim. Behav. 76, 1343–1355 10.1016/j.anbehav.2008.04.023 (doi:10.1016/j.anbehav.2008.04.023) [DOI] [Google Scholar]
- 40.Fruteau C, Voelkl B, van Damme E, Noë R. 2009. Supply and demand determine the market value of food providers in wild vervet monkeys. Proc. Natl Acad. Sci. USA 106, 12 007–12 012 10.1073/pnas.0812280106 (doi:10.1073/pnas.0812280106) [DOI] [PMC free article] [PubMed] [Google Scholar]


