Abstract
Adult social behaviour can be persistently modified by early-life social experience. In rodents, such effects are induced by tactile maternal stimulation resulting in neuroendocrine modifications of the hypothalamic–pituitary–adrenal axis involved in stress responsiveness. Whether similar long-term alterations can occur in the hypothalamic–pituitary–interrenal (HPI) axis of poikilothermic vertebrates is unknown. We compared the expression of four genes of the HPI axis in adults of the cooperatively breeding cichlid Neolamprologus pulcher, which had been exposed to two early-life social treatments 1.5 years prior to brain sampling. Fish reared with parents and siblings had less brain expression of corticotropin-releasing factor and of the functional homologue of the mammalian glucocorticoid receptor (GR1) than individuals reared with same-age siblings only. Expression of the mineralocorticoid receptors (MR) did not differ between treatments, but the MR/GR1 expression ratio was markedly higher in fish reared with parents and siblings. Thus, we show here that early social experience can alter the programming of the stress axis in poikilothermic vertebrates, suggesting that this mechanism is deeply conserved within vertebrates. Moreover, we show for the first time that reprogramming of the stress axis of a vertebrate can be induced without tactile stimulation by parents.
Keywords: early social environment, gene expression, stress response, glucocorticoid receptor, corticotropin-releasing factor, fish
1. Introduction
Persistent influences of the early social environment on social behaviour expressed later in life have been demonstrated in a number of vertebrate taxa. Social enrichment or deprivation experienced shortly after birth can affect, for instance, the performance in dominance relationships and hierarchy formation (laboratory mice [1,2]; rhesus macaques, Macaca mulatta [3]), adult brood care behaviour (laboratory mice [4]; laboratory rats [5,6]) and mate choice (zebra finch, Teaniopygia guttata [7]). In the cooperatively breeding African cichlid Neolamprologus pulcher, altering the structure of social groups during the first two months of life affects the social behaviour expressed across different social contexts and life stages [8,9]. Young reared among their siblings in the absence of dominant adults respond less appropriately to social challenges during the juvenile stage, with ensuing negative effects on the outcome of social interactions [8]. Competitive contests between fish reared without dominants take longer to resolve, and losers of a contest reared without dominants face a higher risk of eviction from the winner's territory. The association between early social environment and behaviour persists until adulthood, as fish reared among siblings only are less likely to be accepted in an unfamiliar social group than fish reared in the presence of both adults and siblings [9]. The social environment thus seems to be an essential input in the development of a behavioural repertoire needed to navigate life in complex social groups (i.e. ‘social competence’ [10]).
How are these effects of the early-life environment on behaviour maintained into adult life? In several mammals and birds, variation in early-life parental care and the ensuing long-term modification of behaviours are related to neurobiological modifications, in particular of the hypothalamic–pituitary–adrenal hormonal axis (HPA) involved in regulating stress responsiveness [11,12]. Epigenetic reprogramming (i.e. a ‘[change] of gene expression that does not involve change in the DNA’ [13], p. 346) has been identified as a key mechanism underlying these effects in rodents (reviewed in earlier studies [11,14–16]). For example, in rats, higher intensities of maternal tactile stimulation result in higher expression levels of genes coding for glucocorticoid receptors implicated in the stress response. These expression changes have been shown to be caused by modifications of DNA methylation patterns [11,16]. However, whether the modification of the early social environment can induce similar long-term alterations in the hormonal stress axis of poikilothermic vertebrates, and whether the reprogramming of the hormonal stress response by brood care behaviour indeed always requires tactile stimulation, is so far unknown.
We investigated whether the early social environment persistently alters the brain expression of genes involved in the poikilotherm hormonal stress axis (that is the hypothalamic–pituitary–interrenal axis, or HPI axis) in the cooperatively breeding cichlid fish N. pulcher. We compared transcription profiles of adult N. pulcher at about 1.5 years after they had been exposed to two different social conditions: for the first two months of life they had been reared either in the presence of parents and siblings, which corresponds to a normal social setting in this species, or only in the presence of same-aged siblings [8,9]. We quantified the expression level of four genes coding for neuropeptides and receptors implicated in the release and binding of corticoids as part of the vertebrate stress response: (i) corticotropin-releasing factor (CRF, the fish homologue of corticotropin-releasing hormone, CRH [17]), (ii) the two glucocorticoid receptors found in N. pulcher (GR1 and GR2 [18,19]), and (iii) the mineralocorticoid receptor (MR [18]).
In rodents, prenatal or postnatal signals of stress alter the expression of genes coding for CRH and GR. Male mice exposed to prenatal stress via placental signals increase CRH expression and decrease GR expression [20]. Likewise, lab rats experiencing more stressful stimuli and/or longer absences of mothers shortly after birth have a higher activation of the HPA axis, including upregulated CRH and downregulated GR expression [11,21]. Based on this evidence, if the absence of guarding adults in N. pulcher increases the stress levels of young (e.g. in nature this situation would be accompanied by higher predation risk), we should expect that gene expression in these fish is altered such that they can respond more readily to stressors. Therefore, we expected that basal CRF would be upregulated in individuals raised without adult fish, whereas GR1, the functional homologue of the mammalian GR, would be downregulated in these fish.
2. Material and methods
(a). Study species
Neolamprologus pulcher (synonymous with Neolamprologus brichardi [22]) is a highly social cichlid endemic to Lake Tanganyika, east Africa, living in social groups that defend small territories around breeding cavities [23]. These groups can consist of up to 40 fish, typically including a breeding pair, offspring from a recent brood, and immature and mature brood care helpers [24,25]. Helpers may be related or unrelated to the breeders [26,27], and often stay at their natal territory far beyond sexual maturity [26,28]. Helpers join in cleaning eggs and larvae produced by breeders, maintenance of the territory, and defence against space competitors and predators [23,28–30]. In turn, helpers benefit from predator defence by other group members, access to high-quality shelters [23,31,32] and a certain probability of inheritance of a breeder position [33].
(b). Experimental fish
We obtained the fish for this study from six different broods consisting of an average of 70 eggs. These broods were split in two equal halves at an age of 10 days (i.e. shortly after larvae reach their free-swimming stage). During the following two months, half of each brood was reared in the presence of their parents or parents plus two helpers (+F treatment), whereas the other half was reared among their same-aged siblings only (−F treatment). After these two months, the treatment phase was terminated, parents and helpers were removed, and the fish were kept separated by sibship and social experience under standard housing conditions described in Taborsky et al. [9] until they were collected for this study. A few fish sampled for our study may have been involved in experiments performed to characterize the behavioural effects of the early social environment (see Arnold & Taborsky [8] and Taborsky et al. [9] for details), because the fish could not be individually marked at the time of these experiments. As we sampled a similar number of fish per sibship and treatment (mean: 3.3 −F fish, 2.7 +F fish), and as the group sizes of +F and −F sibships did not differ significantly at any life stage [9], fish from both treatments had an equal chance of having been used for a behavioural test before. The last behavioural test was done an average of 8.5 months (i.e. at a mean age of 319 days [9]) before the fish were sampled for gene expression analysis.
(c). Sample collection
Brain samples were taken from 36 individuals (16 +F fish and 20 −F fish). At sampling, these fish were on average 574.5 days old (range 512–657), which is about 1.5 years after they received the early-life social treatment. They differed in sex (20 males and 16 females) and social status (10 breeders, 9 helpers, 17 not accepted as helpers; for details on these variables see the electronic supplementary material, table S1). Although all fish kept in the same tank had the same age, they had grown apart in size with time, as dominants grow faster relative to subordinates [34], and males grow faster than females. Individuals were killed by an overdose of Tricaine methanesulfonate (MS 222; Sandoz, Switzerland) within 2 min after catching. All brains were dissected within 8 min after death and immersed in RNALater (Ambion, Inc., Austin, TX). Samples were kept at 4°C for 24 h and then transferred to −20°C until further processing.
(d). Candidate genes and analysis of gene expression levels
We investigated the expression profiles of four genes implicated in the HPI axis of N. pulcher.
(i). Corticotropin-releasing factor
CRF, the fish homologue of CRH [17], is a key neuropeptide in the organization of the behavioural and neuroendocrine response to stress. In fish, it regulates adrenocorticotropic hormone (ACTH) secretion, which in turn stimulates the secretion of glucocorticoids from the interrenal system into the circulatory system [17].
(ii). Glucocorticoid receptors
The two glucocorticoid receptors found in N. pulcher and many other fish species (GR1 and GR2 [18,19]) are ligand-driven transcription factors functioning to control a diverse array of components of the stress response, such as metabolism, energy balance and behaviour. Mammals have only one GR, which exclusively binds glucocorticoids [18]. This receptor is part of the negative feedback loop controlling the duration and amplitude of the stress response [17]. In most fish, GR1 is homologue to the mammalian GR, as it only binds to cortisol, whereas GR2 can bind to both glucocorticoids and mineralocorticoids [18]. The N. pulcher GR1 was originally named GR2 (and GR2 named GR1), which has been shown to be incorrect through functional and sequence analyses [18]. Here, we use the corrected nomenclature.
(iii). Mineralocorticoid receptor
MR binds to glucocorticoids, mineralocorticoids and progesterone, and acts as a transcription factor. As MR usually has a very high affinity to cortisol (10–100 times higher than to GRs in the cichlid fish Astatotilapia burtoni [18]), it remains tonically activated by basal cortisol levels.
Whole-brain RNA extraction and preparation was performed according to Aubin-Horth et al. [35]. Reverse transcription was performed using 500 ng of RNA and a standard SuperScript II protocol (Invitrogen). Primers for the four genes were designed based on sequences available for N. pulcher in the NCBI database for GR1 (formerly GR2; NCBI GenBank: EF661652.1; for-GCTGATCAAGATGAAAGTGC; rev-AGAGTAGACATGAGCCGTGA), GR2 (formerly GR1; EF661651.1; for-TCGTTCCAACAATGTTATCC; rev-GCAGAGTCATCTGATCATCC) and MR (EF661650.1; for-CTCATGGATGTGTCTGTCCT; rev-GAGAGGAACTCGTCGTAGGT). No sequence was available for N. pulcher for CRF. We used the CRF sequence from another African cichlid, A. burtoni (EF363131.1), to design primers (for-TGATTCTGCTAGTTGCCTTC; rev-GACTGGTTGCACATAAGTTT) and amplify a 837 bp region in N. pulcher using cDNA. The resulting PCR amplification product was purified using a standard ExoSAP-IT reagent protocol (USB corporation) and sequenced. The N. pulcher-specific sequence was submitted to NCBI (JX134406) and used to design qPCR-specific primers (for-ATCCCTTCCATCTTCAACAG; rev-CTGGACATCTCCATCATCTC). The resulting primers (Integrated DNA Technologies, USA) and 5 µl of cDNA (25 ng µl−1) were used in a quantitative real-time PCR experiment following a scaled-down version of the Quantitect SYBRGreen PCR kit manufacturer's protocol (Qiagen) using a 384-well plate qRT-PCR machine (Light Cycler, Roche). Standard curves consisting of 6 × 10-fold dilutions were run in duplicates to determine amplification efficiency [36] and determine gene expression levels for each individual, as a fold change relative to the individual with the lowest gene expression value for that gene [35]. Fold change describes by how many times mRNA levels differ in an individual relative to a fixed value (here, the individual with the lowest mRNA level). All samples for a given gene were prepared together on a 384-well plate using an epmotion liquid handler (Eppendorf). Each fish sample was run in triplicates for a given gene along with no primers and no template controls. A melting curve was performed to verify that a single amplified product was present and that no primer dimers were present.
(e). Data analysis
The individual fold change data were used to test for statistical differences between the two social treatments. Besides the expression of each of the four genes, we also compared the ratio of MR to GR1, as in mammals it has been proposed that homeostasis, adaptation and health depend on the balanced interaction between mediators of the onset (membrane and nuclear MR) and termination (nuclear GR) of the stress response (MR/GR balance hypothesis [37]). In this ratio we only included GR1, the functional homologue of the mammalian GR in this cichlid species [18].
We fitted generalized linear mixed models with identity-link functions and ‘family of origin’ as a random factor. Full models included ‘treatment’, ‘sex’ and ‘breeding status’ as fixed factors, but sex and breeding status had no significant effects and were removed from the final models. Being the focal, experimentally manipulated variable, ‘treatment’ was retained in the model even if not significant. The error structure of the models did not deviate from normality and the variances did not deviate from homogeneity. All data were analysed using SPSS v. 17.0 for Windows (SPSS Inc., Chicago, IL).
3. Results
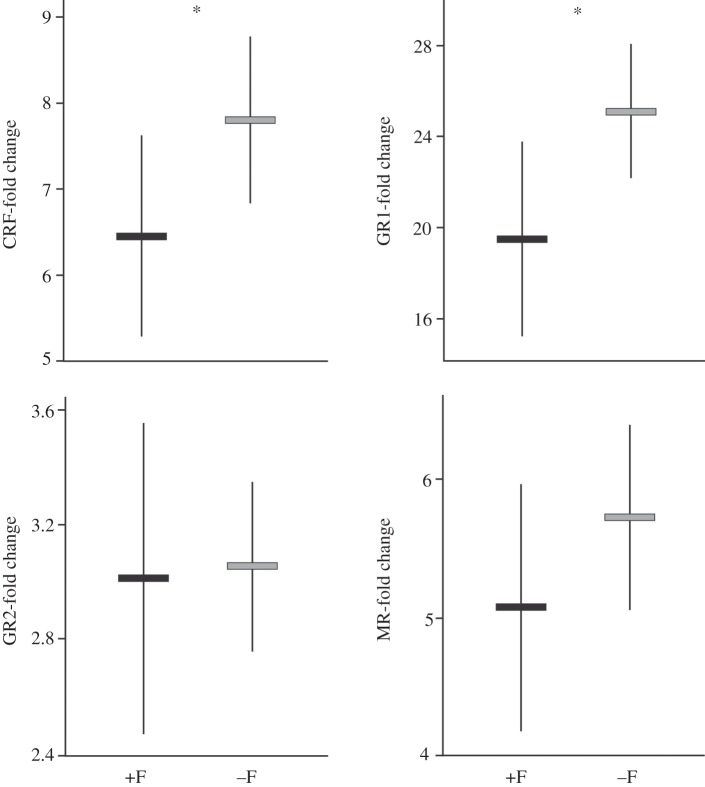
The levels of mRNA transcripts of all four genes were positively correlated across the total sample of 36 fish (table 1). Fish that had been reared together with parents (+F fish) had a significantly lower expression of CRF (F1,33.8 = 4.11, p = 0.050) and of GR1 transcripts (figure 1; F1,34 = 5.52, p = 0.025) compared with −F fish. By contrast, the expression levels of GR2 and MR were not influenced by the social experience treatment (GR2: F1,34 = 0.02, p = 0.88; MR: F1,33.9 = 1.50, p = 0.23). The MR/GR1 ratio was markedly higher in +F fish than in −F fish (figure 2; F1,32.8 = 8.85, p = 0.005).
Table 1.
Correlations of mRNA transcripts of the four tested genes. Pearson product–moment correlation coefficients (r) and p-values are shown (all n = 36). All comparisons are significant after false discovery rate (FDR) control after Benjamini & Hochberg [38].
| GR1 | GR2 | MR | ||
|---|---|---|---|---|
| CRF | r | 0.68 | 0.33 | 0.68 |
| p | <0.001 | 0.047 | <0.001 | |
| GR1 | r | 0.64 | 0.90 | |
| p | <0.001 | <0.001 | ||
| GR2 | r | 0.65 | ||
| p | <0.001 |
Figure 1.
Expression levels of the four target genes in fish reared with (+F) or without (−F) dominant adults during the first two months of life. Graphs show means and s.d. Asterisks indicate significant differences between treatments.
Figure 2.
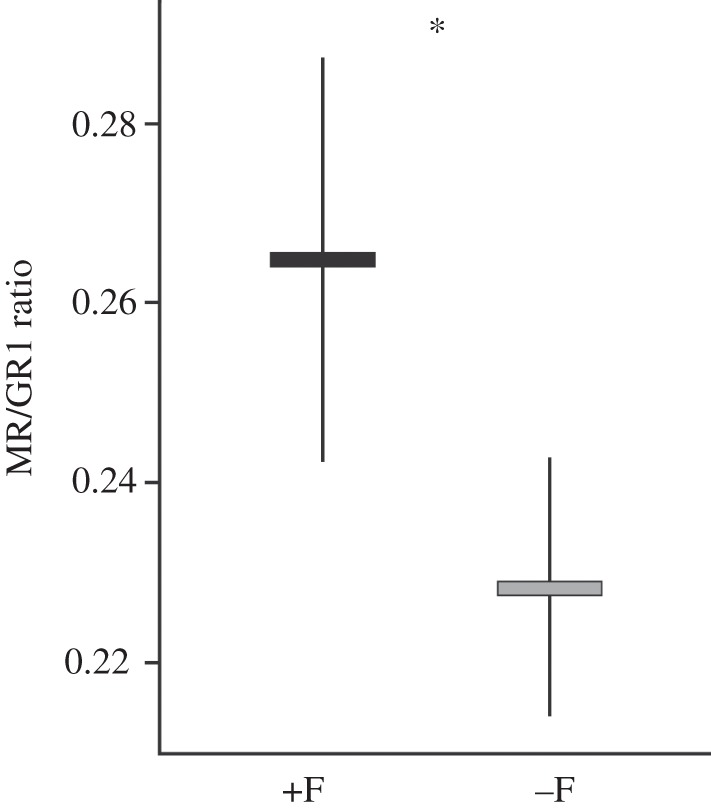
Comparison of the MR/GR1 expression ratio between the two social treatments. For explanation of graphs, see figure 1.
4. Discussion
Studies in mammals showed that the early social environment persistently affects behaviour, and that this is associated with a changed expression of genes implicated in the HPA axis. We hypothesized that similar joint effects on behaviour and gene expression may exist in fish. More specifically, we predicted that expression of the neuropeptide CRF and of the steroid hormone receptor GR1 would be differentially regulated in N. pulcher, a cichlid fish showing lasting alterations of its social behaviour depending on its social rearing environment. We found that early-life social experience indeed significantly altered the expression of these two key gene products of the stress response of teleost fish [17,19]. Our results suggest that the differential expression of these genes results from irreversible reprogramming of the brain that occurred during the social treatment the fish were exposed to in the first two months of their life. Irreversible programming of the brain is suggested because (i) the gene expression differences induced during early life were still detected at ages of 1.5 years and older (that is, about half a year after sexual maturity), and (ii) previous behavioural experiments revealed that social behaviour was persistently affected by the early social treatments, suggesting underlying irreversible differences in the mechanisms controlling behaviour: +F fish performed better than –F fish in social challenge tests across three different life stages (early juvenile [8], late juvenile and early adult stages [9]). Also, (iii) differences in gene expression induced during early life were remarkably robust, as they were detected in adults despite sex differences and differential trajectories of social status occurring among fish belonging to the same treatment group. Alternatively, cascading effects caused by the persistently differing social behaviour of the two treatment groups might have influenced gene expression. This possibility is unlikely, however, as the different social experiences individuals faced after the early social treatment (differences in rank, sex and breeding status) and the ensuing altered frequencies of expressed behaviours are by far stronger than the behavioural differences detected in our behavioural experiments comparing the two treatments groups [8,9]. If social experience gained after the early-life treatments had strongly influenced gene expression of the HPI axis in our study, then this later-life experience should have entirely masked all early-life treatment effects.
CRF is the primary hypothalamic neurohormone regulating the activity of the HPA/HPI axis in vertebrates [39]. Mammalian studies showed that the regulation of CRF expression can be modulated by experiences early in life, and is therefore thought to be involved in setting the ‘tone’ of individual stress responsiveness [40]. Rodent pups exposed to low stress levels and/or to high levels of maternal care typically express low basal CRF levels and an attenuated stress responsiveness [11,13,21]. In line with these findings, CRF gene expression was lower in the fish reared together with older family members (+F fish). The presence of guarding adults may have signalled to the developing young that they lived in a secure environment relative to a −F environment, with little exposure to stressful experiences (see [8] for discussion). Juveniles may thus have responded to this environment by developing a lower HPI reactivity.
In mammals, an attenuated HPA reactivity induced by early experience typically involves a higher expression of GR in the hippocampus and several other brain regions [41]. These receptors mediate an earlier termination of a stress response by a negative feedback on CRF [42]. Therefore, we had expected that GR1 would be expressed more in +F fish than in −F fish. The opposite was found to be true. There are several possible reasons for this deviation from expectation. First, as we analysed total brain samples, we could not identify the specific brain regions where GR1 was differentially expressed between treatments. Second, the role of GR signalling in teleost fish is likely to differ from mammals as even between fish species the specificity and sensitivity of the different corticoid receptors differ [17,18]. Pharmacologically, knocking down both GR receptors in rainbow trout reduces basal CRF transcript abundance, suggesting a role for GR signalling in maintaining basal gene expression of this peptide [19]. The positive correlation between basal CRF and basal GR expression found in our study is in line with this result. Based on their findings, Alderman et al. [19] hypothesized that genomic signalling mediated by GRs in fish may be important for maintaining the transcription of basal CRF, while CRF suppression by cortisol through negative feedback control may involve other unidentified pathways. By contrast to GR1, the expression of GR2 did not differ between early social conditions. GR2 sensitivity to corticoids in N. pulcher is high, such that activation of this receptor by corticosteroids at basal levels can be expected [18]. Therefore, the absence of expression differences in this receptor would indicate that epigenetic reprogramming as a consequence of early-life social experience occurs specifically in genes involved in the stress response rather than in general corticosteroid signalling.
MRs are known to contribute to the negative feedback on CRF [11,43] and are actively involved in stress responsiveness in mammals. While in our study MR transcript levels did not differ between treatments, +F fish had a markedly higher MR/GR1 ratio. The physiological relevance of this ratio in fish has not been investigated so far, but mammalian models suggest its possible implication for human health [37]. Recent findings suggest that the mammalian MR takes a much more active role in the control of stress responses than previously thought: MRs bound to membranes in the hippocampus amplify the onset of a stress response, and thereby control the initial non-genomic stress reaction, which is important for appraisal and coping with a stressor, whereas GRs are essential for management of the later adaptive phase [37]. Thus the balance of MR versus GR may be more relevant for the understanding of HPA/HPI reactivity than the basal levels of each receptor in isolation [44]. We present this ratio analysis while keeping in mind that the functional significance of such a ratio may differ in fish, as (i) aldosterone is not present as a ligand for MR; (ii) two different GRs are found; (iii) non-genomic action of steroid receptors (i.e. membrane-bound steroid receptors not acting as transcription factors) is known to exist as in mammals [45], but has not yet been thoroughly studied in link with the stress response; and (iv) these ratios may be specific to certain brain regions [37].
Epigenetic reprogramming of the stress axis after experimental variation of early-life social experience has so far only been shown in a number of mammalian species, particularly in laboratory rodents, and more recently also in a bird species (zebra finch, T. guttata [12]). Here, we show for the first time that the early social environment can alter the programming of the HPI axis in poikilothermic vertebrates. This suggests that the mechanism for reprogramming the stress axis via early social experience is deeply conserved within vertebrates. In order to fully understand possible homologies, convergences and differences between signalling pathways between homeothermic and poikilothermic vertebrates, future research should (i) localize where in the fish brain corticoid receptors are differentially expressed in response to early-life experience, (ii) identify other possible candidate genes involved in the regulation of the HPI axis, and (iii) explore the mechanisms (e.g. epigenetic marks such as DNA methylation) that allow the long-term maintenance of these differences.
Tactile stimulation has been proposed as a key proximate mechanism inducing a persistent reprogramming of the stress axis in rats and mice (reviewed in earlier studies [11,14–16]). Unlike the brood care of mammals and birds, which involves direct tactile contact during grooming and feeding of young, in N. pulcher, brood care does not involve body contact during the social experience phase [8]. Thus, our results demonstrate that at least in some vertebrates (fish), a reprogramming of the stress axis is possible without direct tactile contact between parents and offspring. Rather than parental tactile stimulation, epigenetic changes in N. pulcher might possibly be induced by the higher frequency of social interactions between siblings that was observed in those fish reared together with adults [8]. Whether epigenetic changes in DNA methylation as detected in rodents are also involved in the reprogramming of the N. pulcher HPI axis is currently under investigation.
Acknowledgements
The experiment was conducted at the Institute of Ecology and Evolution, University of Bern, Switzerland, under licences no. 40/05 and 16/09 of the Veterinary Office of the Kanton Bern.
We thank Sophie Lanahan-Tremblay and Sergio Cortez Ghio for help in the laboratory and sequence analysis. B.T. was financially supported by the Swiss National Science Foundation (SNF, projects 3100A0-111796 and 31003A_133066) and the Austrian Science Fund (FWF, project 18647-B16). We are grateful to the Berne University Research Foundation for funding the laboratory visit of L.T. for genomic analyses at Université Laval, Canada. N.A.H. was financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery grant), and the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT Nouveau chercheur).
References
- 1.Branchi I, D'Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. 2006. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol. Psychiatry 60, 690–696 10.1016/j.biopsych.2006.01.005 (doi:10.1016/j.biopsych.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 2.Branchi I, D'Andrea I, Gracci F, Santucci D, Alleva E. 2009. Birth spacing in the mouse communal nest shapes adult emotional and social behavior. Physiol. Behav. 96, 532–539 10.1016/j.physbeh.2008.12.003 (doi:10.1016/j.physbeh.2008.12.003) [DOI] [PubMed] [Google Scholar]
- 3.Bastian ML, Sponberg AC, Sponberg AC, Suomi SJ, Higley JD. 2003. Long-term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta). Dev. Psychobiol. 42, 44–51 10.1002/dev.10091 (doi:10.1002/dev.10091) [DOI] [PubMed] [Google Scholar]
- 4.Musi B, Deacetis L, Alleva E. 1993. Influence of litter gender composition on subsequent maternal behavior and maternal aggression in female house mice. Ethology 95, 43–53 10.1111/j.1439-0310.1993.tb00455.x (doi:10.1111/j.1439-0310.1993.tb00455.x) [DOI] [Google Scholar]
- 5.Levy F, Melo AI, Galef G, Madden M, Fleming AS. 2003. Complete maternal deprivation affects social but not spatial learning in adult rats. Dev. Psychobiol. 43, 177–191 10.1002/dev.10131 (doi:10.1002/dev.10131) [DOI] [PubMed] [Google Scholar]
- 6.Curley JP, Davidson S, Bateson P, Champagne FA. 2009. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front. Behav. Neurosci. 3, 25. 10.3389/neuro.08.025.2009 (doi:10.3389/neuro.08.025.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adkins-Regan E, Krakauer A. 2000. Removal of adult males from the rearing environment increases preference for same-sex partners in the zebra finch. Anim. Behav. 60, 47–53 10.1006/anbe.2000.1448 (doi:10.1006/anbe.2000.1448) [DOI] [PubMed] [Google Scholar]
- 8.Arnold C, Taborsky B. 2010. Social experience in early ontogeny has lasting effects on social skills in cooperatively breeding cichlids. Anim. Behav. 79, 621–630 10.1016/j.anbehav.2009.12.008 (doi:10.1016/j.anbehav.2009.12.008) [DOI] [Google Scholar]
- 9.Taborsky B, Arnold C, Junker J, Tschopp A. 2012. The early social environment affects social competence in a cooperative breeder. Anim. Behav. 83, 1067–1074 10.1016/j.anbehav.2012.01.037 (doi:10.1016/j.anbehav.2012.01.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taborsky B, Oliveira RF. 2012. Social competence: an evolutionary approach. Trends Ecol. Evol. 27, 679–688 10.1016/j.tree.2012.09.003 (doi:10.1016/j.tree.2012.09.003) [DOI] [PubMed] [Google Scholar]
- 11.Meaney MJ, Szyf M. 2005. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 28, 456–463 10.1016/j.tins.2005.07.006 (doi:10.1016/j.tins.2005.07.006) [DOI] [PubMed] [Google Scholar]
- 12.Banerjee SB, Arterbery AS, Fergus DJ, Adkins-Regan E. 2012. Deprivation of maternal care has long-lasting consequences for the hypothalamic–pituitary–adrenal axis of zebra finches. Proc. R. Soc. B 279, 759–766 10.1098/rspb.2011.1265 (doi:10.1098/rspb.2011.1265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crews D. 2008. Epigenetics and its implications for behavioral neuroendocrinology. Front. Neuroendocrinol. 29, 344–357 10.1016/j.yfrne.2008.01.003 (doi:10.1016/j.yfrne.2008.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champagne FA, Curley JP. 2005. How social experiences influence the brain. Curr. Opin. Neurobiol. 15, 704–709 10.1016/j.conb.2005.10.001 (doi:10.1016/j.conb.2005.10.001) [DOI] [PubMed] [Google Scholar]
- 15.Champagne FA, Curley JP. 2011. Epigenetic influence of the social environment. In Brain, behavior and epigenetics, (eds Petronis A, Mill J.), pp. 185–208 Berlin, Germany: Springer [Google Scholar]
- 16.Fagiolini M, Jensen CL, Champagne FA. 2009. Epigenetic influences on brain development and plasticity. Curr. Opin. Neurobiol. 19, 207–212 10.1016/j.conb.2009.05.009 (doi:10.1016/j.conb.2009.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernier NJ, Flik G, Klaren PHM. 2009. Regulation and contribution of the corticotropic, melanotropic and thyrotropic axes to the stress response in fishes. In Fish neuroendocrinology (ed Bernier NJ.), pp. 235–311 New York, NY: Academis Press [Google Scholar]
- 18.Arterbery AS, Fergus DJ, Fogarty EA, Mayberry J, Deitcher DL, Kraus WL, Bass AH. 2011. Evolution of ligand specificity in vertebrate corticosteroid receptors. BMC Evol. Biol. 11, 14. 10.1186/1471-2148-11-14 (doi:10.1186/1471-2148-11-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alderman SL, McGuire A, Bernier NJ, Vijayan MM. 2012. Central and peripheral glucocorticoid receptors are involved in the plasma cortisol response to an acute stressor in rainbow trout. Gen. Comp. Endocrinol. 176, 79–85 10.1016/j.ygcen.2011.12.031 (doi:10.1016/j.ygcen.2011.12.031) [DOI] [PubMed] [Google Scholar]
- 20.Mueller BR, Bale TL. 2008. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 28, 9055–9065 10.1523/JNEUROSCI.1424-08.2008 (doi:10.1523/JNEUROSCI.1424-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macri S, Wuerbel H. 2006. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm. Behav. 50, 667–680 10.1016/j.yhbeh.2006.06.015 (doi:10.1016/j.yhbeh.2006.06.015) [DOI] [PubMed] [Google Scholar]
- 22.Duftner N, Sefc KM, Koblmuller S, Salzburger W, Taborsky M, Sturmbauer C. 2007. Parallel evolution of facial stripe patterns in the Neolamprologus brichardil/pulcher species complex endemic to Lake Tanganyika. Mol. Phylogenet. Evol. 45, 706–715 10.1016/j.ympev.2007.08.001 (doi:10.1016/j.ympev.2007.08.001) [DOI] [PubMed] [Google Scholar]
- 23.Taborsky M. 1984. Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim. Behav. 32, 1236–1252 10.1016/S0003-3472(84)80241-9 (doi:10.1016/S0003-3472(84)80241-9) [DOI] [Google Scholar]
- 24.Balshine S, Leach B, Neat F, Reid H, Taborsky M, Werner N. 2001. Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher). Behav. Ecol. Sociobiol. 50, 134–140 10.1007/s002650100343 (doi:10.1007/s002650100343) [DOI] [Google Scholar]
- 25.Heg D, Brouwer L, Bachar Z, Taborsky M. 2005. Large group size yields group stability in the cooperatively breeding cichlid Neolamprologus pulcher. Behaviour 142, 1615–1641 10.1163/156853905774831891 (doi:10.1163/156853905774831891) [DOI] [Google Scholar]
- 26.Dierkes P, Heg D, Taborsky M, Skubic E, Achmann R. 2005. Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol. Lett. 8, 968–975 10.1111/j.1461-0248.2005.00801.x (doi:10.1111/j.1461-0248.2005.00801.x) [DOI] [PubMed] [Google Scholar]
- 27.Stiver KA, Dierkes P, Taborsky M, Gibbs HL, Balshine S. 2005. Relatedness and helping in fish: examining the theoretical predictions. Proc. R. Soc. B 272, 1593–1599 10.1098/rspb.2005.3123 (doi:10.1098/rspb.2005.3123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taborsky M, Limberger D. 1981. Helpers in fish. Behav. Ecol. Sociobiol. 8, 143–145 10.1007/BF00300826 (doi:10.1007/BF00300826) [DOI] [Google Scholar]
- 29.Bruintjes R, Taborsky M. 2008. Helpers in a cooperative breeder pay a high price to stay: effects of demand, helper size and sex. Anim. Behav. 75, 1843–1850 10.1016/j.anbehav.2007.12.004 (doi:10.1016/j.anbehav.2007.12.004) [DOI] [Google Scholar]
- 30.Bruintjes R, Taborsky M. 2011. Size-dependent task specialization in a cooperative cichlid in response to experimental variation of demand. Anim. Behav. 81, 387–394 10.1016/j.anbehav.2010.10.004 (doi:10.1016/j.anbehav.2010.10.004) [DOI] [Google Scholar]
- 31.Balshine-Earn S, Neat FC, Reid H, Taborsky M. 1998. Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav. Ecol. 9, 432–438 10.1093/beheco/9.5.432 (doi:10.1093/beheco/9.5.432) [DOI] [Google Scholar]
- 32.Heg D, Bachar Z, Brouwer L, Taborsky M. 2004. Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc. R. Soc. Lond. B 271, 2367–2374 10.1098/rspb.2004.2855 (doi:10.1098/rspb.2004.2855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiver KA, Dierkes P, Taborsky M, Balshine S. 2004. Dispersal patterns and status change in a co-operatively breeding cichlid Neolamprologus pulcher: evidence from microsatellite analyses and behavioural observations. J. Fish Biol. 65, 91–105 10.1111/j.0022-1112.2004.00427.x (doi:10.1111/j.0022-1112.2004.00427.x) [DOI] [Google Scholar]
- 34.Heg D, Bender N, Hamilton I. 2004. Strategic growth decisions in helper cichlids. Proc. R. Soc. Lond. B 271, S505–S508 10.1098/rsbl.2004.0232 (doi:10.1098/rsbl.2004.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aubin-Horth N, Deschenes M, Cloutier S. 2012. Natural variation in the molecular stress network correlates with a behavioural syndrome. Horm. Behav. 61, 140–146 10.1016/j.yhbeh.2011.11.008 (doi:10.1016/j.yhbeh.2011.11.008) [DOI] [PubMed] [Google Scholar]
- 36.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. 10.1093/nar/29.9.e45 (doi:10.1093/nar/29.9.e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joels M, Karst H, DeRijk R, de Kloet ER. 2008. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 31, 1–7 10.1016/j.tins.2007.10.005 (doi:10.1016/j.tins.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B 57, 289–300 [Google Scholar]
- 39.Yao M, Denver RJ. 2007. Regulation of vertebrate corticotropin-releasing factor genes. Gen. Comp. Endocrinol. 153, 200–216 10.1016/j.ygcen.2007.01.046 (doi:10.1016/j.ygcen.2007.01.046) [DOI] [PubMed] [Google Scholar]
- 40.Korosi A, Baram TZ. 2008. The central corticotropin releasing factor system during development and adulthood. Eur. J. Pharmacol. 583, 204–214 10.1016/j.ejphar.2007.11.066 (doi:10.1016/j.ejphar.2007.11.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curley JP, Jensen CL, Mashoodh R, Champagne FA. 2011. Social influences on neurobiology and behavior: epigenetic effects during development. Psychoneuroendocrinology 36 , 352–371 10.1016/j.psyneuen.2010.06.005 (doi:10.1016/j.psyneuen.2010.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. 1998. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301 10.1210/er.19.3.269 (doi:10.1210/er.19.3.269) [DOI] [PubMed] [Google Scholar]
- 43.Gass P, Reichardt HM, Strekalova T, Henn F, Tronche F. 2001. Mice with targeted mutations of glucocorticoid and mineralocorticoid receptors: models for depression and anxiety? Physiol. Behav. 73, 811–825 10.1016/S0031-9384(01)00518-2 (doi:10.1016/S0031-9384(01)00518-2) [DOI] [PubMed] [Google Scholar]
- 44.Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. 2010. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci. Biobehav. Rev. 34, 853–866 10.1016/j.neubiorev.2009.07.006 (doi:10.1016/j.neubiorev.2009.07.006) [DOI] [PubMed] [Google Scholar]
- 45.Borski RJ, Hyde GN, Fruchtman S, Tsai WS. 2001. Cortisol suppresses prolactin release through a non-genomic mechanism involving interactions with the plasma membrane. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 129, 533–541 10.1016/S1096-4959(01)00358-X (doi:10.1016/S1096-4959(01)00358-X) [DOI] [PubMed] [Google Scholar]