Abstract
The pygmy right whale, Caperea marginata, is the most enigmatic of the living baleen whales (Mysticeti). Its highly disparate morphology and the virtual absence of a described fossil record have made it extremely difficult to place Caperea into a broader evolutionary context, and molecular and morphological studies have frequently contradicted each other as to the origins and phylogenetic relationships of the species. Our study of a wealth of material from New Zealand collections, representing a wide range of ontogenetic stages, has identified several new features previously unreported in Caperea, which suggest that the pygmy right whale may be the last survivor of the supposedly extinct family Cetotheriidae. This hypothesis is corroborated by both morphology-based and total evidence cladistic analyses, including 166 morphological characters and 23 taxa, representing all the living and extinct families of toothless baleen whales. Our results allow us to formally refer Caperea to Cetotheriidae, thus resurrecting the latter from extinction and helping to clarify the origins of a long-problematic living species.
Keywords: pygmy right whale, Caperea marginata, Cetotheriidae, baleen whales, taxonomy, phylogenetics
1. Introduction
The pygmy right whale, Caperea marginata, is the smallest, most cryptic and least known of the living baleen whales (Mysticeti). Little is known of Caperea in terms of musculoskeletal functional systems, feeding and social behaviour, and distribution in relation to oceanic conditions [1]. Caperea thus contrasts sharply with other living species of baleen whales (in families Balaenidae, Eschrichtiidae and Balaenopteridae), whose biology is much better known from direct observation of living, stranded and captured individuals. Infrequent strandings of mainly single Caperea, and the dearth of sightings, suggest cryptic and semi-solitary habits around the Southern Ocean, yet large pelagic clusters of individuals have been reported [1]. Early records of the species were based on beach-cast fragments in the mid-1800s, producing a complex history of names that led to the present use of C. marginata [2,3]. From the beginning, the pygmy right whale has been known for its unusual skeletal form (figure 1) [4], and indeed its skeletal disparity explains why the species is the sole member of one (Neobalaenidae) of the four families of living mysticetes [2,4–6].
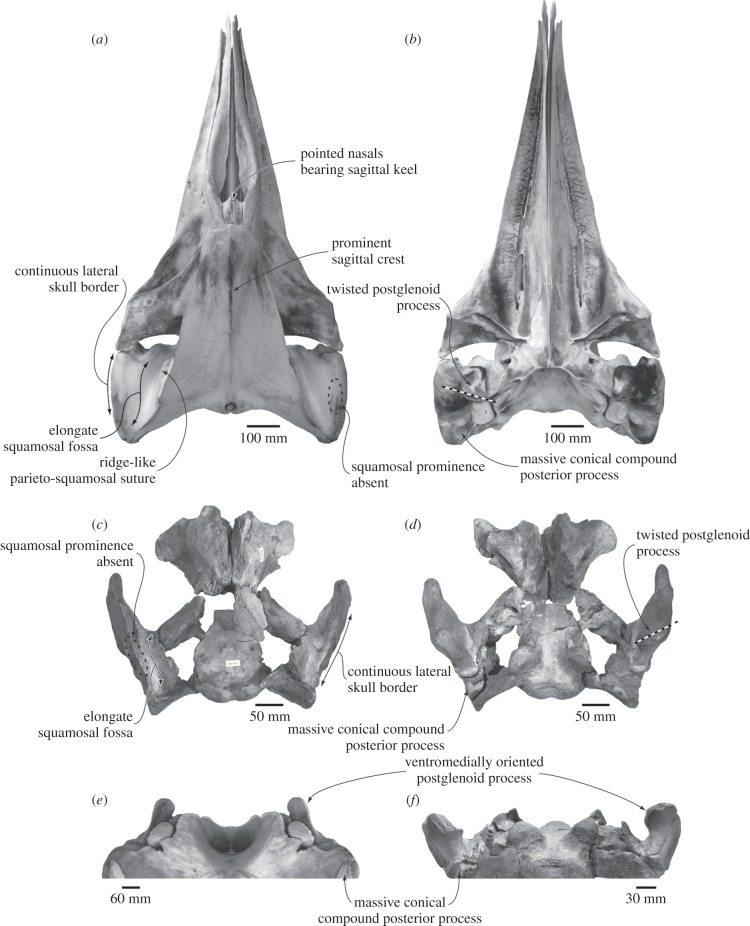
Figure 1.
Comparison and shared features of the skulls of (a,b,e) the living pygmy right whale Caperea marginata (Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand, MM002235) and (c,d,f) the extinct cetothere Herpetocetus transatlanticus (United States National Museum of Natural History, Washington DC, USA, 182962) in (a,c) dorsal, (b,d) ventral and (e,f) posterior views.
In spite of its common name, the pygmy right whale differs from right whales (Balaenidae) in its external form and osteological features in all parts of the skeleton [1,4]. Indeed, despite concentrated recent research on the phylogenetics of mysticetes [7–15], the evolutionary relationships of Caperea have remained largely elusive. Molecular and morphological studies have regularly championed markedly different hypotheses, allying Caperea either with rorquals (Balaenopteridae) on the basis of molecular evidence [9–11], or right whales on the basis of morphology [7,8,12–14]. Controversy over relationships has been compounded by a lack of published reports of unambiguous fossil neobalaenids (which might otherwise provide hard evidence of ancient evolutionary history) [16–18], the relative scarcity of specimens of Caperea in Northern Hemisphere natural history collections, and what we interpret as the likely evolutionary convergence of the feeding apparatus with that of right whales.
The wealth of material housed in New Zealand collections, ranging from neonate and stillborn calves to mature individuals, provides an opportunity to amend this situation. Detailed observations and comparisons revealed a series of ontogenetically variable and phylogenetically significant features previously unreported in Caperea (figures 1 and 2; see also the electronic supplementary material), including some in the feeding apparatus (the juvenile presence of a well-developed, parallel-sided ascending process of the maxilla, and a well-developed, long and low coronoid process on the mandible) and in the ear region (a reduced caudal tympanic process and the presence of a large, hypertrophied, shelf-like lateral tuberosity of the periotic). Among mysticetes, Caperea shares the latter two ear features exclusively with members of the fossil subfamily Herpetocetinae [19], in turn a subdivision of the reportedly extinct Cetotheriidae sensu stricto [7,8,15] (hereafter, Cetotheriidae is used in this sense). Interestingly, these observations add to a range of other similarities between Caperea and cetotheres, including the presence of a hypertrophied, conical compound posterior process of the tympanoperiotic, a poorly developed conical process of the tympanic bulla, pointed nasals and a well-developed sagittal crest on the supraoccipital, thus hinting at the entirely novel possibility of a an evolutionary relationship between these superficially different taxa. In this study, we test whether these similarities might indicate an evolutionary link between Caperea and cetotheres, by assessing them within a broader phylogenetic framework.
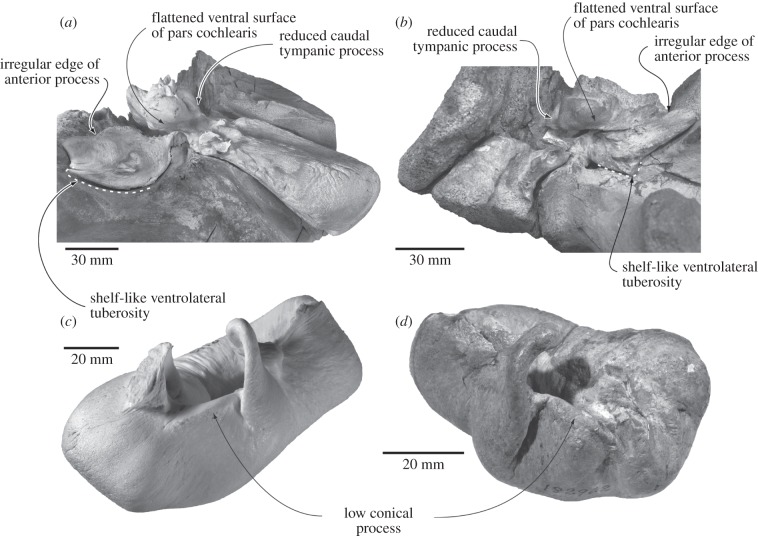
Figure 2.
Comparison and shared features of (a,b) the periotics and (c,d) the bullae of (a,c) C. marginata and (b,d) H. transatlanticus in ventromedial and posterolateral views, respectively. (a) Museum Victoria, Melbourne, Australia, C28531; (c) Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand, MM002119; (b,d) United States National Museum of Natural History, Washington DC, USA, 182962.
2. Material and methods
We constructed an illustrated morphological data matrix of 23 taxa and 166 unordered osteological characters (MorphoBank project 578), drawing on several previously published analyses [7–9,12–15]. To test the effect of morphological versus molecular data, we combined our dataset with the molecular dataset of McGowen et al. [10]. Taxa were chosen so as to represent all major mysticete families and cover as much of their temporal range as possible, ranging from archaic, via relatively recently extinct, to extant. Thus, in addition to a well-sampled Cetotheriidae, our matrix includes living representatives of all of the extant families, as well as the oldest undisputed balaenid (Morenocetus parvus [20]) two of the oldest known balaenopterids (‘Megaptera’ miocaena [21] and Plesiobalaenoptera quarantellii [22]) and a range of other balaenids and balaenopteroids. The extinct archaeocete Zygorhiza kochii and the extant sperm whale Physeter macrocephalus were chosen as outgroups.
We performed two separate analyses: (i) a parsimony-based analysis of the morphological data only; and (ii) a Bayesian total evidence analysis including both molecular and morphological data. For the morphological analysis, branch support was assessed through Bremer support values [23], as well as symmetric resampling (2000 replicates), recorded as GC values [24]. Full details of the cladistic methodology employed here are provided in the electronic supplementary material.
3. Results
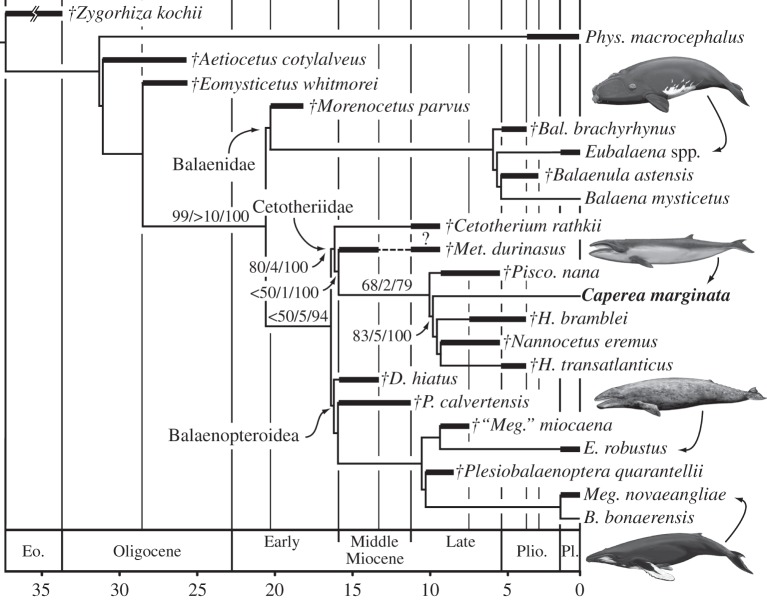
The results of the two analyses were similar (figure 3; electronic supplementary material), and confirmed previous molecular studies in finding Caperea to form a well-supported clade with balaenopterids and eschrichtiids, rather than with balaenids (right whales). In terms of morphology, this solution was seven steps shorter than one enforcing the long-held morphological view of a clade comprising Caperea and balaenids [6–8,12–14]. Living mysticetes fell into three mostly well-supported clades: (i) a monophyletic Balaenidae; (ii) balaenopteroids, including several hitherto supposed ‘cetotheres’ [7,19], Eschrichtius robustus and the living balaenopterids; and (iii) a monophyletic Cetotheriidae, including C. marginata. Within cetotheres, Caperea was most closely related to a herpetocetine clade [8,19], comprising Herpetocetus and Nannocetus.
Figure 3.
The evolutionary relationships of C. marginata based on the results of the parsimony-based morphological and Bayesian total evidence analyses. The tree shown represents the single tree recovered by the parsimony-based analysis of the morphological data only (426 steps, CI = 0.509, RI = 0.655). The tree recovered by the Bayesian analysis was very similar to the one shown here, and differed only in the detailed intra-relationships of the balaenids and balaenopterids, as well as a slightly lower degree of resolution (see the electronic supplementary material). Branch support represents GC values arising from symmetric resampling [24] and Bremer support values [23], as well as posterior probabilities arising from the Bayesian analysis (see the electronic supplementary material for full details). Stratigraphic ranges are based on Uhen [18] and additional information provided in the electronic supplementary material, and should be interpreted as conservative range estimates, rather than actually demonstrated ranges. B., Balaenoptera; Bal., Balaenella; D., Diorocetus; E., Eschrichtius; H., Herpetocetus; Meg., Megaptera; Met., Metopocetus; P., Pelocetus; Pisco., Piscobalaena; Phys., Physeter. Drawings of living mysticetes by Carl Buell. Daggers indicate extinct species.
Caperea unequivocally shares with herpetocetines a continuous lateral skull border (char. 42); a triangular and ventromedially oriented postglenoid process (chars. 63, 65); a postglenoid process located in line with the anterior half of the tympanic bulla (char. 77); an irregular, L-shaped anterior edge of the anterior process of the periotic (char. 85); a broad, elongate, shelf-like lateral tuberosity of the periotic articulating with the squamosal (char. 92); a flattened ventral surface of the pars cochlearis (char. 94); a greatly reduced caudal tympanic process of the periotic (char. 98); and a low conical process of the tympanic bulla (char. 120) (figures 1 and 2). Caperea, furthermore, shares with both Piscobalaena and herpetocetines the presence of anteriorly pointed and sagittally keeled nasals (chars. 38, 39), and with all cetotheres except Cetotherium the presence of a strikingly conical, plug-like compound posterior process of the tympanoperiotic (char. 110). Finally, Caperea shares with all cetotheres the presence of a ridge-like parieto-squamosal suture (char. 59; reversed in Herpetocetus), as well as a posterior process of the tympanoperiotic broadly exposed on the lateral skull wall (char. 111).
Additional synapomorphies of either of the former two clades are ambiguous owing to the non-preservation of the relevant parts of the skull in Metopocetus, and include the absence of a squamosal prominence (char. 60); an anteroposteriorly elongate squamosal fossa (char. 62); the presence of a well-developed sagittal crest of the supraoccipital (char. 70); a straight, rather than ventrally bowed tympanic sulcus (char. 131); and broad, plate-like transverse processes of the lumbar vertebrae (char. 155). Finally, the presence of a parallel-sided ascending process of the maxilla (char. 13) and a twisted postglenoid process (char. 67) may also support either Cetotheriidae as a whole, or the clade including Caperea, Piscobalaena and herpetocetines, but the optimization of these features is ambiguous owing to their apparent absence in Metopocetus.
Based on these shared features and our cladistic analysis, we therefore refer C. marginata to Cetotheriidae, retaining Neobalaenidae as a subfamily in recognition of the disparate morphology of Caperea relative to other cetotheres:
Cetacea Brisson, 1762
Mysticeti Gray, 1864
Cetotheriidae Brandt, 1872; resurrected from extinction
Neobalaeninae Gray, 1873; new rank.
4. Discussion and conclusions
Our results for the first time place Caperea conclusively in a broader evolutionary framework and resolve several problems that have vexed the systematics of this whale to date. Previous morphological analyses [7,8,12–14] emphasized anatomical similarities of Caperea with right whales (Balaenidae). Such comparisons contradict molecular interpretations, and further, in the absence of almost any Caperea-like fossils (but see [16]), imply a surprisingly long ghost lineage for Caperea from the earliest Miocene, at least 20 Ma (based on the oldest described balaenid, M. parvus) [20]. Molecular results have also pointed to an early divergence date for Caperea, Miocene–Oligocene in the range of 17.6–26 Ma [10,11] but, unsurprisingly, have not identified likely fossil relatives of the pygmy right whale. Our analysis now resolves all these issues by (i) reconciling morphological and molecular phylogenies; (ii) identifying cetotheres as previously unrecognized fossil relatives (including sister taxa) of Caperea; and (iii) closing much of the gap between the living pygmy right whale and its assumed early divergence during the Early Miocene or Late Oligocene.
The oldest reliable records of the Caperea–cetotheriid clade are Cetotherium rathkii and Joumocetus shimizui, both probably dating to the Early Tortonian (9.4–11.6 Ma; see the electronic supplementary material) [25,26]. Metopocetus durinasus may be even older, dating to the Langhian (13.8–16 Ma), but there is some uncertainty as to which formation the only known specimen was originally retrieved from (electronic supplementary material). A further specimen (aff. Herpetocetus) dating to the Langhian has been reported from northern Baja California, Mexico, but has yet to be described [27]. The reported Langhian records of Cetotheriidae fall within the 95 per cent highest posterior distribution ranges of some recent molecular clock studies [10], despite being at least 2–10 Myr younger than all previously proposed molecular divergence estimates for Caperea. Because both M. durinasus and Herpetocetus show several disparate and derived characters, such as a conical compound posterior process of the tympanoperiotic, we expect that Cetotheriidae had a substantial earlier history, and that new finds will extend the record of cetotheres closer to the estimated molecular split of Caperea from other living baleen whales.
Within Cetotheriidae, the oldest occurrence of Nannocetus eremus from the Late Miocene (approx. 9 Ma) of California (electronic supplementary material) provides a minimum age estimate for the divergence of Caperea from other cetotheres (figure 3). The virtual absence of described Caperea-like fossils (but see [16]) from the otherwise relatively well-sampled interval of 0–9 Ma could reflect a largely austral distribution of the clade, as for the living pygmy right whale. Compared with the Northern Hemisphere, the austral record of Late Miocene and Pliocene cetaceans is extremely patchy, and mainly limited to fossils from South America [7,12,18]. We therefore expect that fossil relatives of Caperea will be found through increased sampling of the Neogene of South America, Africa and Australasia.
Despite the similarities highlighted by our study, a morphological gulf remains between Caperea and other cetotheriids, which is likely to be related to fundamentally different feeding strategies. It is not inconceivable that the larger size and superficially balaenid-like skull architecture of Caperea may have helped it to survive the Pliocene demise of the last of its cetothere relatives. Given the patchy fossil record, there is presently no evidence as to when the ecomorphological divergence of neobalaenines from other cetotheriids is likely to have started, or indeed what might have driven it. Insight from the living species, such as the recent discovery of visual pigment-associated deep-diving behaviour in Caperea [28], might hold potential clues, but any further analyses of these issues will have to await the description of more informative fossil material.
Acknowledgements
We thank Erich Fitzgerald and Mark Uhen for their insightful reviews, which greatly improved the paper; Nicholas Pyenson, Robert Boessenecker and Gabriel Aguirre Fernández for discussions and comments on an earlier version of the paper; Mónica Buono, Pavel Gol'din, David Janiger, Constantine Tarasenko, Mark Bosselaers, Erich Fitzgerald, Chiara Sorbini and Giovanni Bianucci for providing photographs; Anton Van Helden, Emma Burns, Albert Sanders, Giovanni Bianucci, David Bohaska, James Mead, Charles Potter, Nicholas Pyenson, Tadasu Yamada, Marie Cecile van de Wiel, Pat Holroyd, Mark Goodwin, Christian de Muizon, Peter Howlett and Gianluca Raineri for access to collections and allowing photography; and Carl Buell for providing illustrations of living mysticetes. F.G.M. was supported by a University of Otago Postgraduate Scholarship and Publication Bursary, as well as the Geoscience Society of New Zealand Wellman Research Award, the Systematics Association/Linnean Society of London Systematic Research Fund, a Scottish Association for Marine Science Research Bursary and the Paleontological Society Stephen Jay Gould Award.
References
- 1.Kemper CM. 2009. Pygmy right whale Caperea marginata. In Encyclopedia of marine mammals, 2nd edn (eds Perrin WF, Würsig B, Thewissen JGM.), pp. 939–941 Burlington, IA: Academic Press [Google Scholar]
- 2.Gray JE. 1873. Remarks on some of the species in the forgoing paper. Ann. Mag. Nat. Hist. ser. 4 11, 107–112 [Google Scholar]
- 3.Rice DW. 1998. Marine mammals of the world. Soc. Mar. Mamm. Spec. Publ. 4, 1–231 [Google Scholar]
- 4.Beddard FE. 1901. Contribution towards a knowledge of the osteology of the pigmy whale (Neobalaena marginata). Trans. Zool. Soc. Lond. 16, 87–110 10.1111/j.1096-3642.1901.tb00027.x (doi:10.1111/j.1096-3642.1901.tb00027.x) [DOI] [Google Scholar]
- 5.Miller GS., Jr 1923. The telescoping of the cetacean skull. Smithson. Miscellaneous Collect. 76, 1–70 [Google Scholar]
- 6.Barnes LG, McLeod SA. 1984. The fossil record and phyletic relationships of gray whales. In The gray whale Eschrichtius robustus (eds Jones ML, Swartz SJ, Leatherwood S.), pp. 3–32 New York, NY: Academic Press [Google Scholar]
- 7.Bouetel V, de Muizon C. 2006. The anatomy and relationships of Piscobalaena nana (Cetacea, Mysticeti), a Cetotheriidae s.s. from the early Pliocene of Peru. Geodiversitas 28, 319–395 [Google Scholar]
- 8.Steeman ME. 2007. Cladistic analysis and a revised classification of fossil and recent mysticetes. Zool. J. Linn. Soc. 150, 875–894 10.1111/j.1096-3642.2007.00313.x (doi:10.1111/j.1096-3642.2007.00313.x) [DOI] [Google Scholar]
- 9.Deméré TA, McGowen MR, Berta A, Gatesy J. 2008. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Syst. Biol. 57, 15–37 10.1080/10635150701884632 (doi:10.1080/10635150701884632) [DOI] [PubMed] [Google Scholar]
- 10.McGowen MR, Spaulding M, Gatesy J. 2009. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Mol. Phylogenet. Evol. 53, 891–906 10.1016/j.ympev.2009.08.018 (doi:10.1016/j.ympev.2009.08.018) [DOI] [PubMed] [Google Scholar]
- 11.Steeman ME, et al. 2009. Radiation of extant cetacean driven by restructuring of the oceans. Syst. Biol. 58, 573–585 10.1093/sysbio/syp060 (doi:10.1093/sysbio/syp060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisconti M. 2010. New description of ‘Megaptera’ hubachi Dathe, 1983 based on the holotype skeleton held in the Museum für Naturkunde, Berlin. Quad. Mus. Stor. Nat. Livorno 23, 37–68 [Google Scholar]
- 13.Churchill M, Berta A, Deméré TA. 2011. The systematics of right whales (Mysticeti: Balaenidae). Mar. Mamm. Sci. 28, 497–521 10.1111/j.1748-7692.2011.00504.x (doi:10.1111/j.1748-7692.2011.00504.x) [DOI] [Google Scholar]
- 14.Ekdale EG, Berta A, Deméré TA. 2011. The comparative osteology of the petrotympanic complex (ear region) of extant baleen whales (Cetacea: Mysticeti). PLoS ONE 6, e21311. 10.1371/journal.pone.0021311 (doi:10.1371/journal.pone.0021311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx FG. 2011. The more the merrier? A large cladistic analysis of mysticetes, and comments on the transition from teeth to baleen. J. Mamm. Evol. 18, 77–100 10.1007/s10914-010-9148-4 (doi:10.1007/s10914-010-9148-4) [DOI] [Google Scholar]
- 16.Fitzgerald EMG. 2012. Possible neobalaenid from the Miocene of Australia implies a long evolutionary history for the pygmy right whale Caperea marginata (Cetacea, Mysticeti). J. Vertebr. Paleontol. 32, 976–980 10.1080/02724634.2012.669803 (doi:10.1080/02724634.2012.669803) [DOI] [Google Scholar]
- 17.Graf J, Jacobs L, Polcyn M, Mateus O, Schulp A. 2011. New fossil whales from Angola. J. Vertebr . Paleontol. Abstracts 119 [Google Scholar]
- 18.Uhen MD. 2012. Cetacea. Paleobiology Database Online Systematics Archive 9. See www.pbdb.org
- 19.Whitmore FC, Jr, Barnes LG. 2008. The Herpetocetinae, a new subfamily of extinct baleen whales (Mammalia, Cetacea, Cetotheriidae). Virginia Mus. Nat. Hist. Spec. Publ. 14, 141–180 [Google Scholar]
- 20.Cabrera A. 1926. Cetáceos fósiles del Museo de La Plata. Rev. Mus. La Plata 29, 363–411 [Google Scholar]
- 21.Kellogg AR. 1922. Description of the skull of Megaptera miocaena, a fossil humpback whale from the Miocene diatomaceous earth of Lompoc, California. Proc. USA Natl Mus. 61, 1–18 10.5479/si.00963801.61-2435.1 (doi:10.5479/si.00963801.61-2435.1) [DOI] [Google Scholar]
- 22.Bisconti M. 2010. A new balaenopterid whale from the Late Miocene of the Stirone River, northern Italy (Mammalia, Cetacea, Mysticeti). J. Vertebr. Paleontol. 30, 943–958 10.1080/02724631003762922 (doi:10.1080/02724631003762922) [DOI] [Google Scholar]
- 23.Bremer K. 1994. Branch support and tree stability. Cladistics 10, 295–304 10.1111/j.1096-0031.1994.tb00179.x (doi:10.1111/j.1096-0031.1994.tb00179.x) [DOI] [Google Scholar]
- 24.Goloboff PA, Farris JS, Kallersj M, Oxleman B, Ramirez MJ, Szumik CA. 2003. Improvements to resampling measures of group support. Cladistics 19, 324–332 10.1111/j.1096-0031.2003.tb00376.x (doi:10.1111/j.1096-0031.2003.tb00376.x) [DOI] [Google Scholar]
- 25.Brandt JF. 1873. Untersuchungen über die fossilen und subfossilen Cetaceen Europas. Mém. Acad. Imp. Sci. St. Pétersbourg. 20, 1–371 [Google Scholar]
- 26.Kimura T, Hasegawa Y. 2010. A new baleen whale (Mysticeti: Cetotheriidae) from the earliest late Miocene of Japan and a reconsideration of the phylogeny of cetotheres. J. Vertebr. Paleontol. 30, 577–591 10.1080/02724631003621912 (doi:10.1080/02724631003621912) [DOI] [Google Scholar]
- 27.Barnes LG. 1998. The sequence of fossil marine mammal assemblages. In Avances en investigacion: paleontología de vertebrados, vol. 1 (eds México O, Córdaba-Méndez DA.), pp. 26–79 Pachuca, Mexico: Universidad Autónoma des Estado de Hidalgo [Google Scholar]
- 28.Bischoff N, Nickle B, Cronin TW, Velasquez S, Fasick JI. 2012. Deep-sea and pelagic rod visual pigments identified in the mysticete whales. Vis. Neurosci. 29, 95–103 10.1017/S0952523812000107 (doi:10.1017/S0952523812000107) [DOI] [PubMed] [Google Scholar]