Abstract
Independent or convergent evolution can underlie phenotypic similarity of derived behavioural characters. Determining the underlying neural and neuromuscular mechanisms sheds light on how these characters arose. One example of evolutionarily derived characters is a temporally simple advertisement call of male African clawed frogs (Xenopus) that arose at least twice independently from a more complex ancestral pattern. How did simplification occur in the vocal circuit? To distinguish shared from divergent mechanisms, we examined activity from the calling brain and vocal organ (larynx) in two species that independently evolved simplified calls. We find that each species uses distinct neural and neuromuscular strategies to produce the simplified calls. Isolated Xenopus borealis brains produce fictive vocal patterns that match temporal patterns of actual male calls; the larynx converts nerve activity faithfully into muscle contractions and single clicks. In contrast, fictive patterns from isolated Xenopus boumbaensis brains are short bursts of nerve activity; the isolated larynx requires stimulus bursts to produce a single click of sound. Thus, unlike X. borealis, the output of the X. boumbaensis hindbrain vocal pattern generator is an ancestral burst-type pattern, transformed by the larynx into single clicks. Temporally simple advertisement calls in genetically distant species of Xenopus have thus arisen independently via reconfigurations of central and peripheral vocal neuroeffectors.
Keywords: frog, vocalization, neural circuits, evolution
1. Introduction
Phylogenetic surveys of communication signals characteristically reveal marked diversity in both signal form and complexity [1–6]. Given historically imposed morphological and physiological constraints on the neural and muscular systems that generate those signals [7–9], how does this diversity arise? The advertisement calls of male African clawed frogs (Xenopus and Silurana) provide an opportunity to explore the evolution of vocal signals at the neural and neuromuscular levels. The advantages of this group include a robust phylogeny [10,11] onto which the acoustic features of calls have been mapped [12]. In addition, the neural and neuromuscular mechanisms that underlie vocal production can be dissected in ex vivo preparations [13,14].
Xenopus and Silurana males produce courtship songs with species-specific temporal and spectral features [12]. The basic unit for all such advertisement calls is a brief click, produced when muscles of the vocal organ (larynx) contract in response to nerve activity [14,15]. Male advertisement calls sort into one of four temporal patterns: single clicks, short bursts (2–14 clicks per burst), longer trills (43–127 clicks per trill) or temporally complex biphasic patterns (figure 1a) [12]. Click-type calls are the least temporally complex and the rarest call type, present in just three currently described species: Xenopus borealis, Xenopus new tetraploid and Xenopus boumbaensis (figure 1b). The first two species share a most recent common ancestor, but X. boumbaensis resides in a different species group, suggesting that click-type calls arose at least twice. A parsimony analysis of changes in character state suggests that the click-type call is derived from a moderately complex, burst-type ancestral call (figure 1b) [12]. Thus, click-type calls in Xenopus allow us to investigate evolutionary trajectories leading to derived, temporally simplified behaviours.
Figure 1.
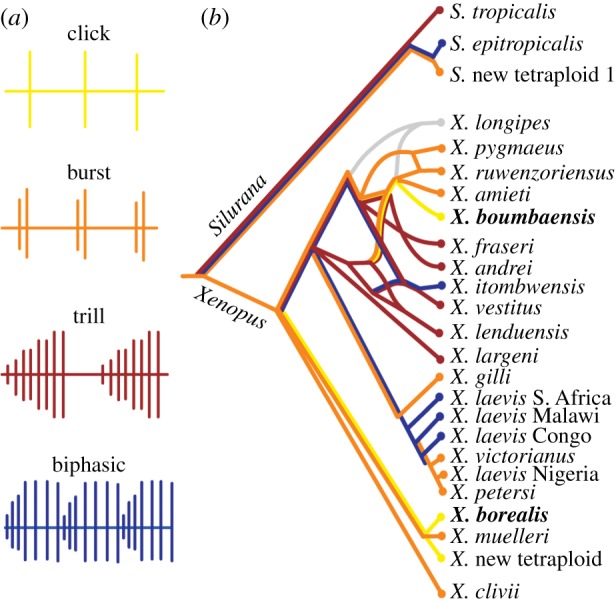
The vocal phylogeny of Xenopus (adapted from Tobias et al. [12]). (a) Xenopus advertisement calls can be classified as one of four types: click (yellow), burst (orange), trill (red) and biphasic (blue), based on the number of clicks per call, interval between calls, intensity modulation of clicks within a call and number of phases in a call. (b) Call types mapped onto the Xenopus phylogeny (colour scheme as in panel (a); grey indicates no data). Reticulation in the phylogeny (e.g. in the species group containing X. boumbaensis) reflects past instances of speciation via hybridization [10,11]. Maximum parsimony analysis suggests that the ancestral call type is a burst, and the simplified click call type is evolutionarily derived [12]. Xenopus borealis and X. boumbaensis (in bold) are two distantly related click-type callers examined in this study.
The neural and neuromuscular mechanisms of calling have been well studied in Xenopus laevis, which has a complex, biphasic advertisement call. In vivo activity recorded from the laryngeal motor nerve during advertisement calling reveals highly synchronized compound action potentials (CAPs), each corresponding to a single click of sound [16]. Mechanisms of call generation can be studied using two informative ex vivo preparations: the isolated brain (which produces fictive calls in the presence of serotonin) and the isolated larynx (which produces sound clicks in response to laryngeal nerve stimulation). Fictive calls recorded from the laryngeal nerve in the isolated brain are composed of CAPs whose temporal patterns match both calls and CAPs recorded in vivo during calling [13,16]. The isolated larynx produces clicks when the laryngeal nerves are stimulated at call-like tempos. Clicks are preceded by muscle contractions that produce tension transients on the tendon inserting into the arytaenoid discs, the sound-initiating components of the larynx [14,15]. While these preparations have informed the neuromuscular bases of sex differences in vocal signalling within a single species, very little is known about how the hindbrain circuit and larynx have been modified to allow for species-specific calls of varying temporal complexity.
The goal of this study was to ask how—at a physiological level—simple click-type calls evolved from a more complex call type. Is the apparently independent appearance of click-type calls in X. borealis and X. boumbaensis owing to shared or distinct neuromuscular mechanisms? One possible scenario is that the vocal pattern generated in the hindbrain was simplified from an ancestral burst-type pattern, and the larynx faithfully converts this simple pattern into a click-type call. Alternatively, the hindbrain may have continued to produce a more complicated, ‘burst-type’ neural pattern, but the larynx converts this pattern into single clicks of sound. To distinguish between these possibilities, we evoked fictive calling from the isolated brain and muscle activity and sounds from the larynx in males of both species.
2. Material and methods
(a). Animals
Sexually mature male X. borealis (n = 15; mean±s.e.m. 18.04±1.10 g) and X. boumbaensis (n = 13; 5.03±0.29 g) were purchased from Xenopus Express (Brooksville, FL) or obtained from the Kelley laboratory Xenopus colony (X. boumbaensis were descendants of a collection originating at the University of Geneva). Animals were group housed in polycarbonate tanks containing 10 l of filtered water, changed twice weekly, and fed frog brittle (Nasco) at least 3 h in advance of changing. Animals were maintained at 18°C (X. borealis) or 23°C (X. boumbaensis) in 12L : 12D.
(b). Behavioural recordings
Frogs were primed for calling with injections of human chorionic gonadotropin (CG; 100 IU X. borealis; 50 IU X. boumbaensis; Sigma, product CG10-10VL) 24–48 and 6 h prior to recording. Each frog was placed with an unreceptive female conspecific in a glass aquarium (60 × 15 × 30 cm; water depth = 25 cm; temperature = 20–22°C) in a dimly lit room. Vocalizations were recorded with a hydrophone (High Tech, Gulfport, MI; output sensitivity: −164.5 dB at 1 V μPa−1, 127 frequency sensitivity = 0.015–10 kHz) and written to disk with a Marantz CD recorder (CDR300, Mahwah, NJ; 44.1 kHz sampling rate). The call for both species is a single click [12]; inter-call intervals (the time between clicks) were measured with the sound analysis program Signal (Engineering Design, Berkeley, CA) from recordings made during the present study and previously (2004–2008) under the same conditions.
(c). Isolated brain preparation
We adapted the isolated brain preparation [13,17] for X. borealis and X. boumbaensis. Frogs were deeply anaesthetized via subcutaneous injection of 1.2 per cent methanesulphonate (MS-222; Sigma, product A5040; 0.01 ml per g body weight), placed on ice and decapitated. The skull was placed in a Sylgard-lined dish filled with 4°C oxygenated (99% O2, 1% CO2) saline composed of (in mM) 96 NaCl, 20 NaHCO3, 2 CaCl2, 2 KCl, 0.5 MgCl2, 10 HEPES, 11 glucose, at pH 7.8 (modified from Luksch et al. [18]).
The brain was removed, maintained in a Sylgard-lined dish filled with approximately 240 ml of constantly oxygenated saline and brought to 21°C over 1 h. Brain recordings were made in a Sylgard-lined chamber filled with 50 ml of 21°C oxygenated saline, with continuous superfusion. The fourth rootlet of nerve IX–X, whose axons innervate the larynx [19], was isolated and fitted with a suction electrode to record population activity of laryngeal motor neurons. Recordings were amplified 10 000 times (AM-Systems Model 1800; Carlsborg, WA), digitized at 20 kHz (Axon Digidata), and stored using pClamp software (version 9.2; Axon Instruments/Molecular Devices, Sunnyvale, CA). Baseline neural activity was recorded for at least 5 min, during which brains often produced spontaneous, long (approx. 1 s), unpatterned activity, corresponding to fictive breathing [17].
Exogenous serotonin was used to induce fictive calling [13]. Serotonin (Sigma; H-9523) stock solution (30 mM) was prepared before each experiment and kept on ice. Fifty μl of stock was diluted in 950 μl bath saline; this volume was applied to the dish to reach a final serotonin concentration of 30 μM. Saline superfusion was suspended during serotonin application. Fictive calling usually began within 40–60 s of serotonin application. After 5–10 min of serotonin treatment, saline superfusion resumed (>250 ml h−1) to completely wash out the serotonin (volume of dish replaced at least four times). Serotonin application was repeated hourly up to three times throughout the recording.
(d). Analysis of vocal and nerve recordings
Clicks and CAPs were identified in Signal and Clampfit (using the threshold search function, triggered at 3σ noise level), respectively. Mean inter-call (vocal) and inter-CAP (nerve) intervals were calculated from bouts of vocal or nerve activity. First, a mean reference interval was calculated across the first five intervals within the first observed instance of regular activity. Regular activity was defined as a bout of activity in which no interval exceeded twice the length of any other interval in the bout. Subsequent bouts were included in analysis if the mean interval of the bout was within 2σ of the reference bout; this provision excluded extremely long intervals that represented breaks in calling (during vocal recordings) or decay in nerve activity over time (nerve recordings). Finally, the mean for the individual was taken by averaging all bouts included for analysis; the sum of all bouts for an individual included at least seven nerve intervals or 30 vocal intervals. Other features of X. boumbaensis nerve patterns (CAPs/burst, intra-burst interval) were calculated from all intervals, regardless of whether they fell into regular bouts of activity.
Not all animals produced extended bouts of both vocal and nerve activity. We thus made unpaired comparisons between intervals calculated from nerve recordings and vocal recordings from separate populations. A one-way ANOVA with post hoc Bonferroni multiple comparisons tests was used to compare mean intervals across four X. borealis patterns in total: two vocal patterns and two nerve patterns. An unpaired t-test was used to compare intervals between one call pattern and one nerve pattern in X. boumbaensis. For both species, one set of paired behavioural and fictive recordings was obtained from the same individual; the paired sound recordings were not included in the analysis, but were consistent with the larger unpaired dataset. Unless otherwise specified, data are expressed as mean±s.e.m.
(e). Isolated larynx preparation
We adapted the isolated larynx preparation described previously [14]. Larynges were removed from the animal and pinned to a Sylgard-lined dissection dish filled with 4°C oxygenated saline. Larynges were pinned dorsal side up, and connective tissue was cleared, exposing the laryngeal muscle and nerve. In some X. boumbaensis preparations, the lungs were left attached in order to maintain air within the lumen, which enhances the quality of sound production (M. Tobias 2011, personal communication). Recordings took place in an 80 ml Sylgard-lined recording chamber filled with room temperature (21°C) oxygenated saline without superfusion.
The laryngeal nerves were stimulated bilaterally with 0.5 ms square pulses (150 μA), delivered to the laryngeal nerves via glass suction electrodes (as described above) attached to a Grass S8800 Stimulator via stimulus isolation units (Grass Technologies, West Warwick, RI).
Sounds, when produced, were recorded with a small hydrophone (Knowles, Inc., Itasca, IL) placed in the dish. Isometric tension was recorded on the tendon connecting the laryngeal muscle to the sound-producing cartilage discs with a force transducer (model FT03; Grass Technologies) attached to a DC amplifier (model P16; Grass Technologies). Electromyogram (EMG) potentials were recorded with bipolar silver-wire electrodes insulated to the tip and amplified with an extracellular amplifier (AM-Systems Model 1800). All signals were digitized and saved either with a MacLab digitizer and Chart software (v. 3.5/3.6; AD Instruments, Colorado Springs, CO) running on a G3 Macintosh computer or (in most cases) with a DigiData digitizer running pClamp software on a Dell computer.
In each species, we presented stimuli approximating observed laryngeal nerve activity: X. borealis larynges were stimulated with single pulses spaced 450 ms apart and X. boumbaensis larynges were stimulated with bursts of single, doublet and triplet nerve stimuli—15 ms inter-stimulus interval (ISI), 1000 ms inter-burst interval (IBI).
X. boumbaensis isolated larynges did not reliably produce sound when connected to EMG electrodes and the force transducer (n = 5), so we recorded only sound in additional (n = 7) larynges. We delivered bursts of single, doublet and triplet stimuli to these larynges via the laryngeal nerves (five trains of 10 stimuli each; 15 ms ISI; 1000 ms IBI; trains delivered in random order). By progressively lengthening the ISI until no clicks were observed, we determined the range of ISIs required for click production (ISIs ranged from 17 to 300 ms; 1000 ms IBI; blocks of five stimuli; n = 5). Click heights were measured to assess relative changes in sound intensity [16]. When no clicks were produced in response to nerve stimulation, the laryngeal nerves were stimulated with shorter (50 ms) intervals to ensure that the larynges were still capable of producing clicks. All data were analysed with Prism (GraphPad) and deposited in the Dryad Repository (http://dx.doi.org/10.5061/dryad.mn324).
3. Results
Xenopus borealis males produce two calls while actively courting [20]. The advertisement call (figure 2a) consists of single clicks repeated at long inter-click intervals (ICI; this study: 458 ± 24 ms). When males approach and attempt to clasp other frogs they produce an approach call (figure 2b), bouts of 2–29 clicks with a faster ICI (112 ± 5 ms; figure 2b).
Figure 2.
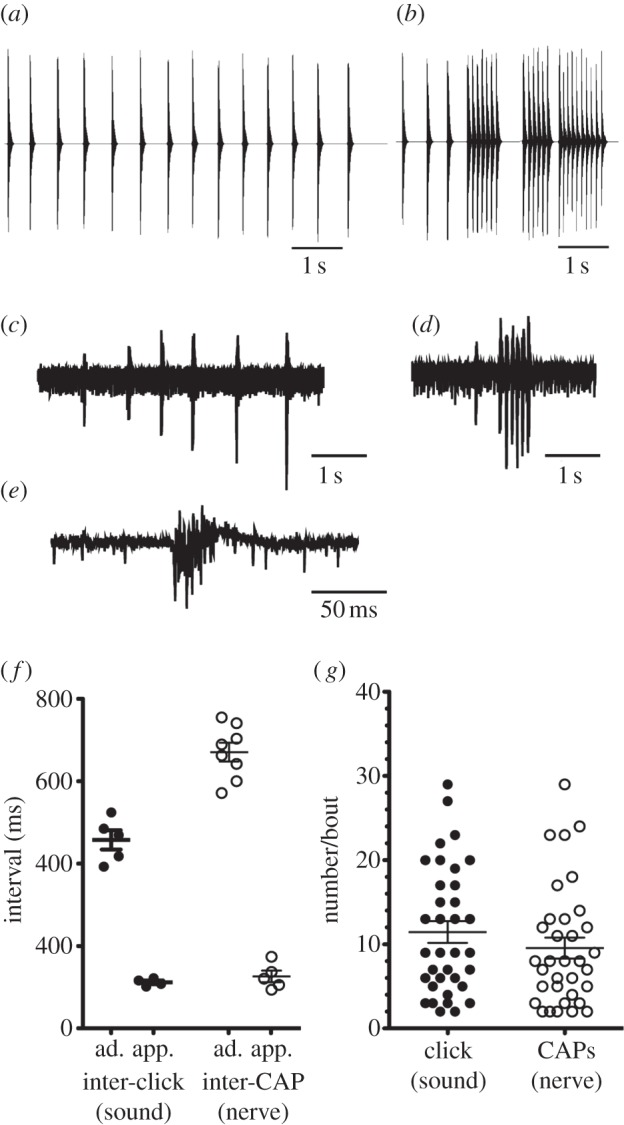
Comparing vocalizations with fictive activity from the isolated X. borealis brain. (a) Waveform of the advertisement call: single clicks repeated at a species-specific interval of approximately 460 ms. (b) Waveform of the approach call: rapid trains of 2–20 clicks at shorter intervals of approximately 112 ms, typically preceded by advertisement calls. (c) Nerve recordings from the isolated X. borealis brain (n = 8) are typically slow trains of CAPs. (d) Trains of CAPs at more rapid intervals, typically preceded by at least one CAP of a longer interval, are less common (n = 5). (e) Enlarged waveform of a single CAP. CAPs are brief (less than 50 ms) and complex, crossing the baseline multiple times. (f) X. borealis advertisement and approach call types can be readily distinguished based on inter-click and inter-CAP interval in both vocal and isolated brain recordings respectively. The rapid pattern of nerve activity is indistinguishable from approach calling, based on interval. The slower pattern of nerve activity is slower than, but resembles, advertisement calling. Each point represents an individual's mean. (g) The number of clicks or CAPs per bout does not differ between approach call and the faster nerve pattern. Each point represents single bouts from a vocal (n = 5) or nerve (n = 5) recording. Horizontal lines represent means; error bars represent s.e.m.
(a). Serotonin-evoked activity in the isolated Xenopus borealis brain
Within 1 minute (48.6±17.9 ms) of serotonin application to the isolated brain, laryngeal nerve activity became highly regular and rhythmic, lasting 1–5 min. Nerve activity comprised a series of CAPs, brief (less than 50 ms) high-frequency events that cross the baseline multiple times (figure 2e).
Two distinct CAP patterns resembled temporal features of calls (figure 2c,d). In one (figure 2c), nerve recordings were made up of slower CAP trains (mean inter-CAP interval of 671±24 ms; n = 8). Five brains (including three that produced the prior pattern) produced a faster pattern: bouts of 2–29 CAPs with a shorter inter-CAP interval (126±14 ms; figure 2d).
(b). Comparing Xenopus borealis neural patterns to vocal patterns
To determine whether these two patterns of nerve activity represent fictive advertisement and approach calling, we compared inter-CAP intervals and ICIs. An ANOVA revealed significant differences in interval across two vocal and two nerve patterns (F3,18 = 176.6, p < 0.001). Post hoc Bonferroni multiple comparisons tests revealed that the mean inter-CAP interval in the slower nerve pattern (670.9±22.9 ms; means taken from 53.6±36.5 intervals per brain; total 429 intervals) was clearly distinguishable from the mean inter-CAP interval in the more rapid pattern (126.3±13.97 ms; t = 19.21, p < 0.05). The inter-CAP interval, however, is longer than the mean advertisement call ICI (457.9 ± 23.55 versus 670.9±22.86 ms; Bonferroni multiple comparisons test; t = 10.37, p < 0.05; figure 2f). Elongated inter-CAP intervals relative to ICIs also characterize fictive advertisement calls recorded from the isolated brain of X. laevis [13]. The inter-CAP intervals (figure 2f) of the more rapid nerve pattern could not be distinguished from the ICIs of approach calling: though slightly longer, the mean inter-CAP interval was not significantly different from the mean approach call ICI (t = 0.43, p > 0.05). The number of CAPs per nerve activity bout also did not differ significantly from the number of clicks per approach calling bout (figure 2g).
(c). Xenopus boumbaensis male vocalizations
The X. boumbaensis advertisement call (figure 3a) is a single click (figure 3b) repeated at a species-specific interval of 1021±305 ms [12].
Figure 3.
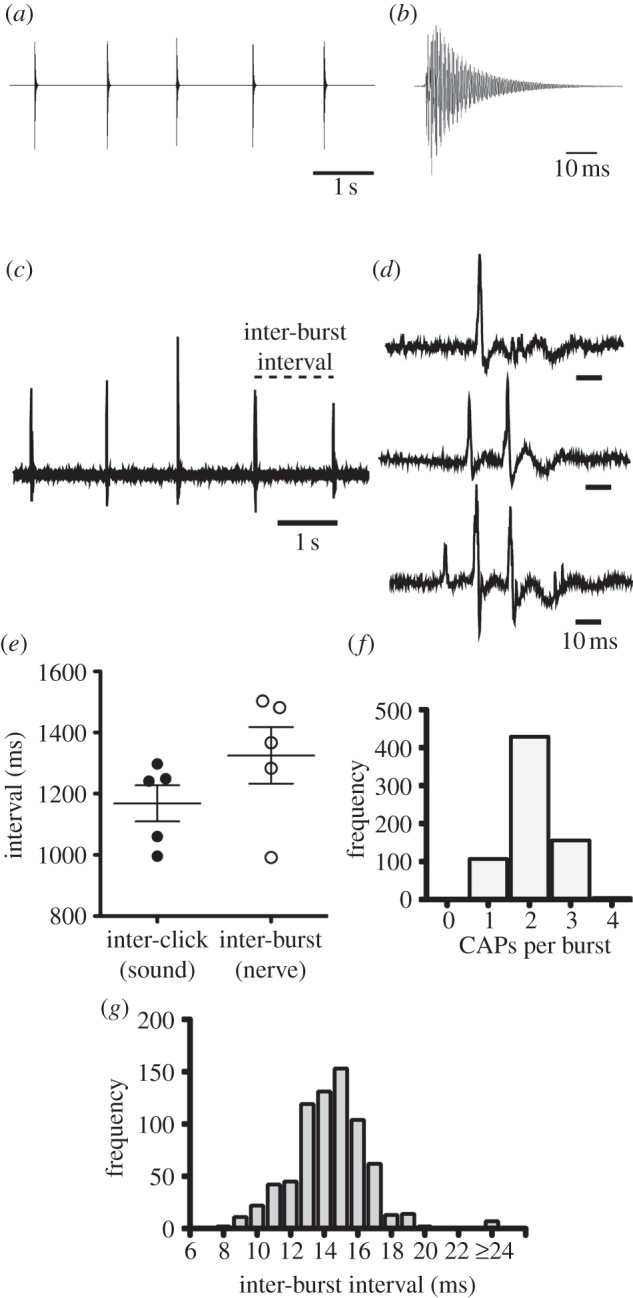
Comparing vocalizations with fictive activity from the X. boumbaensis isolated brain. (a) Waveform of the X. boumbaensis advertisement call: single clicks of sound repeated at approximately 117 ms intervals. (b) Enlarged waveform of a single click; compare this single click with nerve activity in part (d) at the same time scale. (c) X. boumbaensis nerve activity is composed of bursts of CAPs separated by longer inter-burst intervals. (d) Bursts consist of one, two or three CAPs. Quadruplets (not shown) were rare. (e) The mean inter-burst interval recorded from the X. boumbaensis laryngeal nerve in the isolated brain was not significantly different from inter-click intervals from vocal recordings. Each point represents an individual mean. Horizontal lines represent group means, and error bars represent s.e.m. (f) The majority (65%) of all recorded bursts were doublets. (g) Frequency distribution of inter-CAP intervals within all bursts (intra-burst interval) reveals intervals of less than 20 ms (mean 14.4 ± 2.4 (s.d.) ms), which have no behavioural correlate.
(d). Serotonin-evoked activity in the isolated Xenopus boumbaensis brain
Within 8 to 40 s of exogenous serotonin application, X. boumbaensis isolated brains (n = 5) produced periods of regular, rhythmic CAP activity that persisted for 13–500 s (figure 3c). In comparison to X. borealis CAPs, X. boumbaensis CAPs are sharp and short in duration (less than 10 ms) (compare figures 2e with 3e). CAPs occurred in bursts (figure 3c). The predominant form of nerve activity was a CAP doublet (66% of all bursts; figure 3f). Single, triplet and quadruplet CAPs (figure 3d) were less frequent (18%, 15.5% and less than 1%, respectively; figure 3f). The mean (±s.d.) inter-CAP interval within bursts was 14.4±2.4 ms (figure 3g).
Does this pattern of nerve activity resemble the X. boumbaensis advertisement call? Intervals within bursts were very short at 14.4 ms. None of the described X. boumbaensis calls have ICIs that are this short. However, the interval between bursts (figure 3c,d; 1330±92 ms) is not significantly different from mean ICIs of advertisement calls (1169±59 ms; t = 1.474, d.f. = 8, p = 0.18). We conclude that bursts of CAPs underlie each single click in the X. boumbaensis call.
(e). The larynx: peripheral contributions to vocal output
To determine how nerve activity is translated into sound production, we delivered nerve stimulus trains that replicated nerve activity patterns from fictively calling brains. Muscle activity, tension transients from the tendon inserting onto the arytaenoid discs and sounds were recorded from larynges ex vivo.
(f). Xenopus borealis isolated larynx produces clicks in response to single stimuli
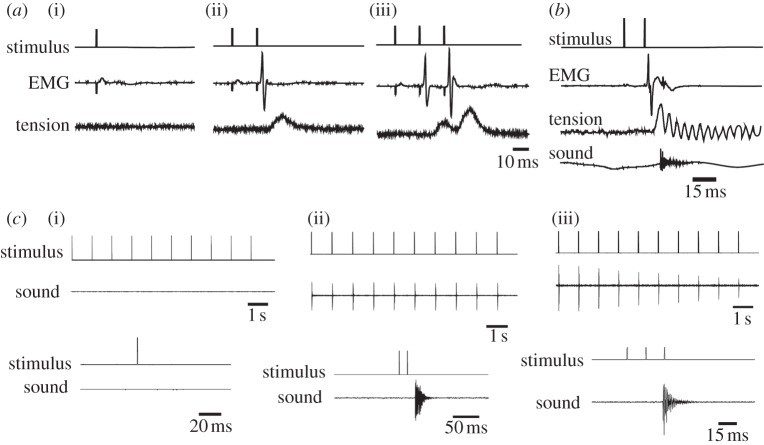
In response to each stimulus in a train, X. borealis larynges (n = 9) produced a single muscle EMG potential and tension transient. In larynges that produced sound (n = 4), a click accompanied each tension transient (figure 4a). Clicks accompanied the rising phase of the tension transient, suggesting that partial shortening of the laryngeal muscle is sufficient for click production (figure 4b).
Figure 4.
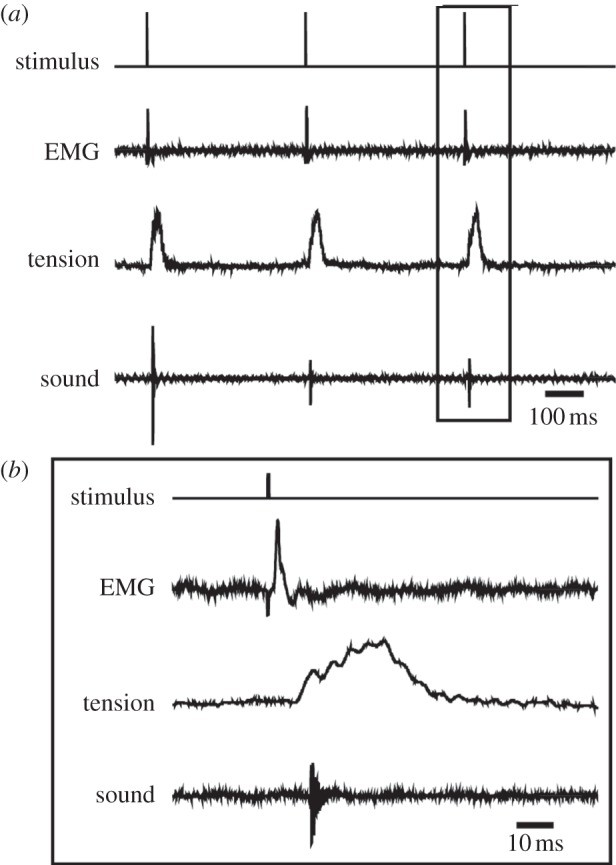
Response of the isolated X. borealis larynx to stimulation. (a) In response to each stimulus (top trace), X. borealis laryngeal muscle produces one EMG potential (second trace) and tension transient (third trace), resulting in one click of sound (bottom trace). (b) Enlarged portion of (a) reveals that sound is produced early in the rising phase of the tension transient.
(g). Xenopus boumbaensis isolated larynx requires doublet stimuli for click production
We recorded EMG, tension and sound in response to single (single stimuli 1000 ms apart), doublet and triplet nerve stimuli (15 ms ISI) in the isolated X. boumbaensis larynx. Single stimuli were ineffective in producing tension transients (n = 5; figure 5a, top). Doublet stimuli produced a potentiated EMG followed by one tension transient (n = 5; figure 5a, middle). Triplet stimuli produced potentiation of the EMG following the second stimulus, resulting in two progressively enhanced tension transients (n = 2; bottom of figure 5a). Most larynges did not produce sound, so the relation between EMG potentiation, tension transient sizes and click production could not be determined systematically. However, in the one larynx that did produce sound (figure 5b), doublet stimuli were sufficient to produce one click.
Figure 5.
Response of the isolated X. boumbaensis larynx to stimulation. (a) In a X. boumbaensis larynx that did not produce sound, (i) single stimuli were not sufficient to produce a tension transient. (ii) Doublet stimuli yielded a single tension transient following EMG potentiation. (iii) Triplet stimuli resulted in EMG potentiation and two tension transients with some maintained tension. (b) In a X. boumbaensis larynx that did produce sound, laryngeal muscle responded to doublet stimuli with a highly potentiating EMG, a single tension transient following the second stimulus, and a single click of sound that occurred during peak muscle tension. Fluctuations at the end of the tension record in this recording were due to continued vibration of the thread attaching the tendon to the force transducer. (c) The isolated X. boumbaensis larynx produces no clicks in response to trains of (i) single stimuli presented 1000 ms apart, but reliably produces one click after each (ii) doublet and (iii) triplet stimulus. Top traces show the whole train of 10 stimuli; bottom traces show enlargements of single, doublet and triplet stimuli.
Because attaching EMG electrodes and the force transducer to X. boumbaensis larynges impeded sound production, we recorded sound alone in additional larynges. Single, doublet and triplet stimulus bursts were presented in blocks of 10 bursts at 1000 ms intervals; five blocks of each type were presented in random order. Doublet and triplet—but not single—stimuli reliably yielded one click of sound (n = 7; figure 5c). Rarely (twice in five of 35 total trials), single stimuli produced small-intensity clicks that were not audible to us (data not shown). In sum, the X. boumbaensis isolated larynx capably converts doublet and triplet—but not single—stimuli into robust tension transients and clicks.
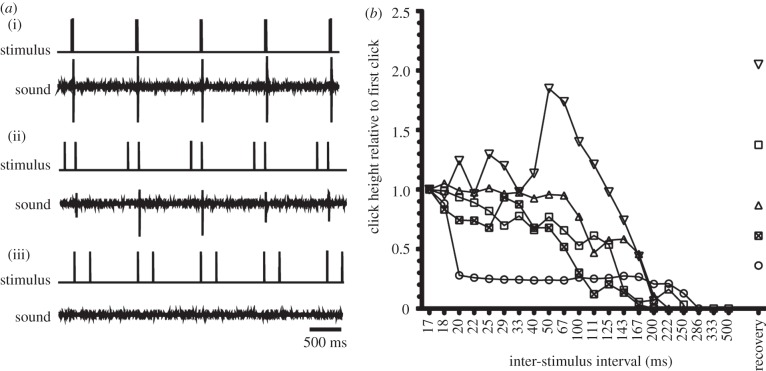
How stringent is the requirement of a 15 ms ISI for click production? We delivered blocks of doublet stimuli containing progressively longer ISIs (n = 5 of seven larynges where only sound was recorded), and measured the resulting clicks (figure 6a). Inter-stimulus intervals above 150 ms (range 167–290 ms) did not result in clicks. Three larynges showed a progressive decrease in click height as ISI increased. One larynx exhibited a sudden decrease in click height; another showed an increase, followed by a decline (figure 6b). We conclude that the larynx is capable of converting doublet stimuli into robust single clicks at intervals between 15 and 100 ms, suggesting that the parameters for potentiation are broad.
Figure 6.
The X. boumbaensis isolated larynx produces clicks in response to stimulus doublets over a range of intervals. (a) An isolated X. boumbaensis larynx produces robust clicks in response to doublet stimuli with a (i) 17 ms ISI, softer clicks in response to doublet stimuli with a (ii) 167 ms ISI, and no clicks in response to doublet stimuli with a (iii) 250 ms ISI. (b) Click amplitude diminishes as ISI increases. Each series represents a larynx mean: mean click height, averaged over the block of five stimuli. The mean height for each block was divided by the mean click height obtained during the first block (17 ms). At the end of the experiment, larynges were stimulated with 50 ms intervals to confirm they were still capable of clicks (‘recovery’).
4. Discussion
The click-type advertisement calls used by X. borealis and X. boumbaensis are temporally simple and evolutionarily derived; a parsimonious interpretation of the phylogeny of call types suggests that click-type calls evolved twice independently from a burst-type call [12]. How was this achieved at the level of the vocal circuit? We entertained two hypotheses: (i) the brain might produce a ‘click-type’ vocal pattern, which would then be faithfully converted into sound by the larynx, or (ii) the larynx might convert a more complex, burst-type vocal pattern into single clicks. Recordings from isolated brains and larynges reveal that X. borealis generates its call via the first mechanism, a click-type nerve pattern and a competent larynx. By contrast, X. boumbaensis generates its call via the second mechanism, a burst-type nerve pattern and laryngeal EMG potentiation.
The nerve pattern of X. boumbaensis comprises small bursts of CAPs (usually doublets) repeated at regular inter-burst intervals. The mean inter-CAP interval (14.4 ms) falls within the range of inter-click intervals of burst-type callers (range = 7–30 ms), whereas the mean inter-burst interval (1300 ms) resembles the inter-burst interval of closely related species, such as X. amieti [12]. Thus, the X. boumbaensis brain produces a burst-type pattern; simply decreasing the number of CAPs in a burst would be sufficient to precipitate a switch from an ancestral burst to a click-type call. The independent evolution of click-type calling in these species thus represents convergence on a similar behavioural phenotype or parallel evolution [21]. Our findings provide an example of behavioural similarity that occurred via distinct reconfigurations of neural circuits, similar to the case of swimming behaviours and neural circuits of nudibranchs [22].
(a). Features of compound action potentials in Xenopus borealis and Xenopus boumbaensis
CAPs recorded from the laryngeal nerve represent population activity of laryngeal motor neurons. CAP durations and shapes probably reflect the extent to which motor neuron activity is synchronous. CAP shape differs between X. borealis and X. boumbaensis. Xenopus boumbaensis CAPs are short (<10 ms) and sharp (figure 3e), whereas X. borealis CAPs are longer (20–50 ms), have a smaller signal : noise ratio and cross the baseline multiple times (figure 2e). Intra-species differences in CAP shape according to call type and sex have been observed in vivo [16] and ex vivo [13] in X. laevis. CAPs recorded from the nerve during slow female (ticking, rapping) and male (ticking, amplectant call) calls in X. laevis resemble X. borealis CAPs described here. Yamaguchi & Kelley [16] suggested that, within a species, short-duration CAPs needed for rapid male calls arise from highly synchronized motor pools, and long-duration CAPs that occur during slower male and female calls arise from relatively less synchronized motor pools. The current study suggests that similar relationships between CAP shape and inter-CAP interval also exist across species. Synchronized motor neuron pools (and their underlying mechanisms) may have been conserved in X. boumbaensis, but lost in X. borealis; such a loss could impose a central constraint on call rapidity in X. borealis.
(b). Species differences in laryngeal processing of neural signals
Isolated larynges from X. borealis and X. boumbaensis require different patterns of stimulation to produce clicks, which explains the observed species differences in fictive vocal patterns. Species differences in muscle fibre recruitment (i.e. the highly potentiating EMGs in the X. boumbaensis larynx) probably reflect differences in the strength of the synapse between vocal motor neurons and their target laryngeal muscles. In the isolated male X. laevis larynx, EMGs potentiate over repeated stimulations; male synapses are mostly weak and potentiate with use, leading to muscle fibre recruitment [23,24]. As a result, click intensity progressively increases over time (intensity modulation). The female larynx exhibits very little EMG potentiation because neuromuscular synapses are strong; this guarantees reliable production of clicks at slower intervals but prevents intensity modulation [14,23].
All Xenopus burst- and trill-type male advertisement calls have some degree of intensity modulation [12], raising the possibility that a weak male laryngeal synapse is a widely conserved feature. For most Xenopus species, which produce short calls separated by long intervals, a weak laryngeal synapse might not convert the first one or two CAPs in a bout of activity into a click, resulting in a slightly shorter call than nerve bout. The overall call pattern (burst or trill) would not be changed. In the case of X. boumbaensis, the consequence of converting two (or three) CAPs into one click is a change in advertisement call temporal structure, from a burst-type to a click-type call. Sound recordings from the isolated X. boumbaensis larynx showed that stimulus doublets and triplets both resulted in a single click, which explains how a somewhat variable fictive pattern (CAP doublets and triplets) can underlie an invariant click-type call. Muscle tension records following triplet stimuli revealed two tension transients on top of a small amount of maintained tension. Maintained tension prevents the sound-producing discs from returning to rest, thus preventing production of subsequent clicks [14]. The click was most likely to have been produced following the second stimulus, and the muscle still experienced some maintained tension when the third stimulus was delivered. If the muscle had not relaxed completely, the third stimulus would not produce a click.
In contrast to X. boumbaensis, the X. borealis vocal pattern is composed of single CAPs at long intervals, necessitating a high safety factor at the laryngeal synapse, so that single CAPs can be reliably converted into sound. Did a strong vocal synapse evolve before a click-type brain pattern? It seems likely, as patterns of single CAPs at long intervals in the presence of a weak synapse would result in a failure of laryngeal muscles to contract at all, resulting in an absence of sound.
(c). On the evolution of simplicity
The idea that behavioural complexity is derived and simplicity is ancestral has been articulated in a number of systems, such as evolution of complexity in bird song structure [25], and nest [26] and burrow [27] architecture. In other systems, phylogenetic analysis reveals ancestral states that are more complex than some extant states, as in the reproductive social behaviour of fiddler crabs [28] and rodent play behaviour [29]. In some cases, complexity arose quite early in the phylogeny, followed by reversals back to a simpler state, as in the electric organ discharge of gymnotiform and mormyrid weakly electric fish [1,30], and the songs of orioles [31] and warblers [32]. Reversals to simpler derived states have also been found at the neural level, in studies of arthropod brain anatomy [33,34].
This study is the first to probe the evolution of neural and neuromuscular bases of Xenopus vocalization, with an emphasis on the production of temporally simplified signals. We found that two distantly related species produce their click-type call using different modifications of central (hindbrain) and peripheral (larynx) components of the vocal circuit. Are there any differences in the hindbrain central pattern generator (CPG) responsible for ‘click-type’ fictive patterns (X. borealis) and those responsible for ‘burst-type’ fictive patterns (X. boumbaensis)? Zornik et al. [35] proposed that the X. laevis vocal CPG contains two circuits working in concert to achieve a temporally complex biphasic call. A circuit that resides in the premotor nucleus dorsal tegmental area of the medulla (DTAM) controls the global timing of the alternation between the fast and slow call phases. The onset of fast trill is accompanied by a slow (approx. 1 Hz) local field potential in DTAM; blocking excitatory neurotransmission disrupts fast trill patterning [35]. Fine-scale timing of inter-click intervals within a call phase is controlled by a circuit distributed between DTAM and its target motor nucleus IX–X [35]. Xenopus borealis calls have one temporal unit; the inter-click interval is the inter-call interval. Which neural strategy does X. borealis use to produce these intervals? The presence of a DTAM wave would suggest a global timing mechanism of inter-call intervals; the absence of a DTAM wave would support a fine-scale mechanism for timing inter-click intervals. By virtue of its burst-type fictive activity, which has both global (the greater than 1 s inter-burst interval) and fine-scale (the 14.4 ms inter-CAP interval) features, we expect to see a DTAM wave during nerve activity in X. boumbaensis and would also expect to find the distributed circuitry supporting ICIs. The diversity of Xenopus advertisement calls provides a favourable arena to study the evolution of neural circuits by probing the structure, function and interactions of hindbrain CPGs and their target effectors.
Acknowledgements
All experimental procedures conformed to guidelines set by the National Institutes of Health and were approved by the Columbia University Institutional Animal Care and Use Committee (protocol no. AC-AAAA8203).
We thank Candace Barnard and Jeremy Korsh for some of the behavioural recordings analysed in this study, Erik Zornik and Martha Tobias for experimental advice, and Erik Zornik, Andrew Bass, Martha Tobias, Ian Hall, Charlotte Barkan, Irene Ballagh as well as two anonymous reviewers for helpful comments on earlier drafts of the manuscript. Support provided by NIH NS23684 (D.B.K.) and Columbia University (research support for the Weintraub Chair).
References
- 1.Lovejoy NR, Lester K, Crampton WGR, Marques FPL, Albert JS. 2010. Phylogeny, biogeography, and electric signal evolution of Neotropical knifefishes of the genus Gymnotus (Osteichthyes: Gymnotidae). Mol. Phylogenet. Evol. 54, 278–290 10.1016/j.ympev.2009.09.017 (doi:10.1016/j.ympev.2009.09.017) [DOI] [PubMed] [Google Scholar]
- 2.Ord TJ, Martins EP. 2006. Tracing the origins of signal diversity in anole lizards: phylogenetic approaches to inferring the evolution of complex behaviour. Anim. Behav. 71, 1411–1429 10.1016/J.Anbehav.2005.12.003 (doi:10.1016/J.Anbehav.2005.12.003) [DOI] [Google Scholar]
- 3.Kusmierski R, Borgia G, Uy A, Crozier RH. 1997. Labile evolution of display traits in bowerbirds indicates reduced effects of phylogenetic constraint. Proc. R. Soc. Lond. B 264, 307–313 10.1098/rspb.1997.0044 (doi:10.1098/rspb.1997.0044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez DM, Rosenberg MS, Pie MR. 2012. The evolution of waving displays in fiddler crabs (Uca spp, Crustacea: Ocypodidae). Biol. J. Linn. Soc. 106, 307–315 10.1111/J.1095-8312.2012.01860.X (doi:10.1111/J.1095-8312.2012.01860.X) [DOI] [Google Scholar]
- 5.Desutter-Grandcolas L, Robillard T. 2003. Phylogeny and the evolution of calling songs in Gryllus (Insecta, Orthoptera, Gryllidae). Zool. Scr. 32, 173–183 10.1046/j.1463-6409.2003.00107.x (doi:10.1046/j.1463-6409.2003.00107.x) [DOI] [Google Scholar]
- 6.Gumm JM, Mendelson TC. 2011. The evolution of multi-component visual signals in darters (genus Etheostoma). Curr. Zool. 57, 125–139 [Google Scholar]
- 7.Podos J, Huber SK, Taft B. 2004. Bird song: the interface of evolution and mechanism. Annu. Rev. Ecol. Evol. Syst. 35, 55–87 10.1146/annurev.ecolsys.35.021103.105719 (doi:10.1146/annurev.ecolsys.35.021103.105719) [DOI] [Google Scholar]
- 8.Podos J. 1997. A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae). Evolution 51, 537–551 10.2307/2411126 (doi:10.2307/2411126) [DOI] [PubMed] [Google Scholar]
- 9.Ryan MJ. 1986. Factors influencing the evolution of acoustic communication: biological constraints. Brain Behav. Evol. 28, 70–82 10.1159/000118693 (doi:10.1159/000118693) [DOI] [PubMed] [Google Scholar]
- 10.Evans BJ, Kelley DB, Tinsley RC, Melnick DJ, Cannatella DC. 2004. A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol. Phylogenet. Evol. 33, 197–213 10.1016/j.ympev.2004.04.018 (doi:10.1016/j.ympev.2004.04.018) [DOI] [PubMed] [Google Scholar]
- 11.Evans BJ, Kelley DB, Melnick DJ, Cannatella DC. 2005. Evolution of RAG-1 in polyploid clawed frogs. Mol. Biol. Evol. 22, 1193–1207 10.1093/molbev/msi104 (doi:10.1093/molbev/msi104) [DOI] [PubMed] [Google Scholar]
- 12.Tobias ML, Evans BJ, Kelley DB. 2011. Evolution of advertisement calls in African clawed frogs. Behaviour 148, 519–549 10.1163/000579511X569435 (doi:10.1163/000579511X569435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes HJ, Yu HJ, Yamaguchi A. 2007. Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator. J. Neurosci. Off. J. Soc. Neurosci. 27, 1485–1497 10.1523/JNEUROSCI.4720-06.2007 (doi:10.1523/JNEUROSCI.4720-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobias ML, Kelley DB. 1987. Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. J. Neurosci. Off. J. Soc. Neurosci. 7, 3191–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yager DD. 1992. A Unique Sound Production Mechanism in the pipid anuran Xenopus borealis. Zool. J. Linn. Soc. 104, 351–375 10.1111/j.1096-3642.1992.tb00927.x (doi:10.1111/j.1096-3642.1992.tb00927.x) [DOI] [Google Scholar]
- 16.Yamaguchi A, Kelley DB. 2000. Generating sexually differentiated vocal patterns: laryngeal nerve and EMG recordings from vocalizing male and female African clawed frogs (Xenopus laevis). J. Neurosci. Off. J. Soc. Neurosci. 20, 1559–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zornik E, Kelley DB. 2008. Regulation of respiratory and vocal motor pools in the isolated brain of Xenopus laevis. J. Neurosci. Off. J. Soc. Neurosci. 28, 612–621 10.1523/JNEUROSCI.4754-07.2008 (doi:10.1523/JNEUROSCI.4754-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luksch H, Walkowiak W, Munoz A, ten Donkelaar HJ. 1996. The use of in vitro preparations of the isolated amphibian central nervous system in neuroanatomy and electrophysiology. J. Neurosci. Methods 70, 91–102 10.1016/S0165-0270(96)00107-0 (doi:10.1016/S0165-0270(96)00107-0) [DOI] [PubMed] [Google Scholar]
- 19.Simpson HB, Tobias ML, Kelley DB. 1986. Origin and identificaiton of fibers in the cranial nerve IX/X complex of Xenopus laevis: lucifer yellow backfills in vitro. J. Comp. Neurol. 244, 430–444 10.1002/cne.902440403 (doi:10.1002/cne.902440403) [DOI] [PubMed] [Google Scholar]
- 20.Yager DD. 1992. Underwater acoustic communication in the african pipid frog, Xenopus borealis. Bioacoustics 4, 1–24 10.1080/09524622.1992.9753201 (doi:10.1080/09524622.1992.9753201) [DOI] [Google Scholar]
- 21.Katz PS. 2011. Neural mechanisms underlying the evolvability of behaviour. Phil. Trans. R. Soc. B 366, 2086–2099 10.1098/rstb.2010.0336 (doi:10.1098/rstb.2010.0336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai A, Newcomb JM, Lillvis JL, Katz PS. 2011. Different roles for homologous interneurons in species exhibiting similar rhythmic behaviors. Curr. Biol. 21, 1036–1043 10.1016/j.cub.2011.04.040 (doi:10.1016/j.cub.2011.04.040) [DOI] [PubMed] [Google Scholar]
- 23.Tobias ML, Kelley DB. 1988. Electrophysiology and dye-coupling are sexually dimorphic characteristics of individual laryngeal muscle fibers in Xenopus laevis. J. Neurosci. Off. J. Soc. Neurosci. 8, 2422–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobias ML, Kelley DB, Ellisman M. 1995. A sex difference in synaptic efficacy at the laryngeal neuromuscular junction of Xenopus laevis. J. Neurosci. Off. J. Soc. Neurosci. 15, 1660–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price J, Lanyon S. 2002. Reconstructing the evolution of complex bird song in the oropendolas. Evolution 56, 1514–1529 10.1111/j.0014-3820.2002.tb01462.x (doi:10.1111/j.0014-3820.2002.tb01462.x) [DOI] [PubMed] [Google Scholar]
- 26.Winkler D, Sheldon F. 1993. Evolution of nest construction in swallows (Hirundinidae): a molecular phylogenetic perspective. Proc. Natl Acad. Sci. USA 90, 5705–5707 10.1073/pnas.90.12.5705 (doi:10.1073/pnas.90.12.5705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber JN, Hoekstra HE. 2009. The evolution of burrowing behaviour in deer mice (genus Peromyscus). Anim. Behav. 77, 603–609 10.1016/j.anbehav.2008.10.031 (doi:10.1016/j.anbehav.2008.10.031) [DOI] [Google Scholar]
- 28.Sturmbauer C, Levinton J, Christy J. 1996. Molecular phylogeny analysis of fiddler crabs: test of the hypothesis of increasing behavioral complexity in evolution. Proc. Natl Acad. Sci. USA 93, 10 855–10 857 10.1073/pnas.93.20.10855 (doi:10.1073/pnas.93.20.10855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellis S, Iwaniuk A. 1999. The roles of phylogeny and sociality in the evolution of social play in muroid rodents. Anim. Behav. 58, 361–373 10.1006/anbe.1999.1141 (doi:10.1006/anbe.1999.1141) [DOI] [PubMed] [Google Scholar]
- 30.Sullivan J, Lavoué S, Hopkins C. 2000. Molecular systematics of the African electric fishes (Mormyroidea: Teleostei) and a model for the evolution of their electric organs. J. Exp. Biol. 203, 665. [DOI] [PubMed] [Google Scholar]
- 31.Price JJ, Friedman NR, Omland KE. 2007. Song and plumage evolution in the new world orioles (Icterus) show similar lability and convergence in patterns. Evolution 61, 850–863 10.1111/J.1558-5646.2007.00082.X (doi:10.1111/J.1558-5646.2007.00082.X) [DOI] [PubMed] [Google Scholar]
- 32.Cardoso GC, Hu Y. 2011. Birdsong performance and the evolution of simple (rather than elaborate) sexual signals. Am. Nat. 178, 679–686 10.1086/662160 (doi:10.1086/662160) [DOI] [PubMed] [Google Scholar]
- 33.Ma X, Hou X, Edgecombe GD, Strausfeld NJ. 2012. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–261 10.1038/nature11495 (doi:10.1038/nature11495) [DOI] [PubMed] [Google Scholar]
- 34.Andrew DR, Brown SM, Strausfeld NJ. 2012. The minute brain of the copepod Tigriopus californicus supports a complex ancestral ground pattern of the tetraconate cerebral nervous systems. J. Comp. Neurol. 520, 3446–3470 10.1002/cne.23099 (doi:10.1002/cne.23099) [DOI] [PubMed] [Google Scholar]
- 35.Zornik E, Katzen AW, Rhodes HJ, Yamaguchi A. 2010. NMDAR-dependent control of call duration in Xenopus laevis. J. Neurophysio. 103, 3501–3515 10.1152/jn.00155.2010 (doi:10.1152/jn.00155.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]