Abstract
Prey species possess a variety of morphological, life history and behavioural adaptations to evade predators. While specific evolutionary conditions have led to the expression of permanent, non-plastic anti-predator traits, the vast majority of prey species rely on experience to express adaptive anti-predator defences. While ecologists have identified highly sophisticated means through which naive prey can deal with predation threats, the potential for death upon the first encounter with a predator is still a remarkably important unresolved issue. Here, we used both laboratory and field studies to provide the first evidence for risk-induced neophobia in two taxa (fish and amphibians), and argue that phenotypically plastic neophobia acts as an adaptive anti-predator strategy for vulnerable prey dealing with spatial and temporal variation in predation risk. Our study also illustrates how risk-free maintenance conditions used in laboratory studies may blind researchers to adaptive anti-predator strategies that are only expressed in high-risk conditions.
Keywords: phenotypic plasticity, predator recognition, experience, predator–prey, predator avoidance, innate recognition
1. Introduction
The ability of prey to survive predator encounters is one of the strongest selective forces affecting the spatial and temporal distributions of animals, and shaping their behaviour, morphology and life-history traits [1,2]. However, predation risk is rarely predictable in space and time [3,4], resulting in considerable variation and uncertainty in threat levels [3,5,6]. Ecologists studying predation have tried to understand the many ways in which prey attempt to maximize their chances of surviving predators, particularly under conditions of uncertain risks. Such strategies and traits used to increase survival are costly; hence, the vast majority of techniques used by prey to evade predators are plastic [7–9]. For example, the presence of predators targeting prey at a specific life stage can lead prey to either speed up or delay the onset of their life-history transitions to minimize the risk on that particular life stage [10]. Prey can also change their morphology in response to predation risk [11]. In some instances, these morphological adaptations may even be reversible [12,13]. Compared with life-history switches and morphological adaptations, behaviour is far more plastic [1,14]. Numerous studies have looked at ways in which prey alter their behavioural decisions when faced with predation risk [15–18].
A prerequisite for such plasticity is the ability of prey to successfully identify predators [19]. Predator recognition among prey species has typically been viewed as a dichotomy of learned versus innate mechanisms. Predator recognition by naive individuals can be achieved through direct and/or indirect learning mechanisms [6,19]. A rich literature exists demonstrating that prey can acquire the recognition of novel predators through personal interactions (direct experience) or by observing prey guild members responding to predators (indirect experience) [19–21]. Both are known to be highly effective mechanisms; however, learning is costly as prey must survive their initial encounter with predators.
While learning opportunities may be numerous for some, situations exist in which all the individuals may be naive to the novel predators in their environment. Such a situation may arise when young are left without parental care (i.e. no knowledge potential), when individuals are exposed to a new or highly variable predator community during a shift in life history, when individuals are exposed to invasive predators or when prey are translocated in a new environment. How can those species cope with this unknown threat? Some prey species possess fixed defensive spines, armour and other morphological adaptations that they have acquired over evolutionary time. This genetic adaptation usually occurs in species that are exposed to a high intensity and predictable risk of predation, for which alternative cheap anti-predator options are not available. For those species, the cost of carrying these defences was overridden by the constant benefit they provided. Similarly, some species have been shown to have an innate recognition of their predators [22–25]. In this case, one can assume that the ability to innately recognize predators was a trait that would increase survival, and hence would spread in the population. Under which circumstances would such a trait appear and provide such a great benefit so as to be maintained in the population? Certainly in populations where the predator community is constant over evolutionary time (i.e. the same predator species are found consistently generation after generation) and the predator diversity is quite low (i.e. the benefit of recognizing one or a few species provides a great survival benefit). Such innate recognition has been shown in closed, evolutionary stable ecosystems [26]. While innate predator recognition could be an ideal trait for populations of naive prey, it seems as if the conditions of its appearance do not allow prey exposed to highly variable predation risk over evolutionary time to benefit from it. Consequently, dealing with first-time predator encounters is likely to be an issue for many naive prey species. Despite the unforgiving nature of predation and the sophistication of many anti-predator strategies, ecologists have yet to unravel the ways in which naive prey can survive this crucial moment.
One way to avoid deadly encounters with novel predators is simply being frightened of everything ‘new’. Neophobia, or the generalized avoidance response to novel stimuli, has been described as a simple mechanism to regulate ecological plasticity [27]. The vast majority of studies of neophobia have focused on birds, and their responses to novel objects and food [27–29]. For example, the ‘dangerous niche hypothesis’ [30] argues that animals should exhibit greater caution while foraging in risky or unfamiliar habitats. Foragers encountering novel situations would benefit from increased caution when exploring new habitats or food types, especially under conditions of high predation risks. Few examples of neophobia are found in the context of predator avoidance. Young turkeys, for instance, often show a fearful response to large silhouettes passing above them [31], a response pattern that is attributed to unfamiliarity. Given the acknowledged ecological role of food-oriented neophobia, it is surprising that virtually no studies exist on the ecological role of predator-oriented neophobia (defined here as the increased predator avoidance response towards any novel cue).
Here, we argue that neophobic responses towards unknown predators may provide naive prey with an adaptive mechanism to avoid those dangerous first encounters. Displaying neophobia has its costs (missed opportunities owing to incorrect avoidance of non-predatory species). Thus, we also argue that, to be adaptive, neophobia needs to be context-dependent, and the trait expressed under conditions of high and/or variable predation risk only. We first tested this hypothesis by testing prey populations with well-known predation histories, both under controlled laboratory conditions and in situ. We used wild-caught Trinidadian guppies (Poecilia reticulata) as our test system. For both experiments, we predicted that fish originating from high-risk environments would respond to the novel predator odour with an anti-predator response, whereas those from a low-risk environment would not.
Neophobia would be beneficial to prey exposed to evolutionary variable predation risk, if its expression was plastic. In others words, neophobia could be expressed in high-risk environments, while it could be suppressed in the absence of any risk indicators. If that is the case, prey detecting environmental cues indicative of a high-risk environment should exhibit neophobia. We investigated the existence of neophobia as a phenotypically plastic trait by maintaining cichlids (Amatitlania nigrofasciata) or wood frog tadpoles (Rana sylvatica) in either a low- or a high-risk environment and comparing their responses to the odour of a novel predator. If neophobia is plastic, we would expect individuals from the high-risk environment to display a fearful response to the novel predator odour.
2. Material and methods
(a). Experiment 1: laboratory comparisons of neophobia in wild populations of guppies
Here, we exposed adult non-gravid female guppies from known low-predation (Upper Aripo) and high-predation (Lower Aripo) populations to paired stimuli in a 2 × 2 design. We tested the strength of the predator avoidance response towards a known risk (conspecific alarm cue versus water) paired with an unknown cue (tilapia odour versus water). Full details of collection sites and stimulus preparation are given in the electronic supplementary material. Shoals of three non-gravid adult female Upper or Lower Aripo river guppies were placed into glass test aquaria (20 l) and allowed to acclimate for at least 4 h. Mean (±s.d.) SL at time of testing was 23.41 ± 2.96 and 20.54 ± 2.76 mm (Upper and Lower Aripo guppies, respectively). Trials consisted of a 5 min pre-stimulus and a 5 min post-stimulus observation period. Immediately following the pre-stimulus observation period, we injected 10 ml of tilapia odour (TO) or water, immediately followed by 10 ml of conspecific alarm cue (AC) or water and began the post-stimulus observation period. During both the observation periods, we recorded an index of area use and a shoaling index, well-documented anti-predator responses in Trinidadian guppies [32]. Area used was recorded every 15 s as the position of each guppy within the tank (1 = bottom third of the tank, 3 = top third of the tank). Area use scores ranged between 3 (all fish near the substrate) to 9 (all fish near the surface). We recorded shoaling index scores every 15 s, which ranged from 1 (no guppies within one body length of each other) to 3 (all guppies within one body length of each other). Behavioural measures were assessed visually and all observations were made blind to treatment.
We calculated the change in shoaling index and area use scores between the observation periods (post–pre). Because the change in shoaling and area use scores are likely to be highly correlated, we analysed both simultaneously using a MANOVA. We tested for the effects of population (Upper Aripo versus Lower Aripo), known risk cue (AC versus water) and unknown risk cue (TO versus water). Given the significant interaction between population and predator odour (see below), we performed one-way MANOVA on each of the cue combinations (water only, predator odour only, AC only and predator odour + ACs). We further analysed the data using separate MANOVAs for each population, including the AC treatment and the novel predator odour treatment as independent variables. All data were normally distributed and variances were equal across treatments, and thus met the assumptions for parametric testing. We tested a total of 112 shoals (n = 14 per treatment combination).
(b). Experiment 2: field comparisons of neophobia in guppies
Here, we conduct in situ observations of guppies exposed to known and novel cues in order to validate the results of our laboratory trials (experiment 1). We tested guppies, in situ, in high-, intermediate- and low-risk streams, for responses to a model pike cichlid paired with one of five cues: stream water (SW), AC (known risk cue), pike cichlid odour (known predator to the high and intermediate-risk populations; CO), TO (novel predator) and lemon oil (unknown ecologically irrelevant odour; LO). Our field trials were conducted in the Upper Aripo (low predation), Tacarigua (intermediate predation) and Lower Aripo rivers. Full details of the sites and stimulus preparation are given in the electronic supplementary material. The predator model was a cast of a freshly killed pike cichlid (14 cm, SL), which was realistically painted, fitted with glass eyes and coated with fibreglass resin. Previous studies have shown that free-ranging guppies respond to this model and live predators in a similar fashion [33].
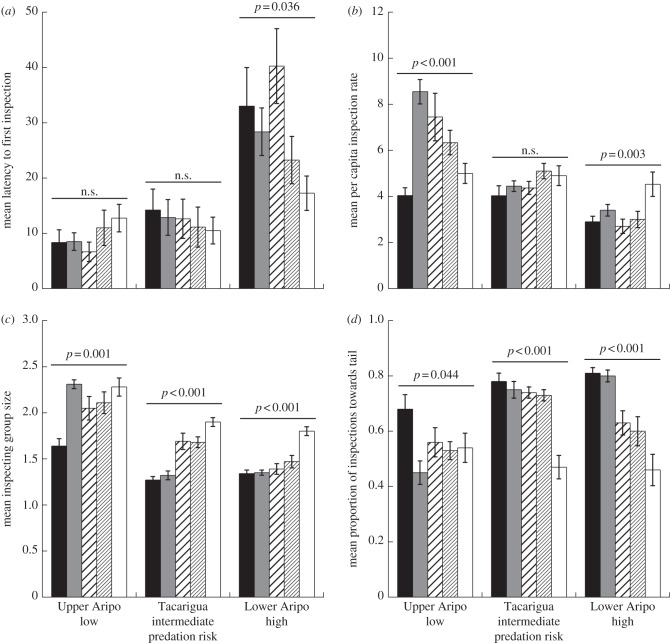
Observations were conducted from the shoreline of slow-flowing pools within each of the three study streams. Observation sites were at least 10 m apart and we moved upstream between observations to reduce the likelihood of repeated exposures to chemical cues. Prior to an observation, we positioned a 3 m length of airline tubing (used to present the chemical stimuli) anchored to a rock (approx. 5 cm in diameter) at least 75 cm from the stream edge. We then positioned the model predator immediately above the end of the stimulus injection tube and waited until at least five guppies were visible within a 50 cm radius of the model. As trials began, we injected 25 ml of one of the five chemical stimuli at a rate of approximately 5 ml min−1. Trials lasted 5 min, during which we recorded the: (i) number of guppies within a 25 cm radius of the model at 15 s intervals, (ii) total number of inspection visits, (iii) number of guppies per inspection visit, (iv) latency from the onset of a trial until the first inspection visit and (v) location of each inspection visit (towards the head versus tail of the model). Reductions in the rate of inspection behaviour and the number of guppies per inspection visit (inspecting group size), and an increase in the proportion of inspections towards the tail (attack cone avoidance), are indicative of risk-averse predator inspection behaviour [33].
To account for differences in the number of guppies present during an observation, we converted the number of inspection visits to a per capita rate (number of inspections divided by the mean number of guppies present). We tested for the effects of stream and chemical stimulus, and the interaction, using two-way ANOVAs for: (i) mean latency to initiate an inspection visit, (ii) per capita inspection rate, (iii) inspecting group size (number of inspectors per visit) and (iv) attack cone avoidance. In the event of significant stream by cue interactions, we analysed each stream separately. We tested a total of eight pools for each stimulus type within each river, except for the Upper Aripo population exposed to AC, where we tested six pools (n = 118). All observations were made blind to treatment.
(c). Experiment 3: inducing neophobia in cichlids and tadpoles
To further test the hypothesis that neophobia is an inducible response to elevated predation risks, we pre-exposed juvenile convict cichlids and wood frog tapoles to high- versus low-risk conditions and tested their response to novel predator cues. Rain-bowtrout (Oncorhynchus mykiss) and tiger salamander (Ambystoma tigrinum) were used as novel predator odours as they are phylogenetically distant (from cichlids and tadpoles, respectively) and represent novel predation threats. Full details of test animals and stimulus preparation are given in the electronic supplementary material.
(i). Cichlid trials
Prior to testing, we exposed cichlids twice per day (approx 10.00 and 16.00 h) for 7 days to either 10 ml of conspecific AC (high risk) or 10 ml of distilled water (low risk). Groups of 20 cichlids were placed into glass aquaria (37 l), containing a gravel substrate. Tank water was continuously filtered and held at approximately 26°C. Partial water changes (approx. 50%) were conducted approximately 30 min after the stimulus injection. Cichlids were fed ad libitum with brine shrimp and commercial flake food at mid-day (i.e. not within 2 h of exposure to the stimulus). Following the pre-exposure phase, we transferred pairs of size-matched cichlids to test tanks; the testing phase began 2 days following the pre-exposure phase. Testing tanks were equipped similarly to those from experiment 1, but were larger (37 l aquaria, filled with 35 l of dechlorinated tap water, approx. 26°C, pH∼7.2). We tested pairs of cichlids as singletons are generally inactive [33]. Cichlids were fed commercial flake food the evening before testing and again 1 h before testing, ensuring that some flakes, once saturated, settled to the substrate, providing foraging opportunities during observations. Each round of pre-exposure (AC or distilled water) yielded five replicates per treatment combination (pre-exposed to high risk and tested to trout odour versus distilled water and pre-exposed to low risk and tested to trout odour versus distilled water). We conducted a total of 20 replicates for each treatment combination (n = 80). All observations were made blind to treatment.
Trials consisted of a 5 min pre-stimulus and a 5 min post-stimulus observation period. Immediately after the pre-stimulus observation, we injected either 10 ml of trout predator odour or distilled water. During each observation period, we recorded time spent moving and number of foraging attempts of the focal fish (randomly selected before the start of the trial). Time spent moving was recorded as the total time, in seconds, that the focal fish was not stationary [34]. We defined a foraging attempt as the pecking towards the substrate with the body inclined at an angle greater than 45° to the substrate [34]. Changes in time moving and foraging attempts (post–pre) were used as dependent variables. We ran a 2 × 2 MANOVA to test the effect of risk level (high versus low) and cue (trout odour versus distilled water) on the behaviour of cichlids. Conditioning block was included as a random factor to account for the non-independence of cichlids from the same pre-exposure group.
(ii). Tadpole trials
Groups of 20 wood frog tadpoles were maintained in 3.7 l pails filled with 3 l of well water and provided with rabbit chow. We exposed tadpoles to two pulses—15 min apart—of injured tadpole cues (high risk) or water (low risk), twice a day for 7 days. On the last day, tadpoles were only treated once. We treated nine pails with injured tadpole cues and nine pails with water. Following the treatment, 4–6 tadpoles from each pail were tested for their response to well water or tiger salamander odour.
We followed a well-established assay [35,36]. Individual tadpoles were placed in a 0.5 l cup filled with well water and left to acclimate for 40 min. Trials consisted of a 4 min pre-stimulus and 4 min post-stimulus observation period, during which we counted the number of times the tadpoles crossed the medial line of the cup. In larval amphibians, line crossing is often used as a proxy for activity, and a decrease in activity is a well-established anti-predator behaviour in this taxon. Following the pre-stimulus observation, we injected either 5 ml of water or 5 ml of tiger salamander odour, and recorded, once again, the behaviour of the tadpole (post-stimulus injection period). The change in activity between the pre- and post-stimulus period indicates the response of the tadpole of the cue. All observations were made blind to treatment. For tadpole trials, percentage change in activity from the pre-stimulus baseline was used as raw data in our analysis. We ran a 2 × 2 nested ANOVA to test the effect of risk level (high versus low) and cue (water versus salamander odour) on the behaviour of tadpoles. Pail was introduced as a nested factor, to account for the non-independence of tadpoles coming from the same pre-exposure group.
3. Results
(a). Experiment 1: laboratory comparisons of neophobia in wild populations of guppies
We found a significant population × novel odour interaction (p = 0.017; table 1). High- and low-risk populations did not differ in their response to water (W+W: F2,25 = 0.79, p = 0.47), to known risk cues (AC+W: F2,25 = 0.53, p = 0.59) or a mixture of known and unknown cues (AC+TO: F2,25 = 0.77, p = 0.48), but differed in their responses to unknown risk cues (W+TO: F2,25 = 6.58, p = 0.005). When considering the Lower Aripo population alone, we found a significant effect of AC (F2,51 = 14.41, p < 0.001; grey solid bars, figure 1) and predator odour (F2,51 = 7.39, p = 0.002; figure 1). There was no significant interaction (F2,51 = 0.43, p = 0.66; figure 1). For the Upper Aripo population, we found a significant effect of cue (AC versus water; F2,51 = 30.80, p < 0.001; white bars, figure 1), but no significant effect of predator odour (F2,51 = 0.12, p = 0.88) nor a predator odour × cue interaction (F2,51 = 0.009, p = 0.99, figure 1).
Table 1.
Results of overall MANOVA testing effects of population (Lower versus Upper Aripo), cue (alarm cue versus water) and predator odour (tilapia versus water) on the observed change in anti-predator behaviour (shoaling index and area use). n = 14 per treatment combination. Significant differences are in bold type.
| F | d.f. | p | |
|---|---|---|---|
| population | 2.23 | 2, 103 | =0.12 |
| cue | 42.97 | 2, 103 | <0.001 |
| predator odour | 3.81 | 2, 103 | =0.025 |
| population × cue | 1.68 | 2, 103 | =0.19 |
| population × predator odour | 4.26 | 2, 103 | =0.017 |
| cue × predator odour | 0.27 | 2, 103 | =0.76 |
| population × cue × predator odour | 0.16 | 2, 103 | =0.85 |
Figure 1.
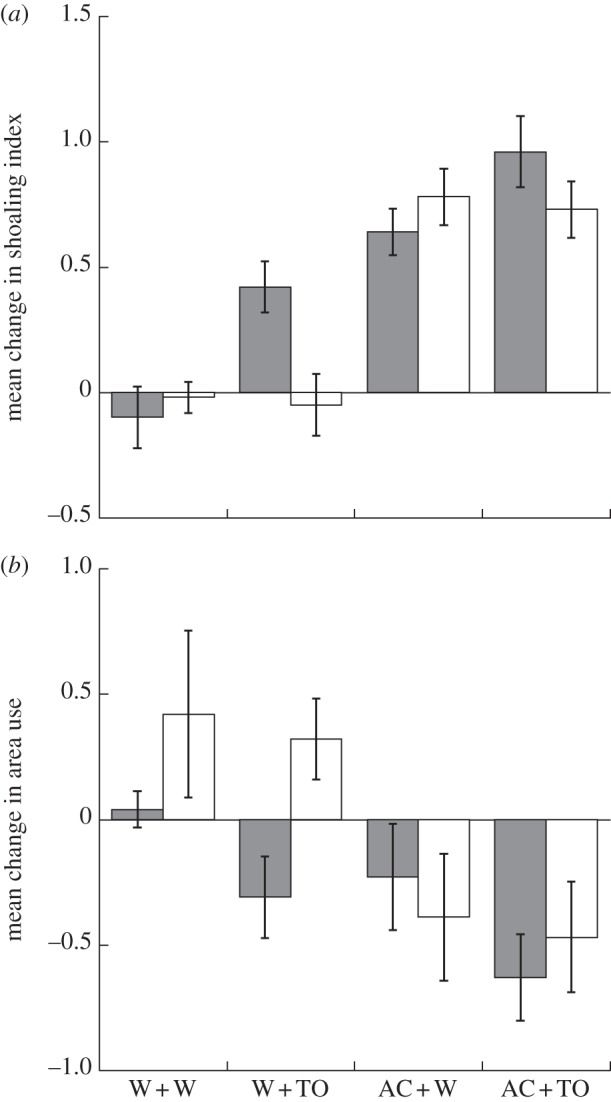
(a) Mean (±s.e.) change in shoaling index and (b) area use for Lower Aripo (grey bars) and Upper Aripo (white bars) guppies. W+W = double water control; W+TO = guppies exposed to tilapia odour (TO) paired with water; AC+W = guppies exposed to conspecific alarm cue (AC) paired with water; AC+TO = guppies exposed to both AC and TO. n = 14 per treatment combination.
(b). Experiment 2: field comparisons of neophobia in guppies
Overall, we found that each measure of predator inspection was affected by both stream and cue (table 2a). There was no effect of cue on the latency to initiate an inspection visit in the Upper Aripo or Tacarigua (table 2b and figure 2a), but there was an effect of cue for guppies tested in the Lower Aripo (table 2b and figure 2a). In the Lower Aripo, guppies exposed to the model predator paired with SW exhibited the shortest latency to inspect, whereas those exposed to AC, CO or TO took longer to inspect. The latency to inspect the model paired with LO was intermediate.
Table 2.
(a) Results of two-way ANOVAs testing the effects of stream (Upper Aripo, Tacarigua or Lower Aripo) and cue (alarm cue, pike cichlid odour, tilapia odour, lemon oil or stream water). (b) Results of one-way ANOVAs for individual streams given to explore significant stream×cue interactions. n = 8 per treatment combination, except Upper Aripo exposed to alarm cue, where n = 6. Significant differences are in bold type.
| F | d.f. | p | |
|---|---|---|---|
| (a) two-way ANOVAs | |||
| latency to inspect | |||
| stream | 35.30 | 2, 101 | <0.001 |
| cue | 1.32 | 4, 101 | =0.27 |
| stream×cue | 2.29 | 8, 101 | =0.027 |
| per capita inspection rate | |||
| stream | 63.77 | 2, 101 | <0.001 |
| cue | 6.91 | 4, 101 | <0.001 |
| stream×cue | 4.49 | 8, 101 | <0.001 |
| inspecting group size | |||
| stream | 107.6 | 2, 101 | <0.001 |
| cue | 35.60 | 4, 101 | <0.001 |
| stream×cue | 7.05 | 8, 101 | <0.001 |
| attack cone avoidance | |||
| stream | 18.08 | 2, 101 | <0.001 |
| cue | 19.49 | 4, 101 | <0.001 |
| stream×cue | 5.12 | 8, 101 | <0.001 |
| (b) one-way ANOVAs | |||
| latency to inspect | |||
| Upper Aripo | 1.19 | 4, 31 | =0.34 |
| Tacarigua | 0.20 | 4, 35 | =0.94 |
| Lower Aripo | 2.90 | 4, 35 | =0.036 |
| per capita inspection rate | |||
| Upper Aripo | 8.81 | 4, 31 | <0.001 |
| Tacarigua | 2.32 | 4, 35 | =0.08 |
| Lower Aripo | 4.89 | 4, 35 | =0.003 |
| inspecting group size | |||
| Upper Aripo | 6.04 | 4, 31 | =0.001 |
| Tacarigua | 34.65 | 4, 35 | <0.001 |
| Lower Aripo | 16.59 | 4, 35 | <0.001 |
| attack cone avoidance | |||
| Upper Aripo | 2.78 | 4, 31 | =0.044 |
| Tacarigua | 19.78 | 4, 35 | <0.001 |
| Lower Aripo | 13.33 | 4, 35 | <0.001 |
Figure 2.
(a) Mean (±s.e.) latency to first inspection visit, (b) per capita inspection rate, (c) inspecting group size and (d) proportion of inspections towards the tail for guppies exposed to conspecific ACs (black bars), pike cichlid odour (OC, dark grey bars), TO (thick-striped bars), lemon oil (fine-striped bars) or stream water (white bars) in each of the three streams. n = 8 per treatment combination, except Upper Aripo guppies exposed to AC (n = 6).
For the per capita inspection rate, we found significant effects of cue for the Upper Aripo and Lower Aripo, but not for the Tacarigua River (table 2b and figure 2b). For the Upper Aripo population, the rate of inspection was lowest in response to the model paired with AC compared with the SW control. Interestingly, when paired with CO, TO or LO, per capita inspection rates increased relative to the SW control. For the Lower Aripo population, inspection rates towards the model paired with all cues were all reduced relative to the SW control.
For the number of inspectors per visit, cue was significant for all three populations (table 2b and figure 2c). In the Upper Aripo, inspecting group size was reduced when the model was paired with AC only. The Tacarigua population exhibited the lowest inspecting group size in response to AC and CO, and the largest groups towards the model paired with SW. The Lower Aripo population exhibited significantly reduced inspecting group sizes in response to all cues relative to the SW control.
Finally, all populations exhibited significant effects of cue for attack cone avoidance (table 2b and figure 2d). The Upper Aripo guppies exhibited increased attack cone avoidance (relative to the SW control) only when the model was paired with AC. Both the Tacarigua and Lower Aripo populations exhibited significant increases in attack cone avoidance (relative to SW) when the model was paired with AC, CO, TO or LO.
(c). Experiment 3: inducing neophobia in cichlids and tadpoles
The behaviour of cichlids was affected by both risk level and cue (interaction: F2,75 = 4.05, p = 0.021; electronic supplementary material, figure S1). There was no effect of conditioning block (F2,74 = 0.35, p = 0.71). Further analysis demonstrated that risk level had no effect on the response of cichlids to distilled water (F2,37 = 0.06, p = 0.94), but high-risk cichlids exhibited an increased predator avoidance response (reduced time moving and foraging attempts) towards trout odour, whereas low-risk cichlids did not (F2,37 = 7.09, p = 0.002).
The behaviour of tadpoles was affected by both risk level and cue (interaction: F1,11.3 = 8.10, p = 0.015; electronic supplementary material, figure S2). Neither pail (F16,10.3 = 1.40, p = 0.30) nor pail × cue (F16,36 = 0.80, p = 0.70) had a significant influence on behaviour. Further analysis revealed that risk did not affect the responses of tadpoles to water (F1,31 = 0.80, p = 0.40), but high-risk tadpoles responded to the salamander with a stronger anti-predator response than low-risk ones (F1,37 = 7.40, p = 0.01).
4. Discussion
The results of our first experiment indicated that guppies collected from a high-predation-risk stream display avoidance responses to novel predator odour, whereas the response of guppies from a low-risk stream were not different from controls. Interestingly, the two populations did not differ in their response to water control or known risk cues, and their response to a mix of known and unknown cues were also similar. This is the first demonstration that different populations of the same species may display variation in neophobia towards novel predator cues. While we cannot rule out population-specific differences, it is unlikely that our results represent a fixed adaptation, as the two populations are not genetically isolated [37,38]. Our second experiment provided further support for in situ population variation in neophobic tendencies, with high- and intermediate-risk populations displaying stronger anti-predator responses to a model predator paired with novel risk cues than a low-risk population.
How might we explain the observed population difference in neophobic responses? We initially hypothesized that predation risk would be a good candidate for the difference seen above. While we sampled different shoals of guppies within each stream, our power of inference is limited, as each stream represents only one population, and the streams themselves differ in a number of parameters besides predation risk level. It is possible that under conditions of high risk, prey may generalize anti-predator responses to any novel predator odour, as the cost of ignoring a predator is greater that the cost of responding to a non-predator. This is unlikely, as the results of experiment 2 showed increased avoidance of an ecologically irrelevant odour (LO). In addition, the results of experiment 3 provide unequivocal evidence of the role played by predation risk with respect to the ability of prey to display neophobic responses. Increasing the background level of risk for one week was enough to alter the response patterns of prey in two taxa (fish and amphibians) to novel predator cues. To our knowledge, this is the first demonstration of context-dependent, phenotypically plastic neophobic responses of prey towards novel predators.
(a). Cost–benefit trade-offs of neophobic responses
The presence of strong neophobic responses in populations characterized as ‘high’ versus ‘low’ predation pressure suggests a balance between the benefits of learned versus genetically fixed predator recognition. The chief benefit of learned predator recognition is likely to be associated with the high degree of behavioural plasticity, which comes from direct and/or indirect experience [6,19]. Predator avoidance is costly as it represents time and energy lost to other activities, such as foraging, courtship and territorial defence [1,5]. Relying on learned information should allow prey to avoid responding to ecologically irrelevant cues, ensuring more accurate risk assessment and threat-sensitive behavioural decisions [6,39]. But this flexibility comes at a cost [40], including the cost of having to survive the initial encounter with a potential predator [26]. By relying on neophobic responses under high-predation-pressure conditions, prey could reduce the initial learning cost while still maintaining sufficient behavioural plasticity to deal with variable predation pressure. We would predict that the subsequent response to novel cues would be shaped through direct experience. The initial neophobic response could either be reinforced, if the prey responds to a novel cue in the presence of an actual threat, or eliminated if the prey responds to a novel cue in the absence of an ecologically relevant threat. Responding to a novel cue in the absence of a realistic threat would constitute a cost, though this would be considerably less than the cost incurred by prey not responding in the presence of an actual predation threat. As such, neophobia should allow prey to reduce their risk of predation by responding to novel cues and adjusting subsequent responses based on direct experience. Together, our current results suggest that neophobic responses to novel predator cues may function as a compensatory mechanism under conditions of increased and/or variable predation pressure.
(b). Rethinking predator recognition
Aquatic species have been widely studied in the context of predator recognition. For a long time, scientists have categorized their response patterns using the dichotomy of learned versus innate predator recognition [23,41,42]. However, recent evidence suggests that predator recognition is much more sophisticated. For instance, prey species can display an anti-predator response towards a novel predator upon their first encounter if the predator species is related to a species already recognized as dangerous by the prey [26,43]. In amphibians, occurrences of the so-called ‘innate’ predator recognition in wild populations sympatric with predators (e.g. ringed salamanders, Ambystoma annulatum) may be the result of embryonic learned predator recognition [44]. Thus, when researchers want to test for innate predator recognition, they need to collect eggs from laboratory-reared individuals, given that eggs collected from the wild may contain highly educated embryos, knowing the identity and the risk posed by their predators [44], and the time of day at which they are most dangerous [45]. Tadpoles can also display generalization of predator recognition based on information learned as embryos [46]. Phenotypically plastic neophobia appears as another sophisticated mechanism to allow naive prey to survive the first encounter.
But how widespread is risk-induced neophobia? We can only speculate about the multiple selective forces that may be imposed on prey species for the successful spread of risk-induced neophobia. The costs of neophobia (missed foraging or mating opportunities owing to unnecessary anti-predator responses) are not negligible. We speculate that neophobia may not be found in social species with overlapping generation or those with extended periods of parental care. Naive individuals, usually young, may have ample opportunities to learn about predators from knowledgeable individuals. Naive prey individuals finding themselves in a highly diverse community (e.g. coral reefs) may not benefit from neophobia, as responding to everything new may carry significant costs that may override benefits from neophobia. Our results provide the first step towards a new phenomenon that should be taken into consideration by those studying predator avoidance. A number of studies have used a novel odour (predator or not) as a negative control in predation studies: to show that an individual's experience changes its response to a given stimulus, you need to show that the change is limited to that particular stimulus, and not to any stimulus. However, laboratory studies often require acclimation of their test species, which is certainly associated with a drop in predation risk. Thus, guppies, cichlids and many other species have been shown not to respond to novel predator cues. However, as we have demonstrated, this lack of response may just be the reflection of local conditions. More studies are needed to investigate the spread of risk-induced neophobia in prey species.
A number of questions arise from our results. If increased local predation risk leads to neophobic responses towards novel odours, how does this neophobia translate to other contexts, such as foraging and/or habitat selection? Individuals are naive to predators in a high-risk environment usually as a result of being born or other major life-history shifts (e.g. migration, metamorphosis). Those life-history transitions are often associated with a change in feeding habits and the need to find new suitable habitat to reproduce. Also, is neophobia an all-or-nothing phenomenon, or can prey display risk-sensitive neophobia (neophobic intensity propositional to the level of risk they are exposed to in their new environment)? How long is the neophobic response maintained in the absence of reinforcement? Our study raised many more questions that we can answer, and future work on this phenomenon should prove fruitful.
This study provides a new adaptive framework for the concept of neophobia and solves a long-standing issue on the way naive prey can increase their chance of survival with novel predators. In addition, we argue that the nature of experimental testing, including long risk-free acclimation periods for test animals, is the reason why this aspect of predation ecology was previously ignored. A few ecologists have already illustrated how the artificial absence of predation can bias our understanding of prey anti-predator responses [14] and have pushed for the return of predation risk in predator–prey studies [47]. Our study also illustrates how the lack of predation in laboratory studies may blind researchers to adaptive anti-predator strategies that are only expressed in high-risk conditions.
Acknowledgements
We thank Dr Jean-Guy Godin and Dr James Grant for fruitful discussions, Jean and Glen Chivers for allowing access to the field site, and Christopher Jackson, Patrick Malka, Heather Auld, Jill and Jonathan Henklestone and Chloé for laboratory and field assistance. Financial support was provided by the Natural Science and Engineering Research Council of Canada and Concordia University to G.E.B., and NSERC and the University of Saskatchewan to D.P.C. and M.C.O.F. All work reported herein was conducted in accordance with Concordia University Animal Research Ethics Protocol AREC-2010-BROW and the University of Saskatchewan Animal Care Protocol 20060014.
References
- 1.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 10.1139/z90-092 (doi:10.1139/z90-092) [DOI] [Google Scholar]
- 2.Peacor SD, Werner EE. 2004. Context dependence of nonlethal effects of a predator on prey growth. Isr. J. Zool. 50, 139–167 10.1560/KPRR-X1C3-5NHE-QV2N (doi:10.1560/KPRR-X1C3-5NHE-QV2N) [DOI] [Google Scholar]
- 3.Sih A. 1992. Prey uncertainty and the balancing of antipredator and feeding needs. Am. Nat. 139, 1052–1069 10.1086/285372 (doi:10.1086/285372) [DOI] [Google Scholar]
- 4.Sih A, Ziemba R, Harding KC. 2000. New insights on how temporal variation in predation risk shapes prey behavior. Trends Ecol. Evol. 15, 3–4 10.1016/S0169-5347(99)01766-8 (doi:10.1016/S0169-5347(99)01766-8) [DOI] [PubMed] [Google Scholar]
- 5.Lima SL, Steury TD. 2005. Perception of predation risk: the foundation of nonlethal predator–prey interactions. In Ecology of predator–prey interactions (eds Barbosa P, Castellanos I.), pp. 166–188 Oxford, UK: Oxford University Press [Google Scholar]
- 6.Dall S, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 10.1016/j.tree.2005.01.010 (doi:10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 7.Tollrian R. 1993. Neckteeth formation in Daphnia pulex as an example of continuous phenotypic plasticity: morphological effects of Chaoborus kairimone concentration and their quantification. J. Plankton Res. 15, 1309–1318 10.1093/plankt/15.11.1309 (doi:10.1093/plankt/15.11.1309) [DOI] [Google Scholar]
- 8.Relyea RA. 2002. Local population differences in phenotypic plasticity: predator-induced changes in wood frog tadpoles. Ecol. Monogr. 72, 77–93 10.1890/0012-9615(2002)072[0077:LPDIPP]2.0.CO;2 (doi:10.1890/0012-9615(2002)072[0077:LPDIPP]2.0.CO;2) [DOI] [Google Scholar]
- 9.Brönmark C, Lakowitz T, Hollander J. 2011. Predator-induced morphological plasticity across local populations of a freshwater snail. PLoS ONE 6, e21773. 10.1371/journal.pone.0021773 (doi:10.1371/journal.pone.0021773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chivers DP, Kiesecker JM, Marco A, Wildy EL, Blaustein AR. 1999. Shifts in life history as a response to predation in western toads (Bufo boreas). J. Chem. Ecol. 25, 2455–2463 10.1023/A:1020818006898 (doi:10.1023/A:1020818006898) [DOI] [Google Scholar]
- 11.Brönmark C, Pettersson LB. 1994. Chemical cues from piscivores induce a change in morphology in crucian carp. Oikos 70, 396–402 10.2307/3545777 (doi:10.2307/3545777) [DOI] [Google Scholar]
- 12.Relyea RA. 2003. Predators come and predators go: the reversibility of predator-induced traits. Ecology 84, 1840–1848 10.1890/0012-9658(2003)084[1840:PCAPGT]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[1840:PCAPGT]2.0.CO;2) [DOI] [Google Scholar]
- 13.Chivers DP, Zhao X, Brown GE, Marchant TA, Ferrari MCO. 2008. Predator-induced changes in morphology of a prey fish: the effects of food level and temporal frequency of predation risk. Evol. Ecol. 22, 561–574 10.1007/s10682-007-9182-8 (doi:10.1007/s10682-007-9182-8) [DOI] [Google Scholar]
- 14.Lima SL, Bednekoff PA. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659 10.1086/303202 (doi:10.1086/303202) [DOI] [PubMed] [Google Scholar]
- 15.Ghalambor CK, Martin TE. 2002. Comparative manipulation of predation risk in incubating birds reveals variability in the plasticity of responses. Behav. Ecol. 13, 101–108 10.1093/beheco/13.1.101 (doi:10.1093/beheco/13.1.101) [DOI] [Google Scholar]
- 16.Laurila A, Pakkasmaa S, Crochet PA, Merilä J. 2002. Predator-induced plasticity in early life history and morphology in two anuran amphibians. Oecologia 132, 524–530 10.1007/s00442-002-0984-7 (doi:10.1007/s00442-002-0984-7) [DOI] [PubMed] [Google Scholar]
- 17.Foam PE, Harvey MC, Mirza RS, Brown GE. 2005. Heads up: juvenile convict cichlids switch to threat-sensitive foraging tactics based on chemosensory information. Anim. Behav. 70, 601–607 10.1016/j.anbehav.2004.12.011 (doi:10.1016/j.anbehav.2004.12.011) [DOI] [Google Scholar]
- 18.Ireland DH, Wirsing AJ, Murray DL. 2007. Phenotypically plastic responses of green frog embryos to conflicting predation risk. Oecologia 152, 162–168 10.1007/s00442-006-0637-3 (doi:10.1007/s00442-006-0637-3) [DOI] [PubMed] [Google Scholar]
- 19.Brown GE, Ferrari MCO, Chivers DP. 2011. Learning about danger: chemical alarm cues and threat-sensitive assessment of predation risk by fishes. In Fish cognition and behavior, 2nd edn (eds Brown C, Laland K, Krause J.), pp. 59–80 Oxford, UK: Blackwell [Google Scholar]
- 20.Mathis A, Chivers DP, Smith RJF. 1996. Cultural transmission of predator recognition in fishes: intraspecific and interspecific learning. Anim. Behav. 51, 185–201 10.1006/anbe.1996.0016 (doi:10.1006/anbe.1996.0016) [DOI] [Google Scholar]
- 21.Griffin AS. 2004. Social learning about predators: a review and prospectus. Anim. Learn. Behav. 32, 131–140 10.3758/BF03196014 (doi:10.3758/BF03196014) [DOI] [PubMed] [Google Scholar]
- 22.Vilhunen S, Hirvonen H. 2003. Innate antipredator responses of Arctic charr (Salvelinus alpinus) depend on predator species and their diet. Behav. Ecol. Sociobiol. 55, 1–10 10.1007/s00265-003-0670-8 (doi:10.1007/s00265-003-0670-8) [DOI] [Google Scholar]
- 23.Hawkins LA, Magurran AE, Armstrong JD. 2004. Innate predator recognition in newly-hatched Atlantic salmon. Behaviour 141, 1249–1262 10.1163/1568539042729694 (doi:10.1163/1568539042729694) [DOI] [Google Scholar]
- 24.Dalesman S, Rundle SD, Cotton PA. 2007. Predator regime influences innate anti-predator behaviour in the freshwater gastropod Lymnaea stagnalis. Freshwater Biol. 52, 2134–2140 10.1111/j.1365-2427.2007.01843.x (doi:10.1111/j.1365-2427.2007.01843.x) [DOI] [Google Scholar]
- 25.Gall BG, Mathis A. 2010. Innate predator recognition and the problem of introduced trout. Ethology 116, 47–58 10.111/j.1439-0310-2009.01718.x (doi:10.111/j.1439-0310-2009.01718.x) [DOI] [Google Scholar]
- 26.Ferrari MCO, Gonzalo A, Messier F, Chivers D. 2007. Generalization of learned predator recognition: an experimental test and framework for future studies. Proc. R. Soc. B 274, 1853–1859 10.1098/rspb.2007.0297 (doi:10.1098/rspb.2007.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg R. 1990. Ecological plasticity, neophobia, and resource use in birds. Stud. Avian Biol. 13, 431–437 [Google Scholar]
- 28.Greenberg R, Mettke-Hofmann C. 2001. Ecological aspects of neophobia and neophilia in birds. In Current ornithology (eds Nolan V, Thompson CF.), pp. 119–178 New York, NY: Springer [Google Scholar]
- 29.Biondi LM, Bó MS, Vassallo AI. 2010. Inter-individual and age differences in exploration, neophobia and problem-solving ability in a Neotropical raptor (Milvago chimango). Anim. Cogn. 13, 701–710 10.1007/s10071-010-0319-8 (doi:10.1007/s10071-010-0319-8) [DOI] [PubMed] [Google Scholar]
- 30.Greenberg R. 2003. The role of neophobia and neophilia in the development of innovative behaviour of birds. In Animal innovation (eds Reader SM, Laland KN.), pp. 175–196 Oxford, UK: Oxford University Press [Google Scholar]
- 31.Schleidt VWM. 1961. Reaktionen von truthühern auf fliegende rabvögel und versuch zur analyse inhrer AAM's. Z. Tierpsychol. 18, 534–560 10.1111/j.1439-0310.1961.tb00241.x (doi:10.1111/j.1439-0310.1961.tb00241.x) [DOI] [Google Scholar]
- 32.Brown GE, Macnaughton CJ, Elvidge CK, Ramnarine I, Godin J-GJ. 2009. Provenance and threat-sensitive predator avoidance patterns in wild-caught Trinidadian guppies. Behav. Ecol. Sociobiol. 63, 699–706 10.1007/s00265-008-0703-4 (doi:10.1007/s00265-008-0703-4) [DOI] [Google Scholar]
- 33.Brown GE, Elvidge CK, Macnaughton CJ, Ramnarine I, Godin J-GJ. 2010. Cross-population responses to conspecific chemical alarm cues in wild Trinidadian guppies, Poecilia reticulata: evidence for local conservation of cue production. Can. J. Zool. 88, 139–147 10.1139/Z09-127 (doi:10.1139/Z09-127) [DOI] [Google Scholar]
- 34.Brown GE, Bongiorno T, Dicapua DM, Ivan LI, Roh E. 2006. Effects of group size on the threat-sensitive response to varying concentrations of chemical alarm cues by juvenile convict cichlids. Can. J. Zool. 84, 1–8 10.1139/Z05-166 (doi:10.1139/Z05-166) [DOI] [Google Scholar]
- 35.Ferrari MCO, Messier F, Chivers DP. 2008. Larval amphibians learn to match antipredator response intensity to temporal patterns of risk. Behav. Ecol. 19, 980–983 10.1093/beheco/arn056 (doi:10.1093/beheco/arn056) [DOI] [Google Scholar]
- 36.Ferrari MCO, Brown GE, Messier F, Chivers DP. 2009. Threat-sensitive generalization of predator recognition by larval amphibians. Behav. Ecol. Sociobiol. 63, 1369–1375 10.1007/s00265-009-0779-5 (doi:10.1007/s00265-009-0779-5) [DOI] [Google Scholar]
- 37.Crispo E, Bentzen P, Reznick DN, Kinnison MT, Hendry AP. 2006. The relative influence of natural selection and geography on gene flow in guppies. Mol. Ecol. 15, 49–62 10.1111/j.1365-294X.2005.02764.x (doi:10.1111/j.1365-294X.2005.02764.x) [DOI] [PubMed] [Google Scholar]
- 38.van Oosterhout C, et al. 2006. Balancing selection, random genetic drift, and genetic variation at the major histocompatibility complex in two wild populations of guppies (Poecilia reticulata). Evolution 60, 2562–2574 10.1554/06-286.1 (doi:10.1554/06-286.1) [DOI] [PubMed] [Google Scholar]
- 39.Ferrari MCO, Trowell JJ, Brown GE, Chivers DP. 2005. The role of learning in the development of threat-sensitive predator avoidance by fathead minnows. Anim. Behav. 70, 777–784 10.1016/j.anbehav.2005.01.009 (doi:10.1016/j.anbehav.2005.01.009) [DOI] [Google Scholar]
- 40.Mery F, Kawecki TJ. 2003. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 270, 2465–2469 10.1098/rspb.2003.2548 (doi:10.1098/rspb.2003.2548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheurer JA, Berejikian BA, Thrower FP, Ammann ER, Flagg TA. 2007. Innate predator recognition and fright response in related populations of Oncorhynchus mykiss under different predation pressure. J. Fish. Biol. 70, 1057–1069 10.1111/j.1095-8649-2007.01367.x (doi:10.1111/j.1095-8649-2007.01367.x) [DOI] [Google Scholar]
- 42.Epp KJ, Gabor CR. 2008. Innate and learned predator recognition mediated by chemical signals in Eurycea nana. Ethology 114, 607–615 10.1111/j.1439-0310.2008.01494.x (doi:10.1111/j.1439-0310.2008.01494.x) [DOI] [Google Scholar]
- 43.Brown GE, Ferrari MCO, Malka PH, Russo S, Tressider M, Chivers DP. 2011. Generalization of predators and nonpredators by juvenile rainbow trout: learning what is and is not a threat. Anim. Behav. 81, 1249–1256 10.1016/j.anbehav.2011.03.013 (doi:10.1016/j.anbehav.2011.03.013) [DOI] [Google Scholar]
- 44.Mathis A, Ferrari MCO, Windel N, Messier F, Chivers DP. 2008. Learning by embryos and the ghost of predation future. Proc. R. Soc. B 275, 2603–2607 10.1098/rspb.2008.0754 (doi:10.1098/rspb.2008.0754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrari MCO, Manek AK, Chivers DP. 2010. Temporal learning of predation risk by embryonic amphibians. Biol. Lett. 6, 308–310 10.1098/rsbl.2009.0798 (doi:10.1098/rsbl.2009.0798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrari MCO, Chivers DP. 2009. Sophisticated early life lessons: threat-sensitive generalization of predator recognition by embryonic amphibians. Behav. Ecol. 20, 1295–1298 10.1093/beheco/arp135 (doi:10.1093/beheco/arp135) [DOI] [Google Scholar]
- 47.Lima SL. 2002. Putting predators back into behavioral predator-prey interactions. Trends Ecol. Evol. 17, 70–75 10.1016/S0169-5347(01)02393-X (doi:10.1016/S0169-5347(01)02393-X) [DOI] [Google Scholar]