Abstract
Mitochondria are crucial to the hypoxia response of aerobic organisms. However, mitochondrial mechanisms for hypoxia adaptation remain largely unknown. We conducted a comparative study on the mitochondrial hypoxia response and adaptation of the Tibetan Plateau and North China lowland populations of migratory locusts, Locusta migratoria. Compared with lowland locusts, Tibetan locusts presented significantly higher hypoxia tolerance and a better-maintained mitochondrial structure in flight muscles under oxygen partial pressure of 1.6 kPa. The hypoxic treatment inhibited the NADH-linked oxidative phosphorylation (OXPHOS) significantly in both locust populations, but to a less extent in Tibetan locusts. Among the critical components of OXPHOS, only cytochrome c oxidase (COX) exhibited significantly higher activity in Tibetan locusts under normoxia and hypoxia. Pharmacological interventions using NaN3 confirmed that COX activity inhibition reduced hypoxia tolerance by downregulating OXPHOS in both locust populations. The enhanced COX activity was caused not by protein content, but by elevated catalytic efficiency resulting from the increased ferrocytochrome c affinity of COX and the increased electron transport rate via catalytic redox centres. These findings reveal a novel mechanism that confers mitochondrial robustness against hypoxia by modulating the COX activity, which represents an adaptation to permanent hypoxia in the Tibetan Plateau.
Keywords: Tibetan plateau, mitochondria, hypoxia response, hypoxia adaptation, catalytic efficiency
1. Introduction
Hypoxia is important in the regulation of cellular function, the pathological processes of many human diseases, and even in the evolutionary process for speciation [1–3]. Hypoxia response mechanisms have been extensively studied in the past decades. For example, hypoxia-inducible factor-1 has been demonstrated to act as the central regulator during hypoxia responses [4]. However, the mechanisms by which aerobic organisms adapt to hypoxia remain poorly understood. One avenue for developing an improved understanding of hypoxia response and adaptation mechanisms is to compare the functional responses of animal populations in hypoxic and normoxic environments. Such comparisons have the potential to reveal important specific alterations induced by natural selection, which allow an organism to function in hypoxic conditions (such as high altitude) [5]. The Tibetan Plateau is one of the most extreme environments for high absolute elevation (with an average elevation of more than 4000 m above sea level (a.s.l.)), which consequently induces hypoxia. Previous studies have demonstrated that Tibetan birds can improve their physiological performance by enhancing the oxygen transport capacity, and have yielded important insights into the genetic basis of adaptation involving the critical oxygen carrier, haemoglobin [6]. However, the mitochondrial mechanisms for adaptation to long-term hypoxia in indigenous animals in Tibet have not been sufficiently investigated.
Mitochondria are crucial to cellular hypoxia responses because of their central role in energy production, reactive oxygen species (ROS) homeostasis and cell death [7,8]. During hypoxic exposure, the oxidative phosphorylation (OXPHOS) for ATP generation is downregulated, the activities of respiratory chain complexes are reduced, and the ROS produced from the respiratory chain during OXPHOS is increased to cause cellular oxidative damage [8–10]. Cytochrome c oxidase (COX), the terminal complex in the respiratory chain, is the only site for oxygen access and utilization for electron transport during OXPHOS. COX consists of 9–13 subunit polypeptides in eukaryotic cells. The three mitochondria-encoded subunits, i.e. COX I–III, form the catalytic core, which contains all of four redox centres, namely, CuB, haem a, and haem a3 in COX I and CuA in COX II, and functions to catalyse the electronic transfer from cytochrome c to molecular oxygen. The other subunits are nuclear-encoded and functionally important for assembling and stabilizing the holoenzyme and regulating COX activity [11]. For example, the largest nuclear-encoded subunit, COX IV, can affect the catalytic constant by altering the internal step of electron transfer between the redox centres via an oxygen concentration-regulated isoform substitution [12]. The mitochondria-encoded subunits (i.e. COX I and II) and the nuclear-encoded subunits (i.e. COX IV and Vb) can coordinate their expression to repress COX activity in response to short-term hypoxia [13]. Given the functional importance of COX in life, adaptive changes in COX activity can improve the OXPHOS for ATP supply during hypoxia. Several sequence variations of COX subunits have been reported in the mitochondrial genomes of high-altitude animals [14]. Functional divergence of COX, which is in conjunction with single nonsynonymous substitution, has also been discovered between geese at different altitudes [15]. Thus, functional variation in COX is an important mechanism for hypoxia resistance and adaptation to a long-term hypoxic environment. However, evidence for the causal link between COX function and hypoxia tolerance in high-altitude animals and the involved mechanism for regulating this enzymatic activity is inadequate.
The mechanism of hypoxia adaptation can be investigated by studying the insect species living in anoxic or hypoxic habitats [16]. Numerous behavioural, morphological and physiological adaptation mechanisms have been discovered in hypoxia-tolerant insects [16,17]. For example, the hairy gene in fruit flies acts as a metabolic switch that regulates hypoxia tolerance [18]. However, the mitochondrial mechanisms for insect hypoxia response and adaptation remain largely unknown. The migratory locust, Locusta migratoria, is the most widespread locust species in the world. The highest distribution of this species has been reported in the Yarlung Tsangpo River (Brahmaputra) basin, an important agricultural zone in Tibet. This basin is more than 3600 m a.s.l. and the oxygen partial pressure (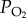 ) is less than 65 per cent of that at sea level. Phylogenetic studies based on mitochondrial genome sequences revealed genetic differences between the Tibetan and lowland populations of migratory locusts in China [19]. Therefore, locust populations that have adapted to the Tibetan Plateau and the lowlands can be used to study the natural inheritable mechanisms for hypoxia tolerance developed in their long-term adaptation to hypoxic environment.
) is less than 65 per cent of that at sea level. Phylogenetic studies based on mitochondrial genome sequences revealed genetic differences between the Tibetan and lowland populations of migratory locusts in China [19]. Therefore, locust populations that have adapted to the Tibetan Plateau and the lowlands can be used to study the natural inheritable mechanisms for hypoxia tolerance developed in their long-term adaptation to hypoxic environment.
In the present study, locust samples from the Tibetan Plateau (more than 3600 m) and North China lowlands (less than 70 m) were used to investigate the biochemical mechanisms for hypoxia response and adaptation. We hypothesized that mitochondrial OXPHOS regulation, particularly, the COX activity modulation, is involved in the hypoxia adaptation of Tibetan locusts. We first conducted a comparative study on the hypoxia response of the two locust populations in terms of physiology, mitochondrial morphology and OXPHOS. The critical components for OXPHOS related to electron transport were then examined. Finally, we confirmed through pharmacological intervention using the COX-specific inhibitor NaN3, that the inheritable functional divergence of COX contributed to the difference in hypoxia tolerance between the Tibetan and lowland locusts.
2. Material and methods
(a). Locust samples
High-altitude migratory locust populations were collected from three suburban localities in Lhasa in the Tibetan Plateau, i.e. Nêdong (SNND; adults living at an elevation of about 3700 m a.s.l.), Maizhokunggar (MZKG; 4000 m), and Doilungdêqên (DLDQ; 4000 m). Low-altitude populations from the North China lowlands were collected from Wudi (SDWD; 67 m), Dagang (TJDQ; less than 10 m) and Huanghua (HBHH; less than 10 m). These locusts were maintained in the laboratory at the Institute of Zoology, Chinese Academy of Sciences in Beijing (less than 50 m). All locust colonies were reared in well-ventilated cages (35 × 35 × 35 cm) at a density of more than 150 individuals per cage, and fed with a diet of fresh greenhouse-grown wheat seedlings and wheat bran. The culturing environment was kept constant with a 14 L : 10 D photo regime at 30±1°C. The cultures were maintained in the laboratory for at least five generations prior to the experiments to eliminate plastic traits derived from environmental heterogeneity and not inherited. Female adults, approximately 14–15-days old, were collected for all assays.
Representative locust geographical populations from the Tibetan Plateau and North China lowland regions were selected through a preliminary investigation on the physiological and biochemical hypoxia responses of the three geographical populations in each region. The interpopulation variation of these traits was analysed by one-way analysis of variance (ANOVA) followed by Tukey post hoc test. No significant difference in hypoxia tolerance, OXPHOS-related respiration activity, or respiratory chain complex activities was observed among the three geographical populations in either the Tibetan or lowland locusts under normoxic and hypoxic conditions (all p > 0.05, one-way ANOVA; all p > 0.05, Tukey post hoc test; see detailed results in the electronic supplementary material, table S1). Therefore, the three geographical populations in each of the altitudes were considered equal for hypoxia-responsive traits. One of the geographical populations, i.e. SDWD for lowland populations and SNND for Tibetan populations, was selected to represent each altitude population in the subsequent assays.
(b). Normobaric hypoxic treatment and hypoxic stupor detection
Normobaric hypoxic treatment was performed in a hypoxic chamber (FLYDWC-50; Fenglei Co., Ltd, China) with programmed airflow and pressure. Eight locusts were placed in a small plastic cage (15 × 15 × 10 cm) as a replicate. The cages were kept in the chamber, into which air was blown and balanced with pure nitrogen to achieve the required 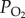 levels.
levels.
The locust hypoxic stupor percentage was measured under extremely hypoxic conditions, i.e. at five different 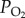 levels (1.2, 1.6, 2.0, 2.4 and 2.8 kPa) for 6 h at 30°C. During the hypoxic treatments, several locusts were paralysed in the first 15 min, and did not recover their abdomen and antennae movement after the first 45 min. Locusts were defined as ‘in stupor’ if no visible motion of the abdomen and the antennae were exhibited at the end of the hypoxic treatments. The control group was kept at 30°C under normal atmospheric conditions.
levels (1.2, 1.6, 2.0, 2.4 and 2.8 kPa) for 6 h at 30°C. During the hypoxic treatments, several locusts were paralysed in the first 15 min, and did not recover their abdomen and antennae movement after the first 45 min. Locusts were defined as ‘in stupor’ if no visible motion of the abdomen and the antennae were exhibited at the end of the hypoxic treatments. The control group was kept at 30°C under normal atmospheric conditions.
(c). Mitochondria isolation and submitochondrial particle preparation
Immediately after normoxic or hypoxic treatment at 1.6 kPa  for 6 h, flight muscles, including the dorsoventral, basilar and dorsal longitudinal muscles, were excised and placed in ice-cold isolation buffer (300 mM sucrose, 15 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid (HEPES), 2 mM ethylenediaminetetraacetic acid (EDTA), and 2.5 mg ml−1 bovine serum albumin (BSA); pH 7.4). After one wash with the isolation buffer, the tissues were 4-stroke homogenated with a glass homogenizer. The homogenates were then filtered through two layers of medical gauze. A fraction of the filtrate was stored at −76°C as the muscle homogenate sample, and the remainder was used for intact mitochondria isolation via differential centrifugation method using optimized centrifugal conditions (500g for 10 min followed by 5000g for 10 min at 4°C). The final flight muscle mitochondrial pellets were used for mitochondrial morphology detection or resuspended in isolation buffer for respiration assay. The transmission electron microscopy (TEM) images showed that the purity of the isolated mitochondria was above 95 per cent. Submitochondrial particles (SMPs) of flight muscle were prepared by sonicating the isolated mitochondria using an Ultrasonic Process analyzer (Sonics, USA) as follows: after resuspension in the sonicating buffer (isolation buffer without BSA), the mitochondria were sonicated in ice water for 6 min (20 s of sonication and 40 s of rest period per cycle, 150 W output power). The lysates were then centrifuged at 5000g at 4°C for 10 min, and then the supernatants were stored at −76°C until use.
for 6 h, flight muscles, including the dorsoventral, basilar and dorsal longitudinal muscles, were excised and placed in ice-cold isolation buffer (300 mM sucrose, 15 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid (HEPES), 2 mM ethylenediaminetetraacetic acid (EDTA), and 2.5 mg ml−1 bovine serum albumin (BSA); pH 7.4). After one wash with the isolation buffer, the tissues were 4-stroke homogenated with a glass homogenizer. The homogenates were then filtered through two layers of medical gauze. A fraction of the filtrate was stored at −76°C as the muscle homogenate sample, and the remainder was used for intact mitochondria isolation via differential centrifugation method using optimized centrifugal conditions (500g for 10 min followed by 5000g for 10 min at 4°C). The final flight muscle mitochondrial pellets were used for mitochondrial morphology detection or resuspended in isolation buffer for respiration assay. The transmission electron microscopy (TEM) images showed that the purity of the isolated mitochondria was above 95 per cent. Submitochondrial particles (SMPs) of flight muscle were prepared by sonicating the isolated mitochondria using an Ultrasonic Process analyzer (Sonics, USA) as follows: after resuspension in the sonicating buffer (isolation buffer without BSA), the mitochondria were sonicated in ice water for 6 min (20 s of sonication and 40 s of rest period per cycle, 150 W output power). The lysates were then centrifuged at 5000g at 4°C for 10 min, and then the supernatants were stored at −76°C until use.
(d). Mitochondrial morphology examination
Two types of samples, including dorsoventral muscle strips and isolated mitochondria, were used to detect mitochondrial morphology via TEM. The muscle strips or isolated mitochondrial pellets were fixed overnight in 2.5 per cent glutaraldehyde, post-fixed in 1 per cent osmium tetroxide for 1.5 h, dehydrated in 50–100% ethanol, soaked in a 1 : 1 epon and acetone mixture for 2 h, and then soaked in epon for embedding and cutting. Ultrathin sections were counterstained with uranyl acetate and imaged with a JEM-2100 LaB6 TEM (JEOL Ltd, Japan). The mitochondria were considered injured if their outer membranes were clearly broken, with more than 10 per cent of their cross-section missing. The total mitochondrial cross-sectional area in each image was determined using the NIH ImageJ software (http://rsb.info.nih.gov/ij/index.html) to calculate the mean of the individual mitochondrial cross-sectional area. Sample preparation for TEM and image analysis was performed via a double-blinded approach.
(e). Oxidative phosphorylation determination
NADH-linked OXPHOS of the isolated mitochondria was determined via the polarographic method using a Clark oxygen electrode (Strathkelvin Instruments, UK) under an ambient condition at 25°C, as previously described [20]. First, 75 μM of ADP was added in the modified respiration buffer (250 mM sucrose, 15 mM HEPES, 15 mM KH2PO4, 2 mM EDTA, and 4 mg ml−1 fatty acid-free BSA; pH 7.2) with 2 mM of pyruvate and 0.2 mM of malate. After the ADP was consumed, another 250 μM of ADP was mixed to initiate the state 3 stage, followed by the state 4 stage when the ADP was completely depleted. Afterwards, the uncoupler, carbonyl cyanide m-chlorophenyl hydrazone (CCCP, 1 μM), was added to initiate the maximal oxygen consumption. Respiratory control ratio (RCR) was calculated as the ratio of state 3 to state 4 respiration rate, whereas ADP : O (P : O) ratio was determined as the ratio of the added ADP micromoles to the consumed microatoms of oxygen during state 3 respiration. Mitochondrial membrane potentials (MMPs) were determined via a fluorometric method using rhodamine 123, as previously described [21]. Fluorometric density was monitored at an excitation/emission wavelength of 502 ± 2.5 nm/525 ± 5 nm using an F-4500 Fluorescence Spectrophotometer (HITACHI, Japan).
(f). Enzymatic activity assays
All enzymatic activities were measured via spectrophotometric methods using a microplate reader (VERSAmax, Molecular Devices, USA) at 30°C. The COX activities in SMPs were measured by the decreased absorbance owing to the oxidation of ferrocytochrome c (45 μM, reduced by sodium dithionite) in the reaction buffer (30 mM KH2PO4 and 1 mM EDTA; pH 7.2) at 550 nm (ɛ = 18.5 mM−1 cm−1), as previously described [20]. The activity of the COX molecules released from the respiratory chain supercomplex structure [22] in SMP was determined using the SMP treated with 3% (v/v) of Triton X-100 by mixing for at least 15 min at 4°C before enzymatic assay. This detergent treatment did not significantly affect the active core centre of COX (Z. Zhang and L. Kang 2012, unpublished data). The COX activities in SMPs were detected using a series of ferrocytochrome c starting concentrations (5, 10, 20, 30, 45, 65, 90 and 135 μM) to determine the ferrocytochrome c kinetics of COX. The Michaelis constant (Km) and the maximal enzymatic activity (Vmax) were then calculated by fitting the least-squares Michaelis–Menten regressions to the data.
The activities of NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), ubiquinol-cytochrome c reductase (complex III), NADH-cytochrome c reductase (complex I + III), pyruvate dehydrogenase complex (PDHc) and catalase were determined as described in the electronic supplementary material, supplementary methods.
(g). Determination of cytochrome c oxidase content and turnover number
The haem aa3 concentration in SMPs was calculated from the difference spectra of dithionite-reduced minus air-oxidized absorbance at 605 to 630 nm (ɛ = 24 mM−1 cm−1), as previously described [13]. The mRNA level of cox1 and cox4 as well as the protein content of cytochrome c and COX IV were measured by standard method of quantitative real-time PCR and Western blot, respectively, as described in the electronic supplementary material, supplementary methods. The COX turnover number was calculated by dividing the COX activity in the SMPs by the haem aa3 concentration.
(h). Cytochrome c oxidase inhibition assay
NaN3 treatment can inhibit COX activity with high specificity, but causes limited mitochondrial and cellular injuries [23]. A series of NaN3 doses (0, 9, 18, 27, 36 and 45 μg NaN3 per gram of locust) was used to inhibit COX. The locusts were injected twice with NaN3 at the second ventral segment of the abdomen at 12 h intervals. At about 12 h after the second injection, the activities of COX, PDHc and complexes I, II, and I + III in the SMPs, the state 3 respiration rate and ROS generation rate of the NADH-linked OXPHOS in the isolated mitochondria, and catalase activity in the homogenate of the flight muscle were measured. The stupor percentage in response to hypoxia was determined after treatment at 2.4 kPa 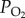 for 6 h.
for 6 h.
(i). Miscellaneous
The ATP concentration was determined using a modified method [24]. The samples (i.e. muscle mitochondria) were first fixed on ice with a 0.5 vol of 2.3 M perchloric acid for 30 min. The insoluble material was removed via centrifugation, and the supernatant was neutralized with 2.5 M KHCO3. Afterwards, the supernatant was filtered through a 0.45 μm membrane (Agilent, USA) and analysed via high-performance liquid chromatography (HPLC) at 254 nm using an Agilent 1100 HPLC system (Agilent, USA). The mitochondrial ROS generation rate was measured as described in the electronic supplementary material. Protein concentration was determined via bicinchoninic acid protein assay (Pierce, USA) and used to normalize the mitochondrial respiration, enzymatic activities and ATP concentration.
(j). Statistical analysis
All data were reported as mean ± s.e. The difference of stupor percentage across populations or a series of 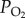 treatments was analysed using two-way ANOVA followed by Tukey post hoc test. The differences between the data obtained from two factors (i.e. populations ×
treatments was analysed using two-way ANOVA followed by Tukey post hoc test. The differences between the data obtained from two factors (i.e. populations ×  ), each of which having only two levels, were first analysed via two-way ANOVA. When a significant effect of any factor or an interaction between factors was detected, the Student's unpaired t-test was then performed between two populations after certain
), each of which having only two levels, were first analysed via two-way ANOVA. When a significant effect of any factor or an interaction between factors was detected, the Student's unpaired t-test was then performed between two populations after certain 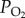 treatment or two
treatment or two 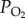 treatments in certain population. The data were log-transformed prior to two-way ANOVA when the variances across groups were not equal (p < 0.05, Levene's test). The stupor percentages were converted by the arcsin square root prior to analysis. Linear regressions were performed using ordinary least squares regression model, and the slopes of the linear regressions of the two locust populations were compared using analysis of covariance [25]. The differences between the mean values were considered significant when p < 0.05. All statistical analyses were performed using SPSS 13.0.
treatments in certain population. The data were log-transformed prior to two-way ANOVA when the variances across groups were not equal (p < 0.05, Levene's test). The stupor percentages were converted by the arcsin square root prior to analysis. Linear regressions were performed using ordinary least squares regression model, and the slopes of the linear regressions of the two locust populations were compared using analysis of covariance [25]. The differences between the mean values were considered significant when p < 0.05. All statistical analyses were performed using SPSS 13.0.
3. Results
(a). Locust hypoxia response in physiology, mitochondrial morphology and ATP concentration
We first compared the hypoxia tolerance of the Tibetan and lowland populations by exposing adult locusts to a series of low 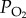 levels and recording the number of individuals going into stupor after 6 h of treatment. The hypoxic stupor percentage of both populations significantly increased with decreasing
levels and recording the number of individuals going into stupor after 6 h of treatment. The hypoxic stupor percentage of both populations significantly increased with decreasing 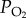 (p < 0.001, two-way ANOVA; all p < 0.05, Turkey post hoc test for factor of
(p < 0.001, two-way ANOVA; all p < 0.05, Turkey post hoc test for factor of  ). The Tibetan locusts were significantly less immobilized by hypoxia than the lowland ones (p < 0.001, two-way ANOVA; figure 1a). At 1.6 kPa
). The Tibetan locusts were significantly less immobilized by hypoxia than the lowland ones (p < 0.001, two-way ANOVA; figure 1a). At 1.6 kPa 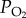 , the Tibetan and the lowland locust populations exhibited the largest difference in stupor percentage at 25.8 ± 3.3% and 85.4 ± 2.8%, respectively (p < 0.001, t-test). Thus, 6 h exposure to 1.6 kPa
, the Tibetan and the lowland locust populations exhibited the largest difference in stupor percentage at 25.8 ± 3.3% and 85.4 ± 2.8%, respectively (p < 0.001, t-test). Thus, 6 h exposure to 1.6 kPa  was used as the hypoxic treatment condition in all subsequent experiments.
was used as the hypoxic treatment condition in all subsequent experiments.
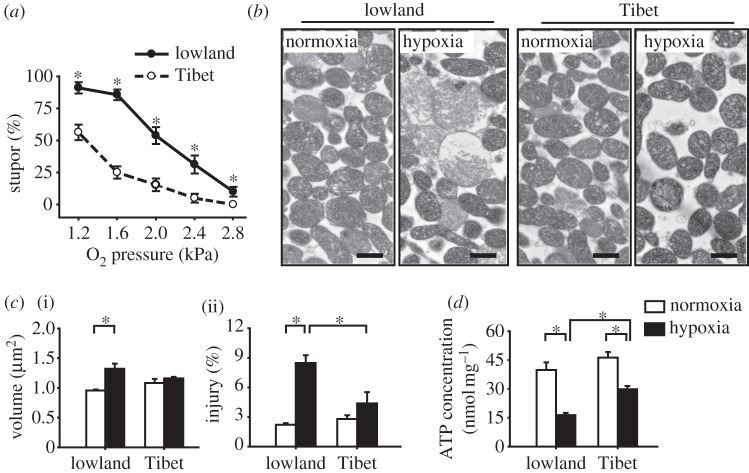
Figure 1.
Differences between the hypoxia responses of Tibetan and lowland migratory locust populations in terms of stupor percentage, mitochondrial morphology, and ATP concentration. (a) Percentages of locusts in stupor in the two locust populations after various oxygen partial pressure (PO2) treatments for 6 h. The most significant difference was detected under 1.6 kPa PO2; thus, this hypoxic treatment was used in all subsequent assays. All samples have six replicates (n = 6), except n = 21 for treatment under 1.6 kPa PO2. (b,c) Mitochondrial morphology in the ultrathin section of the isolated mitochondrial pellet in the two locust populations under normoxic and hypoxic treatments. Representative micrographs of the mitochondria are shown in (b), as well as the volume of individual mitochondrion in (c, (i) n = 7) and the percentages of injured mitochondria in (c, (ii) n = 7). The black bars represent 1 μm. (d) ATP concentrations in the flight muscle mitochondria after normoxic and hypoxic treatments. Normoxia: 21 kPa PO2. Hypoxia: 1.6 kPa PO2. *p < 0.05 (t-test) for comparisons between the Tibetan and lowland locusts under the same PO2 treatment in (a), as well as for the represented comparisons in (c) and (d).
We then examined the structural changes in the isolated mitochondria after hypoxic exposure by observing the mitochondrial morphologies via TEM. For the lowland locusts, hypoxia caused observable morphological changes in the flight muscle mitochondria (figure 1b) by increasing the mitochondrial volume (1.38-fold; p = 0.005, t-test) and the percentage of injured mitochondria (3.83-fold; p < 0.001, t-test) (figure 1c). By contrast, the mitochondrial morphology of the Tibetan locusts was unaffected by hypoxia. We also examined the mitochondrial morphology in the dorsoventral muscle tissue to confirm that our method involving isolated mitochondria has a minor effect on the mitochondrial structure alteration caused by hypoxia. Consistent with the results in the isolated mitochondrial samples, only the mitochondria in the muscle samples of lowland locusts became swollen after hypoxic treatment (see the electronic supplementary material, figure S1a).
Finally, we examined the effects of hypoxia on energy supply from mitochondrial respiration by determining the ATP concentrations in the isolated muscle mitochondria via HPLC. Hypoxia significantly decreased the ATP concentration of both Tibetan and lowland populations (p < 0.001, two-way ANOVA; all p < 0.001, t-test). However, the extent of the decrease was larger in the lowland locusts, with 58.9 per cent decrease versus the 35.5 per cent decrease in the Tibetan specimens, respectively. Thus, ATP in Tibetan locusts was significantly higher than that of lowland locusts after hypoxic treatment (p < 0.001, t-test), although no significant difference was detected under normoxic conditions (figure 1d). Similar results for ATP were observed in the muscle homogenates (see the electronic supplementary material, figure S1b).
(b). Hypoxia-induced oxidative phosphorylation alterations in flight muscle mitochondria
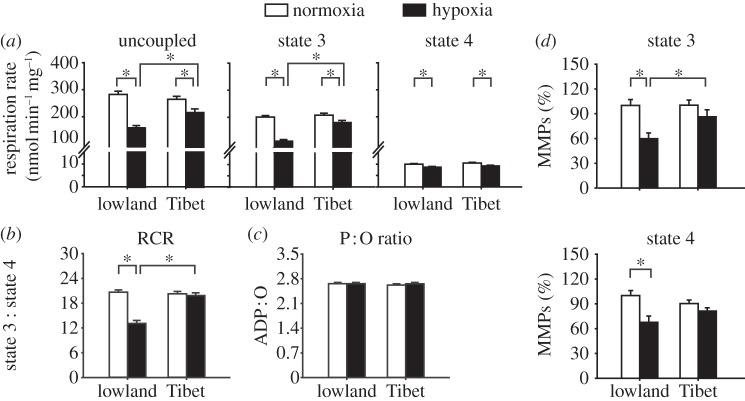
We determined whether the decreased ATP content under hypoxia was because of the altered mitochondrial respiration by investigating NADH-linked OXPHOS. The rates of uncoupled respiration by CCCP and the states 3 and 4 respirations significantly decreased in the muscle mitochondria of both populations after hypoxic treatment (all p < 0.001, two-way ANOVA; all p < 0.05, t-test). However, the reductions in the uncoupled and state 3 respiration rates were significantly greater in the lowland locusts than those in the Tibetan specimens. In particular, the uncoupled respiration in the lowland and Tibetan populations decreased by 44.0 and 19.4 per cent, respectively, whereas state 3 respiration decreased by 44.5 and 13.1 per cent, respectively. Thus, uncoupled and state 3 respiration rates in the Tibetan locusts were significantly higher than that in the lowland locusts after hypoxic treatment (p = 0.005 and p < 0.001, respectively; t-test). However, no difference was observed between the two populations under normoxic conditions (figure 2a). The RCR significantly decreased in the lowland locusts (p < 0.001, t-test), but not in the Tibetan locusts, upon hypoxic exposure (figure 2b). The P : O ratios of both populations were not affected by the hypoxic treatment (figure 2c). The MMPs in states 3 and 4 respirations in the lowland locusts significantly decreased after the hypoxic treatments (p = 0.002 and 0.007, respectively; t-test); this result was not observed in the Tibetan specimens (figure 2d).
Figure 2.
Hypoxia downregulates NADH-linked OXPHOS in the flight muscle mitochondria of Tibetan and lowland locusts. (a–c) The rates of uncoupled, state 3, and state 4 respirations (a), as well as the RCR (b) and ADP : O (P : O) ratio (c) (n = 24, 22, 22, and 21 for lowland-normoxia, lowland-hypoxia, Tibet-normoxia, and Tibet-hypoxia, respectively). (d) Mitochondrial membrane potentials (MMPs); data are expressed as percentages of the respective lowland-normoxia sample (n = 6). Normoxia: 21 kPa PO2. Hypoxia: 1.6 kPa PO2. *p < 0.05 (t-test).
(c). Enhancement of cytochrome c oxidase activity promotes hypoxia tolerance in Tibetan locusts
We investigated the critical components of OXPHOS that underlie the higher hypoxia tolerance of the Tibetan locusts. These components include complexes I, I + III, III (electronic supplementary material, figures S2a–S2c), COX (figure 3a), and mitochondrial cytochrome c contents (see the electronic supplementary material, figure S2d). Only the COX activity in the flight muscle SMPs was significantly higher in the Tibetan locusts than in the lowland ones (p < 0.001, two-way ANOVA) under normoxia (1.30-fold) and hypoxia (1.28-fold) (both p < 0.001, t-test), and was not affected by hypoxic exposure (p = 0.586, two-way ANOVA) (figure 3a, left panel). The SMPs were treated with 3 per cent Triton X-100 to detect the activities of free COX molecule released from respiratory chain supercomplexes embedded in the mitochondrial inner membrane. The COX activities of Tibetan locusts were still significantly higher (1.35-fold) than that of the lowland ones under nomoxia (p = 0.018, t-test) (figure 3a, right panel). The similarity of interpopulation differences in COX activities between detergent-treated and untreated SMPs suggests that the enhanced COX activity was not caused by the respiratory chain supercomplex structure through its effect on the interaction between COX and ferrocytochrome c [26].
Figure 3.
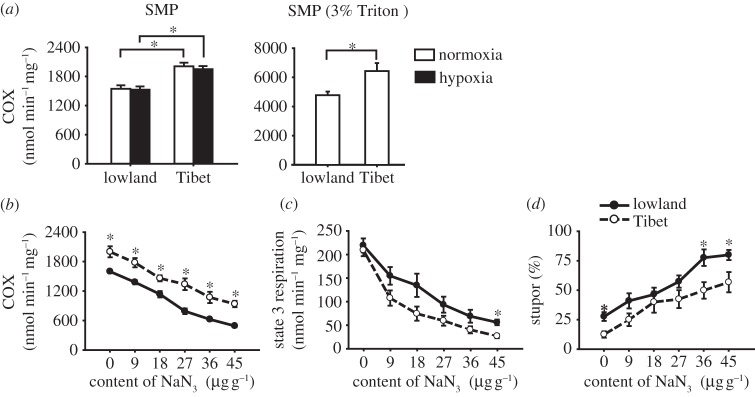
Changes in cytochrome c oxidase (COX) activity affect the hypoxia tolerance in Tibetan and lowland locusts. (a) Difference in the COX activities between the flight muscle mitochondria of Tibetan and lowland locusts under both hypoxic and normoxic conditions. Two types of samples were used: SMPs (n = 17, 16, 17 and 18 for lowland-normoxia, lowland-hypoxia, Tibet-normoxia, and Tibet-hypoxia, respectively) and SMPs with 3 per cent Triton X-100 (n = 7). Normoxia: 21 kPa PO2. Hypoxia: 1.6 kPa PO2. (b–d) Changes in the COX activity (b) in the SMP, the state 3 respiration rate of NADH-linked OXPHOS (d) in the mitochondria, as well as the changes in the hypoxic stupor percentage (d) caused by different NaN3 concentrations (n = 7). *p < 0.05 (t-test) for the represented comparison in (a), and for the comparisons between Tibetan and lowland locusts under the same NaN3 injection concentration in (b–d).
We examined the effect of COX activity inhibition on the hypoxia sensitivity of the migratory locusts using the COX-specific inhibitor NaN3 to establish the causal relationship between COX activity and hypoxia tolerance. The gradual increase in the injected NaN3 concentrations (from 0 to 45 μg NaN3 per gram of locust) resulted in a linear decrease in the COX activities in the SMPs (figure 3b) in both locust populations. The state 3 respiration rate of the NADH-linked OXPHOS of both populations also linearly decreased with increasing NaN3 concentrations (figure 3c), resulting in a gradual increase in the percentage of locusts in hypoxic stupor (figure 3d). Further linear regression analysis showed that the state 3 respiration rate was positively correlated with COX activity (Tibetan: r2 = 0.450, s.e. of slope = 0.018; lowland: r2 = 0.583, s.e. of slope = 0.017). However, the stupor percentage was negatively correlated with the state 3 respiration rate (Tibetan: r2 = 0.406, s.e. of slope = 0.041; lowland: r² = 0.368, s.e. of slope = 0.045) and with the COX activity (Tibetan: r2 = 0.344, s.e. of slope = 0.007; lowland: r2 = 0.612, s.e. of slope = 0.006) (see the electronic supplementary material, figures S3a–S3c). We investigated the different sensitivities of hypoxia tolerance to the variations in COX activity between the Tibetan and lowland locusts by comparing the slopes of regression lines for every two of the three factors (COX activity, state 3 respiration rate of the NADH-linked OXPHOS, and hypoxic stupor percentage) as described above between the two locust populations. No significant difference between the slopes of the two populations was found (all p > 0.05, t-test). Thus, decreased COX activity can reduce the hypoxia tolerance of both locust populations by reducing OXPHOS in a similar pattern and sensitivity.
NaN3 injection may affect hypoxia tolerance by aggravating oxidative stress. This possible effect was excluded by determining the rates of mitochondrial ROS generation and total catalase in the flight muscles of both locust populations after H2O and NaN3 injection (45 μg NaN3 per gram of locust). The results indicated that mitochondrial ROS generation and catalase were not affected by NaN3 injection (p = 0.157 and 0.089, two-way ANOVA, respectively) in both locust populations (electronic supplementary material, figure S3d,e). The activities of other crucial enzymes for OXPHOS, including PDHc and complexes I, I + III, and II, after H2O and NaN3 injections (45 μg NaN3 per gram of locust) were compared to identify the inhibitory specificity of NaN3 to COX activity. No significant change was observed in any detected enzyme activity, except for the enhanced complex II activity in both lowland and Tibetan populations (p < 0.001 and p = 0.001, respectively; t-test) (see the electronic supplementary material, figures S3f–S3i).
(d). Enhanced cytochrome c oxidase activity owing to increased catalytic efficiency
We determined whether increased enzymatic content or catalytic efficiency contributed to enhanced COX activity in the Tibetan locusts. For COX content, no significant difference was observed in the haem aa3 concentration between the Tibetan and lowland locusts under normoxic or hypoxic conditions (figure 4a). The gene expression of two of the COX subunits, namely, cox1 and cox4, showed no significant differences between the two populations under normoxic conditions, except for the significant decrease upon hypoxic treatment in the lowland population (p = 0.027 and 0.002, respectively; t-test) (see the electronic supplementary material, figure S4a). The COX IV peptide expression showed no significant difference between the two populations after normoxic or hypoxic treatments (see the electronic supplementary material, figure S4b). However, an investigation of the COX catalytic efficiency revealed that the turnover number for COX was significantly higher in the Tibetan locusts than that in the lowland specimens (p = 0.001, two-way ANOVA) under both normoxic and hypoxic treatments (p = 0.015 and 0.013, respectively; t-test). The relative difference was not affected by the hypoxic treatment (p = 0.574, two-way ANOVA; figure 4b).
Figure 4.

Increased enzymatic catalytic efficiency enhances the COX activity in the flight muscle of Tibetan locusts under normoxic and hypoxic conditions. (a) Haem aa3 concentrations (n = 7). (b) COX turnover number (n = 7). Normoxia: 21 kPa PO2. Hypoxia: 1.6 kPa PO2. (c) Plot of Michaelis–Menten enzyme kinetics; the data points indicate the average value of each SMP sample. (d) Ferrocytochrome c kinetics of COX in SMPs, including the Michaelis constant (Km, the concentration of ferrocytochrome c at half-maximal activity) (i) and the maximum COX activity (Vmax) (ii), were calculated from the Michaelis–Menten regressions (n = 7 and 6 for Tibet and lowland, respectively). *p < 0.05 (t-test).
We subsequently determined whether the enzymatic affinity for ferrocytochrome c and the electron transport capability via the catalytic redox centre contributed to enhanced COX catalytic efficiency. We investigated the ferrocytochrome c kinetics of COX by measuring the COX activity in flight muscle SMPs under ferrocytochrome c gradient initial concentrations (figure 4c). Lower Km and higher Vmax of COX (p = 0.027 and 0.018, respectively; t-test) were obtained in the Tibetan locusts than in the lowland samples under normoxic conditions (figure 4d).
4. Discussion
The Tibetan Plateau is one of the most extreme environments in the world, where animals have adapted and evolved distinctive traits to cope with local hypoxic stress. Compared with lowland populations, Tibetan locusts exhibit distinct adaptive physiological traits in terms of hypoxia response, including lower incidence of stupor, better-maintained mitochondrial structure, higher ATP content, and less OXPHOS interference. Migratory locusts are generally highly tolerant to hypoxia compared with mammals [8]. Most locusts remain lively under severe hypoxic exposure at 2.8 kPa 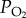 (figure 1a). Their flight muscle mitochondria also exhibit limited inhibition and uncoupling of OXPHOS function. These characteristics may be because of their tracheal system that enables efficient oxygen transport and diffusion [27]. Thus, dramatic functional divergence in the mitochondrial hypoxia response of individuals can only be clearly revealed by comparing Tibetan locusts, which are uniquely adaptive to extreme high-altitude environments, with lowland locusts, which thrive at sea-level conditions. By investigating two populations from different altitudes, our study has confirmed, for the first time to our knowledge, that enhanced COX activity increases the mitochondrial robustness of Tibetan locusts to hypoxic stress. The mechanistic study on COX activity regulation reveals the significance of the catalytic efficiency of COX for OXPHOS, which further explains the regulatory mechanism involved in hypoxia adaptation.
(figure 1a). Their flight muscle mitochondria also exhibit limited inhibition and uncoupling of OXPHOS function. These characteristics may be because of their tracheal system that enables efficient oxygen transport and diffusion [27]. Thus, dramatic functional divergence in the mitochondrial hypoxia response of individuals can only be clearly revealed by comparing Tibetan locusts, which are uniquely adaptive to extreme high-altitude environments, with lowland locusts, which thrive at sea-level conditions. By investigating two populations from different altitudes, our study has confirmed, for the first time to our knowledge, that enhanced COX activity increases the mitochondrial robustness of Tibetan locusts to hypoxic stress. The mechanistic study on COX activity regulation reveals the significance of the catalytic efficiency of COX for OXPHOS, which further explains the regulatory mechanism involved in hypoxia adaptation.
Our comparative study revealed that Tibetan locusts have an inherently higher hypoxia tolerance in terms of multiple physiological traits. This higher hypoxia tolerance of the Tibetan locusts is contributed by the constitutively enhanced COX activity that results from the improved catalytic efficiency. Unlike the COX functional modulation response to short-term hypoxia [12,13], enhanced COX activity in Tibetan locusts was still retained even after five generations of rearing under normoxic conditions and was not affected by the severe hypoxic treatments used (figure 3a). COX activity enhancement helps locusts maintain high aerobic respiration (figure 3c). Thus, Tibetan locusts can maintain high energy supply with limited mitochondrial damage under hypoxic conditions (figure 1). Such physiological alterations can benefit the locusts' organismal performance in the permanent hypoxic environment of the Tibetan Plateau. This observation is consistent with the findings obtained from observing other animals that adapted to high altitude [15,28]. These results suggest that the higher COX activity in Tibetan locusts is an inheritable phenotype that evolved via natural selection in the Tibetan Plateau environment. The comparison between high- and low-altitude goose species shows a striking alteration in the cytochrome c kinetics of COX in high-altitude bar-headed geese [15]. These high-altitude geese had an exceptionally high metabolic rate in conditions of severe hypoxia evolved for their migration over the Himalayas [29]. Accordingly, adaption to high-altitude hypoxia by enhancing COX activity may be a common mechanism developed in animals through convergent evolution. However, unlike previous studies, our work establishes the causality between COX activity and hypoxia resistance of animals that adapted to long-term hypoxia. To our knowledge, this study is the first to propose a mechanism that considers COX functional changes in the hypoxia adaptation of insects at areas of high altitude such as the Tibetan Plateau.
Our study reveals two regulatory mechanisms for the enhanced COX catalytic efficiency in Tibetan locusts, namely, enhanced COX affinity for ferrocytochrome c and improved capability of electron transport via the catalytic redox centre. The limited oxygen supply in hypoxia can shift the redox status of the respiratory chain towards a more-reduced status that increases mitochondrial ROS generation [30]. Thus, high-altitude animals can reduce ROS production by decreasing the reduced status of the respiratory chain [15]. In Tibetan locusts, the two regulatory mechanisms that enhance COX catalytic efficiency allow the mitochondria to maintain a certain COX activity with less reduced cytochrome c, which causes a more-oxidized redox state in the electron transport chain and thus decreases the ROS production. Meanwhile, the improved ferrocytochrome c kinetics of COX can increase the COX activity of Tibetan locusts at a certain redox status of cytochrome c to maintain high electron flux through the respiratory chain for aerobic respiration in a hypoxic environment. Thus, Tibetan locusts can maintain a high energy supply without the cellular oxidative damage caused by aggressive ROS generation. The enhancement of COX catalytic efficiency may also be affected by increased COX affinity for oxygen. However, previous studies revealed that the kinetic value of COX for oxygen is extremely low in organisms, from yeasts to birds and mammals, living in normoxic conditions, e.g. as low as 0.025 kPa 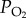 in isolated rat liver mitochondria [31]. Consequently, the COX in animals living in low-altitude areas can react with oxygen even under extremely low oxygen pressure because of the extremely high affinity of COX for oxygen. Thus, we do not expect to see any significant improvement in the COX affinity for oxygen in Tibetan locusts.
in isolated rat liver mitochondria [31]. Consequently, the COX in animals living in low-altitude areas can react with oxygen even under extremely low oxygen pressure because of the extremely high affinity of COX for oxygen. Thus, we do not expect to see any significant improvement in the COX affinity for oxygen in Tibetan locusts.
The difference in the cytochrome c kinetics of COX between altitudes may have evolved through changes in the COX subunit composition or structure. Previous studies showed that human and yeast cells can respond to hypoxia by altering the relative expression of two cox4 isoforms. The reciprocal patterns of the COX isoform expressions may alter the Vmax of COX by affecting the electron transfer through catalytic redox centre [9,12]. In this study, only one cox4 gene was detected in the locusts based on the locust transcriptome [32] and expressed sequence tags database (http://locustdb.genomics.org.cn/). The gene expression analysis of cox1 and cox4 did not reveal any difference between the Tibetan and lowland locusts, and neither did the peptide level analysis of COX IV. Sequence variation in the COX genes may also be responsible for the alterations in the COX structure and activity. For example, an amino acid substitution (Trp-116 → Arg) in COX III was correlated with the alteration cytochrome c kinetics of COX in bar-headed geese [15]. We cloned 13 COX subunit genes of the locusts and compared the sequence differences of the locust populations in the two altitudes. Two and three nonsynonymous fixed mutations were observed in subunit cox1 and cox3, respectively (Z. Zhang and L. Kang 2012, unpublished data). However, considering the different phylogenetic history of the two locust populations [19], more study is needed to dissect the evolutionary origins of these genetic changes including selection, genetic drift and ancestry.
The present study suggests two physiological strategies that are important for the high-altitude adaptation of the Tibetan locusts. The first strategy is to maintain aerobic respiration, which is essential for the ATP supply in normal physiological activities. Aside from the mechanism that enhances COX activity for oxygen use in Tibetan locusts living in hypoxic conditions, several other physiological mechanisms critical for oxygen supply have also been reported. For example, bar-headed geese have exhibited significant improvement through a series of cascading physiological steps of oxygen transport during hypoxia [15,33]. The genetic basis of the improved haemoglobin–oxygen affinity has been correlated with the substitutions in five exterior amino acid residues in high-altitude deer mice [34]. The second strategy is to repress the hypoxia response, e.g. in the Tibetan locusts. Hypoxia response may benefit temporary adaptive adjustment to short-term hypoxia, but can cause harm to the normal survival and reproductive physiology of animals, such us humans [3], under long-term hypoxia in high-altitude regions. Thus, the high-altitude animals tend to eliminate their response to the permanent hypoxia. For example, the bar-headed geese presented less hypoxia response in terms of ventilatory, cardiac and erythropoietic performance compared with the lowland ducks [35]. These findings about indigenous insects, birds and mammals suggest that animals living in high altitudes have developed hypoxia adaptation strategies to maintain aerobic respiration for continuous ATP supply and reduce hypoxia responses for preventing mitochondrial and cellular injuries [8]. Thus, our results have shed light on the adaptive divergence in the mitochondrial function of organisms living in hypoxic and normoxic environments, and elucidated the evolutionary patterns of hypoxia adaptation in indigenous highland animals.
Acknowledgements
This study was supported by grants from the State Key Basic Research and Development Project (2012CB114103), and the National Natural Science Foundation of China (31070336 and 31172148).
Reference
- 1.Zhou J, Schmid T, Schnitzer S, Brune B. 2006. Tumor hypoxia and cancer progression. Cancer Lett. 237, 10–21 10.1016/j.canlet.2005.05.028 (doi:10.1016/j.canlet.2005.05.028) [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. 2007. Life with oxygen. Science 318, 62–64 10.1126/science.1147949 (doi:10.1126/science.1147949) [DOI] [PubMed] [Google Scholar]
- 3.Hackett PH, Roach RC. 2001. Current concepts: high-altitude illness. N. Engl. J. Med. 345, 107–114 10.1056/NEJM200107123450206 (doi:10.1056/NEJM200107123450206) [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. 2007. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, m8. 10.1126/stke.4072007cm8 (doi:10.1126/stke.4072007cm8) [DOI] [PubMed] [Google Scholar]
- 5.Storz JF. 2010. Genes for high altitudes. Science 329, 40–41 10.1126/science.1192481 (doi:10.1126/science.1192481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Hua Z, Tame JR, Lu G, Zhang R, Gu X. 1996. The crystal structure of a high oxygen affinity species of haemoglobin (bar-headed goose haemoglobin in the oxy form). J. Mol. Biol. 255, 484–493 10.1006/jmbi.1996.0040 (doi:10.1006/jmbi.1996.0040) [DOI] [PubMed] [Google Scholar]
- 7.Scheffler IE. 2001. A century of mitochondrial research: achievements and perspectives. Mitochondrion 1, 3–31 10.1016/S1567-7249(00)00002-7 (doi:10.1016/S1567-7249(00)00002-7) [DOI] [PubMed] [Google Scholar]
- 8.Magalhaes J, Ascensão A, Soares JMC, Ferreira R, Neuparth MJ, Marques F, Duarte JA. 2005. Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J. Appl. Physiol. 99, 1247–1253 10.1152/japplphysiol.01324.2004 (doi:10.1152/japplphysiol.01324.2004) [DOI] [PubMed] [Google Scholar]
- 9.Fukuda R, Zhang HF, Kim JW, Shimoda L, Dang CV, Semenza GL. 2007. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129, 111–122 10.1016/j.cell.2007.01.047 (doi:10.1016/j.cell.2007.01.047) [DOI] [PubMed] [Google Scholar]
- 10.Solaini G, Harris DA. 2005. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem. J. 390, 377–394 10.1042/BJ20042006 (doi:10.1042/BJ20042006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capaldi RA. 1990. Structure and function of cytochrome c oxidase. Annu. Rev. Biochem. 59, 569–596 10.1146/annurev.bi.59.070190.003033 (doi:10.1146/annurev.bi.59.070190.003033) [DOI] [PubMed] [Google Scholar]
- 12.Burke PV, Poyton RO. 1998. Structure/function of oxygen-regulated isoforms in cytochrome c oxidase. J. Exp. Biol. 201, 1163–1175 [DOI] [PubMed] [Google Scholar]
- 13.Vijayasarathy C, Damle S, Prabu SK, Otto CM, Avadhani NG. 2003. Adaptive changes in the expression of nuclear and mitochondrial encoded subunits of cytochrome c oxidase and the catalytic activity during hypoxia. Eur. J. Biochem. 270, 871–879 10.1046/j.1432-1033.2003.03447.x (doi:10.1046/j.1432-1033.2003.03447.x) [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Gao W, Gao Y, Tang S, Huang Q, Tan X, Chen J, Huang T. 2008. Mitochondrial genome analysis of Ochotona curzoniae and implication of cytochrome c oxidase in hypoxic adaptation. Mitochondrion 8, 352–357 10.1016/j.mito.2008.07.005 (doi:10.1016/j.mito.2008.07.005) [DOI] [PubMed] [Google Scholar]
- 15.Scott GR, Schulte PM, Egginton S, Scott AL, Richards JG, Milsom WK. 2011. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol. 28, 351–363 10.1093/molbev/msq205 (doi:10.1093/molbev/msq205) [DOI] [PubMed] [Google Scholar]
- 16.Hoback WW, Stanley DW. 2001. Insects in hypoxia. J. Insect Physiol. 47, 533–542 10.1016/S0022-1910(00)00153-0 (doi:10.1016/S0022-1910(00)00153-0) [DOI] [PubMed] [Google Scholar]
- 17.Harrison J, Frazier MR, Henry JR, Kaiser A, Klok CJ, Rascon B. 2006. Responses of terrestrial insects to hypoxia or hyperoxia. Respir. Physiol. Neurobiol. 154, 4–17 10.1016/j.resp.2006.02.008 (doi:10.1016/j.resp.2006.02.008) [DOI] [PubMed] [Google Scholar]
- 18.Zhou D, Xue J, Lai J, Schork NJ, White KP, Haddad GG. 2008. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 4, e1000221. 10.1371/journal.pgen.1000221 (doi:10.1371/journal.pgen.1000221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma C, Yang P, Jiang F, Chapuis M-P, Shali Y, Sword GA, Kang L. 2012. Mitochondrial genomes reveal the global phylogeography and dispersal routes of the migratory locust. Mol. Ecol. 21, 4344–4358 10.1111/j.1365-294X.2012.05684.x (doi:10.1111/j.1365-294X.2012.05684.x) [DOI] [PubMed] [Google Scholar]
- 20.Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. 2005. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 390, 501–511 10.1042/BJ20042130 (doi:10.1042/BJ20042130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scaduto RC, Jr, Grotyohann LW. 1999. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 76, 469–477 10.1016/S0006-3495(99)77214-0 (doi:10.1016/S0006-3495(99)77214-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. 2008. Respiratory active mitochondrial supercomplexes. Mol. Cell 32, 529–539 10.1016/j.molcel.2008.10.021 (doi:10.1016/j.molcel.2008.10.021) [DOI] [PubMed] [Google Scholar]
- 23.Bennett MC, Mlady GW, Kwon YH, Rose GM. 1996. Chronic in vivo sodium azide infusion induces selective and stable inhibition of cytochrome c oxidase. J. Neurochem. 66, 2606–2611 10.1046/j.1471-4159.1996.66062606.x (doi:10.1046/j.1471-4159.1996.66062606.x) [DOI] [PubMed] [Google Scholar]
- 24.Schweinsberg PD, Loo TL. 1980. Simultaneous analysis of ATP, ADP, AMP, and other purines in human erythrocytes by high-performance liquid chromatography. J. Chromatogr. 181, 103–107 10.1016/S0378-4347(00)81276-1 (doi:10.1016/S0378-4347(00)81276-1) [DOI] [PubMed] [Google Scholar]
- 25.Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. 2005. CSF iron, ferritin and transferrin levels in restless legs syndrome. J. Sleep Res. 14, 43–47 10.1111/j.1365-2869.2004.00403.x (doi:10.1111/j.1365-2869.2004.00403.x) [DOI] [PubMed] [Google Scholar]
- 26.Schagger H. 2001. Respiratory chain supercomplexes. IUBMB Life 52, 119–128 10.1080/15216540152845911 (doi:10.1080/15216540152845911) [DOI] [PubMed] [Google Scholar]
- 27.Burmester T, Hankeln T. 2007. The respiratory proteins of insects. J. Insect Physiol. 53, 285–294 10.1016/j.jinsphys.2006.12.006 (doi:10.1016/j.jinsphys.2006.12.006) [DOI] [PubMed] [Google Scholar]
- 28.Hochachka PW, Stanley C, Merkt J, Sumar-Kalinowski J. 1983. Metabolic meaning of elevated levels of oxidative enzymes in high altitude adapted animals: an interpretive hypothesis. Respir. Physiol. 52, 303–313 10.1016/0034-5687(83)90087-7 (doi:10.1016/0034-5687(83)90087-7) [DOI] [PubMed] [Google Scholar]
- 29.Ward S, Bishop CM, Woakes AJ, Butler PJ. 2002. Heart rate and the rate of oxygen consumption of flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus). J. Exp. Biol. 205, 3347–3356 [DOI] [PubMed] [Google Scholar]
- 30.Dosek A, Ohno H, Acs Z, Taylor AW, Radak Z. 2007. High altitude and oxidative stress. Respir. Physiol. Neurobiol. 158, 128–131 10.1016/j.resp.2007.03.013 (doi:10.1016/j.resp.2007.03.013) [DOI] [PubMed] [Google Scholar]
- 31.Scandurra FM, Gnaiger E. 2010. Cell respiration under hypoxia: facts and artefacts in mitochondrial oxygen kinetics. Adv. Exp. Med. Biol. 662, 7–25 10.1007/978-1-4419-1241-1_2 (doi:10.1007/978-1-4419-1241-1_2) [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Yang P, Jiang F, Wei Y, Ma Z, Kang L. 2010. De novo analysis of transcriptome dynamics in the migratory locust during the development of phase traits. PLoS ONE 5, e15633. 10.1371/journal.pone.0015633 (doi:10.1371/journal.pone.0015633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott GR, Egginton S, Richards JG, Milsom WK. 2009. Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc. R. Soc. B 276, 3645–3653 10.1098/rspb.2009.0947 (doi:10.1098/rspb.2009.0947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storz JF, Sabatino SJ, Hoffmann FG, Gering EJ, Moriyama H, Ferrand N, Monteiro B, Nachman MW. 2007. The molecular basis of high-altitude adaptation in deer mice. PLoS Genet. 3, e45. 10.1371/journal.pgen.0030045 (doi:10.1371/journal.pgen.0030045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Black CP, Tenney SM. 1980. Oxygen transport during progressive hypoxia in high-altitude and sea-level waterfowl. Respir. Physiol. 39, 217–239 10.1016/0034-5687(80)90046-8 (doi:10.1016/0034-5687(80)90046-8) [DOI] [PubMed] [Google Scholar]