Abstract
It is well established that the expression of many ornamental traits is dependent on the current condition of the bearer. However, conditions experienced in early life are also known to be important for an individual's subsequent fitness and therefore, directly or indirectly, for the fitness of their mate. Specifically, a recent hypothesis suggests that sexually selected traits might be sensitive to conditions experienced during early-life development and thereby function as honest indicators of developmental history. Whether this applies to colourful male plumage, however, is largely unknown. We tested this idea with a field experiment by manipulating neonatal nutrition in a sexually dichromatic passerine, the hihi (Notymystis cincta). We found that carotenoid supplementation increased nestling plasma carotenoid concentration, which was in turn correlated with increased yellow saturation in male breeding plumage after moulting. We also found that the post-moult luminance (lightness) of the white ear-tufts tended to be reduced in males that had received an all-round nutritional supplement as nestlings. Black breeding plumage was not affected by neonatal nutritional treatment. Although the mechanisms that generate colourful plumage are evidently diverse, our results show that at least some parts of this display are accurate indicators of environmental conditions during development.
Keywords: hihi, developmental stress hypothesis, nutrition, carotenoid-based plumage, melanin-based plumage, structural colours
1. Introduction
Environmental conditions during development are of crucial importance for an individual's subsequent life history [1]. In particular, nutritional deficiencies during prenatal and/or early postnatal development can have subtle long-term consequences beyond obvious effects on immediate growth rates. For example, some adult secondary sexual traits, such as song repertoire size in great reed warblers (Acrocephalus arundinaceus) [2] and eye-span in stalk eyed flies (Cyrtodiopsis dalmanni) [3], are detrimentally affected by a poor nutritional start in life. The developmental stress hypothesis (DSH) was originally proposed to explain how song complexity might honestly indicate male quality by reflecting an individual's developmental history [4]. More recently, the DSH has been used to explain condition dependence of a broader range of sexually selected traits, particularly in terms of the nutritional constraints experienced as neonates [5]. By heeding these signals, females stand to receive direct benefits via superior traits correlated with good developmental conditions and/or indirect genetic benefits if developmental stability is heritable.
Colourful avian ornaments are also susceptible to perturbations in developmental environment [6–8] but their potential role as developmental indicators, particularly in natural populations, has received limited attention. It is reasonable to expect a link between developmental environment and colourful traits. The long-term effects of developmental nutrition on morphology, physiology and metabolism can influence adult ability to assimilate the dietary pigments necessary for pigment-based coloration [9,10], and potentially also to form feather nanostructures necessary for structural coloration [11]. A number of studies have considered the immediate effects of developmental environment on nestling plumage colour [12–15], demonstrating, for example, the importance of maternally invested egg yolk carotenoids for yellow plumage in nestling blue tits [16]. In addition, some studies have tracked the downstream effects of developmental environment on colourful adult traits. However, these have tended to focus on non-plumage-based ornaments, such as the fleshy red wattles of ring-necked pheasants (Phasianus colchicus) [8,17], and the carotenoid-based bills of zebra finches (Taeniopygia guttata) [7,10,18,19] and mallard ducks (Anas platyrhynchos) [20]. Despite this important work, the relevance of developmental conditions for colourful adult plumage is largely unknown.
The sensitivity of colourful adult plumage to developmental conditions may depend on the mechanism of colour production, which varies with the particular colour displayed. In adult birds, different mechanisms of colour production are affected by condition during moult to varying extents, and consequently signal different information. For example, colourful plumage resulting from the deposition of carotenoid pigments is often strongly influenced by environmental factors [21–23]. Melanin-pigmented plumage, in contrast, is traditionally considered to be under stronger genetic than environmental control [24–26], and therefore less likely to be influenced by the developmental environment. Meanwhile, there is increasing evidence that structural colours (e.g. blues and whites) show condition dependence [27–30]. In a similar way, colourful ornaments produced by different mechanisms may respond to different aspects of the developmental environment, or show differing degrees of developmental sensitivity, and consequently signal different information about developmental history.
The aim of this study was to investigate experimentally the downstream effects of neonatal nutrition on male breeding plumage in the New Zealand hihi (Notiomystis cincta), a sexually dichromatic passerine. Adult males display a black melanin-pigmented head, back and breast, yellow carotenoid-pigmented shoulders and wings [31], and structurally coloured white ear tufts. We provided nestlings with nutritional and carotenoid supplements in a balanced and fully crossed experimental design, and subsequently examined the consequences for male breeding plumage. We predicted that properties of the carotenoid-based yellow plumage would be enhanced in nutritionally and carotenoid-supplemented males, that properties of the white ear tufts would be enhanced in nutritionally supplemented males, and that the melanin-pigmented black would not be affected by either nestling treatment.
2. Material and methods
(a). Study species and site
Hihi are an endemic species of New Zealand. They are cavity nesters and lay clutches of three to five eggs [32]. All nestlings develop female-like plumage initially and fledge at the age of around 30 days. Juveniles then undergo a partial moult of body feathers, starting about six weeks after fledging and finishing about three months after fledging (L. Walker 2011, unpublished data), during which time males obtain their breeding plumage. All birds reach breeding age in their first year and do not undergo a subsequent pre-breeding moult. Therefore, body feathers grown during the post-fledging moult will last until after their first breeding season.
Adult hihi eat nectar, fruits and invertebrates. Nectar provides a source of carbohydrates [33]; fruits provide a source of carbohydrates, lipids, fibre and carotenoids (Walker, unpublished data); and invertebrates provide a source of protein [34] and probably carotenoids [35]. The proportion of each food type in the adult diet varies with season and population. Estimates for summer, when adults are feeding nestlings, range from a high proportion of nectar (83% nectar, 6% fruit and 11% invertebrates; [36]) to a high proportion of invertebrates (18% nectar, 13% fruit and 70% invertebrates; [33]). Nestling hihi are fed a combination of nectar, fruit and invertebrates by their parents [37]. The precise proportion of these food types in the nestling diet is unknown, although invertebrates are believed to be the dominant food source up to the age of 8–10 days [38].
We studied a hihi population of approximately 100 pairs on 220 ha Tiritiri Matangi Island (36°36′ S, 174°53′ E). All adults were ringed, all nesting attempts occurred in nest boxes and all nestlings were ringed prior to fledging. Supplementary food (sugar water; 20% by mass) was provided year-round at six feeding stations across the island.
(b). Experimental design
All first-clutch nests (n = 84) in the 2010–2011 austral breeding season were randomly assigned to one of two nutritional treatments; a ‘nutritionally supplemented’ treatment (N+, n = 42) or a ‘control’ treatment (N−, n = 42). Nestlings in N+ nests were hand-fed Wombaroo Lorikeet & Honeyeater Food, a dietary supplement typically used to maintain nectar-eating birds in captivity (nutritional composition: protein 140 g kg−1, fat 60 g kg−1, fibre 6 g kg−1; provided in 30% by mass solution). As a control, nestlings in N− nests were hand-fed a sugar water solution (20% by mass, as provided year round at feeding stations and known to be provisioned to nestlings by parents [39]). Using a graduated plastic syringe, nestlings were fed every second day between four and 20 days of age. Volume fed increased incrementally from 0.2 ml at the age of four days to 3.0 ml at 20 days (see the electronic supplementary material, table S1). The resulting amount of supplement (and therefore amount of constituent nutrients) consequently increased as nestlings grew, and was based on Wombaroo manufacturer recommendations (see the electronic supplementary material for details).
Within all N+ and N− nests, some nestlings had their nutritional treatment enhanced with carotenoids (C+) and some did not (C−). The carotenoids lutein and zeaxanthin were provided in the form of OroGLO liquid (Kemin Industries) at a final concentration of 100 μg ml−1 (based on recommendations in [40]; see the electronic supplementary material for details), and at volumes specified previously. Lutein and zeaxanthin are the principle carotenoids used to pigment yellow feathers in hihi, and their ratio in OroGLO closely matches that found in the circulating plasma of un-supplemented birds [31].
This design created four treatment groups (N+C+, N+C−, N−C+, N−C−), with a nestling-oriented carotenoid supplement overlaid on a brood-oriented nutritional supplement. Treatment group was randomly assigned to the heaviest nestling in a nest, and carotenoid treatment was then alternated down weight rank while nutritional treatment was held constant. Thus, nestlings within a brood shared the same nutritional treatment but could each differ in carotenoid treatment, allowing for within-brood comparisons of carotenoid effect. All nestlings within a brood received a treatment and so in the case of uneven brood sizes the number of C+ and C− nestlings was unbalanced.
Parents continued to provision nestlings throughout the treatment period. Individual nestlings were identified by uniquely trimmed body down when less than or equal to 8 days, and by colour rings when more than 8 days. Nestlings were weighed each time they were hand fed. At 21 days nestlings were ringed with a unique combination of one metal ring and three colour rings, and a blood sample was taken by brachial venipuncture. Blood samples were centrifuged within 3 h to separate plasma, which was stored at −20°C for later analysis of plasma carotenoid concentration. A total of 287 nestlings were treated, across four treatment groups (68 N+C+, 76 N+C−, 72 N−C+, 71 N−C−).
(c). Plasma carotenoid concentration
Plasma samples were analysed for total carotenoid concentration by high-performance liquid chromatography (HPLC), as described previously [41]. Further details are given in the electronic supplementary material.
(d). Plumage colour measurement
Treated nestlings from first clutches fledged throughout December 2010. In March and April 2011, at the end of moult, juvenile males were caught for plumage colour measurement. Reflectance spectra were recorded using a USB-2000 spectrometer (Ocean Optics Inc., Dunedin, FL, USA), DT-Mini Lamp (Deuterium Tungsten Halogen source) and a reflectance probe. The probe was held at 90° to the surface being measured. Each spectrum was an average of four scans and was calculated relative to a diffuse reflectance standard (WS-1, Ocean Optics Inc., The Netherlands). The equipment was calibrated immediately prior to each bird being measured. Repeated measurements were taken from the left and right yellow shoulder patches in all males caught (n = 49 males), and from the black head (n = 41 males) and the white ear tufts (n = 35 males) in those males that were advanced enough in moult. The probe was lifted and replaced between repeat measurements within a body region (six repeats each for yellow and white regions, three for black region). Since a minority of birds had not finished moulting, body moult was scored at the time of colour measurement as ‘finished’ or ‘not finished’. Treated males were captured again for plumage colour measurement in October 2011 (n = 25 males), at the start of their first breeding season. In addition to the measurements taken immediately post-moult, measurements of yellow and white patch size were made (this was not possible until all individuals had moulted the full extent of their yellow shoulders and white ear tufts). Two repeat measurements of white ear tuft length were taken using digital calipers. Digital photographs (Olympus Mju 300) were taken of the ventral surface, the left flank and the right flank of each bird to capture the entire yellow area. Two repeat photographs were taken of each region. A scale rule was included in each image, and yellow patch size was calculated in Image J by calibrating the scale and outlining yellow area using the freehand tool. Measurements of reflectance spectra and patch size were made blind with respect to nestling treatment.
(e). Plumage colour analysis
Reflectance spectra were analysed using models in tetrahedral colour space to extract hue, saturation and luminance variables for each colour patch [42–44] (see the electronic supplementary material for full details). Briefly, we first calculated photon catch values for the four single cones, used in colour vision, and the double cones, used in luminance vision, based on the reflectance spectra and measures of irradiance [44]. Values generated using ‘d65’ irradiance levels and with blue tit spectral sensitivities are presented. Our measure of luminance, the perceived lightness of a patch, was simply the double cone photon catch values. To calculate saturation, the amount of colour compared with white light, we plotted the standardized single cone catch data for each individual in avian tetrahedral colour space [44] and calculated the distance from the centre of the colour space. To calculate hue, the colour type (e.g. blue versus red), we used ratios based on photon catch outputs that are broadly inspired by the way that opponent colour channels work, based on performing a principal component analysis (PCA) on a covariance matrix of the standardized single cone data [45–47].
(f). Data analysis
One-way ANOVAs were used to assess repeatability of the different plumage colour variables (following Lessells & Boag [48]). All plumage colour variables demonstrated significantly higher between individual than within individual variation (p < 0.0001). Repeatability was relatively high in most cases, with R values more than 0.73 for patch size measurements (yellow area and white length), and R values ranging from 0.40 to 0.62 for yellow descriptors (hue, saturation and luminance), 0.25–0.90 for black descriptors and 0.32–0.64 for white descriptors. These values are consistent with other published repeatability values for colourful traits [16,49,50], justifying the use of these colour variables. Repeated measures were averaged per individual for use in subsequent analyses. We chose to use saturation and luminance (not hue) for each colour patch in subsequent analyses. Yellow hue and saturation were highly correlated (r = 0.99, p < 0.001), and we chose to use saturation rather than hue as it most consistently reflects feather carotenoid content across species [51,52]. Hue and saturation were also correlated in black (r = −0.29, p = 0.07) and white (r = 0.48, p = 0.004) plumage, and we again used saturation rather than hue. Luminance is encoded independently of colour and is analysed separately by visual systems, justifying its use for yellow, black and white patches.
Statistical analyses were carried out using R v. 2.13.0 [53]. The effects of nestling treatment and covariates on nestling plasma carotenoid concentration were investigated by fitting a linear mixed effects model using restricted maximum likelihood. Nest identity was included as a random effect in all models, to control for the fact that multiple nestlings from the same brood were sampled (details provided in the electronic supplementary material), and an induced covariance matrix was used to estimate the similarity among nestlings from the same nest. Nutritional treatment (N+/N−), carotenoid treatment (C+/C−), brood size (number hatched), hatch date (centred Julian date), and an interaction between nutritional and carotenoid treatments were included as fixed effects. Carotenoid concentration was Box–Cox transformed to meet the assumptions of normality and homogeneity.
The effects of nestling treatment and covariates on colour variables (saturation and luminance for all patches, plus patch size and ear–tuft length for yellow and white patches, respectively) were investigated by fitting linear models. Separate models were run with each colour variable as the response. Nutritional treatment, carotenoid treatment, brood size, hatch date, and, where relevant, moult score (finished/not finished) were included as explanatory variables. A random nest effect was not appropriate because most nests were represented by only one nestling by the time of colour measurement. To control for the minority of cases where siblings from the same brood were measured, nests were re-sampled 1000 times (details provided in the electronic supplementary material). The assumptions of normality and homogeneity were checked by the examination of residual plots. In cases where residual plots revealed substantial heterogeneity the linear model was fitted using generalized least squares, which allows the variance structure to be specified (details provided in the electronic supplementary material). In cases of non-normality the response variable was Box–Cox transformed.
3. Results
(a). Effect of treatments and covariates on plasma carotenoid concentration
Our carotenoid treatment significantly increased levels of carotenoids circulating in the blood. Nestlings treated with carotenoids (C+) had significantly higher plasma carotenoid concentration at 21 days than nestlings not treated with carotenoids (C−) (10.21 μg ml−1 ± 0.48 s.e. versus 6.07 μg ml−1 ± 0.32 s.e., d.f. = 113, t = 4.86, p ≤ 0.0001). Nutritional treatment (N+/N−), brood size and hatch date did not explain a significant amount of variance in plasma carotenoid concentration (nutritional treatment: d.f. = 69, t = 0.12, p = 0.90; brood size: d.f. = 69, t = 0.32, p = 0.75; hatch date: d.f. = 69, t = 0.88, p = 0.38), and there was no interaction between nutritional treatment and carotenoid treatment (d.f. = 113, t = 0.81, p = 0.42). The variance explained by the random nest effect was 0.03 (53.3%) and the residual variance was 0.02 (46.7%).
(b). Effects of treatments and covariates on yellow plumage colour after moult
Neither nutritional treatment nor carotenoid treatment directly affected properties of yellow plumage, either immediately following juvenile moult or at the start of the subsequent breeding season (see the electronic supplementary material, table S2). We investigated whether individual variation in carotenoid concentration could have masked any treatment effects by searching for a correlation between plasma carotenoid concentration and plumage colour. We found a significant positive correlation between plasma carotenoid concentration at 21 days and yellow saturation immediately following juvenile moult (Kendall's rank correlation, r = 0.23, p = 0.03; figure 1). Nestlings that hatched later in the year moulted breeding plumage that was less intensely yellow, having lower saturation values (t =−2.34, p < 0.05 for 69.1% of re-samples with n = 35 nests; electronic supplementary material, table S2). This effect was however no longer present by the start of the breeding season. The size of the yellow plumage patch at the start of the breeding season was smaller when males developed in larger broods (t =−2.68, p < 0.02 for 64.7% of re-samples with n = 22 nests; electronic supplementary material, table S2). Brood size and moult score did not have a significant effect on yellow plumage properties (see the electronic supplementary material, table S2).
Figure 1.
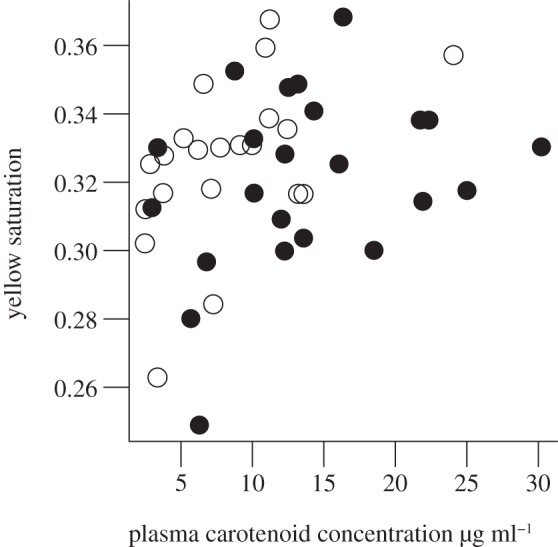
Relationship between plasma carotenoid concentration at 21-days old and yellow saturation immediately after juvenile moult. Plasma carotenoid concentration was significantly elevated in C+ males (filled circles) compared with C− males (open circles).
(c). Effects of treatments and covariates on black plumage colour after moult
Properties of black plumage were not affected by carotenoid or nutritional treatment at either time point that plumage was measured (see the electronic supplementary material, table S3). Males that hatched later in the season displayed breeding plumage that was less intensely black (i.e. that had higher luminance; t = 2.52, p < 0.03 for 62% of re-samples with n = 22 nests; electronic supplementary material, table S3). Brood size and moult score did not affect properties of black plumage colour (see the electronic supplementary material, table S3).
(d). Effects of treatments and covariates on white plumage colour after moult
Supplementation with nutritional treatment influenced the eventual colour of the white ear tufts, but not in the way that we predicted: post-moult white luminance tended to be reduced as a consequence of supplementation (t =−2.15, p < 0.05 for 52.1% of re-samples with n = 27 nests; figure 2; electronic supplementary material, table S4). This trend was no longer present when plumage was measured pre-breeding (t =−0.32, p < 0.05 for 0% of re-samples with n = 22 nests; electronic supplementary material, table S4). Carotenoid treatment, hatch date and brood size did not influence properties of white plumage at either time point (see the electronic supplementary material, table S4).
Figure 2.
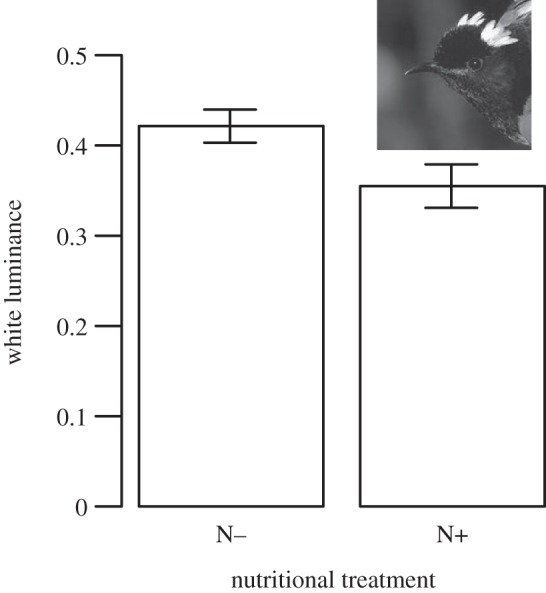
Mean (±s.e.) white luminance values immediately after juvenile moult for N− and N+ treated males (inset: male displaying erect white ear tufts against black head, photo: Brent Stephenson).
4. Discussion
Our experiment shows that some (though not all) components of a male's colourful breeding plumage can accurately indicate the quality of the environment in which he developed, thus providing support for predictions of the developmental stress hypothesis. Previously, the importance of developmental conditions has been demonstrated for some colourful adult integuments [8,10,20], and for nestling plumage [12,14], but never for adult plumage. The effect was most pronounced for yellow carotenoid-based plumage, which reflected multiple attributes of the nestling environment, such as brood size, hatch date and nutritional conditions. In particular, supplementation of the nestling diet with carotenoids increased plasma carotenoid concentration, and individuals with higher plasma carotenoid concentration, independent of treatment, went on to moult into breeding plumage that was coloured more intensely yellow.
There are at least two mechanistic explanations for how early-life carotenoid access might influence carotenoid-based adult plumage. First, carotenoids provided to nestlings may be stored, for example, in the liver [54], and later deposited in the integument during moult (in this case 1.5–3 months after fledging). Second, individuals with increased access to carotenoids as young birds may have an improved ability to assimilate carotenoids as adults [10]. That we find a correlation between nestling carotenoid concentration and adult plumage colour, but no effect of nestling carotenoid treatment on adult plumage colour, suggests that there is individual variation in nestling response to supplementation. Therefore, yellow plumage indicates not only the nature of the nutritional environment in which a male developed, but his intrinsic capacity to exploit those conditions as well.
We also found that the white ear tufts, whose colour is determined structurally, were influenced by conditions experienced during development, although not in a way we expected. Individuals that received the nutritional supplement as nestlings then displayed less bright white feathers after moulting into their breeding plumage. Previous studies have demonstrated positive effects of a high-protein diet on iridescent [29] and white [55] structural plumage and we assumed that our nutritional supplement, which contains proteins, would be beneficial for males. However, our results challenge this assumption. For example, we have found that nestling males whose diet was supplemented with Wombaroo suffered lower survival as a result [56]. Here, we show that males given Wombaroo also end up producing less brightly white plumage. Perhaps there is an optimal level of protein in the diet for developing males [57], which was exceeded in our treatments, incurring both survival and signalling costs. Alternatively, or perhaps additionally, the lipid component of the nutritional treatment may have had a detrimental impact.
Since white plumage is not pigmented it was traditionally considered relatively cheap to produce [58]. However, evidence is accumulating that producing white feathers might be costly after all. For example, dark-eyed juncos (Junco hyemalis) maintained on enriched diets during moult grow larger, brighter white tail patches [55], and house sparrows that undergo an accelerated moult develop less bright white wing-bars [59]. White feather barbs consist of a central air vacuole surrounded by a keratin cortex and typically lack nanostructural organization [60,61]. The white appearance of feathers is a result of the incoherent scattering of all wavelengths of light by feather keratin [58], and it has been suggested that a thicker keratin cortex may reduce the amount of white light reflected [61]. Potentially, this mechanism might be at work in hihi, with early nutritional stress (such as too much protein) impairing a male's future ability to produce a keratin cortex of the appropriate thickness.
Finally, and consistent with previous work suggesting melanin-based traits are largely free from nutritional influence [22,26,62], we detected no effect of our nestling feeding treatments on black breeding plumage. Nevertheless, after controlling for any dietary manipulation, we found that earlier-hatched males had darker black plumage by the breeding season than later-hatched males. We cannot tell from our data whether this correlation is driven by the environment or by intrinsic qualities of the parents (because, for example, individuals that are more melanic might breed earlier in the season and pass on any genetic predisposition to be melanic to their young).
All of the effects of the developmental environment on male breeding plumage found immediately after moult had declined by the time the breeding season began. The most likely explanation is that colour changes occur in the feathers during this five to six month period, perhaps as a result of feather degradation, feather soiling and/or pigment degradation [63]. Our data therefore show that it is unwise to assume that the extent of coloration is fixed once feather pigments have been deposited and/or the feather microstructures determining any structural colours are grown. What are the implications for the potential signalling function of this colourful plumage? At this stage, we have no definite answers because it is not yet known whether male hihi plumage functions to attract mates, or to repel rival males [64], or both. Nevertheless, during the winter months following the moult, hihi aggregate in social groups providing plenty of opportunity for plumage assessment—whether by rival males or potential mates. In other species, plumage displayed well before the breeding season begins can still influence reproductive behaviours many months later [65] and it would be interesting to determine whether the same is true for hihi.
In summary, we have shown that colourful plumage grown after leaving the nest is a window on the developmental history of the male [5], in common with other avian integuments that are known to be affected by neonatal environmental conditions [8,10]. Although the mechanisms that link developmental history with colourful plumage have yet to be identified, our study adds to the diversity of secondary sexual traits whose expression is strongly linked to the environment in which they developed.
Acknowledgements
We thank the New Zealand Department of Conservation and the Supporters of Tiritiri Matangi for allowing us to conduct this study on Tiritiri Matangi Island, and for providing logistical support. We are grateful to Annette Fayet for help with fieldwork, Phill Cassey for advice with data analysis, Matthew Shawkey for helpful discussion and Rose Thorogood for advice with data analysis and for providing helpful comments on a draft. The comments of two anonymous reviewers greatly improved this manuscript. Kemin Industries donated carotenoids. Animal ethics permission was granted by the Zoological Society of London. L.K.W. was supported by a NERC Research Studentship, M.S. was supported by a Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship (BB/G022887/1) and J.G.E. was supported by an RCUK Fellowship. This research project was supported with funding from the Leverhulme Trust.
References
- 1.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260 10.1016/S0169-5347(01)02124-3 (doi:10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 2.Nowicki S, Hasselquist D, Bensch S, Peters S. 2000. Nestling growth and song repertoire size in great reed warblers: evidence for song learning as an indicator mechanism in mate choice. Proc. R. Soc. Lond. B 267, 2419–2424 10.1098/rspb.2000.1300 (doi:10.1098/rspb.2000.1300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David P, Bjorksten T, Fowler K, Pomiankowski A. 2000. Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature 406, 186–188 10.1038/35018079 (doi:10.1038/35018079) [DOI] [PubMed] [Google Scholar]
- 4.Nowicki S, Peters S, Podos J. 1998. Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 38, 179–190 10.1093/icb/38.1.179 (doi:10.1093/icb/38.1.179) [DOI] [Google Scholar]
- 5.Spencer KA, MacDougall-Shackleton SA. 2011. Indicators of development as sexually selected traits: the developmental stress hypothesis in context. Behav. Ecol. 22, 1–9 10.1093/beheco/arq068 (doi:10.1093/beheco/arq068) [DOI] [Google Scholar]
- 6.Gustafsson L, Qvarnstrom A, Sheldon BC. 1995. Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature 375, 311–313 10.1038/375311a0 (doi:10.1038/375311a0) [DOI] [Google Scholar]
- 7.De Kogel CH, Prijs HJ. 1996. Effects of brood size manipulations on sexual attractiveness of offspring in the zebra finch. Anim. Behav. 51, 699–708 10.1006/anbe.1996.0073 (doi:10.1006/anbe.1996.0073) [DOI] [Google Scholar]
- 8.Ohlsson T, Smith HG, Råberg L, Hasselquist D. 2002. Pheasant sexual ornaments reflect nutritional conditions during early growth. Proc. R. Soc. Lond. B 269, 21–27 10.1098/rspb.2001.1848 (doi:10.1098/rspb.2001.1848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blount JD, Metcalfe NB, Arnold KE, Surai PF, Devevey GL, Monaghan P. 2003. Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proc. R. Soc. Lond. B 270, 1691–1696 10.1098/rspb.2003.2411 (doi:10.1098/rspb.2003.2411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGraw KJ, Adkins-Regan E, Parker RS. 2005. Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenscheften 92, 375–380 10.1007/s00114-005-0003-z (doi:10.1007/s00114-005-0003-z) [DOI] [PubMed] [Google Scholar]
- 11.Maia R, Macedo RHF, Shawkey MD. 2012. Nanostructural self-assembly of iridescent feather barbules through depletion attraction of melanosomes during keratinization. J. R. Soc. Interface 9, 734–43 10.1098/rsif.2011.0456 (doi:10.1098/rsif.2011.0456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hõrak P, Vellau H, Ots I, Møller AP. 2000. Growth conditions affect carotenoid-based plumage coloration of great tit nestlings. Naturwissenscheften 87, 460–464 [DOI] [PubMed] [Google Scholar]
- 13.Fargallo JA, Laaksonen T, Korpimäki E, Wakamatsu K. 2007. A melanin-based trait reflects environmental growth conditions of nestling male Eurasian kestrels. Evol. Ecol. 21, 157–171 10.1007/s10682-006-0020-1 (doi:10.1007/s10682-006-0020-1) [DOI] [Google Scholar]
- 14.Peters A, Delhey K, Johnsen A, Kempenaers B. 2007. The condition-dependent development of carotenoid-based and structural plumage in nestling blue tits: males and females differ. Am. Nat. 169, S122–S136 10.1086/510139 (doi:10.1086/510139) [DOI] [PubMed] [Google Scholar]
- 15.Arriero E, Fargallo JA. 2006. Habitat structure is associated with the expression of carotenoid-based coloration in nestling blue tits Parus caeruleus. Naturwissenscheften 93, 173–80 10.1007/s00114-006-0090-5 (doi:10.1007/s00114-006-0090-5) [DOI] [PubMed] [Google Scholar]
- 16.Biard C, Surai PF, Møller AP. 2005. Effects of carotenoid availability during laying on reproduction in the blue tit. Oecologia 144, 32–44 10.1007/s00442-005-0048-x (doi:10.1007/s00442-005-0048-x) [DOI] [PubMed] [Google Scholar]
- 17.Orledge JM, Blount JD, Hoodless AN, Royle NJ. 2012. Antioxidant supplementation during early development reduces parasite load but does not affect sexual ornament expression in adult ring-necked pheasants. Funct. Ecol. 26, 688–700 10.1111/j.1365-2435.2012.01977.x (doi:10.1111/j.1365-2435.2012.01977.x) [DOI] [Google Scholar]
- 18.Birkhead TR, Fletcher F, Pellatt EJ. 1999. Nestling diet, secondary sexual traits and fitness in the zebra finch. Proc. R. Soc. Lond. B 266, 385–390 10.1098/rspb.1999.0649 (doi:10.1098/rspb.1999.0649) [DOI] [Google Scholar]
- 19.Tschirren B, Rutstein AN, Postma E, Mariette M, Griffith SC. 2009. Short- and long-term consequences of early developmental conditions: a case study on wild and domesticated zebra finches. J. Evol. Biol. 22, 387–395 10.1111/j.1420-9101.2008.01656.x (doi:10.1111/j.1420-9101.2008.01656.x) [DOI] [PubMed] [Google Scholar]
- 20.Butler MW, McGraw KJ. 2012. Differential effects of early- and late-life access to carotenoids on adult immune function and ornamentation in mallard ducks (Anas platyrhynchos). PLoS ONE 7, e38043. 10.1371/journal.pone.0038043 (doi:10.1371/journal.pone.0038043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGraw KJ, Ardia DR. 2003. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 162, 704–712 10.1086/378904 (doi:10.1086/378904) [DOI] [PubMed] [Google Scholar]
- 22.Hill GE. 2000. Energetic constraints on expression of carotenoid-based plumage coloration. J. Avian Biol. 31, 559–566 10.1034/j.1600-048X.2000.310415.x (doi:10.1034/j.1600-048X.2000.310415.x) [DOI] [Google Scholar]
- 23.Hõrak P, Saks L, Karu U, Ots I, Surai PF, McGraw KJ. 2004. How coccidian parasites affect health and appearance of greenfinches. J. Anim. Ecol. 73, 935–947 10.1111/j.0021-8790.2004.00870.x (doi:10.1111/j.0021-8790.2004.00870.x) [DOI] [Google Scholar]
- 24.Hill GE, Brawner WR. 1998. Melanin-based plumage coloration in the house finch is unaffected by coccidial infection. Proc. R. Soc. Lond. B 265, 1105–1109 10.1098/rspb.1998.0405 (doi:10.1098/rspb.1998.0405) [DOI] [Google Scholar]
- 25.McGraw KJ, Hill GE. 2000. Differential effects of endoparasitism on the expression of carotenoid- and melanin-based ornamental coloration. Proc. R. Soc. Lond. B 267, 1525–1531 10.1098/rspb.2000.1174 (doi:10.1098/rspb.2000.1174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senar JC, Figuerola J, Domènech J. 2003. Plumage coloration and nutritional condition in the great tit Parus major: the roles of carotenoids and melanins differ. Naturwissenscheften 90, 234–237 10.1007/s00114-003-0414-7 (doi:10.1007/s00114-003-0414-7) [DOI] [PubMed] [Google Scholar]
- 27.Hill GE, Doucet SM, Buchholz R. 2005. The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim. Behav. 69, 387–394 10.1016/j.anbehav.2004.03.013 (doi:10.1016/j.anbehav.2004.03.013) [DOI] [Google Scholar]
- 28.McGraw KJ, Mackillop EA, Dale J, Hauber ME. 2002. Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J. Exp. Biol. 205, 3747–3755 [DOI] [PubMed] [Google Scholar]
- 29.Meadows MG, Roudybush TE, McGraw KJ. 2012. Dietary protein level affects iridescent coloration in Anna's hummingbirds, Calypte anna. J. Exp. Biol. 215, 2742–2750 10.1242/jeb.069351 (doi:10.1242/jeb.069351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keyser AJ, Hill GE. 2000. Structurally based plumage coloration is an honest signal of quality in male blue grosbeaks. Behav. Ecol. 11, 202–209 10.1093/beheco/11.2.202 (doi:10.1093/beheco/11.2.202) [DOI] [Google Scholar]
- 31.Ewen JG, Surai PF, Stradi R, Møller AP, Vittorio B, Griffiths R, Armstrong DP. 2006. Carotenoids, colour and conservation in an endangered passerine, the hihi or stitchbird (Notiomystis cincta). Anim. Conserv. 9, 229–235 10.1111/j.1469-1795.2006.00028.x (doi:10.1111/j.1469-1795.2006.00028.x) [DOI] [Google Scholar]
- 32.Oliver WRB. 1955. New Zealand birds. Wellington, New Zealand: AH and AW Reed [Google Scholar]
- 33.Rasch G. 1985. The behavioural ecology and management of the stitchbird. MSc thesis, University of Auckland, Auckland, New Zealand [Google Scholar]
- 34.Oliver D. 1998. The importance of insects and lerp in the diet of juvenile regent honeyeaters, Xanthomyza phrygia: implications for the conservation of an endangered woodland bird. Wildl. Res. 25, 409–417 10.1071/WR97078 (doi:10.1071/WR97078) [DOI] [Google Scholar]
- 35.Eeva T, Helle S, Salminen J-P, Hakkarainen H. 2010. Carotenoid composition of invertebrates consumed by two insectivorous bird species. J. Chem. Ecol. 36, 608–613 10.1007/s10886-010-9796-0 (doi:10.1007/s10886-010-9796-0) [DOI] [PubMed] [Google Scholar]
- 36.Gravatt D. 1971. Aspects of habitat use by New Zealand honeyeaters, with reference to other forest species. Emu 71, 65–72 10.1071/MU971065 (doi:10.1071/MU971065) [DOI] [Google Scholar]
- 37.Lovegrove TG. 1985. The introduction of stitchbird (Notiomystis cincta) to the Kapiti Island nature reserve. Unpublished report to the Wellington District Office of the Department of Lands and Survey
- 38.Higgins PJ, Peter JM, Steele WK. 2001. Handbook of Australian, New Zealand and Antarctic birds. Volume 5: tyrant-flycatchers to chats. Melbourne, Australia: Oxford University Press [Google Scholar]
- 39.Thorogood R, Kilner RM, Karadaş F, Ewen JG. 2008. Spectral mouth colour of nestlings changes with carotenoid availability. Funct. Ecol. 22, 1044–1051 10.1111/j.1365-2435.2008.01455.x (doi:10.1111/j.1365-2435.2008.01455.x) [DOI] [Google Scholar]
- 40.Biard C, Surai PF, Møller AP. 2006. Carotenoid availability in diet and phenotype of blue and great tit nestlings. J. Exp. Biol. 209, 1004–1015 10.1242/jeb.02089 (doi:10.1242/jeb.02089) [DOI] [PubMed] [Google Scholar]
- 41.Ewen JG, Thorogood R, Karadaş F, Pappas AC, Surai PF. 2006. Influences of carotenoid supplementation on the integrated antioxidant system of a free living endangered passerine, the hihi (Notiomystis cincta). Comp. Biol. Physiol. A 143, 149–154 10.1016/j.cbpa.2005.11.006 (doi:10.1016/j.cbpa.2005.11.006) [DOI] [PubMed] [Google Scholar]
- 42.Stoddard MC, Prum RO. 2011. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052 10.1093/beheco/arr088 (doi:10.1093/beheco/arr088) [DOI] [Google Scholar]
- 43.Stevens M, Stoddard MC, Higham JP. 2009. Studying primate color: towards visual system-dependent methods. Int. J. Primatol. 30, 893–917 10.1007/s10764-009-9356-z (doi:10.1007/s10764-009-9356-z) [DOI] [Google Scholar]
- 44.Endler JA, Mielke PW. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431 10.1111/j.1095-8312.2005.00540.x (doi:10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 45.Komdeur J, Oorebeek M, Van Overveld T, Cuthill IC. 2005. Mutual ornamentation, age, and reproductive performance in the European starling. Behav. Ecol. 16, 805–817 10.1093/beheco/ari059 (doi:10.1093/beheco/ari059) [DOI] [Google Scholar]
- 46.Spottiswoode CN, Stevens M. 2011. How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc. R. Soc. B 278, 3566–3573 10.1098/rspb.2011.0401 (doi:10.1098/rspb.2011.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens M. 2011. Avian vision and egg colouration: concepts and measurements. Avian Biol. Res. 4, 190–206 10.3184/175815511X13207790177958 (doi:10.3184/175815511X13207790177958) [DOI] [Google Scholar]
- 48.Lessells CM, Boag PT. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 10.2307/4087240 (doi:10.2307/4087240) [DOI] [Google Scholar]
- 49.Saino N, Stradi R, Ninni P, Pini E, Møller AP. 1999. Carotenoid plasma concentration, immune profile, and plumage ornamentation of male barn swallows (Hirundo rustica). Am. Nat. 154, 441–448 10.1086/285036 (doi:10.1086/285036) [DOI] [PubMed] [Google Scholar]
- 50.Budden AE, Dickinson JL. 2009. Signals of quality and age: the information content of multiple plumage ornaments in male western bluebirds Sialia mexicana. J. Avian Biol. 40, 18–27 10.1111/j.1600-048X.2008.04344.x (doi:10.1111/j.1600-048X.2008.04344.x) [DOI] [Google Scholar]
- 51.Saks L, McGraw KJ, Hõrak P. 2003. How feather colour reflects its carotenoid content. Funct. Ecol. 17, 555–561 10.1046/j.1365-2435.2003.00765.x (doi:10.1046/j.1365-2435.2003.00765.x) [DOI] [Google Scholar]
- 52.McGraw KJ, Gregory AJ. 2004. Carotenoid pigments in male American goldfinches: what is the optimal biochemical strategy for becoming colourful? Biol. J. Linn. Soc. 83, 273–280 10.1111/j.1095-8312.2004.00388.x (doi:10.1111/j.1095-8312.2004.00388.x) [DOI] [Google Scholar]
- 53.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 54.Martucci RB, Ziulkoski AL, Fortuna VA, Guaragna RM, Guma FCR, Trugo LC, Borojevic R. 2004. β-carotene storage, conversion to retinoic acid, and induction of the lipocyte phenotype in hepatic stellate cells. J. Cell. Biochem. 92, 414–23 10.1002/jcb.20073 (doi:10.1002/jcb.20073) [DOI] [PubMed] [Google Scholar]
- 55.McGlothlin JW, Duffy DL, Henry-Freeman JL, Ketterson ED. 2007. Diet quality affects an attractive white plumage pattern in dark-eyed juncos (Junco hyemalis). Behav. Ecol. Sociobiol. 61, 1391–1399 10.1007/s00265-007-0370-x (doi:10.1007/s00265-007-0370-x) [DOI] [Google Scholar]
- 56.Walker LK, Armstrong DP, Brekke P, Chauvenet ALM, Kilner RM, Ewen JG. In press Giving hihi a helping hand: assessment of alternative rearing diets in food supplemented populations of an endangered bird. Anim. Conserv. 10.1111/acv.12026 (doi:10.1111/acv.12026) [DOI] [Google Scholar]
- 57.Raubenheimer D, Lee KP, Simpson SJ. 2005. Does Bertrand's rule apply to macronutrients? Proc. R. Soc. B 272, 2429–2434 10.1098/rspb.2005.3271 (doi:10.1098/rspb.2005.3271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prum RO. 2006. Anatomy, physics and evolution of structural colours. In Bird colouration. vol. I. Mechanisms and measurements (eds Hill GE, McGraw KJ.), Cambridge, MA: Harvard University Press [Google Scholar]
- 59.Vágási CI, Pap PL, Barta Z. 2010. Haste makes waste: accelerated molt adversely affects the expression of melanin-based and depigmented plumage ornaments in house sparrows. PLoS ONE 5, e14215. 10.1371/journal.pone.0014215 (doi:10.1371/journal.pone.0014215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shawkey MD, Hill GE. 2005. Carotenoids need structural colours to shine. Biol. Lett. 1, 121–124 10.1098/rsbl.2004.0289 (doi:10.1098/rsbl.2004.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shawkey MD, Hill GE. 2006. Significance of a basal melanin layer to production of non-iridescent structural plumage color: evidence from an amelanotic Steller's jay (Cyanocitta stelleri). J. Exp. Biol. 209, 1245–50 10.1242/jeb.02115 (doi:10.1242/jeb.02115) [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez G, Sorci G, Møller AP, Ninni P, Haussy C, De Lope F. 1999. Immunocompetence and condition-dependent sexual advertisement in male house sparrows (Passer domesticus). J. Anim. Ecol. 68, 1225–1234 10.1046/j.1365-2656.1999.00364.x (doi:10.1046/j.1365-2656.1999.00364.x) [DOI] [Google Scholar]
- 63.McGraw KJ, Hill GE. 2004. Plumage color as a dynamic trait: carotenoid pigmentation of male house finches (Carpodacus mexicanus) fades during the breeding season. Can. J. Zool. 82, 734–738 10.1139/Z04-043 (doi:10.1139/Z04-043) [DOI] [Google Scholar]
- 64.Pryke SR, Andersson S. 2003. Carotenoid-based epaulettes reveal male competitive ability: experiments with resident and floater red-shouldered widowbirds. Anim. Behav. 66, 217–224 10.1006/anbe.2003.2193 (doi:10.1006/anbe.2003.2193) [DOI] [Google Scholar]
- 65.Mulder RA, Magrath MJL. 1994. Timing of prenuptial molt as a sexually selected indicator of male quality in superb fairy-wrens (Malurus cyaneus). Behav. Ecol. 5, 393–400 10.1093/beheco/5.4.393 (doi:10.1093/beheco/5.4.393) [DOI] [Google Scholar]


