Abstract
Recent studies have documented effects of plant viruses on host plants that appear to enhance transmission by insect vectors. But, almost no empirical work has explored the implications of such apparent manipulation for interactions among co-infecting pathogens. We examined single and mixed infections of two potyviruses, watermelon mosaic virus (WMV) and zucchini yellow mosaic virus (ZYMV), that frequently co-occur in cucurbitaceae populations and share the same aphid vectors. We found that ZYMV isolates replicated at similar rates in single and mixed infections, whereas WMV strains accumulated to significantly lower levels in the presence of ZYMV. Furthermore, ZYMV induced changes in leaf colour and volatile emissions that enhanced aphid (Aphis gossypii) recruitment to infected plants. By contrast, WMV did not elicit strong effects on plant–aphid interactions. Nevertheless, WMV was still readily transmitted from mixed infections, despite fairing poorly in in-plant competition. These findings suggest that pathogen effects on host–vector interactions may well influence competition among co-infecting pathogens. For example, if non-manipulative pathogens benefit from the increased vector traffic elicited by manipulative competitors, their costs of competition may be mitigated to some extent. Conversely, the benefits of manipulation may be limited by free-rider effects in systems where there is strong competition among pathogens for host resources and/or access to vectors.
Keywords: plant viruses, chemical ecology, host manipulation, plant volatiles, vector transmission
1. Introduction
The transmission of vector borne parasites is strongly influenced by the frequency and nature of interactions between hosts and vectors [1,2], and a growing number of studies in both animal and plant disease systems document parasite-induced changes in host phenotypes that appear conducive to vector transmission [3–6]. In plant pathogen systems, such effects include changes in the quality of the host plant as a resource for herbivorous arthropod vectors and in plant visual and olfactory characteristics that serve as arthropod foraging cues [7–10]. Host manipulation by parasites is a paradigmatic example of extended phenotypic effects [11], and some plant pathogens elicit functionally complex changes in host phenotypes that bear the clear hallmarks of adaptation—for example, pollinator-borne pathogens that induce the production of false flowers or elicit flower-like characteristics in foliar tissues [12]. Where pathogen-induced effects are less dramatic, it may be difficult to distinguish adaptive manipulation from by-product effects of infection. However, it seems likely that natural selection will rarely be indifferent to pathogen effects that significantly impact vector transmission [13,14]. Furthermore, at least in the case of plant viruses, there is some evidence that variation in patterns of pathogen effects on host plants corresponds to variation in vector transmission mechanisms [7,15] in ways consistent with the predictions of epidemiology models. This applies, for example, to patterns of vector attraction and subsequent dispersal from host plants that are conducive to pathogen spread [1,2].
The evolution of plant pathogen effects on host traits influencing interactions with insect vectors is furthermore likely to be influenced by complex ecological interactions in natural systems where multiple pathogens, hosts, insect vectors and non-vector herbivores and predators coexist and interact. For example, pathogen-induced changes in host phenotype that enhance vector recruitment may also thereby increase the total virulence of the pathogen via increased herbivory, and may also increase the probability of host colonization by competing pathogens. Moreover, where co-infection does occur, competing genotypes or other pathogens with similar modes of transmission, can potentially profit from the modified host phenotype without paying the (presumed) costs of manipulation, creating free-rider problems that often accompany the provision of ‘public goods’ [16]. The outcomes of such interactions are likely to strongly influence the fitness of ‘manipulative’ pathogens, which ultimately depends not on how many vectors visit or reside on host plants, but on how many disperse and carry parasites bearing the genotype responsible for the manipulation to new hosts.
To date, however, little work has directly explored pathogen effects on host–vector interactions in complex pathosystems. In particular, few studies have explored interactions between potentially competing plant pathogens (but see [16]). Somewhat more work has been done in animal systems, where studies have addressed parasites with complex life cycles [14,17,18], and examined mixed parasite infections [17,18] and within-population variation in manipulative traits [19]. To address the relative lack of information about co-occurring plant pathogens, the current study documented effects of isolates of two frequently co-occurring plant viruses, watermelon mosaic virus (WMV, formerly WMV-2) and zucchini yellow mosaic virus (ZYMV), on host-plant phenotypes and host interactions with a shared aphid vector (Aphis gossypii) as well as the outcomes of competition between these two viruses.
The interaction of WMV and ZYMV, two closely related potyviruses (Potyviridae), is of particular interest because these genetically distinct virus species belong to the same subgroup [20] and appear to have similar ecological niches, suggesting that competition between these viruses might be an important factor influencing their evolution. WMV and ZYMV have overlapping host ranges (primarily comprising cucurbits [21,22]), cause similar symptoms [22] and are transmitted (non-persistently) by the same aphid species, although with differing efficiency [23]. Like other potyviruses, WMV and ZYMV have unipartite single-stranded positive sense RNA genomes (similar in size and organization [20,24]) encoding a polyprotein, and both use a helper-component protein for attachment to the aphid stylet [25]. The co-occurrence of WMV and ZYMV in the same host populations—and in individual plants—has been reported frequently for both cultivated and wild hosts [26–28]. But co-infection does not appear to cause severe synergistic effects on virulence [29] such as those reported for double infections of some viruses in the genus Potyvirus together with viruses from other genera such as Cucumovirus (family Bromoviridae [30]) or Potexvirus (family Alphaflexiviridae [31])—such effects in fact appear to be relatively rare, and co-occurring viruses in natural landscapes more typically form complex communities displaying weaker interactions [32,33].
The current study specifically examined (i) how different isolates of WMV and ZYMV accumulate within-host plant tissues in single and mixed-inoculation treatments, and (ii) how these viruses (occurring in single and mixed infections) affect host-plant traits that influence plant quality for and attractiveness to aphids.
2. Material and methods
(a). Materials
All experiments used a cultivated inbred line of squash (Cucurbita pepo cv. ‘Dixie’, Willhite Seed Co.), allowing us to focus on variation between virus isolates rather than plants. A single A. gossypii colony, reared on squash, supplied individuals for all experiments. Isolates of WMV and ZYMV were collected from wild cucurbits. ZYMV-RSHS and WMV-RS22 were both collected from C. pepo subsp. texana grown in an experimental plot in Rocksprings, Pennsylvania, USA (in 2007 and 2010, respectively). WMV-HQ11 was collected in 2009 from a symptomatic C. pepo subsp. ozarkana alongside the Illinois River near Saint Louis (Missouri, USA). ZYMV-HBCF was collected in 2009 from a symptomatic Cucurbita foetidissima in Hornsby Bend near Austin (Texas, USA). All isolates (and ZYMV–WMV combinations) were used in experiments assessing virus replication rates and in-plant competition between ZYMV and WMV in co-infection. Isolates WMV-HQ11 and ZYMV-HBCF were further used in assays examining the effect of mixed infections on diseased host plant phenotypes compared with single infections (in preliminary assays these isolates produced somewhat more apparent changes in host plants than their respective conspecific variants).
Sequences obtained for the coat protein from these ZYMV [34] and WMV isolates (see the electronic supplementary material, table S1) ensured that they were genetically distinct, and that samples used for stock tissues did not harbour mixed infections of these viruses. All isolates were mechanically propagated for two generations on C. pepo from a single initially collected leaf stored at −80°C. In all experiments, seven-day-old seedlings were inoculated using a potassium-phosphate buffer of frozen standardized amount of ground infected leaf tissue, or clean buffer for mock-inoculated controls (see the electronic supplementary material and [7]), as is commonly done in mixed-infection experiments with potyviruses [30,31]. Although this method did not allow precise control of the quantity of infectious particles to which the experimental plants were exposed, all individual isolates produced symptomatic infections in more than 73 per cent of cases in three independent series of inoculations performed using the same protocol and inoculum stocks (ZYMV-HBCF: 96.5 ± 3.4% (n = 29) ; ZYMV-RSHS: 100% (n = 26); WMV-HQ11: 83.3 ± 6.8% (n = 30); WMV-RS22: 73.1 ± 8.7% (n = 26)); these infection rates represent a minimal estimate for infectivity, as asymptomatic infections are known to occur frequently with these viruses and cucurbit hosts [35]. Furthermore, an initial experiment revealed no significant reduction in virus RNA accumulation in symptomatic plant tissues two weeks after inoculation with serial dilutions (1, 1/2, 1/4 and 1/8) of the standardized inoculum, indicating that the final carrying capacity of the host was not sensitive to this range of variation in inoculum concentration (regression of viral accumulation against dilution factor, n = 8; Pearson's correlation coefficient = −0.21 and −0.02, p-values = 0.62 and 0.96 for ZYMV-HBCF and WMV-HQ11, respectively). In all the following experiments, only host plants presenting mosaic symptoms were included.
(b). Data dissemination and analyses
Viral sequences were deposited in Genbank (accession nos JX028592-JX028595). Other data are available online via Dryad Digital Repository (doi:10.5061/dryad.7db1n). All statistical analyses were performed using JMP software (SAS institute).
(c). Viral replication in plant tissues
Virus RNA amounts were estimated by RT-qPCR assays quantifying WMV and ZYMV coat protein RNA sequences independently (see the electronic supplementary material). As viral RNA serves as mRNA for translation of a single polyprotein, the copy number of a viral gene should reflect RNA copy number in the host, although without discriminating between encapsidated or non-encapsidated viral genomes. Seven-day-old C. pepo plants were either mock inoculated, singly inoculated with one of our four viral isolates, or inoculated with a 1 : 1 inoculum mixture of two isolates (each of four possible cross-species combinations). For single inoculations, the inoculum was diluted by half with buffer compared with mixed inoculations, ensuring all plants received consistent inoculum amounts of each individual viral isolate. Between eight and 12 replicates of each treatment were inoculated then randomized and maintained in a growth chamber. Five plants of each treatment (chosen randomly from plants showing mosaic symptoms) were used for analyses. Two weeks after inoculation, a standardized sample of leaf tissue was collected by punching a disk of 14 mm diameter from the left side of the last expanded leaf of each plant, immediately frozen in liquid nitrogen, and stored in −80°C until processing for RNA extraction and RT-qPCR (see the electronic supplementary material). The absolute number of virus coat protein RNA copies per ng of RNA was determined for all WMV and ZYMV treatments with the WMV coat protein or ZYMV coat protein assays, respectively. The samples were also run with a C. pepo NADH gene assay to ensure that cDNA synthesis had been successful. The entire experiment was performed twice, and these replicate experiments were treated as blocks in the statistical analyses (described below).
(d). Transmission from single and mixed infections
At the end of the second replicate experiment above, aphid transmission trials were performed by placing 30 adult wingless aphids on the last expanded leaf of each of the 40 experimental plants for 15 min, then transferring them to the first true leaves of three young C. pepo ‘Dixie’ plants (ten aphids per recipient plant), where they were allowed to feed overnight and then removed. The recipient plants were kept in a greenhouse until the appearance of any mosaic symptoms. After two weeks, if at least one of the three recipient plants was showing symptoms, a piece of the symptomatic leaf was collected and the presence and identity of virus RNA was determined as above.
(e). Statistical analyses for virus RNA quantities in plant tissues and aphids
Virus RNA levels in plant tissues and aphids were analysed in separate ANOVAs for WMV treatments (in single and mixed infections with ZYMV) and ZYMV treatments (in single and mixed infections with WMV) on log-transformed values to homogenize the variance and normality of the data. The number of WMV RNA copies was analysed with an ANOVA testing the effect of experimental blocks, WMV isolate (HQ11 or RS22), ZYMV co-infecting isolate (HBCF, RSHS or none) and their interaction. Similarly, the ANOVA on ZYMV RNA copy number tested the effects of experimental blocks, ZYMV isolate (HBCF or RSHS), WMV co-infecting isolate (HQ11, RS22 or none) and their interaction.
(f). Effects on plant size (virulence) and leaf coloration
As host growth reduction is a common estimate for virulence in plant pathology [36], the impact of WMV and ZYMV and their co-infection on host plant fitness was estimated as the change in plant size compared with mock-inoculated plants in the virus quantification experiments. The area of exposed plant surface can also be of importance from the point of view of aphid localization by visual cues and was highly correlated with dry weight in a subset of plants (Pearson's correlation = 0.94, p-value < 0.0001, n = 49 in the first block if experimental plants). Photographs (taken two weeks after inoculation) of all plants in the virus replication experiments described above, including 17 mock-inoculated plants, were analysed using ImageJ software to determine the total exposed leaf surface area. Using the same software, we also quantified the mean red, green, and blue colour components of the leaf surface. The surface-area data were Box-Cox transformed and analysed with an ANOVA testing the effects of ‘inoculation type’ (mock-inoculated plants, single WMV infections, single ZYMV infections and mixed infections), with ‘isolates’ nested within ‘inoculation type’ and block as explanatory variables. The three colour variables (RGB 0–255) were analysed jointly via MANOVA using the same model. A principal component analysis (PCA) was also performed to detail effects on each component of total plant coloration.
(g). Organic volatile collection and quantification
Volatile organic compounds emitted by whole plants mock-inoculated, singly inoculated with either WMV-HQ11, ZYMV-HBCF, or inoculated with a 1 : 1 mixture of these two isolates were collected onto adsorbent filters via a push–pull volatile-collection system and analysed via GC–MS (see the electronic supplementary material methods). Six-h collections (09.00 to 15.00) were performed on seven replicates of each treatment distributed in five consecutive blocks/days. Total volatile production for each treatment was log-transformed and compared by ANOVA with treatment and block as explanatory variables. The 16 main individual compounds collected (log-transformed) were analysed by MANOVA, again using treatment and block as explanatory variables. The contribution of all these compounds to the total variance of the experiment was investigated via PCA.
(h). Aphid performance experiment
Population growth was assessed among mock-inoculated plants, plants singly inoculated with WMV-HQ11 or ZYMV-HBCF, and plants inoculated with a 1 : 1 mixture of these two isolates. Aphid populations were established on ten replicates of 3.5-week-old plants of each treatment by transferring 12 young (first- and second-instar) A. gossypii onto the last fully expanded leaf—to minimize maternal effects these aphids were born and reared on a plant of the same inoculation status as the recipient plant. The youngest three leaves and apex of each plant were then caged with a fine mesh bag, and all 40 plants were randomized in a greenhouse under natural light. Cages were checked after one day, and starting populations adjusted to 10 (if necessary). The size of aphid populations was compared after 14 days, using a one-way ANOVA with inoculation treatment as the explanatory variable.
(i). Aphid preference experiment
Aphid preferences for infected versus mock-inoculated plants were tested in an arena where aphids had access to both visual and olfactory cues. Live (attached) leaves were presented in pairs (one of each treatment) in a closed rectangular box (15.5 × 26 cm). Leaves passed through openings in the side of the box and lay flat on its floor, equidistant from the median line of the arena. Thirty wingless adult aphids, starved for 24 h prior, were then released along the median line (we previously reported very similar responses of winged and wingless A. gossypii odour cues elicited by cucumber mosaic virus (CMV) [7]). The number of aphids present on either leaf was recorded after 1 h. Three combinations were tested: mock-inoculated versus WMV-HQ11-inoculated plants; mock-inoculated versus ZYMV-HBCF-inoculated plants; and mock-inoculated versus plants inoculated with a 1 : 1 mixture of these isolates. Dual choice tests for all three comparisons were run in parallel, using new plants for each of the 10 replications and systematically alternating the spatial orientations of infected and mock-inoculated plants. Paired t-tests were used to compare the number of aphids on each of the two leaves.
3. Results
(a). Within-host accumulation of watermelon mosaic virus and zucchini yellow mosaic virus isolates in single and mixed infections
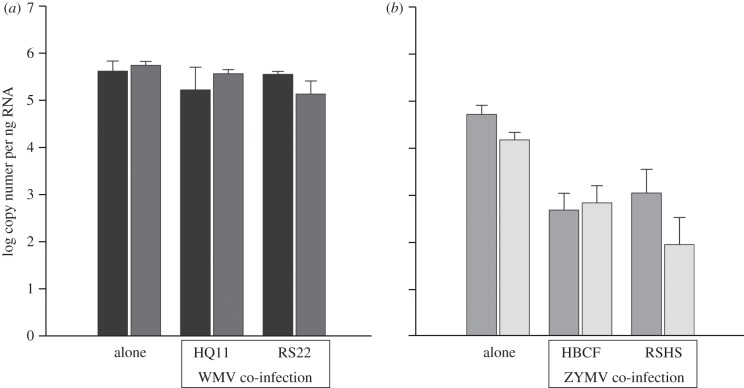
Of 40 symptomatic plants in the four mixed-inoculation treatments of both blocked experiments, 36 (90%) had WMV and ZYMV levels indicative of co-infection two weeks after inoculation, whereas levels of WMV in three plants were below sensitivity of the assay (so that single infection by ZYMV could not be ruled out), and the opposite situation occurred in one plant. Numbers of ZYMV virus RNA copies did not differ between the two isolates tested (ANOVA on log-transformed data, F1,53 = 0.005, p-value = 0.94), nor between single infections and co-infections with either WMV isolate (F2,53 = 1.1, p-value = 0.34, figure 1a). Furthermore, there was no difference between the two WMV isolates in single infections (F1,53 = 2.41, p-value = 0.12). However, the number of WMV copies differed significantly among singly infected plants and co-infections with either ZYMV isolate (ANOVA on log-transformed values, F2,53 = 14.71, p-value < 0.0001), and co-infections yielded significantly fewer WMV genome copies (contrast of single infection versus co-infection treatments, F1,53 = 28.9, p-value <0.0001, figure 1b). Interaction effects were never significant. The same pattern of reduced WMV RNA in co-infection with ZYMV was observed in both blocks of the experiment (entailing two entirely independent series of inoculations).
Figure 1.
RNA copy numbers in plant tissues in single and co-infections by ZYMV and WMV isolates. RNA copy numbers in plants tissues estimated for each virus in the eight different virus-inoculated treatments (log-transformed values, ±s.e.). (a) The estimates obtained for the two isolates of ZYMV, HBCF (black) and RSHS (dark grey), with the ZYMV coat protein assay. The first two bars correspond to the two single-inoculation treatments, the four following bars to co-infections with the WMV isolates HQ11 and RS22. (b) The estimates obtained for the two isolates of WMV, HQ11 (dark grey) and RS22 (light grey), with the WMV coat protein assay. The first two bars correspond to the two single-inoculation treatments, the four following bars to co-infections with the ZYMV isolates HBCF and RSHS.
(b). Aphid transmission of watermelon mosaic virus and zucchini yellow mosaic virus in single and mixed infections
While the scope of the current study does not allow us to make statistically rigorous comparisons of virus transmission rates for individual isolates, our transmission assays revealed intriguing patterns between the two viruses. Of 40 total trials, aphids successfully transmitted viruses to at least one recipient plant in 22 cases, corresponding to a general transmission rate of 55 per cent (table 1). WMV in single infections was successfully transmitted in eight out of 10 trials, compared with four out of 10 for ZYMV, all involving isolate ZYMV-RSHS. Half of the 20 plants in the mixed infection treatments transmitted at least one virus species. One symptomatic recipient plant in each replicate was analysed by RT-qPCR assays for WMV and ZYMV coat protein RNA to determine the presence of each virus in these secondary infected plants: one transmission was WMV only, four were ZYMV only and five had typical co-infection levels of both WMV and ZYMV viruses. The global transmission success from mixed infections of 30 per cent (n = 20) for WMV did not significantly deviate from the 45 per cent (n = 20) success observed for ZYMV from the same plants (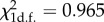 , p-value = 0.32). The latter result suggests that WMV isolates can frequently be transmitted from mixed infections despite the low accumulation for WMV in plant tissues in the presence of ZYMV. Interestingly, although no successful transmission of single infections with the ZYMV isolate HBCF was observed, two of the successful transmissions from mixed infections involved this isolate.
, p-value = 0.32). The latter result suggests that WMV isolates can frequently be transmitted from mixed infections despite the low accumulation for WMV in plant tissues in the presence of ZYMV. Interestingly, although no successful transmission of single infections with the ZYMV isolate HBCF was observed, two of the successful transmissions from mixed infections involved this isolate.
Table 1.
Aphis gossypii transmission assays. (Numbers of successful transmissions of each virus species, i.e. transmitted to at least one of the triplicate recipient plants, from all possible combinations of single and mixed-virus infection treatments.)
| treatment of the infectious plant | virus species in the recipient plant |
|||
|---|---|---|---|---|
| WMV | ZYMV | co-infection | no symptoms | |
| WMV isolates | ||||
| WMV-HQ11 | 3/5 | — | — | 2/5 |
| WMV-RS22 | 5/5 | — | — | 0/5 |
| total WMV | 8/10 | — | — | 2/10 |
| ZYMV isolates | ||||
| ZYMV-HBCF | — | 0/5 | — | 5/5 |
| ZYMV-RHSH | — | 4/5 | — | 1/5 |
| total ZYMV | — | 4/10 | — | 6/10 |
| co-infections | ||||
| HQ11 versus HBCF | 1/5 | 1/5 | 0/5 | 3/5 |
| HQ11 versus RSHS | 0/5 | 1/5 | 2/5 | 2/5 |
| RS22 versus HBCF | 0/5 | 0/5 | 1/5 | 4/5 |
| RS22 versus RSHS | 0/5 | 2/5 | 2/5 | 1/4 |
| total co-infections | 1/20 | 4/20 | 5/20 | 10/20 |
(c). Virulence of watermelon mosaic virus and zucchini yellow mosaic virus in single and mixed infections
Plant surface area differed significantly among the four basic types of inoculation, with mock-inoculated plants being larger than all classes of virus infected plants, and plants infected with ZYMV in either single or co-infections being smaller than those with single infections of WMV (ANOVA on Box-Cox transformed plant surface, ‘inoculation type’ effect F3,87 = 36.53, p-value <0.0001). Thus, all isolates influenced plant performance, but ZYMV appeared more virulent than WMV. There was no significant variation in surface area between isolates within each virus species, or between the four mixed species co-infections (ANOVA on Box-Cox transformed plant surface, ‘isolate’ nested within ‘inoculation type’ effect F5,87 = 1.12, p-value = 0.35). Among all plants from single ZYMV inoculation treatments, there was a slightly non-significant negative correlation (Pearson's correlation coefficient = –0.44, p-value = 0.053, n = 20) between plant surface and ZYMV accumulation (log-transformed), whereas no correlation was detected with WMV levels in single WMV inoculation plants (Pearson's correlation coefficient = –0.20, p-value = 0.39, n = 20).
(d). Changes in leaf coloration
Inoculation type had a significant effect on plant colour of symptomatic plants (Wilk's λ = 0.36, approximate F9,207.02 = 11.99, p-value < 0.0001). Single WMV, single ZYMV and mixed infections were all significantly different from mock-inoculated plants (MANOVA contrasts, F5,83 = 14.87–28.17–27.66, respectively, all p-values < 0.0001). WMV single infections also differed from ZYMV or mixed ZYMV–WMV infections (MANOVA contrasts, F5,83 = 8.04 and 8.80, respectively, both p-values < 0.0001), which did not differ from one another (MANOVA contrasts, F5,83 = 0.79, p-value > 0.50). The first PCA component—combining red and green—explained 63.5 per cent of the variance and separated the ZYMV and co-infected plants from mock-inoculated and WMV-infected ones (see the electronic supplementary material, figure S1). This reflects an increase in yellow coloration in ZYMV and mixed-infection plants. The second component—representing mainly the blue colour component—explained 32.9 per cent or the remaining variance and separated all virus-infected plants from the mock-inoculated controls. There was no significant difference in mean values of red, green and blue colour components between isolates of the same virus or between the different mixed infections (Wilk's λ = 0.81, approximate F15,235.05 = 1.24, p-value = 0.24).
(e). Emissions of volatile organic compounds
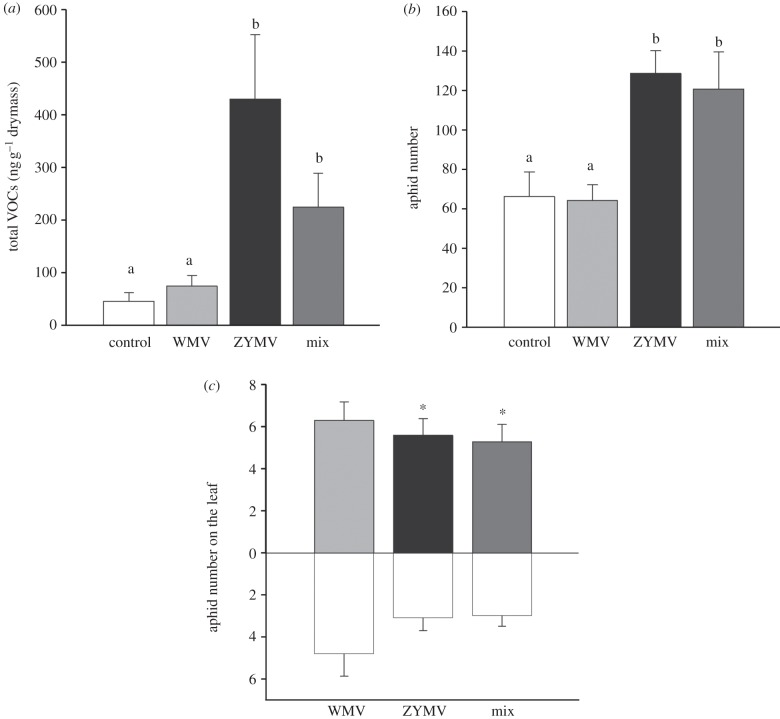
Sixteen volatile compounds were consistently observed across treatments, with overall higher amounts of total volatiles observed for plants infected with ZYMV-HBCF, either alone or in co-infection with WMV-HQ11, compared with mock-inoculated plants or plants infected by WMV-HQ11 alone (ANOVA on log-transformed values of total volatiles emitted, F3,20 = 22.82, p-value < 0.001; figure 2a). A MANOVA on all 16 compounds (after log-transformation) confirmed that the four treatments differed significantly (Wilk's λ = 2.95.10−4, approximate F48,15.6 = 4.69, p-value = 0.0008). PCA revealed that 42.3 per cent of the variance between the samples was explained by a first component combining the compounds limonene, linalool, benzaldehyde, benzyl alcohol, linalool oxide, E-β-ocimene, nonatriene, methyl-salicylic acid and germacrene D, which were all elevated in ZYMV-infected plants of both single and mixed treatments. The second principal component, explaining 25 per cent of the variance, included the compounds 1-octen-3-ol, two unidentified compounds (Unk2 and Unk3), and α-humulene and β-sesquiphellandrene, two sesquiterpenes that were absent or very rare in ZYMV-infected plants (see the electronic supplementary material, table S2 and figure S2).
Figure 2.
Effects of single and mixed infections on plant volatiles, aphid performance and aphid preference. Results of assays bearing on plant–aphid interactions for mock-inoculated plants (‘controls’, white bars), plants inoculated with the isolates WMV-HQ11 (‘WMV’, light grey bars) or ZYMV-HBCF (‘ZYMV’, black bars) and plants co-inoculated with both viruses (‘mix’, dark grey bars). (a) Average of the cumulated emission of the 16 main organic volatile compounds (±s.e., seven replicates of each treatment); (b) Aphid performance estimated as the average number of aphids in colonies after two weeks (±s.e., ten replicates); (c) Aphid preference in choice arenas for control or virus-infected plants estimated as the average numbers of aphids (±s.e., 10 replicates of each test) on exposed leaves for each of the three types of dual choice tests. Different letters above the bars indicate groups that are significantly different (p-value <0.05). *Paired t-test significant with p-value <0.05.
(f). Aphid performance
Two weeks after establishment, the number of aphids was on average twice as high in both ZYMV-HBCF-infected and mixed-infection treatments compared with mock-inoculated and WMV-HQ11-infected plants (One-way ANOVA, F3,35 = 6.60, p-value = 0.0012, figure 2b).
(g). Aphid choice between mock- and virus-inoculated plants
Significantly more aphids were found on infected leaves than on control leaves both for ZYMV-HBCF infections (paired t-test, t-ratio9d.f. = 2.39, p-value = 0.04) and mixed WMV–ZYMV infections (t-ratio9d.f. = 2.41, p-value = 0.039). In contrast, aphids did not discriminate leaves from plants infected with WMV-HQ11 alone from control leaves (t-ratio9d.f. = 0.96, p-value = 0.36, figure 2c)—though higher overall numbers of aphids were found on both leaves of this test compared with other assays, likely because the WMV-infected plants had larger leaves and were paired with similar sized control leaves.
4. Discussion
Our results suggest that within host replication in C. pepo plants manually co-inoculated with WMV and ZYMV was dominated by the latter. We measured similar final RNA copy numbers for both of the ZYMV isolates examined, and these levels were little influenced by the presence of WMV isolates in co-infection (figure 1a). In contrast, both of the WMV isolates examined experienced significantly reduced replication in the presence of either ZYMV isolate, compared with their accumulation in symptomatic plants with single infections (figure 1b), and there was no significant genotype-by-genotype interactions between treatments. Despite the likelihood of some differences in inoculum infectivity among ZYMV and WMV isolates (our WMV strains exhibited a 20% lower overall probability of developing symptomatic infections compared with ZYMV strains), it is unlikely that such effects can explain the two order of magnitude reduction observed for WMV in the presence of ZYMV—particularly as preliminary serial-dilution assays indicated that final virus population sizes were resilient to changes in initial inoculum concentrations. It is certainly possible that the outcome of interactions between ZYMV and WMV might be sensitive to the initial conditions under which in-plant competition takes place (e.g. the relative timing of infection by each virus and/or differences in population size during the early stages of co-infection) so that different alternative scenarios might yield different outcomes. Nevertheless, under the conditions tested here, there is little doubt that the presence of ZYMV exerted a negative effect on the replication of WMV in co-infection, while no similar reciprocal effect was observed.
Despite their lower accumulation levels in mixed-infected plants, however, WMV isolates were still readily transmitted from these plants to new hosts by aphids. On the other hand, the ZYMV-HBCF isolate failed to be transmitted from single infections in any of our subsequent assays, while it was transmitted from mixed infections with WMV isolates (table 1). This pattern suggests that this ZYMV-HBCF may have a deficiency with respect to aphid transmission that is mitigated by the presence of its closely related competitor. Similar transmission rescue has previously been observed in plant viruses [37,38], including another isolate of ZYMV [39] possessing a deficient helper-component protein that functions in virion attachment to aphid mouth parts [25].
In addition to being the major virus accumulating in infected tissues, we found that ZYMV induces significant alteration of host plant traits mediating interactions with aphid vectors. ZYMV-infected plants, and plants with mixed ZYMV–WMV infections, supported significantly higher levels of aphid population growth than plants with WMV infections or uninfected controls (figure 2b). The finding that ZYMV enhances plant quality for aphids runs counter to the apparent tendency of non-persistently transmitted viruses such as ZYMV (and WMV), which can be readily acquired and transmitted by aphids during brief probes of infected tissues and are thus thought to benefit from frequent aphid movement among plants, to more often reduce host plant quality for vectors than persistently transmitted viruses, whose acquisition typically requires sustained aphid feeding on the phloem of infected plants [15]. But these results are consistent with those of a previous study reporting higher reproductive rates for the same aphid species on similar young ZYMV-infected plants [40], and higher vector load on host plants can also permit a larger transmission of viruses when it favours the presence of predators, which may trigger alate dispersal [41,42].
In keeping with the enhanced quality of plants harbouring ZYMV for aphids, a preferential aphid attraction to the leaves of plants with ZYMV or mixed infections was observed, whereas plants harbouring single WMV infections were not significantly more attractive than uninfected controls (figure 2c). These aphid preferences were likely mediated by plant-derived olfactory and visual cues, as aphids are known to use both to locate host plants while walking [43–45]. Both plant volatile emissions and leaf-colour profiles were also altered to a much greater extent by ZYMV than by WMV, whereas mixed infections elicited a phenotype similar to that exhibited by plants with single ZYMV infections (see figure 2a and electronic supplementary material, S1 and S2). Plants harbouring ZYMV in single or mixed infections emitted significantly higher total amounts of organic volatile compounds than controls or WMV plants (figure 2a). Furthermore, there were characteristic qualitative differences in the blends of these plants, as the emission of some sesquiterpenes compounds was reduced in plants harbouring ZYMV, despite the overall elevation of emissions (see the electronic supplementary material, table S2 and figure S2). We previously hypothesized that qualitative changes in volatile emissions (or other cues), which consequently produce a characteristic signature of infection, might be commonly induced by pathogens that enhance host quality for vectors, while pathogens that reduce host quality for vectors might more frequently exaggerate existing host location cues [7], a pattern consistent with some recent empirical findings [3,7,15]. Plants with ZYMV and mixed infections also exhibited significant changes in leaf coloration compared with controls, with elevation on the green and red components (i.e. more intense yellow coloration) and reduction of the blue component (which was also observed for single WMV infections; electronic supplementary material, figure S1). Aphids typically exhibit a preference for yellow and an aversion to blue to ultraviolet coloration (reviewed in Doering & Chittka [43]).
The aphid-attractive changes in plant visual and olfactory cues induced by ZYMV infection may facilitate transmission—vector attraction to infected leaves is theorized to favour the spread of non-persistent viruses, particularly during the early stages of epidemics [2]—and thus may be examples of host manipulation. Alternatively, these effects might be fortuitous by-products of infection, as ZYMV exhibited the highest virulence although with little correlation with its in planta accumulation. The fact that similar effects are not observed for WMV, despite the close relationship between these two viruses, however, suggests these changes of key host traits mediating vector attraction are not unavoidable aspects of viral pathology. In any event, the dramatic differences in the effects elicited by these two closely related and frequently co-occurring viruses raise interesting questions about the potential selection pressures bearing on the evolution of such effects in natural environments.
For example, the fact that plants with mixed infections closely resemble those infected by ZYMV alone suggests that WMV present in co-infected plants might benefit from any enhancement of aphid-transmission opportunities created by ZYMV-driven changes in host traits. Such an ability to increase transmission between hosts by free-riding on the ‘manipulative’ effects of ZYMV might to some extent mitigate the reduced rates of within-host replication that we consistently observed for WMV in mixed infections—such compensatory effects are certainly plausible, since as noted above we observed WMV to be readily transmitted from plants with mixed infections (more than 50% of new infections arising via aphid transmission from mixed-infection source plants included this virus). This observation may be relevant in understanding how these two viruses coexist while inhabiting very similar ecological niches (i.e. overlapping host and vector ranges), as previous work suggests that differential competitive ability for within-host replication and/or for vector transmission between hosts can lead to strain or species displacement [46,47]. Furthermore, although ZYMV did not appear to suffer any significant reduction in within-plant replication in the presence of WMV, free-riding by WMV might reduce the potential benefits of host manipulation for ZYMV to the extent that these viruses are in competition with one another for access to aphid vectors, which currently remains unclear—transmission of each virus requires the binding of virions to the aphid stylet, but little is currently known about the specific sites of attachment for these viruses or whether they are shared [25].
These observations reflect more general questions about the ecology and evolution of interactions among manipulative and non-manipulative pathogens. For example, if access to vectors is a limiting factor on pathogen transmission opportunities, then sharing a vector (and/or optimal patterns of vector behaviour with respect to hosts) with non-manipulative competitors may reduce the fitness advantage of host manipulations that influence the frequency and nature of host–vector interactions. By analogy with free-rider strategies impeding the evolution of stable cooperation, host manipulation might thus tend to be an unstable strategy in the presence of non-manipulative ‘cheaters’ [48], especially if they are unrelated [16]. However, if competition for access to vectors is not zero-sum, then free-riding by non-manipulative pathogens may have limited impacts on the evolutionary dynamics of the manipulators. Such factors influencing competition for between-host transmission may interact with those influencing within-host competition and effects on host virulence to determine the ultimate trajectory of coevolutionary interactions among co-occurring pathogens.
While it is beyond the aims of the current study to determine exactly how these factors have shaped the ecology and evolution of WMV and ZYMV, our findings suggest that variation in viral ‘strategies’ bearing on aphid–host interactions and transmission exists in natural populations of closely related and frequently co-occurring viruses. Thus, the community of potyviruses consisting of WMV and ZYMV appears to be a useful system in which to further explore the dynamics of these interactions and their impact on pathogen evolution. Previous research on virus interactions in co-infection has largely focused on somewhat atypical virus associations exhibiting large synergistic virulence effects (e.g. drastic changes in host size or longevity [49]), and comparisons of their impact on plant-aphid relationships are quite rare. In one example, mixed infections of potato leafroll virus (PLRV; a persistently transmitted virus) with the potyvirus potato virus Y exhibited an increase in virulence symptoms compared with single infections, along with an increase in aphid attraction and performance [50]. On the other hand, in another well described synergy between CMV and potyviruses [30], aphid transmission of either virus was less efficient in double infections compared with single infections [51]. To our knowledge, the current study is the first to investigate the consequences of co-infection on aphid-related plant traits for viruses exhibiting antagonistic competition (at least at the level of in-plant replication), which likely reflects many of the interactions between viral pathogens occurring in nature [52,53].
In addition to providing insight into the evolutionary dynamics of co-occurring pathogens, further exploration of this and similar systems may yield insights with significant implications for the management of vectored plant pathogens in natural and agricultural systems. The evolution of intricate resistance and infectivity mechanisms through the constant co-evolution of plants and their pathogens is now well described and has been immensely useful in providing tools for breeding resistant crops, but more stable or new control strategies are always needed to keep up with pathogen evolution. Expanding the knowledge of other battlefields of plant–pathogen interactions such as the chemical ecology of their relationship with insect vectors should thus inform the development of novel strategies for sustainable management.
Acknowledgements
We thank Heike Betz, Erica Smyers and Janet Saunders for their technical assistance, Irmgard Seidl-Adams, Cristina Rosa, M. Fernanda Penaflor and Kerry Mauck for their help and advice in designing the experiments. Jacqui Shykoff helped to improve earlier versions of the manuscript. We also thank Dr Hector Quemada for his help in the field collection of virus isolates. Financial support for this research was provided by the USDA National Institute of Food and Agriculture (grant no. 2008-35302-04577) and the David and Lucile Packard Foundation. L.S. is financially supported by the European Communities seventh Framework Programme (FP7/2007-2013) under grant agreement no. PIOF-GA-2009-236011.
References
- 1.McElhany P, Real LA, Power AG. 1995. Vector preference and disease dynamics: a study of barley yellow dwarf virus. Ecology 76, 444–457 10.2307/1941203 (doi:10.2307/1941203) [DOI] [Google Scholar]
- 2.Sisterson MS. 2008. Effects of insect-vector preference for healthy or infected plants on pathogen spread: insights from a model. J. Econ. Entomol. 101, 1–8 10.1603/0022-0493(2008)101[1:EOIPFH]2.0.CO;2 (doi:10.1603/0022-0493(2008)101[1:EOIPFH]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 3.Mann RS, Ali JG, Hermann SL, Tiwari S, Pelz-Stelinski KS, Alborn HT, Stelinski LL. 2012. Induced release of a plant-defense volatile ‘deceptively’ attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog. 8, e1002610. 10.1371/journal.ppat.1002610 (doi:10.1371/journal.ppat.1002610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLeod G, Gries R, von Reuss SH, Rahe JE, McIntosh R, Konig WA, Gries G. 2005. The pathogen causing Dutch elm disease makes host trees attract insect vectors. Proc. R. Soc. B 272, 2499–2503 10.1098/rspb.2005.3202 (doi:10.1098/rspb.2005.3202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacroix R, Mukabana WR, Gouagna LC, Koella JC. 2005. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 3, 1590–1593 10.1371/journal.pbio.0030298 (doi:10.1371/journal.pbio.0030298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eigenbrode SD, Ding HJ, Shiel P, Berger PH. 2002. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. Lond. B 269, 455–460 10.1098/rspb.2001.1909 (doi:10.1098/rspb.2001.1909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauck KE, De Moraes CM, Mescher MC. 2010. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl Acad. Sci. USA 107, 3600–3605 10.1073/pnas.0907191107 (doi:10.1073/pnas.0907191107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro L, De Moraes CM, Stephenson AG, Mescher MC. 2012. Pathogen effects on vegetative and floral odours mediate vector attraction and host exposure in a complex pathosystem. Ecol. Lett. 15, 1430–1438 10.1111/ele.12001 (doi:10.1111/ele.12001) [DOI] [PubMed] [Google Scholar]
- 9.Stafford CA, Walker GP, Ullman DE. 2011. Infection with a plant virus modifies vector feeding behavior. Proc. Natl Acad. Sci. USA 108, 9350–9355 10.1073/pnas.1100773108 (doi:10.1073/pnas.1100773108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belliure B, Janssen A, Maris PC, Peters D, Sabelis MW. 2005. Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 8, 70–79 10.1111/j.1461-0248.2004.00699.x (doi:10.1111/j.1461-0248.2004.00699.x) [DOI] [Google Scholar]
- 11.Dawkins R. 1983. The extended phenotype: the long reach of the gene. Oxford, UK: Oxford University Press [Google Scholar]
- 12.Roy BA. 1994. The use and abuse of pollinators by fungi. Trends Ecol. Evol. 9, 335–339 10.1016/0169-5347(94)90154-6 (doi:10.1016/0169-5347(94)90154-6) [DOI] [PubMed] [Google Scholar]
- 13.Anderson RM, May RM. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press [Google Scholar]
- 14.Thomas F, Adamo S, Moore J. 2005. Parasitic manipulation: where are we and where should we go? Behav. Process 68, 185–199 10.1016/j.beproc.2004.06.010 (doi:10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 15.Mauck KE, Bosque-Pérez NA, Eigenbrode SD, De Moraes CM, Mescher MC. 2012. Transmission mechanisms shape pathogen effects on host-vector interactions: evidence from plant viruses. Funct. Ecol. 26, 1162–1175 10.1111/j.1365-2435.2012.02026.x (doi:10.1111/j.1365-2435.2012.02026.x) [DOI] [Google Scholar]
- 16.Vickery WL, Poulin R. 2010. The evolution of host manipulation by parasites: a game theory analysis. Evol. Ecol. 24, 773–788 10.1007/s10682-009-9334-0 (doi:10.1007/s10682-009-9334-0) [DOI] [Google Scholar]
- 17.Cezilly F, Gregoire A, Bertin A. 2000. Conflict between co-occurring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology 120, 625–630 10.1017/s0031182099005910 (doi:10.1017/s0031182099005910) [DOI] [PubMed] [Google Scholar]
- 18.Thomas F, Mete K, Helluy S, Santalla F, Verneau O, DeMeeus T, Cezilly F, Renaud F. 1997. Hitch-hiker parasites or how to benefit from the strategy of another parasite. Evolution 51, 1316–1318 10.2307/2411060 (doi:10.2307/2411060) [DOI] [PubMed] [Google Scholar]
- 19.Thomas F, Brodeur J, Maure F, Franceschi N, Blanchet S, Rigaud T. 2011. Intraspecific variability in host manipulation by parasites. Infect. Genet. Evol. 11, 262–269 10.1016/j.meegid.2010.12.013 (doi:10.1016/j.meegid.2010.12.013) [DOI] [PubMed] [Google Scholar]
- 20.Desbiez C, Lecoq H. 2004. The nucleotide sequence of watermelon mosaic virus (WMV, Potyvirus) reveals interspecific recombination between two related potyviruses in the 5‘ part of the genome. Arch. Virol. 149, 1619–1632 10.1007/s00705-004-0340-9 (doi:10.1007/s00705-004-0340-9) [DOI] [PubMed] [Google Scholar]
- 21.Desbiez C, Lecoq H. 1997. Zucchini yellow mosaic virus. Plant Pathol. 46, 809–829 10.1046/j.1365-3059.1997.d01-87.x (doi:10.1046/j.1365-3059.1997.d01-87.x) [DOI] [Google Scholar]
- 22.Lecoq H, Desbiez C. 2008. Watermelon mosaic virus and zucchini yellow mosaic virus. In Encyclopedia of virology (eds Mahy BWJ, van MHV. Regenmortel), pp. 433–440 Oxford, UK: Elsevier [Google Scholar]
- 23.Castle SJ, Perring TM, Farrar CA, Kishaba AN. 1992. Field and laboratory transmission of watermelon mosaic virus-2 and zucchini yellow mosaic virus by various aphid species. Phytopathology 82, 235–240 10.1094/Phyto-82-235 (doi:10.1094/Phyto-82-235) [DOI] [Google Scholar]
- 24.Gal-On A. 2007. Zucchini yellow mosaic virus: insect transmission and pathogenicity—the tails of two proteins. Mol. Plant Pathol. 8, 139–150 10.1111/j.1364-3703.2007.00381.x (doi:10.1111/j.1364-3703.2007.00381.x) [DOI] [PubMed] [Google Scholar]
- 25.Brault V, Uzest M, Monsion B, Jacquot E, Blanc S. 2010. Aphids as transport devices for plant viruses. C R Biol. 333, 524–538 10.1016/j.crvi.2010.04.001 (doi:10.1016/j.crvi.2010.04.001) [DOI] [PubMed] [Google Scholar]
- 26.Jossey S, Babadoost M. 2008. Occurrence and distribution of pumpkin and squash viruses in Illinois. Plant Dis. 92, 61–68 10.1094/pdis-92-1-0061 (doi:10.1094/pdis-92-1-0061) [DOI] [PubMed] [Google Scholar]
- 27.Koklu G, Yilmaz O. 2006. Occurrence of cucurbit viruses on field-grown melon and watermelon in the Thrace region of Turkey. Phytoprotection 87, 123–130 10.7202/015854ar (doi:10.7202/015854ar) [DOI] [Google Scholar]
- 28.Quemada H, Strehlow L, Decker-Walters DS, Staub JE. 2008. Population size and incidence of virus infection in free-living populations of Cucurbita pepo. Environ. Biosafety Res. 7, 185–196 10.1051/ebr:2008022 (doi:10.1051/ebr:2008022) [DOI] [PubMed] [Google Scholar]
- 29.Fletcher JD, Wallace AR, Rogers BT. 2000. Potyviruses in New Zealand buttercup squash (Cucurbita maxima Duch.): yield and quality effects of ZYMV and WMV 2 virus infections. N Z J. Crop Hort. 28, 17–26 10.1080/01140671.2000.9514118 (doi:10.1080/01140671.2000.9514118) [DOI] [Google Scholar]
- 30.Wang YZ, Gaba V, Yang J, Palukaitis P, Gal-On A. 2002. Characterization of synergy between cucumber mosaic virus and potyviruses in cucurbit hosts. Phytopathology 92, 51–58 10.1094/PHYTO.2002.92.1.51 (doi:10.1094/PHYTO.2002.92.1.51) [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Jara P, Tenllado F, Martinez-Garcia B, Atencio FA, Barajas D, Vargas M, Diaz-Ruiz J, Diaz-Ruiz JR. 2004. Host-dependent differences during synergistic infection by Potyviruses with potato virus X. Mol. Plant Pathol. 5, 29–35 10.1046/j.1364-3703.2004.00202.x (doi:10.1046/j.1364-3703.2004.00202.x) [DOI] [PubMed] [Google Scholar]
- 32.Seabloom EW, Borer ET, Mitchell CE, Power AG. 2010. Viral diversity and prevalence gradients in North American Pacific Coast grasslands. Ecology 91, 721–732 10.1890/08-2170.1 (doi:10.1890/08-2170.1) [DOI] [PubMed] [Google Scholar]
- 33.Seabloom EW, Hosseini PR, Power AG, Borer ET. 2009. Diversity and composition of viral communities: coinfection of barley and cereal yellow dwarf viruses in California grasslands. Am. Nat. 173, E79–E98 10.1086/596529 (doi:10.1086/596529) [DOI] [PubMed] [Google Scholar]
- 34.Simmons HE, Holmes EC, Stephenson AG. 2011. Rapid turnover of intra-host genetic diversity in zucchini yellow mosaic virus. Virus Res. 155, 389–396 10.1016/j.virusres.2010.11.007 (doi:10.1016/j.virusres.2010.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prendeville HR, Ye XH, Morris TJ, Pilson D. 2012. Virus infections in wild plant populations are both frequent and often unapparent. Am. J. Bot. 99, 1033–1042 10.3732/ajb.1100509 (doi:10.3732/ajb.1100509) [DOI] [PubMed] [Google Scholar]
- 36.Sacristan S, Garcia-Arenal F. 2008. The evolution of virulence and pathogenicity in plant pathogen populations. Mol. Plant Pathol. 9, 369–384 10.1111/j.1364-3703.2007.00460.x (doi:10.1111/j.1364-3703.2007.00460.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Froissart R, Michalakis Y, Blanc S. 2002. Helper component-transcomplementation in the vector transmission of plant viruses. Phytopathology 92, 576–579 10.1094/PHYTO.2002.92.6.576 (doi:10.1094/PHYTO.2002.92.6.576) [DOI] [PubMed] [Google Scholar]
- 38.Rochow WF. 1972. The role of mixed infections in the transmission of plant viruses by aphids. Annu. Rev. Phytopathol. 10, 101–124 10.1146/annurev.py.10.090172.000533 (doi:10.1146/annurev.py.10.090172.000533) [DOI] [Google Scholar]
- 39.Bourdin D, Lecoq H. 1991. Evidence that heteroencapsidation between two potyviruses is involved in aphid transmission of a non-aphid-transmissible isolate from mixed infections. Phytopathology 81, 1459–1464 10.1094/Phyto-81-1459 (doi:10.1094/Phyto-81-1459) [DOI] [Google Scholar]
- 40.Blua MJ, Perring TM. 1992. Alatae production and population increase of aphid vectors on virus-infected host plants. Oecologia 92, 65–70 10.1007/BF00317263 (doi:10.1007/BF00317263) [DOI] [PubMed] [Google Scholar]
- 41.Hodge S, Hardie J, Powell G. 2011. Parasitoids aid dispersal of a nonpersistently transmitted plant virus by disturbing the aphid vector. Agric. Forest Entomol. 13, 83–88 10.1111/j.1461-9563.2010.00493.x (doi:10.1111/j.1461-9563.2010.00493.x) [DOI] [Google Scholar]
- 42.Jeger MJ, Chen Z, Powell G, Hodge S, van den Bosch F. 2011. Interactions in a host plant–virus–vector–parasitoid system: modelling the consequences for virus transmission and disease dynamics. Virus Res. 159, 183–193 10.1016/j.virusres.2011.04.027 (doi:10.1016/j.virusres.2011.04.027) [DOI] [PubMed] [Google Scholar]
- 43.Doering TF, Chittka L. 2007. Visual ecology of aphids: a critical review on the role of colours in host finding. Arthropod. Plant Interact. 1, 3–16 10.1007/s11829-006-9000-1 (doi:10.1007/s11829-006-9000-1) [DOI] [Google Scholar]
- 44.Nottingham SF, Hardie J, Dawson GW, Hick AJ, Pickett JA, Wadhams LJ, Woodcock CM. 1991. Behavioral and electrophysiological responses of aphids to host and nonhost plant volatiles. J. Chem. Ecol. 17, 1231–1242 10.1007/bf01402946 (doi:10.1007/bf01402946) [DOI] [PubMed] [Google Scholar]
- 45.Webster B. 2012. The role of olfaction in aphid host location. Physiol. Entomol. 37, 10–18 10.1111/j.1365-3032.2011.00791.x (doi:10.1111/j.1365-3032.2011.00791.x) [DOI] [Google Scholar]
- 46.Lecoq H, Fabre F, Joannon B, Wipf-Scheibel C, Chandeysson C, Schoeny A, Desbiez C. 2011. Search for factors involved in the rapid shift in watermelon mosaic virus (WMV) populations in south-eastern France. Virus Res. 159, 115–123 10.1016/j.virusres.2011.05.004 (doi:10.1016/j.virusres.2011.05.004) [DOI] [PubMed] [Google Scholar]
- 47.Power AG. 1996. Competition between viruses in a complex plant–pathogen. Ecology 77, 1004–1010 10.2307/2265571 (doi:10.2307/2265571) [DOI] [Google Scholar]
- 48.Brown SP. 1999. Cooperation and conflict in host-manipulating parasites. Proc. R. Soc. Lond. B 266, 1899–1904 10.1098/rspb.1999.0864 (doi:10.1098/rspb.1999.0864) [DOI] [Google Scholar]
- 49.Syller J. 2011. Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol. Plant Pathol. 13, 204–216 10.1111/j.1364-3703.2011.00734.x (doi:10.1111/j.1364-3703.2011.00734.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srinivasan R, Alvarez JM. 2007. Effect of mixed viral infections (potato virus Y–potato leafroll virus) on biology and preference of vectors Myzus persicae and Macrosiphum euphorbiae (Hemiptera : Aphididae). J. Econ. Entomol. 100, 646–655 10.1603/0022-0493(2007)100[646:eomvip]2.0.co;2 (doi:10.1603/0022-0493(2007)100[646:eomvip]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 51.Pinto ZV, Marques Rezende JA, Yuki VA, de Stefano Piedade SM. 2008. Ability of Aphis gossypii and Myzus persicae to transmit cucumber mosaic virus in single and mixed infection with two potyviruses to zucchini squash. Summa Phytopathol. 34, 183–185 10.1590/S0100-54052008000200016 (doi:10.1590/S0100-54052008000200016) [DOI] [Google Scholar]
- 52.Martin S, Elena SF. 2009. Application of game theory to the interaction between plant viruses during mixed infections. J. Gen. Virol. 90, 2815–2820 10.1099/vir.0.012351-0 (doi:10.1099/vir.0.012351-0). [DOI] [PubMed] [Google Scholar]
- 53.Roossinck MJ. 2005. Symbiosis versus competition in plant virus evolution. Nat. Rev. Microbiol. 3, 917–924 10.1038/nrmicro1285 (doi:10.1038/nrmicro1285) [DOI] [PubMed] [Google Scholar]