Abstract
Efforts to restore ecosystems often focus on reintroducing apex predators to re-establish coevolved relationships among predators, herbivores and plants. The preponderance of evidence for indirect effects of predators on terrestrial plant communities comes from ecosystems where predators have been removed. Far less is known about the consequences of their restoration. The effects of removal and restoration are unlikely to be symmetrical because removing predators can create feedbacks that reinforce the effects of predator loss. Observational studies have suggested that the reintroduction of wolves to Yellowstone National Park initiated dramatic restoration of riparian ecosystems by releasing willows from excessive browsing by elk. Here, we present results from a decade-long experiment in Yellowstone showing that moderating browsing alone was not sufficient to restore riparian zones along small streams. Instead, restoration of willow communities depended on removing browsing and restoring hydrological conditions that prevailed before the removal of wolves. The 70-year absence of predators from the ecosystem changed the disturbance regime in a way that was not reversed by predator reintroduction. We conclude that predator restoration may not quickly repair effects of predator removal in ecosystems.
Keywords: trophic cascade, alternative states, resilience
1. Introduction
The loss of predators from food webs has degraded ecosystems throughout the world [1–3]. Predators can affect food web structure by reducing the abundance of herbivores or by changing their behaviour in ways that weaken top-down controls on the abundance of plants and the composition of plant communities [4–7]. Although reintroduction of predators can reverse effects of their loss [8,9], this reversal will not occur when the loss of predators from food webs gives rise to an ecosystem state that is resilient to the effects of predator restoration [10,11]. A central challenge for restoring ecosystems is to understand when and why the loss of predators initiates feedbacks that stabilize the ecosystem state created by their absence [12].
Many examples of trophic cascades have come from studies documenting the effects of predator removal [2,3,13,14]. Far fewer examples demonstrate the effects of restoring predators to their native ecosystems. It is vital to distinguish between the effects of removing predators and the effects of restoring them because these effects may not be symmetrical: predator removal may create conditions that resist the effects of their restoration. For example, although restoring extirpated bass to temperate lakes caused a smooth and rapid return of the original food web structure [15], reintroducing arthropod predators to old field mesocosms failed to recover the original ecosystem state [10]. Instead, predator removal from old fields caused changes in herbivory that allowed the competitively dominant plant species to proliferate to the point that predator reintroduction could not restore the original ecosystem configuration. These studies showed that predicting ecosystem response to perturbation of the food web requires understanding feedbacks that create resilience. Understanding these feedbacks often requires manipulation of a food web.
By dramatically restructuring the food web, the reintroduction of the grey wolf (Canis lupus) to the landscapes of the northern range of Yellowstone National Park created an unprecedented opportunity to understand how restoring predators acts to change the state of an ecosystem. Wolves were extirpated from Yellowstone during the early twentieth century. It is clear that the loss of wolves from the ecosystem caused a state change in riparian zones as a result of excessive browsing on the dominant shrub, willow (Salix spp.), by a population of elk (Cervus elaphus) released from control by predators [16–20]. However, it remains unclear if restoration of wolves has restored the riparian zone via a trophic cascade [21–24].
The trophic effects of predator reintroduction may be opposed by changes in the disturbance regime that occurred when wolves were absent from the ecosystem. Beaver (Castor canadensis) disturb small streams by building dams. Willow communities and populations of beaver interact symbiotically in ecologically complete riparian ecosystems. By offering essential food and dam building materials for beaver, willow forms a critical link in the riparian disturbance regime [25]. Disturbance by beaver, in turn, creates conditions particularly well-suited to the life histories of willows [26].
Beaver dams were ubiquitous features of the stream network on the northern range during the 1920s [27]. A third of mainstream reaches show evidence of sediment deposition related to beaver dams [28], a process that has been occurring for millennia [18]. Excessive browsing of willows was implicated in the disappearance of beavers from small streams during the twentieth century [29–31]. The loss of beavers from the northern range created indirect feedbacks on willows, amplifying the direct effects of herbivory by elk. The loss of beaver ponds from the stream network lowered water tables and compressed the area of bare, moist substrate needed for willow establishment [18]. Unimpeded by beaver dams, many northern range streams increased in velocity, downcutting channels and disconnecting flood plains from their adjacent streams [18,28]. Thus, the loss of wolves from the food web caused multiple changes in the ecosystem's biological and physical processes, creating an alternative state where herbaceous vegetation dominated riparian corridors, where willows were predominately sparse in distribution and short in stature, and where beaver, once abundant, were absent [18,32].
Wolves were reintroduced to the northern range of Yellowstone in 1995 to restore a complete food web. The growth of the wolf population during 1995–2010 coincided with a 70 per cent decline in elk numbers [33]. Observations suggest that restoration of wolves altered plant communities in some parts of the northern range via a trophic cascade [34–36], (but also see [37,38]). The trophic cascade hypothesis holds that by modifying foraging behaviour of elk and by reducing their abundance, wolves released plants from top-down control by elk, allowing dramatic recovery in communities degraded by historically excessive herbivory. In particular, it has been argued that trophic effects of wolves have restored willow communities by moderating browsing and allowing willows to grow tall [21,22].
Restoring an ecologically complete ecosystem on the northern range requires the return of tall willow communities to riparian zones and the re-establishment of disturbance by beaver. There is a clear threshold of willow height needed for ecosystem recovery—willow stands must exceed 2 m in height [22,39]. This threshold is important because stems taller than 2 m exceed the reach of browsing ungulates, thereby providing a reliable seed source for willow establishment and preventing complete consumption of the plant during severe winters [39]. Moreover, beavers need tall willows in stands with high biomass to provide food and structural materials for dam building [26].
Here, we test the hypothesis that moderating browsing alone allows willows to recover to their threshold heights relative to the alternative hypothesis that recovery depends on moderating browsing and restoring a disturbance by beaver. These competing hypotheses are motivated by the following ideas. Although excessive browsing by elk released from predation by wolves was responsible for degrading willow communities, the attendant changes in the ecosystem, notably the loss of beaver dams, may have created conditions resilient to restoring wolves to the food web. If reducing top-down effects of browsing is sufficient to allow willows to reach the height threshold for recovery, then there is evidence for the operation of a linear trophic cascade allowing the conclusion that restoring wolves restores riparian ecosystems. Alternatively, if recovery depends on the interplay of trophic and hydrological effects, there is evidence that a complete ecosystem cannot be quickly restored solely by reintroduction of wolves. This alternative hypothesis holds that the current state of the riparian ecosystem is determined by the interaction of top-down control from herbivory and bottom-up control by hydrological changes caused by the loss of disturbance by beaver. This would provide evidence that riparian ecosystems were resilient to the effects of predator restoration; moderating elk herbivory by restoring wolves may be a necessary but insufficient condition for ecosystem restoration.
2. Material and methods
To test these hypotheses, we conducted a 10-year, factorial experiment on the northern range (figure 1; electronic supplementary material, figure S1). Our experiment was designed to represent the attenuation of browsing created by trophic effects of wolves, as well as the modification of stream hydrological processes created by beaver dams. We measured willow height growth and biomass accumulation in response to two levels of herbivory (browsed and unbrowsed; figure 1; electronic supplementary material, figure S2) and two levels of water table depth (dammed and undammed; electronic supplementary material, figure S3) at four sites that were historically dammed by beaver on Yellowstone's northern range (see the electronic supplementary material, figure S1) [23]. Experimental units were four plots per site, each 200 m2 in area. Browsing was eliminated from half of the plots by surrounding them with fences 2.4 m high. Availability of water was enhanced on half of the plots by constructing dams that raised water tables adjacent to streams, simulating the effects of beaver dams that were historically present throughout the northern range. The two treatments (exclosures and dams) were crossed to total four plots per site, including a control representing ambient conditions. Additional details on experimental design may be found in the electronic supplementary material.
Figure 1.

Study system and overview. (a) We built fences to exclude browsing and (b) artificial beaver dams to raise water tables.
In each plot, we quantified willow growth and overwinter losses to browsing and twig shedding. Approximately 10–20 individual plants per plot, representing three species (Salix boothii, Salix geyeriana and Salix bebbiana), were marked permanently and monitored each year. In spring and autumn, willow stature was recorded as height of the tallest stem perpendicular to the ground surface. We annually assessed annual above-ground net primary production at peak standing crop for each plant at the end of the growing season. Current annual growth (CAG) was estimated by measuring lengths of a subset of shoots of the current year and converting them to mass using a length–mass regression fit to 1200 shoots from untagged plants: log(mass) = −3.88+1.18 × log(length) (R2 = 0.92; following methods in [23]).
The preponderance of browsing occurs during winter on the northern range. We estimated overwinter losses of willow tissue from plants using biomass comparison [23,40]. During spring, tagged stems were assessed for biomass remaining to estimate overwinter tissue loss from browsing and twig shedding [40] (electronic supplementary material, figure S2). We defined net accumulated biomass in each year as the CAG remaining after overwinter tissue losses. In browsed plots, overwinter losses included mass lost to browsing and to shedding of new shoots [41]. In unbrowsed plots, overwinter losses consisted of shedding only. Loss (L) was defined as 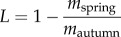 , where mautumn was the sum of CAG on all tagged stems in a plot assessed at the end of the growing season and mspring was the portion of that growth that remained on the plant the following spring [40]. We used the autumn equation for unbrowsed shoots, and converted browsed stem measurements to mass using an equation developed from 180 browsed shoots: log(mass) = −4.52 + 2.54 × log(base diameter) + 0.95 × log(base diameter − browse diameter) (R2 = 0.85). We differentiated between shedding and browsing in browsed plots by estimating the proportion of CAG shed from unbrowsed stems. The unbrowsed subset of stems in browsed plots changed each year depending on which stems ungulates selected. Together, browsed and unbrowsed stems in browsed plots and stems protected from browsing in unbrowsed plots represented the effects of experimental treatments on shoot shedding and browsing (see the electronic supplementary material, figure S2).
, where mautumn was the sum of CAG on all tagged stems in a plot assessed at the end of the growing season and mspring was the portion of that growth that remained on the plant the following spring [40]. We used the autumn equation for unbrowsed shoots, and converted browsed stem measurements to mass using an equation developed from 180 browsed shoots: log(mass) = −4.52 + 2.54 × log(base diameter) + 0.95 × log(base diameter − browse diameter) (R2 = 0.85). We differentiated between shedding and browsing in browsed plots by estimating the proportion of CAG shed from unbrowsed stems. The unbrowsed subset of stems in browsed plots changed each year depending on which stems ungulates selected. Together, browsed and unbrowsed stems in browsed plots and stems protected from browsing in unbrowsed plots represented the effects of experimental treatments on shoot shedding and browsing (see the electronic supplementary material, figure S2).
We monitored the effects of dams on water table levels in wells in dammed and undammed plots. Water tables in undammed plots averaged 121±6 (±1 s.e.m.) cm below ground in July. Dams increased water tables to 88 ± 6 cm in July, a difference of 33 ± 6 cm on average (see the electronic supplementary material, figure S3). Presence of dams did not affect water table levels in adjacent undammed plots. Undammed July water table depths were not significantly different from pre-treatment depth (α = 0.05) during all years except 2008 and 2009. We attribute differences observed during these two years to high flows in streams and late run-off.
(a). Treatment effects after 10 years
We measured two responses: willow height (for years 2001–2010) and accumulated biomass (for years 2002–2005 and 2007–2010). We estimated accumulated biomass of individual plants by summing net CAG in each year: 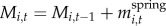 , where Mi,t is the mass of plant i at time t and equals the sum of its mass in the previous year and the net CAG after winter browsing and twig shedding in the current year,
, where Mi,t is the mass of plant i at time t and equals the sum of its mass in the previous year and the net CAG after winter browsing and twig shedding in the current year, 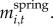
We examined the effects of our experimental treatments using generalized linear mixed-effects models. Using mixed effects allowed us to focus our inference on the effects of the experimental treatments. We included the number of years post-treatment as a continuous predictor and also took into account variation in the effects of the experimental treatments between sites or across years. We constructed models for each response (height and accumulated biomass). We fitted models with random effects for year and site on the intercept, individual treatment effects and interaction effects. We included a three-way interaction among the dam treatment, browsing removal treatment and years since the experiment began (plus all two-way interactions and singular effects) in the fixed effects. We fitted all models using the lme4 package in the R program [42,43]. We selected the best-fit random effects structure for each model using Akaike's information criterion [44], fitting models with restricted maximum likelihood and keeping the fixed effects constant across models [45]. All model selection results and best-fit model coefficients and variance parameters are presented in the electronic supplementary material, tables S1–S4.
We represented uncertainty in regression coefficients by simulating from the best-fit model and calculating 95% prediction intervals for the mean of each treatment across all sites (as described by Gelman & Hill [46]). We calculated effect size for each treatment in 2010 as the natural log of mean height of plants in the treatment divided by mean height of controls, where both means were distributions simulated from the best-fit model. The interaction effect was calculated as the marginal effect of the combined treatment: the natural log of the mean height of plants in the dammed, unbrowsed plots divided by the sum of the difference in height between unbrowsed and control, and the difference between dammed and control. We note that species-specific responses were important in previous analyses of willow height [23]; however, our current analyses indicated that prediction intervals on the species effect overlapped zero for all species pairs.
(b). Comparing treatment effects to observational willow height
We compared heights of willows in experimental plots in 2010 with willow heights across the northern range in the same year to ensure that our experimental results were reasonably representative of conditions on the landscape. We also compared willow heights measured prior to wolf reintroduction with heights measured 15 years after their reintroduction. We used the distributions of model-predicted mean height from each treatment (described earlier) to represent our experiment-derived heights. We measured 113 willow heights at 23 sites at the end of the 2010 growing season.
Sites were selected randomly from a population of stream reaches that were either known to have been occupied by beaver [18,27,28] or were of appropriate size and gradient for damming by beaver [47]. Potential streams were selected based on gradient (greater than 10%) and stream order (third and fourth order). We used a spatially balanced random sampling algorithm (RRQRR) to select from the population of potential sites [48]. Willows within plots were randomly selected. Singer and co-workers measured willow heights across the northern range in 1990, prior to wolf reintroduction [16]. We limited comparison of Singer's data with those species that appeared in our 2010 dataset, resulting in 164 willows at 14 different sites on the northern range.
3. Results
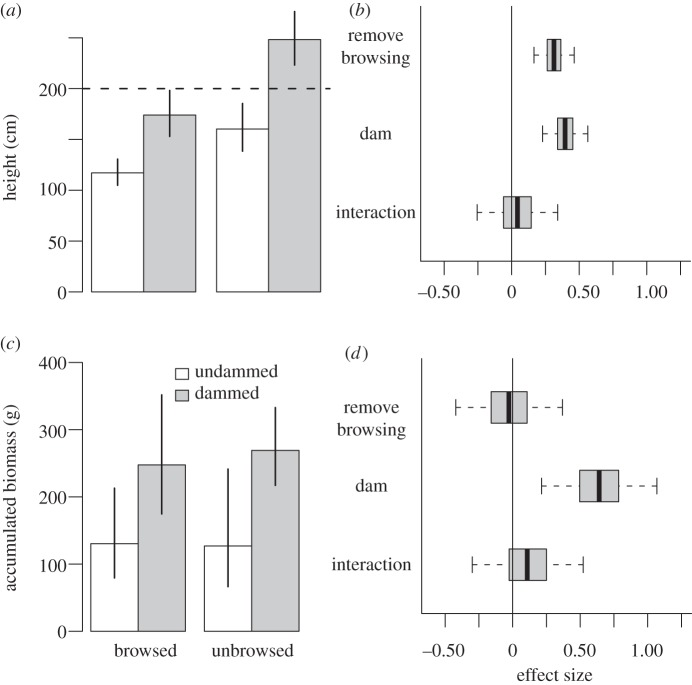
A decade of total protection from ungulate browsing was not sufficient to allow willows to surpass the 2 m threshold height for recovery unless the water table was also raised by simulated beaver dams. Mean heights of plants within exclosures and experiencing ambient stream conditions were well below the recovery threshold (figure 2a). Exclosed, undammed willows were only nominally taller than browsed, undammed willows (mean = 160, 95% prediction interval = [138, 185] cm versus 117 (105, 131) cm) and accumulated no additional net production relative to browsed, undammed willows (130 [65, 242] g versus 127 [78, 214] g; figure 2a,c; electronic supplementary material, figure S3). This result indicates removing browsing had no effect on willow productivity in the absence of dams. The effect of exclosures on height of undammed willows was small, and the effect on their biomass was undetectable (figure 2b,d).
Figure 2.
Willow responses after 10 years. (a) Unbrowsed willows grew taller than browsed willows, and dammed willows grew taller than undammed plants (bars = mean heights from the fit model). Unbrowsed, dammed willows exceeded the 2 m height threshold (dashed line). (b) Dams and browsing removal had similar effects on height after 10 years, but prediction intervals on the interaction between the two treatments overlapped zero. (c) Biomass accumulated at similar rates whether or not willows were browsed, but damming plots increased biomass accumulation through time. (d) Dams had a large positive effect on biomass, whereas removing browsing had no effect. (a,c) White bars, undammed; grey bars, dammed. Error bars indicate 95% prediction intervals. (b,d) Heavy line, mean; horizontal bars, 50% prediction intervals; whiskers, 95% prediction intervals.
Modifying stream conditions by constructing dams without modifying browsing was not sufficient to induce a state change. Heights of dammed plants exceeded the recovery threshold when browsing was removed (248 [223, 276] cm; figures 2a and 3), but dams caused increased growth. After 10 years, browsed willows along dammed streams were nearly 50 per cent taller and accumulated 90 per cent more biomass than browsed willows with ambient stream conditions (174 [153, 199] cm and 248 [175, 352] g versus 117 [105, 131] cm and 130 [79, 214] g; figures 2a,c and 3). The effect size of the dam treatment was stronger on willow biomass accumulation than on willow height (figure 2b,d). Although the interaction term between exclosures and dams remained in the final model for willow height and biomass, the predicted effect size overlapped zero (0.042 [−0.25, 0.338] and 0.11 [−0.30, 0.52]). According to the trophic cascade hypothesis, willows in our control plots should have grown rapidly because they were released from effects of browsing by behavioural and numerical effects of wolves on elk. However, average heights of control plants increased only 39 [30, 48] cm in 10 years and remained far below the recovery threshold (figure 3).
Figure 3.
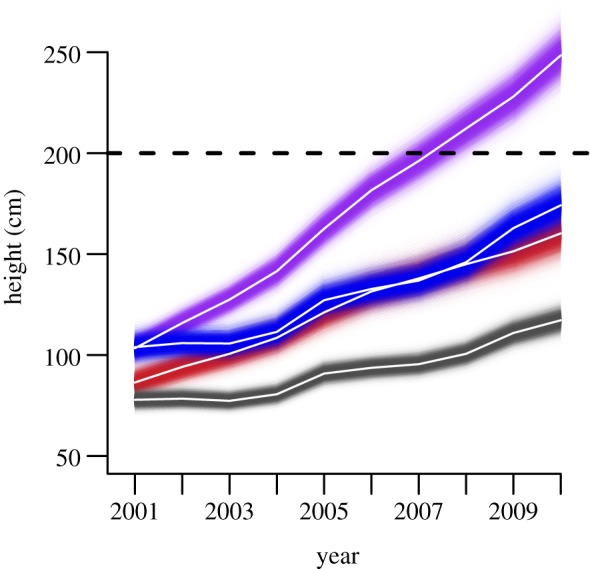
Willow heights from 2001 to 2010 by experimental treatment. White lines represent predicted mean heights for each treatment, dashed line indicated 200 cm height threshold, shading indicates 95% prediction intervals. Colours are as follows: grey, control; red, unbrowsed; blue, dammed; violet, dammed plus unbrowsed.
Willow heights in our experimental plots were representative of current variability in heights observed across sites historically occupied by beaver on the northern range (figure 4). The frequency distribution of 2010 willow heights from 23 sites across the northern range was indistinguishable from the distribution of heights observed on the northern range prior to wolf reintroduction (figure 4). Some tall willows have always existed on the northern range [16], but their abundance relative to short willows has not changed following wolf reintroduction.
Figure 4.
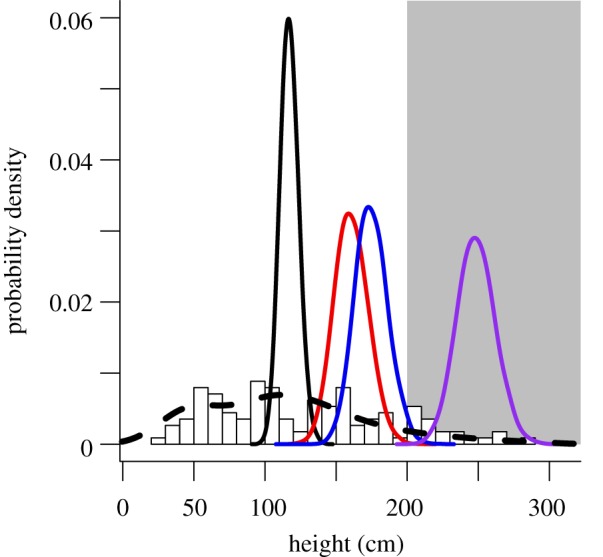
Distributions of willow height on the northern range. Normalized histogram represents end-of-growing-season heights of 113 permanently marked willows observed on the landscape at 23 randomly selected sites with historical or potential for beaver damming. Dashed line represents kernel density of end-of-growing-season heights for 164 willows at 14 different sites measured in 1990 (before wolf reintroduction) by Singer et al. [16]. Solid lines indicate distributions of mean height at the end of 2010 growing season in experimental plots, simulated from the model for willow height; colours as in figure 3. Grey shaded area indicates 200 cm recovery threshold.
4. Discussion
The importance of apex predators to the structure and function of ecosystems has been clearly demonstrated for aquatic and terrestrial systems throughout the world [2,3]. Virtually all previous documentation of the effects of apex predators in large-scale, natural ecosystems occurred following the removal of a large predator [1–3]. Our study of the effects of the recent reintroduction of wolves to the greater Yellowstone ecosystem is novel in that (i) the top predator has been reintroduced after being regionally extinct for nearly 70 years, and (ii) the state of the ecosystem shifted when the predator was removed but has not returned to its original configuration following predator reintroduction.
Landscape-level restoration of riparian zones on Yellowstone's northern range requires restoring physical structure contributed by tall willows as well as restoring the historical disturbance and hydrological regimes created by beaver damming of stream channels. Using a replicated experimental design over 10 years, we showed that the top-down effects of wolves alone were insufficient to restore riparian ecosystems. Restoration was possible, but only when ungulate browsing was eliminated and streams were dammed. The loss of beaver from the network of small streams has lowered water tables, hampering recovery of willows. We showed clear evidence that bottom-up control of willow productivity by hydrological conditions created by beaver dams exceeded top-down control by herbivory. The current state of the landscape is resilient to the trophic effects of wolf restoration because the absence of beaver opposes the return of tall willows and the absence of tall willows opposes the return of beaver.
Our experimental results demonstrate resilience of the alternative state for riparian willows along small streams on the northern range. The initial perturbations to the ecosystem occurred when wolves were extirpated, ungulates increased and beavers abandoned the northern range, which coincided with the loss of willows from riparian zones [18] (figure 5). Seventeen years after wolf reintroduction, willow heights in control plots illustrate that top-down trophic effects alone have not caused willow recovery (figure 5). Removing the initial perturbation of heavy browsing should have allowed willows to recover if the alternative state was not resilient. Instead, willows completely protected from browsing for 10 years showed only moderate height gains. The ecosystem remains in the alternative, short willow state (figure 5).
Figure 5.
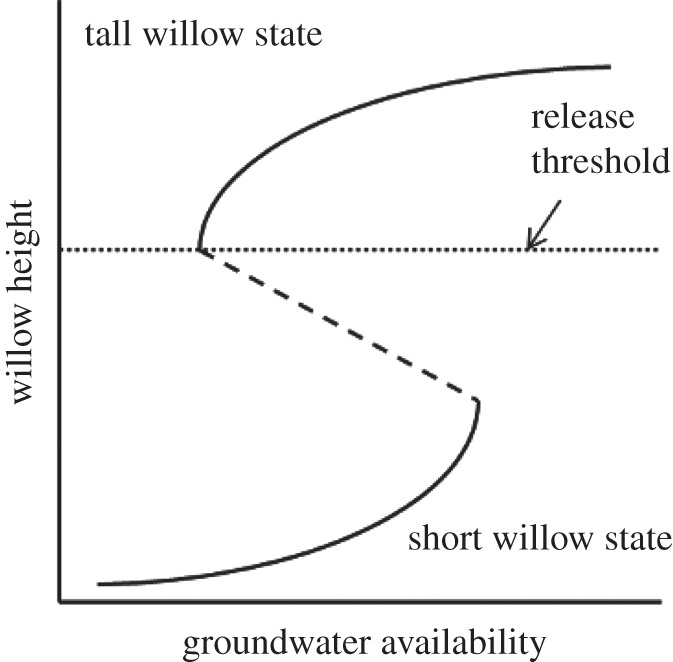
Hypothesized stability landscape for northern range willows as a function of groundwater availability. In areas with high groundwater availability, the tall willow community is the only potential stable state, and is resilient to perturbations owing to browsing. When groundwater availability is very low, a short willow state is the only possibility. In between these two extremes, tall or short willow communities are possible, depending on initial conditions. Historically, willows were probably tall on streams historically occupied by beavers (high groundwater availability). Wolf extirpation and increased ungulate populations led to heavy browsing and beaver abandonment that caused a shift in ecosystem state from the upper curve to the lower curve. In our experiment, all willows began in the short-willow state in 2001. Simulated beaver dams raised water tables, shifting the stable state to the right on the bottom curve. Removing browsing when water tables were high provided a strong enough perturbation to shift the state past the threshold of height recovery to the top curve.
Our dam treatment probably modified a broad suite of stream conditions, including availability of water and nutrients to willows, so we do not argue that elevated water tables alone caused the responses we observed. However, increased availability of water offers a plausible mechanism for these responses for two reasons. Photosynthetic rates of willows on undammed plots with deep water tables were limited by water availability [49], and isotopic analysis of water use by willows adjacent to our plots showed clear positive correlations between height and access to groundwater [24]. Tercek et al. [50] observed that tall willows had twice the net nitrogen mineralization rates of short willows in sites with ambient stream conditions, and suggested that nutrient changes were due to positive feedbacks from increased shading and leaf litter.
Our experimental results shed new light on an on-going debate about the role of wolf-driven trophic cascades in the greater Yellowstone ecosystem [33,37,51]. It is a basic tenet of food web theory that cascading trophic effects of predators on vegetation can only occur when plants are not limited from the bottom up by water, nutrients or other resources (reviewed by Schmitz [52]). These conditions of resource availability are likely to vary across the northern range, leading to spatial variation in the potential to observe trophic effects. It follows that we should expect that indirect effects of wolves on willows will be patchy across the northern range as a result of spatial heterogeneity in access to groundwater and other resources. However, our landscape-level observations suggest that recovery of tall willows following reintroduction of wolves is the exception rather than the norm (figure 4).
The extirpation of wolves from Yellowstone nearly a century ago caused an alternative state to develop in riparian zones across the northern range landscape, but wolf reintroduction has not uniformly restored the historical state of the ecosystem. Beaver have not recolonized any of the sites that were active complexes in the 1920s [27,32]. Seventeen years after restoration of wolves, heights of willows in our control plots (figure 2) and at similar sites across the northern range (figure 4) remain far below the 2 m threshold needed for restoration. The frequency distribution of willow heights closely resembles the distribution observed before wolves were reintroduced (figure 4). Landscape-level restoration of historical conditions in willow communities is opposed by hydrological changes in the riparian zone caused by beaver's continued absence. Our results amplify the fundamental importance of conserving intact food webs because changes in ecosystems caused by removal of apex predators may be resilient to predator restoration.
Acknowledgements
This research was funded by the United States National Science Foundation (DEB-0717367 and DEB-1147369). We thank the staff of the Yellowstone Center for Resources for logistical and technical support, and K. Schoenecker and L. Ziegenfuss for providing F. J. Singer's raw data from his 1994 paper. Many individuals helped with fieldwork. Thanks to D. Theobald and J. Salo for assistance with observational site selection. This manuscript was improved by comments from D. Binkley, W. Romme, M. Kauffman, J. Hoeting and two anonymous reviewers.
References
- 1.Terborgh J, et al. 2001. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 10.1126/science.1064397 (doi:10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- 2.Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH. 2007. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315, 1846–1850 10.1126/science.1138657 (doi:10.1126/science.1138657) [DOI] [PubMed] [Google Scholar]
- 3.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306 10.1126/science.1205106 (doi:10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 4.Carpenter SR, Brock WA, Cole JJ, Kitchell JF, Pace ML. 2008. Leading indicators of trophic cascades. Ecol. Lett. 11, 128–138 10.3410/f.1097684.553926 (doi:10.3410/f.1097684.553926) [DOI] [PubMed] [Google Scholar]
- 5.Chase JM. 1996. Abiotic controls of trophic cascades in a simple grassland food chain. Oikos 77, 495–506 10.2307/3545939 (doi:10.2307/3545939) [DOI] [Google Scholar]
- 6.Frank K, Petrie B, Choi J, Leggett W. 2005. Trophic cascades in a formerly cod dominated ecosystem. Science 308, 1621–1623 10.1126/science.1113075 (doi:10.1126/science.1113075) [DOI] [PubMed] [Google Scholar]
- 7.Pace ML, Cole JJ, Carpenter SR, Kitchell JF. 1999. Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 14, 483–488 10.1016/S0169-5347(99)01723-1 (doi:10.1016/S0169-5347(99)01723-1) [DOI] [PubMed] [Google Scholar]
- 8.Estes JA, Duggins DO. 1995. Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecol. Monogr. 65, 75–100 10.2307/2937159 (doi:10.2307/2937159) [DOI] [Google Scholar]
- 9.Paine RT, Castillo J, Cancino J. 1985. Perturbation and recovery patterns of starfish dominated intertidal assemblages in Chile, New Zealand, and Washington state. Am. Nat. 125, 679–691 10.1086/284371 (doi:10.1086/284371) [DOI] [Google Scholar]
- 10.Schmitz OJ. 2004. Perturbation and abrupt shift in trophic control of biodiversity and productivity. Ecol. Lett. 7, 403–409 10.1111/j.1461-0248.2004.00592.x (doi:10.1111/j.1461-0248.2004.00592.x) [DOI] [Google Scholar]
- 11.Chase J. 2003. Experimental evidence for alternative stable equilibria in a benthic pond food web. Ecol. Lett. 6, 733–741 10.1046/j.1461-0248.2003.00482.x (doi:10.1046/j.1461-0248.2003.00482.x) [DOI] [Google Scholar]
- 12.Suding KN, Gross KL, Houseman GR. 2004. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 19, 46–53 10.1016/j.tree.2003.10.005 (doi:10.1016/j.tree.2003.10.005) [DOI] [PubMed] [Google Scholar]
- 13.Paine RT. 1980. Food webs: linkage, interaction strength, and community infrastructure. J. Anim. Ecol. 49, 666–695 10.2307/4220 (doi:10.2307/4220) [DOI] [Google Scholar]
- 14.Schmitz OJ, Hamback PA, Beckerman AP. 2000. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 155, 141–153 10.1086/303311 (doi:10.1086/303311) [DOI] [PubMed] [Google Scholar]
- 15.Mittelbach GG, Turner AM, Hall DJ, Rettig JE, Osenberg CW. 1995. Perturbation and resilience: a long-term, whole-lake study of predator extinction and reintroduction. Ecology 76, 2347–2360 10.2307/2265812 (doi:10.2307/2265812) [DOI] [Google Scholar]
- 16.Singer FJ, Mark LC, Cates RC. 1994. Ungulate herbivory of willows on Yellowstone's northern winter range. J. Range Manage. 47, 435–443 10.2307/4002993 (doi:10.2307/4002993) [DOI] [Google Scholar]
- 17.Singer FS, Cates RG. 1995. Ungulate herbivory on willows on Yellowstone's northern winter range: response. J. Range Manage. 48, 563–565 10.2307/4003072 (doi:10.2307/4003072) [DOI] [Google Scholar]
- 18.Wolf EC, Cooper DJ, Hobbs NT. 2007. Hydrologic regime and herbivory stabilize an alternative state in Yellowstone National Park. Ecol. Appl. 17, 1572–1587 10.1890/06-2042.1 (doi:10.1890/06-2042.1) [DOI] [PubMed] [Google Scholar]
- 19.Beschta RL, Ripple WJ. 2006. River channel dynamics following extirpation of wolves in northwestern Yellowstone National Park, USA. Earth Surf. Process. 31, 1525–1539 10.1002/esp.1362 (doi:10.1002/esp.1362) [DOI] [Google Scholar]
- 20.Beschta RL. 2005. Reduced cottonwood recruitment following extirpation of wolves in Yellowstone's northern range. Ecology 86, 391–403 10.1890/04-0964 (doi:10.1890/04-0964) [DOI] [Google Scholar]
- 21.Beschta RL, Ripple W. 2010. Recovering riparian plant communities with wolves in northern Yellowstone, USA. Restor. Ecol. 18, 380–389 10.1111/j.1526-100X.2008.00450.x (doi:10.1111/j.1526-100X.2008.00450.x) [DOI] [Google Scholar]
- 22.Beschta RL, Ripple WJ. 2007. Increased willow heights along northern Yellowstone's Blacktail Deer Creek following wolf reintroduction. West North Am. Nat. 67, 613–617 10.3398/1527-0904(2007)67[613:IWHANY]2.0.CO;2 (doi:10.3398/1527-0904(2007)67[613:IWHANY]2.0.CO;2) [DOI] [Google Scholar]
- 23.Bilyeu DM, Cooper DJ, Hobbs NT. 2008. Water tables constrain height recovery of willow on Yellowstone's northern range. Ecol. Appl. 18, 80–92 10.1890/07-0212.1 (doi:10.1890/07-0212.1) [DOI] [PubMed] [Google Scholar]
- 24.Johnston DB, Cooper D, Hobbs N. 2011. Relationships between groundwater use, water table, and recovery of willow on Yellowstone's northern range. Ecosphere 2, 1–11 10.1890/ES10-00150.1 (doi:10.1890/ES10-00150.1) [DOI] [Google Scholar]
- 25.Baker BW, Cade BS. 1995. Predicting biomass of beaver food from willow stem diameters. J. Range Manage. 48, 322–326 10.2307/4002484 (doi:10.2307/4002484) [DOI] [Google Scholar]
- 26.Baker BW, Ducharme HC, Mitchell DCS, Stanley TR, Peinetti HR. 2005. Interaction of beaver and elk herbivory reduces standing crop of willow. Ecol. Appl. 15, 110–118 10.1890/03-5237 (doi:10.1890/03-5237) [DOI] [Google Scholar]
- 27.Warren R. 1926. A study of beaver in the Yancey region of Yellowstone National Park. Roosevelt Wildl. Ann. 1, 13–191 [Google Scholar]
- 28.Persico L, Meyer G. 2009. Holocene beaver damming, fluvial geomorphology, and climate in Yellowstone National Park, Wyoming. Q. Res. 71, 340–353 10.1016/j.yqres.2008.09.007 (doi:10.1016/j.yqres.2008.09.007) [DOI] [Google Scholar]
- 29.Jonas R. 1955. A population and ecological study of beaver (Castor canadensis) of Yellowstone National Park. PhD thesis, University of Idaho, Moscow, ID [Google Scholar]
- 30.Kay CE. 1997. Viewpoint: ungulate herbivory, willows, and political ecology in Yellowstone. J. Range Manage. 50, 139–145 10.2307/4002370 (doi:10.2307/4002370) [DOI] [Google Scholar]
- 31.Ripple WJ, Beschta RL. 2004. Wolves, elk, willows, and trophic cascades in the upper Gallatin range of southwestern Montana, USA. Forest Ecol. Manag. 200, 161–181 10.1016/j.foreco.2004.06.017 (doi:10.1016/j.foreco.2004.06.017) [DOI] [Google Scholar]
- 32.Smith D, Tyers D. 2012. The history and current status and distribution of beavers in Yellowstone National Park. Northwest Sci. 86, 276–288 10.3955/046.086.0404 (doi:10.3955/046.086.0404) [DOI] [Google Scholar]
- 33.Ripple W, Beschta R. 2011. Trophic cascades in Yellowstone: the first 15 years after wolf reintroduction. Biol. Conserv. 145, 205–213 10.1016/j.biocon.2011.11.005 (doi:10.1016/j.biocon.2011.11.005) [DOI] [Google Scholar]
- 34.Frank DA. 2008. Evidence for top predator control of a grazing ecosystem. Oikos 117, 1718–1724 10.1111/j.1600-0706.2008.16846.x (doi:10.1111/j.1600-0706.2008.16846.x) [DOI] [Google Scholar]
- 35.Ripple WJ, Beschta RL. 2004. Wolves and the ecology of fear: can predation risk structure ecosystems? Bioscience 54, 755–766 10.1641/0006-3568(2004)054[0755:WATEOF]2.0.CO;2 (doi:10.1641/0006-3568(2004)054[0755:WATEOF]2.0.CO;2) [DOI] [Google Scholar]
- 36.Beyer HL, Merrill EH, Varley N, Boyce MS. 2007. Willow on Yellowstone's northern range: evidence for a trophic cascade? Ecol. Appl. 17, 1563–1571 10.1890/06-1254.1 (doi:10.1890/06-1254.1) [DOI] [PubMed] [Google Scholar]
- 37.Kauffman MJ, Brodie JF, Jules ES. 2010. Are wolves saving Yellowstone's aspen? A landscape-level test of a behaviorally mediated trophic cascade. Ecology 91, 2742–2755 10.1890/09-1949.1 (doi:10.1890/09-1949.1) [DOI] [PubMed] [Google Scholar]
- 38.Creel S, Christianson D. 2009. Wolf presence and increased willow consumption by Yellowstone elk: implications for trophic cascades. Ecology 90, 2454–2466 10.1890/08-2017.1 (doi:10.1890/08-2017.1) [DOI] [PubMed] [Google Scholar]
- 39.Keigley RB, Frisina MR, Fager CW. 2002. Assessing browse trend at the landscape level. I: Preliminary steps and field survey. Rangelands 24, 28–33 [Google Scholar]
- 40.Bilyeu DM, Cooper DJ, Hobbs NT. 2007. Assessing impacts of large herbivores on shrubs: tests of scaling factors for utilization rates from shoot-level measurements. J. Appl. Ecol. 44, 168–175 10.1111/j.1365-2664.2006.01245.x (doi:10.1111/j.1365-2664.2006.01245.x) [DOI] [Google Scholar]
- 41.Raven J. 1992. The physiology of Salix. Proc. R. Soc. Lond. B 98, 49–62 10.1017/S0269727000007442 (doi:10.1017/S0269727000007442) [DOI] [Google Scholar]
- 42.Bates D, Maechler M, Bolker B. 2012. lme4: linear mixed-effects models using S4 classes. R package version 0.999999-0 See http://CRAN.R-project.org/package=lme4 [Google Scholar]
- 43.R Development Core Team 2011. R: a Language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/ [Google Scholar]
- 44.Burnham K, Anderson D. 1998. Model selection and inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 45.Zuur A, Ieno E, Walker N, Saveliev A, Smith G. 2009. Mixed effects models and extensions in ecology with R. Berlin, Germany: Springer [Google Scholar]
- 46.Gelman A, Hill J. 2006. Data analysis using regression and multilevel/hierarchical models. Cambridge, UK: Cambridge University Press [Google Scholar]
- 47.Baker BW, Hill E. 2003. Beaver (Castor canadensis). In wild mammals of North America: biology, management, and conservation, 2nd edn. (eds Feldhamer G, Thompson B, Chapman J.), pp. 288–310 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 48.Theobald D, Stevens DJ, White D, Urquhart N, Olsen A, Norman J. 2007. Using GIS to generate spatially-balanced random survey designs for natural resource applications. Environ. Manage. 40, 134–146 10.1007/s00267-005-0199-x (doi:10.1007/s00267-005-0199-x) [DOI] [PubMed] [Google Scholar]
- 49.Johnston DB, Cooper DJ, Hobbs NT. 2007. Elk browsing increases aboveground growth of water-stressed willows by modifying plant architecture. Oecologia 154, 467–478 10.1007/s00442-007-0854-4 (doi:10.1007/s00442-007-0854-4) [DOI] [PubMed] [Google Scholar]
- 50.Tercek M, Stottlemyer R, Renkin R. 2010. Bottom-up factors influencing riparian willow recovery in Yellowstone National Park. West North Am. Nat. 70, 387–399 10.3398/064.070.0311 (doi:10.3398/064.070.0311) [DOI] [Google Scholar]
- 51.Mech D. 2012. Is science in danger of sanctifying the wolf? Biol. Conserv. 150, 143–149 10.1016/j.biocon.2012.03.003 (doi:10.1016/j.biocon.2012.03.003) [DOI] [Google Scholar]
- 52.Schmitz OJ. 2010. Resolving ecosystem complexity. Princeton, NJ: Princeton University Press [Google Scholar]