Abstract
Artificial light at night is a rapidly increasing phenomenon and it is presumed to have global implications. Light at night has been associated with health problems in humans as a consequence of altered biological rhythms. Effects on wild animals have been less investigated, but light at night has often been assumed to affect seasonal cycles of urban dwellers. Using light loggers attached to free-living European blackbirds (Turdus merula), we first measured light intensity at night which forest and city birds are subjected to in the wild. Then we used these measurements to test for the effect of light at night on timing of reproductive physiology. Captive city and forest blackbirds were exposed to either dark nights or very low light intensities at night (0.3 lux). Birds exposed to light at night developed their reproductive system up to one month earlier, and also moulted earlier, than birds kept under dark nights. Furthermore, city birds responded differently than forest individuals to the light at night treatment, suggesting that urbanization can alter the physiological phenotype of songbirds. Our results emphasize the impact of human-induced lighting on the ecology of millions of animals living in cities and call for an understanding of the fitness consequences of light pollution.
Keywords: urbanization, light pollution, artificial light at night, timing of reproduction, testicular growth, light loggers
1. Introduction
All over the world, urban areas are growing faster than any other land cover type [1]. As cities spread, rural and natural areas are converted into urban landscapes, reducing the native habitat of many animal and plant species. However, there is an increasing number of species across many taxa that manage to successfully colonize and reproduce in urban environments and are, therefore, exposed to novel environmental conditions. Urban ecology is an established field of research: ecologists have long shown the impact of urbanization on population and community dynamics [2], and in the last two decades new interest has been arising around the mechanisms of individuals' response to urbanization [3], e.g. stress and reproductive physiology [4,5], temporal and spatial activity patterns [6], metabolism [7] and behaviour [8]. Moreover, while the effects of noise at the community and individual level have started to be elucidated [9,10], knowledge about the ecological and evolutionary consequences of artificial night lighting is still limited (but see [11]). This is remarkable given the ample evidence that light through its diel changes and/or seasonal fluctuations (changes in day length) has strong biological relevance for the daily and annual rhythms of life [12].
Seasonal functions, such as reproduction, are thought to be under natural selection because optimal timing, i.e. when environmental conditions are most favourable, ensures best survival of both parents and offspring [13–15]. Because seasonal functions require relatively long periods of development, most of them cannot be initiated instantaneously when ultimate factors become optimal. This is especially true for reproduction, for which the main ultimate factor, food, becomes crucial when offspring require an increasing amount of food. Thus, responding to proximate cues is vital [16]. In birds, species living in temperate zones use photoperiod to predict optimal timing of reproduction. The increase in day length in early spring initiates a cascade of neuroendocrine events thah leads to the development of the gonads [17]. If photoperiod is the main predictive cue for temperate birds, how do these animals cope in a brightly illuminated habitat such as a city? There is increasing evidence that urban birds have an extended breeding season, mainly because of an advanced onset of reproduction. In European blackbirds (Turdus merula), for instance, onset of gonadal recrudescence is advanced up to three weeks in a city population, in both males and females [18]. Several potential factors have been considered to explain the observed differences in the timing of reproduction in urban areas, such as anthropogenic food supply [19], warmer microclimate and more intense social stimulation [18]. In addition, artificial light at night has been hypothesized already by Rowan [20] to stimulate early breeding in London starlings (Sturnus vulgaris). Poultry scientists have long known that exposing birds to long days can stimulate reproduction outside the natural breeding period [21]. However, the effects of light at night on gonadal recrudescence have been investigated using light intensities higher than 15 lux (reviews in [20,22,23]), a value likely to be far above the intensity birds are exposed to in urban areas. Indeed, one of the major challenges for experimental studies investigating the impact of light pollution on wild animals is the lack of knowledge about what levels of light at night animals actually experience in cities. We approached this problem by tagging wild-caught European blackbirds with micro-light loggers and releasing them in their native urban and rural habitats. The field data allowed us to experimentally simulate light intensities that free-roaming blackbirds can normally be exposed to in their natural urban environment, and to test whether these low light intensities at night may cause changes in the timing of reproductive physiology.
2. Material and methods
(a). Measurement of natural exposure to light at night on free-living birds
In order to estimate the light intensity to which free-living blackbirds are exposed at night, we used micro-light loggers (Sigma Delta Tech., Australia, weight = 3 grams). Loggers contained a photodiode (TSL 235, TAOS, USA) whose spectral responsivity ranged from 300 to 1100 nm (peak at 780 nm). Each logger was calibrated during morning twilight against a photometer (LI-1400 and LI-2100, LI-COR, USA) to calculate illuminance (lux) from frequency values. Recordings ranged from 0.00004 to 40000 lux. Loggers recorded and stored light intensity every 2 min. We deployed the loggers between March and June in 2 years (2009 and 2010) on 15 male blackbirds captured in the city of Munich (Germany, 48° 07′ N, 11° 34′ E; 518 m asl) and in a rural forest near the village of Raisting (47°53′ N, 11°04′ E, 553 m asl), 40 km southwest of Munich. Birds were then immediately released on their territory. We recaptured the birds whenever possible after a week of recording. We only analysed data recorded between 10.00 and 16.00, because we wanted to avoid including the twilight phases in the analysis. For forest males (n = 7), light intensity at night was always the lowest detectable value by each light logger, so we simply averaged those values for all birds. For each city male (n = 8), we calculated the median and the maximum of the third quartiles of all its night measures, and then averaged those values for all birds (figure 1).
Figure 1.
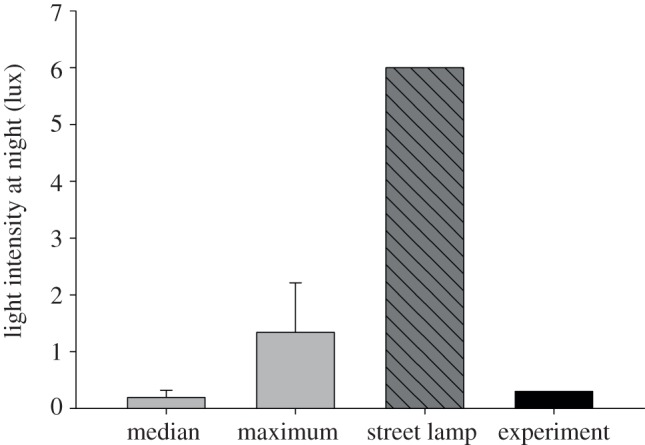
Natural exposure to light at night of free-living city European blackbirds. Light grey bars represent data obtained from loggers deployed on individual birds (n = 8) in their natural urban environment. For each city bird we calculated the median and maximum of the third quartiles of all nights, and used the mean of those values from all birds for presentation in the figure. Data are represented as means ± s.e.m. Dark grey striped bar represents the light intensity measured under a representative street lamp of our study site (6 lux). Black bar represents the light intensity (0.3 lux) we used in the experiment to simulate urban exposure to light at night in the experimental group.
(b). Animals
In July 2010, we captured 40 new adult male blackbirds (20 urban and 20 rural) in the same rural and urban sites described in the previous section. Birds were transported in cloth cages to our facilities in Radolfzell (47°44′ N, 8°58′ E, 404 m asl), and kept individually in outdoor aviaries until 26 Novemberth 2010, when they were moved indoors into individual cages in two separate rooms. Each room contained 10 city and 10 forest birds, all being initially exposed to light/dark (LD) cycles simulating the natural variation of photoperiod in Radolfzell. Birds could hear but not see each other. Food (Granvit, Chemi-Vit, Italy) and drinking water were available ad libitum. We assessed body mass and fat score of birds on the day they were moved indoor, and thereafter, every month. Birds were weighted with a laboratory balance (KERN PCB 1000–2, precision 0.01 g, KERN, Germany) and the amount of subcutaneous fat was scored on a 0–8 scale following [24]. Moult was checked on a weekly basis starting from March 2011. To determine the onset of moult, we used the flight feather moult. To this end, we recorded the state of flight feather moult on a weekly basis using a method modified from [25]. As soon as a bird shed or re-grew the first inner primary (primary 1) of both the left and right wing we defined this date as the onset of moult.
(c). Light treatment
The experiment started on 18 December 2010. Photoperiod followed the local natural variation of day length in both treatment groups and throughout the experiment. Control birds stayed under an LD cycle. Experimental birds were exposed to light/dim light cycles (LLdim). Day-time light intensity in both groups ranged from 250 to 1250 lux, and was provided by dimmable fluorescent white bulbs (Biolux 36 W, Osram, Germany) emitting light at wavelengths covering the visible spectrum. Because lowest light intensity of dimmable fluorescent bulbs were still very high (approx. 20 lux), we used a dimmable incandescent light bulb (SLV Elektronic, Germany, with a wavelength range of 450–950 nm, measured with a Red Tide USB650 spectrometer (OceanOptics, USA)) to simulate the low light intensities which free-roaming blackbirds experienced at night. Incandescent light bulbs were installed so that birds could not see the light bulbs directly, but only received the indirect light. We chose this type of light bulb because first it is a common light source in urban areas, e.g. for outdoor light decoration of houses and second it is representative of the spectrum of lights deployed in the city of Munich (yellow–red lights). Light intensity at night in the experimental group was set at 0.3 lux. Control birds were exposed to a light intensity of approximately 0.0001 lux during the night, provided by the same light bulb type as that used for the experimental group. This light intensity of approximately 0.0001 lux was used to allow birds to orientate in the cage during the night. Each group was exposed to a twilight phase of 35 min both in the morning and evening. Light programmes of both rooms were controlled by Gira Homeserver (Germany). Light intensity in the cages was quantified at all four perches in a cage using a LI-1400 data logger and LI-210 photometric sensor. Values are given as the mean of measurements at all four perches.
(d). Hormone analysis
In order to quantify the effects of light at night on plasma testosterone (T) secretion, we collected blood samples. Blood sampling was conducted on 8 December 2010, before the start of the experiment. Thereafter, we obtained a blood sample from every individual every three to four weeks. We punctured the brachial vein with a 25 g needle and collected 200 μl of blood in a haematocrit tube. Plasma was separated from red blood cells by centrifugation for 10 min (G-force = 18.63) within 30 min after the end of the sampling and then the plasma fraction was stored at −80°C. We determined plasma T concentration using a commercial enzyme immunoassay (EIA) kit (no. 901–065, Enzo Life Sciences, NY, USA), following a double diethyl-ether extraction of 20 μl sample volume. After drying the extract under N2 stream, 400 μl of assay buffer (Tris-buffered saline) was added and the samples were allowed to reconstitute overnight. From each reconstituted sample we used 200 μl for the EIA, separated equally in two adjacent wells. We followed the instructions provided by the manufacturer throughout. Levels of T were calculated using a 5-parameter logistic curve-fitting programme (Microplate Manager; Bio-Rad Laboratories, Inc., CA, USA). Plasma samples from each individual for the entire reproductive cycle (nine samples per bird) were analysed on the same plate. Samples from two individuals were included on each plate. A total of 20 assays were run. Assay lower sensitivity was 5.67 pg ml−1 plasma T. The mean intra-assay coefficient of variation of two replicate standards per plate was 6.9 per cent and the inter-assay coefficient of variation was 11.9 per cent.
(e). Assessment of testicular size
Testicular size was assessed through laparotomy [26]. Incisions were made under Isoflurane anaesthesia (CP-Pharma, Germany). The width of the left testis was measured to the nearest 0.1 mm. Incisions were treated with Actihaemyl gel (Meda Pharma GmbH, Germany) and sealed with Histoacryl (Braun, Germany). All birds recovered rapidly from the procedure. Laparotomies were conducted one week after the blood sampling described in the previous paragraph. We obtained baseline testicular width from all birds on 15 December 2010, before the start of the experiment. Thereafter, laparatomies were conducted monthly.
(f). Song recordings
Song activity was recorded every three to four weeks. An EM-9600 omnidirectional microphone (T-BONE, Australia) was fixed on the ceiling of each room pointing down to the cages. That is, song recording was made on a per room basis (control versus experimental), so standard errors were not quantifiable. The microphone was connected to a Tascam DR-08 digital recorder (TEAC Corporation, Japan). Recordings started in the afternoon and lasted for 24 h. For this study, only recordings from five hours before morning twilight onwards were analysed. The time the first song occurred was recorded and is reported in minutes before twilight.
(g). Statistical analysis
All statistical analysis were performed with software R v. 2.13.0 [27]. All tests were two-tailed and significance was accepted at α = 0.05.
Variation in testicular size was analyzed by general additive mixed-effect models (GAMMs, R package mgcv [28]). Log-transformed testicular width was included as response variable to reach normality and homogeneity of variance. Treatment, origin and interaction between treatment and origin were included as parametric terms. The four possible interactions between factors' levels and date were modelled as smoothed terms. To account for non-independency of repeated measures, subjects were included as random intercepts in the model. In addition, we used threshold values of testicular width to estimate duration of the ‘reproductive season’. The onset and end of ‘functional testes’ were defined as the dates at which testicular width reached a value of 5 mm. This threshold was selected on the assumption that testes start producing sperm at half-maximum volume [18]. The exact date at which testicular growth and regression passed the threshold value was extrapolated for each individual from a four parameters logistic equation (GraphPad Software, USA). The equation used was: testicular size = B + (A − B)/1 + exp((C − date)/D), where A = lower asymptote of the curve, B = upper asymptote of the curve, C = response half way between bottom and top and D = slope of the curve at half way between bottom and top. Length of the ‘reproductive season’ was defined as the number of days for which an individual had functional testes.
Testosterone (T) data were analysed with linear mixed models (LMMs, R package lme4 [29]). Log-transformed T was included as response variable. Date, second-order polynomial date, origin, treatment and all possible interactions were modelled as fixed factors. Subjects were included as random intercepts. Models were evaluated by comparing their Akaike information criterias (AICs). We considered as best model that with the lowest AIC, which included date, origin, treatment and the interaction between treatment and date and treatment and second-order polynomial date. The p-value for each estimate was calculated by Monte Carlo Markow-Chain (MCMC) using the function pvals.fnc in the R package languageR [30]. The interactions were evaluated by comparing the 95 per cent Bayesian credible intervals (CI) of the estimates for each treatment group at each time point of the reproductive cycle. CI were calculated using the function sim in the R package arm [31]. We considered two groups to be significantly different if the CI of the estimate for one group did not include the estimates of the other groups. We used the same type of models and procedure to analyse the variation in body weight and fat scores. In all these models the best AIC was always given by including the linear effects of date, origin and treatment, but no interactions.
The time of initiation of moult was compared between treatments and population by using generalized linear models (GLMs) with a Poisson error structure and a log-link. The date of moult start was included as response variable, and treatment, origin and their interaction were modelled as explanatory variables. The interaction was not significant, therefore, we removed it.
3. Results
In the field, free-living forest birds (n = 7) were exposed on average to 0.00006 lux at night. By contrast, free-living city birds (n = 8) were exposed to highly variable light levels at night (range 0.07–2.2 lux, average 0.2 lux, figure 1).
Our experimental city and forest birds did not differ in body mass (LMM, MCMC estimate = −0.40, pMCMC = 0.67) or fat score (LMM, MCMC estimate = 0.06, pMCMC = 0.70) before and during the entire course of the experiment. Furthermore, light treatment did not have an effect on body weight (LMM, MCMC estimate = 0.08, pMCMC = 0.93) or fat score (LMM, MCMC estimate = 0.17, pMCMC = 0.35) during the reproductive cycle.
Experimental birds exposed to a light intensity of 0.3 lux at night developed functional testes on average 26 days (interpolation from logistic model; table 1 and figure 2a) earlier than control birds kept under dark nights (GAMM, p < 0.001, table 2). City birds became reproductively active on average 13 days earlier than forest individuals (table 1). After taking into account the effect attributable to the different light protocol, this difference was significant (GAMM, p = 0.025, table 2), confirming previous results on wild male blackbirds from the same populations [18]. The outcome of our model revealed a significant interaction between treatment and origin effect (GAMM, p < 0.041, table 2). This interaction was evident during the testicular regression phase. City birds exposed to light at night regressed their testes 14 days earlier than forest conspecifics exposed to artificial light at night, whereas forest and city birds under dark nights differed only by three days in the timing of testicular regression (table 1). Overall experimental birds maintained functional testes 12 days longer than control birds. In particular, experimental forest and city birds had, respectively, 9 and 12 days longer duration of the reproductive season than their counterparts in the control group (table 1).
Table 1.
Effect sizes of light treatments on timing of testicular growth and regression. Calendar dates (1 = 1 Jan) of threshold (5 mm in testicular width) crossing during testicular growth (a) and regression (b), interpolated after fitting logistic growth curves to each individual. (c) Duration of functional testes, calculated as number of days between (a) and (b).
| trait | treatment | origin | dates (s.e.m.) | days (s.e.m.) |
|---|---|---|---|---|
| (a) testicular growth | control | forest | 72.97 (4.07) | |
| city | 64.97 (3.59) | |||
| experimental | forest | 50.63 (5.20) | ||
| city | 33.33 (6.87) | |||
| (b) testicular regression | control | forest | 144.08 (4.92) | |
| city | 142.46 (3.56) | |||
| experimental | forest | 134.27 (8.27) | ||
| city | 120.71 (9.89) | |||
| (c) duration of functional testes | control | forest | 74.41 (6.33) | |
| city | 75.58 (3.07) | |||
| experimental | forest | 83.63 (7.81) | ||
| city | 87.39 (7.66) |
Figure 2.
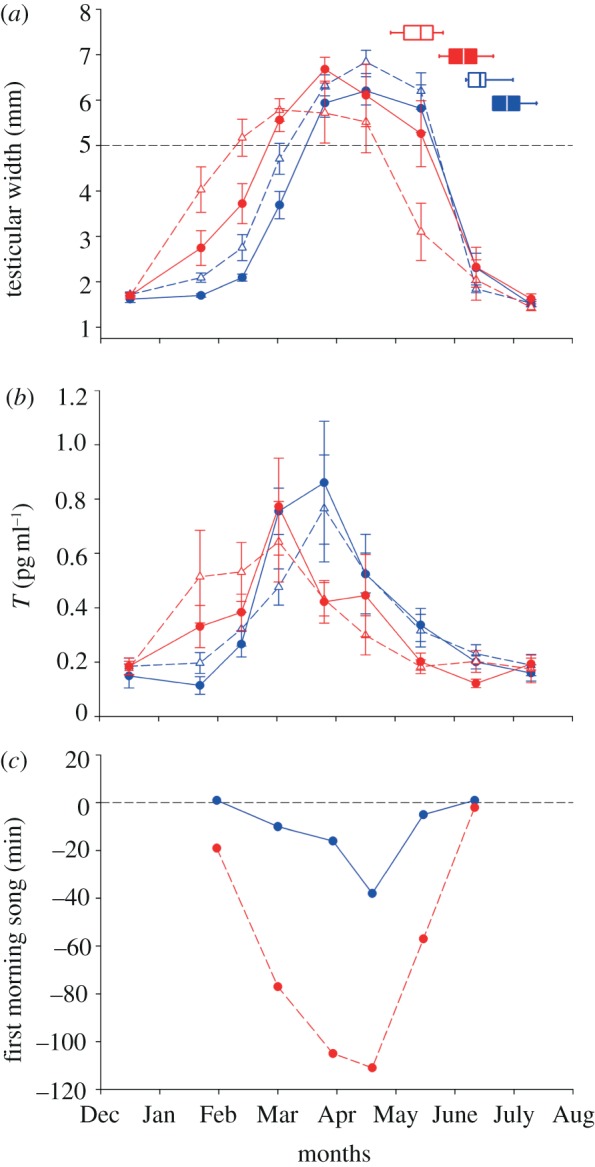
(a) Effects of light at night on seasonal variation in testicular width, testosterone production (b), moult initiation (a) and (c) first morning song in adult male European blackbirds (Turdus merula). (a) City (triangles, dashed lines) and forest (circles, solid lines) adult male European blackbirds were tested to simulated natural photoperiods but with different light intensities at night. Control birds (blue) experienced nights without any light, whereas experimental birds (red) were exposed to constant light of 0.3 lux at night. Dashed horizontal line represents testicular size (width of 5 mm) above which males are assumed to be able to produce fertile sperms. Data represent mean ± s.e.m. In addition, we show time of moult initiation as horizontal box plots on the top right of the (a, control/experimental, blue/red; city/forest, filled/blank background). Sample sizes: control = 20 (10 forest and 10 city birds), experimental = 20 (10 forest and 10 city birds). (b) Seasonal variation in plasma testosterone production. For symbols legend see (a). (c) Time of onset of morning chorus in the two experimental groups (blue circles/solid lines, control, dark night; red circles/dashed lines, experimental/light at night) measured every three to four weeks during testicular cycle. Horizontal dashed line indicates beginning of morning twilight phase. Each circle represents first song of first individual in each group.
Table 2.
Effect of treatment, origin and date on testicular size. Model is generalized additive mixed model (GAMM ) with treatment and origin as fixed effects, and interactions between treatment (c, control; e, experimental), origin (f, forest; c, city) and date as smooth terms.
| parametric terms | estimates | s.e.m. | t-value | p-value |
|---|---|---|---|---|
| intercept | 1.05 | 0.06 | 35.22 | <0.001 |
| treatment | 0.16 | 0.042 | 3.81 | <0.001 |
| origin | 0.1 | 0.042 | 2.24 | 0.025 |
| treatment × origin | 0.36 | 0.06 | −2.05 | 0.041 |
| smooth terms | d.f. | F | p-value | |
| date : treatment (c) : origin (f) | 5.22 | 65.42 | <0.001 | |
| date : treatment (c) : origin (c) | 5.71 | 63.52 | <0.001 | |
| date : treatment (e) : origin (f) | 4.85 | 63.05 | <0.001 | |
| date : treatment (e) : origin (c) | 5.04 | 62.75 | <0.001 |
Levels of plasma T also differed between treatment groups. Birds which were exposed to light at night started to increase the secretion of plasma testosterone earlier than control birds (significant effect of treatment × date interaction: LMM, MCMC estimate =−0.6, pMCMC = 0.0001 and significant effect of treatment × 2nd order date interaction: LMM, MCMC estimate =−0.05, pMCMC = 0.0002). In particular, we found significant differences in the measurements done on 21 January (control: mean =−1.76, CI =−2.02, −1.50; experimental: mean =−1.24, CI =−1.50, −0.99). Although urban birds tended to increase their T concentration earlier than forest birds, these effects are not significant nor is the interaction between origin and treatment significantly different (figure 2b).
Light at night also had an effect on the timing of moult (figure 2a, horizontal box plots). Birds under light at night started their moult on average 22 days before birds under dark nights (GLM, d.f. = 38, z =−5.59, p < 0.001). In addition, we detected within-treatment differences: in both treatment groups city birds started moult on average 13 days before forest birds (GLM, d.f. = 38, z =−3.28, p = 0.001).
Both control and treatment groups showed seasonal variation in the onset of morning song. The experimental group showed an earlier onset of dawn chorus than control group, advancing song onset most dramatically in March and April (figure 2c).
4. Discussion
Here, we show that even extremely low light intensities at night are able to alter the timing of reproductive physiology in songbirds. Although the effect of light stimulation at night on the reproductive system of birds has long been known and effectively used in poultry science to increase egg production, previous studies used light at night of intensities unrealistic of what animals experienced in cities [22,23]. Our results are novel because we used a very low light intensity at night which was determined from data recorded on individual free-roaming urban blackbirds. We used a light intensity at night of 0.3 lux, a value 20 times lower than the light intensity emitted by a typical street lamp in our study site (approx. 6 lux).
There is ample evidence that urban populations of a variety of bird species living in urban areas exhibit an earlier onset of breeding compared with their rural conspecifics [18,19]. Several factors have often been stated to be responsible for this advanced onset of breeding, such as warmer microclimate, increased food availability and higher social stimulation [4]. In addition, light pollution was always listed as an alternative among those possible causes [11,32], but experimental evidence was lacking. The results of the present study confirm the idea of a stimulatory effect by artificial light at night on the timing of reproduction in urban birds. In particular, our study highlights the fact that low intensities of artificial light at night affects reproductive timing not only during the phase of egg-laying, as suggested before [32], but it can advance gonadal growth and testosterone production by up to one month, a considerable amount of time given the length of the breeding season in European blackbirds (three to four months). However, the ecological consequences of the effect of light at night on the timing of reproduction are still unknown. Early breeders could potentially gain a fitness advantage by increasing the number of broods per season and, therefore, reproductive output. However, early breeding in males could also result in decreased fitness if females were not ready to breed. We do not have data on females' reproductive timing in the year we conducted our experiment, but a previous field study from the same populations found that gonadal growth was advanced in both urban males and females blackbirds compared with forest conspecifics [18]. Therefore, early gonadal recrudescence could be advantageous for males. However, to grow the reproductive system early in the spring when weather conditions and food availability are not optimal might come at a metabolic cost. Lack of energy stores might in turn negatively affect the response of the immune system eventually reducing reproductive success and survival [33,34].
The mechanisms through which low light intensities at night may have affected the reproductive system are still unclear. Photoperiodic time measurement in birds depends on a circadian oscillator. If light hits the sensitive phase of the circadian rhythm (12–16 h after dawn), luteinizing hormones are produced and gonadal growth is eventually stimulated [17]. However, light at night may not just have a direct effect on timing of reproduction, but also indirect effects may exist. Two possible indirect effects may be conceivable. First, there is evidence that light at night can alter metabolic processes in mice leading to excess fat storage [35], and high energy stores might speed up reproduction [36,37]. For example, Wikelski et al. [38] have recently shown that the speed of the avian circannual clock may be modified by energy expenditure: house sparrows (Passer domesticus) with higher daily expenditure showed faster circannual reproductive rhythms than birds with lower energy turnover. Because in our experiment birds exposed to light at night had higher levels of daily activity (D. Dominoni 2010, unpublished data), we suggest that the earlier onset of testicular growth in this group may have been a result of changes in metabolic signals. A second hypothesis involves the hormone melatonin. Although melatonin has been generally thought to play little role in avian seasonal biology, recent findings suggest that (i) constant melatonin administration can delay lay date of wild great tits (Parus major) [39] and (ii) melatonin can induce gonadotropin-inhibitory hormone expression (GnIH) [40], whose effect in can directly be seen at the level of the gonads [41]. Because dim light can reduce melatonin production at night [42,43], it is possible that in our experiment birds under light at night had lower level of melatonin and this in turn could have accelerated testicular growth. These direct or indirect effects of light at night are not mutually exclusive hypotheses.
The spectrum of light at night in cities is very diverse resulting in a mosaic-like spatial distribution of different wavelengths of artificial light systems. We are aware that not only light intensity, but also the spectrum of light can affect the photoperiodic response of birds. We designed our experiment to test for the effect of low light intensity alone using a light bulb system which is commonly found as decoration on houses in cities. Furthermore, we controlled for the influence of light spectra by using the same type of light bulbs in both treatment groups. Thus the only difference between the two experimental groups was light intensity during the night. It is remarkable that a light intensity of only 0.3 lux was able to produce such a strong response in the reproductive system. In chickens, the spectral sensitivity of the deep-brain photoreceptors involved in seasonal breeding (VA-opsins) peaks at 492 nm [44], a considerably lower wavelength than what used in our experiment. Moreover, Menaker et al. [45] found that in sparrows the light intensity required for a photoperiodic response is approximately 10 lux. This may highlight the possibility that other indirect effects of light at night might have sped up gonadal growth (see above).
As often the case in laboratory studies, we designed our experiment with only one control and one experimental room. We cannot exclude the possibility that by chance some individuals in the experimental room, that initiated their gonadal growth earlier owing to their high photosensitivity to low light intensities at night, could have stimulated the other animals in the same group resulting in an overall earlier gonadal recrudescence [46,47]. However, we believe that social stimulation cannot explain the observed difference alone since we found within-treatment population differences in reproductive timing. Indeed, our captive city birds showed earlier testicular growth compared with forest individuals in both treatment groups, independently of the light at the night stimulation. This result is in contrast to our previous findings from the same populations. In the field, both male and female urban blackbirds showed a three weeks earlier gonadal growth than forest conspecifics [18], but this pronounced difference in the timing of gonadal recrudescence was strongly reduced in hand-reared birds under laboratory conditions [4], suggesting that the effect in the wild is primarily owing to phenotypic flexibility in physiological responses to urban-specific environmental conditions. One obvious difference between the previous and current study is that the experimental birds of this present study were wild-caught and not hand-reared. Thus, the difference in the response between the two populations could be due to experience to different environmental characteristics of urban and forest habitats during their previous life. For example, urban birds could be more used to humans and, therefore, less stressed, which could translate into earlier gonadal growth. We do not have data to explicitly test this idea. However, urban and forest captive males did not differ in body weight and fat score before and during the entire experiment. Hence, these results argue against the possibility that urban birds were in a better condition compared with their forest conspecifics and thus better body condition alone cannot explain the earlier gonadal development of urban blackbirds in the present study. Moreover, age is known to be one of the factors affecting reproductive timing, because usually older birds grow their gonads earlier than first-year individuals [48,49]. If, for instance, the forest population has a higher mortality rate, then there could be a sampling that is biased towards birds that are younger, and hence less likely to grow their testes at an early date compared with the urban males. To minimize this bias, we only used adult birds (two years old or older). Nevertheless, ageing European blackbirds after the first post-breeding moult is impossible, thus it could be that by chance we had a bias of older urban birds. We cannot directly address this hypothesis. However, we have evidence from our study population that adult male European blackbirds do not grow their testes earlier in consecutive years. This may argue against a confounding age effect in our results. Our captive city and forest birds did not only differ in the timing of gonadal growth but even within the experimental group exposed to light at night city birds showed different gonadal cycles compared with their forest conspecifics, mostly in the timing of testicular regression. These data may indicate that city birds have a different photosensitivity to light at night compared with forest birds, but this hypothesis requires further direct testing. All together our findings suggest that urbanization can modify the physiological phenotype of songbirds.
Although our song recordings were not performed on an individual basis, these data indicate that light at night may also affect the timing of dawn song. Light pollution has been repeatedly associated with advanced daily cycles in songbirds [32,50,51], and here we show a potential direct association between light at night and onset of dawn singing. The effect was season dependent, that is, blackbirds under light at night started to sing earlier than birds under dark nights in the middle of the reproductive cycle, when testes were largest. At the beginning and end of the cycle, when testes were smaller, the effect of light at night was drastically reduced. Because the group of birds under light at night showed seasonal variation in timing of dawn song we suspect that birds were still interpreting the light schedule as a day/night cycle and not a 24 h day.
Our study confirms the hypothesis that artificial light can have a major impact on life-history traits of animals that have colonized urban areas. Hundreds of millions of birds live in cities, but our knowledge of the fitness consequences of light pollution for these animals is still very limited (but see [52]). Light at night has been recently considered to be a relevant issue not just for biodiversity [53], but also for human health and economy [54,55]. We suggest that in the urban millennium, the age of cities and urbanization, it is high time for scientists to intensify research on light pollution and for policy makers to start discussing solutions to mitigate the effects of urbanization on wild animals.
Acknowledgements
All experimental procedures were carried out in accordance with the guidelines of the relevant German agencies.
The original idea of this study and first trials of micro-light logger technique were initiated by Ebo Gwinner. J.P. was financially supported by a grant (Initiative Evolutionary Biology) of the Volkswagen Foundation. Additional funding to D.D. was provided by the International Max Planck Research School for Organismal Biology. This study would have never been conducted without the help of many animal caretakers. We thank K. Mortega, M. Hau, B. Helm, T. Greives, K. Safi, M. Wikelski, D. Dechmann, W. Goymann, J. Greives, who provided useful comments on earlier drafts. This paper is dedicated to the memory of Dr Bjoern Siemers, who unexpectedly died on 23 May 2012.
References
- 1.Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM. 2008. Global change and the ecology of cities. Science 319, 756–760 10.1126/science.1150195 (doi:10.1126/science.1150195) [DOI] [PubMed] [Google Scholar]
- 2.McKinney ML. 2006. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260 10.1016/j.biocon.2005.09.005 (doi:10.1016/j.biocon.2005.09.005) [DOI] [Google Scholar]
- 3.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191 10.1016/j.tree.2005.11.019 (doi:10.1016/j.tree.2005.11.019) [DOI] [PubMed] [Google Scholar]
- 4.Partecke J, Van't Hof TJ, Gwinner E. 2004. Differences in the timing of reproduction between urban and forest European blackbirds (Turdus merula): result of phenotypic flexibility or genetic differences? Proc. R. Soc. Lond. B 271, 1995–2001 10.1098/rspb.2004.2821 (doi:10.1098/rspb.2004.2821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partecke J, Schwabl I, Gwinner E. 2006. Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87, 1945–1952 10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 6.Riley SPD, Sauvajot RM, Fuller T, York EC, Kamradt DA, Bromley C, Wayne RK. 2003. Effects of urbanization and habitat fragmentation on bobcats and coyotes in Southern California. Conserv. Biol. 17, 566–576 10.1046/j.1523-1739.2003.01458.x (doi:10.1046/j.1523-1739.2003.01458.x) [DOI] [Google Scholar]
- 7.Liker A, Papp Z, Bókony V, Lendvai AZ. 2008. Lean birds in the city: body size and condition of house sparrows along the urbanization gradient. J. Anim. Ecol. 77, 789–795 10.1111/j.1365-2656.2008.01402.x (doi:10.1111/j.1365-2656.2008.01402.x) [DOI] [PubMed] [Google Scholar]
- 8.Rees M, Roe JH, Georges A. 2009. Life in the suburbs: behavior and survival of a freshwater turtle in response to drought and urbanization. Biol. Conserv. 142, 3172–3181 10.1016/j.biocon.2009.08.019 (doi:10.1016/j.biocon.2009.08.019) [DOI] [Google Scholar]
- 9.Siemers BM, Schaub A. 2011. Hunting at the highway: traffic noise reduces foraging efficiency in acoustic predators. Proc. R. Soc. B 278, 1646–1652 10.1098/rspb.2010.2262 (doi:10.1098/rspb.2010.2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber JR, Crooks KR, Fristrup KM. 2010. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 25, 180–189 10.1016/j.tree.2009.08.002 (doi:10.1016/j.tree.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 11.Longcore T, Rich C. 2006. Ecological consequences of artificial night lighting. Washington, DC: Island Press [Google Scholar]
- 12.Bradshaw WE, Holzapfel CM. 2007. Evolution of animal photoperiodism. Annu. Rev. Ecol. Syst. 38, 1–25 10.1146/annurev.ecolsys.37.091305.110115 (doi:10.1146/annurev.ecolsys.37.091305.110115) [DOI] [Google Scholar]
- 13.Lyon BE, Chaine AS, Winkler DW. 2008. Ecology: a matter of timing. Science 321, 1051–1052 10.1126/science.1159822 (doi:10.1126/science.1159822) [DOI] [PubMed] [Google Scholar]
- 14.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Methuen & Co. Ltd [Google Scholar]
- 15.Daan S, Dijkstra C, Drent R, Meijer T. 1989. Food supply and the annual timing of avian reproduction. Acta Congr. Int. Ornithol. 19, 392–407 [Google Scholar]
- 16.Farner DS, King JR, Parks KC. 1975. Avian biology, vol. 5 New York, NY: Academic Press [Google Scholar]
- 17.Dawson A, King VM, Bentley GE, Ball GF. 2001. Photoperiodic control of seasonality in birds. J. Biol. Rhythms 16, 365–380 10.1177/074873001129002079 (doi:10.1177/074873001129002079) [DOI] [PubMed] [Google Scholar]
- 18.Partecke J, Van't Hof TJ, Gwinner E. 2005. Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J. Avian Biol. 36, 295–305 10.1111/j.0908-8857.2005.03344.x (doi:10.1111/j.0908-8857.2005.03344.x) [DOI] [Google Scholar]
- 19.Schoech SJ, Bowman R. 2003. Does differential access to protein influence differences in timing of breeding of Florida scrub-jays (Aphelocoma coerulescens) in suburban and wildland habitats? The Auk 120, 1114–1127 [Google Scholar]
- 20.Rowan W. 1938. London starlings and seasonal reproduction in birds. Proc. Zool. Soc. Lond. A 108, 51–78 10.1111/j.1469-7998.1938.tb00021.x (doi:10.1111/j.1469-7998.1938.tb00021.x) [DOI] [Google Scholar]
- 21.Shoup GP. 1918. Artificial lighting of poultry houses in western Washington. Poult. Sci. s2–4, 44–47 [Google Scholar]
- 22.Burger JW. 1949. A review of experimental investigations on seasonal reproduction in birds. Wilson Bull. 61, 211–230 [Google Scholar]
- 23.Bissonnette T. 1937. Photoperiodicity in birds. Wilson Bull. 49, 241–270 [Google Scholar]
- 24.Kaiser A. 1993. A new multi-category classification of subcutaneous fat deposits of songbirds. J. Field Ornithol. 64, 246–255 [Google Scholar]
- 25.Newton I. 1966. The moult of the bullfinch Pyrrhula pyrrhula. Ibis 108, 41–67 10.1111/j.1474-919X.1966.tb07251.x (doi:10.1111/j.1474-919X.1966.tb07251.x) [DOI] [Google Scholar]
- 26.Wingfield JC, Farner DS. 1976. Avian endocrinology: field investigations and methods. Condor 78, 570–573 10.2307/1367117 (doi:10.2307/1367117) [DOI] [Google Scholar]
- 27.R Development Core Team 2011. R: A language and environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 28.Wood SN. 2006. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall/CRC [Google Scholar]
- 29.Bates DM, Sarkar D. 2007. lme4: Linear mixed-effects models using S4 classes. R package version 0.99875–6. See http://cran.r-project.org/web/packages/Ime4/index.html [Google Scholar]
- 30.Baayen RH. 2007. Analyzing linguistic data: a practical introduction to statistics using R. Cambridge, UK: Cambridge University Press [Google Scholar]
- 31.Gelman A, Hill J. 2006. Data analysis using regression and multilevel/hierarchical models. Cambridge, UK: Cambridge University Press [Google Scholar]
- 32.Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739 10.1016/j.cub.2010.08.028 (doi:10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 33.Gustafsson L, Nordling D, Andersson MS, Sheldon BC, Qvarnström A. 1994. Infectious diseases, reproductive effort and the cost of reproduction in birds. Phil. Trans. R. Soc. Lond. B 346, 323–331 10.1098/rstb.1994.0149 (doi:10.1098/rstb.1994.0149) [DOI] [PubMed] [Google Scholar]
- 34.Martin LB, II, Scheuerlein A, Wikelski M. 2003. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. Lond. B 270, 153–158 10.1098/rspb.2002.2185 (doi:10.1098/rspb.2002.2185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18 664–18 669 10.1073/pnas.1008734107 (doi:10.1073/pnas.1008734107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey C. 1996. Avian energetics and nutritional ecology. New York, NY: Chapman & Hall [Google Scholar]
- 37.Martin TE. 1987. Food as a limit on breeding birds: a life-history perspective. Annu. Rev. Ecol. Syst. 18, 453–487 10.1146/annurev.es.18.110187.002321 (doi:10.1146/annurev.es.18.110187.002321) [DOI] [Google Scholar]
- 38.Wikelski M, Martin LB, II, Scheuerlein A, Robinson MT, Robinson ND, Helm B, Hau M, Gwinner E. 2008. Avian circannual clocks: adaptive significance and possible involvement of energy turnover in their proximate control. Phil. Trans. R. Soc. B 363, 411–423 10.1098/rstb.2007.2147 (doi:10.1098/rstb.2007.2147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greives TJ, Kingma SA, Beltrami G, Hau M. 2012. Melatonin delays clutch initiation in a wild songbird. Biol. Lett. 8, 330–332 10.1098/rsbl.2011.1100 (doi:10.1098/rsbl.2011.1100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. 2005. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Natl Acad. Sci. USA 102, 3052–3057 10.1073/pnas.0403840102 (doi:10.1073/pnas.0403840102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuire NL, Kangas K, Bentley GE. 2011. Effects of melatonin on peripheral reproductive function: regulation of testicular GnIH and testosterone. Endocrinology 152, 3461–3470 10.1210/en.2011-1053 (doi:10.1210/en.2011-1053) [DOI] [PubMed] [Google Scholar]
- 42.Evans JA, Elliott JA, Gorman MR. 2007. Circadian effects of light no brighter than moonlight. J. Biol. Rhythms 22, 356–367 10.1177/0748730407301988 (doi:10.1177/0748730407301988) [DOI] [PubMed] [Google Scholar]
- 43.Meyer WE, Millam JR. 1991. Plasma melatonin levels in Japanese quail exposed to dim light are determined by subjective interpretation of day and night, not light intensity. Gen. Comp. Endocrinol. 82, 377–385 10.1016/0016-6480(91)90313-U (doi:10.1016/0016-6480(91)90313-U) [DOI] [PubMed] [Google Scholar]
- 44.Davies WIL, Turton M, Peirson SN, Follett BK, Halford S, Garcia-Fernandez JM, Sharp PJ, Hankins MW, Foster RG. 2012. Vertebrate ancient opsin photopigment spectra and the avian photoperiodic response. Biol. Lett. 8, 291–294 10.1098/rsbl.2011.0864 (doi:10.1098/rsbl.2011.0864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menaker M, Roberts R, Elliott J, Underwood H. 1970. Extraretinal light perception in the sparrow. 3. The eyes do not participate in photoperiodic photoreception. Proc. Natl Acad. Sci. USA 67, 320–325 10.1073/pnas.67.1.320 (doi:10.1073/pnas.67.1.320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoech SJ, Mumme RL, Wingfield JC. 1996. Delayed breeding in the cooperatively breeding Florida scrub-jay (Aphelocoma coerulescens): inhibition or the absence of stimulation? Behav. Ecol. Sociobiol. 39, 77–90 10.1007/s002650050269 (doi:10.1007/s002650050269) [DOI] [Google Scholar]
- 47.Dufty AM, Wingfield JC. 1986. The influence of social cues on the reproductive endocrinology of male brown-headed cowbirds: field and laboratory studies. Horm. Behav. 20, 222–234 10.1016/0018-506X(86)90020-6 (doi:10.1016/0018-506X(86)90020-6) [DOI] [PubMed] [Google Scholar]
- 48.Nol E, Smith JNM. 1987. Effects of age and breeding experience on seasonal reproductive success in the song sparrow. J. Anim. Ecol. 56, 301–313 10.2307/4816 (doi:10.2307/4816) [DOI] [Google Scholar]
- 49.Forslund P, Pärt T. 1995. Age and reproduction in birds—hypotheses and tests. Trends Ecol. Evol. 10, 374–378 10.1016/S0169-5347(00)89141-7 (doi:10.1016/S0169-5347(00)89141-7) [DOI] [PubMed] [Google Scholar]
- 50.Miller MW. 2006. Apparent effects of light pollution on singing behavior of American robins. The Condor 108, 130–139 10.1650/0010-5422(2006)108[0130:AEOLPO]2.0.CO;2 (doi:10.1650/0010-5422(2006)108[0130:AEOLPO]2.0.CO;2) [DOI] [Google Scholar]
- 51.Nemeth E, Brumm H. 2009. Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Anim. Behav. 78, 637–641 10.1016/j.anbehav.2009.06.016 (doi:10.1016/j.anbehav.2009.06.016) [DOI] [Google Scholar]
- 52.Bedrosian TA, Fonken LK, Walton JC, Nelson RJ. 2011. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol. Lett. 7, 468–471 10.1098/rsbl.2010.1108 (doi:10.1098/rsbl.2010.1108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hölker F, Wolter C, Perkin EK, Tockner K. 2010. Light pollution as a biodiversity threat. Trends Ecol. Evol. 25, 681–682 10.1016/j.tree.2010.09.007 (doi:10.1016/j.tree.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 54.Navara KJ, Nelson RJ. 2007. The dark side of light at night: physiological, epidemiological, and ecological consequences. J. Pineal Res. 43, 215–224 10.1111/j.1600-079X.2007.00473.x (doi:10.1111/j.1600-079X.2007.00473.x) [DOI] [PubMed] [Google Scholar]
- 55.Gallaway T, Ohms VR, Mitchell DM. 2010. The economics of global light pollution. Ecol. Econ. 69, 658–665 10.1016/j.ecolecon.2009.10.003 (doi:10.1016/j.ecolecon.2009.10.003) [DOI] [Google Scholar]


